Abstract
Multiple sclerosis is a disease characterised by axonal demyelination in the central nervous system (CNS). The atypical antipsychotic drug clozapine attenuates experimental autoimmune encephalomyelitis (EAE), a mouse model used to study multiple sclerosis, but the precise mechanism is unknown and could include both peripheral and CNS–mediated effects. To better understand where clozapine exerts its protective effects, we investigated the tissue distribution and localisation of clozapine using matrix-assisted laser desorption ionization imaging mass spectrometry and liquid chromatography-mass spectrometry. We found that clozapine was detectable in the brain and enriched in specific brain regions (cortex, thalamus and olfactory bulb), but the distribution was not altered by EAE. Furthermore, although not altered in other organs, clozapine levels were significantly elevated in serum during EAE. Because clozapine antagonises dopamine receptors, we analysed dopamine levels in serum and brain as well as dopamine receptor expression on brain-resident and infiltrating immune cells. While neither clozapine nor EAE significantly affected dopamine levels, we observed a significant downregulation of dopamine receptors 1 and 5 and up-regulation of dopamine receptor 2 on microglia and CD4+-infiltrating T cells during EAE. Together these findings provide insight into how neuroinflammation, as modelled by EAE, alters the distribution and downstream effects of clozapine.
Subject terms: Biochemistry, Diseases of the nervous system, Molecular neuroscience
Introduction
Clozapine is an atypical antipsychotic agent used for neurological disorders including schizophrenia and Parkinson’s disease. Clozapine reduces psychosis by antagonising both the serotonin 2A and C (5-HT2A and 5-HT2C) and the dopamine receptor 2 but has also been shown to be immunomodulatory in the central nervous system (CNS)1, 2. Given that neuroinflammation occurs during schizophrenia3, clozapine’s immunomodulatory ability may contribute to its efficacy in reducing disease severity.
The ability of clozapine to regulate inflammation has been shown to reduce disease in experimental autoimmune encephalomyelitis (EAE), a mouse model useful for the study of neuroinflammatory aspects of multiple sclerosis (MS), a disease characterised by demyelination of the neurons in the CNS4, 5. EAE is characterised by the infiltration of autoreactive CD4 + T cells and other immune cells (e.g. monocytes) into the CNS causing an inflammatory state. It has been shown previously that innate and adaptive immune cells are increased in the CNS and that pro-inflammatory cytokines, like interferon gamma and interleukin-17, associated with a high level of inflammation are upregulated5–7. We have shown previously that clozapine treatment reduces the expression of pro-inflammatory cytokines and chemokines in the CNS as well as reduction the activation markers (Iba1 and GFAP) on CNS-resident cells5–7. However, the precise mechanism by which clozapine ameliorates EAE has not been identified, and because EAE involves not only myelin-specific CD4 + T cells but other peripheral immune and CNS-resident cells, there are many potential targets for clozapine’s protective effects.
A recent phase 1b, randomized, controlled trial in progressive MS patients using low doses of clozapine (i.e. CRISP trial) found that all participants treated with clozapine were withdrawn within 9 days of starting treatment due to adverse events8. The CRISP trial results highlight that clozapine’s actions may be altered during MS such that people with progressive MS have an enhanced sensitivity to clozapine. Due to the early withdrawal of all participants in the clozapine group, this trial could not determine whether clozapine was able to impact MS disease as suggested by pre-clinical studies4, 5, 7. This surprising finding is at the heart of our rationale for this study, which is to understand why this sensitivity to clozapine occurred (i.e. different pharmacology, metabolism, target expression) so that it can be appropriately used in a therapeutic trial. While EAE does not recapitulate all aspects of MS, it does model CD4 + T cell-driven neuroinflammation9. As such, EAE is a useful model to dissect clozapine’s mechanism of action and to understand how neuroinflammation itself may alter clozapine’s effects. Thus, we investigated which tissues are targeted by clozapine by determining its tissue localisation and distribution as well as the expression of its target receptors.
Clozapine antagonises dopamine receptors (DR), which can be separated into two subtypes, D1 subtypes (DR1 and DR5) and D2 subtypes (DR2, DR3 and DR4), based on modulation of cAMP production10. All DRs are highly expressed in the brain10 and on immune cells11, and have shown to be involved in mental disorders and neuroinflammatory diseases like schizophrenia, depression, Parkinson's and Huntington’s disease12, 13. Given clozapine’s affinity for DRs, it has been reported to displace dopamine from the receptor within less than one minute14, 15, directing it towards other receptors and causing it to have unanticipated effects. Importantly, previous research has shown that selective DR1 antagonism reduces EAE16 while selective DR2 antagonism exacerbates EAE4, 16 indicating that dopamine receptors are involved in EAE. Thus, we have focused our investigation on dopamine.
Dopamine is a neurotransmitter with many distinct functions including in the reward pathway and motor control. Previous studies using the EAE model showed that increasing dopamine levels improved the symptoms of EAE17 while depletion worsened disease18. This could be due to the immunomodulatory effects of dopamine on T cells leading to increased production of tumour necrosis factor-α and interleukin-1019. However, the context of the tissue and the inflammatory state seems to determine the effect dopamine on a given T cell subset and function20. The modulation of the function and phenotype of monocytes and macrophages is another way how dopamine might improve symptom of EAE21. Incubation of monocytes and macrophages in vitro with dopamine improved their phagocytic activity. Thus, these studies highlight an important role for dopamine as immunomodulator in EAE and suggest a change in dopamine levels by clozapine might be one means by which clozapine reduces EAE disease severity.
Liquid chromatography-coupled mass spectrometry (LC–MS) is an important tool for drug metabolism and pharmacokinetic studies and is commonly used for metabolite identification, quantification, toxicology and pharmacokinetics due to its high sensitivity, rapid analysis and high specificity22. However, LC–MS only measures absolute levels without any information about localisation and distribution. In contrast, matrix-assisted laser desorption ionization (MALDI) imaging mass spectrometry (IMS) analyses tissue sections for the distribution of a range of unlabelled targets23, and thus provides spatial information.
In this study, we used MALDI IMS in combination with quantitative LC–MS time of flight analysis to investigate the localisation and distribution of clozapine and dopamine in brain regions and peripheral organs. In addition, we analysed dopamine and DRs in the CNS to better understand clozapine’s actions during EAE-associated neuroinflammation.
Results
Clozapine was detected in the brain by MALDI IMS
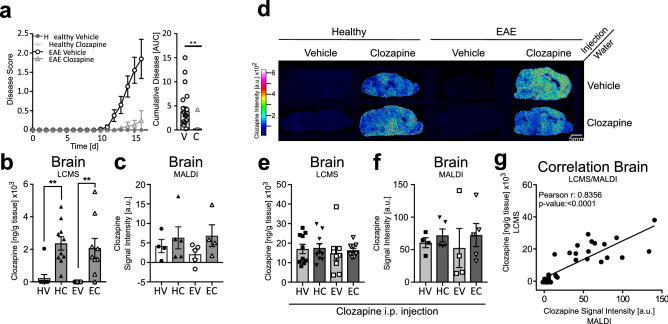
Clozapine readily passes through the blood–brain barrier to mediate its anti-psychotic effects24. Previous studies have shown that clozapine administered orally at a concentration of 60 mg/kg; reduced the onset and the severity of EAE in the mice5, 25; therefore, we assessed the distribution and localisation of clozapine in the brains of healthy and EAE mice treated with vehicle or clozapine. As reported previously, clozapine treatment reduced the severity of disease as measured by the disease score and the area under curve of the disease course (Fig. 1a, **p < 0.0021, V-Vehicle, C-Clozapine). Absolute quantification of clozapine levels from brain homogenates detected by LC–MS showed around 2 µg per gram tissue in the brains of healthy and EAE clozapine-treated mice with no difference detected between healthy and EAE mice (m/z 327.137, Fig. 1b, p < 0.01, HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine). Because we found that clozapine is readily detected by MALDI IMS (Fig. S1c,d), we used MALDI IMS to evaluate clozapine levels in the brain but found that the levels were close to the background (vehicle-treated mice; Fig. 1c, HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine). These low levels may be due to the rapid clearance of clozapine, which has a half-life of approximately 1.5 h in rodents26. Therefore, to increase the detection of clozapine in the mouse brain with MALDI IMS, mice were administered increasing concentrations of clozapine, and the brains were removed 30 min later when clozapine can be detected in all organs by PET scanning26, 27. As seen in Fig. S1b,e, all tested doses of clozapine could be detected in the brains of healthy mice and the intensity of clozapine correlated to the dose administered (Fig. S1b,e). However, at the highest concentration of clozapine, sedation, which is a well-known side effect of clozapine, occurred28. Thus, a lower dose was selected, with 10 mg/kg clozapine giving reliable results. While there could be potential differences in localisation, concentration and distribution between chronic exposure to clozapine in the drinking water and a single injection of clozapine, this method readily enabled detection and quantification of clozapine in the brain (Fig. 1c,f respectively). Figure 1d shows representative images for all eight treatment groups. No differences were detected by LC–MS or MALDI IMS between healthy and EAE mice, with or without clozapine in the drinking water (Fig. 1e,f HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine). A Pearson correlation between the intensity of clozapine (MALDI IMS) and the absolute concentration of clozapine (LC–MS) showed a highly significant correlation (Fig. 1g) supporting the quantitative use of MALDI IMS for these studies.
Figure 1.
Clozapine in the brain of healthy and EAE mice. Mice were treated with clozapine or vehicle 1 day prior to EAE induction in the drinking water and scored daily (0: normal to 5: moribund). 30 min before euthanasia mice were injected i.p. with 10 mg/kg clozapine (a) Disease course of mice and disease burden assessed by area under the curve analysis. Shown are the means and SEM of individual mice from two experiments combined (n = 20 mice/group), Paired t test, two-tailed **p < 0.0021 (b,d) Absolute concentration of clozapine in mouse brain measured with LC–MS (n = 10 mice/group) one-way ANOVA repeated measure followed by Tukey Test, **p < 0.01 (c,e) Quantification of clozapine intensity by MALDI IMS in the whole mouse brain (n = 4–5 mice/group) (f) Positive correlation of absolute clozapine concentration by LC–MS and clozapine intensity by MALDI; Pearson correlation coefficient: 0.8477 with p-value < 0.0001. (g) Representative MALDI images from all eight treatment groups.
Clozapine preferentially localised to specific brain regions, but its distribution was not altered by EAE
The MALDI images showed that clozapine was not evenly distributed in the brain. To evaluate regional differences, we segmented the brain into six regions based on the optical images and specific lipid distributions detected by MALDI IMS (Fig. 2a). Analysis of these brain regions (CBX—Cerebellum; CTX—Cortex; MB—Midbrain; MOB + AON—main and accessory olfactory bulb; MY + P—Medulla and Pons; TH—Thalamus) showed high clozapine intensity in the cortex, the thalamus and the olfactory (Fig. 2b,c,d; *p > 0.05; **p > 0.01; ***p > 0.001, ****p > 0.0001). Clozapine intensity was significantly increased in mice pre-treated with clozapine in the drinking water compared to vehicle in healthy mice (Fig. 2c,d) but the localisation to different brain regions did not change. To determine if neuroinflammation altered clozapine distribution, we compared healthy and EAE mice treated with clozapine in drinking water but found no overall significant difference (Fig. 2e, V-Vehicle, C-Clozapine, S1f). Additionally, no differences were observed between healthy and EAE mice if reinjected with clozapine in the presence or absence of clozapine in drinking water (Fig. 2f, V-Vehicle, C-Clozapine, S1g). Taken together, these data suggest that while clozapine seems to localise to specific brain regions, neuroinflammation in EAE and chronic or acute clozapine exposure do not alter its distribution.
Figure 2.
Segmentation of mouse brain into different brain regions and clozapine intensity. (a) Optical image, clozapine MALDI image and different lipid distributions for the segmentation into different brain regions. CBX cerebellum; CTX cortex; MB midbrain; MOB + AON main and accessory olfactory bulb; MY + P medulla and pons; TH thalamus. Clozapine intensity in the different brain region measured by MALDI in mice with clozapine in the drinking water only (b) and vehicle (c) or clozapine (d) in the drinking water and i.p. re-injection of 10 mg/kg clozapine. (n = 4–5 mice/group), two-way ANOVA repeated measure followed by Sidak’s multiple comparison test, *p > 0.05; **p > 0.01; ***p > 0.001, ****p > 0.0001 (e) Clozapine intensity in midbrain, cortex and thalamus in healthy and EAE mice (f) and after clozapine re-injection C – clozapine; V—vehicle.
During EAE, clozapine and norclozapine levels were significantly elevated in the serum, but not other peripheral organs.
To determine if clozapine levels in peripheral organs were altered by either chronic clozapine exposure or on-going neuroinflammation, we evaluated clozapine levels in the serum, spleen, liver, and lung. The liver is the major site for metabolism of clozapine into norclozapine and other metabolites; furthermore, given the reported immunomodulatory activity of clozapine and its effects in the EAE model, we chose to measure clozapine levels in the spleen, the major secondary lymphoid organ. Continuous treatment with clozapine in the drinking water of mice with EAE showed significantly higher levels of the drug in the serum compared to healthy animals (500 vs 100 ng/mL, EAE vs healthy; Fig. 3a; **p > 0.01; HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine). This effect was lost with re-injection (Fig. S2a). Absolute quantification of clozapine by LC–MS in the liver and lung and MALDI IMS analysis of the splenic red and white pulp showed no difference between healthy and EAE mice (Fig. 3b,c, HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine, R-red, W-white S2b to d). Together these results indicate that the distribution of clozapine in serum but not other tissues is altered by EAE.
Figure 3.
Clozapine and norclozapine quantification in the serum and liver and clozapine intensity in the spleen by MALDI. (a,b) Mice were treated with clozapine or vehicle 1 day prior to EAE induction in the drinking water and absolute clozapine concentration measured by LC–MS was analysed in the serum (a) and liver (b) (n = 10 mice/group), two-way ANOVA repeated measure followed by Sidak’s multiple comparison test, ** p > 0.01; (c-e) Mice were treated with clozapine or vehicle 1 day prior to EAE induction in the drinking water and clozapine signal intensity by MALDI IMS was analysed in the spleen, separated by red and white matter (n = 4–5 mice/group) (c) and norclozapine concentration measured by LC–MS was analysed in the serum (d) and liver (e) (n = 10 mice/group) in healthy and EAE mice treated with clozapine in the drinking water and reinjection of clozapine, two-way ANOVA repeated measure followed by Sidak’s multiple comparison test, *p > 0.05; **p > 0.01; ***p > 0.001.
Because the higher serum levels could reflect changes in clozapine’s metabolism, we measured the primary metabolite of clozapine, norclozapine (m/z 312.1142). No significant differences between groups was seen in the brain or the lung (Fig. S2e,f), but interestingly, a significant difference between healthy and EAE animals was detected in the serum, where significantly higher norclozapine levels were detected during EAE after chronic or single exposure to clozapine (Fig. 3d **p > 0.01). Furthermore, although no difference between healthy and EAE animals was observed in the liver samples, significantly higher levels of norclozapine were detected when clozapine was chronically administered before the single clozapine injection (Fig. 3e *p > 0.05; ***p > 0.001). Even though, hypermetabolism and changes in the metabolism of clozapine into other metabolites, like clozapine-N-oxide, cannot be ruled out completely, these findings indicate that the higher levels of clozapine in the serum during EAE were not due to impaired metabolism of clozapine to norclozapine in the liver.
Clozapine does not alter dopamine levels or its localisation
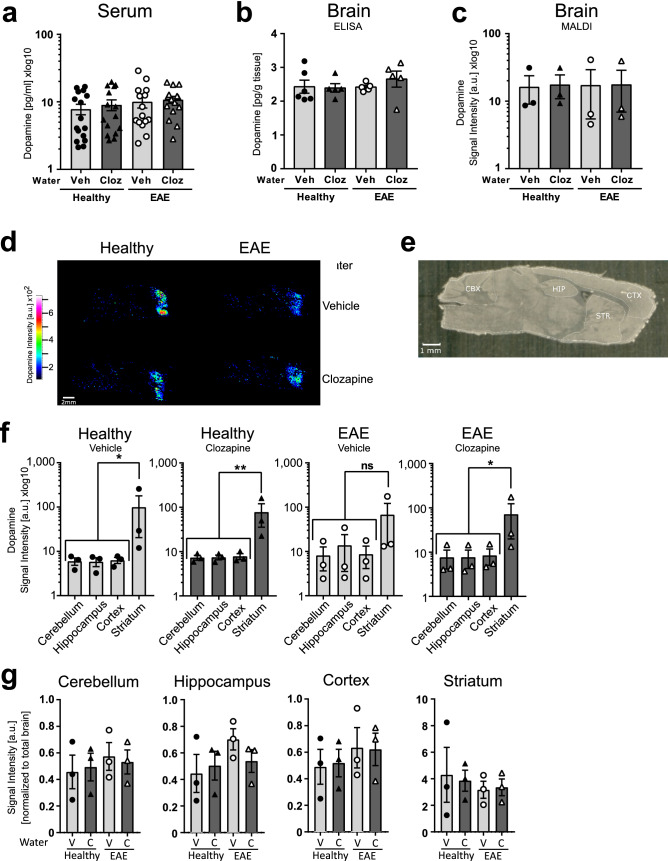
One of clozapine’s mechanisms of action is through antagonism of serotonin and dopamine receptors, which could change in vivo dopamine levels. The contribution of targeting the serotonin receptors, 5-HT2A/2C by clozapine is unclear, but there is evidence that it might have an effect on forebrain norepinephrine and dopamine neurotransmission21, which could change in vivo dopamine levels. Therefore, we analysed the concentration of dopamine in the serum and brain of healthy and EAE mice with or without clozapine treatment in the drinking water, because dopamine exerts its main effect as a hormone and neurotransmitter in the brain. Interestingly, we found that serum levels were similar between all groups (Fig. 4a) as were the levels in whole brain lysates (Fig. 4b). Since this analysis was done on the whole brain, we analysed brains by MALDI IMS to determine if clozapine treatment altered dopamine localisation (Fig. 4c–g). In all animals, dopamine intensity was highest in the striatum compared to the hippocampus, cortex or cerebellum while looking at each treatment individually (Fig. 4f; *p > 0.05; **p > 0.01). Showing the same data from Fig. 4f but sorted by the different brain regions, we could see that this distribution did not change with clozapine treatment or during EAE (Fig. 4g V-Vehicle, C- Clozapine) suggesting that clozapine does not significantly alter dopamine levels or distribution in the serum or CNS.
Figure 4.
Dopamine quantification in the serum and brain. Mice were treated with clozapine or vehicle 1 day prior to EAE induction in the drinking water and different organs were collected and analysed for absolute concentration of dopamine in the serum (n = 15 mice/group) (a) or brain (n = 5 mice/group) (b) by ELISA. (c) Quantification of dopamine intensity by MALDI IMS in the whole mouse brain (n = 3 mice/group) (d) Representative MALDI images from all four treatment groups (e) Optical image for the segmentation into different brain regions. CBX cerebellum; CTX cortex; HIP hippocampus; STR striatum (f) clozapine intensity in the different brain region measured by MALDI separated by treatment group. (n = 3 mice/group) RM-one way ANOVA comparing Striatum to the others regions, *p > 0.05; **p > 0.01 (g) Clozapine intensity by different treatment in the different brain region measured by MALDI separated by brain regions. C clozapine; V Vehicle (n = 3 mice/group).
EAE altered the expression of dopamine receptors on immune cells in the CNS
Since antagonism of dopamine receptors (DR) by clozapine29 has immunomodulatory effects30–32, the expression of all five DRs was analysed on brain-resident microglia and brain-infiltrating immune cells (CD4+T cells, CD11b+Ly6C+ monocytes and macrophages, Fig. S3). While CD4 + T cells play a key role in EAE and MS,9 monocytes and macrophages have also been shown to be important in EAE and MS5, 33–36 and to express dopamine receptors21. Moreover, clozapine has been reported to impair monocyte infiltration into the brain and spinal cord6. Thus, we included these myeloid cell types in the analysis of dopamine receptor expression in the CNS.
In healthy mice, DR1 expression (Fig. 5a) was most highly expressed on macrophages (ordinary one-way ANOVA followed by Tukey’s multiple comparison test comparing: microglia vs -macrophage p = 0.002; CD4 T cells vs -macrophage p = 0.005) and monocytes (ordinary one-way ANOVA followed by Tukey’s multiple comparison test: microglia- vs monocytes p = 0.007, CD4 T cells vs -monocytes p = 0.01) while DR5 expression was highest on macrophages (ordinary one-way ANOVA followed by Tukey’s multiple comparison test: microglia vs -macrophage p = 0.003; CD4 T cells vs -macrophage p = 0.04; monocytes vs -macrophage p = 0.002) and DR4 on monocytes (ordinary one-way ANOVA followed by Tukey’s multiple comparison test: microglia vs -monocytes p = 0.0002; CD4 T cells vs -monocytes p = 0.0005; monocytes vs -macrophages p = 0.008) (Fig. 5b,e). DR3 expression was low and DR2 similar on all cell types from healthy animals (Fig. 5c,d).
Figure 5.
Dopamine receptor expression in the brain. Dopamine receptor expression on brain-resident or infiltrating immune cells in healthy mice compared to EAE mice (a) DR1 (b) DR5 (c) DR2 (d) DR3 (e) DR4 (n = 15 mice/group) Ordinary one-way ANOVA followed by sidak’s multiple comparison test comparing each cell type, * p > 0.05, ** p > 0.001. Mice were treated with clozapine or vehicle 1 day prior to EAE induction in the drinking water and immune cells were analysed for expression of dopamine receptors by flow cytometry. (f) DR1 expression on microglia (g) DR1 expression on CD4 + T cells (h) DR1 expression on monocytes (i) DR1 expression on macrophages (j) DR2 expression on microglia (k) DR2 expression on CD4 + T cells (l) DR2 expression on monocytes (m) DR2 expression on macrophages. (n = 10–15 mice/group) HV healthy vehicle; HC healthy clozapine; EV EAE vehicle; EC EAE clozapine, two-way ANOVA followed by Sidak’s multiple comparison test, * p > 0.05.
Interestingly, EAE led to a significant reduction in DR1 on macrophages and monocytes, but the reduction on microglia did not reach significance (Fig. 5a, ordinary one-way ANOVA followed by Sidak’s multiple comparison test comparing healthy vs EAE for each cell type (for all Fig. 5a–e). DR2 expression was significantly increased on microglia and CD4+T cells by EAE while the increase on macrophages did not reach significance (Fig. 5c). Although DR3 expression was low in healthy mice, EAE increased the expression on macrophages significantly (Fig. 5d). No significant EAE-induced changes were detected for DR4 or DR5 (Fig. 5b,e).
Analysing now the same data from the healthy and EAE DR expression analysis in more detail and taking the treatment with clozapine into account, we could see that overall, clozapine treatment significantly enhanced DR1 and DR2 expression on microglia, monocytes and macrophages even during EAE (p < 0.05 and p < 0.01, respectively Fig. 5f–m HV-healthy vehicle, HC-healthy clozapine, EV-EAE vehicle, EC-EAE clozapine, two-way ANOVA followed by Sidak’s multiple comparison test (for all Fig. 5m–f). Whereas no consistent clozapine-mediated effects on DR3, DR4 and DR5 expression was detected (Fig. S2g). These results indicate that EAE reduces the expression of DR1 and enhances the expression of type 2 DR (DR2 and DR3) on immune cells in the brain while clozapine upregulates both DR1 and DR2.
Discussion
MS is characterised by demyelination of neuronal axons targeted by infiltrating autoreactive CD4+T cells. Using the EAE model to study neuroinflammatory MS, we have shown previously that clozapine effectively reduces disease severity4 and that this beneficial effect is not due to altered peripheral responses5, 25. Specifically, while clozapine does not inhibit antigen-specific Th1 or Th17 responses in the periphery, it modestly alters other peripheral T cell responses during EAE and promotes in vitro differentiation into Tregs25. However, these changes to peripheral T cells are not responsible for clozapine’s beneficial effects during EAE25. To determine whether protection was due to a direct action of clozapine in the CNS, we assessed the distribution and concentration of clozapine in the brain and other tissues of healthy and EAE animals by MALDI IMS and LC–MS. We found that although EAE did not alter the localisation or distribution of clozapine in the brain, liver lung, or spleen, it significantly increased the levels in the serum. Additionally, while dopamine levels were not affected by EAE or by clozapine, dopamine receptor (DR) expression on brain-isolated immune cells was significantly and differentially altered by EAE and clozapine suggesting that clozapine may act differently during EAE due to an altered expression of its target receptor. This study is the first in-depth report assessing clozapine, dopamine and dopamine receptors in the brain during EAE and after chronic clozapine administration and highlights how EAE-associated neuroinflammation may alter clozapine’s target pathways.
The finding that clozapine levels were significantly higher in the serum of EAE compared to healthy mice is intriguing because there were no differences detected in any other tissue. The higher levels were not due to impaired metabolism as norclozapine concentrations were also significantly elevated during EAE. Instead, it is possible that clozapine and norclozapine are retained in the serum, and previous research has shown that clozapine and its metabolites are strongly bound to the serum protein, α-1-acid glycoprotein37, with the free fraction of clozapine being less than 10%38. Additionally, higher concentrations of α-1-acid glycoprotein have been reported in the serum and plasma of MS patients with different stages of disease39, 40. Furthermore, a membrane bound α-1-acid glycoprotein can be synthesized by leucocytes and subsequently cleaved and released as the soluble serum form41. Because, leucocytes are activated in EAE, it is possible that immune cells are responsible for an increase in α-1-acid glycoprotein, which binds and retains clozapine in the blood. Peak serum levels of clozapine are reached 1–6 h after oral dosing and the half-life is about 4 to 16 h in healthy adults42, and our findings suggest this half-life may be extended during a neuroinflammatory response.
MALDI IMS was used for the first time to analyse the distribution of unlabelled clozapine within the brain tissue from healthy and EAE mice. Previously, Quiason and Shahidi-Latham used a DHB matrix on brain samples from rats treated orally with clozapine and reported a good detection of the drug in the brain tissue23. We also found DHB gave the best results compared to other matrices (data not shown); however, in our study, the oral-administered clozapine dose was too low to detect consistently in the brain. Therefore, we injected 10 mg/kg clozapine 30 min before euthanasia and were able to detect clozapine reproducibly. Similarly, Hsieh et al. showed by MALDI IMS that intravenous injections of clozapine into rats resulted in a high detection of clozapine in the brain after 45 min43 and Baldessarini et al. showed by reversed-phase liquid chromatographic separation, that detection levels peak at 30 min after 10 mg/kg intraperitoneal injection26.
With the segmentation of the brain into six different brain regions, we showed that clozapine localises to specific brain regions, with highest intensity in the cortex and thalamus and lowest in the cerebellum and medulla. Using desorption electrospray ionization IMS, clozapine was also detected in healthy rat brains after oral dose of 50 mg/kg clozapine, with the highest levels in the cortical regions38. Brain PET scan imaging from healthy humans injected with11C-labelled clozapine showed that clozapine seems to localize to specific brain regions with the highest intensity in the insula and striatum and the lowest in the cerebellum27, and our results are consistent with these findings.
The high concentrations in the liver compared to other organs are most likely because the liver is the major site where clozapine is metabolized. The cytochrome P450 system, in particular CYP1A2, converts clozapine into the two main primary metabolites norclozapine and clozapine-N-oxide44. Experiments in CYP1A2-null mice showed a significant longer half-life in the serum and a reduced time of clearance45. This finding likely explains the high clozapine concentrations in the liver after acute exposure. Over time, clozapine concentrations reduce due to its metabolism and only low levels can be detected in the continuously treated animals. While an ion suitable for clozapine-N-oxide was detected in some liver samples, it’s presence and concentration were inconsistent. Interestingly, we did see increased norclozapine intensity in liver samples from mice continuously treated with clozapine in the drinking water. This leads to the conclusion that clozapine metabolites accumulate in the liver over time and have a longer half-life.
One mechanism of clozapine’s action is through antagonism of serotonin and dopamine receptors (DR). Our choice to investigate dopamine was based upon clozapine’s known targets and the involvement of those targets in EAE. Clozapine was developed to antagonize DR2 but has greater affinity for DR1, serotonin receptors (esp 5-HT2A and C subclasses), alpha adrenergic receptors, and histamine receptor 146. At the time we began this study, previous research had shown that selective DR1 antagonism reduces EAE while selective DR2 antagonism exacerbates EAE indicating that regulation of the dopamine receptor pathways is important16. In contrast, serotonin reuptake inhibitors as well as a novel 5-HT1A/D2 ligand have been shown to ameliorate EAE suggesting a positive role for serotonin in MS47–49. These findings suggest that clozapine’s ability to reduce EAE is not principally through serotonin receptor antagonism although further research to assess the contribution of 5-HT2A and C is warranted, but that dopamine receptors look to be involved. Based upon these studies, we focused our investigation on dopamine and not serotonin.
The specific role of dopamine and its receptors is still unclear but several previous studies have shown that the changes in dopamine might be related to inflammation in autoimmune diseases50. We have shown here that dopamine levels do not change in the serum of healthy or EAE mice despite continuous clozapine treatment in the drinking water. In addition, dopamine levels in the brain tissue measured by ELISA or by MALDI IMS were not different. However, we identified the striatum as the major site of dopamine localisation and it did not change in any condition tested. This finding agrees with several studies where high dopamine levels were detected in the striatum by HPLC51–53. Although Balkowiec-Iskra et al. reported higher concentrations than our study, these reports differ in tissue (isolated striatum vs whole brain) and method (HPLC vs MALDI IMS)44. Increased dopamine levels after clozapine injection were previously reported in the rat caudate, nucleus accumbens and the medial prefrontal cortex measured by HPLC46–48, which is a more sensitive method compared to MALDI IMS. Similarly, these studies assessed specific brain regions compared to our analysis in whole brains, which would dilute the dopamine levels. In addition, most of these studies injected clozapine i.p. and studied the dopamine concentration within one hour after injection while our treatment regime was in a continuous treatment of clozapine in the drinking water. Meltzer et al. have shown that dopamine levels peak 60 min after clozapine injection and then return to baseline levels46. This immediate and high response of dopamine to the clozapine injection may differ from the continuous lower levels of clozapine achieved by administration in the drinking water because it might depend on when the mice were last drinking. Future studies investigating the effect of chronic, low dose clozapine on dopamine, may need to assess not only dopamine but also its metabolites like DOPAC47.
Interestingly, the regions with the highest clozapine levels are known to express a high density of DR1 and DR2, and the cerebellum, with low dopamine levels, has lower DR expression54. All DRs are reported to be widely expressed in the CNS and several studies have reported their expression on immune cells11, which play an important role in MS50. Moreover, DR expression on immune cells is also associated with some neurological and immune-related disorders55, 56. But, to our knowledge, this study is the first to monitor DRs on brain-resident cells and infiltrating immune cells during EAE. Here, we show that all five DR are present on brain-resident microglia, although the levels were lower than on other myeloid cells (i.e. monocytes, and macrophages). Furthermore, we found that EAE upregulated D2 subtypes (DR2 and DR3) and downregulated DR1. Interestingly, clozapine had an enhancing effect on DR1 and DR2 expression on cells from healthy and EAE animals. This study does not exclude any beneficial effects of clozapine on peripheral immune cells although our previous studies have shown that clozapine does not directly alter peripheral CD4+ T cells during EAE25. Instead, we have reported that clozapine modifies peripheral and CNS-resident innate immune cells with the greatest effects found in the CNS6. Therefore, this study has focused on DR expression on infiltrating and CNS-resident cells. Given the fascinating actions of dopamine outside of the CNS, further work needs to be performed to investigate DR expression on immune cells in the periphery and CNS at different stages of EAE, given that DR expression on immune cells is highly dynamic, context-sensitive and variable57.
A solid body of evidence indicates that in MS, dopaminergic pathways are dysregulated in the peripheral immune system. In general, human T cells express all DRs on their surface57, but the level of receptor expression differs between different T cell populations and activation states, and between resting and activated T cells. In healthy donors, resting T cells showed only a minimal expression of DR2-5 and no DR1 expression58. In line with our data, D1 subtypes are decreased on effector T cells from MS patients, and dopamine does not affect these cells57. Additionally, decreased DR5 expression could be detected, and DR5 performs an inhibitory role on T cells during MS56. Besides this evidence that DR are modulated during MS, dopamine showed only very limited therapeutic effects50.
Overall, this study is the first to show that clozapine localises to specific brain regions, which are known for high expression of the DR. While the localisation of clozapine was not altered by EAE, we found that clozapine levels were significantly elevated in the serum along with its metabolite norclozapine. Finally, immune cells in the CNS during EAE had decreased levels of DR1 but increased DR2, both of which were enhanced by clozapine administration. Overall, these findings indicate that clozapine may be retained for longer during neuroinflammation and mediate differential effects on immune cells through its target receptors, and together suggest that clozapine administration may have unexpected actions during MS due to modification of dopamine pathways, which is in line with a recent clinical trial assessing clozapine administration during MS8.
Material and methods
All methods were carried out in accordance with relevant guidelines and regulations.
Animals
Eight-ten-week old female C57BL/6J mice were purchased from the Biomedical Research Unit of the Malaghan Institute of Medical Research (Wellington, NZ) and housed in the Victoria University of Wellington PC2 animal facility at a 12 h dark–light cycle with food and water ad libitum.
Ethics statement
All animal experiments were carried out in the School of Biological Sciences Animal Facility at Victoria University of Wellington and were approved by the Victoria University of Wellington Animal Ethics Committee (2014-R23 and 25295). All methods were carried out in accordance with relevant guidelines and regulations and in compliance with the ARRIVE guidelines.
EAE induction and treatments
Mice were immunized s.c. in the rear flanks with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (50 µg/mouse; Genescript, Piscataway, NJ USA) in complete Freund’s adjuvant (Sigma, St Louis, MO USA) containing 500 µg/mouse Mycobacterium tuberculosis (Fort Richard, Auckland, NZ). In addition, mice were injected i.p. with pertussis toxin (200 ng/mouse; List Biochemicals, Campbell, CA USA) on days 0 and 2. Mice were weighed and scored daily as follows: 0, normal; 1, partial tail paralysis; 2, full tail paralysis; 3, paralysis in one hind limb; 4, paralysis in both hind limbs; and 5, moribund. One day before immunization drinking water was changed to 60 mg/kg/day clozapine (Douglas Pharmaceuticals Ltd., Auckland, NZ) or vehicle (0.1 M acetic acid). Mice were injected i.p. with 10 mg/kg clozapine or vehicle 30 min prior to CO2 euthanasia. Spleen, liver, lung and brain were collected and immediately snap-frozen in liquid nitrogen. Serum was collected as described previously6. Organs and serum were stored at − 80 °C until analysis.
Tissue preparation for HPLC–MS analysis
Liver (70 mg) and lung tissue (20 mg) were homogenized in 1 mL 0.1 M acetic acid using a Polytron tissue homogenizer. Solutions were resuspended and centrifuged for 5 min at 1000 g, supernatant removed and centrifuged a second time (5 min at 14,000×g). Brain sections 10 µm thick) were cut on a Leica CM3050 cryotome (Leica Microsystem, Wetzlar, Germany). Five sections/brain were homogenized in 100 µL 0.1 M acetic acid. Solutions were resuspended using a pipette, centrifuged for 5 min at 1000 g and supernatant was spin filtered through a 30 K Microcon Centrifugal Filter (Millipore, Burlington, MA USA). Serum samples were mixed 1:1 with acetonitrile, vortexed 30 s and centrifuged 5 min at 14,000×g. All sample were transferred to vials containing a glass insert for small volumes (ThermoFisher, Waltham, MA USA). For the clozapine standard curves, clozapine was dissolved in 0.1 M acetic acid and standard curves were spiked in untreated healthy serum or tissue samples before sample preparation. All samples and standards were spiked with quetiapine (m/z 384.174, Douglas Pharmaceuticals Ltd.) as an internal standard.
QTOF LC–MS analysis
An Agilent 1260 HPLC with a refrigerated autosampler (4 °C) equipped with a Synergi Hydro-RP column (50 × 2.0 mm, 2.5 µm, 100 Å, Phenomenex, Torrance, CA USA; Mobile phase in Table S1) was used for chromatography and samples were kept at 4 °C during the time of measurement with column temperature at 35 °C. An Agilent 6530 Accurate Mass Q-TOF LC–MS system (Santa Clara, CA USA) was used for detection with the settings shown in Table S1. Two scans per second were acquired between m/z 100 and 3200 with constant infusion of reference ions to maintain mass accuracy.
Tissue preparation for MALDI IMS
Brain samples were sectioned as above, thaw mounted onto MALDI target plates or indium tin oxide-coated slides and stored at − 80 °C until analysis. Sections for dopamine detection were collected approximately 1 mm away from the midline.
MALDI matrix application for clozapine detection
Tissue sections were dried in a vacuum desiccator for 30 min prior to scanning on a flatbed scanner (Epson Expression 10,000 XL; Suwa, Nagano, Japan) and 0.5 µl calibration mix was added outside of the tissue (containing clozapine, angiotensin II, bradykinin fragment 1–7, P14R and adrenocorticotropic hormone fragment 18–39). A TM-sprayer (HTX Imaging, Chapel Hill, NC USA) was used to spray-coat the sections with the DHB matrix as specified in Table S2. Every second pass the nozzle was rotated 90° to ensure equal distribution of the matrix. The last two passes had a track offset of 1.5 mm.
MALDI matrix application and derivatisation for dopamine detection
TPP-TFB derivatisation matrix was sprayed on the tissue with the TM-sprayer as specified in Table S2. The tissue sections were incubated at RT for 2 h, then spray-coated with detection matrix CHCA as specified in Table S2 and incubated an additional 2 h at RT. The final concentrations of TPP-TFB and CHCA were 47 ng/mm2 and 2.5 µg/mm2, respectively.
MALDI imaging mass spectrometry (IMS)
The spray-coated sections were analysed with an AB Sciex TOF/TOF 5800 mass spectrometer (Framingham, MA USA) operated as specified in Table S3. The laser intensity was optimized for each sample set. The mass spectrometer was externally calibrated using calibration peptides and clozapine as reference masses. The imaging resolution was set to 50 µm for clozapine and 100 µm for dopamine. The imaging data was stored in the Analyze 7.5 format.
Data processing
MALDI IMS data was processed with msIQuant software59. Prior to loading the files into msIQuant, the data files were converted from the Analyze 7.5 format to the imzml format using the converter module from Spectviewer60, kindly provided by Jean-Pierre Both (French Atomic Energy Commission, France). Pre-processing of all images included background correction and spectral alignment. Image normalisation was based on the root mean square in msIQuant. Tissue sections used for clozapine detection were segmented into different brain regions based on three ion signals (m/z 488.01, 848.65 and 872.61), while tissue sections used for dopamine detection were segmented based on the optical image.
QTOF LC–MS data was analysed using the Qualitative Analysis B.06.00 from Agilent.
Dopamine ELISA detection in serum and brain
Serum was collected and used immediately. For the brain, one brain hemisphere was mashed in PBS (0.1 mL PBS per 100 mg tissue). Samples were stored at – 20 °C. Two freeze and thaw cycles were performed, lysates were centrifuged at 5000×g for 5 min at 4 °C and supernatants were assayed immediately. Dopamine was measured using mouse dopamine ELISA kit from CUSABIO (CSB-E08661m; Houston, TX USA) according to manufactures instructions61. Plates were read at 450 nm using a PerkinElmer (Waltham, MA USA) microplate reader.
Flow cytometry
Each brain was processed into a single-cell suspension and stained as described previously6 (Supplementary Table S4), and each sample of isolated cells was split into 5 parts for detection of the different dopamine receptors (Panel design Supplementary Table S5)62–64. After incubation with the primary antibodies, cells were incubated with the secondary antibody, FITC goat anti-rabbit IgG (BD Biosciences, San Jose, CA USA) or isotype control for 25 min. Flow cytometry was performed on a BD FACS Canto II (BD Biosciences) and analysed using FlowJo software version 10.1 (Treestar Inc, Woodburn, OR USA).
Statistical analyses
All graphs and statistical analyses were generated using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA USA). For comparison of more than two groups one-way or two-way analysis of variance (ANOVA) was used with the recommended multiple comparison tests as indicated in the figure legend and as recommended by GraphPad Prism.
Supplementary Information
Acknowledgements
The authors would like to thank Pirooz Zareie (Monash University) for his help and Jan Vorster (Victoria University of Wellington) for his technical advice on the LC-MS.
Abbreviations
- ANOVA
Analysis of variance
- cAMP
Cyclic adenosine monophosphate
- CD4
Cluster of differentiation 4
- DHB
2,5-Dihydroxybenzoic acid
- DR
Dopamine receptors
- EAE
Experimental autoimmune encephalomyelitis
- HPLC
High performance liquid chromatography
- IMS
Imaging mass spectrometry
- LC–MS
Liquid chromatography coupled mass spectrometry
- MALDI
Matrix-assisted laser desorption ionization
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Mass spectrometry
- PET
Positron emission tomography
- PBS
Phosphate-buffered saline
- QTOF
Quadrupole time-of-flight mass spectrometry
- RRID
Research resource identifier (see scicrunch.org)
Author contributions
K.R. performed all animal experiments, LC–MS work, ELISA and flow cytometry, analysed the data, and wrote the manuscript. S.S. performed the MALDI IMS and analysed them. RAK assisted with the LC–MS work and T.W.J. assisted with the MALDI IMS. B.C. contributed to the experimental design. A.C.L. contributed to the experimental design, data analysis and edited the final manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Ministry of Business, Innovation, and Employment (RTVU1503).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Anne Camille La Flamme and Bronwen Connor have a patent for the use of clozapine during multiple sclerosis. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katharina Robichon and Sven Sondhauss.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82667-6.
References
- 1.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 2.Li X, Frye MA, Shelton RC. Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology. 2012;37:77–101. doi: 10.1038/npp.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song X, et al. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naive, first-episode schizophrenia. Psychopharmacology. 2014;231:319–325. doi: 10.1007/s00213-013-3382-4. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan D, et al. Treatment with the antipsychotic agent, risperidone, reduces disease severity in experimental autoimmune encephalomyelitis. PLoS ONE. 2014;9:e104430. doi: 10.1371/journal.pone.0104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green LK, et al. Enhanced disease reduction using clozapine, an atypical antipsychotic agent, and glatiramer acetate combination therapy in experimental autoimmune encephalomyelitis. Mult. Scler. J. Exp. Transl. Clin. 2017;3:2055217317698724. doi: 10.1177/2055217317698724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robichon K, Patel V, Connor B, La Flamme AC. Clozapine reduces infiltration into the CNS by targeting migration in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2020;17:53. doi: 10.1186/s12974-020-01733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton N, Kivell B, McCaughey-Chapman A, Connor B, La Flamme AC. Clozapine administration enhanced functional recovery after cuprizone demyelination. PLoS ONE. 2019;14:e0216113. doi: 10.1371/journal.pone.0216113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Flamme A, et al. Safety and acceptability of clozapine and risperidone in progressive multiple sclerosis: A phase I, randomised, blinded, placebo-controlled trial. BMJ Neurol. Open. 2020;2:e000060. doi: 10.1136/bmjno-2020-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br. J. Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 11.Arce-Sillas A, et al. Expression of dopamine receptors in immune regulatory cells. NeuroImmunoModulation. 2019;26:159–166. doi: 10.1159/000501187. [DOI] [PubMed] [Google Scholar]
- 12.Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin. Ther. Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaus S, Antke C, Muller HW. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav. Brain Res. 2009;204:1–31. doi: 10.1016/j.bbr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol. Psychiatry. 2001;50:873–883. doi: 10.1016/S0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- 15.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J. Psychiatry Neurosci. 2000;25:161–166. [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano K, et al. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: Preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 2008;373:286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra CD, et al. Therapeutic effect of the D2-dopamine agonist bromocriptine on acute and relapsing experimental allergic encephalomyelitis. Psychoneuroendocrinology. 1994;19:135–142. doi: 10.1016/0306-4530(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 18.Balkowiec-Iskra E, et al. MPTP-induced central dopamine depletion exacerbates experimental autoimmune encephalomyelitis (EAE) in C57BL mice. Inflamm. Res. 2007;56:311–317. doi: 10.1007/s00011-007-6128-0. [DOI] [PubMed] [Google Scholar]
- 19.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J. Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr. Opin. Pharmacol. 2008;8:460–471. doi: 10.1016/j.coph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Arreola R, et al. Immunomodulatory effects mediated by dopamine. J. Immunol. Res. 2016;2016:3160486. doi: 10.1155/2016/3160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korfmacher WA. Principles and applications of LC-MS in new drug discovery. Drug Discov. Today. 2005;10:1357–1367. doi: 10.1016/S1359-6446(05)03620-2. [DOI] [PubMed] [Google Scholar]
- 23.Quiason CM, Shahidi-Latham SK. Imaging MALDI MS of dosed brain tissues utilizing an alternative analyte pre-extraction approach. J. Am. Soc. Mass Spectrom. 2015;26:967–973. doi: 10.1007/s13361-015-1132-z. [DOI] [PubMed] [Google Scholar]
- 24.Naheed M, Green B. Focus on clozapine. Curr. Med. Res. Opin. 2001;17:223–229. doi: 10.1185/03007990152673864. [DOI] [PubMed] [Google Scholar]
- 25.Zareie P, Connor B, La Flamme AC. Amelioration of experimental autoimmune encephalomyelitis by clozapine is not associated with defective CD4 T cell responses. J. Neuroinflamm. 2017;14:68. doi: 10.1186/s12974-017-0842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldessarini RJ, et al. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology. 1993;9:117–124. doi: 10.1038/npp.1993.50. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, et al. In vivo tissue pharmacokinetics of carbon-11-labeled clozapine in healthy volunteers: A positron emission tomography study. CPT Pharmacomet. Syst. Pharmacol. 2015;4:305–311. doi: 10.1002/psp4.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olesen OV, Poulsen B. On-line fully automated determination of clozapine and desmethylclozapine in human serum by solid-phase extraction on exchangeable cartridges and liquid chromatography using a methanol buffer mobile phase on unmodified silica. J. Chromatogr. 1993;622:39–46. doi: 10.1016/0378-4347(93)80247-2. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: Past, present and future. Curr. Top. Med. Chem. 2016;16:3385–3403. doi: 10.2174/1568026616666160608084834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon S, Kim SH, Shin SY, Lee YH. Clozapine reduces Toll-like receptor 4/NF-kappaB-mediated inflammatory responses through inhibition of calcium/calmodulin-dependent Akt activation in microglia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;81:477–487. doi: 10.1016/j.pnpbp.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Hinze-Selch D, et al. Effects of clozapine on in vitro immune parameters: A longitudinal study in clozapine-treated schizophrenic patients. Neuropsychopharmacology. 1998;19:114–122. doi: 10.1016/S0893-133X(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 32.Maes M, et al. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res. 1997;26:221–225. doi: 10.1016/S0920-9964(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 33.Weiner HL. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008;255(Suppl 1):3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 34.Tierney JB, Kharkrang M, La Flamme AC. Type II-activated macrophages suppress the development of experimental autoimmune encephalomyelitis. Immunol. Cell Biol. 2009;87:235–240. doi: 10.1038/icb.2008.99. [DOI] [PubMed] [Google Scholar]
- 35.Stone S, La Flamme AC. Type II activation of macrophages and microglia by immune complexes enhances Th17 biasing in an IL-6-independent manner. PLoS ONE. 2016;11:e0164454. doi: 10.1371/journal.pone.0164454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuluundorj D, Harding SA, Abernethy D, La Flamme AC. Glatiramer acetate treatment normalized the monocyte activation profile in MS patients to that of healthy controls. Immunol. Cell Biol. 2016;95:297–305. doi: 10.1038/icb.2016.99. [DOI] [PubMed] [Google Scholar]
- 37.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab. Rev. 2001;33:161–235. doi: 10.1081/DMR-100104402. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman JM, et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc. Natl. Acad. Sci. USA. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adam P, et al. CSF and serum orosomucoid (alpha-1-acid glycoprotein) in patients with multiple sclerosis: A comparison among particular subgroups of MS patients. Clin. Chim. Acta. 2003;334:107–110. doi: 10.1016/S0009-8981(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 40.Rithidech KN, et al. Protein expression profiles in pediatric multiple sclerosis: Potential biomarkers. Mult. Scler. 2009;15:455–464. doi: 10.1177/1352458508100047. [DOI] [PubMed] [Google Scholar]
- 41.Gahmberg CG, Andersson LC. Leukocyte surface origin of human alpha1-acid glycoprotein (orosomucoid) J. Exp. Med. 1978;148:507–521. doi: 10.1084/jem.148.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao LV, Snyder ML, Vallaro GM. Rapid liquid chromatography/tandem mass spectrometer (LCMS) method for clozapine and its metabolite N-desmethyl clozapine (norclozapine) in human serum. J. Clin. Lab. Anal. 2009;23:394–398. doi: 10.1002/jcla.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh Y, et al. Matrix-assisted laser desorption/ionization imaging mass spectrometry for direct measurement of clozapine in rat brain tissue. Rapid Commun. Mass Spectrom. 2006;20:965–972. doi: 10.1002/rcm.2397. [DOI] [PubMed] [Google Scholar]
- 44.Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: A review of their clinical utility. J. Psychopharmacol. 2003;17:234–238. doi: 10.1177/0269881103017002014. [DOI] [PubMed] [Google Scholar]
- 45.Aitchison KJ, et al. Clozapine pharmacokinetics and pharmacodynamics studied with Cyp1A2-null mice. J. Psychopharmacol. 2000;14:353–359. doi: 10.1177/026988110001400403. [DOI] [PubMed] [Google Scholar]
- 46.Kapur S, Remington G. Atypical antipsychotics: New directions and new challenges in the treatment of schizophrenia. Annu. Rev. Med. 2001;52:503–517. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- 47.Simmons RD, Buzbee TM, Linthicum DS. Methysergide, a serotonin antagonist, does not inhibit the expression of autoimmune encephalomyelitis in the rabbit. J. Neuroimmunol. 1989;22:77–79. doi: 10.1016/0165-5728(89)90012-X. [DOI] [PubMed] [Google Scholar]
- 48.Bhat R, Mahapatra S, Axtell RC, Steinman L. Amelioration of ongoing experimental autoimmune encephalomyelitis with fluoxetine. J. Neuroimmunol. 2017;313:77–81. doi: 10.1016/j.jneuroim.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Popovic M, et al. Neuroprotective arylpiperazine dopaminergic/serotonergic ligands suppress experimental autoimmune encephalomyelitis in rats. J. Neurochem. 2015;135:125–138. doi: 10.1111/jnc.13198. [DOI] [PubMed] [Google Scholar]
- 50.Rangel-Barajas C, Coronel I, Floran B. Dopamine receptors and neurodegeneration. Aging Dis. 2015;6:349–368. doi: 10.14336/AD.2015.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chartoff EH, Marck BT, Matsumoto AM, Dorsa DM, Palmiter RD. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. Proc. Natl. Acad. Sci. USA. 2001;98:10451–10456. doi: 10.1073/pnas.181356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balkowiec-Iskra E, et al. Dopamine, serotonin and noradrenaline changes in the striatum of C57BL mice following myelin oligodendrocyte glycoprotein (MOG) 35–55 and complete Freund adjuvant (CFA) administration. Acta Neurobiol. Exp. (Wars) 2007;67:379–388. doi: 10.55782/ane-2007-1655. [DOI] [PubMed] [Google Scholar]
- 53.Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc. Natl. Acad. Sci. USA. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001;22:127–137. doi: 10.1016/S0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 55.Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T-cells and dendritic cells. J. Neuroimmunol. 2009;216:8–19. doi: 10.1016/j.jneuroim.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Giorelli M, Livrea P, Trojano M. Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: Effects of IFN-beta. J. Interferon Cytokine Res. 2005;25:395–406. doi: 10.1089/jir.2005.25.395. [DOI] [PubMed] [Google Scholar]
- 57.Levite M, Marino F, Cosentino M. Dopamine, T cells and multiple sclerosis (MS) J. Neural Transm. (Vienna) 2017;124:525–542. doi: 10.1007/s00702-016-1640-4. [DOI] [PubMed] [Google Scholar]
- 58.McKenna F, et al. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: A flow cytometric study. J. Neuroimmunol. 2002;132:34–40. doi: 10.1016/S0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- 59.Kallback P, Nilsson A, Shariatgorji M, Andren PE. msIQuant–quantitation software for mass spectrometry imaging enabling fast access, visualization, and analysis of large data sets. Anal. Chem. 2016;88:4346–4353. doi: 10.1021/acs.analchem.5b04603. [DOI] [PubMed] [Google Scholar]
- 60.Robbe MF, et al. Software tools of the Computis European project to process mass spectrometry images. Eur. J. Mass Spectrom. (Chichester) 2014;20:351–360. doi: 10.1255/ejms.1293. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Lee CH, Kim HG, Kim HR. Decreased dopamine in striatum and difficult locomotor recovery from MPTP insult after exposure to radiofrequency electromagnetic fields. Sci. Rep. 2019;9:1201. doi: 10.1038/s41598-018-37874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mignini F, et al. Dopamine receptor immunohistochemistry in the rat choroid plexus. J. Auton. Pharmacol. 2000;20:325–332. doi: 10.1046/j.1365-2680.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, et al. Dopamine D1 and D3 receptors modulate heroin-induced cognitive impairment through opponent actions in mice. Int. J. Neuropsychopharmacol. 2017;20:257–268. doi: 10.1093/ijnp/pyw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keeler BE, Lallemand P, Patel MM, de Castro Bras LE, Clemens S. Opposing aging-related shift of excitatory dopamine D1 and inhibitory D3 receptor protein expression in striatum and spinal cord. J. Neurophysiol. 2016;115:363–369. doi: 10.1152/jn.00390.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.