Abstract
Material hardship, or difficulty affording basic resources such as food, housing, utilities, and health care, increases children’s risk for internalizing problems. The uncinate fasciculus (UNC) and two of the gray matter regions it connects–the orbitofrontal cortex (OFC) and amygdala--may play important roles in the neural mechanisms underlying these associations. We investigated associations among material hardship, UNC microstructure, OFC and amygdala structure, and internalizing symptoms in children. Participants were 5- to 9-year-old children (N = 94, 61% female) from socioeconomically diverse families. Parents completed questionnaires assessing material hardship and children’s internalizing symptoms. High-resolution, T1-weighted magnetic resonance imaging (n = 51) and diffusion tensor imaging (n = 58) data were acquired. UNC fractional anisotropy (FA), medial OFC surface area, and amygdala gray matter volume were extracted. Greater material hardship was significantly associated with lower UNC FA, smaller amygdala volume, and higher internalizing symptoms in children, after controlling for age, sex, and family income-to-needs ratio. Lower UNC FA significantly mediated the association between material hardship and internalizing symptoms in girls but not boys. These findings are consistent with the notion that material hardship may lead to altered white matter microstructure and gray matter structure in neural networks critical to emotion processing and regulation.
Keywords: material hardship, uncinate fasciculus, amygdala, white matter, anxiety, depression
Internalizing disorders, including anxiety disorders and major depressive disorder (MDD), are highly prevalent and a major source of disability worldwide (LeWinn et al., 2014; Tromp et al., 2019). Socioeconomic disadvantage during childhood leads to an enduring risk for internalizing problems that persists into adulthood (Gilman et al., 2002; Wadsworth et al., 2016). Although previous studies have shed light on the neural mechanisms underlying these associations (Farah, 2017), this work has focused primarily on composites of socioeconomic status (SES) or broad indicators such as family income and parental education. Although it is now recommended that socioeconomic factors be examined separately (Duncan & Magnuson, 2012; Schenck-Fontaine & Panico, 2019), the studies that have done so have focused primarily on conventional indicators and not on other, more proximal indices, such as material hardship (Schenck-Fontaine & Panico, 2019). Research on these more proximal measures is needed to fully disentangle how socioeconomic disadvantage may impact the developing brain in ways that increase risk for internalizing problems (Gershoff et al., 2007; Neckerman et al., 2016).
Material hardship captures the lived conditions of economic hardship and refers to difficulty affording basic resources, such as food, housing, utilities, and health care (Mayer & Jencks, 1989). Although families living in poverty are more likely to experience material hardship, families with incomes above the poverty threshold also experience material hardship (Gershoff et al., 2007; Zilanawala & Pilkauskas, 2012). Additionally, not all families living in poverty experience material hardship, in part because of variability in the generosity of the social safety net (Beverly, 2001; Iceland & Bauman, 2007). Thus, material hardship has been documented as a correlated but distinct construct from income and a more proximal reflection of lived economic hardship than family income. Although material hardship has been significantly associated with higher internalizing symptoms in children, even after accounting for family income (Shankar et al., 2017; Slopen et al., 2010; Sun et al., 2015; Zilanawala & Pilkauskas, 2012), the neural mechanisms underlying these associations are not well understood. As such, the goal of this study was to examine the associations among material hardship, brain structure and connectivity, and internalizing symptoms in children.
Conceptual Model Guiding This Study
Material hardship has been theorized to influence children’s social-emotional development and risk for internalizing problems by increasing exposure to chronic stress (Chien & Mistry, 2013; Conger & Donnellan, 2007; Gershoff et al., 2007; Huang et al., 2017; Sun et al., 2015). Consistent with the family stress model (Conger & Donnellan, 2007), material hardship has been found to increase parental stress, leading to negative parenting behavior, which in turn increases children’s exposure to chronic stress and interferes with the development of social-emotional skills, such as emotion processing and regulation (Ashiabi & O’Neal, 2007; Gershoff et al., 2007; Huang et al., 2017; Sun et al., 2015; Wu & Schimmele, 2005). Difficulties with emotion processing and regulation have been found to link exposure to chronic stressors with internalizing problems. Taken together, material hardship may alter the development of frontolimbic circuitry underlying emotion processing and regulation and in turn lead to increases in children’s internalizing symptoms (see Figure S1).
Key components of frontolimbic circuitry underlying emotion regulation include the uncinate fasciculus (UNC) and two of the gray matter regions it connects—namely, the medial orbitofrontal cortex (OFC) and amygdala (Catani et al., 2002; Schmahmann et al., 2007). Variability in UNC microstructure has been associated with emotion regulation (Hein et al., 2018; Swartz et al., 2014; Zuurbier et al., 2013) and implicated in internalizing disorders (Adluru et al., 2017; Ho et al., 2017; Tromp et al., 2019; Vilgis et al., 2017). The amygdala is a subcortical structure centrally involved in reactivity to emotional stimuli and threat detection (Davidson & Irwin, 1999; Davis & Whalen, 2001) and strongly implicated in internalizing disorders (Warnell et al., 2018). The medial OFC (vmPFC) is heavily involved in emotion regulation and has been consistently implicated in internalizing disorders (Merz et al., 2018; Schmaal et al., 2017). However, no work to date has examined these core components of frontolimbic circuitry in children in relation to both material hardship and internalizing symptoms.
Material Hardship, UNC Microstructure, and OFC and Amygdala Structure
In diffusion tensor imaging (DTI) studies, fractional anisotropy (FA) reflects the degree of directionality of water diffusion in white matter tracts, which indicates the microstructural properties of white matter tracts and in turn capacity for functional communication between connected brain regions (Beaulieu, 2002; Thomason & Thompson, 2011). Several studies have found associations between socioeconomic circumstances and FA in the UNC. However, findings have been mixed. Socioeconomic disadvantage has been associated with reduced FA in the UNC in children (Dufford & Kim, 2017) and adults (Gianaros et al., 2013), whereas greater food insecurity was associated with higher FA in the UNC in children and adolescents (Dennison et al., 2019). Yet another study failed to find a link between socioeconomic factors and UNC FA in a large sample of children and adolescents (Ursache & Noble, 2016).
In neuroanatomical studies of subcortical gray matter, socioeconomic factors have been similarly variably linked with amygdala volume. Socioeconomic disadvantage has been associated with smaller amygdala volume (Brody et al., 2017; Hanson et al., 2015; Luby et al., 2013; McDermott et al., 2019; Merz et al., 2017), larger amygdala volume (Noble et al., 2012), or no difference in amygdala volume (Hanson et al., 2011; Noble et al., 2015). One possibility is that these associations vary as a function of the timing or duration of socioeconomic disadvantage (McEwen, 2003; Merz et al., 2017).
Although socioeconomic factors have been consistently associated with PFC structure, the specific PFC regions where differences have been found have varied (Farah, 2017). Recent studies have examined cortical thickness and surface area separately, given that they are genetically and developmentally distinct (Panizzon et al., 2009; Raznahan et al., 2011). Two major studies have linked socioeconomic disadvantage with reduced OFC surface area in children and adolescents (McDermott et al., 2019; Noble et al., 2015). Findings for OFC thickness have been more mixed (Lawson et al., 2013; Mackey et al., 2015; McDermott et al., 2019; Noble et al., 2015). To our knowledge, no work has focused on associations of material hardship with these indices of PFC-amygdala structure and microstructure in children.
UNC Microstructure and OFC and Amygdala Structure and Internalizing Problems
Lower FA in the UNC has been significantly associated with internalizing disorders in children and adolescents (Adluru et al., 2017; Cullen et al., 2010; Ho et al., 2017; LeWinn et al., 2014; Tromp et al., 2019; Vilgis et al., 2017). Although most studies have used clinical samples and compared those with and without an internalizing disorder, some studies have focused on typically-developing children and adolescents (Mohamed Ali et al., 2018). It is also noteworthy that some studies have found that higher FA in the UNC is associated with adolescent depression (Aghajani et al., 2014; Bracht et al., 2015; Kircanski et al., 2019).
Similarly, internalizing disorders or symptoms have been linked to amygdala size, though the directionality is again unclear. Both larger (Albaugh et al., 2017; De Bellis et al., 2000; Qin et al., 2014; van der Plas et al., 2010) and smaller amygdala volumes (Merz et al., 2017; Milham et al., 2005; Mueller et al., 2013; Rosso et al., 2005; Strawn et al., 2015; Warnell et al., 2018) have been associated with internalizing symptoms and disorders, and some studies have failed to find significant associations (Koolschijn et al., 2013; Merz et al., 2018). Duration of the internalizing disorder or the number of episodes may partially explain these discrepancies (McEwen, 2003).
Internalizing problems tend to be associated with structural differences in the medial OFC (vmPFC), although the specific patterns of these associations are inconsistent. A large meta-analysis found that adolescents with MDD had reduced surface area in the medial OFC (Schmaal et al., 2017), and another study linked general anxiety symptoms with decreased surface area in OFC (Newman et al., 2015). Pediatric and adolescent MDD (or depressive symptoms) have been associated with reduced OFC thickness in some studies (Marrus et al., 2015; Merz et al., 2018; Peterson et al., 2009) but not others (Schmaal et al., 2017; Whittle et al., 2014), and pediatric anxiety disorders have been associated with increased OFC thickness (Gold et al., 2017; Strawn et al., 2014). Given that most studies have focused on adults and adolescents with internalizing disorders, more research is needed that focuses on typically-developing children to understand patterns of associations that may precede the onset of an internalizing disorder (Keenan et al., 2008).
Taken together, altered structural development of PFC-amygdala circuitry underlying emotion processing and regulation may represent one mechanism through which material hardship increases risk for internalizing disorders in children (see Figure S1). More specifically, material hardship may alter UNC microstructure and medial OFC structure, leading to weaker downregulation of emotional reactivity governed by the amygdala (Hein et al., 2018; Swartz et al., 2014; Zuurbier et al., 2013) and in turn greater vulnerability to internalizing disorders. Material hardship may simultaneously increase amygdala reactivity to negative emotional stimuli, concomitant with altered amygdala volume, also resulting in greater risk for internalizing problems (Gaffrey et al., 2013). To our knowledge, no work has examined whether these core components of PFC-amygdala circuitry mediate associations between material hardship and internalizing symptoms in children.
Sex Differences in These Associations
During adolescence, internalizing disorders become much more prevalent in girls compared to boys (Hankin et al., 1998). The degree to which stressors increase anxiety and depressive symptoms has been found to vary by sex, with girls more likely to show stress-related increases in these symptoms (Hodes & Epperson, 2019; Oldehinkel & Bouma, 2011). Sex differences in the effects of stressors, such as material hardship, on PFC-amygdala circuitry are less well understood. Sex differences have been found in the associations between adverse, stressful experiences and PFC-amygdala structure and connectivity (Burghy et al., 2012; Whittle et al., 2009, 2016) and between PFC-amygdala structure and connectivity and internalizing disorders (Rubinow & Schmidt, 2019; Tromp et al., 2019; Whittle et al., 2014), although consistent patterns have not yet been identified. Thus, research is needed that investigates sex differences in associations among material hardship, PFC-amygdala circuitry and internalizing symptoms in children.
Current Study
The goal of this study was to investigate associations among material hardship, core components of PFC-amygdala circuitry underlying emotion processing and regulation (UNC FA, medial OFC surface area, amygdala volume), and internalizing symptoms, and whether these indices mediated the association between material hardship and internalizing symptoms. Participants were 5- to 9-year-old children (N = 94; 61% female) from socioeconomically diverse families. Parents completed questionnaires assessing material hardship and children’s internalizing symptoms. Children participated in an MRI scanning session that included T1- and diffusion-weighted sequences. UNC FA (n = 58), medial OFC surface area (n = 51), and amygdala gray matter volume (n = 51) were extracted. Given evidence that medial OFC surface area is associated with both socioeconomic disadvantage (McDermott et al., 2019; Noble et al., 2015) and internalizing disorders (Newman et al., 2015; Schmaal et al., 2017), analyses of medial OFC structure focused on cortical surface area rather than thickness.
We hypothesized that higher material hardship would be significantly associated with greater internalizing symptoms in children, replicating past work (Sun et al., 2015; Zilanawala & Pilkauskas, 2012). At the neural level, our a priori hypotheses centered on the UNC and two of the gray matter regions it connects, namely the medial OFC and amygdala. We expected that material hardship would be associated with UNC FA, medial OFC surface area, and amygdala volume, which would in turn be associated with internalizing symptoms. Directionality could not be specified for these hypotheses due to inconsistencies in the existing research, as detailed above (Dennison et al., 2019; Dufford & Kim, 2017; Kircanski et al., 2019; LeWinn et al., 2014). Finally, we expected these associations to be stronger in girls compared to boys (Hodes & Epperson, 2019; Oldehinkel & Bouma, 2011).
To examine whether results were specific to material hardship, we conducted supplemental analyses of family income-to-needs ratio. We expected that material hardship would be more strongly associated with internalizing symptoms, UNC microstructure, and OFC and amygdala structure compared to family income-to-needs ratio. Material hardship reflects the lived conditions of socioeconomic disadvantage which would be expected to more directly increase parental stress. In comparison, lower family income-to-needs ratio may be a more distal influence on parental stress (Conger & Donnellan, 2007). Although our main diffusion measure of interest was FA, given that it is a summary and non-specific measure of white matter microstructure (Song et al., 2002), we also examined axial diffusivity (AD; water diffusivity along the axon), radial diffusivity (RD; water diffusivity perpendicular to the axon) and mean diffusivity (MD; overall magnitude of diffusion) to interpret our results with greater specificity (Budde, Xie, Cross, & Song, 2009; Hatton et al., 2018; Klawiter et al., 2011; Winklewski et al., 2018).
Methods
Participants
Recruitment.
Participants were recruited in New York, New York through local family events and posting flyers in the neighborhood. Families were recruited to generate a socioeconomically diverse sample. Participants were screened for eligibility over the phone. Inclusion criteria for children included: between 5 and 9 years of age, born after 37 gestational weeks, product of a singleton pregnancy, no history of medical or psychiatric conditions, English as the primary language spoken in the home, and no contraindications for MRI scanning.
Sample characteristics.
Participants ranged in age from 5.06 – 9.87 years (N = 94; 61% female). Parental education ranged from 6.50–20.00 years, and family income-to-needs ratio ranged from .17 to 15.21. Fifty percent were Hispanic/Latino; 31% were African American, non-Hispanic/Latino; and 14 % were European American, non-Hispanic/Latino. Thirty percent of the families had household incomes below the U.S. poverty threshold (see Table 1).
Table 1.
Descriptive statistics for sample characteristics (N = 94)
| M | SD | |
|---|---|---|
| Child age (years) | 7.03 | 1.29 |
| Family income-to-needs ratio | 2.68 | 2.79 |
| Parental education (years) | 14.14 | 2.64 |
| % | n | |
| Child sex (female) | 60.64 | 57 |
| Child race/ethnicity | ||
| African American, non-Hispanic/Latino | 30.85 | 29 |
| Hispanic/Latino | 50.00 | 47 |
| European American, non-Hispanic/Latino | 13.83 | 13 |
| Other | 5.32 | 5 |
| Family income below U.S. poverty thresholda | 29.79 | 28 |
Note. Parental education reflects educational attainment averaged across parents.
Income-to-needs ratio < 1.00
Procedure and Sample Sizes
Families took part in two study visits within a month. During the first visit, parents (N = 94) completed questionnaires which included items on socioeconomic background and child internalizing symptoms. Eighty-five children were enrolled in the MRI portion of the study and participated in a mock MRI scan. During the second visit, 66 children participated in an actual MRI scanning session. MRI data were not acquired if the child was afraid, fidgety, or uninterested during the mock scan (n = 7) or if the child or family decided not to participate in the actual MRI scan following the mock scan (n = 12). T1-weighted MRI data were acquired for all 66 children. Diffusion-weighted imaging data were acquired for 61 children, as five children discontinued their scanning session prior to the diffusion-weighted sequence. There were no significant differences in material hardship, t(92) = .46, p = .64, internalizing symptoms, t(82) = −.30, p = .76, or family income-to-needs ratio, t(92) = −.17, p = .87, between participants with and without imaging data. Informed consent/assent was obtained from all families. All procedures were approved by the Institutional Review Boards at the New York State Psychiatric Institute and Teachers College, Columbia University.
MRI Acquisition and Processing
MRI data were acquired on a 3-Tesla General Electric (GE) Discovery MR750 scanner with a 32-channel head coil. Whole brain DTI data were acquired using a single-shot spin echo planar imaging sequence with the following parameters: 60 axial slices, 2.5 mm slice thickness, TR = 15,700 ms, TE = 86.4 ms, FOV (x) = 24 mm, FOV (y) = 24 mm, matrix size = 132 × 128 (machine-interpolated to 256 × 256 for post-processing), voxel size = 0.94 mm × 0.94 mm × 2.5 mm. The diffusion-weighted images were acquired along 15 non-collinear directions with b = 1000 s/mm2. Three baseline images with b = 0 s/mm2 were also acquired. Two trained research assistants visually inspected the raw diffusion-weighted images, eddy current corrected diffusion-weighted images, and color encoded FA images (He et al., 2014). This resulted in the exclusion of three participants’ DTI data due to motion artifacts. Thus, 58 children had usable DTI data.
Images were processed using FMRIB Software Library (FSL) version 5.0.11 (Oxford, UK) (Smith et al., 2004). DTI acquisitions were corrected for subject motion, eddy current-induced distortion, outlier replacement, and within-volume (or “slice-to-volume”) movement using FSL Eddy (Andersson et al., 2016, 2017). Brain Extraction Tool (Smith, 2002) was used to extract a brain mask from the eddy corrected image to exclude non-brain tissue. FSL DTIFIT was used to fit diffusion tensors at each voxel, and the FA image was derived from the fitted diffusion tensors. Data were then processed using tract-based spatial statistics (TBSS) (Smith et al., 2006). Since the subjects were all young children, the adult-derived target image (FMRIB58_FA) was inappropriate for registering the FA image from the subject’s native space to the template space. Therefore, we automatically identified the most representative one as the target image from all subjects, and the target image was then affine-aligned into MNI152 standard space. Each FA image was then transformed into standard space by combining the nonlinear transform to the target FA image with the affine transform from that target to the standard space. A skeleton of white matter tracts that were common to all participants was created by thinning the mean FA image using a threshold of 0.2. Nonlinear warps and skeleton projection were then also applied to MD, RD, and AD images. The Johns Hopkins University (JHU) white matter tractography atlas (Wakana et al., 2007) was used to quantify mean FA for the right and left UNC from the skeletonized FA image. As right and left UNC FA were significantly correlated, r = .80, p < .0001, and we did not have a priori hypotheses about laterality, we averaged UNC FA values across the left and right hemispheres. UNC MD, RD, and AD values were also extracted and averaged across the right and left hemispheres.
T1-weighted.
Anatomical imaging data were acquired using a high-resolution T1-weighted fast spoiled gradient echo sequence (TR = 7.1 ms; TE = 2.7 ms; TI = 500 ms; flip angle = 11 degrees; 176 sagittal slices; 1.0 mm slice thickness; FOV = 25 cm; inplane resolution = 1.0 by 1.0 mm). All images were visually inspected for motion artifacts and ghosting, resulting in the exclusion of 15 participants’ data from analyses. There was no manual editing of imaging data that were deemed usable.
Cortical reconstruction and volumetric segmentation were performed using standard automated procedures in FreeSurfer (version 6.0) (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999; Fischl et al., 2004; Fischl & Dale, 2000). The cortex was parcellated into gyral-based regions based on the Desikan-Killiany atlas (Desikan et al., 2006). Cortical surface area was calculated as the sum of the area of the vertices falling within a given region. Surface area in the left and right medial OFC was extracted. As we did not have a priori hypotheses about laterality, and left and right medial OFC surface area were significantly correlated, r = .72, p < .001, surface area was summed across the left and right hemispheres.
Automated segmentation of subcortical volumes in FreeSurfer has been shown to be robust to anatomic variability and to have accuracy comparable to manual labeling techniques (Fischl et al., 2002; Makowski et al., 2018). All segmentations of the amygdala passed visual inspection for major errors. As right and left amygdala volume were significantly correlated, r = .67, p < .0001, and we did not have a priori hypotheses about laterality, amygdala volume was summed across the right and left hemispheres.
Measures
Income-to-needs ratio.
Parents reported their annual household income and the number of adults and children in the household. The income-to-needs ratio was calculated by dividing household income by the poverty threshold for the size of the family.
Material hardship.
The Material Deprivation Scale (Pilkauskas et al., 2012) is a 14-item survey which asks parents if they have experienced hardships in paying bills (e.g., rent, utilities), providing enough food for their family, affording medical care, and maintaining adequate housing in the last year. Parents responded to each item on a binary scale (yes = 1; no = 0), and affirmative answers were summed to create a total score. Higher scores indicate greater material hardship (Cronbach’s α = .77). Material hardship was significantly inversely associated with income-to-needs ratio, r = −.36, p < .001.
Child internalizing symptoms.
Parents completed the Revised Child Anxiety and Depression Scale–Parent Version (RCADS-P) (Chorpita, Moffitt, & Gray, 2005), a 47-item questionnaire assessing internalizing symptoms in children between 6 and 18 years old. Parents rate each item on a 4-point scale ranging from 0 (never) to 3 (always). The total score (Cronbach’s α = .90) was used in this study. The RCADS-P has been shown to demonstrate adequate internal consistency and validity (Ebesutani et al., 2015).
The RCADS‐P was added to the protocol once a number of families had already participated. More specifically, 36% (n = 30) of parents completed the RCADS-P over the phone on a day after the MRI scan, while 64% (n = 54) of parents did so during the first testing session as described above. All analyses of children’s internalizing symptoms accounted for when the RCADS-P was completed.
Statistical Analyses
Using SAS (version 9.3), multiple linear regression was conducted to examine the associations of material hardship with children’s internalizing symptoms, UNC FA, medial OFC surface area, and amygdala volume. We then used multiple linear regression to examine associations of children’s UNC FA, medial OFC surface area, and amygdala volume with their internalizing symptoms. Mediation models were run to examine whether UNC FA, medial OFC surface area, or amygdala volume mediated the association between material hardship and internalizing symptoms. The significance of the mediated or indirect effect was tested using bias‐corrected bootstrapping via the PROCESS macro in SAS (Hayes, 2013; Preacher & Hayes, 2008). To test the a priori hypothesis that these associations would be stronger in girls, these analyses were then run in boys and girls separately. The threshold for statistical significance was p < .05.
Child age, sex, and family income-to-needs ratio were included as covariates in these regression models. Whole brain volume was also included in analyses of amygdala volume. Time of RCADS-P completion was included as a covariate in analyses of internalizing symptoms. Given that there were no significant racial/ethnic differences in UNC FA, medial OFC surface area, amygdala volume or internalizing symptoms (all p’s = .36–.64), race/ethnicity was not included as a covariate. Data points that were more than 3 SDs above the mean for internalizing symptoms (n = 1) or material hardship (n = 1) were Winsorized. Given that family material hardship and income are correlated but distinct constructs and families with varying household incomes may experience material hardship, analyses of material hardship controlled for family income-to-needs ratio, as recommended in recent publications (Schenck-Fontaine & Panico, 2019). To control for multiple comparisons, false discovery rate (FDR) correction (Benjamini & Hochberg, 1995) was applied (via PROC MULTTEST in SAS) to analyses of associations of material hardship with UNC FA, medial OFC surface area, and amygdala volume (α = .05). When significant differences in UNC FA were found, follow-up analyses of AD, RD, and MD in the UNC were conducted.
Results
Descriptive statistics and zero-order correlations for material hardship, UNC FA, medial OFC surface area, amygdala volume, and internalizing symptoms are provided in Table 2. Material hardship was significantly positively correlated with internalizing symptoms in children.
Table 2.
Descriptive statistics and zero-order correlations for material hardship, uncinate fasciculus FA, medial OFC and amygdala structure, and internalizing symptoms
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| 1 | Material hardship | -- | ||||
| 2 | Uncinate fasciculus FA | −.18 | -- | |||
| 3 | Medial OFC surface area (mm2) | −.02 | .20 | -- | ||
| 4 | Amygdala volume (mm3) | −.25+ | .23 | .65*** | -- | |
| 5 | Internalizing symptoms | .27* | −.01 | .05 | −.06 | -- |
| N | 94 | 58 | 51 | 51 | 84 | |
| M | 2.54 | .52 | 3224.61 | 2820.81 | 22.14 | |
| SD | 2.04 | .03 | 523.17 | 371.91 | 11.42 | |
| Range | 0.00–9.00 | .45–.59 | 2133.00–4287.00 | 1637.80–3563.50 | 3–60 |
FA, fractional anisotropy; OFC, orbitofrontal cortex
p < .10;
p < .05;
p < .001
Material Hardship and Internalizing Symptoms
Greater material hardship was significantly associated with higher internalizing symptoms in children, β = .28, p = .02, ηp2 = .07 (see Figure 1).
Figure 1.
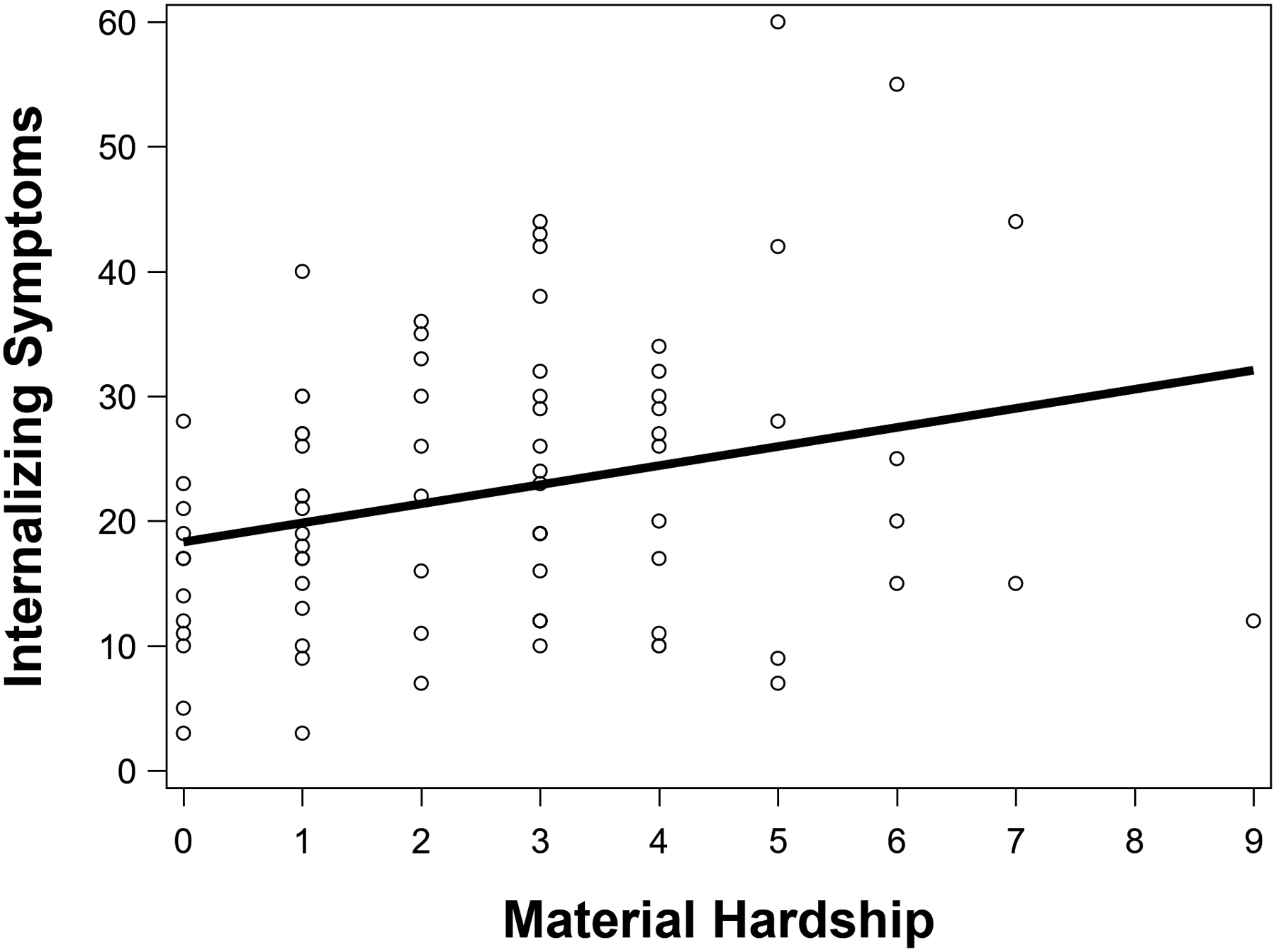
Greater material hardship was significantly associated with higher internalizing symptoms in children. Regression analyses controlled for age, sex, family income-to-needs ratio and when the RCADS-P was completed.
RCADS-P, Revised Child Anxiety and Depression Scale–Parent Version
Material Hardship, UNC Microstructure and Medial OFC and Amygdala Structure
Higher material hardship was significantly associated with lower UNC FA, β = −.29, p = .03, ηp2 = .09 (see Figure 2), and smaller amygdala volume, β = −.29, p = .01, ηp2 = .15 (see Figure 3), but not significantly associated with medial OFC surface area, β = .06, p = .68, ηp2 = .004. After FDR correction for multiple comparisons, material hardship remained significantly associated with UNC FA (FDR-corrected p = .04) and amygdala volume (FDR-corrected p = .02). Figure 4 shows representative images of the uncinate fasciculus and amygdala.
Figure 2.
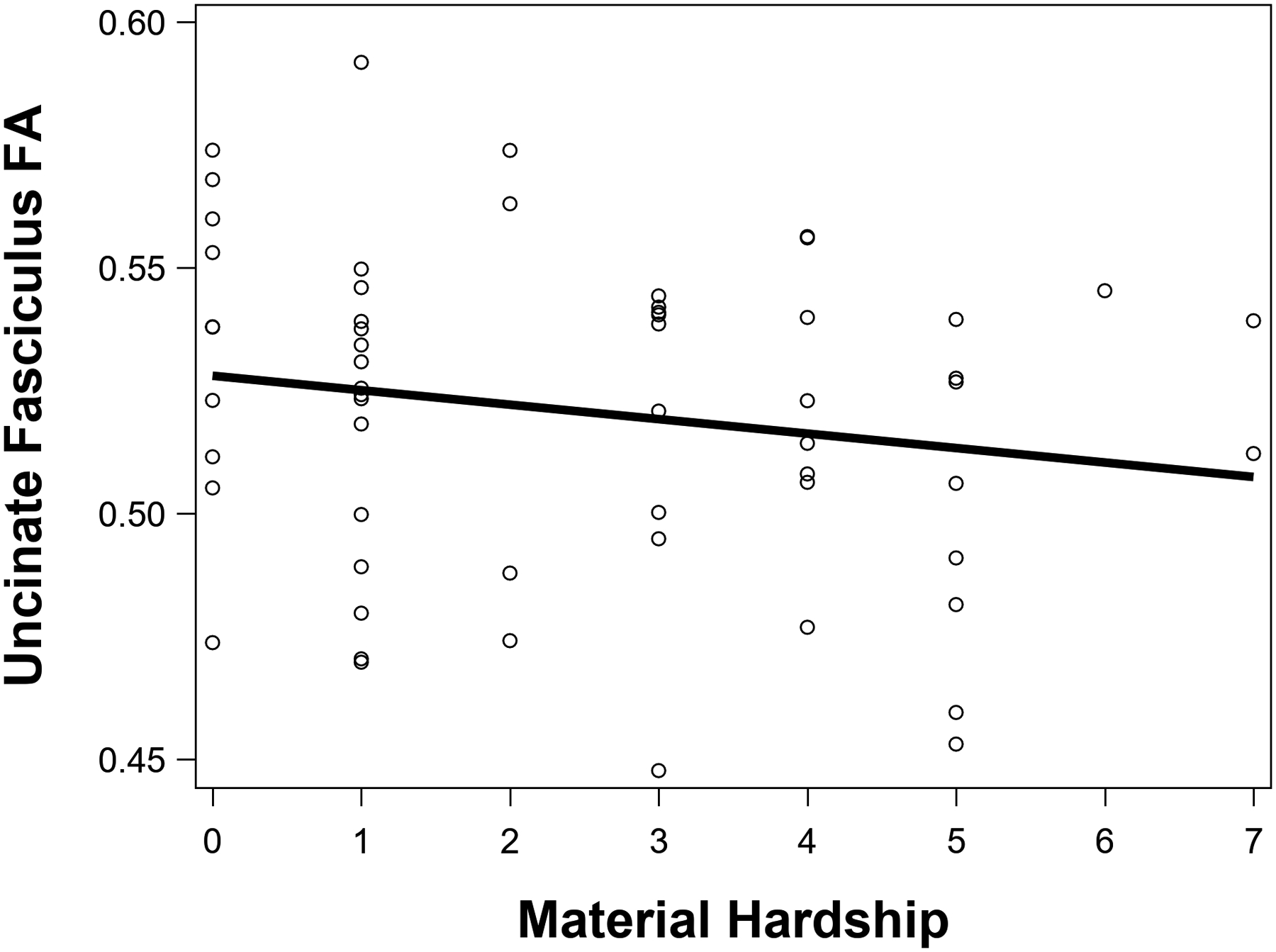
Greater material hardship was significantly associated with lower fractional anisotropy (FA) in the uncinate fasciculus.
Figure 3.
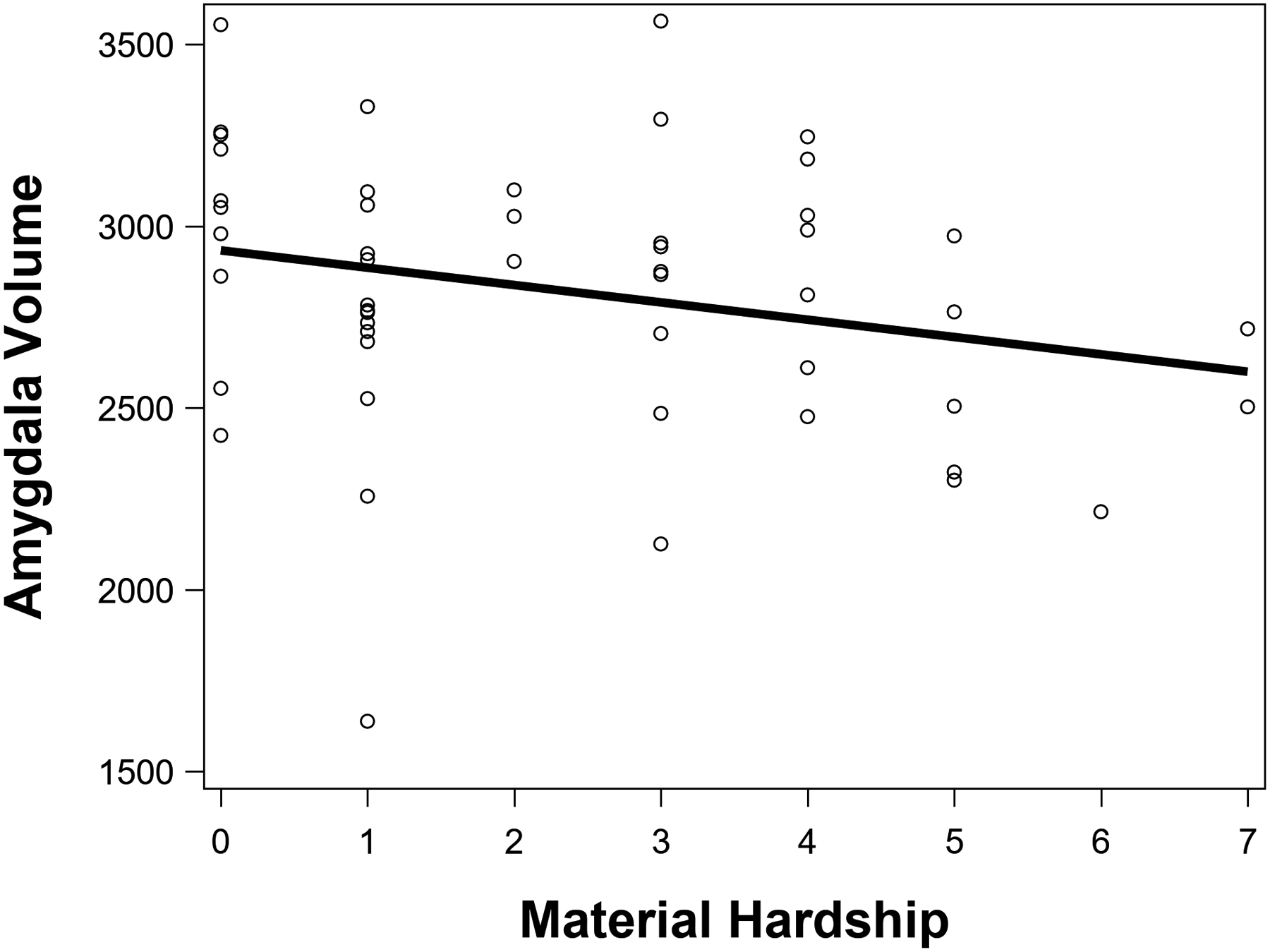
Greater material hardship was significantly associated with smaller amygdala volume (mm3).
Figure 4.
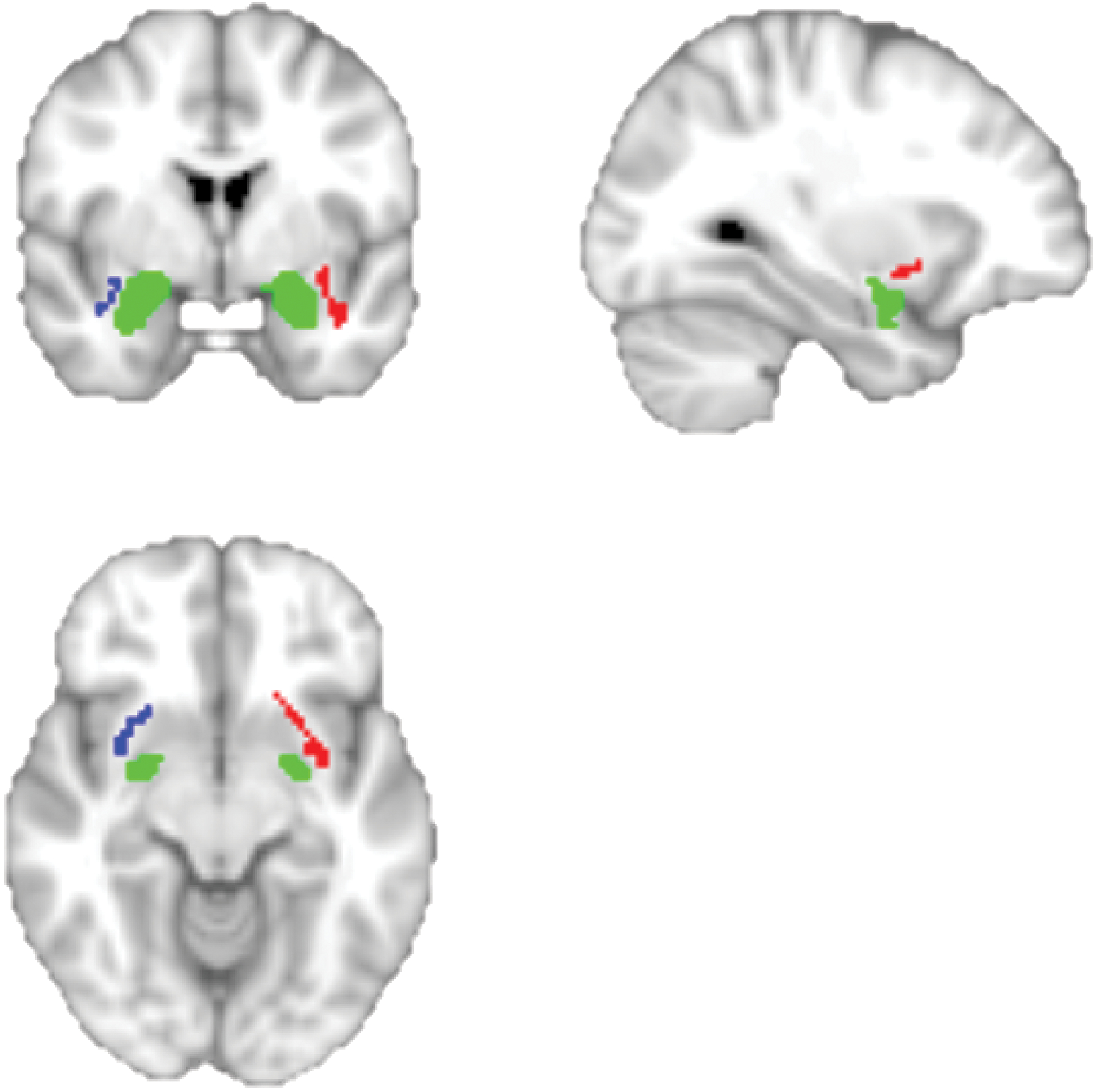
Representative images of the uncinate fasciculus and amygdala overlaid on the MNI152 T1 image are presented for visualization purposes. The amygdala is displayed in green and the uncinate fasciculus is displayed in red and blue. Note that although we extracted amygdala volume in local subject space, we show the amygdala in MNI space for visualization purposes.
UNC Microstructure, Medial OFC and Amygdala Structure, and Internalizing Symptoms
UNC FA, medial OFC surface area, and amygdala volume were not significantly associated with children’s internalizing symptoms (p = .28–.66). UNC FA, medial OFC surface area, and amygdala volume did not significantly mediate the association between material hardship and internalizing symptoms.
Sex Differences
Material hardship-by-sex interactions for indices of PFC-amygdala circuitry were not significant, likely due to the relatively small sample size. However, this interaction for the UNC had a small effect size (ηp2 = .02) whereas for the amygdala and medial OFC it had negligible effect sizes (ηp2 = .00–.001). The UNC FA/OFC surface area/amygdala volume-by-sex interactions were not significant, but the interaction had a medium effect size for the UNC (ηp2 = .05) compared to negligible effect sizes for the amygdala (ηp2 = .01) and medial OFC (ηp2 = .001). These results coupled with our a priori hypotheses about sex differences supported the analysis of associations among material hardship, UNC FA, and internalizing symptoms separately in boys and girls. Such analyses revealed that greater material hardship was significantly associated with lower UNC FA in girls (n = 35), β = −.42, p = .02, ηp2 = .17, but not boys (n = 23), β = −.09, p = .66, ηp2 = .01. UNC FA was not significantly associated with internalizing symptoms in girls or boys. Nonetheless, the indirect effect between material hardship and internalizing symptoms via UNC FA was significant for girls (n = 32), ab = .12, SE = .10, 95% CI: [.004, .42], but not boys. In girls, greater material hardship was associated with lower UNC FA, which was in turn associated with higher internalizing symptoms (see Figure 5). To rule out alternative interpretations, mediation models were run in which the predictor and mediator were switched and the mediator and outcome were switched. Neither of these alternative models yielded significant indirect effects.
Figure 5.
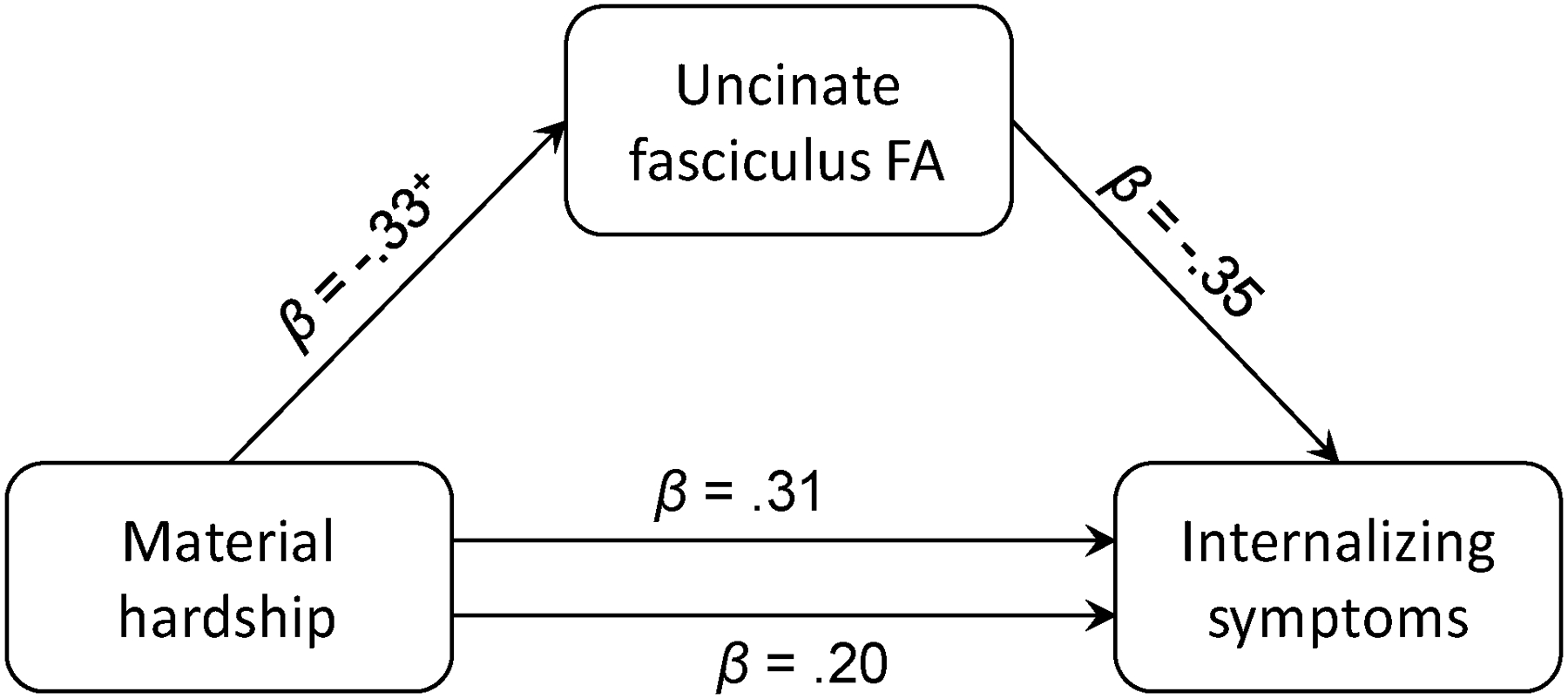
FA in the uncinate fasciculus (UNC) significantly mediated the association between material hardship and internalizing symptoms in girls (n = 32), ab = .12, SE = .10, 95% CI: [.004, .42]. Greater material hardship was associated with lower FA in the UNC, which was in turn associated with higher internalizing symptoms in girls. The solid line between material hardship and internalizing symptoms represents the total effect while the dotted line represents the direct effect after accounting for the mediated or indirect effect.
FA, fractional anisotropy
UNC, uncinate fasciculus
+ p < .10
Specificity of Results to Material Hardship
Family income-to-needs ratio was not significantly associated with internalizing symptoms, β = .11, p = .33, UNC FA, β = −.23, p = .08, medial OFC surface area, β = .26, p = .06, or amygdala volume, β = −.10, p = .34.
Material Hardship and RD, AD, and MD in the UNC
Greater material hardship was significantly associated with lower AD in the UNC, β = −.45, p = .002, ηp2 = .17, but not significantly associated with RD in the UNC, β = .13, p = .33, ηp2 = .02, or MD in the UNC, β = −.17, p = .25, ηp2 = .03. Exploratory whole-brain voxel-based analyses indicated material hardship was not significantly associated with FA values across the brain after correction for multiple comparisons at p < .05.
Discussion
The goals of this study were to examine associations among material hardship, core components of PFC-amygdala circuitry underlying emotion processing and regulation (UNC microstructure, medial OFC surface area, amygdala volume), and internalizing symptoms in children, and whether these indices of PFC-amygdala structure mediated associations between material hardship and internalizing symptoms. Material hardship refers to difficulty affording basic necessities, such as food and housing, capturing the lived conditions of economic hardship (Mayer & Jencks, 1989; Schenck-Fontaine & Panico, 2019). Findings indicated that greater material hardship was significantly associated with higher internalizing symptoms in children, replicating past work (Sun et al., 2015; Zilanawala & Pilkauskas, 2012). Greater material hardship was also significantly associated with lower FA in the UNC, consistent with previous studies of socioeconomic factors (Dufford & Kim, 2017; Gianaros et al., 2013). Lower FA in the UNC may indicate weaker connectivity and communication between PFC regions and anterior temporal regions, such as the amygdala, and lower ability to downregulate amygdala reactivity (Hein et al., 2018; Swartz et al., 2014) and in turn negative emotions (Zuurbier et al., 2013).
Greater material hardship was also significantly associated with smaller amygdala volume in children, consistent with previous work on family income and parental education (Brody et al., 2017; Hanson et al., 2015; Luby et al., 2013; McDermott et al., 2019; Merz et al., 2017). Smaller amygdala volume has been associated with greater reactivity to negative emotional stimuli (e.g., high fearfulness or stress reactivity) in animal models (Pedraza et al., 2014; Yang et al., 2008) and human studies (Foell et al., 2019). Furthermore, there is evidence of an inverse association between amygdala volume and amygdala reactivity to negative emotional stimuli (e.g., threat cues, stressors) in humans (Gianaros et al., 2008; Kalmar et al., 2009) and in animals (Pedraza et al., 2014). Taken together, the combination of heightened emotional reactivity and reduced emotion regulation could lead to more frequent or intense experiences of negative emotion and possibly increased risk for internalizing problems.
Material hardship was more strongly associated with UNC FA and amygdala volume than family income-to-needs ratio, consistent with the possibility that it has more proximal or independent associations with these outcomes (Conger & Donnellan, 2007; Schenck-Fontaine & Panico, 2019). Material hardship may lead to altered UNC microstructure and amygdala volume through several proximal mechanisms. Material hardship has been associated with nutritional deficiencies (Rose, 1999; Rose & Oliveira, 1997), increased exposure to environmental toxins (Chuang et al., 1999; Jerrett, 2009; Mohai et al., 2009; Morello-Frosch et al., 2011), reduced investments in children’s social-emotional development (e.g., high-quality early care and education) (Duncan et al., 2017), and chronic stress (Conger & Donnellan, 2007; Gershoff et al., 2007; Newland et al., 2013; Sun et al., 2015). According to the family stress model (Conger & Donnellan, 2007) and animal models of chronic stress (McEwen et al., 2016), material hardship may increase parental stress, leading to lower quality parental care, which in turn exposes children to chronic stress and alters their PFC-amygdala circuitry (see Figure S1) (Chien & Mistry, 2013; Gershoff et al., 2007; Huang et al., 2017; Sun et al., 2015). Research is needed to tease apart the roles of these different possible mechanisms.
Relatedly, specific types of material hardship (e.g., food insecurity, housing instability, unmet medical needs, utility shut-offs) have been differentially associated with children’s health and developmental outcomes (Yoo et al., 2009; Zilanawala & Pilkauskas, 2012). Research is needed to investigate the roles of these specific types of material hardship and their potentially unique contributions to children’s brain structure and connectivity.
Although significant interactions were not found between material hardship and child sex, we analyzed UNC FA separately in boys and girls to test our a priori hypothesis that associations would be stronger in girls. In girls, UNC FA significantly mediated the association between material hardship and internalizing symptoms. Greater material hardship was associated with lower UNC FA, which was in turn associated with higher internalizing symptoms. It is possible that during middle childhood, girls are particularly susceptible to the effects of chronic stress on PFC-amygdala circuitry, consistent with recent reviews (Hodes & Epperson, 2019; Oldehinkel & Bouma, 2011). In girls, material hardship may reduce FA in the UNC, weakening the ability of the PFC to downregulate amygdala reactivity to negative emotional stimuli and increasing risk for internalizing disorders (Hein et al., 2018; Swartz et al., 2014; Zuurbier et al., 2013). However, it is important to note the small sample size and cross-sectional design of this study.
UNC FA and medial OFC and amygdala structure were not significantly associated with internalizing symptoms in children. Previous research has linked these measures of PFC-amygdala structure with internalizing disorders and symptoms in children and adolescents, although the directionality of these associations has been inconsistent across studies (Adluru et al., 2017; Albaugh et al., 2017; Cullen et al., 2010; De Bellis et al., 2000; Ho et al., 2017; LeWinn et al., 2014; Merz et al., 2017; Milham et al., 2005; Mueller et al., 2013; Qin et al., 2014; Rosso et al., 2005; Strawn et al., 2015; Tromp et al., 2019; van der Plas et al., 2010; Vilgis et al., 2017; Warnell et al., 2018). These associations have been found in clinical samples as well as typically-developing children and adolescents (Merz et al., 2017; Mohamed Ali et al., 2018). It is possible that these associations vary by age (McEwen, 2003) or are stronger and more consistent for biobehavioral indices (e.g., threat sensitivity) associated with internalizing symptoms (Foell et al., 2019). These possibilities should be investigated in future work.
At the cellular level, lower FA may reflect a number of processes, including lower myelination, coherence in orientation, and/or density of fibers (Song et al., 2002). In addition to lower FA, material hardship was significantly associated with lower AD in the UNC. While there is more to understand about the neurobiological underpinnings of the diffusion signal, evidence suggests that decreases in AD may reflect axonal disorganization (Budde et al., 2009; Hatton et al., 2018; Klawiter et al., 2011; Winklewski et al., 2018). DTI methods are limited with regard to specifying the underlying cellular processes (Jones et al., 2013). Future research using complementary MRI techniques is needed to more fully understand the cellular mechanisms that may be driving differences in white matter microstructure.
This study had a number of strengths, including the use of a multi-modal neuroimaging approach, strong psychometric properties of the questionnaire measures, hypotheses derived from prior theoretical and empirical work, focus on novel research questions, and analyses accounting for an array of potentially confounding factors. Several limitations of this study should also be taken into account when interpreting the results. First, given that this study employed a cross-sectional, correlational design, inferences about developmental change or causality cannot be made. Second, researchers have raised concerns regarding potential biases when testing mediation models using data from cross-sectional studies (Cole & Maxwell, 2003; Maxwell & Cole, 2007). Such analyses can still be valuable in terms of revealing possible mechanisms when the mediation model being tested is theoretically and empirically based (Shrout, 2011). Nonetheless, research is needed that tests these mediation models using longitudinal data. Third, small sample size may have limited our power to detect significant interactions for the UNC. Given that effect sizes for those interactions were small to medium, future studies should test such interactions using larger samples. Fourth, material hardship was measured through parent report, rather than an objective assessment of actual lived conditions. Therefore, this measure may confound economic stress and material hardship. Fifth, the strength of the association between material hardship and children’s internalizing symptoms may have been influenced by shared method variance. Sixth, this study focused on the UNC and two of the gray matter regions it connects. Future studies should investigate additional neural networks in terms of their potential mechanistic role in linking material hardship with elevated internalizing symptoms in children. Indeed, other neural systems that have been associated with both socioeconomic disadvantage and internalizing problems could play roles in these mechanisms (Lambert et al., 2017). Future studies should also examine whether associations of material hardship with UNC FA and amygdala volume may be specific to the right or left hemispheres. Such analyses would add to our understanding of how material hardship may affect the neural circuitry underlying emotion processing and regulation.
In conclusion, this study is the first to reveal that material hardship may be associated with lower PFC-amygdala structural connectivity and amygdala volume in children. These neural networks have been linked with reduced emotion regulation and greater emotional reactivity. In girls, lower structural connectivity between PFC regions and medial temporal regions (e.g., amygdala) may partially explain associations between material hardship and internalizing symptoms. These findings have practice and policy implications, including underscoring the importance of continued state and federal funding for income-support and safety net programs, which have been found to reduce families’ experiences of material hardship and improve children’s health outcomes (Black et al., 2004; Frank et al., 2006; Meyers et al., 2005; Pilkauskas et al., 2012). Programs and policies that reduce material hardship during childhood may prevent alterations in the development of emotion processing and regulatory neural networks that increase the risk for mental health problems.
Supplementary Material
Acknowledgements
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant numbers UL1TR001873 and UL1RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided from the Gertrude H. Sergievsky Center, Columbia University Irving Medical Center; Teachers College, Columbia University; Russell Sage Foundation; and National Institute of Mental Health (T32MH13043). We are grateful to the families who participated in this study. We also thank Rachel RouChen Lin, Charles Sisk, Mayra Lemus Rangel, Lexi Paul, Samantha Moffett, Julissa Veras, and Victor Issa Garcia for assisting with data collection. The authors declare no conflicts of interest.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Adluru N, Luo Z, Van Hulle CA, Schoen AJ, Davidson RJ, Alexander AL, & Goldsmith HH (2017). Anxiety-related experience-dependent white matter structural differences in adolescence: A monozygotic twin difference approach. Scientific Reports, 7. 10.1038/s41598-017-08107-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M, Veer IM, van Lang NDJ, Meens PHF, van den Bulk BG, Rombouts S. a. R. B., Vermeiren RRJM, & van der Wee NJ (2014). Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychological Medicine, 44(11), 2287–2298. 10.1017/S0033291713003000 [DOI] [PubMed] [Google Scholar]
- Albaugh MD, Nguyen T-V, Ducharme S, Collins DL, Botteron KN, D’Alberto N, Evans AC, Karama S, Hudziak JJ, & Brain Development Cooperative Group. (2017). Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biological Psychology, 124, 133–140. 10.1016/j.biopsycho.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, & Bastiani M (2017). Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage, 152, 450–466. 10.1016/j.neuroimage.2017.02.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, & Sotiropoulos SN (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141, 556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Ashiabi GS, & O’Neal KK (2007). Children’s Health Status: Examining the Associations among Income Poverty, Material Hardship, and Parental Factors. PLoS ONE, 2(9). 10.1371/journal.pone.0000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. JSTOR. [Google Scholar]
- Beverly SG (2001). Material hardship in the United States: Evidence from the Survey of Income and Program Participation. Social Work Research, 25(3), 143–151. JSTOR. [Google Scholar]
- Black MM, Cutts DB, Frank DA, Geppert J, Skalicky A, Levenson S, Casey PH, Berkowitz C, Zaldivar N, Cook JT, Meyers AF, Herren T, & Children’s Sentinel Nutritional Assessment Program Study Group. (2004). Special Supplemental Nutrition Program for Women, Infants, and Children participation and infants’ growth and health: A multisite surveillance study. Pediatrics, 114(1), 169–176. 10.1542/peds.114.1.169 [DOI] [PubMed] [Google Scholar]
- Bracht T, Linden D, & Keedwell P (2015). A review of white matter microstructure alterations of pathways of the reward circuit in depression. Journal of Affective Disorders, 187, 45–53. 10.1016/j.jad.2015.06.041 [DOI] [PubMed] [Google Scholar]
- Brody GH, Gray JC, Yu T, Barton AW, Beach SRH, Galván A, MacKillop J, Windle M, Chen E, Miller GE, & Sweet LH (2017). Protective Prevention Effects on the Association of Poverty With Brain Development. JAMA Pediatrics, 171(1), 46–52. 10.1001/jamapediatrics.2016.2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, & Song S-K (2009). Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: A quantitative pixelwise analysis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(9), 2805–2813. 10.1523/JNEUROSCI.4605-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, & Birn RM (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. 10.1038/nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, & Jones DK (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage, 17(1), 77–94. [DOI] [PubMed] [Google Scholar]
- Chien NC, & Mistry RS (2013). Geographic Variations in Cost of Living: Associations With Family and Child Well-Being. Child Development, 84(1), 209–225. 10.1111/j.1467-8624.2012.01846.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JC, Callahan PJ, Lyu CW, & Wilson NK (1999). Polycyclic aromatic hydrocarbon exposures of children in low-income families. Journal of Exposure Analysis and Environmental Epidemiology, 9(2), 85–98. [DOI] [PubMed] [Google Scholar]
- Cole DA, & Maxwell SE (2003). Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology, 112(4), 558–577. 10.1037/0021-843X.112.4.558 [DOI] [PubMed] [Google Scholar]
- Conger RD, & Donnellan MB (2007). An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology, 58, 175–199. 10.1146/annurev.psych.58.110405.085551 [DOI] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Kurma S, & Lim KO (2010). Altered White Matter Microstructure in Adolescents with Major Depression: A Preliminary Study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(2), 173–83.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Davidson, null, & Irwin, null. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, & Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. 10.1016/S0006-3223(00)00835-0 [DOI] [PubMed] [Google Scholar]
- Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, & McLaughlin KA (2019). Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Development, 90(1), e96–e113. 10.1111/cdev.13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dufford AJ, & Kim P (2017). Family Income, Cumulative Risk Exposure, and White Matter Structure in Middle Childhood. Frontiers in Human Neuroscience, 11. 10.3389/fnhum.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, & Magnuson K (2012). Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science, 3(3), 377–386. 10.1002/wcs.1176 [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K, & Votruba-Drzal E (2017). Moving Beyond Correlations in Assessing the Consequences of Poverty. Annual Review of Psychology, 68, 413–434. 10.1146/annurev-psych-010416-044224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesutani C, Tottenham N, & Chorpita B (2015). The Revised Child Anxiety and Depression Scale - Parent Version: Extended Applicability and Validity for Use with Younger Youth and Children with Histories of Early-Life Caregiver Neglect. Journal of Psychopathology and Behavioral Assessment, 37(4), 705–718. 10.1007/s10862-015-9494-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2017). The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron, 96(1), 56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Foell J, Palumbo IM, Yancey JR, Vizueta N, Demirakca T, & Patrick CJ (2019). Biobehavioral threat sensitivity and amygdala volume: A twin neuroimaging study. NeuroImage, 186, 14–21. 10.1016/j.neuroimage.2018.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Neault NB, Skalicky A, Cook JT, Wilson JD, Levenson S, Meyers AF, Heeren T, Cutts DB, Casey PH, Black MM, & Berkowitz C (2006). Heat or eat: The Low Income Home Energy Assistance Program and nutritional and health risks among children less than 3 years of age. Pediatrics, 118(5), e1293–1302. 10.1542/peds.2005-2943 [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Singer J, Shenoy R, & Luby JL (2013). Disrupted Amygdala Reactivity in Depressed 4–6-Year-Old Children. Journal of the American Academy of Child and Adolescent Psychiatry, 52(7), 737–746. 10.1016/j.jaac.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, & Lennon MC (2007). Income Is Not Enough: Incorporating Material Hardship Into Models of Income Associations With Parenting and Child Development. Child Development, 78(1), 70–95. 10.1111/j.1467-8624.2007.00986.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, & Verstynen TD (2013). Inflammatory Pathways Link Socioeconomic Inequalities to White Matter Architecture. Cerebral Cortex, 23(9), 2058–2071. 10.1093/cercor/bhs191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, & Hariri AR (2008). Individual Differences in Stressor-Evoked Blood Pressure Reactivity Vary with Activation, Volume, and Functional Connectivity of the Amygdala. The Journal of Neuroscience, 28(4), 990–999. 10.1523/JNEUROSCI.3606-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, & Buka SL (2002). Socioeconomic status in childhood and the lifetime risk of major depression. International Journal of Epidemiology, 31(2), 359–367. [PubMed] [Google Scholar]
- Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, Berman E, Leibenluft E, & Pine DS (2017). Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 10.1038/npp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. 10.1037//0021-843x.107.1.128 [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between Income and the Hippocampus. PLOS ONE, 6(5), e18712. 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, & Davidson RJ (2015). Behavior Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological Psychiatry, 77(4), 314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Panizzon MS, Vuoksimaa E, Hagler DJ, Fennema-Notestine C, Rinker D, Eyler LT, Franz CE, Lyons MJ, Neale MC, Tsuang MT, Dale AM, & Kremen WS (2018). Genetic relatedness of axial and radial diffusivity indices of cerebral white matter microstructure in late middle age. Human Brain Mapping, 39(5), 2235–2245. 10.1002/hbm.24002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press. [Google Scholar]
- He X, Liu W, Li Q, Liu F, Rauh VA, Monk C, Yin D, Bansal R, Duan Y, Kangarlu A, Alayar BS, & Xu D (2014). Automated Assessment of the Quality of Diffusion Tensor Imaging Data Using Color Cast of Color-Encoded Fractional Anisotropy Images. Magnetic Resonance Imaging, 32(5), 446–456. 10.1016/j.mri.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Mattson WI, Dotterer HL, Mitchell C, Lopez-Duran N, Thomason ME, Peltier SJ, Welsh RC, Hyde LW, & Monk CS (2018). Amygdala habituation and uncinate fasciculus connectivity in adolescence: A multi-modal approach. NeuroImage, 183, 617–626. 10.1016/j.neuroimage.2018.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, King LS, Leong JK, Colich NL, Humphreys KL, Ordaz SJ, & Gotlib IH (2017). Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Social Cognitive and Affective Neuroscience, 12(9), 1460–1469. 10.1093/scan/nsx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, & Epperson CN (2019). Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biological Psychiatry. 10.1016/j.biopsych.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Kim Y, & Sherraden M (2017). Material hardship and children’s social-emotional development: Testing mitigating effects of Child Development Accounts in a randomized experiment. Child: Care, Health and Development, 43(1), 89–96. 10.1111/cch.12385 [DOI] [PubMed] [Google Scholar]
- Iceland J, & Bauman KJ (2007). Income poverty and material hardship: How strong is the association? The Journal of Socio-Economics, 36(3), 376–396. 10.1016/j.socec.2006.12.003 [DOI] [Google Scholar]
- Jerrett M (2009). Global geographies of injustice in traffic-related air pollution exposure. Epidemiology, 20(2), 231–233. 10.1097/EDE.0b013e31819776a1 [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, & Turner R (2013). White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, & Blumberg HP (2009). Relation Between Amygdala Structure and Function in Adolescents With Bipolar Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 48(6), 636–642. 10.1097/CHI.0b013e31819f6fbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Feng X, Babinski D, Hinze A, Rischall M, & Henneberger A (2008). Subthreshold Symptoms of Depression in Preadolescent Girls Are Stable and Predictive of Depressive Disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 47(12), 1433–1442. 10.1097/CHI.0b013e3181886eab [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Sisk LM, Ho TC, Humphreys KL, King LS, Colich NL, Ordaz SJ, & Gotlib IH (2019). Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Development and Psychopathology, 1–12. 10.1017/S0954579419000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang H-F, Budde MD, Naismith RT, Song S-K, Cross AH, & Benzinger TL (2011). Radial Diffusivity Predicts Demyelination in ex-vivo Multiple Sclerosis Spinal Cords. NeuroImage, 55(4), 1454–1460. 10.1016/j.neuroimage.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, van IJzendoorn MH, Bakermans-Kranenburg MJ, & Crone EA (2013). Hippocampal volume and internalizing behavior problems in adolescence. European Neuropsychopharmacology, 23(7), 622–628. 10.1016/j.euroneuro.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and Psychopathology, 29(3), 929–940. 10.1017/S0954579416000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between Children’s Socioeconomic Status and Prefrontal Cortical Thickness. Developmental Science, 16(5), 641–652. 10.1111/desc.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, Simmons AN, & Yang TT (2014). White Matter Correlates of Adolescent Depression: Structural Evidence for Frontolimbic Disconnectivity. Journal of the American Academy of Child and Adolescent Psychiatry, 53(8), 899–909.e7. 10.1016/j.jaac.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, & Barch D (2013). The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatrics, 167(12), 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AP, Finn AS, Leonard JA, Jacoby Senghor DS, West MR, Gabrieli CFO, & Gabrieli JDE (2015). Neuroanatomical Correlates of the Income Achievement Gap. Psychological Science, 26(6), 925–933. 10.1177/0956797615572233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski C, Béland S, Kostopoulos P, Bhagwat N, Devenyi GA, Malla AK, Joober R, Lepage M, & Chakravarty MM (2018). Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. NeuroImage, 170, 182–198. 10.1016/j.neuroimage.2017.02.069 [DOI] [PubMed] [Google Scholar]
- Marrus N, Belden A, Nishino T, Handler T, Ratnanather JT, Miller M, Barch D, Luby J, & Botteron K (2015). Ventromedial Prefrontal Cortex Thinning in Preschool-Onset Depression. Journal of Affective Disorders, 180, 79–86. 10.1016/j.jad.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12(1), 23–44. 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- Mayer SE, & Jencks C (1989). Poverty and the Distribution of Material Hardship. The Journal of Human Resources, 24(1), 88–114. JSTOR. 10.2307/145934 [DOI] [Google Scholar]
- McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, Reardon PK, Lalonde F, Greenstein D, Patel R, Chakravarty MM, Lerch JP, & Raznahan A (2019). Longitudinally Mapping Childhood Socioeconomic Status Associations with Cortical and Subcortical Morphology. The Journal of Neuroscience, 39(8), 1365–1373. 10.1523/JNEUROSCI.1808-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. 10.1016/S0006-3223(03)00177-X [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 41(1), 3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, He X, & Noble KG (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage : Clinical, 20, 243–251. 10.1016/j.nicl.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2017). Socioeconomic Status, Amygdala Volume, and Internalizing Symptoms in Children and Adolescents. Journal of Clinical Child and Adolescent Psychology, 1–12. 10.1080/15374416.2017.1326122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers A, Cutts D, Frank DA, Levenson S, Skalicky A, Heeren T, Cook J, Berkowitz C, Black M, Casey P, & Zaldivar N (2005). Subsidized housing and children’s nutritional status: Data from a multisite surveillance study. Archives of Pediatrics & Adolescent Medicine, 159(6), 551–556. 10.1001/archpedi.159.6.551 [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, Charney D, & Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biological Psychiatry, 57(9), 961–966. 10.1016/j.biopsych.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Mohai P, Lantz PM, Morenoff J, House JS, & Mero RP (2009). Racial and Socioeconomic Disparities in Residential Proximity to Polluting Industrial Facilities: Evidence From the Americans’ Changing Lives Study. American Journal of Public Health, 99(Suppl 3), S649–S656. 10.2105/AJPH.2007.131383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed Ali O, Vandermeer MRJ, Sheikh HI, Joanisse MF, & Hayden EP (2018). Girls’ internalizing symptoms and white matter tracts in Cortico-Limbic circuitry. NeuroImage : Clinical, 21. 10.1016/j.nicl.2018.101650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, & Kyle AD (2011). Understanding the cumulative impacts of inequalities in environmental health: Implications for policy. Health Affairs (Project Hope), 30(5), 879–887. 10.1377/hlthaff.2011.0153 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, & Ernst M (2013). Grey Matter Volume in Adolescent Anxiety: An Impact of the Brain-Derived Neurotropic Factor Val66Met Polymorphism? Journal of the American Academy of Child and Adolescent Psychiatry, 52(2), 184–195. 10.1016/j.jaac.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckerman KM, Garfinkel I, Teitler JO, Waldfogel J, & Wimer C (2016). Beyond income poverty: Measuring disadvantage in terms of material hardship and health. Academic Pediatrics, 16(3 Suppl), S52–S59. 10.1016/j.acap.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland RP, Crnic KA, Cox MJ, Mills-Koonce WR, & Family Life Project Key Investigators. (2013). The family model stress and maternal psychological symptoms: Mediated pathways from economic hardship to parenting. Journal of Family Psychology, 27(1), 96–105. 10.1037/a0031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Thompson WK, Bartsch H, Hagler DJ, Chen C-H, Brown TT, Kuperman JM, McCabe C, Chung Y, Libiger O, Akshoomoff N, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Murray SS, … Jernigan TL (2015). Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Structure and Function, 221(6), 3013–3025. 10.1007/s00429-015-1085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Zijl PV, … Sowell ER (2015). Family Income, Parental Education and Brain Structure in Children and Adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, & Bouma EMC (2011). Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neuroscience and Biobehavioral Reviews, 35(8), 1757–1770. 10.1016/j.neubiorev.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, & Kremen WS (2009). Distinct Genetic Influences on Cortical Surface Area and Cortical Thickness. Cerebral Cortex, 19(11), 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza C, Sánchez-López J, Castilla-Ortega E, Rosell-Valle C, Zambrana-Infantes E, García-Fernández M, Rodriguez de Fonseca F, Chun J, Santín LJ, & Estivill-Torrús G (2014). Fear extinction and acute stress reactivity reveal a role of LPA(1) receptor in regulating emotional-like behaviors. Brain Structure & Function, 219(5), 1659–1672. 10.1007/s00429-013-0592-9 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, & Weissman MM (2009). Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6273–6278. 10.1073/pnas.0805311106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkauskas NV, Currie J, & Garfinkel I (2012). The Great Recession, Public Transfers, and Material Hardship. The Social Service Review, 86(3), 401–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, & Menon V (2014). Amygdala Subregional Structure and Intrinsic Functional Connectivity Predicts Individual Differences in Anxiety During Early Childhood. Biological Psychiatry, 75(11), 892–900. 10.1016/j.biopsych.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, & Giedd JN (2011). How Does Your Cortex Grow? The Journal of Neuroscience, 31(19), 7174–7177. 10.1523/JNEUROSCI.0054-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D (1999). Economic determinants and dietary consequences of food insecurity in the United States. The Journal of Nutrition, 129(2S Suppl), 517S–520S. 10.1093/jn/129.2.517S [DOI] [PubMed] [Google Scholar]
- Rose D, & Oliveira V (1997). Nutrient intakes of individuals from food-insufficient households in the United States. American Journal of Public Health, 87(12), 1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, & Yurgelun-Todd DA (2005). Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry, 57(1), 21–26. 10.1016/j.biopsych.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Rubinow DR, & Schmidt PJ (2019). Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology, 44(1), 111–128. 10.1038/s41386-018-0148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck-Fontaine A, & Panico L (2019). Many Kinds of Poverty: Three Dimensions of Economic Hardship, Their Combinations, and Children’s Behavior Problems. Demography, 56(6), 2279–2305. 10.1007/s13524-019-00833-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bülow R, Selonke M, … Veltman DJ (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22, 900–909. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, & Wedeen VJ (2007). Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain: A Journal of Neurology, 130(Pt 3), 630–653. 10.1093/brain/awl359 [DOI] [PubMed] [Google Scholar]
- Shankar P, Chung R, & Frank DA (2017). Association of Food Insecurity with Children’s Behavioral, Emotional, and Academic Outcomes: A Systematic Review. Journal of Developmental and Behavioral Pediatrics: JDBP, 38(2), 135–150. 10.1097/DBP.0000000000000383 [DOI] [PubMed] [Google Scholar]
- Shrout PE (2011). Commentary: Mediation Analysis, Causal Process, and Cross-Sectional Data. Multivariate Behavioral Research, 46(5), 852–860. 10.1080/00273171.2011.606718 [DOI] [PubMed] [Google Scholar]
- Slopen N, Fitzmaurice G, Williams DR, & Gilman SE (2010). Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 49(5), 444–452. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TEJ (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 Suppl 1, S208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, & Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17(3), 1429–1436. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, & Phan KL (2015). Neurostructural Abnormalities in Pediatric Anxiety Disorders. Journal of Anxiety Disorders, 32, 81–88. 10.1016/j.janxdis.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, John Wegman C, Dominick KC, Swartz MS, Wehry AM, Patino LR, Strakowski SM, Adler CM, Eliassen JC, & DelBello MP (2014). Cortical surface anatomy in pediatric patients with generalized anxiety disorder. Journal of Anxiety Disorders, 28(7), 717–723. 10.1016/j.janxdis.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Sun W, Li D, Zhang W, Bao Z, & Wang Y (2015). Family Material Hardship and Chinese Adolescents’ Problem Behaviors: A Moderated Mediation Analysis. PLoS ONE, 10(5). 10.1371/journal.pone.0128024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, & Monk CS (2014). Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage, 86, 212–220. 10.1016/j.neuroimage.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, & Thompson PM (2011). Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology, 7, 63–85. 10.1146/annurev-clinpsy-032210-104507 [DOI] [PubMed] [Google Scholar]
- Tromp DPM, Williams LE, Fox AS, Oler JA, Roseboom PH, Rogers GM, Benson BE, Alexander AL, Pine DS, & Kalin NH (2019). Altered Uncinate Fasciculus Microstructure in Childhood Anxiety Disorders in Boys But Not Girls. The American Journal of Psychiatry, 176(3), 208–216. 10.1176/appi.ajp.2018.18040425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, & Noble KG (2016). Socioeconomic status, white matter, and executive function in children. Brain and Behavior, 6(10). 10.1002/brb3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EAA, Boes AD, Wemmie JA, Tranel D, & Nopoulos P (2010). Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience, 5(4), 424–431. 10.1093/scan/nsq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgis V, Vance A, Cunnington R, & Silk TJ (2017). White matter microstructure in boys with persistent depressive disorder. Journal of Affective Disorders, 221, 11–16. 10.1016/j.jad.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Evans GW, Grant K, Carter JS, & Duffy S (2016). Poverty and the Development of Psychopathology. In Developmental Psychopathology (Vol. 4, pp. 1–44). John Wiley & Sons, Inc. [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, & Mori S (2007). Reproducibility of Quantitative Tractography Methods Applied to Cerebral White Matter. NeuroImage, 36(3), 630–644. 10.1016/j.neuroimage.2007.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnell KR, Pecukonis M, & Redcay E (2018). Developmental relations between amygdala volume and anxiety traits: Effects of informant, sex, and age. Development and Psychopathology, 30(4), 1503–1515. 10.1017/S0954579417001626 [DOI] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, Pantelis C, McGorry P, & Allen NB (2014). Structural Brain Development and Depression Onset During Adolescence: A Prospective Longitudinal Study. American Journal of Psychiatry, 171(5), 564–571. 10.1176/appi.ajp.2013.13070920 [DOI] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Dennison M, Schwartz O, Simmons JG, Sheeber L, & Allen NB (2016). Observed Measures of Negative Parenting Predict Brain Development during Adolescence. PLoS ONE, 11(1). 10.1371/journal.pone.0147774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yap MBH, Yücel M, Sheeber L, Simmons JG, Pantelis C, & Allen NB (2009). Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Social Cognitive and Affective Neuroscience, 4(3), 247–256. 10.1093/scan/nsp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, & Szarmach A (2018). Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes—What Do We Know? Frontiers in Neurology, 9. 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, & Schimmele CM (2005). Food Insufficiency and Depression. Sociological Perspectives, 48(4), 481–504. 10.1525/sop.2005.48.4.481 [DOI] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson R-M, Cameron HA, Williams RW, & Holmes A (2008). Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology, 33(11), 2595–2604. 10.1038/sj.npp.1301665 [DOI] [PubMed] [Google Scholar]
- Yoo JP, Slack KS, & Holl JL (2009). Material Hardship and the Physical Health of School-Aged Children in Low-Income Households. American Journal of Public Health, 99(5), 829–836. 10.2105/AJPH.2007.119776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilanawala A, & Pilkauskas NV (2012). Material Hardship and Child Socioemotional Behaviors: Differences by Types of Hardship, Timing, and Duration. Children and Youth Services Review, 34(4), 814–825. 10.1016/j.childyouth.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier LA, Nikolova YS, Ahs F, & Hariri AR (2013). Uncinate Fasciculus Fractional Anisotropy Correlates With Typical Use of Reappraisal in Women But Not Men. Emotion, 13(3), 385–390. 10.1037/a0031163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


