Abstract
Background & Aims
Acid hypersensitivity is claimed to be a symptomatic trigger in functional dyspepsia (FD); however, the neuroimmune pathway(s) and the mediators involved in this process have not been investigated systematically. Palmitoylethanolamide (PEA) is an endogenous compound, able to modulate nociception and inflammation, but its role in FD has not been assessed.
Methods
Duodenal biopsy specimens from FD and control subjects, and peroxisome proliferator-activated receptor-α (PPARα) null mice were cultured at a pH of 3.0 and 7.4. Mast cell (MC) number, the release of their mediators, and the expression of transient receptor potential vanilloid receptor (TRPV)1 and TRPV4, were evaluated. All measurements also were performed in the presence of a selective blocker of neuronal action potential (tetradotoxin). FD and control biopsy specimens in acidified medium also were incubated in the presence of different PEA concentrations, alone or combined with a selective PPARα or PPAR-γ antagonist.
Results
An acid-induced increase in MC density and the release of their mediators were observed in both dyspeptic patients and controls; however, this response was amplified significantly in FD. This effect was mediated by submucosal nerve fibers and up-regulation of TRPV1 and TRPV4 receptors because pretreatment with tetradotoxin significantly reduced MC infiltration. The acid-induced endogenous release of PEA was impaired in FD and its exogenous administration counteracts MC activation and TRPV up-regulation.
Conclusions
Duodenal acid exposure initiates a cascade of neuronal-mediated events culminating in MC activation and TRPV overexpression. These phenomena are consequences of an impaired release of endogenous PEA. PEA might be regarded as an attractive therapeutic strategy for the treatment of FD.
Keywords: Functional Dyspepsia, Duodenal Mucosa, Mast Cells, Enteric Nervous System, Visceral Hypersensitivity
Abbreviations used in this paper: ALIAmides, autacoid local inflammation antagonism amides; ELISA, enzyme-linked immunosorbent assay; EPS, epigastric pain syndrome; FD, functional dyspepsia; IBS, irritable bowel syndrome; KO, knockout; MC, mast cell; NGF, nerve growth factor; PEA, palmitoylethanolamide; PDS, postprandial distress syndrome; PPARα, peroxisome proliferator-activated receptor-α; TRPV, transient receptor potential vanilloid; TTX, tetradotoxin
Graphical abstract
Summary.
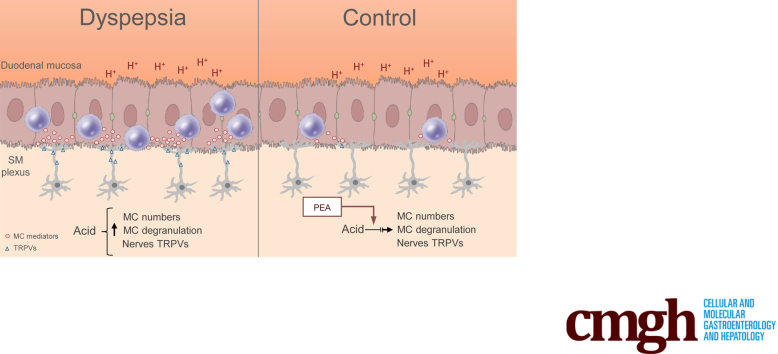
Functional dyspepsia is characterized by duodenal hypersensitivity to acid and low-grade inflammation. An impaired release of palmitoylethanolamide underlies the acid-induced activation of mast cells and sensitization of enteric neurons. Palmitoylethanolamide might be an attractive target in functional dyspepsia.
Functional dyspepsia (FD) is a heterogeneous and highly prevalent gastrointestinal disorder characterized by a plethora of recurrent symptoms located in the epigastrium, in the absence of any underlying organic cause.1,2 In the attempt to stratify dyspepsia patients into pathophysiological and therapeutically meaningful subtypes, the Rome criteria have recognized 2 main FD subgroups: the postprandial distress syndrome (PDS) characterized by meal-related symptoms, such as early satiety and postprandial fullness, and the epigastric pain syndrome (EPS), in which symptoms are mainly unrelated to meals, such as epigastric burning or pain. Traditionally, the stomach was indicated as the major culprit in dyspepsia pathophysiology.1, 2, 3, 4 More recently, a paradigm shift has occurred, with mounting evidence showing that subtle duodenal abnormalities, including low-grade intestinal inflammation, increased mucosal permeability, and increased chemical sensitivity of duodenal mucosa, play a crucial role in the generation of dyspeptic symptoms.5, 6, 7
Among the earlier-described mechanisms, the role of acid hypersensitivity is sustained by the empiric evidence that acid suppression, by either proton pump inhibitors or antihistamines (anti-H2), is effective in improving symptoms in a subsets of FD patients, especially those with EPS,3,8, 9, 10 and that both duodenal acid infusion and delayed acid clearance induces dyspeptic symptoms in healthy subjects.10, 11, 12, 13, 14, 15 Collectively, these results led to the hypothesis that duodenal sensitivity to acid participates in FD pathophysiology; however, the potential pathways underlying this phenomenon in dyspepsia have not been verified conclusively.
Duodenal acid stimuli indeed may activate submucosal nerve endings directly, through the involvement of acid-sensitive receptors, such as the transient receptor potential vanilloid (TRPV) subtypes.5, 6, 7 On the other hand, resembling what has been shown in models of esophagitis, luminal acid also could activate a reflex pathway involving mucosal mast cell (MC) degranulation and the subsequent sensitization and activation of capsaicin-sensitive afferent neurons.16,17 Indeed, preliminary data have shown that acid-suppressive therapy is able to improve low-grade inflammation and impaired mucosal integrity in the duodenum in FD.18 The complexity of the neuroimmune cross-talk responsible for the subtle, but consistently reported, duodenal abnormalities observed in FD patients has led to the hypothesis of a role for inflammatory cells, including MCs and eosinophils and their mediators in FD pathophysiology. It is believed that in functional gastrointestinal disorders, there is a disproportion between the protective and harmful response of mucosal inflammatory cells to subliminal stimuli (such as acid or lipids), ultimately leading to neural excitation (ie, visceral hyperalgesia) owing to the imbalanced release of inflammatory mediators. Nonetheless, many questions remain unresolved and the role of inflammatory cells in acid hypersensitivity and the possible neuroimmune pathways involved have not been investigated.
In this complex scenario, palmitoylethanolamide (PEA), an endogenous N-acylethanolamine, thought to be involved in several protective mechanisms, activated on-demand in response to proinflammatory stimuli.19, 20, 21 PEA belongs to a group of autacoid local inflammation antagonism amides (ALIAmides) involved in many pathophysiological processes, including pain processing and inflammation.20,22, 23, 24 The first described anti-inflammatory effects of PEA, known as the ALIA mechanism, were related mainly to its ability to modulate mast cell activation and degranulation.25,26 In addition, PEA is also a direct agonist of the vanilloid receptor TRPV127 and it has been shown extensively that this compound displays a wide range of anti-inflammatory effects mediated by peroxisome proliferator-activated receptor-α (PPARα) activation,28 a member of the nuclear hormone-receptor superfamily of ligand-activated transcription factors. In irritable bowel syndrome (IBS), lower PEA plasma levels were found to be associated significantly with more severe abdominal pain.29 To date, whether PEA is involved in FD pathophysiology remains uninvestigated.
Our aim was to evaluate the neuroimmune pathways involved in duodenal acid–induced responses ex vivo, and specifically to verify the following: (1) if an acid challenge of duodenal biopsy specimens from FD and control patients is able to recruit and activate mucosal MCs; (2) if acid-induced responses up-regulate the TRPV1- and TRPV4-expressing fibers, known to be involved in nociception; (3) if the release of the endogenous analgesic molecule PEA is impaired in dyspeptic patients; and (4) if the exogenous administration of PEA inhibits the acid-induced responses in duodenal biopsy specimens from FD patients through PPARα involvement.
Results
Acid Exposure Increases Duodenal Mucosa MC Density and Activity in a Nerve-Dependent Fashion
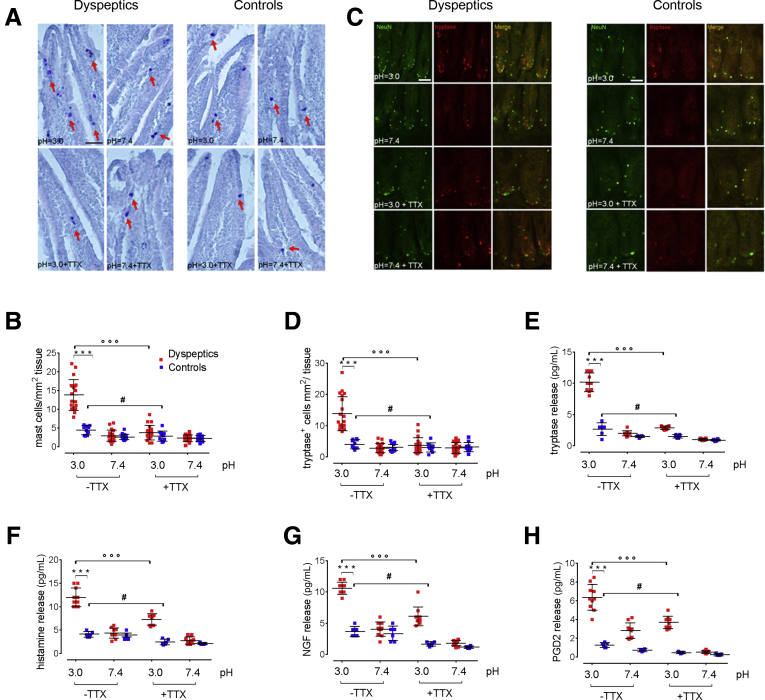
A duodenal acid challenge caused an increase in the density of MCs and tryptase-positive cells in the mucosa of all subjects, although a significantly higher increase in the dyspeptic patients than in the control group was observed (P < .001 vs control) (Figure 1A–D). Similarly, the release of MC mediators such as histamine, nerve growth factor (NGF), Prostaglandin D2 (PGD2), and tryptase were increased significantly in FD (Figure 1E–H). Immunofluorescence analysis showed that tryptase-positive cells were located in close proximity with Neuronal nuclear protein (NeuN)-positive fibers, likely suggesting that a MC–nerve interaction may occur after acid stimulation of the duodenal mucosa (Figure 1C).
Figure 1.
Effects of acid challenge on mucosal MC numbers and activation. (A) Histochemical images showing toluidine-positive cells (arrows) and (B) relative quantification of MCs in duodenal mucosa of dyspeptic and control biopsy specimens cultured at pH 3.0 and 7.4, respectively, and in the presence or absence of TTX. Original magnification: 20×. Data show the number of MCs counted per square millimeter of tissue. (C) Representative immunofluorescence images showing the close proximity of tryptase-immunoreactive cells (red) to NeuN-positive fibers (green). Original magnification: 20×. (D) Relative quantification of tryptase-immunopositive cells. Data show the number of tryptase-positive cells per square millimeter of tissue. (E–H) ELISA assays, respectively, quantifying the release of tryptase, histamine, NGF, and PGD2 in FD and healthy duodenal mucosal biopsy specimens. ∗∗∗P < .001 vs control; °°°P < .001 FD untreated vs pretreatment with TTX; #P < .05 control untreated vs pretreatment with TTX. All results are expressed as means ± SD of 20; n = 20 and 10 (B and D) and 10 and 6 (F–H) dyspeptic and control subjects, respectively.
Interestingly, pretreatment with tetradotoxin (TTX) (10-7 mol/L) significantly inhibited acid-induced recruitment and activation of MCs, and the release of histamine, NGF, PGD2, and tryptase in biopsy specimens of both FD and control subjects (P < .05 and P < .01 vs untreated for controls and FD patients, respectively) (Figure 1). The observation that the number of MCs and the release of their mediators was similar in both dyspeptic patients and controls, at a neutral pH, while they were increased significantly after acid exposure, likely suggests that this represents a physiological response to acid that is amplified significantly in FD patients. Furthermore, the ability of TTX to inhibit such acid-induced effects indicates that this mechanism, at least in part, is mediated by the activity of local nerve circuitry.
Duodenal Acid Exposure Up-regulates TRPV1 and TRPV4 Expression on Submucosal Nerve Endings
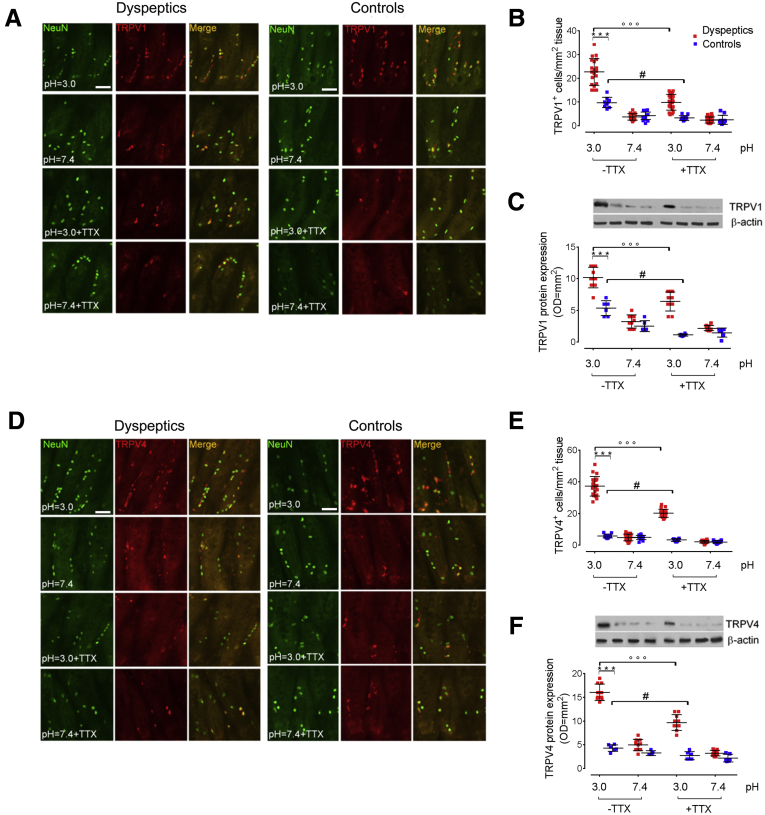
Compared with a neutral pH, the acid challenge resulted in an overall increased expression of TRPV1 and TRPV4 in both dyspeptic patients and controls, however, immunofluorescence quantization showed that the relative increase in immunoreactivity was significantly higher in the mucosa of dyspeptic patients than control subjects (P < .001) (Figure 2A, B, D, and E, respectively).
Figure 2.
Acid challenge up-regulates TRPV1 and TRPV4 expression in submucosal nerve endings. (A) Immunofluorescence staining of NeuN (green) and TRPV1-positive cells (red), and (B) relative graph bars quantifying TRPV1-positive cells in duodenal mucosa of dyspeptic and controls biopsy specimens cultured at pH 3.0 and 7.4, respectively, and in the presence or absence of TTX. Original magnification: 20×. Data show the number of TRPV1-positive cells per square millimeter of tissue. (C) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) quantifying TRPV1 protein expression. (D) Immunofluorescence staining and (E) relative graph bars quantifying TRPV4-positive cells (red). Original magnification: 20×. Data show the number of TRPV4-positive cells per square millimeter of tissue. (F) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) quantifying TRPV4 protein expression. ∗∗∗P < .001 vs control; °°°P < .001 FD untreated vs pretreatment with TTX and #P < .05 control untreated vs pretreatment with TTX. All results are expressed as means ± SD of 20; n = 20 and 10 (B and E) and 10 and 6 (C and F) dyspeptic and control subjects, respectively. OD, optical density.
Although MCs also have been shown to express TRPVs,30 immunofluorescence analysis showed that the acid-induced overexpression of TRPV1 and TRPV4 was located mostly on nerve fibers because it is co-localized with NeuN-positive fibers (Figure 2A and D, respectively). Western blot analysis confirmed that the acid-induced expression of both TRPV1 and TRPV4 was higher in dyspeptic patients than controls (Figure 2C and F). Further supporting the involvement of enteric neurons in duodenal responses, the increased expression of both TRPV1 and TRPV4 was inhibited by TTX pretreatment, and this effect was more evident in dyspeptic than in control subjects (P < .05 and P < .001 vs pretreatment for controls and dyspeptic patients, respectively) (Figure 2).
Acid-Induced Release of Endogenous Palmitoylethanolamide Is Impaired in Dyspepsia Patients
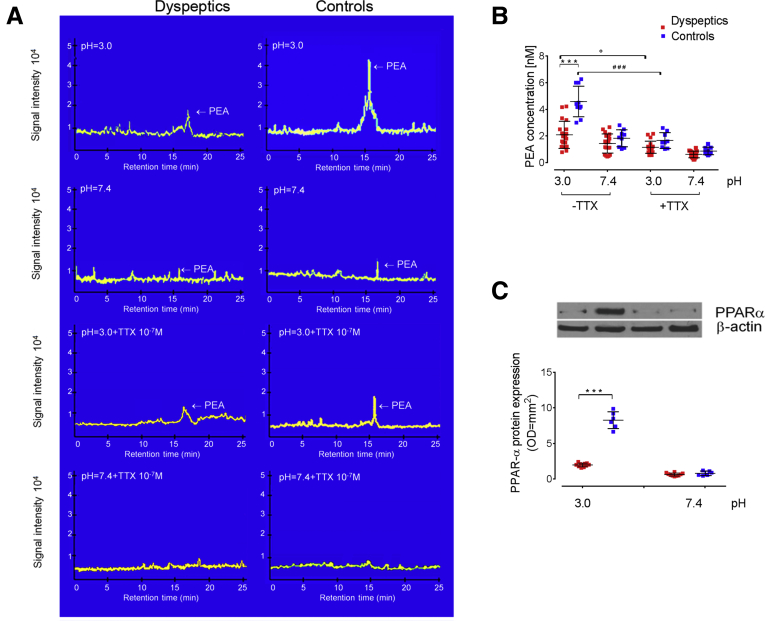
Palmitoylethanolamide is an on-demand, endogenously released molecule that exerts anti-inflammatory and analgesic properties19, 20, 21 and it has been shown to directly inhibit MC activation.24,25 In our experimental setting, at a neutral pH, the release of PEA was virtually absent in both controls and patients. After the acid challenge, the release of PEA was increased significantly in controls, but not in the dyspeptic group (P < .001) (Figure 3A and B). Pretreatment with TTX caused a significant inhibition of acid-induced PEA release (P < .05 vs pretreatment with TTX for both control and dyspeptic subjects) (Figure 3A and B), likely suggesting that the release of PEA is neuronal-dependent. Although the pharmacologic activity of PEA is still not understood completely, it has been clarified that PEA effects partially depend on its ability to activate PPARα receptors.28 We observed that paralleling the release of PEA, the expression of PPARα was increased significantly in controls upon acid stimulation, but not in FD subjects (P < .001 vs control at a pH of 3.0).
Figure 3.
Acid-induced release of PEA and PPARα expression in duodenal mucosa. (A) Representative chromatography coupled to tandem mass spectrometry analysis and (B) relative quantification of PEA levels (expressed as nanomolar concentration in duodenal homogenates) from mucosa of 20 dyspeptic and 10 control biopsy specimens cultured at pH 3.0 and 7.4, respectively, and in the presence or absence of TTX. (C) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) showing PPARα protein expression in tissue homogenates from 10 and 6 dyspeptic and control subjects, respectively. ∗∗∗P < .001 vs control; °P < .05 FD untreated vs pretreatment with TTX; ###P < .05 control untreated vs pretreatment with TTX. All results are expressed as means ± SD.
Exogenous PEA Dose-Dependently Counteracts the Acid-Induced Responses in Cultured Duodenal Biopsy Specimens of Dyspeptic Patients Through a PPARα-Mediated Pathway
We previously showed that exogenous PEA administration was able to reduce intestinal inflammatory responses in colonic biopsy specimens of ulcerative colitis patients31 and we, hence, ran a second set of experiments to test the ability of PEA to counteract the acid-induced responses in the duodenum of dyspeptic patients. We found that PEA significantly reduced the overall number of MCs and tryptase-positive cells and yielded to a consistent reduction of TRPV1 and TRPV4 immunopositivity in the mucosa exposed to acid (P < .001) (Figure 4A–H). Similarly, PEA induced a significant and concentration-dependent down-regulation of TRPV1 and TRPV4 protein expression and of histamine, tryptase, PGD2, and NGF release, (P < .05, P < .01, and P < .001 for PEA at 0.001, 0.01, and 0.1 μmol/L, respectively) (Figure 4I–M).
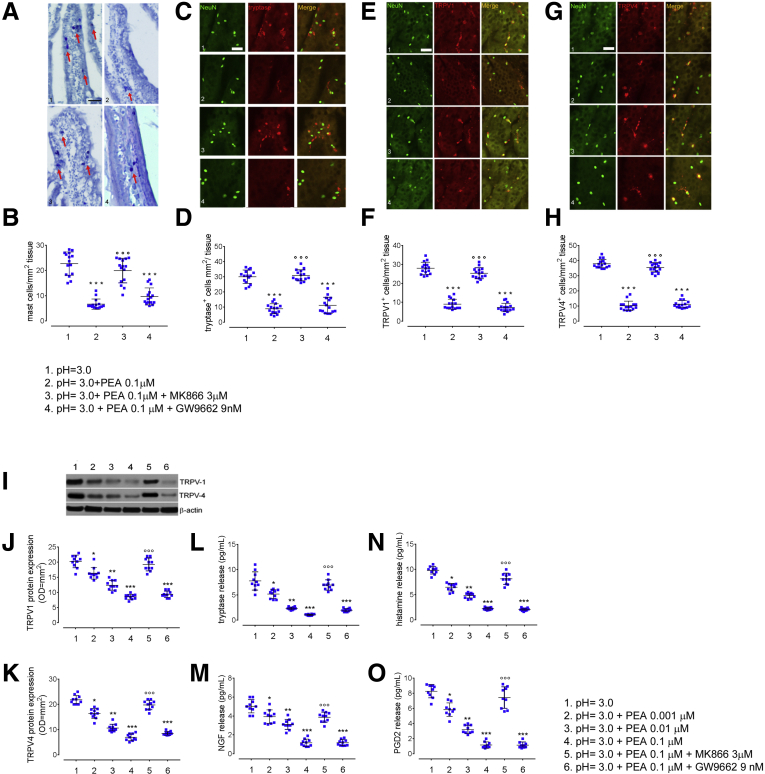
Figure 4.
Effects of exogenous PEA administration on acid-induced MC recruitment, TRPV1 and TRPV4 expression, and inflammatory mediator release in duodenal mucosa from dyspeptic patients. (A) Immunohistochemical images showing toluidine-positive cells (arrows) and (B) relative quantification of MCs in duodenal mucosa deriving from dyspeptic patient cultured biopsy specimens at (1) pH = 3.0, in the presence of (2) exogenous PEA (0.1 μmol/L), co-incubated with either (3) PPARα antagonist MK866 (3 μmol/L), or (4) PPARγ antagonist (GW9662 9 nmol/L). Original magnification: 20×. Data show the number of MCs counted per square millimeter of tissue. Immunofluorescence staining of NeuN (green) and (C) tryptase-, (E) TRPV1-, (G) TRPV4-positive cells (all red) and relative graph bars quantifying (D) tryptase-positive, (F) TRPV1-positive, (H) and TRPV4-positive cells. Data show the number of immune-reactive cells counted per square millimeter of tissue. Original magnification: 20×. (I) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) quantifying (J) TRPV1 and (K) TRPV4 protein expression at (1) pH = 3.0, in the presence of increasing concentrations of exogenous PEA (2) 0.001 μmol/L, (3) 0.01 μmol/L, (4) 0.1 μmol/L alone, or co-incubated with either (5) PPARα antagonist MK866 (3 μmol/L) or (6) PPARγ antagonist (GW9662 9 nmol/L). (L–O) ELISA essays quantifying, respectively, the release of tryptase, NGF (pg/mL), histamine, and PGD2 in dyspeptic biopsy specimens, cultured in the same experimental conditions. ∗P < .05 for PEA 0.001 μmol/L, ∗∗P < .01 for PEA 0.01 μmol/L, and ∗∗∗P < .001 for PEA 0.1 μmol/L vs acid challenge and for co-incubation with PPARγ antagonist GW9662 vs acid challenge; °°°P < .001 for co-incubation with PPARα antagonist MK866 vs acid challenge. All results are means ± SD of n = 20 dyspeptic subjects.
To provide mechanistic insights into PEA pharmacologic activity, we evaluated whether PEA anti-inflammatory effects were dependent on PPAR-receptor activation. We found that in the presence of MK866, a PPARα antagonist, PEA effects were inhibited significantly, whereas they were unchanged after incubation with the PPARγ antagonist GW9662 (P < .001 for PEA 0.1 μmol/L + MK866 3 μmol/L vs PEA 0.1 μmol/L and P < .001 for PEA 0.1 μmol/L + GW9662 9 nmol/L vs acid challenge, respectively) (Figure 4A–M).
Similar results were obtained in acid-treated control biopsy specimens, in which PEA induced a significant overall reduction of MC density and tryptase-positive cells, as well as the number of TRPV1- and TRPV4-expressing cells by a PPARα-mediated pathway (P < .001 vs acid challenge) (Figure 5A–H). In addition, PEA treatments caused a significant and concentration-dependent decrease of TRPV1 and TRPV4 expression and histamine, tryptase, PGD2, and NGF release induced by acid challenge (P < .1 for PEA 0.001 μmol/L, P < .01 for PEA 0.01 μmol/L, and P < .001 for PEA 0.1 μmol/L vs acid challenge) (Figure 5I–M). According to the previous results, we confirmed that PPARα antagonists, but not PPARγ antagonists, abolished PEA effects (P < .001 for PEA 0.1 μmol/L + MK866 3 μmol/L vs PEA 0.1 μmol/L and P < .001 for PEA 0.1 μmol/L + GW9662 9 nmol/L vs acid challenge, respectively) (Figure 5A–M). As summarized in Figure 6, the selective involvement of PPARα was shown further by the observation that PEA had no effect on acid-induced responses in PPARα knockout (KO) mice (P < .001 for untreated vs treated with PEA 10 μmol/L at pH 3.0).
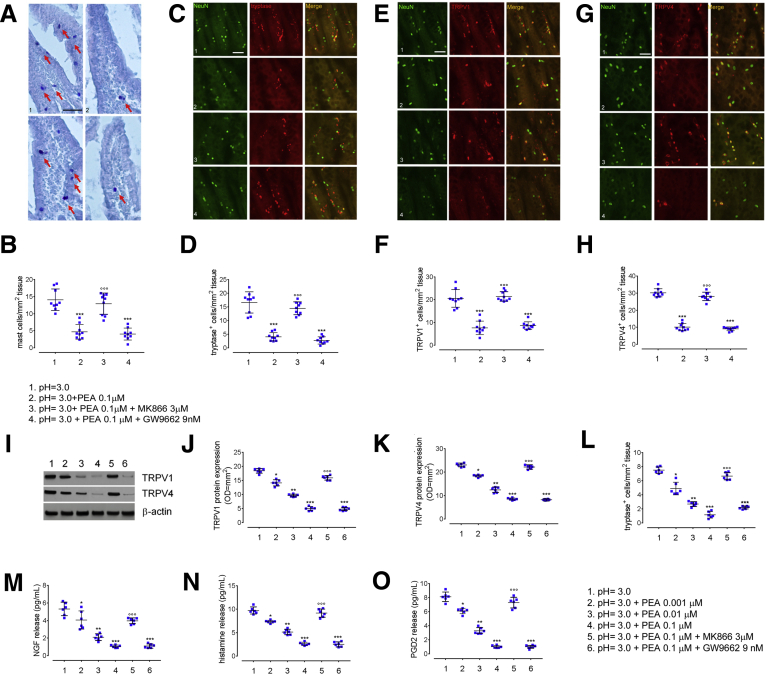
Figure 5.
Effects of increasing concentrations of exogenous PEA in in vitro duodenal biopsy specimens from controls. (A) Immunohistochemical images showing toluidine-positive cells (arrows) and (B) relative quantification of MCs in duodenal mucosa deriving from control cultured biopsy specimens at (1) pH = 3.0, in the presence of (2) exogenous PEA (0.1 μmol/L), co-incubated with either (3) PPARα antagonist MK866 (3 μmol/L) or (4) PPARγ antagonist (GW9662 9 nmol/L). Original magnification: 20×. Data show the number of MCs counted per square millimeter of tissue. Immunofluorescence staining of NeuN (green) and (C) tryptase-positive, (E) TRPV1-positive, and (G) TRPV4-positive cells (all red) and relative graph bars quantifying (D) tryptase-positive, (F) TRPV1-positive, (H) and TRPV4-positive cells deriving from controls biopsy specimens, cultured in the same experimental conditions. Original magnification: 20×. Data show the number of immune-reactive cells counted per square millimeter of tissue. (I) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) quantifying (J) TRPV1 and (K) TRPV4 protein expression in tissue homogenates deriving from control cultured biopsy specimens at (1) pH = 3.0, in the presence of increasing concentrations of exogenous PEA (2) 0.001 μmol/L, (3) 0.01 μmol/L, (4) 0.1 μmol/L alone or co-incubated with either (5) PPARα antagonist MK866 (3 μmol/L) or (6) PPARγ antagonist (GW9662 9 nmol/L). (L–O) ELISA essays quantifying, respectively, the release of tryptase, NGF, histamine, and PGD2 in dyspeptic biopsy specimens, cultured in the same experimental conditions. ∗P < .05 for PEA 0.001 μmol/L, ∗∗P < .01 for PEA 0.01 μmol/L, and ∗∗∗P < .001 for PEA 0.1 μmol/L vs acid challenge and for co-incubation with PPARγ antagonist GW9662 vs acid challenge; °°°P < .001 for co-incubation with PPARα antagonist MK866 vs acid challenge. All results are means ± SD of n = 10 control subjects.
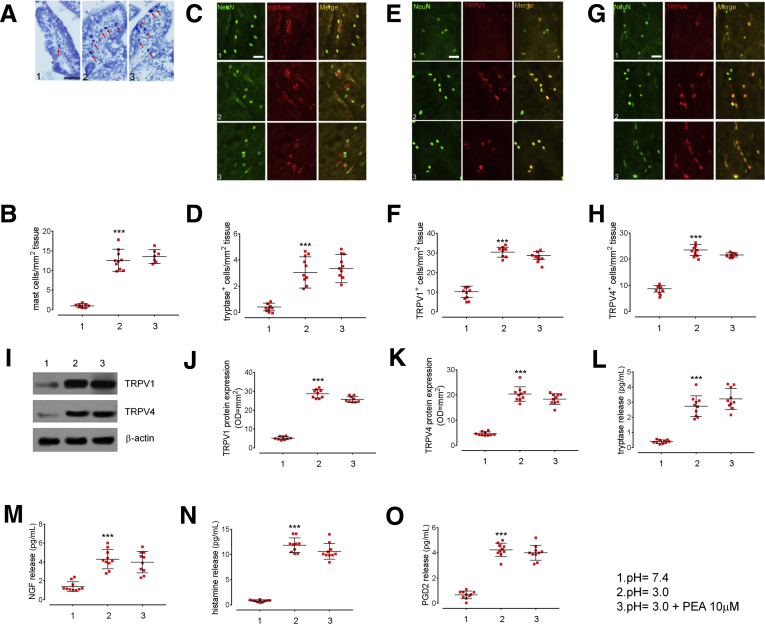
Figure 6.
Effects of acid challenge and exogenous PEA administration in PPARα KO mice. (A) Histochemical images showing toluidine-positive cells (arrows) and (B) relative quantification of MCs in the duodenum of PPARα KO mice at pH 3.0 and 7.4, in the presence or absence of exogenous PEA 10 μmol/L. Original magnification: 20×. Data show the number of MCs counted per square millimeter of tissue. Immunofluorescence staining of NeuN (green) and (C) tryptase-positive, (E) TRPV1-positive, and (G) TRPV4-positive cells (all red) and relative graph bars quantifying (D) tryptase-positive, (F) TRPV1-positive, (H) and TRPV4-positive cells deriving from PPARα KO mice in the same experimental conditions. Original magnification: 20×. Data show the number of immune-reactive cells counted per square millimeter of tissue. (I) Immunoblot analysis and relative densitometric analysis (arbitrary units normalized on the expression of the housekeeping protein β-actin) quantifying (J) TRPV1 and (K) TRPV4 protein expression. (L–O) ELISA essays quantifying, respectively, the release of tryptase, NGF, histamine, and PGD2 in PPARα KO mice, in the same experimental conditions. All results are the means ± SD of n = 10 mice for each experimental group, respectively. ∗∗∗P < .001 vs acid challenge.
Discussion
Visceral hypersensitivity, defined as the heightened perception of subliminal visceral sensations, is a hallmark feature of FD patients. It is well recognized that the duodenal mucosa of dyspepsia patients could over-react to physiological stimuli, and several chemicals had been advocated to induce visceral sensitization through the recruitment of sensory neurons and the reduction of pain threshold.32
Among the chemicals able to prime dyspeptic symptoms, compelling evidence is arising on the role of acid. For instance, duodenal acid infusion promotes the onset of nausea in both healthy subjects and dyspeptic patients; and a higher 24-hour acid exposure was detected in the duodenum of FD patients.13,14 Preliminary data showed that acid-suppressive therapy improves duodenal mucosal integrity and low-grade inflammatory activity in dyspeptic patients.18 However, how the change in pH could interfere with duodenal physiology and the underlying pathways involved have not been investigated systematically.
In our study, we observed that mucosal MCs are recruited and activated by an acid challenge. The observation that this phenomenon occurred both in FD and controls suggests that mucosal MCs potentially participate in the physiological responses to the lowering of pH. Nevertheless, in FD patients, this response was exaggerated, with a 2-fold increase in MC number and a 3-fold increase in the release of their mediators. MCs play a key role in the communication between the environment and enteric neurons and this bidirectional interaction seems to be pivotal in the proper functioning of the gastrointestinal tract.33, 34, 35, 36, 37, 38, 39
Based on this rationale, we evaluated whether nerve fibers participate in MC activation in FD, by blocking the enteric neurotransmission with TTX, before the acid challenge. Although there is evidence that acid stimuli may affect MC function per se,16,17 we could not prove this direct interaction. On the contrary, our results showed that TTX significantly inhibited MC recruitment and the release of their mediators, confirming the hypothesis that the acid-mediated increase in MCs is modulated by enteric neurons.40
Notably, acid exposure also promotes the release of NGF, a neurotrophin produced by both MCs and neurons. This mediator, crucial for neuronal survival, has been involved in neuroplasticity by activating numerous molecular pathways able to permanently induce structural and functional changing in enteric neurons.36,41 This evidence supports the idea that submucosal nerve endings mediate the duodenal response to acid, and that the activation of this pathway persistently may alter the neuronal network, reorganizing their structure, function, and/or connections.42
Interestingly, acid also represents one of the main agonists of the TRP channels, which are intrinsic membrane receptors involved in visceral nociception. These receptors are expressed widely on sensory nerves and viscera43 and both TRPV1 and TRPV4 receptors have been associated strongly with gastrointestinal inflammation and abdominal pain.34,35,43, 44, 45, 46 Hence, we evaluated the expression of TRPV1 and TRPV4 in the duodenum of FD patients and controls at neutral and acid pH, respectively.
After acid exposure, we observed that the expression of both TRPV1 and TRPV4 increased significantly in both dyspeptic patients and controls, but this effect was again amplified significantly in FD patients. The activation of TRPV channels is a key step in visceral nociception and its function is finely regulated.47
Among the endogenous compounds able to modulate the activation of these receptors, PEA is a N-acylethanolamine, released on demand in response to several proinflammatory stimuli.19, 20, 21, 22, 23, 24
Indeed, recent studies have shown that this amide is able to modulate both pain perception and the neuroinflammatory response and might induce TRPV desensitization directly.17,48
In our study, we observed that acid exposure induces the release of PEA in healthy subjects, supporting that this amide takes part in the regulation of the neuroinflammatory response in vivo. On the contrary, after the acid challenge, we observed impaired release of PEA in the duodenum of dyspeptic patients as compared with controls. Because previous studies have shown that PEA also interacts with MCs,49,50 we hypothesized that both the increased number of MCs and the activation of TRPV 1 and TRPV 4 depends, at least in part, on the reduced levels of PEA.
We therefore evaluated whether the exogenous administration of PEA was able to inhibit the acid-induced MC activation and TRPV up-regulation in dyspeptic patients. After the co-incubation with acid and PEA, in duodenal biopsy specimens of FD patients, we observed that the number of MCs, as well as the expression of TRPV1 and TRPV4, were reduced significantly compared with the acid challenge alone.
The reason why dyspeptic patients produce less PEA remains to be established, but this is in line with other signs of decreased activity of endocannabinoid synthesis pathways in these patients, as observed in imaging studies of endocannabinoid receptors in the brain.51
PEA anti-inflammatory properties could be related to several underlying mechanisms, such as the following: (1) as stated earlier, one of the first described anti-inflammatory effects of PEA was related to its ability of directly modulating MC activation, (2) PEA is able to induce TRPV desensitization directly, and (3) PEA can activate PPARα,31 a member of the nuclear hormone-receptor superfamily of ligand-activated transcription factors.
To gain more mechanistic insights into the anti-inflammatory properties of PEA in FD, we decided to investigate the effects of a selective inhibitor of PPARα receptors, MK866, on MC recruitment and TRPV activity in acid-incubated biopsy specimens in the presence of PEA. Pretreatment with MK866 prevented the protective effects of PEA on duodenal tissue, while PEA effects were unaffected by the co-administration of the selective PPARγ antagonist GW9662. Supporting the role of PPARα receptors further, PEA was unable to inhibit the recruitment and activation of mast cells and the up-regulation of TRPV1 and TRPV4 receptors in PPARα KO mice. Altogether, these data support that the effects of PEA are mediated by its agonism on PPARα receptors. Our results are in line with the recent observation that there is a strong interaction between PPARα receptors and TRPV channels, and that this cross-talk plays an important role in pain modulation.52
In conclusion, our observations support that duodenal acid exposure induces a cascade of TTX-dependent events that ultimately lead to MC activation and TRPV overexpression, and that these phenomena are at least partly secondary to an impaired release of endogenous PEA. Because the exogenous administration of PEA was able to counteract the neuroinflammatory response in ex vivo duodenal biopsy specimens of FD patients, PEA might be regarded as a potential, innovative, manageable, and low-cost treatment for FD.
Our study was not without setbacks. First, we did not evaluate mucosal barrier function. In a recent article evaluating the ultrastructural duodenal abnormalities of FD patients, Vanheel et al53 showed an increase in MC and eosinophil density and degranulation in FD patients. Although these investigators failed to observe an association between activation of these cells and impaired mucosal integrity, previous evidence has suggested that increased acid load could disrupt the intestinal barrier and lead to the impairment of duodenal membrane integrity, which in turn correlates with low-grade inflammation.5, 6, 7 On the other hand, acid hypersensitivity itself may be an epiphenomenon related to impaired duodenal integrity and permeability54 by enabling the passage of H+ ions through the epithelium. Second, in this study we only tested the TRP channels, while other acid-sensitive channels, such as Acid Sensitive Ionic Channels, were not assessed. Previous evidence5,55 has described that Acid Sensitive Ionic Channels also are expressed on duodenal visceral afferent nerve endings and they could be involved in acid sensitization; nonetheless, we have not studied their involvement. Third, PEA belongs to the wider family of endocannabinoid-like compounds, which comprise several lipid-derived mediators (including N-oleoylethanolamine) that have been shown to act synergistically with prototypic endocannabinoids by either competing for enzymatic degradation or increasing their receptor-binding affinity.23,24 Our data suggest an impairment of the endocannabinoid system in FD, supporting the renowned, yet unverified, hypothesis of clinical endocannabinoid deficiency in chronic functional pain syndromes.56 It is therefore conceivable that, analogously to PEA, other components of the endocannabinoid system, namely N-oleoylethanolamine or the cannabinoid receptors, also could be involved in FD pathophysiology. Finally, our results could have been strengthened by discriminating patients according to dyspepsia subtyping based on the prevalent symptom pattern (EPS vs PDS) and/or based on the acute postinfectious onset of the symptoms, which has been shown previously to correlate with low-grade inflammatory changes.57 Unfortunately, there was a high degree of overlap between EPS and PDS subgroups, with more than 50% of our population complaining of both meal-related and unrelated symptoms, which, regrettably, often reflects the clinical scenario in everyday clinical practice.58 This together with the small sample size prevented us from performing a post hoc analysis examining the impact of the different FD subgroups on our results. Moving forward, assessing whether these responses are preferentially altered in certain subgroups of dyspeptic patients could provide a better understanding of the pathophysiological mechanisms underlying the genesis of dyspepsia symptoms and allow patient selection that could benefit the most from PEA treatment.
Despite these limitations, we provide evidence here that PEA release is impaired in the duodenal mucosa of FD patients and that its exogenous administration is able to restore MC infiltration and TRPV up-regulation, thus providing the rationale for its use in the pharmacotherapy of FD. Because PEA currently is administered orally as a dietary supplement,19,59 it would be of remarkable clinical interest to test its efficacy in FD patients, given their still-disappointing response to pharmacotherapy.
The treatment with PEA/polydatin was tested in a recent randomized controlled trial in IBS patients,59 further proving that the ALIAmides, the endocannabinoids, or, more likely, both systems are involved in functional disorders featured by chronic pain.
Although in this clinical study we were unable to discern whether PEA effects were related to the modulation of the nervous system, secondary to MC stabilization or to the modulation of the endocannabinoid system, PEA in combination with polydatin was effective in reducing the severity of abdominal pain/discomfort in IBS. The originality of our study stands in the evaluation of the mechanistic pathways involved in PEA release in healthy and dyspeptic patients and in proving that this ALIAmide participates in acid-induced responses in vivo. Hopefully, by providing evidence of an impaired PEA release, this study will prompt future studies that aim to analyze the role of the endocannabinoid and ALIAmides systems in FD systematically, as well as in other functional gastrointestinal disorders.
Material and Methods
Patients and Experimental Design
The experimental group comprised 20 patients diagnosed with FD according to Rome III criteria, referred to our tertiary center for diagnostic esophagogastroduodenoscopy (dyspeptic group; 14 girls; mean age, 42 ± 9.1 y) and 10 control subjects (control group; 7 girls; mean age, 45 ± 9.9 y), undergoing esophagogastroduodenoscopy for gastric cancer screening. All studied subjects gave written informed consent. All procedures were approved by the ethical committee of the University of Naples Federico II. Patients were considered eligible after exclusion of organic causes for dyspeptic symptoms, as assessed by careful history taking, clinical examination, and routine biochemistry. During the consultation, patients’ main symptoms also were noted by using the standardized Patients Assessed GastroIntestinal-symptoms questionnaire60 and patients were classified as having EPS (3 patients, 2 girls) or PDS (6 patients, 4 girls) dyspepsia subtype according to Rome criteria. When complaining of both meal-related and unrelated symptoms, patients were classified as overlapping EPS-PDS subtype (11 patients, 8 girls). During the endoscopy, routine biopsy specimens were taken from the antrum and from the second part of the duodenum. Exclusion criteria were considered as follows: presence of esophagitis, gastric atrophy, Helicobacter pylori infection, erosive gastroduodenal lesions at endoscopy, the use of nonsteroidal anti-inflammatory drugs, drugs affecting gastric acid secretion during the preceding 2 weeks, corticosteroids or other immunosuppressive drugs in the preceding 6 months, diabetes or celiac disease, first-degree family members with type 1 diabetes, history of allergy, or inflammatory bowel disease.
In all eligible patients, 6 biopsy specimens were collected from the second part of the duodenum. All biopsy specimens were oriented with the basolateral membrane cultured in fetal bovine serum–supplemented Dulbecco’s modified Eagle medium (Sigma Aldrich, Milano, Italy) at 37°C in 5% CO2/95% air, while the apical membrane was challenged with normal or acidified Dulbecco’s modified Eagle medium at a pH of 7.4 and 3.0. All biopsy specimens were cultured with or without a selective blocker of neuronal action potential (10-7 mol/L TTX; Tocris Bioscience, Bristol, UK) to assess the enteric neuronal involvement in acid-induced responses. In a subset of experiments, acid-challenged dyspeptic and control biopsy specimens also were incubated with increasing concentrations of PEA (0.001, 0.01, or 0.1 μmol/L) (Tocris Bioscience) alone, or combined with a selective PPARα antagonist (3 μmol/L MK866; Tocris Bioscience) or PPARγ antagonist (9 nmol/L GW9662; Tocris Bioscience). Concentrations of both antagonists were selected based on our previous experiments and studies reported in the literature.61,62 Biopsy specimens then were homogenized and analyzed by Western blot and enzyme-linked immunosorbent assay (ELISA) analysis as described later. In the same experimental conditions, some samples were fixed in paraformaldehyde and used for immunohistochemical or immunofluorescence analysis.
Animals
Six-week-old PPARα KO mice (Taconic, Germantown, New York) were used for the experiments. All procedures were approved by La Sapienza University's Ethics Committee. Animal care was in compliance with the International Association for the Study of Pain and European Community (EC L358/1 18/12/86) guidelines on the use and protection of animals in experimental research. All mice were maintained on a 12-hour light/dark cycle in a temperature-controlled environment with access to food and water ad libitum. PPARα KO mice (n = 16) were killed and the duodenum was carefully isolated and treated according to the earlier-described experimental design.
Protein Extraction and Western Blot Analysis
Human biopsy specimens and mouse tissues were homogenized in ice-cold hypotonic lysis buffer to obtain cytosolic extracts according to a method previously published by our group.63 Extracts underwent electrophoresis through a polyacrylamide minigel. Proteins were transferred onto a nitrocellulose membrane that was saturated with nonfat dry milk and then incubated with either rabbit anti-TRPV1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-TRPV4 (Novus Biological, Ltd, Cambridge, UK), rabbit anti-PPARα (Abcam, Cambridge, UK), or mouse anti–β-actin (Santa Cruz Biotechnology). Membranes then were incubated with the specific secondary antibodies conjugated to horseradish peroxidase (Dako, Milan, Italy). Immune complexes were shown by enhanced chemiluminescence detection reagents (Amersham Biosciences, Milan, Italy). Blots were analyzed by scanning densitometry (GS-700 imaging densitometer; Bio-Rad, Milan, Italy). Results were expressed as optical density (arbitrary units; mm2) and normalized on the expression of the housekeeping protein β-actin.
ELISA for NGF, Prostaglandin 2, Tryptase, and Histamine Release
ELISA for NGF (Novus Biological), Prostaglandin 2 (Cusabio, Wuhan, China), tryptase (Antikorper Online, Aachen, Germany), and histamine (Antikorper Online) was performed on tissue homogenates. For each specific sample, depending on its human or murine origin, according to the provided manufacturer’s protocol a quantification of tissue-released mediators was performed. Absorbance was measured on a microtiter plate reader (Biochrom EZ Read 400 ELISA Microplate Reader; Rodano, Milan, Italy). NGF, Prostaglandin 2, tryptase, and histamine levels were determined using a standard curve method.
Histochemical and Immunohistochemistry Analyses
After the treatment, tissues were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned in 10-μm–thick serial sections, and processed for histologic analysis. To evaluate the MC duodenal infiltration, the samples were stained with 0.5% toluidine blue according to the manufacturer’s protocol (Thermo Scientific Raymond Lamb, Fisher Scientific, Eastobourne, UK). Images of at least 6 representative nonoverlapping fields were recorded by an Optika microscope equipped with a Pro HDMI PC-TV Camera (Optika, Ponteranica, BG, Italy) and toluidine-positive cells were counted in a blinded fashion (by L.S. and G.E.). The data represent the median results of the 2 blinded assessors; in all cases, results of the assessments differed by no more than 5%. Results were quantified by ImageJ software (National Institutes of Health, Bethesda, MD) and are expressed as the number of cells per square millimeter.
Samples for immunohistochemical assessment were fixed in 4% paraformaldehyde, then postfixed overnight with 30% sucrose, and frozen using 2-methylbutane. Tissues then were sectioned in 10-μm slices by cryostat cutting and processed for immunofluorescence. To avoid unspecific staining, slices were pretreated with 10% bovine serum albumin 0.1% Triton (Sigma Aldrich, Milano, Italy) X-100–phosphate-buffered saline solution for 90 minutes at room temperature and subsequently stained for 1 hour with mouse anti-TRPV1 antibody (Alomone Labs, Jerusalem, Israel) and mouse anti-TRPV4 antibody (US Biological, Salem, MA; Life Science, Amsterdam, Netherlands), mouse antitryptase antibody (Abcam), and rabbit anti-NeuN antibody (Merck Millipore, Billerica, MA). Sections then were incubated for 1 hour at room temperature in the dark with the proper secondary antibody: fluorescein isothiocyanate–conjugated anti-rabbit (1:100; Abcam) or Texas Red–conjugated anti-mouse (1:100 and 1:64, respectively; both from Abcam, Cambridge, UK). Slides were analyzed with a microscope (Nikon Eclipse 80i by Nikon Instruments Europe), and images were captured at 10× and 20× magnification by a high-resolution digital camera (Nikon Digital Sight DS-U1). Images were analyzed using ImageJ software (National Institutes of Health), and positive cells in randomly selected areas were counted independently (L.S. and G.E.). Immunofluorescence-positive cells in each square millimeter then were recorded to achieve the average values.
Measurement of PEA Levels in Human and Mouse Tissues
Human and mouse samples were immediately weighed, dipped into liquid nitrogen, and then stored at −70° until analysis. Samples were dried by speed vacuum, redissolved in methanol, vortexed, and centrifuged. The supernatant was analyzed by liquid chromatography coupled to tandem mass spectrometry using a 325-Mass Spectometer liquid chromatography/mass spectrometry Triple Quadrupole Mass Spectrometer (Agilent Technologies Italia, Cernusco s/N, Italy). According to the literature,64 retention time of PEA fractions was detected at approximately 15–18 minutes. To determine PEA concentrations, the mass spectrometer was operated in the positive ion, multiple-reaction monitoring mode. The linearity of the measuring range was assessed with standard curves ranging from 0.01 to 20 nmol/L. Standard curves were generated using linear regression. PEA levels were quantified in both mouse and human samples and expressed as a nanomolar concentration.
Data and Statistical Analysis
Results were expressed as means ± SD of n experiments. Data distribution was checked with the D'Agostino and Pearson normality test. Statistical analysis was performed using parametric 1-way analysis of variance and multiple comparisons were performed by the Bonferroni post hoc test. P values less than .05 were considered significant.
Acknowledgments
The authors thank Professor Dario Bruzzese, Associate Professor in Biostatistics at the Department of Public Health, University “Federico II” of Naples, for his valuable input in revising the statistical methods and the graphic results of our manuscript.
CRediT Authorship Contributions
Giovanni Sarnelli, Prof (Conceptualization: Lead; Supervision: Lead; Validation: Lead; Writing – review & editing: Equal);
Marcella Pesce, MD (Investigation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal);
Luisa Seguella, Dr (Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Writing – original draft: Equal);
Jie Lu, Prof (Formal analysis: Equal; Investigation: Equal);
Eleonora Efficie, Dr (Investigation: Equal); Jan Tack, Prof (Methodology: Equal; Supervision: Equal; Writing – review & editing: Equal);
Fatima Domenica Elisa De Palma, Dr (Formal analysis: Equal); Alessandra D’Alessandro, MD (Data curation: Equal; Formal analysis: Supporting; Writing – original draft: Supporting);
Giuseppe Esposito, Prof (Data curation: Equal; Formal analysis: Equal; Investigation: Lead; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.Tack J., Talley N.J. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013 Mar;10(3):134–141. doi: 10.1038/nrgastro.2013.14. [DOI] [PubMed] [Google Scholar]
- 2.Tack J., Talley N.J., Camilleri M., Holtmann G., Hu P., Malagelada J.R., Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M., Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013 Mar;10(3):187–194. doi: 10.1038/nrgastro.2013.11. [DOI] [PubMed] [Google Scholar]
- 4.Tack J., Bisschops R., Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004 Oct;127(4):1239–1255. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Vanheel H., Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013;10:142–149. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 6.Lee K.J., Tack J. Duodenal Implications in the Pathophysiology of Functional Dyspepsia. J Neurogastroenterol Motil. 2010;16(3 July) doi: 10.5056/jnm.2010.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung H., Talley N.J. Role of the Duodenum in the Pathogenesis of Functional Dyspepsia: A Paradigm Shift. J Neurogastroenterol Motil. 2018;24(3 July) doi: 10.5056/jnm18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talley N.J., Meineche-Schmidt V., Pare P., Duckworth M., Räisänen P., Pap A., Kordecki H., Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials [the Bond and Opera studies] Aliment Pharmacol Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H., Kusunoki H., Kamiya T., Futagami S., Yamaguchi Y., Nishizawa T., Iwasaki E., Matsuzaki J., Takahashi S., Sakamoto C., Haruma K., Joh T., Asakura K., Hibi T. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): A multicentre, prospective, randomized, double-blind, placebo-controlled clinical trial. United European Gastroenterol J. 2013 Dec;1(6):445–452. doi: 10.1177/2050640613510904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz M.P., Samsom M., van Berge Henegouwen G.P., Smout A.J. Effect of inhibition of gastric acid secretion on antropyloroduodenal motor activity and duodenal acid hypersensitivity in functional dyspepsia. Aliment Pharmacol Ther. 2001;15:1921–1928. doi: 10.1046/j.1365-2036.2001.01123.x. [DOI] [PubMed] [Google Scholar]
- 11.Samsom M., Verhagen M.A., van Berge Henegouwen G.P., Smout A.J. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/s0016-5085(99)70171-x. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz M.P., Samsom M., Smout A.J.P.M. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am J Gastroenterol. 2001;96:2596–2602. doi: 10.1111/j.1572-0241.2001.04103.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee K.J., Demarchi B., Demedts I., Sifrim D., Raeymaekers P. Tack JA pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol. 2004;99:1765–1773. doi: 10.1111/j.1572-0241.2004.30822.x. [DOI] [PubMed] [Google Scholar]
- 14.Bratten J., Jones M.P. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J Clin Gastroenterol. 2009;43:527–533. doi: 10.1097/MCG.0b013e31818e37ab. [DOI] [PubMed] [Google Scholar]
- 15.di Stefano M., Vos R., Vanuytsel T., Janssens J., Tack J. Prolonged duodenal acid perfusion and dyspeptic symptom occurrence in healthy volunteers. Neurogastroenterol Motil. 2009;21:712–e740. doi: 10.1111/j.1365-2982.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunne D.P., Paterson W.G. Acid-induced esophageal shortening in humans: A cause of hiatus hernia? Can J Gastroenterol. 2000;14(10):847–850. doi: 10.1155/2000/438981. [DOI] [PubMed] [Google Scholar]
- 17.Barclay R.L., Dinda P.K., Morris G.P., Paterson W.G. Morphological evidence of mast cell degranulation in an animal model of acid-induced esophageal mucosal injury. Dig Dis Sci. 1995;269:G219–G224. doi: 10.1007/BF02212685. [DOI] [PubMed] [Google Scholar]
- 18.Wauters L., Ceulemans M., Frings D., Accarie A., Toth J., Farré R., De Hertogh G., Tack J., Vanuytsel T. Proton pump inhibitors reduce duodenal eosinophilia and symptoms in functional dyspepsia patients by anti-inflammatory rather than acid-suppressive effects abstract DDW 2020, in press. Gastroenterology. 2020;158:S52. [Google Scholar]
- 19.Petrosino S., Iuvone T., Di Marzo V. N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie. 2010;92:724–727. doi: 10.1016/j.biochi.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Pesce M., Esposito G., Sarnelli G. Endocannabinoids in the treatment of gastrointestinal inflammation and symptoms. Current Opinion in Pharmacology. 2018;43:81–86. doi: 10.1016/j.coph.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Pesce M., D'Alessandro A., Borrelli O., Gigli S., Seguella L., Cuomo R., Esposito G., Sarnelli G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell. Mol. Med. 2018;22(2):706–715. doi: 10.1111/jcmm.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau B.K., Vaughan C.W. Targeting the endogenous cannabinoid system to treat neuropathic pain. Front Pharmacol. 2014;5:28. doi: 10.3389/fphar.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler C.J., Jonsson K.O., Tiger G. Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem Pharmacol. 2001;62:517–526. doi: 10.1016/s0006-2952(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 24.Cravatt B.F., Lichtman A.H. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 25.Aloe L., Leon A., Levi-Montalcini R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Action. 1993;39:145–147. doi: 10.1007/BF01972748. [DOI] [PubMed] [Google Scholar]
- 26.Facci L., Dal Toso R., Romanello S., Buriani A., Skaper S.D., Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho W.-S.V., Barrett D.A., Randall M.D. ‘Entourage’ effects of Npalmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005 Jan;67(1):15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 29.Fichna J., Wood J.T., Papanastasiou M., Vadivel S.K., Oprocha P., Sałaga M., Sobczak M., Mokrowiecka A., Cygankiewicz A.I., Zakrzewski P.K., Małecka-Panas E., Krajewska W.M., Kościelniak P., Makriyannis A., Storr M.A. Endocannabinoid and Cannabinoid-Like Fatty Acid Amide Levels Correlate with Pain-Related Symptoms in Patients with IBS-D and IBS-C: A Pilot Study. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0085073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner H., Del Carmen K.A., Stokes A. Link between TRPV channels and mast cell function. Handb. Exp. Pharmacol. 2007;179:457–471. doi: 10.1007/978-3-540-34891-7_27. [DOI] [PubMed] [Google Scholar]
- 31.Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014 Aug;63(8):1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 32.van Boxel O.S., ter Linde J.J., Siersema P.D., Smout A.J.P.M. Role of chemical of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010 Apr;105(4):803–811. doi: 10.1038/ajg.2010.100. [DOI] [PubMed] [Google Scholar]
- 33.Kurashima Y., Goto Y., Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013 Dec;43(12):3108–3115. doi: 10.1002/eji.201343782. [DOI] [PubMed] [Google Scholar]
- 34.Barbara G., Stanghellini V., De Giorgio R., Cremon C., Cottrell G.S., Santini D., Pasquinelli G., Morselli-Labate A.M., Grady E.F., Bunnett N.W., Collins S.M., Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004 Mar;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 35.Barbara G., Wang B., Stanghellini V., de Giorgio R., Cremon C., Di Nardo G., Trevisani M., Campi B., Geppetti P., Tonini M., Bunnett N.W., Grundy D., Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007 Jan;132(1):26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Wood J.D. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Reed D.E., Barajas-Lopez C., Cottrell G., Velazquez-Rocha S., Dery O., Grady E.F., Bunnett N.W., Vanner S.J. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003 Mar1;547(Pt 2):531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjea D., Martinov T. Mast cells: Versatile gatekeepers of pain. Mol Immunol. 2014 Mar 22 doi: 10.1016/j.molimm.2014.03.001. pii: S0161-5890[14]00054-6. doi:10.1016/j.molimm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schemann M., Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013 Apr;144(4):698–704.e4. doi: 10.1053/j.gastro.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buhner S., Schemann M. Mast cell–nerve axis with a focus on the human gut. Biochimica et Biophysica Acta 1822. 2012:85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Vasina V., Barbara G., Talamonti L., Stanghellini V., Corinaldesi R., Tonini R., De Pontia F., De Giorgio R. Enteric neuroplasticity evoked by inflammation. Autonomic Neuroscience. 2006;126-127:264–272. doi: 10.1016/j.autneu.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Christianson J.A., Bielefeldt K., Altier C., Cenac N., Davis B.M., Gebhart G.F., High K.W., Kollarik M., Randich A., Undem B., Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009 Apr;60(1):171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vay L., Gu C., McNaughton P.A. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012 Feb;165(4):787–801. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demir I.E., Schäfer K.H., Tieftrunk E., Friess H., Ceyhan G.O. Neural plasticity in the gastrointestinal tract: chronic inflammation, neurotrophic signals, and hypersensitivity. Acta Neuropathol. 2013 Apr;125(4):491–509. doi: 10.1007/s00401-013-1099-4. [DOI] [PubMed] [Google Scholar]
- 45.Cenac N., Altier C., Motta J.P., d'Aldebert E., Galeano S., Zamponi G.W., Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010 Apr;59(4):481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 46.Brierley S.M., Page A.J., Hughes P.A., Adam B., Liebregts T., Cooper N.J., Holtmann G., Liedtke W., Blackshaw L.A. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008 Jun;134(7):2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jara-Oseguera A., Nieto-Posadas A., Szallasi A., Islas L.D., Rosenbaum T. Molecular Mechanisms of TRPV1 Channel Activation. The Open Pain Journal. 2010;3:68–81. doi: 10.2174/1876386301003010068. [DOI] [Google Scholar]
- 48.Balvers M.G., Verhoeckx K.C., Meijerink J., Wortelboer H.M., Witkamp R.F. Measurement of palmitoylethanolamide and other N-acylethanolamines during physiological and pathological conditions. CNS Neurol Disord Drug Targets. 2013 Feb 1;12(1):23–33. doi: 10.2174/1871527311312010007. [DOI] [PubMed] [Google Scholar]
- 49.Skaper S.D., Facci L., Giusti P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol Neurobiol. 2013 Oct;48(2):340–352. doi: 10.1007/s12035-013-8487-6. [DOI] [PubMed] [Google Scholar]
- 50.De Filippis D., Negro L., Vaia M., Cinelli M.P., Iuvone T. New insights in mast cell modulation by palmitoylethanolamide. CNS Neurol Disord Drug Targets. 2013 Feb 1;12(1):78–83. doi: 10.2174/1871527311312010013. [DOI] [PubMed] [Google Scholar]
- 51.Ly H.G., Ceccarini J., Weltens N., Bormans G., Van Laere K., Tack J., Van Oudenhove L. Increased cerebral cannabinoid-1 receptor availability is a stable feature of functional dyspepsia: a [F]MK-9470 PET study. Psychother Psychosom. 2015;84(3):149–158. doi: 10.1159/000375454. [DOI] [PubMed] [Google Scholar]
- 52.Ambrosino P. Functional and biochemical interaction between PPARα receptors and TRPV1 channels: Potential role in PPARα agonists-mediated analgesia. Pharmacol Res. 2014, Sep;87:113–122. doi: 10.1016/j.phrs.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Vanheel H., Vicario M., Boesmans W., Vanuytsel T., Salvo-Romero E., Tack J., Farré R. Activation of Eosinophils and Mast Cells in Functional Dyspepsia: An Ultrastructural Evaluation. Scientific Reports. 2018;8:5383. doi: 10.1038/s41598-018-23620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanheel H., Vicario M., Vanuytsel T., Van Oudenhove L., Martinez C., Keita A.V., Pardon N., Santos J., Söderholm J.D., Tack J., Farré R. Duodenal low-grade inflammation and impaired mucosal integrity in functional dyspepsia patients. Neurogastroenterol. Motil. 2012 24;(Suppl. s2):17–42. [Google Scholar]
- 55.Page A., Brierley J.S.M., Martin C.M., Price M.P., Symonds E., Butler R., Wemmie J.A., Blackshaw L.A. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo E.B. Clinical endocannabinoid deficiency [CECD]: can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett. 2004;25:31–39. [PubMed] [Google Scholar]
- 57.Kindt S., Tertychnyy A., de Hertogh G., Geboes K., Tack J. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil. 2009;21:832–e856. doi: 10.1111/j.1365-2982.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 58.van Kerkhoven L.A., Laheij R.J., Meineche-Schmidt V., Veldhuyzen-van Zanten S.J., de Wit N.J., Jansen J. Functional dyspepsia: not all roads seem to lead to rome. J Clin Gastroenterol. 2009;43:118–122. doi: 10.1097/MCG.0b013e31815591f7. [DOI] [PubMed] [Google Scholar]
- 59.Cremon C., Stanghellini V., Barbaro R.M., Cogliandro R.F., Bellacosa L., Santos J., Vicario M., Pigrau M., Alonso Cotoner C., Lobo B., Azpiroz F., Bruley des Varannes S., Neunlist M., DeFilippis D., Iuvone T., Petrosino S., Di Marzo V., G Barbara G. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther. 2017 Apr;45(7):909–922. doi: 10.1111/apt.13958. Epub 2017 Feb 6. [DOI] [PubMed] [Google Scholar]
- 60.Rentz A.M., Kahrilas P., Stanghellini V., Tack J., Talley N.J., de la Loge C., Trudeau E., Dubois D., Revicki D.A. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with functional gastrointestinal disease. Qual Life Res. 2004 Dec;13(10):1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 61.Sarnelli G., D'Alessandro A., Iuvone T., Capoccia E., Gigli S., Pesce M., Seguella L., Nobile N., Aprea G., Maione F., de Palma G.D., Cuomo R., Steardo L., Esposito G. Palmitoylethanolamide Modulates Inflammation-Associated Vascular Endothelial Growth Factor (VEGF) Signaling via the Akt/mTOR Pathway in a Selective Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α)-Dependent Manner. Plos one. 2016;11(5) doi: 10.1371/journal.pone.0156198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarnelli G., Gigli S., Capoccia E., Iuvone T., Cirillo C., Seguella L., Nobile N., D'Alessandro A., Pesce M., Steardo L., Cuomo R., Esposito G. Antiproliferative and antiangiogenic effects of palmitoylethanolamide in Caco-2 human colon cancer cell involve a selective PPAR-alpha dependent inhibition of Akt/mTOR pathway. Phytother. Res. 2016;30:963–970. doi: 10.1002/ptr.5601. [DOI] [PubMed] [Google Scholar]
- 63.Turco F., Sarnelli G., Cirillo C., Palumbo I., De Giorgi F., D’Alessandro A., Cammarota M., Giuliano M., Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63:105–115. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 64.Ghafouri N., Ghafouri B., Larsson B., Turkina M.V., Karlsson L., Fowler C.J., Gerdle B. High levels of N-palmitoylethanolamide and N-stearoylethanolamide in microdialysate samples from myalgic trapezius muscle in women. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027257. [DOI] [PMC free article] [PubMed] [Google Scholar]