Abstract
Evolutionary arms races are broadly prevalent among organisms including bacteria, which have evolved defensive strategies against various attackers. A common microbial aggression mechanism is the type VI secretion system (T6SS), a contact-dependent bacterial weapon used to deliver toxic effector proteins into adjacent target cells. Sibling cells constitutively express immunity proteins that neutralize effectors. However, less is known about factors that protect non-sibling bacteria from T6SS attacks independently of cognate immunity proteins. In this study, we observe that human Escherichia coli commensal strains sensitive to T6SS attacks from Vibrio cholerae are protected when co-cultured with glucose. We confirm that glucose does not impair V. cholerae T6SS activity. Instead, we find that cells lacking the cAMP receptor protein (CRP), which regulates expression of hundreds of genes in response to glucose, survive significantly better against V. cholerae T6SS attacks even in the absence of glucose. Finally, we show that the glucose-mediated T6SS protection varies with different targets and killers. Our findings highlight the first example of an extracellular small molecule modulating a genetically controlled response for protection against T6SS attacks. This discovery may have major implications for microbial interactions during pathogen-host colonization and survival of bacteria in environmental communities.
Subject terms: Bacteriology, Bacterial genetics, Microbial ecology
Introduction
Vibrio cholerae is the waterborne enteric pathogen that causes serious, often fatal cholera diarrheal disease when ingested by humans. This ubiquitous microbe is found in dense polymicrobial marine communities on chitinous surfaces and in animal reservoirs like fish, zooplankton or insects1–4. To compete with other cells in densely populated microbial environments, V. cholerae employs a harpoon-like type VI secretion system (T6SS)5–8. The V. cholerae T6SS punctures adjacent cells and delivers toxic effector proteins that disrupt lipid membranes and cell walls of the bacterial envelope9–11. In animal models, V. cholerae uses T6SS effectors to eliminate “target” commensal bacteria like Escherichia coli and Aeromonas veronii12,13. V. cholerae strains with an active T6SS exhibit increased pathogenicity and cause severe cholera-like symptoms in these animal model systems12–14, suggesting the apparatus plays important roles in enhancing the ability of V. cholerae to colonize environmental habitats and infect hosts. While many studies have investigated T6SS offensive abilities, less is known about mechanisms of protection against T6SS aggression.
Bacterial “killer” cells with active T6SSs constitutively express immunity proteins that recognize and neutralize toxic effectors10,11,15. The survival of V. cholerae target cells devoid of immunity proteins is modestly increased by enhanced production of secreted exopolysaccharides, presumably by creating a physical barrier between the killer and target strains16,17. Modifications in the cell wall composition of Acinetobacter baumannii confer protection against T6SS attacks18. Furthermore, envelope stress response systems play important roles in the survival of target cells against T6SS attacks17,19–22.
Environmental conditions and small molecules can modulate T6SS-mediated antagonism by Vibrios, but studies of external conditions that trigger target cells to overcome attacks are lacking21,23,24. Here we report that exogenous glucose protects human commensal E. coli strains from T6SS-mediated aggression by V. cholerae. Protection varies with the target and killer strains tested and is mediated by the target cells’ cyclic adenosine monophosphate (cAMP) receptor protein (CRP) glucose response regulator. Since glucose decreases pathogen colonization of the intestinal tract and alters interactions between V. cholerae and E. coli cells in hosts25,26, induced protection from T6SS attacks by metabolites may be common in microbial communities of environmental and human health importance.
Results
Exogenous glucose protects E. coli target cells against T6SS attacks
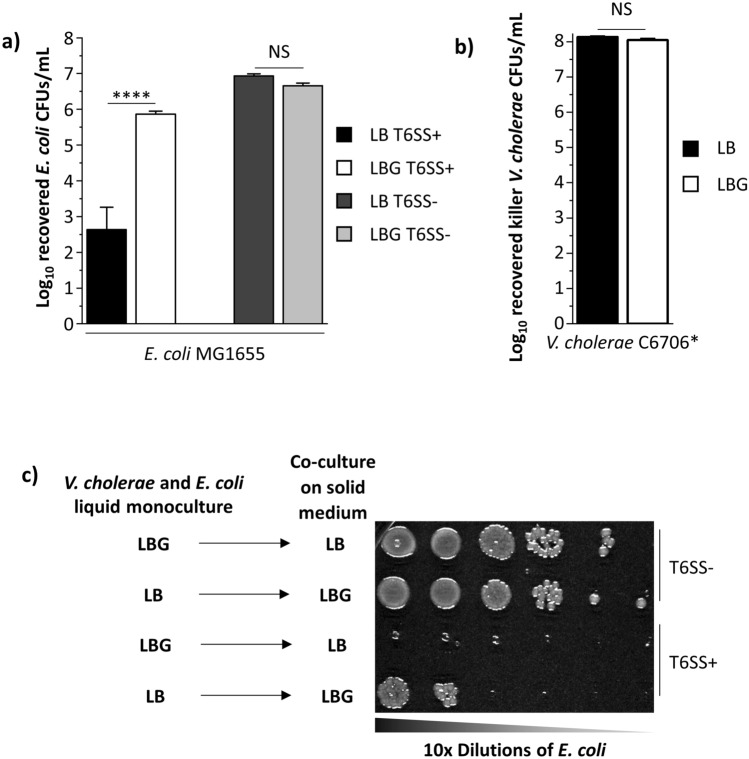
When co-cultured on rich LB medium, V. cholerae pandemic isolate C6706 constitutively expressing the QstR master gene regulator (denoted here as C6706*) kills E. coli MG1655 target cells efficiently (Fig. 1a)27–30. By contrast, a T6SS- V. cholerae C6706* strain with a deletion in the gene encoding the essential T6SS protein VasK is unable to eliminate target cells (Fig. 1a)31. When V. cholerae C6706* and E. coli MG1655 cells are co-cultured on LB medium containing 0.4% glucose (LBG), reflecting physiological levels, the number of recovered E. coli cells is significantly increased (~ 1000-fold) compared to co-cultures on LB with no added glucose (Fig. 1a)32,33. By contrast, the number of recovered V. cholerae killer cells is unaltered when co-cultured for three hours with E. coli MG1655 on LB and LBG (Fig. 1b).
Figure 1.
E. coli cells are protected against V. cholerae T6SS attacks when co-cultured in the presence of glucose. (a) E. coli MG1655 cells were co-cultured for 3 h with either killer T6SS+ or defective T6SS- V. cholerae C6706* cells on LB or LBG plates. The number of recovered chloramphenicol-resistant E. coli cells was determined by counts of colony forming units (CFUs) after co-culture mixtures were diluted and spread on chloramphenicol plates. A two-way ANOVA with a Bonferroni post hoc test was performed to determine significance. A minimum of 3 independent replicates were analyzed. (b) The same competition assay was performed as described above. However, the number of recovered killer V. cholerae C6706* cells was determined instead. A Welch’s t-test was performed to determine significance. A minimum of 3 independent replicates were analyzed. (c) V. cholerae C6706* cells grown overnight in liquid LB or LBG were co-cultured with E. coli on solid LBG or LB media, respectively, followed by dilution and plating as described in the “Methods” section. ****p < 0.0001, NS not significant.
To determine whether E. coli cells require active glucose induction to withstand T6SS attacks, we incubated V. cholerae C6706* and E. coli MG1655 in liquid LBG or LB and then co-cultured the strains on either solid LB or solid LBG medium, respectively (Fig. 1c). E. coli cells overcome T6SS attacks when incubated in liquid LB and co-cultured with V. cholerae C6706* on solid LBG but are poorly recovered when incubated in liquid LBG and co-cultured on solid LB (Fig. 1c). We also hypothesized that glucose could allow E. coli cells to escape T6SS attacks by replicating faster and evading killer cells34. However, when grown individually in monoculture conditions that mimic co-culture assays, recovered E. coli and V. cholerae cell numbers are unaltered on LBG compared to LB (Supplementary Fig. S1). Thus, E. coli cells susceptible to a killer overcome T6SS attacks but do not replicate significantly faster when glucose is present.
The identities of target and killer strains alter protection against T6SS attacks
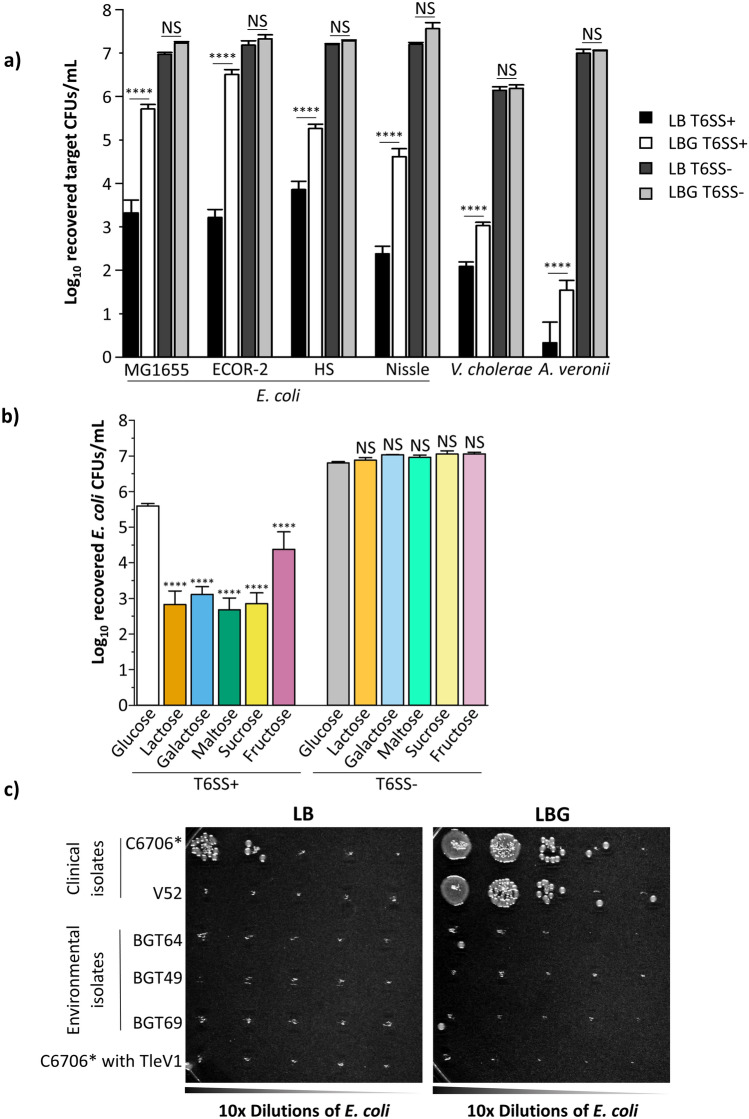
To test whether the robust glucose-mediated protection is specific to E. coli MG1655, we co-cultured killer V. cholerae C6706* with other human E. coli commensal strains (Nissle, HS and ECOR-2), as well as fish symbiotic A. veronii and susceptible V. cholerae that lacks all three immunity proteins for C6706* T6SS effectors13,35–41. All tested target strains have increased recovery when glucose is added to the medium (Fig. 2a). Importantly, the recovery difference on LBG compared to LB is greater than one order of magnitude for all the E. coli target tests, while A. veronii and V. cholerae targets are still efficiently eliminated even in the presence of glucose (Fig. 2a). The number of recovered target cells when competed against C6706* T6SS-deficient killers on LB or LBG medium is unchanged (Fig. 2a).
Figure 2.
Protection against T6SS attacks depends on the target strain, the killer strain and the sugar substrate. (a) Along with MG1655, E. coli commensal strains ECOR-2, HS, Nissle, susceptible V. cholerae and A. veronii target cells were co-cultured with killer V. cholerae C6706* or C6706* T6SS- on LB or LBG. A three-way ANOVA with a Bonferroni post hoc test was performed to determine significance. A minimum of 3 independent replicates were analyzed. (b) Killer V. cholerae C6706* cells were co-cultured with E. coli MG1655 cells on LB medium containing 0.4% of the indicated sugar compounds. A two-way ANOVA with a post hoc Tukey HSD test was used to determine significance. A minimum of 3 independent replicates were analyzed. (c) Clinical killers V. cholerae C6706*, V52 and C6706* with TleV1, as well as environmental strains BGT49, BGT64 and BGT69 were co-cultured with E. coli MG1655 cells on LB medium (left) or LBG medium (right) and then diluted and plated as described in the “Methods” section. ****p < 0.0001, NS not significant.
We next investigated whether the protection of E. coli against T6SS attacks is specific to glucose. We co-cultured killer C6706* and target E. coli MG1655 on LB medium containing different sugars, each at a concentration of 0.4%: fructose and galactose (monosaccharides), as well as sucrose, maltose and lactose (glucose-containing disaccharides). No other sugar permits the recovery observed with glucose, while fructose provides an intermediate level of protection (Fig. 2b, see “Discussion” section). When identical co-culture experiments are conducted using T6SS- defective killer C6706* cells, the number of recovered E. coli MG1655 cells is similar for all tested sugars (Fig. 2b).
To determine whether the effects on E. coli we observed were generalizable to other killers, we examined T6SS attacks from other V. cholerae strains. V. cholerae clinical strain V52 constitutively expresses T6SS genes and encodes the same toxins as C670611,42,43. Like C6706*, V52 poorly eliminates E. coli MG1655 on LBG (Fig. 2c). Strains BGT49, BGT64 and BGT69 were isolated from environmental, rather than clinical, sources44,45. These three strains engage in robust contact killing with effector toxins different from clinical strains and efficiently eliminate E. coli despite glucose supplementation (Fig. 2c)44,45. We recently reported that an engineered C6706* strain encoding the additional TleV1 effector originally found in strain BGT49 (but not in other strains tested here) kills parental V. cholerae C6706 cells lacking the cognate immunity gene44. Surprisingly, C6706* with TleV1 can kill E. coli even in the presence of glucose (Fig. 2c). These results reveal that glucose protection varies with target cells and with effector toxins deployed by different killers.
V. cholerae can efficiently use its T6SS when co-cultured with E. coli cells in the presence of glucose
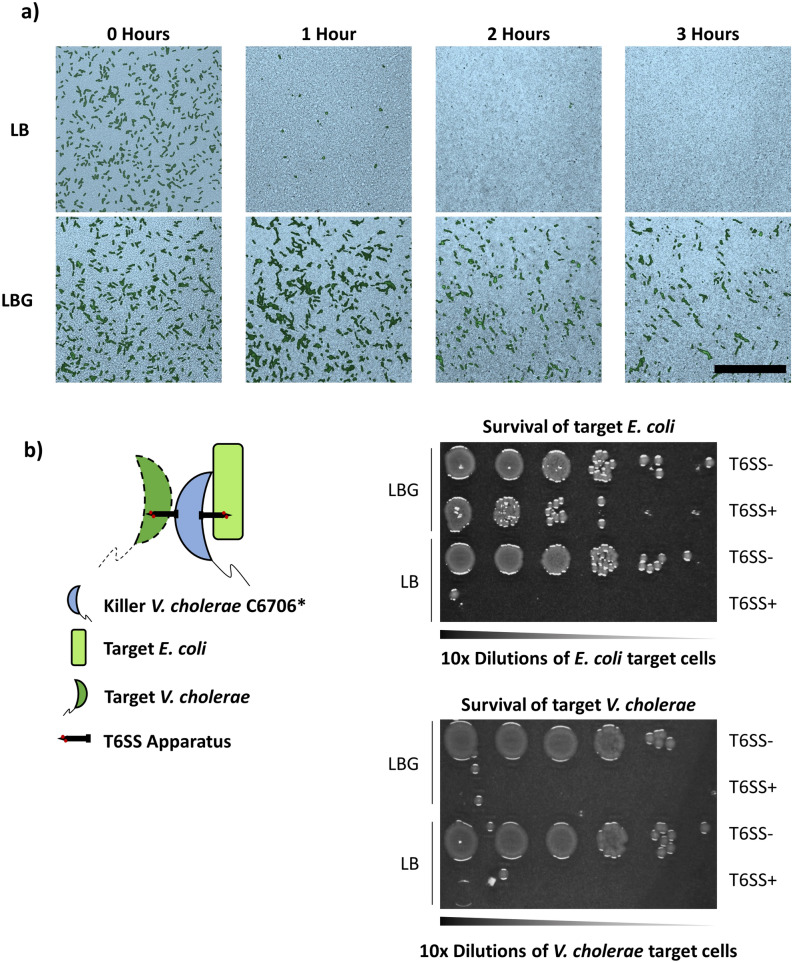
To confirm that individual E. coli cells are protected from T6SS attacks on LBG, we imaged co-cultures of sfGFP-labelled E. coli MG1655 with unlabeled V. cholerae C6706* by confocal microscopy. On LB medium, E. coli cells are eliminated after 3 h (Fig. 3a, top panels). By contrast, on LBG, multi-cell E. coli clusters surrounded by V. cholerae cells form and can be observed after 3 h (Fig. 3a, bottom panels). We also examined the membrane integrity of E. coli cells when competed against V. cholerae on LB or LBG. Propidium iodide (PI) is a DNA-binding dye that exhibits high fluorescence when bound to the DNA of cells with compromised membranes. We observed a PI signal for co-cultures on both LB and LBG (Supplementary Fig. S2). However, on LBG, the PI signal does not overlap with the signal from surviving sfGFP-labelled E. coli cells, indicating that the cells’ membranes remain intact (Supplementary Fig. S2).
Figure 3.
Individual E. coli MG1655 cells are protected against V. cholerae T6SS attacks but do not impair T6SS killing. (a) Fluorescently labeled green E. coli MG1655 cells were densely co-cultured with unlabeled V. cholerae C6706* on LB (top panels) or LBG (bottom panels) and the same frame was imaged for three hours using confocal microscopy. The pseudocolor images depict the progression of T6SS killing at different time points, showing brightfield microscopy images (bright blue) of the dense biofilm overlaid with the fluorescence signal of E. coli cells projected on a plane parallel to the agar substrate. Scale bar is 50 µm. (b) Killer V. cholerae C6706* or C6706* T6SS- was co-cultured with both E. coli MG1655 and susceptible V. cholerae target cells, and then diluted and plated as described in the “Methods” section.
To determine if E. coli can reduce the efficiency of T6SS-mediated killing in the presence of glucose, we performed a polyculture assay in which V. cholerae C6706* killer (or C6706* T6SS-) cells were cultured simultaneously with both E. coli and susceptible V. cholerae that lack immunity proteins for C6706* T6SS effectors and are defective for T6SS killing (Fig. 3b). Under these conditions, E. coli cells are protected from T6SS attacks and replicate as expected, while target V. cholerae cells are efficiently killed by the C6706* but not by C6706* T6SS- killer (Fig. 3b). Taken together, these results reveal that glucose allows individual E. coli cells to survive active T6SS attacks.
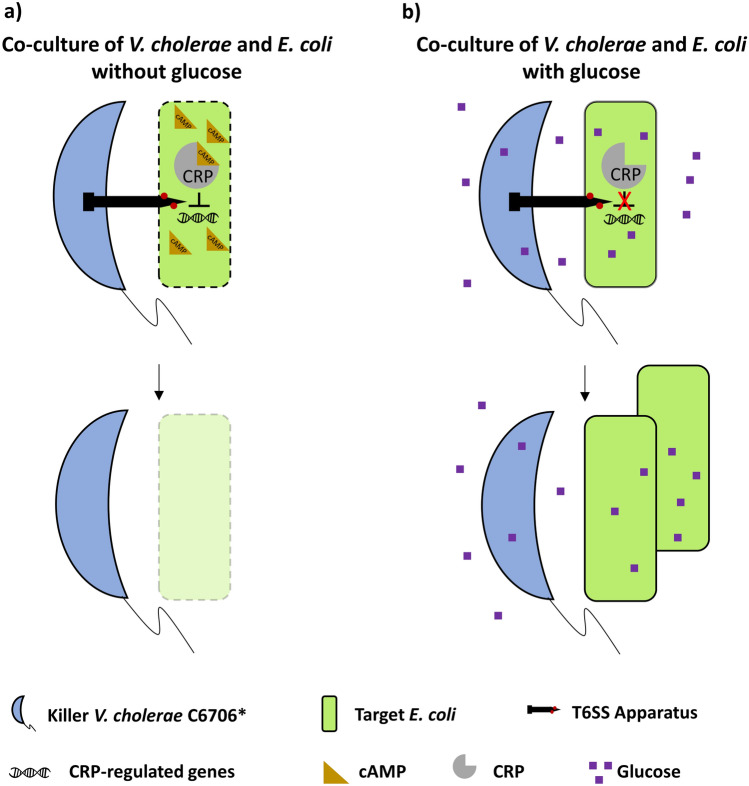
A crp disruption protects E. coli cells against T6SS attacks even in the absence of glucose
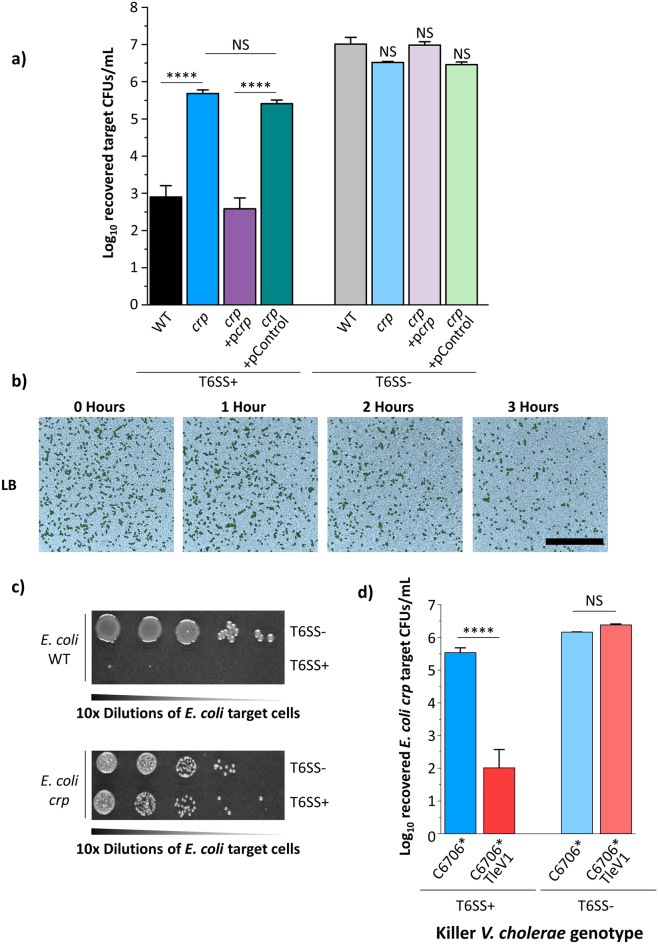
We wondered whether a physiological response to glucose levels could mediate the E. coli protection. In E. coli and other bacteria, low glucose conditions lead to accumulation of intracellular cyclic adenosine monophosphate (cAMP), which binds to and activates the cAMP receptor protein (CRP)46. CRP transcriptionally controls expression of hundreds of genes for varied behaviors46. Since high glucose conditions are mimicked by a crp disruption, we integrated an antibiotic resistance cassette into the crp gene on the chromosome of E. coli MG1655 (E. coli crp). When co-cultured with V. cholerae C6706* on LB lacking glucose, the number of recovered E. coli crp cells is comparable to that of wild type E. coli cells co-cultured with V. cholerae on LBG (Figs. 1a, 4a). Complementation with a plasmid expressing crp restores E. coli susceptibility to T6SS attacks (Fig. 4a). Using confocal microscopy, we confirmed that the crp disruption confers protection against T6SS attack to E. coli cells even in the absence of glucose (Fig. 4b). After 3 h, distinct clusters of sfGFP-labelled E. coli crp cells are visible when co-cultured with V. cholerae C6706* on LB, similar to wild type E. coli on LBG (Figs. 3a, 4b). We also observed that in a polyculture assay where V. cholerae C6706* killer cells are cultured with both the MG1655 crp disruption mutant and wild type MG1655, the MG1655 crp disruption mutant strain is protected against T6SS attacks while wild type MG1655 is successfully eliminated (Fig. 4c). Consistent with results on LBG (Fig. 2c), V. cholerae C6706* (but not C6706* T6SS-) with TleV1 efficiently kills E. coli crp on LB lacking glucose (Fig. 4d). These findings reveal that both exogenous glucose and a loss of CRP confer protection to attacks by some T6SS effectors.
Figure 4.
A crp disruption confers protection to E. coli MG1655 cells in the absence of glucose. (a) E. coli MG1655 cells with a crp disruption harboring the indicated plasmids were co-cultured with V. cholerae C6706* (or V. cholerae C6706* T6SS-) on LB medium. A two-way ANOVA with a post hoc Bonferroni test was used to determine significance. A minimum of 3 independent replicates were analyzed. (b) Fluorescently labeled green E. coli MG1655 crp cells were densely co-cultured with unlabeled killer V. cholerae C6706* on LB and the same frame was imaged for three hours using confocal microscopy. Brightfield images (bright blue pseudocolor) were overlaid with the fluorescence signal of E. coli cells projected on a plane parallel to the agar substrate. Scale bar is 50 µm. (c) Killer V. cholerae C6706* was co-cultured with both wild type E. coli MG1655 and E. coli crp target cells and then diluted and plated as described in the “Methods” section. (d) V. cholerae C6706* or V. cholerae C6706* with TleV1 (or the T6SS- mutant of each one) were co-cultured with E. coli MG1655 crp on LB medium. A two-way ANOVA with a post hoc Bonferroni test was used to determine significance. A minimum of 3 independent replicates were analyzed. ****p < 0.0001, NS not significant.
Discussion
Bacteria living in natural complex polymicrobial communities with limited resources are engaged in evolutionary arms races with other prokaryotes, eukaryotes and viruses47–49. Competition mechanisms include secretion of antibiotics, lysis by phages, and contact-dependent killing via the T6SS50,51. Antibiotic resistance and anti-CRISPR systems have emerged in response to these activities50–52. In addition to immunity proteins that neutralize effectors, it is becoming more apparent that arms races between T6SS killers and target bacterial cells have also led to the emergence of diverse defensive mechanisms we are beginning to identify17,34,53–55.
Cells lacking immunity proteins for cognate effectors are typically susceptible to T6SS attacks, but physical separation, stress response regulators and polysaccharide secretion contribute to protection from T6SS attacks16,17,20–22,34,53. We find that E. coli MG1655 overexpressing the rcsA gene for colanic acid production from a plasmid, though mucoid in appearance, only have a modest improvement in survival against V. cholerae C6706* T6SS killing on LB (Supplementary Fig. S3)17,56. Since this increase is several orders of magnitude lower than the protection induced by glucose, production of colanic acid is unlikely to be the primary factor responsible for the glucose-mediated protection in E. coli against V. cholerae T6SS attacks observed here (Supplementary Fig. S3, Fig. 1). Furthermore, because E. coli growth rates on LB or LBG are not significantly different during a 3 h monoculture growth assay (Supplementary Fig. S1), the glucose-mediated protection is not simply a consequence of initial large target cell domains that outnumber V. cholerae cells as reported previously34. This conclusion is also supported by our confocal microscopy results, where we observe persistence and then replication of single E. coli cells outnumbered and surrounded by V. cholerae cells (Fig. 3a).
Previously, glucose has been shown to alter interactions between pandemic V. cholerae strains and other bacteria25,26. In zebrafish hosts, E. coli cells inhibit growth of V. cholerae by secreting acids in the presence of glucose26. Although acid-induced growth inhibition of V. cholerae can occur after extended co-culture with an acid-producing bacterial species, we observe that the number of recovered V. cholerae cells is unaltered when co-cultured for 3 h with E. coli on LB medium with or without glucose (Fig. 1b)25,26. Acid stress response pathways contribute to protection of E. coli cells against V. cholerae T6SS attacks17,22. Consistent with this observation, we also observe that buffering LBG media at a near-neutral pH of 7.4 enhances killing by approximately tenfold (Supplementary Fig. S4). However, this is again insufficient to explain the 1000-fold protection imparted by glucose (Supplementary Fig. S4, Fig. 1a). It remains possible that acidic conditions created by secreted acetate during aerobic growth on LBG medium could trigger stress response pathways in E. coli cells, but these effects alone likely play a modest role in the protection from T6SS attacks observed here.
The addition of other sugars to LB media results in significantly fewer recovered E. coli cells compared to glucose, with fructose conferring an intermediate level of protection (Fig. 2b). Both fructose and glucose transporters have been shown to alter cAMP levels and CRP activity, which could explain why both sugars confer E. coli cells protection (Fig. 2b)46,57–60. We find that an E. coli crp disruption mutant is protected from V. cholerae C6706* attacks on LB (without glucose induction) in a similar manner to wild type E. coli on LBG (Fig. 4a). This indicates that CRP likely directly or indirectly represses a gene or genes that confers E. coli MG1655 protection against T6SS attacks (Fig. 5). CRP controls expression of genes involved in stress-response pathways61–63. Furthermore, CRP represses expression of antibiotic resistance genes in E. coli64. Since envelope damage, acidic stress and osmotic stress response pathways have been recently shown to increase protection against T6SS attacks, we hypothesize that CRP could also repress one or more stress response genes that confer protection against the T6SS17,20,22.
Figure 5.
Model depicting the proposed E. coli glucose-mediated protection mechanism against the V. cholerae C6706* T6SS. (a) When E. coli cells are grown on rich media in the absence of glucose, CRP is bound by cAMP and represses (directly or indirectly) an unknown gene (or genes). Repression of those genes results in higher susceptibility to T6SS attacks. (b) When E. coli cells are grown on rich media in the presence of glucose, CRP is not bound by cAMP and cannot repress the genes it controls under low glucose conditions. As a result, E. coli cells have increased protection against T6SS attack.
All tested human commensal E. coli strains survive T6SS attacks significantly better on LBG medium compared to LB, suggesting the glucose-mediated protection is widespread among diverse E. coli strains (Fig. 3a)35,37–39,41. Even though susceptible target V. cholerae and A. veronii strains display an increase in the number of recovered cells when the LB medium is supplemented with glucose, these target cells are efficiently eliminated, and the protection is much reduced compared to E. coli strains (Fig. 2a). The glucose-mediated protection also depends on the identity of the killer strain. Clinical strain V52 also kills E. coli MG1655 to a lesser degree with glucose present, similar to pandemic C6706*, although we observed more variation between replicates in the number of recovered E. coli cells when co-cultured with V52 on LBG (Fig. 2c, data not shown). Environmental V. cholerae strains, which encode different effectors than V52 or C6706, efficiently kill E. coli even in the presence of glucose (Fig. 2c)44. These results suggest that the toxicity of T6SS effectors may be differentially affected by glucose-mediated changes in E. coli.
The observation that V. cholerae C6706* killer cells carrying an additional, non-native effector can bypass the protection conferred by glucose and effectively kill E. coli also supports our conclusion that glucose does not prevent effector delivery by the killer into E. coli cells (Fig. 2c). These results suggest that the acquisition of diverse toxins might be an evolutionary adaptive strategy for bypassing protective mechanisms developed in potential target strains under fluctuating external conditions28,36,44,65. It is possible that the ability of certain killer strains to metabolize glucose might contribute to glucose-induced T6SS protection in some killer-target interactions. However, since CRP controls stress response pathways which might increase protection of E. coli against T6SS attacks, we favor a model that CRP-induced changes in the E. coli cell envelope may affect the toxicity of some V. cholerae T6SS effectors17,22,62–64.
To our knowledge, this study highlights the first demonstration that an external molecule can induce a genetic response that increases protection to T6SS attacks in target cells. Zhao et al. have shown that T6SS-mediated killing of E. coli cells by V. cholerae enhances cholera symptoms in mouse model systems12. We speculate that glucose or other external metabolites in the digestive tract could contribute to the neutralization by commensals of T6SS attacks from members of the gut microbiota or foreign pathogens66. Future experiments will identify the roles played by stress response pathways in the glucose-mediated protection to T6SS, determine the role of this protection in vivo, and study the effects glucose has on other killer and target bacterial species, including members of the human gut microbiome.
Methods
V. cholerae and E. coli genetic mutations
All V. cholerae mutant strains were made as previously described using the pKAS allelic exchange methods67. The E. coli crp mutant strain was made using the Lambda Red system68,69. The E. coli strain expressing sfGFP was made using an integration event from a pKAS construct. E. coli strains Nissle, HS and ECOR-2, as well as A. veronii and V. cholerae target cells harbored the pSLS3 plasmid to confer chloramphenicol resistance. Restriction enzymes, polymerases and Gibson mix reagents were used according to the manufacturer’s instructions (Promega and New England Biolabs). Plasmids were verified by PCR and Sanger sequencing (Eurofins). All strains and plasmids are described in Supplementary Table S1.
Bacterial co-culture assays
Bacterial cultures were grown in liquid LB or LB + glucose 0.4% (LBG) media with shaking at 37 °C overnight. Cultures were back-diluted, incubated at 37 °C with shaking for 3 h in the same conditions as the overnight cultures and the absorbance for each sample was set to an OD600 of 1. Strains harboring plasmids were grown in overnight liquid media with the respective antibiotics required to maintain plasmids and were washed three times with fresh media before co-cultured. Killer and target strains were mixed at a 10:1 (killer:target) ratio and 50 µL of the mixture was spotted on a filter paper with a 0.22 µm pore size. For polyculture assays, strains were mixed at a ratio of 10:1:1 (killer:target:target). The filter was placed on LB or LBG agar media and incubated at 37 °C for 3 h. Filters were vortexed in 5 mL of sterile LB medium for 30 s and 100 µL (or 3 μL for spot plating) of serial dilutions were spread on chloramphenicol plates to select for target cells. At least 3 independent replicates were used for statistical comparisons.
Modified co-culture assays were performed as described above for different conditions. Different sugars (fructose, sucrose, lactose, galactose and maltose) were added to LB to a final concentration of 0.4%. Overnight cultures, back-diluted cultures and co-culture experiments were performed on medium containing the respective sugar. Co-culture assays using buffered media were performed on LBG medium adjusted to a pH of 7.4 and buffered with 40 mM MOPS. Experiments were also conducted with buffered LBG medium containing 40 mM HEPES or 100 mM phosphate buffers and similar results were obtained. Overnight cultures, back-diluted cultures and co-culture experiments were performed on pH 7.4 buffered LBG medium. Strains expressing rcsA and crp were induced with 100 µM IPTG during overnight cultures and co-cultures with killer cells.
Confocal microscopy
Confocal microscopy experiments were performed as described previously44. Briefly, overnight cultures were back-diluted 1:100 for 3 h in the same media as the overnights and the concentration of each sample was set to an OD600 of 10. A 4 µL droplet aliquot of propidium iodide (100 µg/mL) was added to an LB or LBG agar pad on a glass slide and allowed to dry. Next, a 1 µL aliquot of a 10:1 killer:target cell mixture was spotted onto the propidium iodide drop on the agar pad. Cells were imaged at 96–100% humidity and 37 °C using a Nikon A1R confocal microscope. At different time points, a three-dimensional stack of the biofilm was recorded with lateral dimensions of 118 µm × 118 µm and a vertical step size of 1 µm. Fluorescence images were obtained by projecting all slices of the three-dimensional stack onto one plane parallel to the agar substrate. Those projected fluorescence images were overlaid onto a cross-sectional bright-field image of the biofilm. A Perfect Focus System with a 40 × objective (Plan Fluor ELWD 40 × DIC M N1) was used to stabilize the focus in the plane of the colony growth. Images were processed using ImageJ.
Supplementary Information
Acknowledgements
We would like to thank Dr. Jacob Thomas and Dr. Will Ratcliff for advice and useful discussions, Megan Dillon for assistance with co-culture assays, Dr. Wai-Leung Ng for providing us the E. coli Nissle strain, Dr. Shannon D. Manning for providing us the E. coli ECOR-2 strain and Dr. Vanessa Sperandio for providing us the E. coli HS strain. BKH would also like to thank funding from the Georgia Institute of Technology School of Biological Sciences, NSF (MCB 1149925 and BMAT-2003721) and BSF (2015103). GS would like to thank the German National Academy of Natural Sciences Leopoldina (LDPS 2017-03).
Author contributions
C.C., H.N., S.W. and G.S. designed and performed experiments. C.C., H.N., S.W., G.S., P.Y. and B.H. analyzed the data. C.C. and B.H. wrote the manuscript. All authors reviewed the manuscript. P.Y. and B.H. provided funding.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81813-4.
References
- 1.Senderovich Y, Izhaki I, Halpern M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE. 2010;5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Magny GC, et al. Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh sundarbans. Appl. Environ. Microbiol. 2011;77:6125–6132. doi: 10.1128/AEM.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huq A, et al. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 1983;45:275–283. doi: 10.1128/AEM.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sela R, Hammer BK, Halpern M. Quorum-sensing signaling by chironomid egg masses’ microbiota, affect Haemagglutinin/Protease (HAP) production by Vibrio cholerae. Mol. Ecol. 2020 doi: 10.1111/mec.15662. [DOI] [PubMed] [Google Scholar]
- 5.Pukatzki S, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unterweger D, et al. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat. Commun. 2014;5:3549. doi: 10.1038/ncomms4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisan CV, Hammer BK. The Vibrio cholerae type VI secretion system: toxins, regulators and consequences. Environ. Microbiol. 2020;22:4112–4122. doi: 10.1111/1462-2920.14976. [DOI] [PubMed] [Google Scholar]
- 9.Russell AB, et al. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature. 2013;496:508–512. doi: 10.1038/nature12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S. Lytic activity of the Vibrio cholerae Type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J. Biol. Chem. 2013;288:7618–7625. doi: 10.1074/jbc.M112.436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata ST, Unterweger D, Rudko SP, Pukatzki S. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog. 2013;9:e1003752. doi: 10.1371/journal.ppat.1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Caro F, Robins W, Mekalanos JJ. Antagonism toward the intestinal microbiota and its effect on Vibrio cholerae virulence. Science. 2018;359:210–213. doi: 10.1126/science.aap8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan SL, et al. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc. Natl. Acad. Sci. 2018;115:E3779–E3787. doi: 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fast D, Kostiuk B, Foley E, Pukatzki S. Commensal pathogen competition impacts host viability. Proc. Natl. Acad. Sci. 2018;115:7099–7104. doi: 10.1073/pnas.1802165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong TG, Ho BT, Yoder-Himes DR, Mekalanos JJ. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. 2013;110:2623–2628. doi: 10.1073/pnas.1222783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toska J, Ho BT, Mekalanos JJ. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. U.S.A. 2018;115:7997–8002. doi: 10.1073/pnas.1808469115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersch SJ, et al. Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat. Microbiol. 2020;5:706–714. doi: 10.1038/s41564-020-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le N-H, et al. Peptidoglycan editing provides immunity to Acinetobacter baumanniiduring bacterial warfare. Sci. Adv. 2020;6:5614. doi: 10.1126/sciadv.abb5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, et al. A high-throughput interbacterial competition screen identifies ClpAP in enhancing recipient susceptibility to type VI secretion system-mediated attack by Agrobacterium tumefaciens. Front. Microbiol. 2020;10:1–14. doi: 10.3389/fcimb.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lories B, et al. Biofilm bacteria use stress responses to detect and respond to competitors. Curr. Biol. 2020;30:1231–1244.e4. doi: 10.1016/j.cub.2020.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong TG, et al. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl. Acad. Sci. U.S.A. 2015;112:2181–2186. doi: 10.1073/pnas.1425007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal F, et al. Differential cellular response to translocated toxic effectors and physical penetration by the type VI secretion system. Cell Rep. 2020;31:107766. doi: 10.1016/j.celrep.2020.107766. [DOI] [PubMed] [Google Scholar]
- 23.Speare L, Smith S, Salvato F, Kleiner M, Septer AN. Environmental viscosity modulates interbacterial killing during habitat transition. MBio. 2020;11:1–14. doi: 10.1128/mBio.03060-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann V, et al. Bile salts modulate the mucin-activated type VI secretion system of pandemic Vibrio cholerae. PLoS Negl. Trop. Dis. 2015;9:e0004031. doi: 10.1371/journal.pntd.0004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon SS, Mekalanos JJ. 2,3-Butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immunol. 2006;74:6547–6556. doi: 10.1128/IAI.00695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nag D, Breen P, Raychaudhuri S, Withey JH. Glucose metabolism by Escherichia coli inhibits Vibrio cholerae intestinal colonization of zebrafish. Infect. Immunol. 2018;86:1–13. doi: 10.1128/IAI.00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Scrudato M, Blokesch M. A transcriptional regulator linking quorum sensing and chitin induction to render Vibrio cholerae naturally transformable. Nucleic Acids Res. 2013;41:3644–3658. doi: 10.1093/nar/gkt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgeaud S, Metzger LC, Scrignari T, Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 29.Watve SS, Thomas J, Hammer BK. CytR is a global positive regulator of competence, type VI secretion, and chitinases in Vibrio cholerae. PLoS ONE. 2015;10:e0138834. doi: 10.1371/journal.pone.0138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaskolska M, Stutzmann S, Stoudmann C, Blokesch M. QstR-dependent regulation of natural competence and type VI secretion in Vibrio cholerae. Nucleic Acids Res. 2018;46:10619–10634. doi: 10.1093/nar/gky717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng J, Ho B, Mekalanos JJ. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS ONE. 2011;6:e23876. doi: 10.1371/journal.pone.0023876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liakat S, et al. In vitro measurements of physiological glucose concentrations in biological fluids using mid-infrared light. Biomed. Opt. Express. 2013;4:1083. doi: 10.1364/BOE.4.001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. Liver Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein DB, Ringel P, Basler M, Wingreen NS. Established microbial colonies can survive type VI secretion assault. PLoS Comput. Biol. 2015;11:e1004520. doi: 10.1371/journal.pcbi.1004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm. Bowel Dis. 2008;14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 36.Thomas J, Watve SS, Ratcliff WC, Hammer BK. Horizontal gene transfer of functional type VI killing genes by natural transformation. MBio. 2017;8:1–11. doi: 10.1128/mBio.00654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine M, et al. Escherichia coli strains that cause diarrhœa but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;311:1119–1122. doi: 10.1016/S0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y, et al. A new immunoglobulin-binding protein, EibG, is responsible for the chain-like adhesion phenotype of locus of enterocyte effacement-negative, shiga toxin-producing Escherichia coli. Infect. Immunol. 2006;74:5747–5755. doi: 10.1128/IAI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochman H, Selander RK. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 1984;157:690–693. doi: 10.1128/JB.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenborn U. Escherichia coli strain Nissle 1917-from bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016;363:212. doi: 10.1093/femsle/fnw212. [DOI] [PubMed] [Google Scholar]
- 41.Leatham MP, et al. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect. Immunol. 2009;77:2876–2886. doi: 10.1128/IAI.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unterweger D, et al. Constitutive type VI secretion system expression gives Vibrio cholerae intra- and interspecific competitive Advantages. PLoS ONE. 2012;7:e48320. doi: 10.1371/journal.pone.0048320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metzger LC, et al. Independent regulation of type VI secretion in Vibrio cholerae by TfoX and TfoY. Cell Rep. 2016;15:951–958. doi: 10.1016/j.celrep.2016.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crisan CV, et al. Analysis of Vibrio cholerae genomes identifies new type VI secretion system gene clusters. Genome Biol. 2019;20:163. doi: 10.1186/s13059-019-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernardy EE, Turnsek MA, Wilson SK, Tarr CL, Hammer BK. Diversity of clinical and environmental isolates of Vibrio cholerae in natural transformation and contact-dependent bacterial killing indicative of type VI secretion system activity. Appl. Environ. Microbiol. 2016;82:2833–2842. doi: 10.1128/AEM.00351-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busby S, Ebright RH. Transcription activation by catabolite activator protein (CAP) J. Mol. Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- 47.Yanni D, Márquez-Zacarías P, Yunker PJ, Ratcliff WC. Drivers of spatial structure in social microbial communities. Curr. Biol. 2019;29:R545–R550. doi: 10.1016/j.cub.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 48.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Torsvik V, Øvreås L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002;5:240–245. doi: 10.1016/S1369-5274(02)00324-7. [DOI] [PubMed] [Google Scholar]
- 50.Cornforth DM, Foster KR. Antibiotics and the art of bacterial war. Proc. Natl. Acad. Sci. 2015;112:10827–10828. doi: 10.1073/pnas.1513608112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hampton HG, Watson BNJ, Fineran PC. The arms race between bacteria and their phage foes. Nature. 2020;577:327–336. doi: 10.1038/s41586-019-1894-8. [DOI] [PubMed] [Google Scholar]
- 52.Pawluk A, et al. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat. Microbiol. 2016;1:1–6. doi: 10.1038/nmicrobiol.2016.85. [DOI] [PubMed] [Google Scholar]
- 53.McNally L, et al. Killing by Type VI secretion drives genetic phase separation and correlates with increased cooperation. Nat. Commun. 2017;8:14371. doi: 10.1038/ncomms14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirchberger PC, Unterweger D, Provenzano D, Pukatzki S, Boucher Y. Sequential displacement of type VI secretion system effector genes leads to evolution of diverse immunity gene arrays in Vibrio cholerae. Sci. Rep. 2017;7:45133. doi: 10.1038/srep45133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross BD, et al. Human gut bacteria contain acquired interbacterial defence systems. Nature. 2019;575:224–228. doi: 10.1038/s41586-019-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottesman S, Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol. Microbiol. 1991;5:1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 57.Escalante A, Cervantes AS, Gosset G, Bolívar F. Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: Peculiarities of regulation and impact on growth and product formation. Appl. Microbiol. Biotechnol. 2012;94:1483–1494. doi: 10.1007/s00253-012-4101-5. [DOI] [PubMed] [Google Scholar]
- 58.Görke B, Stülke J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 59.Kornberg HL. Routes for fructose utilization by Escherichia coli. J. Mol. Microbiol. Biotechnol. 2001;3:355–359. [PubMed] [Google Scholar]
- 60.Tchieu JH, Norris V, Edwards JS, Saier J. The complete phosphotransferase system in Escherichia coli. J. Mol. Microbiol. Biotechnol. 2001;3:329–346. [PubMed] [Google Scholar]
- 61.Gosset G, Zhang Z, Nayyar S, Cuevas WA, Saier MH. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 2004;186:3516–3524. doi: 10.1128/JB.186.11.3516-3524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khankal R, Chin JW, Ghosh D, Cirino PC. Transcriptional effects of CRP* expression in Escherichia coli. J. Biol. Eng. 2009;3:1–14. doi: 10.1186/1754-1611-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franchini AG, Ihssen J, Egli T. Effect of global regulators RpoS and cyclic-AMP/CRP on the catabolome and transcriptome of Escherichia coli K12 during carbon- and energy-limited growth. PLoS ONE. 2015;10:1–24. doi: 10.1371/journal.pone.0133793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishino K, Senda Y, Yamaguchi A. CRP regulator modulates multidrug resistance of Escherichia coli by repressing the mdtEF multidrug efflux genes. J. Antibiot. (Tokyo) 2008;61:120–127. doi: 10.1038/ja.2008.120. [DOI] [PubMed] [Google Scholar]
- 65.LaCourse KD, et al. Conditional toxicity and synergy drive diversity among antibacterial effectors. Nat. Microbiol. 2018;3:440–446. doi: 10.1038/s41564-018-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wexler AG, et al. Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc. Natl. Acad. Sci. 2016;113:3639–3644. doi: 10.1073/pnas.1525637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skorupski K, Taylor RK. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 68.Yu D, et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomason LC, Sawitzke JA, Li X, Costantino N, Court DL. Recombineering: Genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. 2014;106:1–39. doi: 10.1002/0471142727.mb0116s106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.