Abstract
Stroke is still one of the most common causes for mortality and morbidity worldwide. Following acute stroke onset, biochemical and cellular changes induce further brain injury such as neuroinflammation, cell death, and blood-brain barrier disruption. Matricellular proteins are non-structural proteins induced by many stimuli and tissue damage including stroke induction, while its levels are generally low in a normal physiological condition in adult tissues. Currently, a matricellular protein tenascin-C (TNC) is considered to be an important inducer to promote neuroinflammatory cascades and the resultant pathology in stroke. TNC is upregulated in cerebral arteries and brain tissues including astrocytes, neurons, and brain capillary endothelial cells following subarachnoid hemorrhage (SAH). TNC may be involved in blood-brain barrier disruption, neuronal apoptosis, and cerebral vasospasm via the activation of mitogen-activated protein kinases and nuclear factor-kappa B following SAH. In addition, post-SAH TNC levels in cerebrospinal fluid predicted the development of delayed cerebral ischemia and angiographic vasospasm in clinical settings. On the other hand, TNC is reported to promote fibrosis and exert repair effects for an experimental aneurysm via macrophages-induced migration and proliferation of smooth muscle cells. The authors review TNC-induced inflammatory signal cascades and the relationships with other matricellular proteins in stroke-related pathology.
Keywords: biomarker, blood-brain barrier disruption, cerebral vasospasm, matricellular protein, neuroinflammation, neuronal apoptosis, stroke, subarachnoid hemorrhage
Introduction
Stroke is a large public concern in terms of both human and financial resources (1, 2). In the United States, annual stroke expenses have reached approximately 33.9 billion dollars (2). Although recent research has been clarifying pathological changes in the brain following stroke, therapeutic options for these patients remain limited.
Neuroinflammation is a key pathologic change arising from stroke. Findings from both clinical and animal studies have indicated that inflammatory reactions may contribute to the development of brain injury following stroke (3–5). Post-stroke tissue damage releases secondary breakdown products of brain tissue and blood components. Damage-associated molecular patterns (DAMPs) are endogenous molecules released as a result of tissue damage that rapidly activate the innate immune response by interacting with a number of pattern recognition receptors (PRRs) located primarily on microglia and macrophages (6, 7). Activated microglia and macrophages release inflammatory cytokines and mediators via activation of signaling pathways downstream of the PRRs. The PRRs include Toll-like receptors (TLRs), cytosolic NOD-like receptors and inflammasomes, receptors for advanced glycation end products, and other scavenger receptors (8–10). Following stroke, the TLR4 signaling pathway is involved in the initial steps of neuroinflammation cascades, which result in brain injury such as vasogenic and cytotoxic edema and blood-brain barrier (BBB) disruption (11). Furthermore, neuroinflammation recruits more DAMPs, accelerating the inflammatory response. The secondary brain injury includes early brain injury (EBI), cerebral vasospasm (CVS), and delayed cerebral ischemia (DCI) after subarachnoid hemorrhage (SAH). Neuroinflammation is currently considered to be a critical factor contributing to morbidity and mortality in stroke patients who survive the initial brain damage and needs to be addressed in order to improve clinical outcomes (11–13).
Matricellular proteins (MCPs) are extracellular matrix (ECM) components upregulated and released by tissue damage, exerting both beneficial and harmful effects through binding to receptors, other matrix proteins, growth factors (GFs), and cytokines (14). Recent studies have demonstrated the efficacy of treatments targeting MCPs in preclinical stroke neuroinflammation models (15, 16).
In this review, we focus on a MCP tenascin-C (TNC) involved in neuroinflammation following stroke, and highlight current evidence for its use as a clinical biomarker and a therapeutic target.
What Are MCPs?
The concept of MCPs was introduced in 1995 due to their characteristics which differ from classical ECM proteins (17). MCPs are currently considered important inducers that regulate the expression of inflammatory mediators and are involved in diverse pathological changes such as cell death, immunomodulation, inflammation, fibrosis, vascular permeability, and angiogenesis via modulation of the molecular functions or cellular responses to the molecules (18, 19). MCPs can work on the plasma membrane, intracellularly, in body fluids, or in the ECM, and also act as reservoirs of the bioactive molecules (18, 19). The level of protein expression is low in normal physiological conditions in adult tissues in general, and MCP knockout mice undergo normal development (18, 20). Almost all tissues and cell types produce MCPs following various stimuli which disappear after stimulus removal. MCPs do not provide a scaffold for stable cell adhesion, but induce cell motility and tissue remodeling via modulation of cell surface receptors, other matrix proteins, GFs, and cytokines (Table 1) (18). Accumulating evidence suggests that many types of MCPs, such as TNC, periostin, galectin-3, and osteopontin, contribute to aggravation or improvement of neuroinflammation in stroke at least partly by influencing the expression of each other (15, 18–23).
Table 1.
Characteristics of matricellular proteins compared with classical extracellular matrix (ECM) proteins.
| Classical ECM protein | Matricellular protein |
|---|---|
| - Structural protein | - Soluble non-structural protein - Low expression level under normal physiological conditions in adult tissues - Induced in almost any tissue by stimuli and disappear after stimulus removal |
| - Provide a scaffold for stable cell adhesion | - Induce cell motility and tissue remodeling - Various functions via interacting with cell surface receptors, other matrix proteins, growth factors, and cytokines - Knockout mice basically undergo normal development |
TNC: The Structure and Isoforms
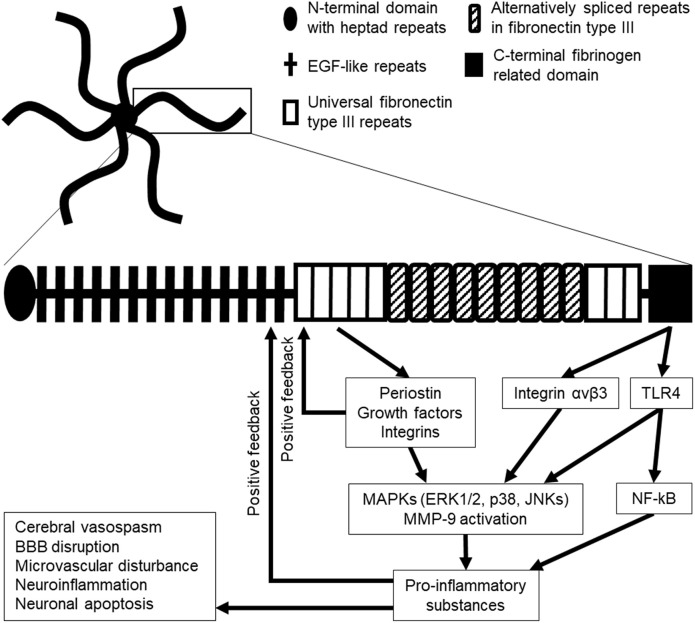
Tenascins (TNs) are representative of MCPs and are comprised of a family of four homologs, that is, TNC, TNR, TNW, and TNX (22–24). Among the TNs, only TNC has been investigated in stroke (20). TNC was discovered in the early 1980s and initially referred to by different terms such as myotendinous antigen, glioma mesenchymal ECM, hexabrachion, TN, J1-200/220, cytotactin, and neuronectin (25, 26). TNC is a pleiotropic ECM glycoprotein with a large molecular weight (180–400 kDa). Its N-terminal contains highly conserved heptad repeats, followed by 14 epidermal growth factor (EGF)-like repeats and up to 15 fibronectin type III (FN III) repeats comprised of universal repeats and alternatively spliced repeats; and a fibrinogen repeat domain is located at the C-terminal (25, 27–30). Alternatively spliced repeats in the FN III domain are comprised of a combination of A1, A2, A3, A4, B, AD2, AD1, C, and D domains in humans and A1, A2, A4, B, C, and/or D domains in mice, which are inserted between domains 5 and 6 in universal FN III repeats (20, 23, 29–34). TNC generally forms a disulfide-linked hexamer mediated by the N-terminal domain in which six flexible arms emanate from a central globular particle (Figure 1) (20, 22, 29, 30, 35). In humans, TNC is encoded at a single gene located at 9q33 (20).
Figure 1.
Hexamer structure of tenascin-C (TNC; upper), monomer structure of TNC (lower), and the possible downstream signaling pathway in stroke. Six TNC monomers combine to a hexamer at their N-terminal domains. EGF, epidermal growth factor; ERK1/2, extracellular signal-regulated kinase 1/2; JNKs, c-Jun N-terminal kinases; MMP-9, matrix metalloproteinase-9; TLR4, Toll-like receptor 4.
TNC exhibits a diverse range of isoforms in various tissues, the splicing of which is regulated by intracellular pH. Under exposure to basic pH ~7.30–7.50 as observed with fetal cells and aggressive tumors, the level of longer or larger TNC isoforms is enhanced (33). Isoforms with a large molecular mass (≥200 kDa) contain at least one alternatively spliced FN III repeat. Each alternatively spliced FN III repeat has unique functions (33). Larger TNC isoforms induce cell proliferation and migration, and control cell spreading, resulting in promotion of destruction or remodeling of local tissues (33). In addition, larger TNC isoforms can be easily degraded by matrix metalloproteinases (MMPs), leukocyte elastase, and possibly other serine proteases (33). MMPs usually cleave the sites located within the alternatively spliced region (23, 33). In contrast, TNC isoforms with a lower molecular mass (<200 kDa) lack A1-D domains in alternatively spliced FN III repeats and seem to be more stable in dense connective tissues and to be expressed at low levels in a physiologically normal tissue (33). Under physiological pH <7.0, the level of small TNC isoforms is increased (33). Different TNC isoforms seem to be produced by proteolytic processing of a large multimodular TNC isoform. The proteolytic destruction may impart novel functions to TNC by destroying existing binding sites or generating smaller fragments with new binding sites: these new functions can drive entirely novel processes compared to the previous or intact form (33). Smaller TNC fragments exert quite different reactions in various cells. Fragmented EGF-like domains of TNC induce apoptotic effects on vascular smooth muscle cells in culture, while intact or full-length TNC does not have the functions (36). Thus, respective TNC isoforms seem to have flexible physiological or pathological functions (20). However, the timing and location of distinct TNC isoforms production during inflammatory reactions have not been completely investigated. In addition, the functions of individual TNC isoforms have not yet been fully clarified (20).
TNC Expression During Developmental Stage
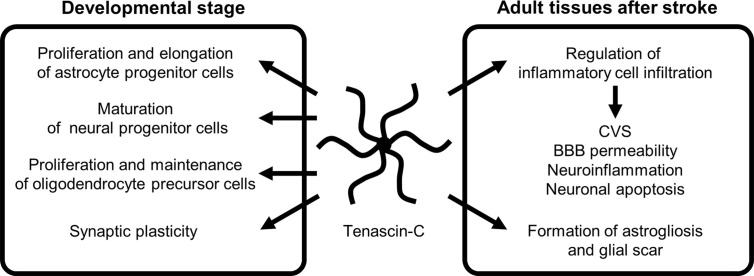
TNC is highly expressed during embryonic development and was first identified in developing astrocytes (37–41). Currently, TNC is considered to be primarily induced by astrocytes and radial glial progenitor cells and to play a crucial role in normal brain development: it serves as a repulsive substrate for neuronal and astrocytic growth and plays a role in proliferation and process elongation of astrocyte progenitor cells, maturation of neural progenitor cells, proliferation and maintenance of oligodendrocyte precursors, and synaptic plasticity through autocrine and paracrine regulatory mechanisms during developing stages (Figure 2) (28, 34, 40–47). In the spinal cord, TNC is synthesized by a subset of gliogenic precursors in the late phase of embryogenesis and influences proliferation and migration of a subpopulation of astrocytes (48). Although the expression of TNC is downregulated in the brain 2–3 weeks after birth, it is involved in hippocampal synaptic plasticity and synchronized neural network activities in the mature brain via control of postsynaptic L-type Ca2+ channels (47). Intrahippocampal injections of recombinant TNC fragments containing the FN III repeats 6–8 block the retention of memory and hippocampal formation in mice, showing the mediation in hippocampus-dependent contextual memory and hippocampal synaptic plasticity (49).
Figure 2.
The role of tenascin-C during developmental stage and following stroke. BBB, blood-brain barrier; CVS, cerebral vasospasm.
Regulation of TNC Expression in Adult Tissues
In adult tissues, the expression and the distribution of TNC are typically limited under normal physiological conditions but transiently upregulated in reaction to inflammatory responses or tissue damages (50, 51). TNC expression is controlled by several transcription factors and intracellular regulators, including T cell factor/lymphoid enhancer-binding factor, nuclear factor (NF)-κB, Notch1 and Notch2, hepatocyte NF-4α, Ets, SP1, c-myc, homeobox transcription factor Prx1, Rho, c-Jun, and extracellular signal-regulated kinases (ERKs) (29, 32). Overexpression of the transcription factors Slug and Sox9 induce TNC and periostin expression (52). However, the involvement of these transcription factors in stroke has not been investigated in vivo. In contrast, micro-ribonucleic acids (RNAs) such as miR-355 downregulate TNC expression in breast cancer metastases (53). Upregulation of TNC appears in reactive astrocytes, injured neurons, and glial scar formation with restricted occurrence in space and time: therefore, these cells are considered to release TNC (20, 22, 29, 47, 50, 54, 55). TNC modulates a variety of cell functions and morphologies (22, 23). Scratch wound assays induce TNC expression by astrocytes in vitro (56). Levels of TNC enhancement following stab-wound injury to cerebellar and cerebral cortical structures depend on the number of glial fibrillary acidic protein (GFAP)-positive cells, which represent reactive astrocytes (55). GFAP was significantly suppressed in TNC-knockout mice compared to wild-type ones one week after stab injury (57); and therefore TNC may be involved in the late acute phase formation of astrogliosis around sites of injury and failed regeneration (55). However, in another mice study, TNC exerted protective effects after brain damage (57). The study demonstrated that extravasated immunoglobulin G was considerably prolonged and RNA levels of proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukins (ILs)-1β and -6 were higher in the cerebral cortex after stab-wound injury in TNC-deficient mice: TNC production might promote BBB repair or maintain the BBB integrity by the reduction of inflammatory cytokine levels (57).
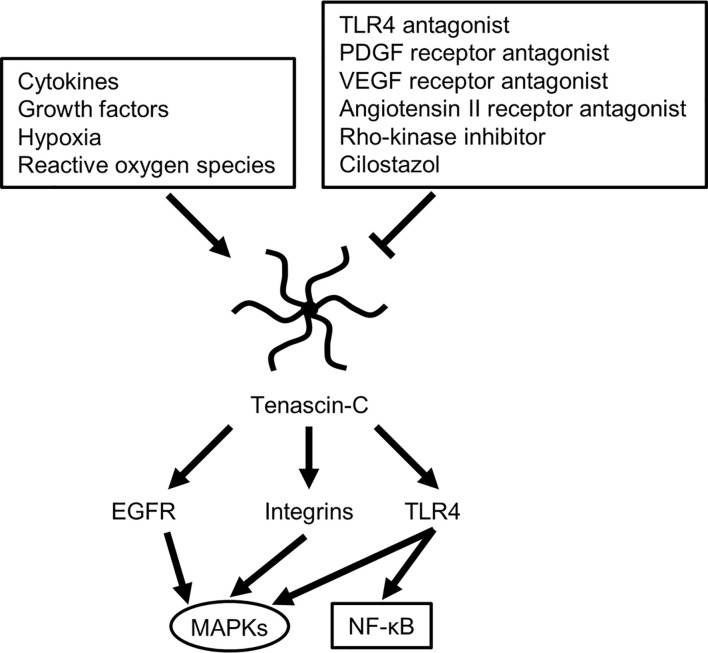
TNC induces MMPs, which seem to result in a positive TNC feedback loop via MMP-induced TNC cleavage (29). In addition, many stimuli, including various pro- and anti-inflammatory cytokines, GFs, hypoxia, reactive oxygen species, and mechanical stress, readily but transiently upregulate TNC within several hours in various pathological conditions such as myocarditis, arteriosclerosis, and cancer, irrespective of the location or type of causative insults (Figure 3) (29, 58). Clinically, TNC has been reported as a plasma biomarker of neurodegenerative diseases, as significantly elevated TNC levels were found in the peripheral blood of patients with Alzheimer’s disease with mild cognitive impairments and in the amniotic fluid of pregnancies affected by Down syndrome (59–61). In addition, TNC expression is induced in the hippocampi of both epileptic rats and human patients with temporal lobe epilepsy (62–64). In the brains of patients with temporal lobe epilepsy, the regions exhibiting diffuse and elevated expression of TNC were characterized by an extended area of reactive gliosis and synaptic reorganization (42). Loss of TNC in transgenic CRND8 mice caused enhanced production of anti-inflammatory cytokines and decreased production of proinflammatory cytokines, associated with reduction of β- and γ-secretase activity, Aβ oligomerization, amyloid plaque load, and synaptic impairments (65). However, another study demonstrated that TNC may be involved in the maintenance of late acute phase astrogliosis surrounding the site of severe injuries, and exert anti-inflammatory and BBB-repairing effects (57). Thus, TNC induced by reactive astrocytes may play neuroprotective, neurotoxic or other diverse roles depending on the context, including regulation of astrocyte reactivity, BBB permeability, and potentiation of inflammatory processes (Figure 2). TNC may also directly affect neuronal plasticity and lead to memory impairments (42).
Figure 3.
Possible molecular mechanisms for regulating tenascin-C expression and downstream signaling cascade. EGFR, epidermal growth factor receptor; MAPKs, mitogen-activated protein kinases; NF-κB, nuclear factor-kappa B; PDGF, platelet-derived growth factor; TLR4, Toll-like receptor 4; VEGF, vascular endothelial growth factor.
TNC in SAH
Many experimental studies as to TNC have been reported in SAH in rats and mice. Some have demonstrated that TNC is expressed in the walls of spastic cerebral arteries (endothelial, smooth muscle, adventitial, and periarterial inflammatory cells) and in brain parenchyma (astrocytes, neurons, and brain capillary endothelial cells), primarily in the surface of the cerebral cortex between 24 and 72 h after SAH by endovascular perforation (22, 37, 66–69). In a clinical setting, TNC levels in the cerebrospinal fluid (CSF) were below the diagnostic threshold level in patients with an unruptured cerebral aneurysm but markedly increased after cerebral aneurysmal rupture (70). Elevation of TNC expression may be affected by several factors, including elevated intracranial pressure as well as brain damage resulting from local or systemic inflammatory reactions (68, 71). A previous experimental study in rats showed that even cisternal saline injections caused elevated intracranial pressure and induced slight subarachnoid inflammatory reactions, which caused TNC upregulation in the basilar artery adventitia (68, 71). TNC is a key pathological factor that promotes activation of inflammatory cell infiltration in the periarterial space, causing EBI in terms of neuroinflammation, BBB disruption, and neuronal apoptosis; and also is involved in CVS and plays an important role in the development of DCI (Figure 2) (3–5, 20, 29, 37, 66, 72). Recent studies demonstrated that intracisternal injections of both intact or full-length TNC and recombinant TNC fragments containing the EGF-like repeats which activate EGF receptors activated mitogen-activated protein kinases (MAPKs) in arterial smooth muscle cells, causing prolonged CVS, but had no effects on neurobehavior, brain water content, and BBB integrity in normal healthy rats; however, in SAH rats, the TNC injections caused neurological impairments (3, 16, 22, 66, 72–77). In addition, TNC-induced activation of MAPKs is considered to upregulate MMP-9 in brain capillary endothelial cells and to cause BBB disruption in mice with SAH, although the mechanisms remain unidentified (29, 72, 73, 75, 77, 78). MAPK activation also results in a release of inflammatory mediators (18, 79). Human studies have repeatedly shown elevation of inflammatory mediators such as endothelin-1, TNF-α, and ILs-1β and -6 in CSF after SAH (37, 80–82). IL-1β induces TNC production via MAPK-dependent or -independent pathways, while TNC stimulates the synthesis of IL-1β (58, 77). This positive feedback mechanism upregulates TNC and the receptors in an early phase of SAH and may cause more activation of TNC signaling transduction and consequently further development or aggravation of EBI including neuronal apoptosis and BBB disruption, as well as prolonged CVS (Figure 1) (66, 69, 73). Post-SAH neuronal apoptosis develops through TNC-induced activation of p38 and ERK1/2 (20, 66), and the EGF-like repeats of TNC have been involved in apoptotic processes in cultured human smooth muscle cells (23). The blockage of TNC induction prevented post-SAH MAPK activation in the brain and suppressed EBI in terms of neuronal apoptosis and BBB disruption (22). Overexpression of ILs-1β and -6 itself is also known to cause apoptosis by triggering caspase cascade reactions (16).
Effects of TNC Knockout on Experimental SAH
Some studies using TNC-knockout mice have reported a relationship between TNC and EBI or CVS. In a filament perforation SAH model, TNC knockout did not change the total volume of SAH (22). However, TNC knockout alleviated neurological impairment and decreased brain water content and Evans blue dye extravasation, which were associated with inactivation of three major MAPKs (c-Jun N-terminal kinase [JNK], p38, and ERK1/2) in brain capillary endothelial cells in the cerebral cortex; and the MAPK inactivation resulted in inhibition of MMP-9 induction and retention of tight junction proteins such as zonula occludens (ZO)-1 (20, 22, 72, 83). In addition, TNC-knockout mice demonstrated prevention of CVS, which was associated with a reduction in periarterial inflammatory cells infiltration and MAPK inactivation in cerebral arterial smooth muscle cells as well as suppression of caspase-dependent neuronal apoptosis in the cerebral cortex with reduction or inactivation of TLR4, NF-κB, and ILs-1β and -6 (37, 50). TNC knockout also inhibited post-SAH upregulation of another MCP, periostin, in brain capillary endothelial cells and neurons (83). In a hepatic ischemia and reperfusion model, the protective effects of TNC knockout have been also shown in terms of a marked decrease in apoptotic hepatic cells via reduction of inflammatory cytokines and MMP-9 (77). Exogenous TNC treatment induced TLR4 and MMP-9 and aggravated EBI in wild-type SAH rats; and abolished the protective effects through induction of TLR4 and MMP-9 in TNC-knockout SAH and transient hepatic ischemic models in mice (20, 22, 66, 73, 76).
TNC in Cerebral Aneurysm, Post-SAH Chronic Hydrocephalus, and Ischemic Stroke
TNC induced potent aneurysm repair through the fibrosis-promoting effects in a rat aneurysm model, possibly by recruiting macrophages, which secrete cytokines to induce migration and proliferation of smooth muscle cells (84). In contrast, the fibrosis-promoting effects of TNC may cause chronic hydrocephalus after SAH due to obstruction of circulation and reabsorption of CSF (70). Therefore, TNC induction may be protective if it is induced in the ruptured cerebral aneurysm wall but detrimental if it is induced in the brain, cerebral arteries, subarachnoid space, or CSF after SAH (22). However, no studies have investigated the role of TNC in cerebral aneurysmal genesis, growth, or rupture and the subsequent hemostasis. In addition, the effects of TNC on a ruptured cerebral aneurysm itself are unknown. Further studies are needed to clarify the role of TNC in intracranial aneurysm animal models (22).
In ischemic stroke in rats, treatment with neurotrophic factor L-serine upregulated TNC at 5 days post-ischemia and exerted neuroprotective effects by inducing the proliferation of neural stem cells and microvessels and the reconstruction of neurovascular units, resulting in neurorepair in the ischemic boundary zone (85). However, the mechanisms have not been investigated.
TLR4 Cascades and TNC in Stroke
TLRs are constituents of the innate immune system that are activated by DAMPs. At present, a total of 11 human and 13 murine TLRs have been identified (86). Since its discovery in 1998, TLR4 has been the most studied TLR family member (16, 87). TLR4 signaling is currently considered an important neuroinflammation therapeutic target because TLR4 has the unique ability to trigger two distinct signaling pathways (16, 71, 79, 86–88), the myeloid differentiation primary response protein 88 (MyD88)-dependent cascade in the acute phase and the Toll receptor-associated activator of interferon (TRIF)-dependent cascade in the late phase (86). TLR4 is expressed on the cell surface of various cells including microglia, neurons, astrocytes, brain capillary endothelial cells, endothelial and smooth muscle cells of the cerebral arteries, as well as peripheral blood cells including leukocytes, macrophages, and platelets (16, 86). TLR4 is activated by numerous DAMPs such as red blood cell breakdown products (heme, hemin, and methemoglobin), extravasated fibrinogen and fibrin, various intracellular components, and MCPs including TNC and galectin-3 (Figure 3) (16, 18). Activation of TLR4 induces the activation of the adaptor molecule MyD88 and subsequently the downstream signaling transcriptional factors NF-κB and activator protein (AP)-1. The process of AP-1 activation is primarily mediated by MAPKs including JNK, p38, and ERK1/2 (16, 79, 86, 89, 90). Both NF-κB and AP-1 upregulate MCPs including TNC, as well as proinflammatory cytokines or mediators such as TNF-α, IL-1β, -6, -8, and -12, intercellular adhesion molecule-1, monocyte chemoattractant protein, and MMP-9 (16, 20). These proinflammatory cytokines and mediators upregulate specific cell adhesion molecules on endothelial cells and induce neuroinflammation as well as the degradation of the inter-endothelial tight junctions and basal membrane in brain capillaries, which leads to BBB disruption and apoptosis of various cells, aggravating tissue damage after stroke (16, 20, 91). MMP-9 is a proinflammatory mediator induced by inflammatory cytokines and reactive oxygen species, and degrades components of the ECM of the cerebral microvessel basal lamina such as collagen IV, laminin, and fibronectin, as well as inter-endothelial tight junction proteins such as ZO-1, causing BBB disruption (92, 93). TNC amplifies the expression levels through positive feedback mechanisms utilizing the TLR4 signaling pathway, leading to further activation of the signaling transduction and the development or aggravation of secondary brain injury, as TNC itself is a ligand of TLR4 (16, 22). Experimental SAH studies have demonstrated that TNC induces CVS via activation of TLR4 and the downstream signaling MAPKs JNKs and p38 for more than 72 h in a rat cerebral artery, and that selective TLR4 antagonists LPS-RS and IAXO-102 inhibit TNC-induced CVS as well as expression of TLR4 in endothelial cells and smooth muscle cells of the arteries (Figure 3) (16, 17, 22, 73, 75, 76, 89). Therefore, targeting TLR4 is a potential therapeutic option against neuroinflammation after stroke. A recent study demonstrated that a selective TLR4 antagonist attenuated neurobehavioral impairments and prevented BBB disruption via suppression of the expression of MAPK JNK, MMP-9, MCPs such as TNC and periostin, as well as inflammatory mediators such as IL-6 and cyclooxygenase-1 in post-SAH mice (79, 89). TNC-knockout post-SAH mice showed less subarachnoid space infiltration of inflammatory cells in association with suppression of TLR4/NF-κB/IL-1β/IL-6 and the MMP-9 signaling pathway (37, 50, 67).
On the other hand, the late phase TRIF-dependent pathway in stroke induces interferon regulatory factor-3 as well as NF-κB and MAPKs, releasing interferon-β (86, 94). Interferon-β also modulates the innate immune response but exerts both anti-inflammatory and anti-apoptotic effects (94). The ligands of TLR4 interact with the receptor without distinction and induce the same downstream signaling pathways. However, the mechanisms to control the activation of respective pathways remains unclear (86).
TNC as a Clinical Biomarker of Stroke
In clinical settings of SAH, EBI is very difficult to be diagnosed precisely. Loss of consciousness at ictus, poor initial clinical grade, a large amount of SAH and/or intraventricular hematoma, presence of global cerebral edema, and inflammatory mediators have been generally used as surrogate markers of EBI (20). However, these markers are neither objective nor specific to EBI (35). Highly specific biomarkers that reflect EBI and predict the development of DCI are needed to enable earlier diagnosis and treatment of EBI and DCI (67). The ideal biomarkers should be easily measured via simple methods and provide accurate and prompt results (95).
If TNC upregulation after stroke reflects secondary brain injury, blood and CSF TNC concentrations can be a candidate for biomarkers: both concentrations are easily measured using an enzyme-linked immunosorbent assay (35). Previous studies have shown that the level of TNC containing alternatively spliced B or C domains in both CSF and peripheral blood may be used as a diagnostic and prognostic biomarker of inflammation and tissue remodeling processes in several diseases such as cardiomyopathy, myocarditis, osteoarthritis, hepatitis, and tumor (58, 96–100).
In patients with SAH, higher plasma and CSF TNC levels may be associated with severe EBI, angiographic CVS, and DCI (22, 101). Plasma TNC level increases independent of serum levels of C-reactive protein and some proinflammatory cytokines (102). Clinically, the peripheral blood level of TNC isoforms containing a C domain in the alternatively spliced FN III repeats at 1 to 3 days from SAH onset could not predict the development of CVS (68). However, the plasma level peaked between 4 and 6 days from SAH onset and was significantly higher in patients who subsequently developed CVS (68). The plasma TNC level increased before 2.4 days of the development of CVS as determined by transcranial Doppler ultrasonography and before 3.6 days of the onset of symptomatic CVS (67, 68, 103). In intracerebral hemorrhage patients, a higher serum level of TNC containing a C domain in the FN III repeats at admission was associated with greater hematoma volume and worse initial neurological status. In addition, the elevation of TNC level was independently correlated with early neurological deterioration, hematoma growth, and worse clinical outcomes defined as modified Rankin scale score >2 at 90 days (104).
In contrast, the CSF level of TNC containing a C domain in the FN III repeats peaked within the first 3 days after SAH onset and correlated with worse neurological status and greater hematoma volume at admission; and additionally, it predicted the development of CVS and shunt-dependent chronic hydrocephalus as well as poorer functional outcomes (22, 35, 70, 101, 105, 106). The differences in the time course of TNC levels between the plasma and CSF may be because TNC in the CSF may be belatedly transferred to the plasma due to its large molecular weight, although the possibility that TNC is released by different cells between the CSF and the plasma cannot be excluded. Although the reason of different time course of peripheral blood and CSF TNC levels after SAH remains unexplained, the findings in previous studies suggest that severe hemorrhagic stroke may induce higher expressions of TNC and that both CSF and peripheral blood TNC levels could be used in predicting or diagnosing the development of CVS and DCI after SAH (20, 22, 35, 103, 107). At present, the most practical clinical application of TNC appears to be its use as a biomarker (67).
Contribution of Other MCPs to Regulating TNC Expression in SAH-Associated Neuroinflammation
Periostin
TNC directly binds to other MCPs periostin and galectin-3, and may regulate the expression levels of each other in stroke, playing diverse roles (29, 42, 54, 66, 101). Periostin is a multimodular N-glycoprotein (93 kDa) with a N-terminal cysteine-rich EMI domain, fourfold repeated fasciclin (FAS) 1 domains in the middle, and a hydrophilic C-terminal region (108). The C-terminal region interacts with other ECM proteins such as TNC, collagen, fibronectin, and heparin (15, 66, 72, 74, 107, 109–111). The FAS1 domain of periostin also directly binds to integrins (αvβ1, αvβ3, αvβ5, and α6β4) and TNC, exerting various functions (112–115). Periostin is secreted by stromal cells, which are stimulated by cytokines, transforming growth factor (TGF)-β, and other GFs which are produced in epithelial cells and other cells (112). In an experimental study, periostin was expressed in brain capillary endothelial cells and neurons in the cerebral cortex at 24 h after SAH induction (83). TNC and periostin may induce expression of each other, forming a positive feedback loop (67, 72, 83, 107, 108). MAPKs are both downstream and upstream of periostin, TNC, and IL-6; and thus activated MAPKs induce periostin, TNC, and IL-6, which in turn activate MAPKs, resulting in a positive feedback to cause and aggravate brain injury via various mechanisms including MMP-9 activation (54, 66, 72, 83, 107, 112). An experimental study using an endovascular perforation SAH model in mice reported that upregulated periostin enhanced the expression of TNC associated with activation of MAPKs p38 and ERK1/2 as well as MMP-9, resulting in ZO-1 degradation in brain capillary endothelial cells and the subsequent aggravation of BBB disruption (83). In addition, recombinant full-length periostin administration exacerbated post-SAH neurobehavioral impairments, brain edema, BBB disruption, and TNC induction in the post-SAH brain (83). In contrast, anti-periostin antibody prevented post-SAH neurobehavioral impairments, brain edema formation, and BBB disruption via downregulation of TNC, inactivation of p38, ERK1/2, and MMP-9, and the resultant retention of ZO-1 (83, 107). These findings suggest that full-length periostin strongly interacts with TNC and contributes to post-SAH BBB disruption and neurobehavioral impairments via the MAPK pathway, and that neutralizing full-length periostin may be an effective novel therapeutic strategy for EBI after SAH (107). TNC-knockout mice also showed the inhibition of periostin induction in the post-SAH brain and exhibited less neurobehavioral impairments (83). The interaction between periostin and TNC may play an important role in post-SAH EBI and provides a new insight for future researches (83).
Periostin also binds to integrins, leading to neuroinflammation and BBB disruption (108). In experimental SAH, the process is at least partly mediated by MAPK activation and upregulation of MMP-9 (108). However, periostin-integrin binding also induces neurogenesis via activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway and upregulation of an anti-inflammatory cytokine TGF-β (18). The apparent discrepancy may be resolved by future studies to clarify how periostin relates with each integrin subtype in cerebrovascular diseases. In a clinical setting, a higher serum periostin level at admission was associated with worse initial neurological status, greater hemorrhage volume, more frequent development of DCI, and worse clinical outcomes in patients with aneurysmal SAH (116). In addition, plasma periostin levels increased before the development of DCI, irrespective of the presence or absence of CVS (108, 117). Therefore, periostin levels in the peripheral blood may be a predictive marker for post-SAH DCI, regardless of CVS development.
Galectin-3
Galectins are a family of MCPs comprised of more than 15 members of the β-galactoside-binding lectins and their conserved peptide sequence elements in the carbohydrate-recognition domains (CRDs) which show high affinities to β-galactoside-containing carbohydrate moieties of glycoconjugates (118). Galectins are classified into three types: proto-type (galectins-1, 2, 5, 7, 10, 11, 13–20), tandem-repeat-type (galectins-4, 6, 8, 9, 12), and chimera-type (galectin-3). Proto-type is comprised of monomers or homodimers with the sole CRD; tandem-repeat-type consists of N- and C- terminal distinct CRDs connected by a single-polypeptide-chain linker; and chimera-type has a C-terminal CRD and a N-terminal non-CRD domain which consists of proline- and glysine-rich short tandem repeats (118). The characteristics of chimera-type galectin-3 is to form a bridge between different ligands and to provide different functions (118). Recently, some studies exhibited that galectin-3 is activated through binding to TNC via its CRD domain (20, 118, 119). Activated galectin-3 possibly causes the development of brain injury including neuroinflammation after stroke (118–120). Galectin-3 induced by pro-inflammatory mediators contributed to brain immune responses via a major inflammatory signaling of Janus kinase/signal transducer and activation of transcription (STAT) and NF-κB pathways (121–123). In addition, galectin-3 is a ligand of TLR4 and activates its downstream signaling pathways as described above (18). In clinical settings, higher acute-stage plasma galectin-3 levels were associated with the development of DCI with no angiographic CVS after SAH (120). An experimental study showed that galectin-3 might cause post-SAH BBB disruption possibly by binding to TLR4 and activating ERK1/2, STAT-3, and MMP-9 (124).
Osteopontin
Osteopontin, another MCP, seems to have inhibitory effects against TNC in the setting of SAH (19). Osteopontin is an acidic phosphoglycoprotein (40–80 kDa) that contains several functional domains, allowing for integrin and CD44 receptor binding (15, 35). Osteopontin is subjected to numerous post-translational modifications including serine/threonine phosphorylation, glycosylation, tyrosine sulfation, and transglutamination, all of which regulate its functions (15). Five distinct isoforms are generated by alternative splicing (15). Thrombin and MMPs-2, -3, -7, -9, and -12 induce proteolytic cleavage of osteopontin (15). Osteopontin regulates homeostasis, angiogenesis, and immune responses through the upregulation in a variety of diverse cell types at the site of injury, stress, and inflammation (125). An intracellular form of osteopontin was expressed in dendritic cells and macrophages of the immune system in response to transient ischemic injury in the brain, and a secreted form of osteopontin promoted remodeling of the ECMs in the brain (15). After SAH induction, osteopontin binds to L-arginyl-glycyl-L-asparate (RGD)-dependent integrins and exerts neuroprotective effects by alleviating CVS and BBB disruption via induction of MAPK phosphatase-1, an endogenous MAPK inhibitor (22). Interestingly, both osteopontin and RGD-dependent integrin receptor antagonists significantly inhibited the vasoconstrictive effect by recombinant TNC fragments containing EGF-like repeats (20). The findings suggest that RGD-dependent integrins may be involved in CVS development, and that TNC binds to the integrins to develop CVS. Although the mechanisms of osteopontin’s anti-TNC effects remain poorly understood in stroke, osteopontin and TNC share some receptors such as RGD-dependent integrins, and therefore at least partly competitive inhibition may be the mechanism (22). A novel multimodal nanoparticle, simultaneous multiple aptamers and RGD targeting, which combines triple affinity for nucleolin, RGD-containing integrins, and TNC, has been reported as a candidate for a targeted therapy against TNC (126): the nanoparticle would be well worth trying in SAH and other stroke types, considering the possible effects on both RGD-dependent integrins and TNC.
Contribution of GFs and Integrins to Regulating TNC Expression in Stroke
The FN III domains 1–5, specifically domain 5 of TNC, have a high binding affinity for multiple GFs, such as platelet-derived GF (PDGF), vascular endothelial GF (VEGF), fibroblast GF (FGF) including FGF-2, and TGF-β1 as well as neurotrophin-3 (Figure 1) (27, 30, 54).
PDGF
PDGF is a homodimeric, non-glycosylated, polypeptide chain GF with a molecular weight of 28-35 kDa (127, 128). In SAH studies, PDGF is upstream of endogenous TNC and interrelated with TNC (66, 67, 69, 74). Exogenous TNC injections induce and activate PDGF receptors (PDGFRs) possibly via interreceptor interactions, which in turn upregulate TNC in the cerebral arteries and brain (66, 69). TNC may be further upregulated by a positive feedback on more PDGF activation via upregulated PDGFRs and crosstalk signaling between receptors, leading to more MAPK activation and consequent development of CVS, neuronal apoptosis, and neurological impairments in SAH rats (22). In rat SAH models, an intraperitoneal injection of imatinib mesylate, a tyrosine kinase inhibitor of PDGFR, showed the suppression of TNC induction and attenuated neurological impairments, the development of CVS and neuronal apoptosis via inactivation of MAPKs such as JNK, p38, and ERK1/2 (22, 66, 69). In addition, a cisternal injection of recombinant TNC to imatinib mesylate-treated experimental SAH rats reactivated MAPKs to abolish the protective effects of imatinib mesylate on neuronal apoptosis and CVS, resulting in neurological aggravation (22, 66, 69). Thus, TNC downregulation was demonstrated to be involved in the neuroprotective effect mechanism of imatinib mesylate (72), and PDGFs and PDGFRs were suggested as a potential therapeutic target to regulate TNC expression and to prevent post-SAH EBI and CVS (Figure 3) (67).
VEGF
VEGF, a member of a family of secreted polypeptides with a highly conserved receptor-binding cystine-knot structure similar to that of the PDGF, is a homodimeric protein (34–46 kDa) that stimulates the formation of blood vessels (129). Although TNC regulates VEGF expression in tumors, no studies have reported if VEGF directly induces TNC (74, 130). In mice, VEGF enhances BBB permeability in normal brain as well as brain with inflammatory diseases (74). Neutralization of VEGF downregulated VEGF receptor-2, a major mediator of the kinase activity effects of VEGF, in association with suppression of TNC expression and MAPKs activation (69, 73, 131). Taken together, TNC may be involved in VEGF-induced BBB disruption in SAH (Figure 3) (72).
FGF-2
FGF-2 belongs to the FGF family and exhibits several isoforms with molecular weights ranging 18–34 kDa (132–135). FGF-2 is highly expressed in the brain and regulates a variety of cell functions including proliferation, morphogenesis, and suppression of apoptosis (27, 136, 137). FGF-2 is secreted by damaged neurons, and the synergistic action with TGF-β1, which is also upregulated in response to an injury, stimulates the expression of TNC (138). TNC binds to FGF-2 and promotes survival of oligodendrocyte precursor cells by enhancing FGF receptor-mediated signaling and blocking bone morphogenic protein signaling (139). A recent study showed that recombinant FGF-2 activated PI3K and Akt, leading to suppression of neuronal apoptosis after SAH (132). Thus, administration or augmentation of FGF-2 may be a promising therapy to reduce post-SAH neuronal apoptosis via activation of the FGF receptor/PI3K/Akt signaling pathway (132). However, the action of FGF-2 on TNC after stroke has not been investigated.
Integrins
Integrins are a superfamily of cell adhesion receptors that primarily recognize ECMs and cell-surface ligands, and are composed of α and β subunits that form 24 known combinations (140). Five members of the integrin family that recognize TNC as a ligand have been identified: isoforms α2β1, α8β1, α9β1, αvβ3, and αvβ6 (140, 141). All the integrins except for α9β1 bind to the FN III repeat sites of TNC, while α9β1 binds to the fibrinogen globe (20, 140). Integrin αvβ3 is expressed on endothelial cells and activates the downstream signaling that involves MAPKs, proinflammatory mediators such as ILs, and MMP-9; however, the role of the integrin αvβ3 signaling pathway in stroke has not been investigated (19, 37, 83, 106, 140, 142–145). Activated integrin αvβ3 induces internalization of ZO-1 and occludin, disrupts vascular endothelial-cadherin localization, and increases expression of MMP-9 (146). Therefore, activation of integrin αvβ3 may be involved in BBB disruption. In contrast, β1 integrins form laminin-binding, collagen-binding (α2β1), RGD-binding (α8β1), or ECMs-binding (α9β1) heterodimers (147–150). The β1 integrins are increased in cerebral blood vessels in ischemic cortex, and induce angiogenesis as well as leukocyte adhesion and migration following ischemic stroke (147, 151). In addition, increased β1 integrins in neuronal cells were associated with neuronal adhesion, and neurite outreach and regeneration (151). Thus, it has been demonstrated that β1 integrin signaling is required for neurovascular formation and recovery as well as endothelial cell migration, proliferation and blood vessel formation following transient ischemic stroke in mice (152). Therefore, β1 integrin may be a therapeutic target for ischemic stroke and other pathological conditions through modulating angiogenesis (152). On the other hand, α2 integrins have been reported to be associated with an increased risk for ischemic stroke (151). Activation of α2β1 integrin prevents endothelial cells from proliferating through binding to laminin (153). In addition, overexpression of integrin α2β1 was associated with ischemic stroke and myocardial infarction by clot formation, while its absence results in a prolonged bleeding time within safe limits (154). Therefore, inhibition of integrin α2β1 may be a potential therapy for ischemic stroke. The expression and the role of integrins α8β1, α9β1, and αvβ6 have not yet been elucidated following stroke (147). At present, it is unknown if integrins influence TNC expression.
Other Therapeutic Candidates for TNC-Induced Brain Injury Following Stroke
TNC expression can be reduced by several medications, including cilostazol, steroids, and non-steroidal anti-inflammatory drugs (NSAIDs) (Figure 3) (32, 103, 155). An in vitro study found that cilostazol, an anti-platelet and peripheral arterial vasodilating agent, is a selective inhibitor of phosphodiesterase type III with pleiotropic actions that include the inhibition of inflammatory reactions (18, 155). Blockage of phosphodiesterase type III can inhibit induction of TNC at the transcriptional level by activating the cyclic adenosine monophosphate–protein kinase A signaling pathway (103, 155). In patients with aneurysmal SAH, 300 mg/day cilostazol treatment almost completely suppressed the elevation of plasma levels of TNC variants containing alternatively spliced FN III B and C domains at days 1–12 after SAH onset, and prevented the development of DCI and chronic shunt-dependent hydrocephalus, resulting in improved clinical outcomes (103, 156). TNC is induced by inflammation, and TNC itself can induce inflammatory reactions (58, 66, 157). Therefore, some anti-inflammatory medications are also associated with reduced TNC expression. For example, steroids and NSAIDs suppressed TNC expression in macrophages and human vascular smooth muscle cells in vitro and in arterial smooth muscle cells in vivo (32). With respect to inflammatory signaling, in a rat model of SAH, a MAPK JNK inhibitor SP600125 reversed the vasoconstrictive effects of TNC, and a MAPK p38 inhibitor SB203580 abolished TNC-induced TLR4 upregulation and TNC’s vasoconstrictive effects (73).
Angiotensin II is a well-known potent inducer of TNC but the potential mechanisms have not been identified (158). Drugs that inhibit the effects of angiotensin II such as angiotensin II receptor blockers (ARBs) may block vascular TNC expression (159). In a model of carotid artery stent implantation in hypercholesterolemic rabbits, an ARB candesartan cilexetil prevented in-stent neointimal hyperplasia, which was associated with a decrease in macrophage infiltration and TNC expression in the arterial wall: the immunostaining study showed that TNC was induced in a limited area around the stent struts, but the expression disappeared by the ARB treatment (109). ARBs may suppress in-stent restenosis after carotid artery stenting via anti-inflammatory effects through TNC inhibition (109). Eplenerone, an aldosterone receptor antagonist, also inhibited the development of inflammation and fibrosis associated with reduced TNC expression in an angiotensin II-induced hypertension model in mice (160).
Inhibition of Rho-kinase also suppressed expression of TNC in smooth muscle cells in hypertensive rat pulmonary arteries (161). In a clinical setting, a Rho-kinase inhibitor hydroxyfasudil is commonly used to prevent CVS after SAH in Japan, although the levels of TNC have not been measured (20).
Currently, Neuradiab® (81C6 anti-TNC antibody; Bradmer Pharmaceuticals, Inc.) and double-stranded RNA directed against TNC have been reported as candidates for anti-TNC directed therapy (126). Further evidence would facilitate the development of therapeutic agents targeting TNC.
Conclusions
TNC potentially plays a key role in pathophysiological changes via neuroinflammation and appears to be a future therapeutic target in patients with stroke. However, the protective and detrimental roles of TNC with respect to each disease and the stage have not been completely unveiled. If TNC is set as a therapeutic molecular target, the therapeutic (time) window should also be addressed. Current evidence shows that TNC can be a biomarker to predict secondary injuries following stroke. Further studies to determine the underlying molecular mechanisms of TNC-induced pathophysiological changes and the regulation of TNC expression are warranted.
Author Contributions
Both authors contributed equally to the planning, preparation, drafting, and writing of the article. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Taiju Life Social Welfare Foundation (Grant Number, N/A) and the JSPS KAKENHI (Grant Number JP20K09346) to HS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res (2017) 120:439–48. 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation (2017) 135:e146–603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller BA, Turan N, Chau M, Pradilla G. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. BioMed Res Int (2014) 2014:384342. 10.1155/2014/384342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Provencio JJ. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: a review. Acta Neurochir Suppl (2013) 115:233–8. 10.1007/978-3-7091-1192-5_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carr KR, Zuckerman SL, Mocco J. Inflammation, Cerebral Vasospasm, and Evolving Theories of Delayed Cerebral Ischemia. Neurol Res Int (2013) 2013:1–12. 10.1155/2013/506584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Rooij NK, Linn FHH, van der Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry (2007) 78:1365–72. 10.1136/jnnp.2007.117655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle (2012) 11:2084–91. 10.4161/cc.20316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shichita T, Ito M, Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Front Cell Neurosci (2014) 8:319. 10.3389/fncel.2014.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaefer L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem (2014) 289:35237–45. 10.1074/jbc.R114.619304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhry SR, Hafez A, Jahromi BR, Kinfe TM, Lamprecht A, Niemelä M, et al. Role of damage associated molecular pattern molecules (DAMPs) in aneurysmal subarachnoid hemorrhage (aSAH). Int J Mol Sci (2018) 19:2035. 10.3390/ijms19072035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayman EG, Wessell A, Gerzanich V, Sheth KN, Simard JM. Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care (2017) 26:301–10. 10.1007/s12028-016-0354-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki H. What is Early Brain Injury? Transl Stroke Res (2015) 6:1–3. 10.1007/s12975-014-0380-8 [DOI] [PubMed] [Google Scholar]
- 13. Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KTS. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth (2012) 109:315–29. 10.1093/bja/aes264 [DOI] [PubMed] [Google Scholar]
- 14. Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signals 0s taht spur autophagy and inmunity. Immunol Rev (2013) 249:158–75. 10.1111/j.1600-065X.2012.01146.x.PAMPs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawakita F, Kanamaru H, Asada R, Suzuki H. Potential roles of matricellular proteins in stroke. Exp Neurol (2019) 322:113057. 10.1016/j.expneurol.2019.113057 [DOI] [PubMed] [Google Scholar]
- 16. Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regener Res (2017) 12:193–6. 10.4103/1673-5374.200795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki H, Shiba M, Fujimoto M, Kawamura K, Nanpei M, Tekeuchi E, et al. Matricellular Protein: A New Player in Cerebral Vasospasm Following Subarachnoid Hemorrhage. Acta Neurochir Suppl (2013) 115:213–8. 10.1007/978-3-7091-1192-5_39 [DOI] [PubMed] [Google Scholar]
- 18. Okada T, Suzuki H. Mechanisms of neuroinflammation and inflammatory mediators involved in brain injury following subarachnoid hemorrhage. Histol Histopathol (2020) 35:18208. 10.14670/HH-18-208 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki H, Fujimoto M, Shiba M, Kawakita F, Liu L, Ichikawa N, et al. The Role of Matricellular Proteins in Brain Edema after Subarachnoid Hemorrhage. Acta Neurochir Suppl (2016) 121:151–6. 10.1007/978-3-319-18497-5_27 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki H, Fujimoto M, Kawakita F, Liu L, Nakatsuka Y, Nakano F, et al. Tenascin-C in brain injuries and edema after subarachnoid hemorrhage: Findings from basic and clinical studies. J Neurosci Res (2020) 98:42–56. 10.1002/jnr.24330 [DOI] [PubMed] [Google Scholar]
- 21. Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol (2014) 37:1–14. 10.1016/j.matbio.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki H, Kawakita F. Tenascin-C in aneurysmal subarachnoid hemorrhage: deleterious or protective? Neural Regener Res (2016) 11:230–1. 10.4103/1673-5374.177721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. J Cell Sci (2016) 129:4321–7. 10.1242/jcs.190546 [DOI] [PubMed] [Google Scholar]
- 24. Roll L, Faissner A. Tenascins in CNS lesions. Semin Cell Dev Biol (2019) 89:118–24. 10.1016/j.semcdb.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 25. Erickson HP. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr Opin Cell Biol (1993) 5:869–76. 10.1016/0955-0674(93)90037-q [DOI] [PubMed] [Google Scholar]
- 26. Rettig WJ, Triche TJ, Garin-Chesa P. Stimulation of human neuronectin secretion by brain-derived growth factors. Brain Res (1989) 487:171–7. 10.1016/0006-8993(89)90954-2 [DOI] [PubMed] [Google Scholar]
- 27. De Laporte L, Rice JJ, Tortelli F, Hubbell JA. Tenascin C Promiscuously Binds Growth Factors via Its Fifth Fibronectin Type III-Like Domain. PLoS One (2013) 8:e62076. 10.1371/journal.pone.0062076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishio T, Kawaguchi S, Iseda T, Kawasaki T, Hase T. Secretion of tenascin-C by cultured astrocytes: regulation of cell proliferation and process elongation. Brain Res (2003) 990:129–40. 10.1016/s0006-8993(03)03448-6 [DOI] [PubMed] [Google Scholar]
- 29. Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal (2009) 3:287–310. 10.1007/s12079-009-0075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reinhard J, Roll L, Faissner A. Tenascins in Retinal and Optic Nerve Neurodegeneration. Front Integr Neurosci (2017) 11:30. 10.3389/fnint.2017.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci (2017) 81:22–31. 10.1016/j.mcn.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 32. Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res (2011) 92:19–28. 10.1093/cvr/cvr183 [DOI] [PubMed] [Google Scholar]
- 33. Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr (2015) 9:48–82. 10.4161/19336918.2014.987587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jayakumar AR, Apeksha A, Norenberg MD. Role of Matricellular Proteins in Disorders of the Central Nervous System. Neurochem Res (2017) 42:858–75. 10.1007/s11064-016-2088-5 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki H, Nishikawa H, Kawakita F. Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regener Res (2018) 13:1175–8. 10.4103/1673-5374.235022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallner K, Li C, Shah PK, Wu K-J, Schwartz SM, Sharifi BG. EGF-Like Domain of Tenascin-C Is Proapoptotic for Cultured Smooth Muscle Cells. Arterioscler Thromb Vasc Biol (2004) 24:1416–21. 10.1161/01.ATV.0000134299.89599.53 [DOI] [PubMed] [Google Scholar]
- 37. Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K, et al. Effects of Tenascin-C Knockout on Cerebral Vasospasm After Experimental Subarachnoid Hemorrhage in Mice. Mol Neurobiol (2018) 55:1951–8. 10.1007/s12035-017-0466-x [DOI] [PubMed] [Google Scholar]
- 38. Keilhauer G, Faissner A, Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature (1985) 316:728–30. 10.1038/316728a0 [DOI] [PubMed] [Google Scholar]
- 39. Kruse J, Keilhauer G, Faissner A, Timpl R, Schachner M. The J1 glycoprotein–a novel nervous system cell adhesion molecule of the L2/HNK-1 family. Nature (1985) 316:146–8. 10.1038/316146a0 [DOI] [PubMed] [Google Scholar]
- 40. Meiners S, Powell EM, Geller HM. A distinct subset of tenascin/CS-6-PG-rich astrocytes restricts neuronal growth in vitro. J Neurosci (1995) 15:8096–108. 10.1523/JNEUROSCI.15-12-08096.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powell EM, Geller HM. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia (1999) 26:73–83. [DOI] [PubMed] [Google Scholar]
- 42. Jones EV, Bouvier DS. Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease. Neural Plast (2014) 2014:321209. 10.1155/2014/321209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faissner A, Kruse J. J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron (1990) 5:627–37. 10.1016/0896-6273(90)90217-4 [DOI] [PubMed] [Google Scholar]
- 44. Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci (2009) 29:5546–57. 10.1523/JNEUROSCI.0759-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meiners S, Powell EM, Geller HM. Neurite outgrowth promotion by the alternatively spliced region of tenascin-C is influenced by cell-type specific binding. Matrix Biol (1999) 18:75–87. 10.1016/s0945-053x(98)00008-0 [DOI] [PubMed] [Google Scholar]
- 46. Rigato F, Garwood J, Calco V, Heck N, Faivre-Sarrailh C, Faissner A. Tenascin-C Promotes Neurite Outgrowth of Embryonic Hippocampal Neurons through the Alternatively Spliced Fibronectin Type III BD Domains via Activation of the Cell Adhesion Molecule F3/Contactin. J Neurosci (2002) 22:6596–609. 10.1523/JNEUROSCI.22-15-06596.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull (2018) 136:101–8. 10.1016/j.brainresbull.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 48. Karus M, Denecke B, Ffrench-Constant C, Wiese S, Faissner A. The extracellular matrix molecule tenascin C modulates expression levels and territories of key patterning genes during spinal cord astrocyte specification. Development (2011) 138:5321–31. 10.1242/dev.067413 [DOI] [PubMed] [Google Scholar]
- 49. Strekalova T, Sun M, Sibbe M, Evers M, Dityatev A, Gass P, et al. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol Cell Neurosci (2002) 21:173–87. 10.1006/mcne.2002.1172 [DOI] [PubMed] [Google Scholar]
- 50. Liu L, Fujimoto M, Nakano F, Nishikawa H, Okada T, Kawakita F, et al. Deficiency of Tenascin-C Alleviates Neuronal Apoptosis and Neuroinflammation After Experimental Subarachnoid Hemorrhage in Mice. Mol Neurobiol (2018) 55:8346–54. 10.1007/s12035-018-1006-z [DOI] [PubMed] [Google Scholar]
- 51. Udalova IA, Ruhmann M, Thomson SJP, Midwood KS. Expression and immune function of tenascin-C. Crit Rev Immunol (2011) 31:115–45. 10.1615/critrevimmunol.v31.i2.30 [DOI] [PubMed] [Google Scholar]
- 52. González-González L, Alonso J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front Oncol (2018) 8:225. 10.3389/fonc.2018.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature (2008) 451:147–52. 10.1038/nature06487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta (2009) 1793:888–92. 10.1016/j.bbamcr.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 55. Laywell ED, Dörries U, Bartsch U, Faissner A, Schachner M, Steindler DA. Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc Natl Acad Sci U S A (1992) 89:2634–8. 10.1073/pnas.89.7.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T, Hase T. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience (2005) 132:87–102. 10.1016/j.neuroscience.2004.12.028 [DOI] [PubMed] [Google Scholar]
- 57. Ikeshima-Kataoka H, Shen J-S, Eto Y, Saito S, Yuasa S. Alteration of inflammatory cytokine production in the injured central nervous system of tenascin-deficient mice. In Vivo (Brooklyn) (2008) 22:409–13. [PubMed] [Google Scholar]
- 58. Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol (2003) 200:488–99. 10.1002/path.1415 [DOI] [PubMed] [Google Scholar]
- 59. Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol (2012) 69:1310–7. 10.1001/archneurol.2012.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall JR, Johnson LA, Barber RC, Vo HT, Winter AS, O’Bryant SE. Texas Alzheimer’s Research and Care Consortium. Biomarkers of basic activities of daily living in Alzheimer’s disease. J Alzheimers Dis (2012) 31:429–37. 10.3233/JAD-2012-111481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cho C-KJ, Smith CR, Diamandis EP. Amniotic fluid proteome analysis from Down syndrome pregnancies for biomarker discovery. J Proteome Res (2010) 9:3574–82. 10.1021/pr100088k [DOI] [PubMed] [Google Scholar]
- 62. Becker AJ, Chen J, Zien A, Sochivko D, Normann S, Schramm J, et al. Correlated stage- and subfield-associated hippocampal gene expression patterns in experimental and human temporal lobe epilepsy. Eur J Neurosci (2003) 18:2792–802. 10.1111/j.1460-9568.2003.02993.x [DOI] [PubMed] [Google Scholar]
- 63. Niquet J, Jorquera I, Faissner A, Ben-Ari Y, Represa A. Gliosis and axonal sprouting in the hippocampus of epileptic rats are associated with an increase of tenascin-C immunoreactivity. J Neurocytol (1995) 24:611–24. 10.1007/BF01257376 [DOI] [PubMed] [Google Scholar]
- 64. Scheffler B, Faissner A, Beck H, Behle K, Wolf HK, Wiestler OD, et al. Hippocampal loss of tenascin boundaries in Ammon’s horn sclerosis. Glia (1997) 19:35–46. [DOI] [PubMed] [Google Scholar]
- 65. Xie K, Liu Y, Hao W, Walter S, Penke B, Hartmann T, et al. Tenascin-C deficiency ameliorates Alzheimer’s disease-related pathology in mice. Neurobiol Aging (2013) 34:2389–98. 10.1016/j.neurobiolaging.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 66. Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W, Suzuki H. Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res (2014) 5:238–47. 10.1007/s12975-014-0333-2 [DOI] [PubMed] [Google Scholar]
- 67. Shiba M, Suzuki H. Lessons from tenascin-C knockout mice and potential clinical application to subarachnoid hemorrhage. Neural Regener Res (2019) 14:262–4. 10.4103/1673-5374.244789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suzuki H, Kanamaru K, Suzuki Y, Aimi Y, Matsubara N, Araki T, et al. Tenascin-C is induced in cerebral vasospasm after subarachnoid hemorrhage in rats and humans: a pilot study. Neurol Res (2010) 32:179–84. 10.1179/174313208X355495 [DOI] [PubMed] [Google Scholar]
- 69. Shiba M, Suzuki H, Fujimoto M, Shimojo N, Imanaka-Yoshida K, Yoshida T, et al. Imatinib mesylate prevents cerebral vasospasm after subarachnoid hemorrhage via inhibiting tenascin-C expression in rats. Neurobiol Dis (2012) 46:172–9. 10.1016/j.nbd.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 70. Suzuki H, Kinoshita N, Imanaka-Yoshida K, Yoshida T, Taki W. Cerebrospinal fluid tenascin-C increases preceding the development of chronic shunt-dependent hydrocephalus after subarachnoid hemorrhage. Stroke (2008) 39:1610–2. 10.1161/STROKEAHA.107.505735 [DOI] [PubMed] [Google Scholar]
- 71. Suzuki H, Fujimoto M, Kawakita F, Liu L, Nakano F, Nishikawa H, et al. Toll-Like Receptor 4 and Tenascin-C Signaling in Cerebral Vasospasm and Brain Injuries After Subarachnoid Hemorrhage. Acta Neurochir Suppl (2020) 127:91–6. 10.1007/978-3-030-04615-6_15 [DOI] [PubMed] [Google Scholar]
- 72. Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K, et al. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg (2016) 124:1693–702. 10.3171/2015.4.JNS15484 [DOI] [PubMed] [Google Scholar]
- 73. Fujimoto M, Suzuki H, Shiba M, Shimojo N, Imanaka-Yoshida K, Yoshida T, et al. Tenascin-C induces prolonged constriction of cerebral arteries in rats. Neurobiol Dis (2013) 55:104–9. 10.1016/j.nbd.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 74. Liu L, Fujimoto M, Kawakita F, Nakano F, Imanaka-Yoshida K, Yoshida T, et al. Anti-Vascular Endothelial Growth Factor Treatment Suppresses Early Brain Injury After Subarachnoid Hemorrhage in Mice. Mol Neurobiol (2016) 53:4529–38. 10.1007/s12035-015-9386-9 [DOI] [PubMed] [Google Scholar]
- 75. Fujimoto M, Shiba M, Kawakita F, Shimojo N, Imanaka-Yoshida K, Yoshida T, et al. Vasoconstrictive effect of tenascin-C on cerebral arteries in rats. Acta Neurochir Suppl (2015) 120:99–103. 10.1007/978-3-319-04981-6_17 [DOI] [PubMed] [Google Scholar]
- 76. Fujimoto M, Shiba M, Kawakita F, Liu L, Nakasaki A, Shimojo N, et al. Epidermal growth factor-like repeats of tenascin-C-induced constriction of cerebral arteries via activation of epidermal growth factor receptors in rats. Brain Res (2016) 1642:436–44. 10.1016/j.brainres.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 77. Kuriyama N, Duarte S, Hamada T, Busuttil RW, Coito AJ. Tenascin-C: a novel mediator of hepatic ischemia and reperfusion injury. Hepatology (2011) 54:2125–36. 10.1002/hep.24639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cho A, Graves J, Reidy MA. Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol (2000) 20:2527–32. 10.1161/01.atv.20.12.2527 [DOI] [PubMed] [Google Scholar]
- 79. Okada T, Kawakita F, Nishikawa H, Nakano F, Liu L, Suzuki H. Selective Toll-Like Receptor 4 Antagonists Prevent Acute Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage in Mice. Mol Neurobiol (2018) 56:1–10. 10.1007/s12035-018-1145-2 [DOI] [PubMed] [Google Scholar]
- 80. Suzuki K, Meguro K, Sakurai T, Saitoh Y, Takeuchi S, Nose T. Endothelin-1 concentration increases in the cerebrospinal fluid in cerebral vasospasm caused by subarachnoid hemorrhage. Surg Neurol (2000) 53:131–5. 10.1016/s0090-3019(99)00179-2 [DOI] [PubMed] [Google Scholar]
- 81. Mathiesen T, Edner G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor—α following subarachnoid hemorrhage. J Neurosurg (1997) 87:215–20. 10.3171/jns.1997.87.2.0215 [DOI] [PubMed] [Google Scholar]
- 82. Hendryk S, Jarzab B, Josko J. Increase of the IL-1 beta and IL-6 levels in CSF in patients with vasospasm following aneurysmal SAH. Neuro Endocrinol Lett (2003) 25:141–7. [PubMed] [Google Scholar]
- 83. Liu L, Kawakita F, Fujimoto M, Nakano F, Imanaka-Yoshida K, Yoshida T, et al. Role of Periostin in Early Brain Injury After Subarachnoid Hemorrhage in Mice. Stroke (2017) 48:1108–11. 10.1161/STROKEAHA.117.016629 [DOI] [PubMed] [Google Scholar]
- 84. Hamada K, Miura Y, Toma N, Miyamoto K, Imanaka-Yoshida K, Matsushima S, et al. Gellan sulfate core platinum coil with tenascin-C promotes intra-aneurysmal organization in rats. Transl Stroke Res (2014) 5:595–603. 10.1007/s12975-014-0352-z [DOI] [PubMed] [Google Scholar]
- 85. Sun L, Qiang R, Yang Y, Jiang Z-L, Wang G-H, Zhao G-W, et al. L-Serine Treatment May Improve Neurorestoration of Rats after Permanent Focal Cerebral Ischemia Potentially Through Improvement of Neurorepair. PLoS One (2014) 9:e93405. 10.1371/journal.pone.0093405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem (2010) 114:13–27. 10.1111/j.1471-4159.2010.06736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science (80- ) (1998) 282:2085–8. 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- 88. Liu L, Suzuki H. “The Role of Matricellular Proteins in Experimental Subarachnoid Hemorrhage-Induced Early Brain Injury”. In: Cell Mol Approaches to Regen Repair. Heidelberg, Germany: Springer; (2018). p. 397–407. 10.1007/978-3-319-66679-2_20 [DOI] [Google Scholar]
- 89. Kawakita F, Fujimoto M, Liu L, Nakano F, Nakatsuka Y, Suzuki H. Effects of Toll-Like Receptor 4 Antagonists Against Cerebral Vasospasm After Experimental Subarachnoid Hemorrhage in Mice. Mol Neurobiol (2017) 54:6624–33. 10.1007/s12035-016-0178-7 [DOI] [PubMed] [Google Scholar]
- 90. Fang H, Wang P-F, Zhou Y, Wang Y-C, Yang Q-W. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation (2013) 10:27. 10.1186/1742-2094-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kanamaru H, Suzuki H. Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regener Res (2019) 14:1138–43. 10.4103/1673-5374.251190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Peeyush Kumar T, McBride DW, Dash PK, Matsumura K, Rubi A, Blackburn SL. Endothelial Cell Dysfunction and Injury in Subarachnoid Hemorrhage. Mol Neurobiol (2018) 56:1992–2006. 10.1007/s12035-018-1213-7 [DOI] [PubMed] [Google Scholar]
- 93. Guo Z, Sun X, He Z, Jiang Y, Zhang X, Zhang JH. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol Res (2010) 32:715–20. 10.1179/016164109X12478302362491 [DOI] [PubMed] [Google Scholar]
- 94. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol (2004) 4:499–511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- 95. Li W, Pan R, Qi Z, Liu KJ. Current progress in searching for clinically useful biomarkers of blood-brain barrier damage following cerebral ischemia. Brain Circ (2018) 4:145–52. 10.4103/bc.bc_11_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aso N, Tamura A, Nasu M. Circulating tenascin-C levels in patients with idiopathic dilated cardiomyopathy. Am J Cardiol (2004) 94:1468–70. 10.1016/j.amjcard.2004.07.156 [DOI] [PubMed] [Google Scholar]
- 97. Hasegawa M, Hirata H, Sudo A, Kato K, Kawase D, Kinoshita N, et al. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. J Rheumatol (2004) 31:2021–6. [PubMed] [Google Scholar]
- 98. Imanaka-Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, et al. Tenascin-C is a useful marker for disease activity in myocarditis. J Pathol (2002) 197:388–94. 10.1002/path.1131 [DOI] [PubMed] [Google Scholar]
- 99. Tanaka H, El-Karef A, Kaito M, Kinoshita N, Fujita N, Horiike S, et al. Circulating level of large splice variants of tenascin-C is a marker of piecemeal necrosis activity in patients with chronic hepatitis C. Liver Int (2006) 26:311–8. 10.1111/j.1478-3231.2005.01229.x [DOI] [PubMed] [Google Scholar]
- 100. Yoshida J, Wakabayashi T, Okamoto S, Kimura S, Washizu K, Kiyosawa K, et al. Tenascin in cerebrospinal fluid is a useful biomarker for the diagnosis of brain tumour. J Neurol Neurosurg Psychiatry (1994) 57:1212–5. 10.1136/jnnp.57.10.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Suzuki H, Kanamaru K, Shiba M, Fujimoto M, Kawakita F, Imanaka-Yoshida K, et al. Tenascin-C is a possible mediator between initial brain injury and vasospasm-related and -unrelated delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl (2015) 120:117–21. 10.1007/978-3-319-04981-6_20 [DOI] [PubMed] [Google Scholar]
- 102. López-Sánchez M, Muñoz-Esquerre M, Huertas D, Montes A, Molina-Molina M, Manresa F, et al. Inflammatory markers and circulating extracellular matrix proteins in patients with chronic obstructive pulmonary disease and left ventricular diastolic dysfunction. Clin Respir J (2017) 11:859–66. 10.1111/crj.12428 [DOI] [PubMed] [Google Scholar]
- 103. Suzuki H, Nakatsuka Y, Yasuda R, Shiba M, Miura Y, Terashima M, et al. Dose-Dependent Inhibitory Effects of Cilostazol on Delayed Cerebral Infarction After Aneurysmal Subarachnoid Hemorrhage. Transl Stroke Res (2019) 10:381–8. 10.1007/s12975-018-0650-y [DOI] [PubMed] [Google Scholar]
- 104. Wang L-G, Huangfu X-Q, Tao B, Zhong G-J, Le Z-D. Serum tenascin-C predicts severity and outcome of acute intracerebral hemorrhage. Clin Chim Acta (2018) 481:69–74. 10.1016/j.cca.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 105. Suzuki H, Kanamaru K, Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, et al. Cerebrospinal fluid tenascin-C in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol (2011) 23:310–7. 10.1097/ANA.0b013e31822aa1f2 [DOI] [PubMed] [Google Scholar]
- 106. Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke (2010) 41:1783–90. 10.1161/STROKEAHA.110.586537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nishikawa H, Suzuki H. Implications of periostin in the development of subarachnoid hemorrhage-induced brain injuries. Neural Regener Res (2017) 12:1982. 10.4103/1673-5374.221150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kawakita F, Suzuki H. Periostin in cerebrovascular disease. Neural Regener Res (2020) 15:63. 10.4103/1673-5374.264456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ichikawa N, Toma N, Kawakita F, Matsushima S, Imanaka-Yoshida K, Yoshida T, et al. Angiotensin II type 1 receptor blockers suppress neointimal hyperplasia after stent implantation in carotid arteries of hypercholesterolemic rabbits. Neurol Res (2015) 37:147–52. 10.1179/1743132814Y.0000000436 [DOI] [PubMed] [Google Scholar]
- 110. Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, et al. Incorporation of Tenascin-C into the Extracellular Matrix by Periostin Underlies an Extracellular Meshwork Architecture. J Biol Chem (2010) 285:2028–39. 10.1074/jbc.M109.051961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, et al. Periostin regulates atrioventricular valve maturation. Dev Biol (2008) 316:200–13. 10.1016/j.ydbio.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol (2014) 37:150–6. 10.1016/j.matbio.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 113. Kudo A. Introductory review: periostin—gene and protein structure. Cell Mol Life Sci (2017) 74:4259–68. 10.1007/s00018-017-2643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and Characterization of a Novel Protein, Periostin, with Restricted Expression to Periosteum and Periodontal Ligament and Increased Expression by Transforming Growth Factor β. J Bone Miner Res (1999) 14:1239–49. 10.1359/jbmr.1999.14.7.1239 [DOI] [PubMed] [Google Scholar]
- 115. Bonnet N, Garnero P, Ferrari S. Periostin action in bone. Mol Cell Endocrinol (2016) 432:75–82. 10.1016/j.mce.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 116. Luo W, Wang H, Hu J. Increased concentration of serum periostin is associated with poor outcome of patients with aneurysmal subarachnoid hemorrhage. J Clin Lab Anal (2018) 32:e22389. 10.1002/jcla.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kanamaru H, Kawakita F, Nakano F, Miura Y, Shiba M, Yasuda R, et al. Plasma Periostin and Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Neurotherapeutics (2019) 16:480–90. 10.1007/s13311-018-00707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nishikawa H, Suzuki H. Possible Role of Inflammation and Galectin-3 in Brain Injury after Subarachnoid Hemorrhage. Brain Sci (2018) 8:30. 10.3390/brainsci8020030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yang R-Y, Rabinovich GA, Liu F-T. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med (2008) 10:e17. 10.1017/S1462399408000719 [DOI] [PubMed] [Google Scholar]
- 120. Nishikawa H, Nakatsuka Y, Shiba M, Kawakita F, Fujimoto M, Suzuki H. Increased Plasma Galectin-3 Preceding the Development of Delayed Cerebral Infarction and Eventual Poor Outcome in Non-Severe Aneurysmal Subarachnoid Hemorrhage. Transl Stroke Res (2018) 9:110–9. 10.1007/s12975-017-0564-0 [DOI] [PubMed] [Google Scholar]
- 121. Yip PK, Carrillo-Jimenez A, King P, Vilalta A, Nomura K, Chau CC, et al. Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci Rep (2017) 7:41689. 10.1038/srep41689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Venkatraman A, Hardas S, Patel N, Singh Bajaj N, Arora G, Arora P. Galectin-3: an emerging biomarker in stroke and cerebrovascular diseases. Eur J Neurol (2018) 25:238–46. 10.1111/ene.13496 [DOI] [PubMed] [Google Scholar]
- 123. Shin T. The pleiotropic effects of galectin-3 in neuroinflammation: a review. Acta Histochem (2013) 115:407–11. 10.1016/j.acthis.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 124. Nishikawa H, Liu L, Nakano F, Kawakita F, Kanamaru H, Nakatsuka Y, et al. Modified citrus pectin prevents blood-brain barrier disruption in mouse Subarachnoid hemorrhage by inhibiting Galectin-3. Stroke (2018) 49:2743–51. 10.1161/STROKEAHA.118.021757 [DOI] [PubMed] [Google Scholar]
- 125. Rogall R, Rabenstein M, Vay S, Bach A, Pikhovych A, Baermann J, et al. Bioluminescence imaging visualizes osteopontin-induced neurogenesis and neuroblast migration in the mouse brain after stroke. Stem Cell Res Ther (2018) 9:182. 10.1186/s13287-018-0927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci (2011) 68:3175–99. 10.1007/s00018-011-0783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Heldin C-H, Westermark B. Mechanism of Action and In Vivo Role of Platelet-Derived Growth Factor. Physiol Rev (1999) 79:1283–316. 10.1152/physrev.1999.79.4.1283 [DOI] [PubMed] [Google Scholar]
- 128. Ying H-Z, Chen Q, Zhang W-Y, Zhang H-H, Ma Y, Zhang S-Z, et al. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics. Mol Med Rep (2017) 16:7879–89. 10.3892/mmr.2017.7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem (1996) 271:603–6. 10.1074/jbc.271.2.603 [DOI] [PubMed] [Google Scholar]
- 130. Tanaka K, Hiraiwa N, Hashimoto H, Yamazaki Y, Kusakabe M. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer (2004) 108:31–40. 10.1002/ijc.11509 [DOI] [PubMed] [Google Scholar]
- 131. Li W, Lu Z-F, Man X-Y, Li C-M, Zhou J, Chen J-Q, et al. VEGF upregulates VEGF receptor-2 on human outer root sheath cells and stimulates proliferation through ERK pathway. Mol Biol Rep (2012) 39:8687–94. 10.1007/s11033-012-1725-6 [DOI] [PubMed] [Google Scholar]
- 132. Okada T, Enkhjargal B, Travis ZD, Ocak U, Tang J, Suzuki H, et al. FGF-2 Attenuates Neuronal Apoptosis via FGFR3/PI3k/Akt Signaling Pathway After Subarachnoid Hemorrhage. Mol Neurobiol (2019) 56:8203–19. 10.1007/s12035-019-01668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sørensen V, Nilsen T, Wiedłocha A. Functional diversity of FGF-2 isoforms by intracellular sorting. BioEssays (2006) 28:504–14. 10.1002/bies.20405 [DOI] [PubMed] [Google Scholar]
- 134. Kardami E, Jiang Z-S, Jimenez SK, Hirst CJ, Sheikh F, Zahradka P, et al. Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc Res (2004) 63:458–66. 10.1016/j.cardiores.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 135. Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol (2000) 32:115–20. 10.1016/S1357-2725(99)00123-5 [DOI] [PubMed] [Google Scholar]
- 136. Noda M, Takii K, Parajuli B, Kawanokuchi J, Sonobe Y, Takeuchi H, et al. FGF-2 released from degenerating neurons exerts microglial-induced neuroprotection via FGFR3-ERK signaling pathway. J Neuroinflammation (2014) 11:1–11. 10.1186/1742-2094-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang X, et al. BFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett (2012) 212:137–46. 10.1016/j.toxlet.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 138. Smith GM, Hale JH. Macrophage/Microglia Regulation of Astrocytic Tenascin: Synergistic Action of Transforming Growth Factor-β and Basic Fibroblast Growth Factor. J Neurosci (1997) 17:9624–33. 10.1523/JNEUROSCI.17-24-09624.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Garcion E, Halilagic A, Faissner A, Ffrench-Constant C. Generation of an environmental niche for neural stem cell development bythe extracellular matrix molecule tenascin C. Development (2004) 131:3423–32. 10.1242/dev.01202 [DOI] [PubMed] [Google Scholar]
- 140. Takada Y, Ye X, Simon S. The integrins. Genome Biol (2007) 8:215. 10.1186/gb-2007-8-5-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Tucker RP, Chiquet-Ehrismann R. Tenascin-C: Its functions as an integrin ligand. Int J Biochem Cell Biol (2015) 65:165–8. 10.1016/j.biocel.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 142. Soung YH, Clifford JL, Chung J. Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep (2010) 43:311–8. 10.5483/BMBRep.2010.43.5.311 [DOI] [PubMed] [Google Scholar]
- 143. Chong HC, Tan CK, Huang R-L, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol (2012) 2012:351089. 10.1155/2012/351089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rivera LB, Bradshaw AD, Brekken RA. The regulatory function of SPARC in vascular biology. Cell Mol Life Sci (2011) 68:3165–73. 10.1007/s00018-011-0781-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Smyth SS, Patterson C. Tiny dancers: The integrin-growth factor nexus in angiogenic signaling. J Cell Biol (2002) 158:17–21. 10.1083/jcb.200202100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Edwards DN, Bix GJ. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am J Physiol Physiol (2019) 316:C252–63. 10.1152/ajpcell.00151.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Edwards DN, Bix GJ. The Inflammatory Response After Ischemic Stroke: Targeting β2 and β1 Integrins. Front Neurosci (2019) 13:540. 10.3389/fnins.2019.00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Caimi G, Canino B, Ferrara F, Montana M, Musso M, Porretto F, et al. Granulocyte integrins before and after activation in acute ischaemic stroke. J Neurol Sci (2001) 186:23–6. 10.1016/s0022-510x(01)00495-6 [DOI] [PubMed] [Google Scholar]
- 149. Campanero MR, Sánchez-Mateos P, del Pozo MA, Sánchez-Madrid F. ICAM-3 regulates lymphocyte morphology and integrin-mediated T cell interaction with endothelial cell and extracellular matrix ligands. J Cell Biol (1994) 127:867–78. 10.1083/jcb.127.3.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell (2002) 110:673–87. 10.1016/s0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- 151. Wu X, Reddy DS. Integrins as receptor targets for neurological disorders. Pharmacol Ther (2012) 134:68–81. 10.1016/j.pharmthera.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lathia JD, Chigurupati S, Thundyil J, Selvaraj PK, Mughal MR, Woodruff TM, et al. Pivotal role for beta-1 integrin in neurovascular remodelling after ischemic stroke. Exp Neurol (2010) 221:107–14. 10.1016/j.expneurol.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 153. Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, et al. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell (2001) 8:115–27. 10.1016/s1097-2765(01)00285-4 [DOI] [PubMed] [Google Scholar]
- 154. Miller MW, Basra S, Kulp DW, Billings PC, Choi S, Beavers MP, et al. Small-molecule inhibitors of integrin α 2 β 1 that prevent pathological thrombus formation via an allosteric mechanism. Proc Natl Acad Sci (2009) 106:719–24. 10.1073/pnas.0811622106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fujinaga K, Onoda K, Yamamoto K, Imanaka-Yoshida K, Takao M, Shimono T, et al. Locally applied cilostazol suppresses neointimal hyperplasia by inhibiting tenascin-C synthesis and smooth muscle cell proliferation in free artery grafts. J Thorac Cardiovasc Surg (2004) 128:357–63. 10.1016/j.jtcvs.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 156. Nakatsuka Y, Kawakita F, Yasuda R, Umeda Y, Toma N, Sakaida H, et al. Preventive effects of cilostazol against the development of shunt-dependent hydrocephalus after subarachnoid hemorrhage. J Neurosurg (2017) 127:319–26. 10.3171/2016.5.JNS152907 [DOI] [PubMed] [Google Scholar]
- 157. Ma S, Yang D, Li D, Tang B, Sun M, Yang Y. Cardiac extracellular matrix tenascin-C deposition during fibronectin degradation. Biochem Biophys Res Commun (2011) 409:321–7. 10.1016/j.bbrc.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 158. Sharifi BG, LaFleur DW, Pirola CJ, Forrester JS, Fagin JA. Angiotensin II regulates tenascin gene expression in vascular smooth muscle cells. J Biol Chem (1992) 267:23910–5. [PubMed] [Google Scholar]
- 159. Fischer JW. Tenascin-C: A key molecule in graft stenosis. Cardiovasc Res (2007) 74:335–6. 10.1016/j.cardiores.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 160. Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, et al. Eplerenone Attenuates Myocardial Fibrosis in the Angiotensin II-Induced Hypertensive Mouse: Involvement of Tenascin-C Induced by Aldosterone-Mediated Inflammation. J Cardiovasc Pharmacol (2007) 49:261–8. 10.1097/FJC.0b013e318033dfd4 [DOI] [PubMed] [Google Scholar]
- 161. Chapados R, Abe K, Ihida-Stansbury K, McKean D, Gates AT, Kern M, et al. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res (2006) 99:837–44. 10.1161/01.RES.0000246172.77441.f1 [DOI] [PubMed] [Google Scholar]