Abstract
Background
Prior studies suggest that referral to genetic counseling and completion of genetic testing vary by race/ethnicity; however, the data are limited.
Objective
We sought to evaluate patterns of genetic testing and clinical outcomes across race/ethnicity at a hereditary breast and ovarian cancer center.
Design
The medical records for all patients undergoing genetic assessment at a hereditary breast and ovarian cancer center were reviewed and stratified by self-reported race/ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, and Asian).
Participants
A total of 1666 patients met inclusion criteria (non-Hispanic Whites, 1367; Hispanics, 85, non-Hispanic Blacks, 101; Asians, 113).
Main Measures
Demographics, patient characteristics, and referral patterns for patients who underwent genetic testing were analyzed using Kruskal-Wallis tests, chi-square test, or Fisher’s exact tests, stratifying by self-reported race/ethnicity. Pathogenic mutations and variants of unknown significance (VUS) were reviewed. Outcomes of patients with genetic mutations and personal history of breast and/or gynecologic malignancies were compared.
Key Results
Non-Hispanic Whites were more likely to be referred due to family cancer history compared to all other ethnicities while Non-Hispanic Blacks, Hispanics, and Asians were more likely to be referred due to personal history of cancer (p < 0.001). Non-Hispanic Blacks and Hispanics were more likely to have advanced-stage cancer at the time of genetic testing (p < 0.02). Rates of mutations did not differ by race/ethnicity when Ashkenazi Jewish patients were excluded (p = 0.08). Among patients found to have a BRCA1/2 mutation, Non-Hispanic Whites were more likely to undergo cancer screening and risk-reducing surgery compared with all other ethnicities (p = 0.04).
Conclusions
Minority patients were more likely to utilize genetic services following a cancer diagnosis and less likely due to family cancer history, suggesting a missed opportunity for mutation detection and cancer prevention in this population. Efforts to eradicate racial/ethnic disparities in early access to genetic testing and guided cancer prevention strategies are essential.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-06064-x) contains supplementary material, which is available to authorized users.
BACKGROUND
In the USA, breast cancer is the most common malignancy and the second most common cause of cancer-related mortality.1 Ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other gynecologic malignancy.2 The incidence and burden of breast and ovarian cancer vary among ethnic groups. Non-Hispanic Whites in the USA have the highest incidence of both breast and ovarian cancer compared with all other ethnic groups.2, 3 Breast cancer mortality remains substantially higher in non-Hispanic Black women compared with that in non-Hispanic White, Hispanic, or Asian women.4–7 Studies suggest that approximately 20–25% of ovarian cancers and 9% of breast cancers are due to inherited germline mutations, highlighting the importance of genetic assessment for women with these cancers.8–10
National guidelines across medical specialties encourage genetic services for women with a diagnosis of breast cancer or gynecologic cancer or a strong cancer family history.11–16 For women with breast cancer, the rate of BRCA1/2 mutations is comparable regardless of race, highlighting the importance of genetic testing based solely on clinical risk factors and not race.17 However, data suggest that patients are not receiving genetics services consistently or equitably. Despite expanding guidelines recommending genetic testing and increasing access to cost-effective and time-efficient sequencing, racial and ethnic disparities in the use of genetic testing persist.18, 19
Genetic testing is critical as it allows for personalized cancer treatment, targeted cancer surveillance, and risk-reducing surgery that can reduce morbidity and mortality and cascade testing of at-risk relatives.20–23 There is scarce literature focused on the downstream implications of genetic testing and whether race or ethnicity are predictive of resulting genetically targeted cancer prevention.16, 24 The Precision Medicine Initiative was launched upon the potential of genomic information to tailor medical care and it is, therefore, imperative that research focuses not only on access to genetic testing but also on resulting utilization of cancer preventative strategies.
OBJECTIVE
The aim of this study is to investigate the patterns of genetic testing and clinical outcomes of patients, stratified by race/ethnicity, who underwent testing, in a large urban academic medical center over a 3-year period.
DESIGN
The study was approved by the New York Presbyterian Hospital/Weill Cornell Medical Center Institutional Review Board. Data was collected on all patients undergoing genetic counseling at the hereditary breast and ovarian cancer center between January 1, 2013, and December 31, 2016. Data included patient demographics, insurance status, genetic testing results, personal and family history of cancer, cancer screening, treatment, and follow up status as of 12/31/2017. Self-reported race/ethnicity was characterized as non-Hispanic White, Hispanic, non-Hispanic Black, and Asian. Patient follow-up status was defined as no evidence of disease, alive with disease, dead of cancer, lost to follow-up, and only seen for testing.
Participants
Patients were referred for genetic counseling from various settings including primary care physicians, obstetrician gynecologists, medical and gynecologic oncologists, breast surgeons, and self-referrals. Reasons for referral included personal history of cancer, family history of cancer(s), personal and family history of cancer, or family history of a known pathogenic mutation. The clinical indication for referral was at the discretion of the referring provider. During genetic counseling, patients provided family history, ancestry, and self-reported race in order to create a pedigree that included at least 3 generations. Recommendations from the genetic counselors on whether to undergo genetic testing were based on the NCCN guidelines at the time of study and were consistent across the cohort.11, 12
Inclusion and Exclusion Criteria
All individuals presenting to the hereditary breast and ovarian cancer for genetic counseling were considered for inclusion in this study. Male patients were excluded as the focus was on breast and ovarian cancer. Patients reporting “other” on self-reported race/ethnicity were also excluded as the aim was to evaluate outcomes based on self-reported race/ethnicity.
Main Measures
Demographics, patient characteristics, and referral patterns for patients who underwent genetic testing were analyzed, stratifying by self-reported race/ethnicity. Pathogenic mutations and variants of unknown significance (VUS) were reviewed. Outcomes of patients with genetic mutations and personal history of breast and/or gynecologic malignancies were compared. We evaluated cancer screening following genetic assessment including breast MRI, breast ultrasound, pelvic ultrasound, annual CA 125, colonoscopies, EGD, and abdominal MRI done more frequently than standard of care. Differences across self-reported race/ethnicity were assessed using the Kruskal-Wallis tests for non-normally distributed continuous variables. Chi-square tests, or Fisher’s exact tests for small sample sizes, were used for categorical variables. The acceptable α error level was set at p = 0.05 using 2-tailed tests. Data were analyzed using SAS statistical software (version 9.4, Cary, NC).
KEY RESULTS
In total, 1864 patients presented to the hereditary breast and ovary cancer center for genetic assessment over the study period. Nienty-two patients declined testing for the following reasons: patient changed mind about the decision to proceed with testing (28), testing not covered by insurance (20), testing previously performed (30), other (12). Reasons for refusing genetic testing did not differ significantly across race/ethnicity (p = 0.99). A total of 1772 patients underwent genetic testing following in-person genetic counseling. Ninety-eight patients were male and excluded from the study. Eight patients reported race/ethnicity as “other” and were excluded. One thousand six hundred sixty-six patients were included in the final analysis. A total of 1527 patients had follow-up data available for review.
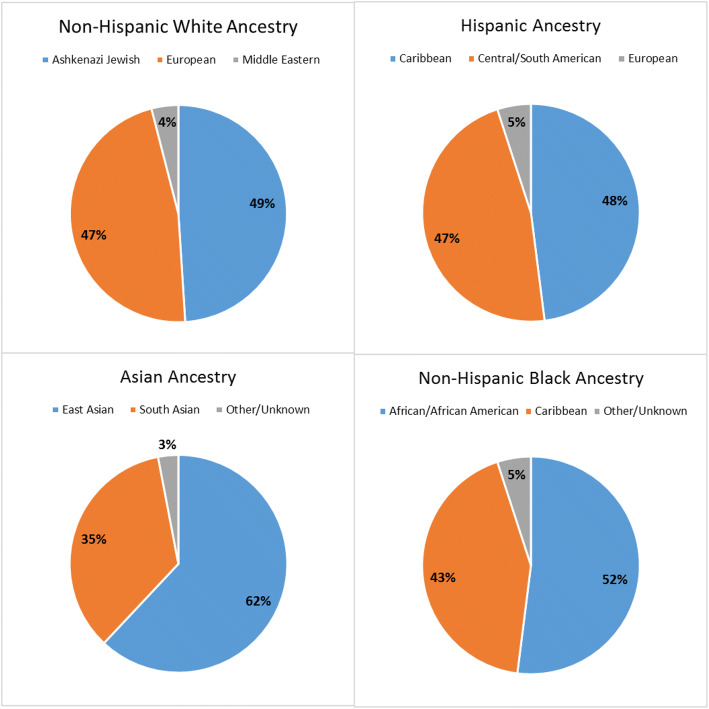
The mean age at testing was 51.3 years (range 18–97) across the entire cohort. Ancestry data obtained from pedigrees can be found in Figure 1. Asians were younger than other groups at the time of testing, with 58.4% under the age of 45. The majority of patients who underwent testing had private insurance (74%) or Medicare (17.8%) as primary coverage. Only 4.1% of patients undergoing genetic testing were uninsured. Non-Hispanic Whites and Asians were more likely to have private insurance compared with non-Hispanic Blacks and Hispanics (p < 0.001). Referral patterns of patients to genetic counseling varied by specialty across race/ethnicity (p < 0.01). Asians were the most likely to be referred by breast surgeons and the least likely to be referred by medical oncologists. Non-Hispanic Whites and Asians were more likely to be referred by obstetricians/gynecologists than Hispanics and non-Hispanic Blacks. Internal medicine/family medicine practitioners were the least common referring specialty across all races/ethnicities (Table 1).
Figure 1.
Ancestry by race/ethnicity among patients undergoing genetic testing.
Table 1.
Characteristics of Patients who Underwent Testing, by Race/Ethnicity (n = 1666)
| Characteristic | Overall (n = 1666) | Non-Hispanic White (n = 1367) | Hispanic (n = 85) | Non-Hispanic Black (n = 101) | Asian (n = 113) | p value |
|---|---|---|---|---|---|---|
| Age at testing (mean, range) | 51.3 (18, 97) | 52.3 (18, 97) | 46.8 (25, 72) | 49.4 (23, 80) | 44.9 (29, 75) | < 0.001 |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Age at testing | < 0.001 | |||||
| < 45 years | 600 (36.0) | 457 (33.4) | 39 (45.9) | 38 (37.6) | 66 (58.4) | |
| 45–59 years | 547 (32.8) | 435 (31.8) | 32 (37.7) | 44 (43.6) | 36 (31.9) | |
| 60+ years | 519 (31.1) | 475 (34.8) | 14 (16.5) | 19 (18.8) | 11 (9.7) | |
| Insurance status | < 0.001 | |||||
| Private | 1232 (74.0) | 1011 (74.0) | 56 (65.9) | 67 (66.3) | 98 (86.7) | |
| Medicaid | 68 (4.1) | 28 (2.1) | 18 (21.2) | 17 (16.8) | 5 (4.4) | |
| Medicare | 297 (17.8) | 274 (20.1) | 4 (4.8) | 14 (13.9) | 5 (4.4) | |
| Uninsured | 68 (4.1) | 53 (3.9) | 7 (8.2) | 3 (3.0) | 5 (4.4) | |
| Referring MD specialty | < 0.01 | |||||
| Breast surgeons | 473 (28.4) | 360 (26.3) | 28 (32.9) | 37 (36.6) | 48 (42.5) | |
| Internal/family medicine | 195 (11.7) | 163 (11.9) | 9 (10.6) | 14 (13.9) | 9 (8.0) | |
| Obstetrics/gynecology | 419 (25.2) | 354 (25.9) | 16 (18.8) | 17 (16.8) | 32 (28.3) | |
| Hematology/oncology | 517 (31.0) | 440 (32.2) | 26 (30.6) | 31 (30.7) | 20 (17.7) | |
| Self-referred | 62 (3.7) | 50 (3.7) | 6 (7.1) | 2 (2.0) | 4 (3.5) | |
| Type of test sent | 0.57 | |||||
| Single | 1091 (65.6) | 904 (66.3) | 54 (63.5) | 65 (64.4) | 68 (60.2) | |
| Panel | 571 (34.3) | 459 (33.7) | 31 (36.5) | 36 (35.6) | 45 (39.8) | |
| Reason for referral | < 0.001 | |||||
| Family history only | 645 (38.7) | 549 (40.5) | 26 (30.6) | 31 (30.7) | 39 (34.5) | |
| Personal history only | 221 (13.3) | 157 (11.6) | 19 (22.4) | 20 (19.8) | 25 (22.1) | |
| Family and personal history | 788 (47.3) | 651 (48.0) | 38 (44.7) | 50 (49.5) | 49 (43.4) | |
| Personal cancer history | ||||||
| Breast | 939 (56.4) | 748 (54.7) | 54 (63.5) | 64 (63.4) | 73 (64.6) | 0.04* |
| Positive receptor | 606 (86.7) | 484 (87.5) | 35 (83.3) | 38 (80.9) | 49 (86.0) | 0.54* |
| Negative receptor | 93 (13.3) | 69 (12.5) | 7 (16.7) | 9 (19.2) | 8 (14.0) | |
| GYN (ovarian) | 53 (3.2) | 44 (3.2) | 2 (2.4) | 5 (5.0) | 2 (1.8) | 0.58* |
| Other† | 70 (4.2) | 57 (4.2) | 7 (8.2) | 2 (2.0) | 4 (3.5) | 0.19* |
| Stage of cancer (among those with any personal history) | 0.02‡ | |||||
| 0 | 225 (24.6) | 176 (24.2) | 13 (25.5) | 13 (20.6) | 23 (31.9) | |
| 1–2 | 581 (63.6) | 471 (64.8) | 30 (58.8) | 36 (57.1) | 44 (61.1) | |
| 3–4 | 107 (11.7) | 80 (11.0) | 8 (15.7) | 14 (22.2) | 5 (6.9) | |
*Comparing frequency of women with history of specific cancer type across race/ethnicity
†Other malignancy include pancreatic n = 2, GI/colorectal n = 5, melanoma n = 12
‡Comparing stages 0–2 versus stages 3–4
The most common indication for referral to genetic counseling across all races and ethnicities was both personal history and family history of malignancy. However, differences in reason for referral were noted across race/ethnicity, with non-Hispanic Whites and Asians more likely than non-Hispanic Blacks and Hispanics to be referred based solely on family history (p < 0.001). When excluding patients of Ashkenazi Jewish (AJ) ancestry from the cohort, non-Hispanic Whites were still more likely to be referred based solely on family history compared with the other race/ethnicities (p = 0.03). The most common personal history of malignancy at the time of consultation was breast cancer (939) followed by gynecologic cancer (53). A personal history of breast cancer was more common in Hispanics, non-Hispanic Blacks, and Asians than in non-Hispanic Whites (p = 0.04).
The majority of patients had early-stage cancer at the time of referral. However, differences in advanced-stage disease (stages III/IV) were noted with non-Hispanic Blacks and Hispanics more likely to have advanced-stage disease than non-Hispanic Whites and Asians (p = 0.02) (Table 1). When evaluating the characteristics of patients who presented with a personal history of breast cancer alone (939), non-Hispanic Blacks, Hispanics, and Asians were significantly younger than non-Hispanic Whites (p < 0.001). Non-Hispanic Blacks were also more likely to die of cancer compared with all other racial/ethnic groups (p = 0.01) (Table 2). There were no significant differences in the timing of genetic testing relative to cancer diagnosis across race/ethnicity in patients with personal history of cancer.
Table 2.
Characteristics of Patients with Personal History of Breast Cancer, by Race/Ethnicity (n = 939)
| Non-Hispanic White (n = 748) | Hispanic (n = 54) | Non-Hispanic Black (n = 64) | Asian (n = 73) | p value | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Characteristics | |||||
| Age at testing (mean, range) | 57.2 (24, 97) | 49.1 (31, 72) | 51.0 (28, 80) | 45.9 (30, 75) | < 0.001 |
| Reason for referral | < 0.001 | ||||
| Personal history only | 145 (19.4) | 17 (31.5) | 18 (28.1) | 25 (34.3) | |
| Family and personal history | 603 (80.6) | 37 (68.5) | 46 (71.9) | 48 (65.8) | |
| Stage of cancera | 0.08 b | ||||
| 0 | 104 (25.4) | 9 (27.3) | 8 (21.1) | 18 (32.7) | |
| 1 | 193 (47.2) | 16 (48.5) | 14 (36.8) | 22 (40.0) | |
| 2 | 73 (17.9) | 5 (15.2) | 7 (18.4) | 13 (23.6) | |
| 3 | 27 (6.6) | 2 (6.1) | 8 (21.1) | 0 | |
| 4 | 12 (2.9) | 1 (3.0) | 1 (2.6) | 2 (3.6) | |
| Status at end of follow-up* | 0.01 | ||||
| NED | 506 (77.0) | 40 (78.4) | 41 (69.5) | 54 (80.6) | |
| AWD | 82 (12.5) | 8 (15.7) | 7 (11.9) | 7 (10.5) | |
| DOC | 7 (1.1) | 1 (2.0) | 5 (8.5) | 1 (1.5) | |
| LTF | 62 (9.4) | 2 (3.9) | 6 (10.2) | 5 (7.5) | |
aStage for women with diagnosis same year of testing
bBased on reclassification of stage of cancer (0, 1–2, 3–4)
*Excludes patients who presented only for testing (n = 105), includes 4-year FU
We evaluated the ethnic minorities (Table 3) referred for genetic testing with a personal history of breast and/or ovarian cancer (200) to determine if any of these patients would have met NCCN guideline criteria for genetic testing based on family history prior to their cancer diagnosis. In total, 39.1% (27) of Non-Hispanic Blacks would have met at least one criterion for family history–based genetic testing, 32.1% (18) of Hispanics, and 25.3% (19) of Asians. A total of 18.8% (13) of Non-Hispanic Blacks would have met 2 or more criteria for family history–based genetic testing, 12.5% (7) of Hispanics, and 2.7% (2) of Asians. Among ethnic minorities with a breast or ovarian cancer at the time of testing, 17.4% (12) of Non-Hispanic Black patients had a mutation or variant of uncertain significance (VUS) on genetic testing, 7.1% (4) of Hispanics, and 9.3% (7) Asians.
Table 3.
Ethnic Minorities Referred for Genetic Testing with a Personal history of Breast and Ovarian Cancer (n = 200)
| Hispanic (n = 56) | Non-Hispanic Black (n = 69) | Asian (n = 75) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Cancer history at the time of genetic counseling | |||
| Personal + family history confirmed at time of consultation | 39 (69.6) | 53 (76.8) | 51 (68.0) |
| Met at least one Criteria for testing based on family history alone | 18 (32.1) | 27 (39.1) | 19 (25.3) |
| Met 2 or more criteria for testing based on family history alone | 7 (12.5) | 13 (18.8) | 2 (2.7) |
| Mutation/VUS identified | 4 (7.1) | 12 (17.4) | 7 (9.3) |
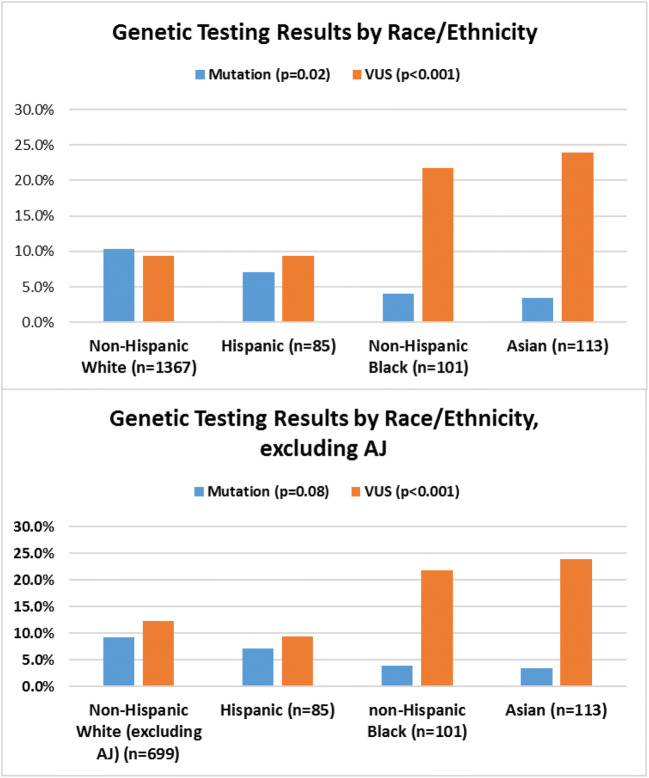
Among the study group, 65.6% (1091) underwent single-gene genetic testing and 34.3% (571) underwent multigene panel testing, with no significant difference between racial/ethnic groups (p = 0.57) (Table 1). Pathogenic mutations were identified in 9.4% (156) of patients, with non-Hispanic Whites having the highest rate compared to other race/ethnicities (p = 0.02) (Fig. 2). However, when excluding patients of AJ ancestry from the non-Hispanic White cohort, there were no significant differences in mutation occurrences across race/ethnicities (p = 0.08) (Fig. 2). Pathogenic mutations in BRCA1/2 genes were most common across all ethnicities. BRCA1 mutations were found in 55 patients (non-Hispanic Whites AJ, 34; non-Hispanic White non-AJ, 17; Hispanics, 1; non-Hispanic Black, 2; Asians, 1). Fifty-six BRCA2 mutations were identified (non-Hispanic White AJ, 23; non-Hispanic White non-AJ, 25; Hispanic, 4; non-Hispanic Black, 2; Asians, 2). Other genetic mutations identified include CHEK2 (23), ATM (3), APC (5), BARD1 (1), BRIP1 (1), FANCC (3), MSH6 (1), PALB2 (2), PMS2 (1), RAD51D (2), and TP53 (1).
Figure 2.
Genetic testing results by race/ethnicity.
VUS were identified in 11.1% (185) of patients. Significant differences in the frequency of VUS were noted across race/ethnicity, with non-Hispanic Blacks (21.8%) and Asians (23.9%) more likely to have VUS than non-Hispanic Whites (9.4%) and Hispanics (9.4%) (p < 0.001). Six percent of VUS detected in the study group were, over time, reclassified as benign and less than 1 % of VUS were reclassified as pathogenic over the study period. Variant reclassification did not differ significantly across race/ethnicities (p = 0.66).
In order to assess the clinical impact of genetic testing, we reviewed cancer screening and risk-reducing surgical interventions following the acquisition of testing results. Among patients with a BRCA1/2 mutation, non-Hispanic Whites were more likely to undergo increased cancer screening (36.6% (37)) and risk-reducing surgery (50.5% (51)) compared with other race/ethnicities (33.3% (4), 25% (3), respectively, p = 0.04). When excluding AJ ancestry, non-Hispanic Whites were still more likely than all other races to undergo risk-reducing screening and/or surgery in the setting of a pathogenic mutation (p < 0.01). There were no differences in prophylactic surgery or screening observed between non-Hispanic Whites and all other races in the setting of the diagnosis of either BRCA1/2 VUS or non-BRCA VUS (p = 0.22 and p = 0.50, respectively).
DISCUSSION
We identified significant ethnic/racial disparities in genetic assessment among a large cohort of women receiving care at an urban academic medical center. Our findings suggest varying thresholds for referral to genetic counselors based on race/ethnicity and potentially missed opportunities for early cancer detection and prevention.
Previous studies demonstrate lack of awareness of genetic testing among women with breast and ovarian cancer, most pronounced among minority populations.25, 26 However, studies also show that once counseled on genetic options, most minority patients pursue genetic testing with cost, not attitude towards testing, being the most important barrier.25, 26 In our study, fewer than 5% of patients undergoing genetic counseling declined testing and rates did not vary by race/ethnicity. Only 1% of the population declined testing due to cost. These findings suggest great acceptability of genetic testing across all races and the accessibility of cost-effective testing platforms and should encourage providers to refer at-risk individuals for genetic counseling.
There were significant differences in referral patterns for genetic counseling in our study population. Primary care physicians are thought to play a major role not only in the referral of patients for effective cancer screening tests but also in the identification of at-risk individuals based on personal and family cancer history. However, in our study population, internal and family medicine doctors referred the fewest patients across all ethnicities, while subspecialty oncologists and surgeons referred the most. Differences across ethnicities were also identified, with non-Hispanic Whites and Asians, more likely to be referred by obstetricians and gynecologist than non-Hispanic Blacks and Hispanics. Our findings are comparable with those reported by Armstrong et al. who found only 36.8% of US women at high risk for breast and ovarian cancer undergo genetic counseling.27 The most common reason cited was lack of a clinical recommendation, with the lowest rate of referral being from obstetrician gynecologists. Cragun et al. showed that discussion of genetic testing with a provider was 16 times less likely among non-Hispanic Black women and nearly 2 times less likely among Spanish-speaking Hispanic women when compared to non-Hispanic White women.24 A similar finding was reported in women of color diagnosed with ovarian cancer who were also significantly less likely to receive genetic counseling referrals.28 Taken together, these studies suggest an opportunity for increased training of primary care–based specialists in the identification of at-risk individuals. This could potentially mitigate unconscious or implicit biases that may play a role in the lack and/or delay of referrals for genetic testing.
The lack of knowledge by providers about the prevalence of hereditary cancer syndromes among ethnic minorities may contribute to referral decision-making practices. Mutation rates reported for women have historically been based on predominantly non-Hispanic White populations. More recently, analysis of non-AJ women who underwent genetic testing through Myriad provided more evidence regarding the prevalence of deleterious mutations across ethnicities. Women of non-European descent had a higher prevalence of pathogenic BRCA1/2 mutations compared with women of Western European descent, with higher rates of BRCA1/2 mutations found among Africans (15.6%), Latin Americans (14.8%), and Asians (12.7%) compared with non-AJ Whites of European ancestry (12.1%).29 Mutation rates in our study population were similar across race/ethnicity after exclusion of AJ women.
We found that family cancer history was more likely to prompt genetic testing in Non-Hispanic Whites compared with other races, even when excluding AJ. A possible explanation is that non-Hispanic Blacks were less likely to have a family history warranting referral; however, in our study population, this was not the case. In fact, the majority of non-Hispanic Blacks, Hispanics, and Asians who were referred due to personal history of malignancy also had a significant self-reported family cancer history. Among ethnic minorities referred for testing for personal diagnosis of breast and/or ovarian cancer, approximately 70% had a significant family history confirmed with genetic counseling. Most concerning is our finding that among these minority women with cancer, many (39% non-Hispanic Black, 32% Hispanic, and 25% Asian) would have met the criteria for genetic testing based solely on family history. In addition, at the time of testing, non-Hispanic Black women were more likely to be younger and have advanced-stage disease. As there are known biologic differences with breast cancer among minority populations, such as younger age at diagnosis (< 50), and higher prevalence of triple-negative breast cancer associated with worse prognosis, these findings highlight the importance of targeting at-risk younger women from all ethnicities for genetic testing based on family history alone.4–7, 30
Among patients with pathogenic mutations in our cohort, minorities were less likely to undergo surgical interventions and increased screening compared with non-Hispanic Whites. Cragun et al. suggest that rates of risk-reducing salpingo-oophorectomy are lower among non-Hispanic Black BRCA1/2 carriers compared with non-Hispanic Whites and Hispanics.24 Reasons for disparities in risk-reducing interventions are likely multifactorial but may include lack of education, poor counseling, cultural differences, and/or presumed and real financial barriers. More research is needed in this area to determine the true causes of the disparity and interventions to eradicate it. We also found higher rates of VUS in minorities compared with non-Hispanic Whites. Fortunately, concerns regarding inappropriate surgical procedures related to increased detection of VUS were not justified in the current study. There was no increase in risk-reducing surgery among non-Hispanic Whites or minorities in our cohort with VUS, but there was a rise in screening uptake that is also demonstrated in previous studies.31
This is one of the largest single-institution experiences exploring genetic testing with high-risk patients in understudied racial and ethnic groups. The diverse study population found in New York City, with a range of ancestry and ethnicities, is a major strength. All patients were seen by the same genetic counseling team, allowing for consistency in counseling across ethnicities and thorough pre- and post-test counseling. Limitations of this study include its retrospective nature, resulting in incomplete identification of all factors potentially affecting reasons for referral as well as self-reported race/ethnicity and patient history. Referral patterns for patients with diagnosis of ovarian cancer changed over time in our institution with many gynecologic oncologists directly testing patients regardless of family history due to changes in the NCCN guidelines. Additional biases may exist in referral patterns of specialists compared with general practitioners due to direct testing done in general obstetrician gynecologists’ offices that would not be captured in our data. Furthermore, we could not assess for knowledge gaps related to risk factors for referrals and patients’ lack of knowledge about family history. In addition, our study population and results may not be representative of findings in other geographic regions which may have varying access to health services including genetic counselors and different patient populations. There are variations in breast and ovarian cancer epidemiology, disease biology, and access to care that may affect race- and ethnicity-specific variability in the women who received genetic testing that may not have been captured.
CONCLUSIONS
In conclusion, there are clear disparities in genetic testing among minority populations. Interventions to reduce disparities in genetic testing should focus on identifying patients at high risk of mutations by obtaining detailed family history, considering risk among younger-aged women who are minorities, and increasing patient and provider awareness and understanding of the benefits of testing regardless of race/ethnicity. It is crucial to provide appropriate follow-up and counseling regarding cancer risk-reduction recommendations once a pathogenic mutation is identified. With the expansion of insurance and increased access to genetic testing platforms, financial barriers are more likely to be eliminated and should not be a justification for the lack of genetic testing. Ensuring that the benefits of cancer prevention and early detection associated with increased genetic testing are accessible to all patients, regardless of race/ethnicity, is imperative for the eradication of existing healthcare disparities.
Electronic Supplementary Material
(DOCX 33 kb)
Authors’ Contribution
None.
Compliance with Ethical Standards
The study was approved by the New York Presbyterian Hospital/Weill Cornell Medical Center Institutional Review Board.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior presentations: Society of Gynecologic Oncology Annual Meeting, 2018
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: Final Data for 2016. Natl Vital Stat Rep. 2018;67(5):1–76. [PubMed] [Google Scholar]
- 2.Statistics, C. Ovarian Cancer Rates by Race and Ethnicity. https://www.cdc.gov/cancer/ovarian/statistics/race.htm (accessed August 16).
- 3.Statistics, C. Breast Cancer Rates by Race and Ethnicity. https://www.cdc.gov/cancer/breast/statistics/race.htm (accessed August 16).
- 4.Dean M, Boland J, Yeager M, Im KM, Garland L, Rodriguez-Herrera M, Perez M, Mitchell J, Roberson D, Jones K, Lee HJ, Eggebeen R, Sawitzke J, Bass S, Zhang X, Robles V, Hollis C, Barajas C, Rath E, Arentz C, Figueroa JA, Nguyen DD, Nahleh Z. Addressing health disparities in Hispanic breast cancer: accurate and inexpensive sequencing of BRCA1 and BRCA2. Gigascience. 2015;4:50. doi: 10.1186/s13742-015-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2010;76(1):44–52. doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D, Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, Mohar A, Arrieta Ó. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658–69. doi: 10.1002/cncr.25961. [DOI] [PubMed] [Google Scholar]
- 8.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Yi Q, Burger RA, Chan JK, Davidson SA, Mannel RS, DiSilvestro PA, Lankes HA, Ramirez NC, King MC, Swisher EM, Birrer MJ. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016;2(4):482–90. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsop K, Fereday S, Meldrum C, de Fazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, Sharma L, Saam J, Lancaster J, Daly MB. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–1730. doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 11.Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD, Friedman S, Garber JE, Goorha S, Gruber SB, Hampel H, Kaklamani V, Kohlmann W, Kurian A, Litton J, Marcom PK, Nussbaum R, Offit K, Pal T, Pasche B, Pilarski R, Reiser G, Shannon KM, Smith JR, Swisher E, Weitzel JN, Network NCC. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8(5):562–94. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 12.Daly MB, Pilarski R, Axilbund JE, Berry M, Buys SS, Crawford B, Farmer M, Friedman S, Garber JE, Khan S, Klein C, Kohlmann W, Kurian A, Litton JK, Madlensky L, Marcom PK, Merajver SD, Offit K, Pal T, Rana H, Reiser G, Robson ME, Shannon KM, Swisher E, Voian NC, Weitzel JN, Whelan A, Wick MJ, Wiesner GL, Dwyer M, Kumar R, Darlow S. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. J Natl Compr Canc Netw. 2016;14(2):153–62. doi: 10.6004/jnccn.2016.0018. [DOI] [PubMed] [Google Scholar]
- 13.Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, Kohlmann W, Kurian A, Litton JK, Madlensky L, Merajver SD, Offit K, Pal T, Reiser G, Shannon KM, Swisher E, Vinayak S, Voian NC, Weitzel JN, Wick MJ, Wiesner GL, Dwyer M, Darlow S. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw. 2017;15(1):9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster JM, Powell CB, Chen LM, Richardson DL, Committee SCP. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3–7. doi: 10.1016/j.ygyno.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Moyer VA, Force USPS. T., Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–81. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 16.Hinchcliff EM, Bednar EM, Lu KH, Rauh-Hain JA. Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol. 2019;153(1):184–191. doi: 10.1016/j.ygyno.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22(1):72–8. doi: 10.1097/GCO.0b013e328332dca3. [DOI] [PubMed] [Google Scholar]
- 18.Kurian AW, Ford JM. Multigene Panel Testing in Oncology Practice: How Should We Respond? JAMA Oncol. 2015;1(3):277–8. doi: 10.1001/jamaoncol.2015.28. [DOI] [PubMed] [Google Scholar]
- 19.Kurian AW, Griffith KA, Hamilton AS, Ward KC, Morrow M, Katz SJ, Jagsi R. Genetic Testing and Counseling Among Patients With Newly Diagnosed Breast Cancer. JAMA. 2017;317(5):531–534. doi: 10.1001/jama.2016.16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Practice C. o. G., ACOG Committee Opinion No. 727: Cascade Testing: Testing Women for Known Hereditary Genetic Mutations Associated With Cancer. Obstet Gynecol. 2018;131(1):e31–e34. doi: 10.1097/AOG.0000000000002457. [DOI] [PubMed] [Google Scholar]
- 21.Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, Bethan Powell C, Smith EB, Robson ME, Boyd J, Coleman RL, Lu K. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. 2017;146(2):217–224. doi: 10.1016/j.ygyno.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Offit K. The future of clinical cancer genomics. Semin Oncol. 2016;43(5):615–622. doi: 10.1053/j.seminoncol.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, Huzarski T, Eisen A, Foulkes WD, Kim-Sing C, Ainsworth P, Tung N, Lynch HT, Neuhausen S, Metcalfe KA, Thompson I, Murphy J, Sun P, Narod SA. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–53. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cragun D, Weidner A, Lewis C, Bonner D, Kim J, Vadaparampil ST, Pal T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497–2505. doi: 10.1002/cncr.30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hann KEJ, Freeman M, Fraser L, Waller J, Sanderson SC, Rahman B, Side L, Gessler S, Lanceley A, team, P. s Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health. 2017;17(1):503. doi: 10.1186/s12889-017-4375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacour RA, Daniels MS, Westin SN, Meyer LA, Burke CC, Burns KA, Kurian S, Webb NF, Pustilnik TB, Lu KH. What women with ovarian cancer think and know about genetic testing. Gynecol Oncol. 2008;111(1):132–6. doi: 10.1016/j.ygyno.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong J, Toscano M, Kotchko N, Friedman S, Schwartz MD, Virgo KS, Lynch K, Andrews JE, Aguado Loi CX, Bauer JE, Casares C, Bourquardez Clark E, Kondoff MR, Molina AD, Abdollahian M, Walker G, Sutphen R. Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study. JAMA Oncol. 2015;1(9):1251–60. doi: 10.1001/jamaoncol.2015.3048. [DOI] [PubMed] [Google Scholar]
- 28.Manrriquez E, Chapman JS, Mak J, Blanco AM, Chen LM. Disparities in genetics assessment for women with ovarian cancer: Can we do better? Gynecol Oncol. 2018;149(1):84–88. doi: 10.1016/j.ygyno.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–33. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturtz LA, Melley J, Mamula K, Shriver CD, Ellsworth RE. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer. 2014;14:62. doi: 10.1186/1471-2407-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culver JO, Brinkerhoff CD, Clague J, Yang K, Singh KE, Sand SR, Weitzel JN. Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clin Genet. 2013;84(5):464–72. doi: 10.1111/cge.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)