Abstract
Introduction
Data on the characteristics of apremilast patients in real-world settings are limited. We assessed the demographics and disease characteristics of apremilast-treated patients in the Corrona Psoriasis Registry overall and by treatment history.
Methods
The Corrona Psoriasis Registry is a large, independent, prospective, observational registry of adult patients (age ≥ 18 years) who initiate an eligible systemic medication for treatment of psoriasis at or after enrollment (incident users) or within 12 months before enrollment (prevalent users). The current analyses included psoriasis patients enrolled in the Corrona Psoriasis Registry between April 1, 2015, and January 7, 2018. Patients were adults (age ≥ 18 years) with psoriasis who were enrolled between April 1, 2015, and January 7, 2018 and initiated apremilast at the time of registry enrollment or a subsequent visit (incident users) or within the 12 months prior to registry enrollment (prevalent users). Patient characteristics were evaluated descriptively at the index date, defined as the enrollment date for prevalent users and the visit when apremilast was initiated for incident users.
Results
Among 660 patients who initiated apremilast at registry enrollment or a visit thereafter, psoriatic arthritis, hypertension, and hyperlipidemia were common. There were more systemic-experienced (61.4%) versus systemic-naive (38.6%) patients; 43.8% had prior biologic exposure. Most patients were not receiving concomitant systemic treatment (70.2%); 27.4% were receiving concomitant biologic therapy. Most patients had mild or moderate disease (psoriasis-involved body surface area ≤ 10% [76.0%], Investigator Global Assessment ≤ 3 [88.3%], Psoriasis Area and Severity Index ≤ 10 [84.5%]). Dermatologist-reported psoriatic arthritis was present in 47.0% of patients; 33.9% of patients had a Psoriasis Epidemiology Screening Tool score of ≥ 3, suggestive of psoriatic arthritis. Systemic-experienced apremilast patients had higher rates of obesity and comorbidities and experienced a greater impact on quality of life (mean Dermatology Life Quality Index, 7.3 vs. 6.5) versus systemic-naive patients.
Conclusion
In this real-world observational study of apremilast users in the Corrona Psoriasis Registry, most patients had less-severe disease and higher rates of prior exposure to biologic treatments compared with patients with moderate-to-severe psoriasis enrolled in phase 3 clinical studies.
Keywords: Apremilast, Corrona Psoriasis Registry, Psoriasis, Observational study, Real-world
Key Summary Points
| Why carry out this study? |
| To determine the demographic and clinical characteristics of patients treated with apremilast in the Corrona Psoriasis Registry. |
| What was learned from the study? |
| In this observational study of apremilast users enrolled in the Corrona Psoriasis Registry, most had less-severe skin disease and greater biologic experience versus trials in patients with moderate-to-severe psoriasis. One-third of patients had a Psoriasis Epidemiology Screening Tool score indicating PsA, and many received concomitant biologics. |
| In real-world clinical practice, physicians prescribed apremilast to patients who had less-severe psoriasis compared with apremilast patients in the phase 3 ESTEEM studies, but apremilast patients in the Corrona Psoriasis Registry had psoriasis severity that was more comparable to patients in the phase 4 UNVEIL study. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13379888.
Introduction
Apremilast, an oral phosphodiesterase-4 inhibitor, is approved for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy and patients with active psoriatic arthritis (PsA). In phase 3 clinical trials, apremilast demonstrated efficacy, a favorable tolerability profile, and improvements in pruritus and quality of life (QOL) versus placebo in patients with moderate-to-severe plaque psoriasis [1–3].
Limited data are available regarding the types of patients with psoriasis who are initiated on apremilast treatment in US dermatology care settings [4–7]. The Corrona Psoriasis Registry was launched in 2015, in collaboration with the National Psoriasis Foundation, to better characterize the epidemiology and natural history of psoriasis and the safety and effectiveness of systemic psoriasis treatments in real-world US patients [8]. To further understand the profile of psoriasis patients treated with apremilast in the United States, demographic, clinical, and treatment characteristics at enrollment or the time of apremilast initiation were evaluated among patients in the Corrona Psoriasis Registry who received apremilast. To elucidate the treatment patterns in the real-world use of apremilast, analyses were performed that compared apremilast users at the time of registry enrollment or apremilast initiation. We hypothesized that characteristics would vary according to psoriasis treatment history.
Methods
Registry Design and Patient Population
The Corrona Psoriasis Registry is a large, independent, prospective, observational registry of adult patients (age ≥ 18 years) with a dermatologist’s diagnosis of psoriasis who initiate an eligible systemic medication for treatment of psoriasis at or after enrollment (incident users) or within 12 months before enrollment (prevalent users) [8]. Eligible treatments approved by the US Food and Drug Administration for psoriasis as of the date of data analysis (January 7, 2018) were biologics (adalimumab, brodalumab, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, and ustekinumab) and nonbiologic systemic agents (acitretin, apremilast, cyclosporine, and methotrexate). The target enrollment for patients initiating a nonbiologic systemic medication (N = 500) was met on June 20, 2016, and patients initiating nonbiologic systemic medications (e.g., apremilast) are no longer being enrolled. After June 20, 2016, the only patients receiving nonbiologics who were enrolled in the registry were those who switched from an eligible medication to a nonbiologic. At the time of data analysis (January 7, 2018), the registry patients were recruited by 443 participating dermatologists from 193 private and academic practice sites in 43 US states and Canada.
Data Collection
The registry collects patient and physician data using questionnaires during routine dermatology office visits approximately every 6 months. All participating investigators were required to obtain full board approval to conduct research involving human subjects. Sponsor approval and approval for continuing review were obtained through a central institutional review board (IRB; IntegReview, Corrona-PSO-500). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs, and approval documentation was submitted to the sponsor before initiating any study procedures. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All registry patients were required to provide written informed consent before participating.
Assessments
Patient characteristics evaluated at visits included demographics (e.g., age, gender, race, ethnicity, health insurance type, employment status), lifestyle characteristics (e.g., body weight, body mass index), and comorbidities (current and previous). Patient disease and treatment profiles were characterized by assessing psoriasis duration and morphology, the diagnosis and duration of PsA, clinical assessments (psoriasis-involved body surface area [BSA]; Psoriasis Area and Severity Index [PASI, 0–72]; Investigator Global Assessment [IGA, 0–4]; Psoriasis Epidemiology Screening Tool [PEST, 0-5]), and prior and concomitant psoriasis treatments. Patient-reported outcome (PRO) measures included Dermatology Life Quality Index (DLQI, 0–30) and visual analog scales (VASs; 0–100 mm) for skin pain, fatigue, and pruritus.
Statistical Analysis
The current analyses represent a cross-sectional descriptive study that included psoriasis patients enrolled in the Corrona Psoriasis Registry between April 1, 2015, and January 7, 2018. Patient characteristics at the index date were evaluated in patients who initiated apremilast at the time of registry enrollment or a subsequent visit (incident users) or who initiated apremilast within the 12 months before registry enrollment (prevalent users). The index date was defined as the enrollment date for prevalent users and the visit when apremilast was initiated for incident users.
Descriptive statistics at index were calculated for categorical (no. [%]) and continuous (mean [SD]) variables in the overall apremilast population, and by apremilast user type (incident or prevalent) and prior systemic experience (systemic-naive or systemic-experienced). Systemic-experienced patients were defined as those who had prior treatment with a biologic (adalimumab, brodalumab, guselkumab, etanercept, infliximab, ixekizumab, secukinumab, and ustekinumab) or nonbiologic (acitretin, apremilast, cyclosporine, and methotrexate) systemic therapy. Data were evaluated as observed; no methodology was used to impute missing values.
Results
Patient Demographics and Comorbidity Profile
Among the 3902 patients enrolled in the Corrona Psoriasis Registry as of January 7, 2018, 660 apremilast-treated patients were included in descriptive analyses of demographic, clinical, and treatment characteristics, including 461 prevalent apremilast users and 199 incident apremilast users. There were also 255 systemic-naive and 405 systemic-experienced patients among the apremilast-treated patients.
Patient demographics and comorbidities at index are summarized in Table 1. Overall, mean (SD) age was 53.7 (14.1) years, half of the patients were female, and almost three-quarters were white. At index, most of the patients were overweight or obese, and the most common comorbidities were PsA, hypertension, and hyperlipidemia. The proportion of prevalent users was higher than that of incident users, and there were more systemic-experienced than systemic-naive patients. More than two-thirds of the patients had private health insurance, and half were employed full time.
Table 1.
Patient demographics, disease characteristics, and treatment profile at index date
| Characteristic | Overall apremilast population N = 660 |
Incident users n = 199 |
Prevalent users n = 461 |
Systemic-naive n = 255 |
Systemic-experienced n = 405 |
|---|---|---|---|---|---|
| Age, mean (SD), years | 53.7 (14.1) | 53.0 (13.9) | 54.0 (14.2) | 53.3 (15.0) | 53.9 (13.5) |
| Female, no. (%) | 332 (50.3) | 99 (49.7) | 233 (50.5) | 125 (49.0) | 207 (51.1) |
| Race, no. (%) | |||||
| White | 477 (72.3) | 157 (78.9) | 320 (69.4) | 175 (68.6) | 302 (74.6) |
| African American | 17 (2.6) | 11 (5.5) | 6 (1.3) | 6 (2.4) | 11 (2.7) |
| Asian | 113 (17.1) | 18 (9.0) | 95 (20.6) | 50 (19.6) | 63 (15.6) |
| Other | 53 (8.0) | 13 (6.5) | 40 (8.7) | 24 (9.4) | 29 (7.2) |
| Body mass index, mean (SD), kg/m2 | 30.2 (7.3) | 31.0 (8.1) | 29.8 (6.9) | 29.2 (6.7) | 30.8 (7.6) |
| Body mass index category, no. (%) | |||||
| Underweight/normal (< 25 kg/m2) | 162 (24.5) | 47 (23.6) | 115 (24.9) | 77 (30.2) | 85 (21.0) |
| Overweight (≥ 25 to < 30 kg/m2) | 218 (33.0) | 60 (30.2) | 158 (34.3) | 85 (33.3) | 133 (32.8) |
| Obese (≥ 30 kg/m2) | 280 (42.4) | 92 (46.2) | 188 (40.8) | 93 (36.5) | 187 (46.2) |
| Body weight, mean (SD), kg | 86.5 (23.2) | 89.2 (23.2) | 85.3 (23.1) | 83.0 (20.9) | 88.6 (24.4) |
| Health insurance type, no. (%) | |||||
| Private | 446 (68.0) | 144 (72.4) | 302 (66.1) | 169 (66.8) | 277 (68.7) |
| Medicare | 123 (18.8) | 31 (15.6) | 92 (20.1) | 49 (19.4) | 74 (18.4) |
| Medicaid | 73 (11.1) | 19 (9.5) | 54 (11.8) | 31 (12.3) | 42 (10.4) |
| No insurance | 14 (2.1) | 5 (2.5) | 9 (2.0) | 4 (1.6) | 10 (2.5) |
| Full-time employment, no. (%) | 332 (50.4) | 105 (52.8) | 227 (49.3) | 126 (49.4) | 206 (51.0) |
| History of comorbiditiesa in ≥ 5%, no. (%) | |||||
| Hypertension | 304 (46.1) | 87 (43.7) | 217 (47.1) | 105 (41.2) | 199 (49.1) |
| Hyperlipidemia | 213 (32.3) | 60 (30.2) | 153 (33.2) | 73 (28.6) | 140 (34.6) |
| Depression | 113 (17.1) | 27 (13.6) | 86 (18.7) | 34 (13.3) | 79 (19.5) |
| Cardiovascular disease | 109 (16.5) | 24 (12.1) | 85 (18.4) | 41 (16.1) | 68 (16.8) |
| Anxiety | 108 (16.4) | 31 (15.6) | 77 (16.7) | 35 (13.7) | 73 (18.0) |
| Diabetes mellitus | 107 (16.2) | 32 (16.1) | 75 (16.3) | 37 (14.5) | 70 (17.3) |
| IBD/other GI disordersb | 95 (14.4) | 29 (14.6) | 66 (14.3) | 35 (13.7) | 60 (14.8) |
| Cancerc | 92 (13.9) | 35 (17.6) | 57 (12.4) | 30 (11.8) | 62 (15.3) |
| Serious infection | 53 (8.0) | 19 (9.5) | 34 (7.4) | 13 (5.1) | 40 (9.9) |
| PsA | 310 (47.0) | 96 (48.2) | 214 (46.4) | 83 (32.5) | 227 (56.0) |
| PsA duration, mean (SD), years | 9.7 (10.6) | 10.0 (11.0) | 9.5 (10.4) | 6.1 (6.9) | 11.0 (11.3) |
| Psoriasis duration, mean (SD), years | 14.3 (13.9) | 13.9 (13.4) | 14.5 (14.1) | 11.9 (13.4) | 15.8 (14.0) |
| Psoriasis morphology, no. (%) | |||||
| Plaque | 632 (95.8) | 193 (97.0) | 439 (95.2) | 244 (95.7) | 388 (95.8) |
| Guttate | 23 (3.5) | 11 (5.5) | 12 (2.6) | 8 (3.1) | 15 (3.7) |
| Erythrodermic | 10 (1.5) | 4 (2.0) | 6 (1.3) | 0 (0.0) | 10 (2.5) |
| Pustular (localized) | 5 (0.8) | 2 (1.0) | 6 (1.3) | 1 (0.4) | 7 (1.7) |
| Pustular (generalized) | 8 (1.2) | 1 (0.5) | 4 (0.9) | 0 (0.0) | 5 (1.2) |
| Inverse/intertriginous | 35 (5.3) | 11 (5.5) | 24 (5.2) | 14 (5.5) | 21 (5.2) |
| Scalp | 210 (31.8) | 69 (34.7) | 141 (30.6) | 77 (30.2) | 133 (32.8) |
| Nail | 74 (11.2) | 22 (11.1) | 52 (11.3) | 16 (6.3) | 58 (14.3) |
| Palmoplantar | 73 (11.1) | 22 (11.1) | 51 (11.1) | 24 (9.4) | 49 (12.1) |
| PEST (0–5) ≥ 3, no. (%) | 222 (33.9) | 62 (31.5) | 160 (34.9) | 50 (19.8) | 172 (42.7) |
| BSA, mean (SD) | 10.3 (14.9) | 12.0 (15.7) | 9.5 (14.5) | 7.7 (10.6) | 11.9 (16.9) |
| PASI (0–72), mean (SD) | 5.7 (6.6) | 6.5 (7.0) | 5.4 (6.5) | 4.8 (6.0) | 6.3 (7.0) |
| PASI > 10, no. (%) | 102 (15.5) | 31 (15.6) | 71 (15.4) | 26 (10.2) | 76 (18.8) |
| IGA category, no. (%) | |||||
| 0 (clear) | 52 (7.9) | 13 (6.5) | 39 (8.5) | 18 (7.1) | 34 (8.4) |
| 1 (almost clear) | 76 (11.5) | 16 (8.0) | 60 (13.0) | 37 (14.5) | 39 (9.6) |
| 2 (mild) | 176 (26.7) | 40 (20.1) | 136 (29.5) | 79 (31.0) | 97 (24.0) |
| 3 (moderate) | 279 (42.3) | 101 (50.8) | 178 (38.6) | 97 (38.0) | 182 (44.9) |
| 4 (severe) | 77 (11.7) | 29 (14.6) | 48 (10.4) | 24 (9.4) | 53 (13.1) |
| DLQI (0–30), mean (SD) | 7.0 (6.0) | 7.2 (6.1) | 6.9 (5.9) | 6.5 (5.6) | 7.3 (6.2) |
| Prior medication use, no. (%) | |||||
| ≥ 1 Systemic | 405 (61.4) | 125 (62.8) | 280 (60.7) | 0 | 405 (100.0) |
| ≥ 1 Biologic | 289 (43.8) | 97 (48.7) | 192 (41.6) | 0 | 289 (71.4) |
| Concomitant systemic therapy, no. (%) | |||||
| Apremilast monotherapy (i.e., without concomitant systemic therapy) | 463 (70.2) | 168 (84.4) | 295 (64.0) | 238 (93.3) | 213 (52.6) |
| Apremilast + any biologic | 181 (27.4) | 24 (12.1) | 157 (34.1) | 16 (6.3) | 165 (40.7) |
| Apremilast + biologic (TNF inhibitor) | 67 (10.2) | 15 (7.5) | 52 (11.3) | 7 (2.7) | 60 (14.8) |
| Apremilast + biologic (other) | 114 (17.3) | 9 (4.5) | 105 (22.8) | 9 (3.5) | 105 (25.9) |
| Apremilast + nonbiologic systemic | 16 (2.4) | 7 (3.5) | 9 (2.0) | 0 | 16 (4.0) |
| Concomitant topical, no. (%) | 460 (69.7) | 111 (55.8) | 349 (75.7) | 188 (73.7) | 272 (67.2) |
| Concomitant phototherapy, no. (%) | 68 (10.3) | 7 (3.5) | 61 (13.2) | 41 (16.1) | 27 (6.7) |
| Patient-reported outcome based on visual analog scaled (0–100 mm), mean (SD) | |||||
| Skin pain | 25.0 (29.3) | 30.1 (31.1) | 22.8 (28.2) | 21.6 (27.6) | 27.1 (30.1) |
| Fatigue | 35.3 (29.8) | 37.5 (29.2) | 34.3 (30.0) | 32.4 (29.2) | 37.1 (30.0) |
| Pruritus | 40.9 (32.2) | 45.8 (33.4) | 38.8 (31.4) | 38.4 (30.9) | 42.5 (32.9) |
Number of patients with data available varied with the characteristic considered
Index date is defined as enrollment date for prevalent users and the visit when apremilast was initiated for incident users
BSA psoriasis-involved body surface area, DLQI Dermatology Life Quality Index, GI gastrointestinal, IBD inflammatory bowel disease, IGA Investigator’s Global Assessment, PASI Psoriasis Area and Severity Index, PEST Psoriasis Epidemiology Screening Tool, PsA psoriatic arthritis, SD standard deviation, TNF tumor necrosis factor
aIncludes comorbidities at or prior to the index visit
bIncludes IBD/other GI disorders, ulcerative colitis, or Crohn’s disease
cExcludes non-melanoma skin cancer
dRange of possible scores: 0 (no pain/fatigue/itch) to 100 (worst imaginable pain/fatigue/itch)
Rates of common comorbidities were generally similar for incident and prevalent users; however, the proportion of obese patients was lower among prevalent than incident users, and prevalent users had lower rates of cancer and higher rates of cardiovascular disease and depression than incident users. Demographic characteristics were mostly similar for systemic-naive versus systemic-experienced patients; however, the proportion of obese patients was higher for systemic-experienced patients. Systemic-naive patients had lower rates of most comorbid diseases compared with systemic-experienced patients, except for cardiovascular disease, which was similar between groups.
Patient Disease and Treatment Profile
Among apremilast users in the Corrona Psoriasis Registry, the mean (SD) psoriasis duration was 14.3 (13.9) years; approximately one-third of patients had scalp psoriasis, and approximately one-third had a PEST score of ≥ 3, suggestive of PsA (Table 1) [9, 10]. Most patients had BSA ≤ 10%, IGA ≤ 3, and PASI ≤ 10 (Fig. 1, Table 1). Many patients had previously been treated with systemic or biologic therapy. The majority were receiving apremilast as monotherapy or concomitantly with topical therapy, and most patients were not receiving concomitant systemic treatment; 27.4% of patients had concomitant treatment with a biologic therapy. The use of apremilast in combination with a biologic was more prevalent in systemic-experienced versus systemic-naive patients, and in prevalent versus incident apremilast users.
Fig. 1.
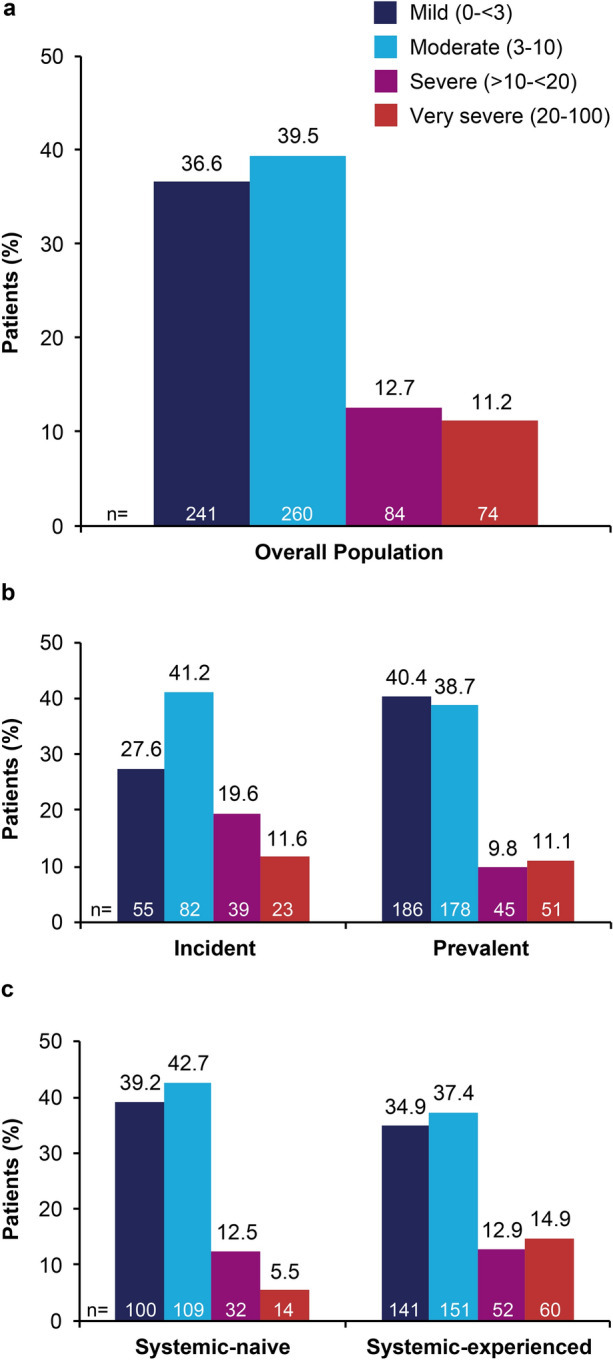
BSA in a the overall apremilast population, b incident versus prevalent users, c systemic-naive versus systemic-experienced users. Incident = initiated apremilast treatment at or after enrollment; prevalent = initiated apremilast treatment within 12 months before enrollment
The mean duration of psoriasis was similar for incident and prevalent users and shorter for systemic-naive versus systemic-experienced patients (Table 1). The proportions of patients with scalp psoriasis were similar among incident versus prevalent users and systemic-naive versus systemic-experienced patients. The proportions of patients with a PEST score of ≥ 3 were similar between incident and prevalent users; however, a PEST score of ≥ 3 was reported in a lower proportion of systemic-naive versus systemic-experienced patients. In general, prevalent users and systemic-naive patients had less-severe disease at the index date (i.e., a lower mean BSA, IGA category, and mean PASI score) than incident users and systemic-experienced patients, respectively, and the proportion of patients with severe to very severe psoriasis (i.e., BSA > 10%) was lower in prevalent and systemic-naive versus incident and systemic-experienced patients (Fig. 1). The proportion of patients with prior use of systemic treatments was similar in incident versus prevalent users, and the proportion of patients with prior biologic use was higher in incident versus prevalent users (Table 1). Apremilast monotherapy was more frequent in incident versus prevalent users and in systemic-naive versus systemic-experienced patients. Concomitant use of topical treatments was less frequent in incident versus prevalent users and more frequent in systemic-naive versus systemic-experienced patients.
PRO Measures
Approximately 25% of patients reported a very large or extremely large impact of psoriasis on QOL at index in the overall apremilast population. The proportion of patients who reported a very large or extremely large impact of psoriasis on QOL was similar for incident and prevalent users and numerically lower in systemic-naive versus systemic-experienced patients (Fig. 2).
Fig. 2.
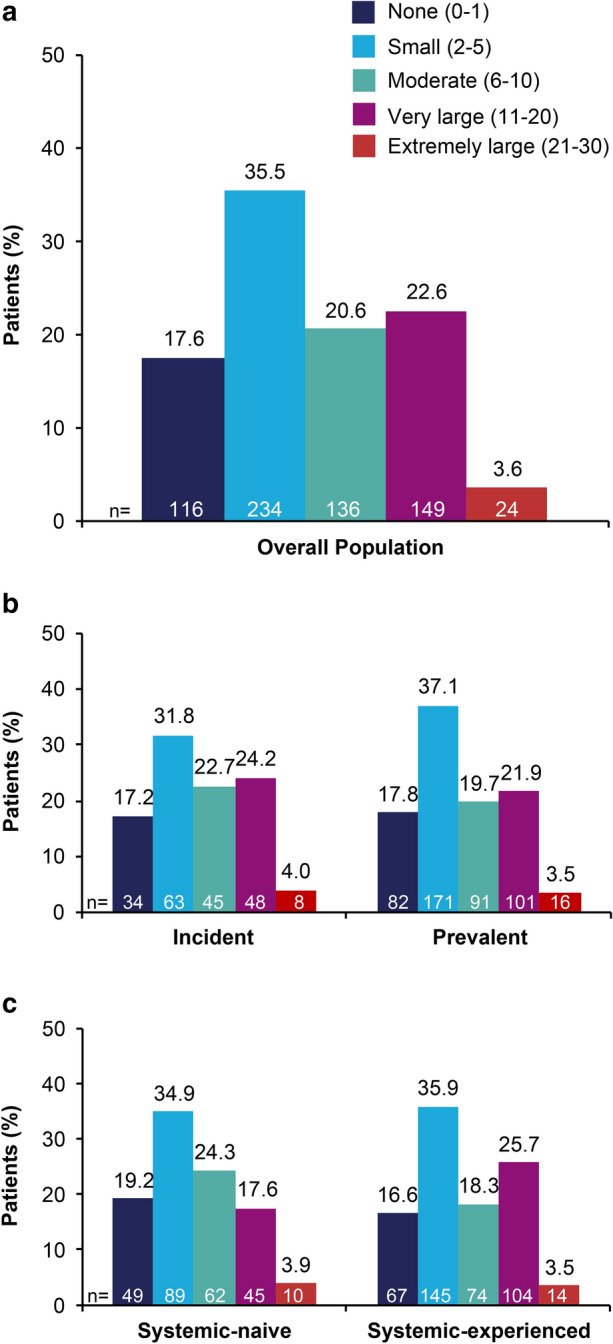
DLQI in a the overall apremilast population, b incident versus prevalent users, c systemic-naive versus systemic-experienced users. Incident = initiated treatment at or after enrollment; prevalent = initiated treatment within 12 months before enrollment
Patient-reported skin pain and pruritus VAS scores were higher in incident versus prevalent users and generally similar in systemic-naive and systemic-experienced patients (Table 1). Fatigue VAS scores were generally similar in the overall apremilast population, incident and prevalent users, and systemic-naive and systemic-experienced patients (Table 1).
Discussion
This report of real-world use of apremilast among patients in the Corrona Psoriasis Registry provides insights into the demographic, disease, and treatment characteristics of apremilast patients in clinical practice settings in the United States. Apremilast users in the Corrona Psoriasis Registry had mean BSA and PASI scores at the index date (10.3% and 5.7, respectively) that were more similar to patients with moderate plaque psoriasis enrolled in the UNVEIL study (7.2% and 8.1)—which required BSA 5–10% for enrollment [11]—than to patients with moderate-to-severe psoriasis in the pooled ESTEEM studies (25.2% and 19.1). The higher baseline PASI scores in the phase 3 ESTEEM studies are a reflection of the inclusion criteria (PASI score ≥ 12), whereas in the real-world Corrona Psoriasis Registry, physicians had the option to initiate apremilast at their discretion based on their clinical experience. PROs, including DLQI and pruritus VAS scores, were lower at index for apremilast users in the Corrona Psoriasis Registry (DLQI: 7.0; pruritus VAS: 40.9 mm) compared with baseline scores for patients in the pooled ESTEEM studies (DLQI: 12.4 with placebo and 12.7 with apremilast; pruritus VAS: 65.1 mm with placebo and 66.6 mm with apremilast) and in the overall study population of UNVEIL (DLQI: 11.0; pruritus VAS: 56.6 mm) [11]. Approximately one-third of apremilast users in the Corrona Psoriasis Registry had scalp psoriasis, which is considerably lower than the proportion of patients with scalp psoriasis (Scalp Physician Global Assessment ≥ 1) in the pooled ESTEEM (93.2%) and UNVEIL (75.6%) studies.
Apremilast-treated patients in the Corrona Psoriasis Registry had a high rate of prior exposure to biologic therapy (43.8%), in contrast with patients in the pooled ESTEEM studies (30.1%) and apremilast-treated patients in a recent health-claims analysis of a large healthcare plan (18.1%) [12], demonstrating that some apremilast users in the Corrona Psoriasis Registry had more severe disease before switching to apremilast. Nearly half of the apremilast users in the Corrona Psoriasis Registry had comorbid PsA, which is higher than the proportion of overall users with concurrent PsA in the Corrona Psoriasis Registry (40%) [8], the pooled ESTEEM studies (20.0%), and the UNVEIL study (14.5%); thus, apremilast users may have had a greater psoriatic disease burden than patients in the general Corrona Psoriasis Registry population. Apremilast users in the Corrona Psoriasis Registry had numerically higher rates of hypertension, hyperlipidemia, and diabetes than the overall Corrona Psoriasis Registry population [8] and patients with moderate psoriasis treated with apremilast in a real-world, prospective, 6-month chart review [6]. It is possible that some patients in the Corrona Psoriasis Registry switched from a biologic to apremilast due to safety concerns, a common patient-reported reason for discontinuing biologics [13].
As hypothesized, we found some key differences between the characteristics of apremilast users who had received prior systemic treatment versus those who were systemic-naive. Systemic-experienced patients had higher comorbidity burden, including higher rates of PsA and PEST score ≥ 3, more severe psoriasis, and a greater impact of psoriasis on QOL compared with systemic-naive patients. The use of apremilast in combination with other systemic treatments was more common among systemic-experienced versus systemic-naive patients, in agreement with a recent analysis of the Corrona Psoriasis Registry, which found that the majority of psoriasis patients receiving systemic treatments in combination had prior experience with systemic agents for psoriasis [14]. More than one-quarter of patients received apremilast along with a biologic therapy. Higher rates of concomitant biologic use were observed among prevalent versus incident users and among systemic-experienced versus systemic-naive patients. Taken together, these findings suggest that some systemic-experienced patients in the Corrona Psoriasis Registry may have received apremilast in combination with other systemic treatments, including biologic therapy, to help manage more severe psoriasis.
Findings of this observational study are limited to physicians and patients who voluntarily enrolled in the Corrona Psoriasis Registry in the United States, and may not be generalizable to all psoriasis patients or psoriasis patients in other regions.
Conclusions
In this real-world observational study of patients treated with apremilast in the Corrona Psoriasis Registry, most patients who received apremilast had less-severe disease than patients in phase 3 clinical trials, which enrolled patients with moderate-to-severe psoriasis. However, apremilast users in the Corrona Psoriasis Registry had higher rates of prior exposure to biologic treatments compared with patients in phase 3 clinical trials of apremilast. Systemic-experienced apremilast patients had a higher comorbidity burden, more severe psoriasis, and experienced a greater impact of psoriasis on QOL compared with systemic-naive patients. Use of apremilast in combination with a biologic therapy was more common among prevalent versus incident users and in systemic-experienced versus systemic-naive patients.
Acknowledgements
We thank the participants of the study.
Funding
This study was sponsored by Corrona, LLC and the analysis was funded by Celgene Corporation. Amgen acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019. Amgen funded the journal’s rapid service fee.
Medical Writing and/or Editorial Assistance
The authors received editorial support in the preparation of this report from Amy Shaberman, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, sponsored by Celgene Corporation, Summit, NJ, USA and Amgen Inc., Thousand Oaks, CA, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this report.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the interpretation of the results.
Disclosures
Dr. Gottlieb has received honoraria as an advisory board member and consultant for Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Incyte, Janssen, LEO Pharma, Novartis, Sun Pharmaceutical Industries, UCB, and Xbiotech (only stock options which she has not used), and has received research/educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis, Sun Pharmaceuticals, UCB, and Xbiotech. Dr. Merola is a consultant and/or investigator for AbbVie, Arena, Avotres, Biogen, Bristol-Myers Squibb, Celgene Corporation, Dermavant, Eli Lilly and Company, EMD Serono, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Cirulli was an employee of Celgene Corporation at the time of the analysis and is currently an employee of Cara Therapeutics, Inc. Ms. Williams, Mr. Linowski, and Dr. Paris are employees of Amgen Inc. Dr. Litman, Ms. Guo, Ms. Emeanuru, Dr. McLean, and Ms. Cronin are employees of Corrona. Dr. Strober has served as a consultant and advisory board member for AbbVie, Almirall, Amgen Inc., Arena, Aristea, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Dermavant, Dermira, Eli Lilly and Company, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Leo Pharma, Meiji Seika Pharma, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi-Genzyme, Sun Pharma, and UCB; has served as an investigator for AbbVie, Corrona Psoriasis Registry, Dermavant, and Dermira; has served as a speaker for AbbVie, Amgen Inc., Eli Lilly and Company, Janssen, and Ortho Dermatologics; and is the Scientific Co-Director for the Corrona Psoriasis Registry.
Corrona has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Eli Lilly and Company, Genentech, Gilead, Janssen, Merck, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi-Genzyme, and Sun Pharma.
Compliance with Ethics Guidelines
All participating investigators were required to obtain full board approval to conduct research involving human subjects. Sponsor approval and continuing review approval was obtained through a central institutional review board (IRB; IntegReview, Corrona-PSO-500). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs, and approval documentation was submitted to the sponsor before initiating any study procedures. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All registry patients were required to provide written informed consent before participating in the registry.
Data Availability
The Corrona dataset is based on a large US multicenter study adhering to a number of institutional review boards, with complex logistics. Patients did not provide their consent for raw data sharing during the data collection for this purpose, and the Corrona data sharing policies do not permit raw data sharing for this purpose. An aggregated limited dataset from the current analyses is available to qualified investigators with an approved protocol. Data requests may be sent to Corrona, represented by Dr. Jeffrey D. Greenberg MD MPH, NYU School of Medicine, New York, NY, e-mail jgreenberg@corrona.org.
References
- 1.Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]) J Am Acad Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 3.Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31:507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;23:1173–1179. [DOI] [PubMed]
- 5.Armstrong A, Levi E. Real-world clinical experience with apremilast in a large US retrospective cohort study of patients with moderate to severe plaque psoriasis. J Drugs Dermatol. 2017;16:1240–1245. [PubMed] [Google Scholar]
- 6.Knuckles MLF, Levi E, Soung J. Treating moderate plaque psoriasis: prospective 6-month chart review of patients treated with apremilast. J Dermatol Treat. 2019;30:430–434. doi: 10.1080/09546634.2018.1528326. [DOI] [PubMed] [Google Scholar]
- 7.Lee EB, Amin M, Egeberg A, Wu JJ. Adverse events associated with apremilast use and withdrawal for psoriasis in a real-world setting. J Eur Acad Dermatol Venereol. 2018;32:e393–e394. doi: 10.1111/jdv.15061. [DOI] [PubMed] [Google Scholar]
- 8.Strober B, Karki C, Mason M, Guo N, Holmgren SH, Greenberg JD, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78:323–332. doi: 10.1016/j.jaad.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469–474. [PubMed] [Google Scholar]
- 10.Mease PJ, Palmer JB, Hur P, Strober BE, Lebwohl M, Karki C, et al. Utilization of the validated Psoriasis Epidemiology Screening Tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: a cross-sectional analysis from the US-based Corrona Psoriasis Registry. J Eur Acad Dermatol Venereol. 2019;33:886–892. doi: 10.1111/jdv.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strober B, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, Chen R, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801–808. [PubMed] [Google Scholar]
- 12.Snow K, Ung B, Pelletier C, Edwards A, Edmonds S, Mehta R, et al. Patient characteristics and treatment patterns of psoriasis (PsO) and psoriatic arthritis (PsA) patients within a health plan [poster]. Presented at: AMCP Managed Care and Specialty Pharmacy Annual Meeting; April 23–26, 2018; Boston, MA.
- 13.Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–881. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo L, Abittan BJ, Hashim PW, Karki C, Mason M, Lebwohl M. Combination use of systemic therapies in psoriasis: baseline characteristics from the Corrona Psoriasis Registry. J Drugs Dermatol. 2019;18:731–740. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Corrona dataset is based on a large US multicenter study adhering to a number of institutional review boards, with complex logistics. Patients did not provide their consent for raw data sharing during the data collection for this purpose, and the Corrona data sharing policies do not permit raw data sharing for this purpose. An aggregated limited dataset from the current analyses is available to qualified investigators with an approved protocol. Data requests may be sent to Corrona, represented by Dr. Jeffrey D. Greenberg MD MPH, NYU School of Medicine, New York, NY, e-mail jgreenberg@corrona.org.


