Background:
Breast-conserving surgery followed by radiotherapy represents the standard of care for early-stage breast cancer. The aim of this article was to provide a review of the literature about the use of the lateral thoracic artery perforator (LTAP) flap, the lateral thoracodorsal (LTD) flap, and the lateral intercostal artery perforator (LICAP) flap in lateral partial breast defect.
Methods:
A literature search was performed via PubMed, Medline, and Cochrane. Patient’s characteristics, topography and size of breast defect, flap size and design, number of perforators, and operative time were analyzed. Aesthetic and patient-reported outcomes, postoperative complications, and donor site morbidity were also registered.
Results:
Thirteen articles fulfilled inclusion criteria, and 432 patients were included. Different flap designs and flap combinations were described. Satisfactory outcomes were reported for between 78% and 100% of cases. Patient satisfaction ranged from 75.8% to 92.5% of cases. The overall complication rate was 9.25%, and donor site morbidity was very low (3.7%).
Conclusions:
A distinct advantage of LTAP, LTD, and LICAP flap reconstruction is that the thoracodorsal pedicle is not sacrificed, not compromising eventual delayed breast reconstruction with TDAP or latissimus dorsi flaps. This staged approach to partial breast reconstruction is especially useful in cases where the oncological margins are uncertain and wider resections (or mastectomies) are secondly required.
INTRODUCTION
Breast-conserving surgery (BCS) followed by radiotherapy represents the standard of care for early-stage breast cancer.1 Oncoplastic breast surgery (OBS), combining the principles of surgical oncology with the aesthetically-derived breast-reduction techniques,2–4 leads to better aesthetic results and quality of life when compared with conservative breast surgery alone.5–13 OBS makes use of either of 2 different approaches: volume displacement or volume replacement.7 Volume displacement approach consists of redistributing the remaining mammary parenchyma, reshaping the breast6 through dermo-glandular flaps, with or without contralateral breast symmetrization procedures. Volume replacement approach consists of filling the breast defect using local flaps where volume is missing, generally without the need to symmetrization of the contralateral breast.7,12,13
Volume replacement techniques have progressed over time, expanding the oncoplastic reconstructive arsenal from the use of a pedicled latissimus dorsi (LD) musculocutaneous flap to the use of several fasciocutaneous flaps available in the thoracic region.14 Although the LD flap has advantages such as reliable vascularity, easy dissection, and high volume availability, it leads to a non-neglectable donor site morbidity, including seroma and limitation of shoulder movements.15,16 Fasciocutaneous flaps, without violating any muscle, result in a significantly minor morbidity at the donor site. These can be based on the same pedicle of the LD flap (thoracodorsal artery perforator flap, which is based on the thoracodorsal artery) or on a different vascular pedicle, such as the intercostal artery or the lateral thoracic artery.14,17 Lateral breast defects can be reconstructed using the lateral thoracic artery perforator (LTAP) flap, the lateral thoracodorsal (LTD) flap, the lateral intercostal artery perforator (LICAP) flap, or the thoracodorsal perforator (TDAP) flap. The latter, being based on the thoracodorsal artery, excludes the possibility of harvesting an LD flap. The LTAP flap is based on a direct cutaneous branch of the lateral thoracic pedicle that generally arises directly from the axillary vessels, and variably from the thoracodorsal vessels (Fig. 1). The LTD flap is a wedge-shaped fasciocutaneous transposition flap, located in the lateral aspect of the thorax. It is based on the lateral intercostal artery and it is harvested along with the muscular fascia without microsurgical perforator dissection. The LICAP flap has the same blood supply but it is harvested via a microsurgical perforator dissection and it should not include the underlying muscular fascia.18 These 3 flaps (LTAP, LTD, and LICAP) have the advantage of preserving the thoracodorsal pedicle without sacrificing the skin paddle of the LD musculocutaneous flap in a scenario of unknown oncological margins. The aim of this article was to provide a review of the literature regarding the use of the LTAP flap, the LTD flap, and the LICAP flap for treating lateral partial breast defects.
Fig. 1.
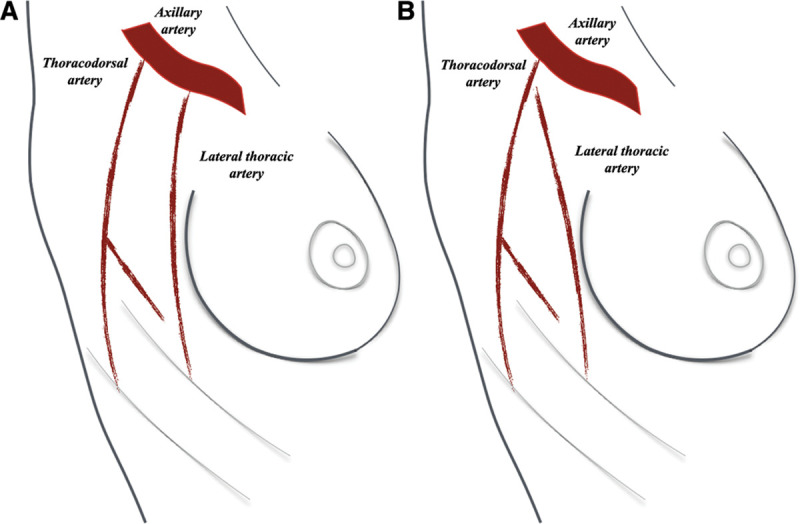
Lateral thoracic artery generally arises directly from the axillary vessels (A) and variably from the thoracodorsal vessels (B).
PATIENTS AND METHODS
A literature research was performed via PubMed, Medline, and Cochrane databases according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis19 (PRISMA) guidelines, with the aim of providing a comprehensive review of the literature about the use of the LTAP flap, the LTD flap, and the LICAP flap in BCS.
The following MeSH terms were used: “lateral thoracic artery perforator flap,” “lateral thoracodorsal flap,” “lateral intercostal artery perforator flap,” “oncoplastic breast local flap,” and “chest wall perforator flap” (period: 2004–2020; last search done on May 18, 2020). Two reviewers performed double screening and data extraction. Abstracts were screened to identify eligible articles. Reference lists of relevant articles were analyzed for supplementary studies.
Inclusion and Exclusion Criteria
The selection of the studies was based on the following inclusion criteria:
studies reporting the use of the LTAP flap, LTD flap, and/or LICAP flap after BCS;
registration of outcomes after surgical treatment;
full text available in English.
The studies were excluded due to any one of the following criteria:
studies reporting the use of the LTAP flap, LTD flap, and/or LICAP flap for total breast reconstruction;
studies reporting the use of the LTAP flap, LTD flap, and/or LICAP flap, combined with implant positioning;
review articles;
case report;
non-referenced articles;
expert opinion or comment (Level V).
Data Collection
Extracted data included type of study, number of patients included, mean age, time of reconstruction, topography of breast defect, specimen weight, preoperative assessment (Doppler mapping), flap design, patient positioning, type of dissection strategy, flap size, number of perforators included in the flap, operative time, mean follow-up time, aesthetic results, patient satisfaction, postoperative complications, and donor site morbidity.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software (version 24.0; IBM Corporation, Somers, N.Y.).
RESULTS
A total of 594 citations from PubMed, Medline, and Cochrane Library were initially identified. After a title and abstract review (analyzed by 2 different reviewers), 39 records were considered relevant. Full-text examination excluded a further 26 articles. Only 13 articles20–32 of the initial research, published between 2006 and 2020, fulfilled inclusion criteria and were included in the systematic review (Fig. 2, PRISMA Guidelines). From the 13 selected studies, 9 were retrospective studies and 3 were prospective studies. In Total, 4 studies described partial breast reconstruction using LTAP flap25,26,29,32 (combined with LICAP flap in some patients in 3 studies25,26,32), 3 studies described partial breast reconstruction using LTD flap,20,23,24 and 6 studies described partial breast reconstruction using LICAP flap.21,22,27,28,30,31 A total of 432 patients were included, and the sample size of each study ranged from 8 to 87 patients. From the 432 included patients, 176 patients underwent an LTAP flap reconstruction (a combined LTAP + LICAP flap reconstruction in 103 cases), 76 patients underwent an LTD flap reconstruction, and 180 patients underwent an LICAP flap reconstruction. The mean age of patients was 43.74. In 10 studies, the local flap was harvested at the time of tumor resection in all cases, but in 3 studies, the oncoplastic procedure was immediate or delayed to a second operation depending on cases. Roy26 performed a one-stage or a two-stage reconstruction depending on the tumor/breast ratio. Patients with a high tumor/breast ratio (bordering on to recommendation for mastectomy) underwent a delayed partial breast reconstruction using a combined LTAP and LICAP flap.
Fig. 2.
PRISMA guidelines flowchart.
Breast Defect
All the studies reported that the defect was located at lateral portion of the breast. Overall, the most common tumor location was the upper outer quadrant (66.66% of cases) followed by the lower outer quadrant (22.22% of cases) and the central aspect of the breast (8.88% of cases). In addition, 3 cases of lateral columnar shape mastectomy (defect on lower and upper outer quadrants) and 1 case of inner and outer upper quadrants excision were described. In these cases, the breast reconstruction was performed with a LICAP flap combined with an LTAP flap.
The mean weight of the resected breast tissue was 100.97 g (range 40–550 g). In detail, the mean weight of the resected breast tissue was 67.2 g, 94.08 g, and 180.37 g in patients undergone LTAP, LTD, and LICAP flap reconstruction, respectively.
Preoperative Planning and Flap Design
LTAP Flap25,26,29,32
Using a Doppler probe, preoperative identification of perforator vessels was performed in all cases, evaluating the area between the anterior border of the LD muscle and the lateral border of the breasts, from the third to the seventh intercostal spaces. With respect to design, the lateral breast crease was used as superior and anterior border of the flap. Flap height varied from 4.4 to 10 cm, and flap length varied from 16 to >30 cm. Flap thickness and flap volume were reported by only 1 study, and ranged from 1.6 to 3.5 cm and from 112.6 to 588 cm3 (mean 309.5 cm3), respectively.
LTAP–LICAP Flaps (Table 1)25,26,32
Table 1.
Summary
| Study | Type | Flap | Sample | Age (y) | Rec Time | Defect | Specimen Weight |
|---|---|---|---|---|---|---|---|
| Munhoz et al20 | R | LTD | 34 | — | Immediate | –20 UOQ–10 Transition of the UOQ and LOQ–4 LOQ | 310 g(215–550)40%–60% of total breast volume |
| Hamdi et al21 | R | LICAP | 8 | — | Immediate | Lateral breast region | — |
| Munhoz et al22 | R | LICAP | 11 | 47.3 | Immediate | –8 LOQ | 164.7 |
| –3 UOQ | |||||||
| Yang et al23 | R | LTD | 20 | 48.5 (39–60) | Immediate | –15 UOQ | 76.8 g |
| –2 LOQ | (40–150 g) | ||||||
| –3 Central | |||||||
| Lee et al24 | R | LTD | 22 | 45.7 (23–65) | Immediate | –38 UOQ | 74.2 g |
| LICAP | 25 | –7 LOQ | 148.4 g | ||||
| (50–408 g) | |||||||
| McCulley et al25 | R | LTAP | 31 | — | Immediate | UOQ, LOQ, central | — |
| LTAP + LICAP | 12 LTAP | Delayed | |||||
| 19 LTAP + LICAP | |||||||
| Roy26 | R | LTAPLICAP | 40 | 49 (42–69) | Immediate →29Delayed (high tumor breast ratio) →11 | — | 96 g |
| (35–193) | |||||||
| >20% | |||||||
| Kim et al27 | P | LICAP | 19 | 47.21 | Immediate | LOQ | 71.18 |
| Martellani et al28 | R | LTAP 1 | 15 | 54 (43–64) | Immediate | –9 UOQ | 53.7 g (29–140) |
| LICAP 14 | –3 LOQ | ||||||
| –2 OOQQ | |||||||
| –1 UUQQ | |||||||
| Hong et al29 | P | LTAP | 58 | 42.9 (35–49) | Immediate | –24 UOQ | 73.8 g |
| 33 | –9 LOQ | (50–100) | |||||
| 25 BCS only | |||||||
| Meybodi et al30 | P | LICAP | 22 | 58 | Immediate 20 | –11 UOQ | 86 g |
| (40–74) | Delayed 2 | –7 LOQ | |||||
| –3 Lateral (3 or 9 o’clock) | |||||||
| –1 OOQQ | |||||||
| Kim et al31 | R | LICAP | 40 | 46.68 | Immediate | UOQ, LOQ | 71.18 g |
| Soumian et al32 | P | LTAP | 3 | 54 | Immediate | UOQ, LOQ, central | 62.5 g |
| (21–231) | |||||||
| LTAP + LICAP | 84 |
LIQ, lower inner quadrant; LOQ, lower outer quadrant; OOQQ, outer quadrants; P, prospective; Pr, prospective randomized; R, retrospective; UIQ, upper inner quadrant; UOQ, upper outer quadrant; UUQQ, upper quadrants.
In studies that described combined reconstructions with LICAP and LTAP flaps, the inframammary sulcus, the lateral breast crease, and the posterior axillary fold were marked. The flaps were marked as turnover flaps in all cases, except for 1 case, for which a propeller flap was designed. However, the authors described that LICAP/LTAP flaps could also be used as propeller flaps, with division of the LTAP pedicle proximally but with preservation of the pedicle distally.
LTD Flap20,23,24
One study described preoperative Doppler tracing. The flap axis was placed along the lateral and the dorsal extensions of the inframammary fold, and the flap base was located on a line extended from the anterior axillary line. This flap design allowed hiding the donor site scar under the bra line. The base of the flap varied between 5 and 7 cm, and it was determined by the pinch test, which is useful to estimate the available volume of the lateral chest wall. The length of the flap was reported by 2 studies, and it ranged from 7 to 20 cm.
A wedge-shaped flap was described in all cases. One study reported that for small breast defects, the flap was planned as a triangle placed exclusively on the lateral part of the thorax, whereas for moderate or large defects, the distal portion of the flap reached the posterior thoracic region. Moreover, in the case of large breast defects, the superior and inferior limits of the flap were marked obliquely to create a convex flap design that allows harvesting a large amount of skin and subcutaneous fat and that narrows the flap base to avoid tension to the lateral region of the breast (Fig. 3). The flap was either advanced into the breast defect or rotated by 90 degrees.
Fig. 3.
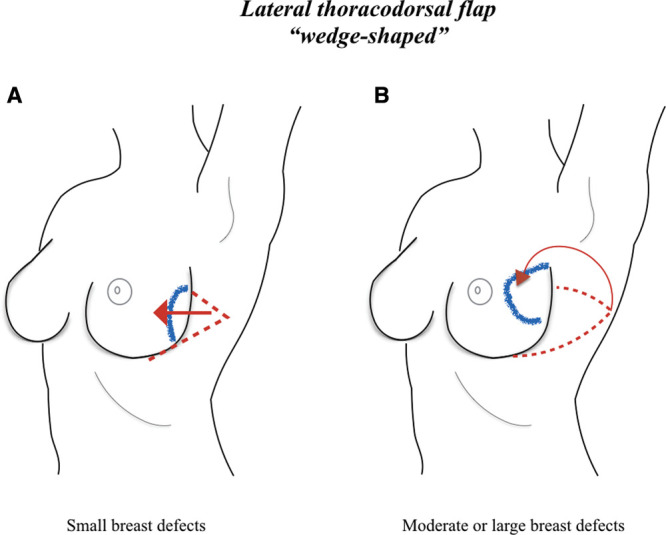
LTD flap design. The flap can be planned as a triangle placed on the lateral part of the thorax in the case of small breast defects; in these cases, the flap can be advanced into the breast defect (A). In the case of moderate or large defects, the distal portion of the flap can reach the posterior thoracic region, and the border of the flap can be marked obliquely to create a convex flap design, which allows harvesting a large amount of skin and subcutaneous fat, and narrows the flap base to avoid tension to the lateral region of the breast. In these cases, the flap can be rotated by 90 degrees into the breast defect (B).
LICAP Flap21,22,27,28,30,31
Preoperative identification of perforator vessels using a Doppler probe was described in all cases. Hamdi21 and Roy26 suggested performing Doppler evaluation, with the patient lying down simulating the intraoperative position. In addition, 2 studies27,31 described that all patients underwent a 3-dimensional chest computed tomography angiography preoperatively to identify the dominant perforator vessel by its relationship with the LD muscle. Based on this location, a Doppler mapping was used to recheck the perforator, and the skin was marked for use during the design phase. The donor site incisions were generally designed horizontally along the skin tension lines, with the posterior tip curving up parallel to the underlying ribs. The inferior incision was planned to position the resultant scar along the inframammary fold, by most authors. Flap width was defined by pinching redundant roll of fat on the lateral chest wall and was adapted to the expected breast defect.
Meybodi et al proposed a modified LICAP flap, which is designed with 2 “lazy S” starting from the inframammary fold line (Fig. 4). Moreover, 1 author31 described that for cases requiring eventual additional volume replacement because of oncologic problems, other flaps (such as the thoracodorsal artery perforator flap) were preoperatively designed.
Fig. 4.
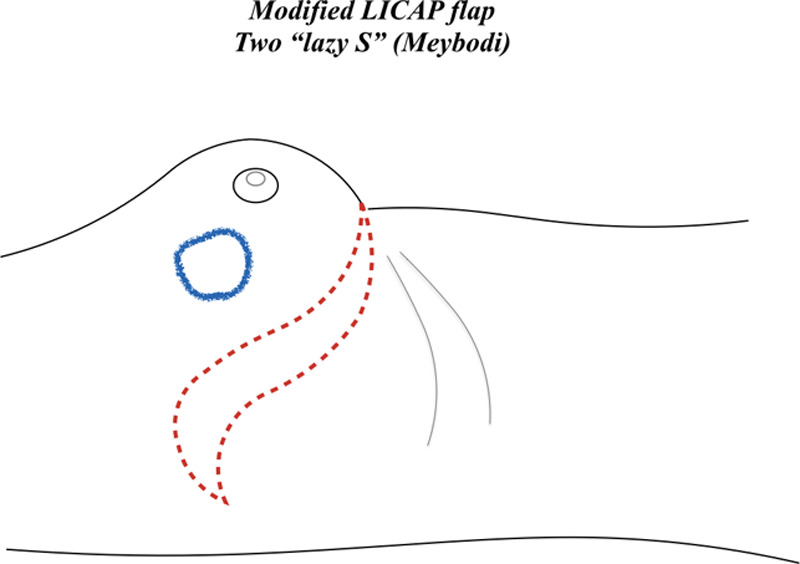
Modified LICAP flap (Meybodi technique), designed with 2 “lazy S” starting from the inframammary fold line.
Harvesting Technique
All patients underwent surgery in a supine position, with the ipsilateral arm abducted for axillary procedure. Some authors described the possibility of placing a small sand bag or an inflatable device (inflated at the end of the oncologic procedure) beneath the ipsilateral paraspinal area to achieve a tilt of patient, which allowed a wide donor-site-area exposure for a comfortable harvesting of the flap and avoided intraoperative position change (Table 2).28–32
Table 2.
Preoperative Assessment, Flap Design and Size, Dissection Techniques, and Operative Times
| Study | Preoperative Assessment | Flap Design | Dissection | Flap Size | Operative Time (minutes) |
|---|---|---|---|---|---|
| Munhoz et al20 | Pinch test | Wedge-shaped | Fascia of the anterior serratus and LD muscles are included in the flap. | Length → 8–20 cm | 77’ (42–100’) |
| –Flap axis in the lateral extensions of the IMF | |||||
| –Flap base on a line extended from the anterior axillary line. | Avoid a wide undermining in the inframammary sulcus. | ||||
| For small defects→a triangle located exclusively on the lateral aspect of the thorax. | Lateral to medial dissection. | ||||
| For moderate– large defects→distal limit can reach the posterior thoracic region, and the inferior and superior limits are designed more obliquely with curved borders. | |||||
| Hamdi et al21 | Doppler mapping | Wedge-shaped | Above the fascia/lateral to medial dissection. | 18 × 8 cm | 45’ |
| After visualization of the anterior border of the LD muscle, the smaller posterior branch of the lateral cutaneous branch is identified and followed to find the bigger anterior branch. | |||||
| Pedicle’s length of 3–5 cm. | |||||
| Munhoz et al22 | — | — | Subfascial dissection. | 13 × 6.5 cm | — |
| Advancement or rotation. | |||||
| Yang et al23 | –Pinch test | Wedge-shaped | Fascia of the anterior serratus and LD muscles are included in the flap. | Flap base→ 5–7 cm | |
| –Doppler mapping | –Flap axis in the lateral extensions of the IMF | ||||
| –Flap base on a line extended from the anterior axillary line. | |||||
| Depending on the shape of the breast defect, a V-Y advancement flap implantation was performed as an adjunct to the existing LTD flap. | Flap length → 7–15 cm | ||||
| Lee et al24 | Pinch test | Wedge-shaped | Fascia of the anterior serratus and LD muscles are included in the flap. | — | — |
| Flap axis →lateral extensions of the IMF | |||||
| McCulley et al25 | Doppler mapping | Lateral breast crease → anterior and superior aspect of the flap marking. The remaining flap is then drawn with account of perforator position, size of flap required, and available skin laxity. | Medial to lateral dissection | Flap height → 8–10 cm | |
| Lateral breast crease incision (breast/axillary and identification of perforators). | |||||
| Flap length → up to 30 cm | |||||
| The caudal and cephalic aspects of the flap can also be mobilized to visualize perforators, but care needs to be taken at the cephalic border to avoid damaging the lateral thoracic pedicle. | |||||
| All combined LICAP/LTAP flaps were designed as turnover flaps, except for one that was better designed as a propeller flap. | |||||
| Once the perforator and pedicle is isolated, then the remaining flap can be raised from lateral to medial. | |||||
| The pure LTAP flaps were used as transposition flaps. | |||||
| Roy26 | –Pinch test | Flap oriented parallel to the skin tension lines with the tip curving up posteriorly parallel to the underlying ribs and following the angiosome. | — | — | — |
| –Doppler mapping | |||||
| Kim et al27 | –3D chest computed tomography angiography | The donor incision was made either horizontally or vertically, in consultation with the patient to ensure that the scar was not visible. | Skin paddle→ propeller flap method. | 4 × 10 to 6 × 17 | 249.3’ (breast and plastic surgery) |
| No skin paddle→ perforator was dissected with the fascial layer and the flap was positioned by using a turnover method. | |||||
| –Doppler mapping | The incision was planned to position the resultant scar along the inframammary fold. | ||||
| Martellani et al28 | –Pinch test | — | All flaps were harvested on several perforators. The perforator that offered the best swing of the flap with minimal rotation of the vessel was finally selected. | — | 63 (50–125) |
| Hong et al29 | Doppler mapping | Round-shaped flap | Longitudinal incision along the anterior axillary line | Mean volume: 309.5 cm3 Length: 16–21 cm | 72’ |
| Subfascial dissection | |||||
| Flap rotation | |||||
| Width: 4.4–8 cm | |||||
| Meybodi et al30 | Doppler mapping | Modified LICAP: | The area between the 2 lazy S lines was de-epithelialized | ||
| Two lazy S lines | |||||
| Breast excision was performed from the anterior border of the flap and axillary surgery was performed from the superior border of the flap | |||||
| The perforators were preserved in a mesentery of tissue around which the flap was either flipped or rotated | |||||
| Kim et al31 | –3D chest computed tomography angiography–Doppler mapping | Flap design began from the IMF line, which is most often located between the 6th and 7th intercostal spaces.In preparation for cases requiring additional tissue excision because of oncologic problems, other flaps techniques (TDAP, muscle-sparing LD) were also designed. | Subfascial dissectionHorizontal incision to the bra lineSkin paddle→ Propeller (29 patients) (clockwise or counterclockwise rotation)No skin paddle→ Turnover (11 patients) | — | 249.3’ |
| Soumian et al32 | –Pinch test | LICAP/LTAP flaps→IMF and posterior axillary fold are marked. | LICAP/LTAP flaps could also be used as propeller flaps (skin paddle). | Flap length: up to 30 cm | — |
| –Doppler mapping | |||||
| No magnification was required during the surgery. |
IMF, inframammary fold; TD, thoracodorsal.
LTAP Flap25,26,29,32
A lateral breast crease incision was used by most authors to perform breast tumor excision, axillary surgery, and identification of perforators. Once the LTAP and the lateral thoracic pedicle were dissected, the remaining flap was raised from lateral to medial direction. The pedicle was completely or partially dissected until enough length was achieved to allow insetting of the flap in the breast defect without tension. De-epithelialization of the skin paddle was performed before placing the flap in the breast defect whenever necessary. The flap was either advanced into the breast defect or rotated by 90 degrees depending on the defect, and was fixed to the remaining breast tissue.
LTAP-LICAP Flaps25,26,32
Axillary surgery and harvesting of flaps were performed through the same incision whenever feasible. If, upon exploration, the dominant perforator vessel was an LTAP perforator, then it was isolated, and the perforator and pedicle were dissected free as far proximal as required to allow flap mobility without tension. If, upon exploration, the dominant perforator vessel was found to be a lateral intercostal artery perforator, a LICAP flap was harvested; if a mixture of small LICAP and LTAP perforators were identified, a combination of LTAP and LICAP flaps were raised. In this last case, perforators needed to be in a similar vertical axis, allowing a “mesentery” of lateral chest wall perforators to be created and moved as a turnover flap. McCulley25 stated that caudal and cephalic aspects of the flap can be mobilized to identify perforator vessels but care needs to be taken at the cephalic border of the flap to avoid damaging the lateral thoracic pedicle. Once the perforator and pedicle are isolated, then the remaining flap can be raised in a lateral to medial direction. Soumian32 reported that LICAP perforators were usually found between the 4th and the 6th intercostal spaces, while the LTAP vessels can be traced vertically along the mid-axillary line for about 2–3 cm, arising between the 3rd and the 5th intercostal spaces in most cases (Fig. 5).
Fig. 5.
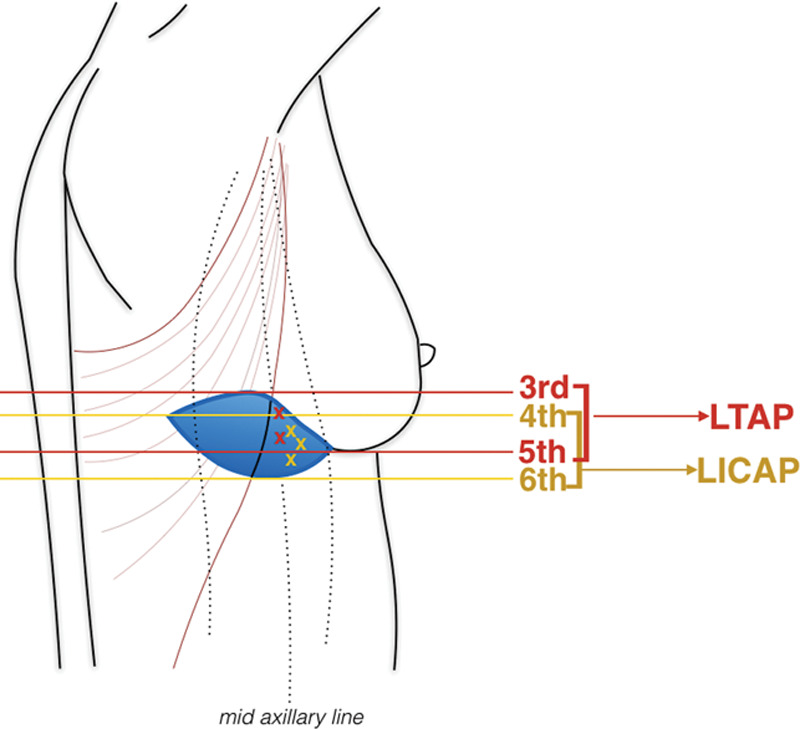
LTAP and LICAP perforators are located in an area between the anterior border of the latissimus dorsi muscle and the lateral border of the breasts from the third to the seventh intercostal spaces (LTAP), and from the fourth to the sixth intercostal spaces (LICAP).
LTD Flap20,23,24
Most authors began the dissection from the lateral border of the flap, progressing in a lateral-to-medial direction for performing a subfascial dissection. The vascular supply of the LTD flap is derived from the lateral intercostal perforators, from the muscular fascia, and from the lateral perforators of the intercostal arteries. For this reason, all authors stressed the concept that this flap should include the fascia of the serratus and LD muscles to provide a reliable vascular supply. Moreover, care must be taken to avoid a wide undermining in the inframammary sulcus. One study reported that, depending on the shape of the breast defect, a V-Y advancement flap was performed in some cases in addition to the LTD flap procedure.
LICAP Flap21,22,27,28,30,31
Margin incision was carried down to the underlying anterior serratus and LD muscles, performing a suprafascial or a subfascial dissection. Hamdi21 reported that pedicle’s length of 3–5 cm is generally adequate to reach a defect over the lateral or superior part of the breast, and that if a longer pedicle is required, the dissection should be carried on within the costal groove. In addition, Hamdi21 underlined that the lateral cutaneous nerve can eventually be stripped from the intercostal nerve to harvest a sensate LICAP flap. The flaps were partially or completely de-epithelialized depending on the defect and then passed into the breast and folded to fill the defect.
Three studies described either turnover or propeller flaps. Kim31 reported 29 cases of “propeller LICAP flap” and 11 cases of “turnover LICAP flap” (Fig. 6). In detail, propeller flaps were harvested when a skin paddle was needed, performing a clockwise or counterclockwise rotation. Turnover flaps were harvested in patients undergone tumor excision without skin inclusion. The flaps were positioned by folding and then were tunneled toward the defect area. Despite 3 studies reporting that, with a proper dissection, it was possible to achieve an adequate perforator length to rotate the LICAP flap as a propeller without causing torsion of the perforator, the authors agreed that turnover flap allowed achieving a more stable circulation.
Fig. 6.
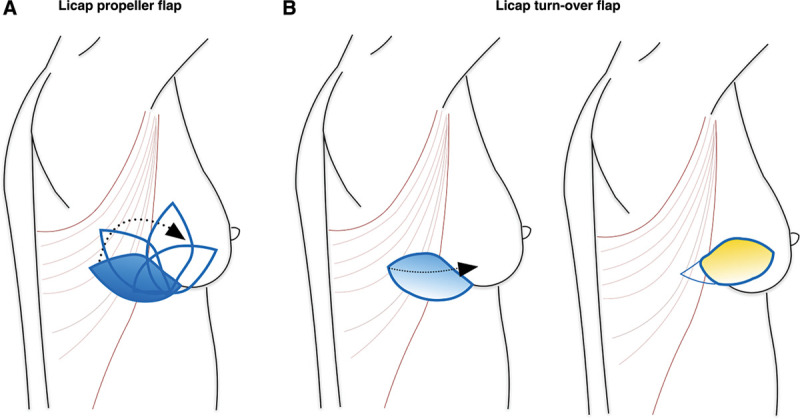
LICAP propeller and turnover flaps. A, Propeller flaps were harvested when a skin paddle was needed, performing a clockwise or counterclockwise rotation. B, Turnover flaps were harvested in patients undergoing tumor excision without skin inclusion.
In the “modified LICAP flap technique” described by Meybodi et al,30 the tumor excision was performed from the anterior border of the flap and the axillary surgery from the superior border of the flap. The area between the 2 “lazy S” lines was de-epithelialized when needed. The perforator vessels were preserved in a mesentery of tissue, around which the flap was either flipped or rotated. The average operative time was reported by only 4 studies, which was 72 minutes for the LTAP flap, 77 minutes (range 42–100 minutes) for the LTD flap, and 156.15 minutes (range 50–249 minutes) for the LICAP flap.
Cosmetic Results
The mean follow-up was 19.48 months. Cosmetic results were evaluated by 11 of 13 studies. Aesthetic outcomes were evaluated using the 5-Point-Likert scale by 3 blinded plastic surgeons, considering the overall aesthetic score in 3 studies (2 studies on LTD flap and 1 study on LTAP flap) (Table 3). One study reported a mean score of 4.13, and the other 2 studies reported a mean score of 4.08. In a study that described 20 cases of LTD flap, the Modified Michigan Breast Reconstruction Outcome Survey (score: 1–5) was adopted, evaluating breast shape (mean score: 4.15) and breast symmetry (mean score: 4.01) separately. Another study about LTD flap considered the mean of the individual scores on nipple–areola complex, breast shape, and symmetry, given by a panel of 3 individuals (patient, surgeon, and independent observer). In this study, the score ranged from 1/4 (poor) to 4/4 (very good); the score for the NAC was good or very good in 90.1% of cases; the score for the breast shape was good or very good in 93%; the score for the breast symmetry was good or very good in 81.2%. All 3 patients with satisfactory results (2/4) and 1 patient with poor result (1/4) showed susbsequent flap complications with unaesthetic scars and marked fibrosis. Moreover, 1 patient presented with breast asymmetry, in which the breast subjected to radiation therapy was more retracted than the contralateral one. Yang23 described the use of several types of local flaps in partial breast defects, and reported comparable results in terms of aesthetic satisfaction, as LD flap and TDAP flap showed higher scores than did the LTD flap.
Table 3.
Outcomes
| Study | Follow-up | Aesthetic Outcomes | Patient Satisfaction | Complications | Donor Site Morbidity |
|---|---|---|---|---|---|
| Munhoz et al20 | 23 (6–71) | NAC→ good or very good in 90.1%, satisfactory in 7.8 % | 88.2→% very satisfied or satisfied with their result. | Total: 5 | Total: 8 |
| Breast shape→ good or very good in 93% satisfactory in 3.8% | 11.8→disappointed and none regretted the surgery | 3 partial flap necrosis | 5 seroma | ||
| Breast symmetry→ good or very good in 81.2%, satisfactory in 14.7%, and poor in 1.9%. | 2 fat necrosis | 3 wound dehiscence | |||
| Overall→ good or very good 88.2%, satisfactory 8.8% and poor in 2.9%. | 1 infection | 1 infection | |||
| Hamdi et al21 | — | — | — | — | None |
| Munhoz et al22 | 32 | All patients achieved a satisfactory breast shape, volume, and symmetry | 92.3% very satisfied or satisfied | Total: 3 | 2 wound dehiscence |
| 1 fat necrosis | |||||
| Yang et al23 | 8 (2–28) | Modified Michigan Breast Reconstruction Outcome Survey (1–5) | Modified Michigan Breast Reconstruction Outcome Survey (1–5) | Total 4: | 1 wound dehiscence |
| Surgeons’ assessment: | Overall satisfaction →79% (score 4–5) | 1 partial flap necrosis | |||
| Overall aesthetic score →4.08 | Aesthetic satisfaction → 69% (score 4–5) | 2 fat necrosis | |||
| Breast shape→4.15 | |||||
| Breast symmetry→ 4.01 | |||||
| Lee et al24 | 11.3 (4–23) | Three blinded plastic surgeons 5-point Likert scale ranging | KNUH breast reconstruction satisfaction questionnaire | Total 3: | None |
| Overall aesthetic score →4.13 | (5-point Likert scale) | 3 fat necrosis (LICAP) | |||
| Satisfactory (mean scores >4) →81.7% LTD →76.2% LICAP | |||||
| McCulley et al25 | None of the patients have required revisional procedures to the reconstructed breast | 1 venous compromise settled spontaneously with complete flap survival | None | ||
| Roy26 | 27 (12–49) | Harris Scale | Body Image scale | 2 hematomas | None |
| Good to excellent in 82% patients | 80% →high satisfaction (<20). | 1 superficial skin | |||
| Any symmetrization | necrosis | ||||
| 2 fat necrosis | |||||
| Kim et al27 | 25.2 | 42% → excellent | Kyungpook National University Hospital modification of the Breast-Q | Total: 7 | 2 wound dehiscence |
| 36% → good | 42% → excellent | 4 partial linear necrosis | |||
| 36% → good | 3 fat necrosis | ||||
| Martellani et al28 | — | 1 symmetrizationIn all cases a good aesthetic result, with good symmetry, was achieved. | — | Total: 1 | Total: 7 |
| 1 hematoma | 3 seromas | ||||
| 4 wound dehiscences | |||||
| Hong et al29 | 21 (13–27) | Three blinded plastic surgeons 5-point Likert scale ranging | Questionnaire Michigan Breast Reconstruction Outcomes Survey (5-point Likert scale): | Total: 4 | None |
| Overall aesthetic score → 4.08 | –General satisfaction→ 81.8% | 2 partial adipose liquefaction | |||
| –Aesthetic satisfaction→75.8% | 2 wound infection | ||||
| Meybodi et al30 | — | No patients had a scar that extended posterior to the posterior axillary line | — | Total: 2 | None |
| 1 axillary seroma | |||||
| 1 infection | |||||
| Kim et al31 | 25.6 | 21 excellent | Kyungpook National University Hospital modification of the Breast-Q | Total: 9 | None |
| 16 good | 21 excellent | 4 linear necrosis (3 propeller/1 turnover) | |||
| No statically significant difference between Propeller and Turnover | 16 good | 3 fat necrosis (2 propeller /1 turnover) | |||
| 2 venous congestion (propeller) | |||||
| Soumian et al32 | 15 | None of the patients needed symmetrization procedure on the contralateral side | — | Total: 8 4 hematoma (2 axillary, 2 breast) |
Aesthetic outcomes following LICAP flap were evaluated subjectively by surgeons as good to excellent (considering symmetry, shape and volume) in 100%, 78%, 100%, and 92.5% of cases in 4 studies.27,28,31,32 In addition, Roy25 reported that 82% of patients achieved good or excellent aesthetic outcomes, using the Harris Scale. Kim31 compared aesthetic results in patients undergone “LICAP propeller” versus “LICAP turnover” flap and reported no significant statistical difference. Lastly, 6 studies reported that none of the patients have required revision procedures to the reconstructed breast or symmetrization procedures on the contralateral side.
Patient Satisfaction
Only 1 study29 reported data on patient satisfaction with LTAP reconstruction, describing that 81.8% of patients were overall satisfied and 75.8% were satisfied with the aesthetic results, using the “Michigan Breast Reconstruction Outcomes Survey.”
In the case of LTD flap reconstruction, 3 studies20,23,24 reported an “overall patient satisfaction” in 79%, 81.7%, and 88.2% of cases using the “Modified Michigan Breast Reconstruction Outcome Survey” or the “KNUH breast reconstruction satisfaction questionnaire.” One study23 compared different types of local flaps in immediate reconstruction of partial breast defects, reporting a relatively higher score in patients undergone LTD flap (88.2%) reconstruction with respect to other local flaps (thoracoepigastric flap: 75.8%, intercostal artery perforator flap: 76.2%, thoracodorsal artery perforator flap: 81.3%).
With respect to LICAP flap, 4 studies reported data on patient satisfaction. One study27 used the “Kyungpook National University Hospital modification of the Breast-Q,” reporting the achievement of a high level of satisfaction in 78% of cases, whereas another study26 used the “Body Image Scale,” reporting the achievement of a high level of satisfaction in 80% of cases. The other 2 studies did not mention any specific questionnaire, reporting the achievement of high or very high levels of patient satisfaction in 92.3% and 92.5% of cases.22,31
Complications
The overall complication rate was 9.25%. In the case of LTAP, the harvesting complication rate was 4.54%. Fat necrosis was described in 2 patients (1.13%), and a partial venous flow disorder spontaneously reversible within 48 hours was reported in 1 case (0.56%). Four cases (2.27%) of postoperative hematomas (2 in axillary region and 2 in the breast) and 1 case of wound infection (0.56%) were also reported.
In the case of LTD flap, the harvesting complication rate was 11.84%. Partial flap necrosis and fat necrosis were described in 4 patients (5.26%) and infection in 1 case (1.31%). In the case of LICAP, the harvesting complication rate was 12.72%. In detail, partial flap necrosis and fat necrosis were described in 17 patients (9.44%), hematoma in 3 cases (1.66%), and partial venous congestion in 2 cases (1.11%).
Donor Site
The overall donor site complication rate was 3.70%. Among all patients, only 10 cases of wound dehiscence were reported (6 after LICAP flap, 3 after LTD flap, and 1 after LTAP flap). In 2 of the 3 patients undergone LTD flap reconstruction, the wound dehiscence required a surgical revision with skin suture. Two studies described the onset of seroma at donor site after LTD flap (5 cases) and LICAP flap (3 cases), which was resolved by serial dorsal punctures and aspirations (overall rate: 1.85%).
DISCUSSION
Oncoplastic techniques consented to expand the indications for BCS to those tumors that otherwise would be treated with a mastectomy followed by autologous or implant-based breast reconstruction,33–38 allowing at the same time greater volume excision without aesthetic compromise. Oncoplastic procedures represent one of the most common operations plastic surgeons face daily. Volume displacement or volume replacement approaches are differently indicated depending on tumor/breast ratio, tumor location, and breast morphology. The predicted percentage of resected breast tissue represents the main factor in moving toward 1 of the 2 techniques: between 10% and 20%, the use of volume displacement techniques should be favored; above 20%, volume replacement is indicated.10 With respect to volume replacement techniques, the introduction of fasciocutaneous flaps allowed minimizing donor site morbidity and offered a wide range of choice, essentially based on the breast defect topography and dimension. Many studies12,14,17 have been published in the attempt to provide a decision-making guide to choose the best local flap, essentially based on the breast defect topography and dimension. This review focused on lateral partial breast reconstruction using 3 types of flap: the LTAP flap, the LTD flap, and the LICAP flap. The common advantageous feature of these three flaps is the preservation of the thoracodorsal pedicle, which leaves the possibility of using an LD flap in the case of unknown oncological margins.
The lateral chest wall has a triple blood supply: from the thoracodorsal artery, from the lateral thoracic artery,39 and from the lateral intercostal artery. This vascular arrangement gives the advantage of harvesting several large or small volume flaps while maintaining the intrinsic vascular supply of the lateral chest wall. The current article describes different types of lateral chest wall flap design and different combinations of flaps, based on the lateral thoracic artery and/or the lateral intercostal artery. According to our analysis, this is the first systematic review on this topic.
The 13 studies included for our review presented at least a level of evidence IV. Breast defects varied from lumpectomy to outer columnar mastectomy, and the average weight of resected breast tissue was relatively moderate (100 g). The available volume of the LTAP, the LTD, and the LICAP flaps is limited, and it is linked to the presence of redundant skin on the lateral chest wall. However, the possibility of easily harvesting combined flaps (LTAP + LICAP) allowed in some cases to safely fill defects > 500 g.
LTAP Flap
The main disadvantage of the LTAP flap is the anatomical variation and the relatively unstable diameter of its perforator vessels. The LTA gives rise to a direct cutaneous branch, which runs inferiorly along the lateral chest wall in approximately 85% of individuals.40,41 Similarly to the superficial circumflex iliac artery perforator, this branch is classified as a “direct cutaneous perforator” and it is located in an area between the 2 muscle borders of the pectoralis major and LD muscles (along the mid-axillary line), at the level of the 3rd–5th intercostal spaces, within 2 cm of the lateral breast crease. LTAP flap harvesting not includes any bothersome intramuscular dissection, making this flap relatively easy. To overcome the inconveniences of anatomical variability and unpredictable size of perforators, Tashiro et al42 suggested performing a preoperative Color Doppler Ultrasound for developing a surgical plan before starting the operation. In our review, a preoperative Doppler mapping was performed in all patients undergone an LTAP flap reconstruction. However, Doppler is unable to precisely locate perforators.42 Even if the Color Doppler Ultrasound requires a longer learning curve than Doppler, we believe that it can find a useful application in the surgical planning of the LTAP. In our review, most of authors reserved the possibility of adapting the reconstructive planning to the intraoperative exploration of perforator vessels, performing eventually combined flaps (LTAP/LICAP) if, upon direct visualization, the dominant perforator vessel was found to be a LICAP or a mixture of small LICAP and LTAP. The LTAP/LICAP combination flaps can be described as “freestyle” perforator flaps. The LTAP flap provides noticeable advantages compared with other local options. First of all, its pedicle can be partially or entirely dissected to allow greater flap mobilization when compared with the LICAP flap (especially for upper outer quadrant reconstruction). Second, avoiding intramuscular dissection, LTAP flap causes minimal donor site morbidity. Third, the possibility of including LICAP perforators provides additional perfusion and volume, improving the versatility of this flap.
LICAP Flap
LICAP perforators tend to be positioned more inferiorly (4th–6th intercostal space) and laterally than LTAP perforators. Although the use of the LICAP flap is well established and reliable, its perforators are variable in size and position, and this constitutes the main disadvantage of this flap. In our review, all authors performed a preoperative Doppler mapping and/or a preoperative CT scan to establish perforator distance from the anterior border of the LD muscle. LTAP perforator incorporation in LICAP flaps was quite common (about 30% of cases in our review) especially in cases of “turnover” flaps. Indeed, the fact that LTAP perforators are generally positioned more medially than the LICAP perforators makes them very simple to incorporate in a LICAP turnover flap. McCulley25 pointed out that the reconstructive surgeon should recognize the option of the LTAP flap so as not to damage the lateral thoracic pedicle when dissecting the superior aspect of the flap.
Differently from Levine’s algorithm17 (in which dissection was performed from lateral to medial, making the use of the thoracodorsal perforator vessels as the first option, and then moving medially onto the LTAP and LICAP vessels), McCulley et al25 believed that the LICAP flap (with a simple turnover design and without the need for pedicle dissection) remains the best option for partial lateral breast defect reconstruction. Because LICAP and LTAP flaps do not sacrifice the main pedicle (thoracodorsal vessels), they are considered expendable. In this way, when a moderate skin paddle is required, it is still feasible to harvest an LD flap for eventual delayed breast reconstruction in the case of positive margins. For this reason, Levine’s algorithms may be more adapted to delayed partial breast reconstruction in which the oncologic features of the cancer are completely known and prior axillary surgery could have compromised lateral thoracic artery.
LTD Flap
LTD flap is considered basically as a variation of the LICAP flap, being based on the same vascular supply. Differently from the LICAP and LTAP flaps, it included LICAP perforators, but perforator skeletonization is not required. For this reason, it can be advanced into the breast defect or can be rotated by 90 degrees, but it cannot be used as a propeller flap. The unlimited degree of rotation of this flap constitutes its main disadvantage. Regarding flap harvesting, all authors agreed on the fact that extreme caution has to be taken to preserve and to include the muscular fascia from the superficial to the deep layer during LTD dissection.
The mean follow-up was relatively long, and most of the studies described cosmetic results. Despite the heterogeneity of the evaluation methods and follow-up, we can conclude that these 3 flaps allowed obtaining satisfactory aesthetic results (mean score > 4/5 for LTAP, LTAP/LICAP, and LTD flaps; between 78% and 100% of good or excellent outcomes for LICAP). Moreover, about half of the studies reported that none of the patients have required revision procedures to the reconstructed breast or symmetrization procedures on the contralateral side.
In total, 7 of 13 studies described patient-reported outcomes measures using the “Michigan Breast Reconstruction Outcomes Survey,” the “Kyungpook National University Hospital modification of the Breast-Q,” the “Body Image Scale,” or an unspecified assessment of subjective satisfaction. Similarly to aesthetic outcomes, despite the heterogeneity, our review suggested that volume replacement with LTAP, LTD, and LICAP flaps allows reaching a high patient satisfaction in about 80% of cases. One of the main causes of aesthetic failure in breast reconstruction is postoperative radiation therapy. Despite the great majority of patients underwent adjuvant radiation treatment, no study reported poor aesthetic results linked with irradiation (except for 1 case of severe asymmetry).
The overall complication rate was 9.25%. The most common complications were partial flap necrosis and fat necrosis followed by hematoma. However, the flap necrosis was managed with simple medications in most of the cases not requiring revision surgery. Overall donor site complication rate was very low (3.7%), including 10 cases of wound dehiscence and 8 cases of seroma resolved by serial aspirations. The main limitations of this systematic review are the retrospective nature of most of the included studies, and the heterogeneity in aesthetic outcomes and patient satisfaction evaluation methods.
CONCLUSIONS
The use of lateral chest wall perforator flaps represents an excellent option for partial breast reconstruction of small to moderate defects in tumors in lateral and inferior quadrants. A distinct advantage of LTAP, LTD, and LICAP flaps is that they do not sacrifice the main pedicle of the lateral chest wall (thoracodorsal artery), not compromising eventual delayed breast reconstruction with TDAP or LD flaps. This staged approach to partial breast reconstruction is especially useful in cases where the oncological margins are uncertain and wider resections or mastectomies are secondly required. The LTAP flap represents a reliable flap when suitable perforators are available and it can improve the reliability of the LICAP flap (combined LTAP/LICAP flap), allowing a greater versatility in partial breast reconstructions. LTD flap has the advantage of avoiding perforator skeletonization but it allows a limited flap mobilization. LICAP flap reconstruction is a well-established and reliable option; this review pointed out the different possible flap designs and the possibility of harvesting combined flaps.
Despite the heterogeneity of evaluation methods, both aesthetic and patient-reported outcomes were satisfactory for the 3 flaps. In addition, the donor site morbidity was very low.
Footnotes
Published online 14 January 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol. 2013;20:3469–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grieco MP, Simonacci F, Bertozzi N, et al. Breast reduction: a case series analysis. EuroMed Biomed J. 2016;11:157–164. [Google Scholar]
- 3.Grieco MP, Bertozzi N, Grignaffini E, et al. A three-year experience with medial-pedicle-based breast reduction for different mammary hypertrophy. Acta Biomed. 2018;89:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polotto S, Grieco MP, Simonacci F, et al. Reduction mammoplasty techniques in post-bariatric patients: our experience. Acta Biomed. 2017;88:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosasih S, Tayeh S, Mokbel K, et al. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis of 18,103 patients. Am J Surg. 2020;220:385–392. [DOI] [PubMed] [Google Scholar]
- 6.Arndt V, Stegmaier C, Ziegler H, et al. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. J Cancer Res Clin Oncol. 2008;134:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertozzi N, Pesce M, Santi PL, et al. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci. 2017;21:2572–2585. [PubMed] [Google Scholar]
- 8.Raposio E, Belgrano V, Santi P, et al. Which is the ideal breast size?: Some social clues for plastic surgeons. Ann Plast Surg. 2016;76:340, 345. [DOI] [PubMed] [Google Scholar]
- 9.Asgeirsson KS, Rasheed T, McCulley SJ, et al. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2005;31:817–823. [DOI] [PubMed] [Google Scholar]
- 10.Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2014;72:145–149. [DOI] [PubMed] [Google Scholar]
- 11.Chen JY, Huang YJ, Zhang LL, et al. Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: a meta-analysis. J Breast Cancer. 2018;21:321, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Losken A, Hamdi M. Partial breast reconstruction: current perspectives. Plast Reconstr Surg. 2009;124:722–736. [DOI] [PubMed] [Google Scholar]
- 13.White J, Achuthan R, Turton P, et al. Breast conservation surgery: state of the art. Int J Breast Cancer. 2011;2011:107981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdi M, Van Landuyt K, Van Hedent E, et al. Advances in autogenous breast reconstruction: the role of preoperative perforator mapping. Ann Plast Surg. 2007;58:18–26. [DOI] [PubMed] [Google Scholar]
- 15.Koh CE, Morrison WA. Functional impairment after latissimus dorsi flap. ANZ J Surg. 2009;79:42–47. [DOI] [PubMed] [Google Scholar]
- 16.Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134:303–314. [DOI] [PubMed] [Google Scholar]
- 17.Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg. 2005;116:762–767. [DOI] [PubMed] [Google Scholar]
- 18.Sjøberg T, de Weerd L. Lateral thoracodorsal flap or lateral intercostal artery perforator flap. What is in the name? Ann Plast Surg. 2017;78:600. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munhoz AM, Montag E, Arruda EG, et al. The role of the lateral thoracodorsal fasciocutaneous flap in immediate conservative breast surgery reconstruction. Plast Reconstr Surg. 2006;117:1699–1710. [DOI] [PubMed] [Google Scholar]
- 21.Hamdi M, Van Landuyt K, De Frene B, et al. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg. 2006;59:644–652. [DOI] [PubMed] [Google Scholar]
- 22.Munhoz AM, Montag E, Arruda E, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast. 2011;20:233–240. [DOI] [PubMed] [Google Scholar]
- 23.Yang JD, Ryu DW, Lee JW, et al. Usefulness of a lateral thoracodorsal flap after breast conserving surgery in laterally located breast cancer. Arch Plast Surg. 2013;40:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Kim MC, Park HY, et al. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg. 2014;3:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:686–691. [DOI] [PubMed] [Google Scholar]
- 26.Roy PG. One-stage vs. two-stage approach for partial breast reconstruction with lateral chest wall perforator flaps. Cancer Treat Res Commun. 2016;9:56–61. [Google Scholar]
- 27.Kim JB, Kim DK, Lee JW, et al. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg. 2018;45:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martellani L, Manara M, Renzi N, et al. Use of LICAP and LTAP flaps for breast reconstruction. Acta Chir Plast. 2019;60:4–8. [PubMed] [Google Scholar]
- 29.Hong S, Wang S, Liu J, et al. Usefulness of lateral thoracic adipofascial flaps after breast-conserving surgery in small-to moderate-sized breasts. Clin Breast Cancer. 2019;19:370–376. [DOI] [PubMed] [Google Scholar]
- 30.Meybodi F, Cocco AM, Messer D, et al. The modified lateral intercostal artery perforator flap. Plast Reconstr Surg Glob Open. 2019;7:e2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JB, Eom JR, Lee JW, et al. Utility of two surgical techniques using a lateral intercostal artery perforator flap after breast-conserving surgery: a single-center retrospective study. Plast Reconstr Surg. 2019;143:477e–487e. [DOI] [PubMed] [Google Scholar]
- 32.Soumian S, Parmeshwar R, Chandarana M, et al. Chest wall perforator flaps for partial breast reconstruction: surgical outcomes from a multicenter study. Arch Plast Surg. 2020;47:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raposio E, Cicchetti S, Adami M, et al. Computer planning for breast reconstruction by tissue expansion: an update. Plast Reconstr Surg. 2004;113:2095–2097. [DOI] [PubMed] [Google Scholar]
- 34.Bertozzi N, Pesce M, Santi P, et al. Tissue expansion for breast reconstruction: Methods and techniques. Ann Med Surg (Lond). 2017;21:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellini E, Pesce M, Santi P, et al. Two-stage tissue-expander breast reconstruction: a focus on the surgical technique. Biomed Res Int. 2017;2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardani M, Bertozzi N, Grieco MP, et al. Breast reconstruction with anatomical implants: a review of indications and techniques based on current literature. Ann Med Surg (Lond). 2017;21:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertozzi N, Pesce M, Santi P, et al. One-stage immediate breast reconstruction: a concise review. Biomed Res Int. 2017;2017:6486859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grieco MP, Simonacci F, Bertozzi N, et al. Breast reconstruction with breast implants. Acta Biomed. 2019;89:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JT, Kim SW. Another option of perforator flap in the lateral thoracic area: lateral thoracic perforator flap. J Reconstr Microsurg. 2014;30:443–450. [DOI] [PubMed] [Google Scholar]
- 40.Harii K, Torii S, Sekiguchi J. The free lateral thoracic flap. Plast Reconstr Surg. 1978;62:212–222. [DOI] [PubMed] [Google Scholar]
- 41.Taylor GI, Daniel RK. The anatomy of several free flap donor sites. Plast Reconstr Surg. 1975;56:243–253. [DOI] [PubMed] [Google Scholar]
- 42.Tashiro K, Harima M, Mito D, et al. Preoperative color Doppler ultrasound assessment of the lateral thoracic artery perforator flap and its branching pattern. J Plast Reconstr Aesthet Surg. 2015;68:e120–e125. [DOI] [PubMed] [Google Scholar]


