Abstract
Hypoplastic left heart syndrome is a constellation of malformations which result from the severe underdevelopment of any left-sided cardiac structures. Once considered to be universally fatal, the prognosis for this condition has tremendously improved over the past four decades since the work of William Norwood in the early 1980s. Today, a staged surgical approach is applied for palliating this distinctive cohort of patients, in which they undergo three operative procedures in the first 10 years of their life. Advancements in medical technologies, surgical techniques, and our growing experience in the management of HLHS have made survival into adulthood a possibility. Through this review, we present the different phases of the staged approach with primary focus on stage 1—its modifications, current technique, alternatives, and latest outcomes.
Keywords: Congenital heart defects, Hypoplastic left heart syndrome, Norwood procedure, Modifications, Interventions, Treatment outcomes
Introduction
The term hypoplastic left heart syndrome (HLHS) was coined by Noonan and Nadas in 1958 to describe a constellation of malformations resulting from severe underdevelopment of any left-sided cardiac structures [1]. It includes valvular stenosis (aortic and/or mitral) and atresia or hypoplasia of left ventricle (LV), ascending aorta, and the aortic arch. Although rare in incidence, it is the greatest culprit for congenital heart disease (CHD)–related mortality in infants. Since its initial description by Lev, we have come a long way in the management of HLHS from compassionate care being the only possibility in 1970s to these children now surviving to adulthood [2]. Based on the current consensus, a neonate with HLHS undergoes a 3-staged surgical palliation although variations in practices exist. With this review, we primarily focus on stage 1 with a brief description of the other aspects of the palliative process.
Pre-stage 1: Fetal diagnosis and intervention
In a fetus with critical aortic stenosis, Allan et al. were the first to observe and study the intrauterine development of HLHS using serial echocardiography [3]. Since then, not only the delineation of fetal cardiac anatomy has improved but also our understanding of fetal cardiovascular physiology and flow patterns has greatly evolved. An association between the posterior deviation of septum primum and HLHS has been identified [4]. During fetal period, LV is mainly filled by the flow through foramen ovale and any restriction at this atrial level shunting may lead to growth impairment of left-sided cardiac structures. Consequently, such alterations in LV outflow or inflow tracts lead to progressive development of LV hypoplasia throughout the fetal life [5, 6]. Constellations of anatomical lesions suggestive of HLHS can be identified as early as 18th week of gestation, which enables us to determine the prognosis of the affected fetus and modify treatment accordingly.
Upon diagnosis of HLHS prenatally, the parents must be counseled regarding the possible outcomes and management options, ranging from alteration in perinatal care to fetal interventions or even termination of pregnancy. In milder forms of HLHS, the family must be referred to a hospital with a Norwood program for delivery. This facilitates immediate stabilization and preoperative management of a fragile newborn. However, in moderate forms, a more invasive approach may be required such as fetal valvuloplasty or atrial septoplasty.
Most fetuses with aortic stenosis progress to develop HLHS and require a multi-stage univentricular repair after birth. If a fetal aortic valvuloplasty (FAV) is performed, the progressing underdevelopment of LV can be decelerated, thus improving chances for a biventricular repair postnatally. FAV has also been shown to improve fetal hemodynamics and growth of cardiac structures resulting in a significantly better survival [7]. A study reporting data from 18 centers registered in the International Fetal Cardiac Intervention Registry (IFCIR) demonstrated that biventricular circulation was almost twice as likely to be achieved in children who underwent FAV [8]. The procedure, however, comes with an increased risk of fetal demise and premature delivery [8, 9].
In restricted foramen ovale morphology, there is a double jeopardy in terms of suboptimal LV filling and growth as well as back pressure changes in the lungs, putting the fetus at risk of intrauterine demise or making it an extremely high-risk candidate for Norwood procedure postnatally. In such situations, interventions such as fetal atrial septoplasty and/or atrial septum stenting can be undertaken to improve chances of survival by virtue of left atrial decompression, which diminishes left atrial hypertension and hence prevents further damage to the developing lungs [9]. As per a recent report from IFCIR, the feasibility of performing these interventions was as close as 77% [10]. It also showed that the need for cesarean delivery and immediate neonatal resuscitation was lower in the group who underwent fetal septal intervention; however, these interventions have not yet been shown to improve survival.
In addition to these invasive interventions, maternal hyperoxygenation therapy (MHT) is a non-invasive approach to improve prenatal and postnatal hemodynamics in some HLHS substrates. The therapy consists of supplementing 100% oxygen to the mother during the later period of pregnancy to increase fetal pulmonary blood flow, thus increasing the venous return to the left atrium and stimulating growth of hypoplastic left heart structures. However, the effectiveness of MHT still remains controversial [11]. Though there is a theoretical risk of cerebral underdevelopment by MHT, it has not been observed in reported studies [12].
In severe forms of HLHS such as substrates with diminutive ascending aorta (< 2 mm), aortic atresia with retrograde coronary flow, mitral valve atresia with concomitant restricted foramen ovale, severe tricuspid regurgitation, or right ventricular dysfunction, parents may be counseled regarding the termination of pregnancy as a last resort.
Stage 1 palliation
Norwood procedure is now considered the gold standard for the first step in the staged palliation of HLHS, which is usually undertaken around 2 weeks after birth.
Preoperative stabilization
The goals of preoperative stabilization include maintaining ductal patency, balancing pulmonary flow (Qp) to systemic flow (Qs) ratio, and achieving adequate tissue oxygenation [13]. Early administration of prostaglandin E1 is necessary to maintain ductal patency for supporting the systemic circulation of the neonate. The second aspect of preoperative care includes manipulation of pulmonary (PVR) and systemic vascular resistance (SR) aimed at preventing pulmonary overcirculation by use of hypoxic gases (14–20% FiO2) along with nitrogen or ambient gas and deliberate hypoventilation or use of 2–5% CO2 to increase arterial CO2. Alternatively, sodium nitroprusside and phenoxybenzamine can be administered to manipulate SR.
Norwood procedure
Cayler et al. theorized that there were five main hemodynamic requirements for a successful surgical palliation—a large intra-atrial communication, a large arterio-ventricular communication, an adequately sized aorta, high pulmonary vascular resistance, and sufficient coronary perfusion [14]. Norwood improved on the initial palliative attempts by introducing a staged surgical program for HLHS aimed at establishing the right ventricle (RV) as the chamber supporting the systemic circulation. In 1983, he described his breakthrough technique for the first palliative stage of the three-staged approach, which now bears the name of “Norwood procedure” [15].
The components of the Norwood procedure are as follows:
-
(i)
Atrial septectomy - creation of an unrestricted interatrial communication to allow pulmonary venous return to flow into the RV. This is achieved by performing complete excision of atrial septum primum.
-
(ii)
Aortic arch reconstruction - establishment of a permanent connection between the RV and aorta. This step involves constructing a neo-aorta by utilizing the proximal main pulmonary artery (MPA), ascending aorta, aortic arch, and descending aorta. The aim is to provide unobstructed, low resistance, systemic outflow.
-
(iii)
Establishing pulmonary circulation - a new source for the pulmonary circulation is developed using either a modified Blalock-Taussig shunt (mBTS) or a right ventricle-pulmonary artery shunt (RVPAS). It also aimed at optimizing the pulmonary flow and vasculature to enable the neonate to progress to subsequent palliative stages (Figs. 1 and 2).
Fig. 1.
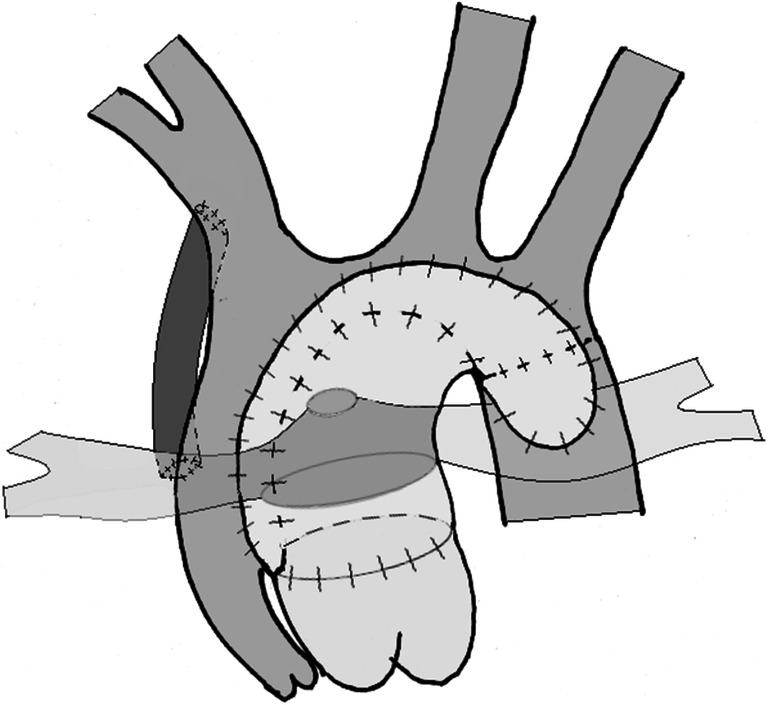
Illustration of Norwood procedure with a modified Blalock-Taussig Shunt
Fig. 2.
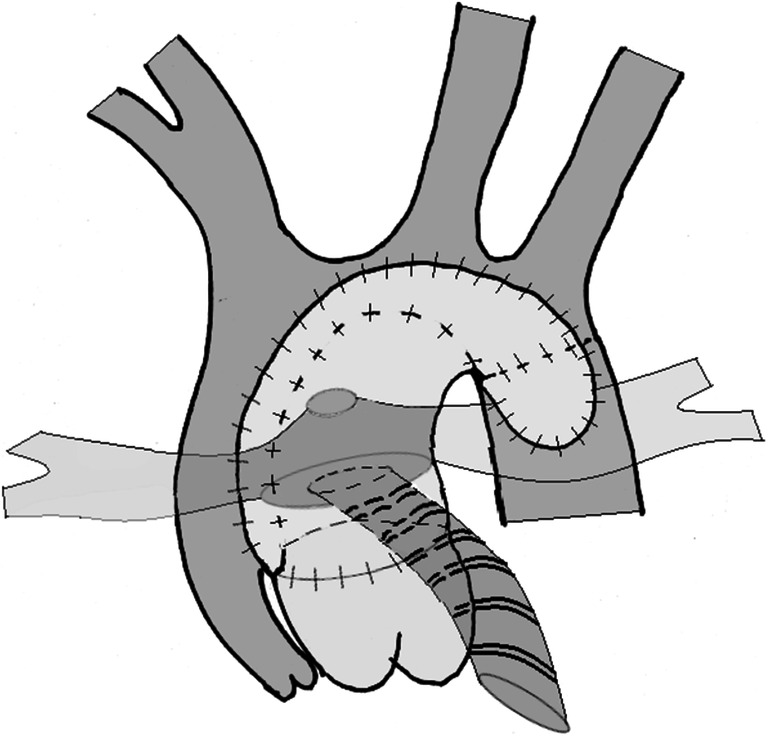
Illustration of Norwood procedure with a right ventricle-pulmonary artery shunt using a ring-enforced conduit
With this modified anatomy, the RV receives both the systemic and pulmonary venous returns, which it then simultaneously pumps into both the circulations in a parallel fashion. Based on concepts of flow dynamics, this single ventricular heart and its parallel cardiac output will eject more blood into the vascular bed (pulmonary or systemic) with the least resistance. This phenomenon has important implications in the postoperative management after stage 1 palliation (S1P), which is not discussed in detail here as it is not the focus of our review.
Past and current approach
Since the conception of the Norwood procedure, the prognosis of once extremely fatal diagnosis of HLHS has dramatically improved. The groundbreaking work of Norwood and his team planted the seeds for realizing the goal of providing these children a chance at long-term survival. As the Norwood procedure gained recognition throughout the world, several surgeons reproduced the operation and identified technical complications. They incorporated novel innovations and modifications to the original procedure in hopes of further enhancing the technique and improving outcomes. Over the past four decades, several modifications to the neo-aortic construction and establishment of pulmonary circulation, evidently the most crucial aspects of the Norwood procedure, have been proposed as outlined in (Tables 1 and 2).
Table 1.
Modifications in neo-aorta reconstruction
| Study | Modification | Comments |
|---|---|---|
| Patch variants | ||
| Pigott et al., 1988 [16] | Pulmonary homograft patch | Pulmonary homograft is a pliable material which provided good hemostatic results in comparison to prosthetic conduits originally proposed by Norwood. |
| Gargiulo et al., 1999 [17] | Bovine pericardial patch | This material provided advantages similar to the pulmonary homograft while being easier to modify, readily available and having better growth potential. |
| Burkhart et al., 2005 [18] | Interdigitating reconstruction | This approach was primarily aimed at reducing aortic arch stenosis. A longitudinal incision in the anterior and posterior walls of the descending aorta was carried out, then, an “interdigitating” anastomosis of the distal aortic arch with the posterior wall and of the pulmonary homograft with the anterior wall was performed to construct the neo-aorta. The results were exceptional with 0% postoperative obstruction of the aortic arch. |
| Healy et al., 2007 [19] | Bovine jugular vein graft | Owing to its intrinsic conduit-like shape among other benefits such as easier availability, wide-size spectrum, and cost-effectiveness, a vascular graft was proposed as an alternative to pulmonary homograft. |
| Sinha et al., 2009 [20] | Femoral vein homograft | Another vascular graft was suggested for neo-aortic construction emphasizing on its suitability for HLHS with extremely diminutive ascending aorta (< 2 mm). This graft was also suitable for a conventional reconstruction as a patch. |
| Bernabei et al., 2013 [21] | Autologous pericardial patch | The distinctive benefit of this material was its “ready-to-use” nature in the surgical field. It also eliminated the drawbacks of exogenous materials such as costs and risk of infection. |
| Jacobsen et al., 2018 [22] | Porcine intestinal submucosal patch | This material is reported to undergo remodeling in response to local proliferative signals eventually integrating with the local tissue. The report presents it as a non-inferior alternative to other materials. |
| Non-patch variants | ||
| Bu’Lock et al., 1995 [23] | Modified Norwood | This approach involved utilizing the MPA, aortic arch, and descending aorta for constructing the neo-aorta. An anastomosis between the posterior walls of aortic arch and descending aorta was performed to form a confluence. The MPA was transected and its proximal portion was sutured to this confluence with the suture line extending down to ascending aorta. This technique eliminates exogenous material and provides better neo-aorta growth potential. |
| Sung et al., 2009 [24] | U-shaped excision of branch PAs | The “Modified Norwood” procedure may cause narrowing of the space for PAs resulting in left bronchial compression. As a solution, a U-shaped division of the branch PAs from the posterior wall of MPA was suggested. It spares more MPA wall anteriorly to be used for neo-aortic augmentation. This approach was also expected to improve vascular growth, but, pulmonary angioplasty was necessary in majority cases. |
| Asada et al., 2017 [25] | Chimney reconstruction | Chimney reconstruction is a modification of the technique described by Sung et al. It starts with a U-shaped separation of branch PAs from MPA and is followed by a direct longitudinal closure of the MPA wall instead of a patch. This forms a conical MPA which is then used to reconstructed the aorta as in the “modified Norwood”. This technique potentially allows a more tension-free anastomosis, further reducing the risk of PA compression. |
Table 2.
Modification for re-establishing pulmonary flow
| Study | Modification | Comments |
|---|---|---|
| Systemic to pulmonary artery shunt | ||
| Plunkett et al., 1998 [26] | Chimney patch | A “chimney patch” using homograft tissue and a PTFE shunt was prepared prior to the operation. The homograft tissue sealed the defect in the branch PAs created subsequent to MPA transection. The PTFE shunt is anastomosed to innominate artery to establish systemic-pulmonary connection. Their cohort had widely patent shunts with no need for intervention after S1P. |
| Tam et al., 2001 [27] | Saphenous vein homograft | In an effort to reduce shunt thrombosis, biological saphenous vein homograft was used experimentally for mBTS. The results were discouraging as majority of patients required intervention because of shunt stenosis. Expensive costs and lack of pediatric-sized grafts were other disadvantages. |
| Right ventricle to pulmonary artery shunt | ||
| Kishimoto et al., 1999 [28] | Xenopericardial bicuspid-valved conduit | Kishimoto revisited the RVPAS after Norwood abandoned it owing to dismal outcomes with this technique. They used a 6-mm xenopericardial bicuspid-valved conduit in contrast to 12 mm conduits utilized by Norwood. The smaller conduit restricted pulmonary overcirculation resulting in better hemodynamics and survival. |
| Sano et al., 2003 [29] | Non-valved “sano shunt” | Sano suggested a further reduction in conduit size proposing a 4–5-mm non-valved conduit depending of patient’s weight. The conduit was first anastomosed on the distal stump of the transected MPA and then to a ventriculotomy about 2 cm below the pulmonary valve. |
| Barron et al., 2009 [30] | Left-sided vs right-sided placement | Sano described a left-sided placement of the RVPAS; however, it was found frequently require intervention which was difficult as it necessitated mobilization of PA across to the left side. In view of this, a right-sided placement was examined and found to be a safe technique with improved survival outcomes and PA growth. |
| Schreiber et al., 2009 [31] | Ring-enforced PTFE conduit | A ring-enforced PTFE conduit was developed to tackle a fairly common issue of proximal PTFE conduit stenoses. The rings provide a more stable structure to the conduit and prevents its substernal compression, consequently, it was found to reduce the incidence of stenoses and positively impact interstage mortality. |
| Tweddell et al., 2012 [32] | Dunk technique | Due to high work load of RV, its muscle may become hypertrophied resulting in stenosis of the RVPAS conduit. In efforts to prevent this, a ventriculotomy with a stab incision and no addition muscle resection was performed and about three rings of an enforced conduit are placed inside the RV cavity. Reintervention rates were higher with this technique, with potentially detrimental impact on long-term survival. |
| Saito et al., 2019 [33] | Anterior translocation of PA | A novel technique was recently proposed with prospects of providing a better surgical access to the PAs, which may facilitate intervention in case of poor PA growth after Norwood. |
These modifications have their pros and cons which have made them useful in certain circumstances. The current technique of neo-aortic reconstruction starts with meticulous excision of ductal tissue, complete mobilization of the descending aorta, and an incision performed in the lesser curvature of aorta until the level of transected MPA. An interdigitating anastomosis is utilized to join distal arch with descending aorta, ascending aorta and MPA are amalgamated with few interrupted sutures, and the entire neo-aorta is reconstructed with a hemi-pulmonary homograft [16, 18].
With respect to the pulmonary blood flow, mBTS was initially used by Norwood and still remains the preferred approach in several high-volume centers. It is a Gore-Tex graft (size based on weight 3 mm (< 2.5 kg), 3.5 mm (2.5–3.5 kg), and 4 mm (> 3.5 kg)) which connects the innominate artery to the right pulmonary artery (RPA). However, the seminal work of Sano et al. re-popularized the RVPAS modification, which Norwood described in his first report but soon abandoned it due to dismal results [29]. In the present day, RVPAS is accomplished with a ring-enforced polytetrafluoroethylene (PTFE) conduit (size based on weight 4 mm (< 2.5 kg), 5 mm (2.5–3.5 kg), and 6 mm (> 3.5 kg)) using the “dunk-technique” [31, 32]. These are crucial modifications to Sano’s RVPAS technique which have shown to reduce the incidence of proximal conduit stenosis and improve interstage mortality.
Single ventricle reconstruction trial
In the present day, there remains much debate regarding the “shunt of choice.” Theoretically, RVPAS is advantageous in that it abolishes “diastolic runoff,” a phenomenon which occurs post-mBTS Norwood. Diastolic runoff is the continuous antegrade flow into the pulmonary circulation during both systolic and diastolic cardiac cycle, thereby compromising coronary filling during the diastole resulting in impairment of myocardial perfusion. This phenomenon is known as “coronary steal” [34]. The elimination of diastolic runoff has several advantages including, but not limited to, stable postoperative period and decreased interstage mortality.
Several reports have compared the short- and long-term outcomes of mBTS and RVPAS in order to identify the best choice. However, these studies have been mainly non-randomized reports with limited follow-up and demonstrated conflicting results. To solve this issue, Ohye and several collaborators within the Pediatric Heart Network Investigators of North America organized the “single ventricle reconstruction” (SVR) trial. In which, 549 HLHS patients were randomized to undergo Norwood procedure with either mBTS or RVPAS in 15 centers with the primary outcome measure as death or cardiac transplantation at 12 months. In 2010, Ohye and colleagues published the initial data derived from the SVR trial [34]. Their analysis demonstrated that patients with RVPAS had significantly better transplant-free survival at 12 months than with mBTS. However, no such difference between the two arms was noticed after the 12-month period or even at 3-year follow-up [35]. Additionally, mBTS was found to be more beneficial with regard to lower postoperative complications and better pulmonary artery (PA) growth and preservation of right ventricular function. In 2018, the same collaborative published the results of their cohort after a follow-up period of 6 years—SVR extension study (SVR II) [36]. The primary findings were identical to SVR I such that transplant-free survival at 6 years was not statistically significant in favor of either shunt type. However, they found that the RVPAS cohort had a 5% higher transplant-free survival and a survival advantage prior to stage 2, albeit, needing more catheter interventions compared with mBTS group. Interestingly, this recent analysis highlighted an interaction between shunt type and annual Norwood volume of the performing surgeon, with more experienced surgeons producing better outcomes with mBTS. The SVR collaborative intends to continue their surveillance of this cohort and report their even longer term outcomes and outline suggestions accordingly, truly, intending to be a “gift that keeps on giving” [37].
Latest S1P outcomes
Since the conception of the Norwood procedure, the survival of neonates with HLHS into infancy has upgraded with every era. A recently published article analyzed the institution’s experience with 1663 neonates who underwent Norwood procedure from 1984 to 2014 [38]. They divided this period into 6 eras and presented that the mortality rates had dropped from 40.4% in 1984–1988 era to 15.7% in 2009–2013 era. Although these results have been mainly promising, the survival rates in the past 15 years have plateaued despite improvement in treatment and management modalities. Mascio and associates also expressed that having > 3 patient-specific risk factors were the major determinants of post-S1P mortality in the latest era. The flattening of survival rates in the recent eras is probably due to more high-risk cases undergoing surgical palliation as compared with the older era when these cases may have succumbed to the disease before such referral. The latest analysis of the “Society of Thoracic Surgeons”(STS) congenital heart surgery database revealed that mortality subsequent to Norwood procedure is at 15% [39]. To deal with these high-risk cases hindering further improvement in survival, some alternatives to Norwood procedure have been proposed and utilized.
Alternatives for S1P
As an alternative to Norwood operation, a hybrid procedure combining interventional catheterization and off-pump surgery was proposed by Gibbs et al. in 1993 [40]. It involves Bilateral PA banding, stenting of ductus arteriosus and atrial septectomy. Its primary rationale was to avoid cardiopulmonary bypass (CPB), the use of which has been shown to inflict ischemic damage to vital organs when used in the neonatal period. It was considered to be less intensive, thereby, being a better approach for neonates at high risk of developing ischemic sequelae. The data from some single-center studies has suggested the hybrid procedure as an alternative to the standard Norwood [41, 42]. This has led to the adoption of the hybrid procedure as the primary approach in several centers irrespective of risk stratification, based on the reasoning that it postpones major surgery to an older age and avoids CPB insults in early life. This theoretical advantage, however, comes with drawback of a technically difficult and comprehensive stage 1 + 2, during which removal of the ductal stent becomes the cause of major concern along with the need for pulmonary arterioplasty subsequent branch PA banding. In addition, current evidence shows that the hybrid approach is associated with higher reintervention rates and smaller PA growth in comparison with Norwood while not significantly altering survival [43].
Nevertheless, the principle of the hybrid procedure may be utilized as a bridge to stage 1 Norwood in high-risk HLHS neonates, who are deemed extremely unstable to undergo surgical and CPB insult. “Hybrid-bridge-to-Norwood” or “salvage-bridge-to-Norwood” is a sequence which begins with bilateral PA banding soon after birth to delay the Norwood procedure for a more opportune time. It is accompanied by administration of prostaglandin E1 to maintain ductal patency if necessary. In contrast to the hybrid procedure, ductal stenting and atrial septostomy is usually not performed in this modified sequence. Once the neonate is hemodynamically stable, the standard Norwood procedure is undertaken, usually after the neonatal period. This approach has shown promising results in cases where the only other alternative would be definite demise. It is also being utilized as the primary approach, especially in Japan, where bilateral PA banding is offered to > 50% of cases. The analysis of a Japanese database revealed that 5-year survival with primary PA banding was comparable with Norwood but only in high-volume centers [44].
Interstage period
With increasing experience, improvements in surgical techniques and perioperative care, and introduction of prenatal diagnosis, in-hospital survival following S1P has dramatically improved [37]. Nevertheless, the period of even greater concern for these ailing children comes after they have been successfully discharged post-S1P.
The period of time from hospital discharge after S1P until readmission for stage II surgery is commonly referred to as the interstage period (IP). The risk of mortality during this period is considerably high (2.7–16%) [45, 46]. To reduce interstage mortality (ISM), research has focused on determining risk factors (patient-specific, clinical, surgical, and socioeconomic) which may be targeted to decrease ISM. These risk factors are outlined in (Table 3).
Table 3.
Risk factors for interstage mortality (ISM)
| Risk factors for ISM | |
|---|---|
| Patient-specific risk factors |
Low birth weight (< 2.5 kg) Prematurity (born at ≤ 37 weeks’ gestation) Presence of genetic syndromes |
| Clinical factors |
Postoperative complications (arrhythmias, RV dysfunction) Unplanned intervention during IP Prolonged ICU stay (> 30 days) following S1P Longer duration of ventilation |
| Surgical factors |
Presence of mBTS Greater age at S1P Low weight at S1P Longer aortic cross clamp time |
| Socioeconomic factors |
Teenage mother Single adult caregiver Low education or income level |
Yet another major contribution in management of HLHS patients was made by Ghanayem et al., who reported their experience with a coordinated home monitoring program (HMP) during the IP [47]. They demonstrated an exceptional survival advantage following the initiation of their program, reporting a decline in ISM from 15 to 0%. A review on the role of HMPs indicated that they have the potential to reduce ISM to > 3% [48]. To the contrary, however, a large, multicenter study conducted through the National Pediatric Cardiology Quality Improvement Collaborative (NPC-QIC) database was unable to establish such association of HMPs with mortality, heart transplantation, or unscheduled readmissions [49], although they did notice that HMPs were beneficial in improving weight gain in this period which is sensitive to growth failure. The available literature regarding HMPs is suggestive that they have a definite place in HLHS care [50]. The essential aspects of HMP and other IP interventions to reduce ISM are highlighted in (Table 4).
Table 4.
Interventions for decreasing interstage mortality (ISM)
| Interventions for decreasing ISM | |
|---|---|
|
Home nursing visits Effective care transition - Parental education initiatives prior to hospital discharge - Providing parents with written plan regarding feeding, medications, red flags. - Establishing contact of the family with the care team (cardiologist, nutritionist, speech therapists, specialist nurse) Home monitoring program - Use of infant weight scale and pulse oximeter - Informing parents about the following: o How to use these tools? o How to identify signs of growth failure, dehydration, or desaturation (“red flags”)? o Who to contact in case of change in clinical status of the child? - High degree of parental involvement o Daily measurement and recording of weight and SpO2 in a log book. |
What is After Norwood?
Stage 2 palliation
After successful survival through the IP, children with HLHS require additional 2 stages of surgical intervention for easing the work load on RV of supporting both pulmonary and systemic circulations in parallel. Stages 2 and 3 procedures are aimed at converting the post-Norwood physiology such that blood flows in series through both circulatory systems, effectively, “unloading” the RV and leaving it to support only the systemic circulation.
In the staged surgical approach, superior cavopulmonary anastomosis constitutes the second stage. It reduces the work load of the RV by diverting venous return from the upper body to flow directly into the pulmonary circulation. The optimal time for undertaking this procedure is usually at 4–6 months of age [51]. By then, the high perinatal PVR drops down to low enough levels which would permit systemic venous blood to passively flow through the cavopulmonary anastomosis. Currently, the bi-directional Glenn (BDG) and the hemi-Fontan (HF) are two surgical approaches used for creating this connection. BDG involves dividing the superior vena cava (SVC) at the SVC-right atrium (RA) junction, closing off the atrial end and anastomosing SVC to the right PA (RPA) in an end-to-side manner. In the HF approach, the SVC-RA junction is occluded with a patch and the junction is connected to the RPA such that SVC return flows directly into the PAs. The choice of approach is left at the surgeon’s discretion as excellent results are achievable with either approach.
Following the completion of the second palliative stage, a few crucial improvements in the cardiac physiology are expected to occur. Firstly, the RV “unloading” leads to a reduced end diastolic volume, which in turn decreases ventricular wall stress and results in improved ventricular function with reduced atrioventricular regurgitation. Secondly, the systemic-to-pulmonary shunt is taken down during stage 2 palliation (S2P) which reduces the potential diastolic runoff further improving coronary perfusion and diastolic pressure. The present-day outcomes for S2P have been predominantly excellent with several institutions reporting long-term survival at almost 87% with either approaches [52, 53].
Stage 3 palliation
There are two surgical approaches routinely used for the Fontan procedure—lateral tunnel Fontan (LTF) and extracardiac cardiac Fontan (ECF). It is important to note that S2P determines the approach used in stage 3. HF requires construction of an LTF at stage 3—which involves excising the SVC-RA junction occlusion patch and sewing a PTFE in RA to reroute venous return from inferior vena cava (IVC) towards PA. If a BDG is performed at S2P, stage 3 is accomplished with an ECF which connects the IVC to the RPA using a PTFE conduit. The occurrence of arrhythmias and other long-term complications remains the major cause of concern with these approaches with some reports suggesting that ECF may be more advantageous that LTF in this regard [54, 55]. However, there is no significant difference in early or late mortality between ECF and LTF [55, 56]. In patients at high risk of Fontan failure, small fenestrations may be created in the Fontan circuit to provide another source to fill the single ventricle, although with desaturated venous blood [57]. The application of fenestration is especially beneficial in patients with higher pulmonary vascular pressures; however, its use in standard-risk patients is still controversial.
Stage 4—transplant
In the management of HLHS, cardiac transplantation may be utilized at any stage of palliation as a rescue therapy. Initially, some institutions offered the option of cardiac transplantation as the primary approach for severe variants of HLHS with poor RV function [58]. But shortages of donor hearts and improved survival with staged reconstruction have hindered the acceptance of this approach as the standard. Cardiac transplantation may also be indicated in cases where reconstructive surgery fails, patient deteriorates between stages or risk factors such as poor RV function, and moderate-severe tricuspid regurgitation among others prevents progression to the next stage. However, the need for transplant mainly arises several years after Fontan completion owing to the late complications of the Fontan physiology [59]. Cardiac transplantation may be used as last report in these cases, although mortality during the waiting period and 1 year after transplantation remains considerably high for HLHS recipients in comparison with other pediatric recipients [60].
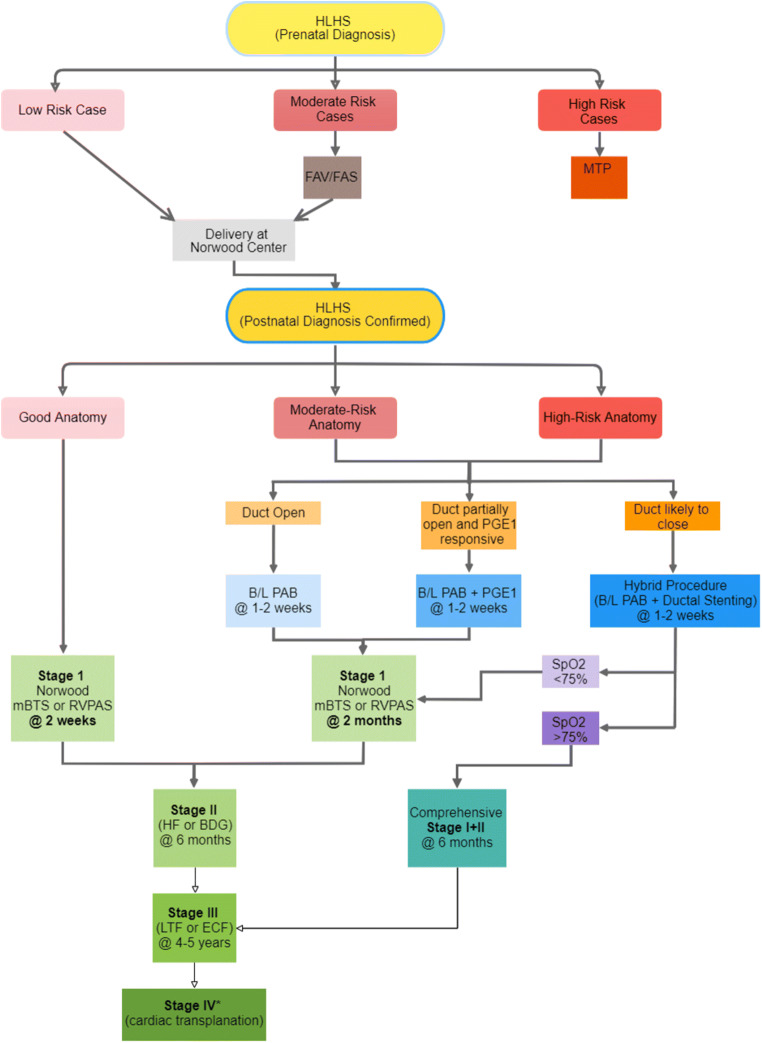
The current recommendation of surgical algorithm based on risk stratification and HLHS morphology are summarized in Fig. 3.
Fig. 3.
Staged palliative approach based on risk stratification and HLHS morphology. MTP, medical termination of pregnancy; PAB, pulmonary artery banding; PGE1, prostaglandin E1; SpO2, oxygen saturation; the asterisk indicates that cardiac transplantation may also be used as an option in case of patient’s clinical deterioration in between stages
Future directions
Even though CHD has become a leading indication for cardiac transplant, the waiting period tends to be longer for these patients than non-CHD cases. Therefore, as a bridge-to-transplantation, options such as ventricular assist device (VAD) are being considered [61]. In the CHD cohort, several factors such as complex anatomy, multiple prior surgeries, significant comorbidities, and an inherent risk of heart failure present significant challenges in the application of VAD. Nonetheless, VAD support offers advantages such as extended palliative support, possibility of extubation and hospital discharge, and prospects of rehabilitation. Berlin EXCOR, a VAD, has been shown to be safe and effective in the pediatric patients with multiple reports investigating its utility in this population demonstrating longer duration of support [62].
With the ever-advancing field of medicine, futuristic options for tackling concerns of cardiac dysfunction and heart failure are being introduced in HLHS management such as autologous stem cell therapy [63]. A clinical trial initiated by the University of Oklahoma has shown encouraging results with implantation of autologous umbilical cord blood-derived mononuclear cells directly into RV myocardium. In phase 1 trial, 10 HLHS patients received this experimental treatment during their S2P. The results have been promising with preservation of RV function and normal growth during the follow-up period suggesting a possibly safe and feasible modern modality to further enhance the survival of HLHS children [64].
Conclusion
The medical fraternity has come a long way since the initial description of the HLHS spectrum. Prominent contributions from exceptional surgeons such as William Norwood, Shunji Sano, Edward Bove, and Thomas Spray have paved the way for the continuously enhancing care and management of these children, besides completely transforming the prognosis of HLHS. These children are now such enabled that survival into 40s–50s is no more a far-fetched dream but a reality, with their life quality being more remarkable than would have been expected in the past. This feat is further emphasized by a report of HLHS patients who underwent the grueling staged palliation process and was able to have successful pregnancies in their 20s. At the same time, our objective should still focus on further advancing our techniques and modalities as well as towards identifying the most effective management approaches to achieve even better results in this extraordinary cohort.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Noonan JA, Nadas AS. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am. 1958;5:1029–1056. doi: 10.1016/s0031-3955(16)30727-1. [DOI] [PubMed] [Google Scholar]
- 2.Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest. 1952;1:61–70. [PubMed] [Google Scholar]
- 3.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–343. doi: 10.1016/0167-5273(89)90226-x. [DOI] [PubMed] [Google Scholar]
- 4.Chin AJ, Weinberg PM, Barber G. Subcostal two-dimensional echocardiographic identification of anomalous attachment of septum primum in patients with left atrioventricular valve underdevelopment. J Am Coll Cardiol. 1990;15:678–681. doi: 10.1016/0735-1097(90)90645-6. [DOI] [PubMed] [Google Scholar]
- 5.Makikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- 6.Graupner O, Enzensberger C, Wieg L, et al. Evaluation of right ventricular function in fetal hypoplastic left heart syndrome by color tissue Doppler imaging. Ultrasound Obstet Gynecol. 2016;47:732–738. doi: 10.1002/uog.14940. [DOI] [PubMed] [Google Scholar]
- 7.Kovacevic A, Öhman A, Tulzer G, et al. Fetal hemodynamic response to aortic valvuloplasty and postnatal outcome: a European multicenter study. Ultrasound Obstet Gynecol. 2018;52:221–229. doi: 10.1002/uog.18913. [DOI] [PubMed] [Google Scholar]
- 8.Moon-Grady AJ, Morris SA, Belfort M, et al. International fetal cardiac intervention registry: A worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol. 2015;66:388–399. doi: 10.1016/j.jacc.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Schidlow DN, Freud L, Friedman K, Tworetzky W. Fetal interventions for structural heart disease. Echocardiography. 2017;34:1834–1841. doi: 10.1111/echo.13667. [DOI] [PubMed] [Google Scholar]
- 10.Jantzen DW, Moon-Grady AJ, Morris SA, et al. Hypoplastic left heart syndrome with intact or restrictive atrial septum: A report from the international fetal cardiac intervention registry. Circulation. 2017;136:1346–1349. doi: 10.1161/CIRCULATIONAHA.116.025873. [DOI] [PubMed] [Google Scholar]
- 11.Lara DA, Morris SA, Maskatia SA, et al. Pilot study of chronic maternal hyperoxygenation and effect on aortic and mitral valve annular dimensions in fetuses with left heart hypoplasia. Ultrasound Obstet Gynecol. 2016;48:365–372. doi: 10.1002/uog.15846. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph AM. Maternal hyperoxygenation for the human fetus: should studies be curtailed? Pediatr Res. 2019. 10.1038/s41390-019-0604-4. [DOI] [PubMed]
- 13.Theilen U, Shekerdemian L. The intensive care of infants with hypoplastic left heart syndrome. Arch Dis Child Fetal Neonatal Ed. 2005;90:F97–102. doi: 10.1136/adc.2004.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayler GG, Smeloff EA, Miller GE., Jr Surgical palliation of hypoplastic left side of the heart. N Engl J Med. 1970;282:780–783. doi: 10.1056/NEJM197004022821405. [DOI] [PubMed] [Google Scholar]
- 15.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–26. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 16.Pigott JD, Murphy JD, Barber G, Norwood WI. Palliative reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg. 1988;45:122–128. doi: 10.1016/s0003-4975(10)62420-4. [DOI] [PubMed] [Google Scholar]
- 17.Gargiulo G, Pace Napoleone C, Solinas M, Frascaroli G, Pierangeli A. A new patch for the Norwood procedure. Ann Thorac Surg. 1999;68:1873–1874. doi: 10.1016/s0003-4975(99)01013-9. [DOI] [PubMed] [Google Scholar]
- 18.Burkhart HM, Ashburn DA, Konstantinov IE, et al. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:61–65. doi: 10.1016/j.jtcvs.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Healy DG, Nolke L, Wood AE. Bovine jugular vein as a shaped alternative patch material for aortic augmentation in the Norwood procedure. J Thorac Cardiovasc Surg. 2007;133:567–568. doi: 10.1016/j.jtcvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 20.Sinha P, Moulick A, Jonas RA. Femoral vein homograft for neoaortic reconstruction in Norwood stage 1 operation. Ann Thorac Surg. 2009;87:1309–1310. doi: 10.1016/j.athoracsur.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Bernabei M, Margaryan R, Arcieri L, Bianchi G, Pak V, Murzi B. Aortic arch reconstruction in newborns with an autologous pericardial patch: Contemporary Results. Interact Cardiovasc Thorac Surg. 2013;16:282–285. doi: 10.1093/icvts/ivs510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen RM, Mitchell ME, Woods RK, Loomba RS, Tweddell JS. Porcine small intestinal submucosa may be a suitable material for Norwood arch reconstruction. Ann Thorac Surg. 2018;106:1847–1852. doi: 10.1016/j.athoracsur.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Bu’Lock FA, Stumper O, Jagtap R, et al. Surgery for infants with a hypoplastic systemic ventricle and severe outflow obstruction: Early results with a modified Norwood procedure. Br Heart J. 1995;73:456–461. doi: 10.1136/hrt.73.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung SC, Chang YH, Lee HD, Ban JE, Choo KS. Technical modification of the neoaortic reconstruction in Norwood procedure: Sparing of anterior wall of the main pulmonary artery. J Card Surg. 2009;24:437–439. doi: 10.1111/j.1540-8191.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- 25.Asada S, Yamagishi M, Itatani K, Yaku H. Chimney reconstruction of the aortic arch in the Norwood procedure. J Thorac Cardiovasc Surg. 2017;154:e51–e54. doi: 10.1016/j.jtcvs.2017.04.079. [DOI] [PubMed] [Google Scholar]
- 26.Plunkett MD, Bond LM, Geiss DM. Use of the “chimney patch” technique for Norwood stage I procedures. Ann Thorac Surg. 1998;66:1438–1439. doi: 10.1016/s0003-4975(98)00743-7. [DOI] [PubMed] [Google Scholar]
- 27.Tam VK, Murphy K, Parks WJ, et al. Saphenous vein homograft: A superior conduit for the systemic arterial shunt in the Norwood operation. Ann Thorac Surg. 2001;71:1537–1540. doi: 10.1016/s0003-4975(01)02467-5. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto H, Kawahira Y, Kawata H, Miura T, Iwai S, Mori T. The modified norwood palliation on a beating heart. J Thorac Cardiovasc Surg. 1999;118:1130–1132. doi: 10.1016/S0022-5223(99)70118-2. [DOI] [PubMed] [Google Scholar]
- 29.Sano S, Ishino K, Kawada M, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–509. doi: 10.1016/s0022-5223(02)73575-7. [DOI] [PubMed] [Google Scholar]
- 30.Barron DJ, Brooks A, Stickley J, et al. The Norwood procedure using a right ventricle-pulmonary artery conduit: Comparison of the right-sided versus left-sided conduit position. J Thorac Cardiovasc Surg. 2009;138:528–537. doi: 10.1016/j.jtcvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber C, Kasnar-Samprec J, Hörer J, et al. Ring-enforced right ventricle-to-pulmonary artery conduit in Norwood stage I reduces proximal conduit stenosis. Ann Thorac Surg. 2009;88:1541–1545. doi: 10.1016/j.athoracsur.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 32.Tweddell JS, Mitchell ME, Woods RK, Spray TL, Quintessenza JA. Construction of the right Ventricle-to-PA conduit in the Norwood: the “Dunk” technique. Oper Tech Thorac Cardiovasc Surg. 2012;17:81–98. [Google Scholar]
- 33.Saito T, Aoki M, Hagino I. Norwood operation with anterior translocation of pulmonary artery. Ann Thorac Surg. 2019;108:e387–e388. doi: 10.1016/j.athoracsur.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 34.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newburger JW, Sleeper LA, Frommelt PC, et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newburger JW, Sleeper LA, Gaynor JW, et al. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. doi: 10.1161/CIRCULATIONAHA.117.029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaquiss RDB. The single ventricle reconstruction trial: The gift that keeps on giving. J Thorac Cardiovasc Surg. 2016;151:676–677. doi: 10.1016/j.jtcvs.2015.09.095. [DOI] [PubMed] [Google Scholar]
- 38.Mascio CE, Irons ML, Ittenbach RF, et al. Thirty years and 1663 consecutive Norwood procedures: Has survival plateaued? J Thorac Cardiovasc Surg. 2019;158:220–229. doi: 10.1016/j.jtcvs.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs JP, Mayer JE, Pasquali SK, et al. The Society of Thoracic Surgeons congenital heart surgery database: 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:691–704. doi: 10.1016/j.athoracsur.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: A new approach to palliation for the hypoplastic left heart syndrome. Br Heart J. 1993;69:551–555. doi: 10.1136/hrt.69.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd DF, Cutler L, Tibby SM, et al. Analysis of preoperative condition and interstage mortality in Norwood and hybrid procedures for hypoplastic left heart syndrome using the Aristotle scoring system. Heart. 2014;100:775–780. doi: 10.1136/heartjnl-2013-304759. [DOI] [PubMed] [Google Scholar]
- 42.Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2070. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Sower CT, Romano JC, Yu S, Lowery R, Pasquali SK, Zampi JD. Early and mid-term outcomes in high-risk single-ventricle patients: hybrid versus Norwood palliation. Ann Thorac Surg. 2019;108:1849–1855. doi: 10.1016/j.athoracsur.2019.06.061. [DOI] [PubMed] [Google Scholar]
- 44.Hirata Y, Miyata H, Hirahara N, et al. Long-term results of bilateral pulmonary artery banding versus primary Norwood procedure. Pediatr Cardiol. 2018;39:111–119. doi: 10.1007/s00246-017-1735-1. [DOI] [PubMed] [Google Scholar]
- 45.Pizzuto M, Patel M, Romano J, et al. Similar interstage outcomes for single ventricle infants palliated with an aortopulmonary shunt compared to the Norwood procedure. World J Pediatr Congenit Heart Surg. 2018;9:407–411. doi: 10.1177/2150135118768720. [DOI] [PubMed] [Google Scholar]
- 46.Alsoufi B, McCracken C, Kochilas LK, Clabby M, Kanter K. Factors associated with interstage mortality following neonatal single ventricle palliation. World J Pediatr Congenit Heart Surg. 2018;9:616–623. doi: 10.1177/2150135118787723. [DOI] [PubMed] [Google Scholar]
- 47.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 48.Ugonabo N, Hirsch-Romano JC, Uzark K. The role of home monitoring in interstage management of infants following the Norwood procedure. World J Pediatr Congenit Heart Surg. 2015;6:266–273. doi: 10.1177/2150135114563771. [DOI] [PubMed] [Google Scholar]
- 49.Oster ME, Ehrlich A, King E, et al. Association of interstage home monitoring with mortality, readmissions, and weight gain: a multicenter study from the national pediatric cardiology quality improvement collaborative. Circulation. 2015;132:502–508. doi: 10.1161/CIRCULATIONAHA.114.014107. [DOI] [PubMed] [Google Scholar]
- 50.Anderson JB, Beekman RH, Kugler JD, et al. Improvement in interstage survival in a national pediatric cardiology learning network. Circ Cardiovasc Qual Outcomes. 2015;8:428–436. doi: 10.1161/CIRCOUTCOMES.115.001956. [DOI] [PubMed] [Google Scholar]
- 51.Meza JM, Hickey EJ, Blackstone EH, et al. The optimal timing of stage 2 palliation for hypoplastic left heart syndrome :an analysis of the pediatric heart network single ventricle reconstruction trial public data set. Circulation. 2017;136:1737–1748. doi: 10.1161/CIRCULATIONAHA.117.028481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pająk J, Buczyński M, Stanek P, et al. Preoperative single ventricle function determines early outcome after second-stage palliation of single ventricle heart. Cardiovasc Ultrasound. 2017;15:21. doi: 10.1186/s12947-017-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichay NR, Gorbatykh YN, Kornilov IA, et al. Risk factors for unfavorable outcomes after bidirectional cavopulmonary anastomosis. World J Pediatr Congenit Heart Surg. 2017;8:575–583. doi: 10.1177/2150135117728505. [DOI] [PubMed] [Google Scholar]
- 54.Zheng J, Li Z, Li Q, Li X. Meta-analysis of Fontan procedure: Extracardiac conduit vs. intracardiac lateral tunnel. Herz. 2018;43:238–245. doi: 10.1007/s00059-017-4553-6. [DOI] [PubMed] [Google Scholar]
- 55.Lin Z, Ge H, Xue J, et al. Comparison of extracardiac conduit and lateral tunnel for functional single-ventricle patients: A meta-analysis. Congenit Heart Dis. 2017;12:711–720. doi: 10.1111/chd.12503. [DOI] [PubMed] [Google Scholar]
- 56.Rijnberg FM, Blom NA, Sojak V, et al. A 45-year experience with the Fontan procedure: tachyarrhythmia, an important sign for adverse outcome. Interact Cardiovasc Thorac Surg. 2019;29:461–468. doi: 10.1093/icvts/ivz111. [DOI] [PubMed] [Google Scholar]
- 57.Lemler MS, Scott WA, Leonard SR, Stromberg D, Ramaciotti C. Fenestration improves clinical outcome of the Fontan procedure: a prospective, randomized study. Circulation. 2002;105:207–212. doi: 10.1161/hc0202.102237. [DOI] [PubMed] [Google Scholar]
- 58.Razzouk AJ, Chinnock RE, Gundry SR, et al. Transplantation as a primary treatment for hypoplastic left heart syndrome: Intermediate-term results. Ann Thorac Surg. 1996;62:1–7. doi: 10.1016/0003-4975(96)00295-0. [DOI] [PubMed] [Google Scholar]
- 59.Frigiola A, Lo RM. Late complications of Fontan’s operation. G Ital Cardiol. 2017;18:625–630. doi: 10.1714/2741.27945. [DOI] [PubMed] [Google Scholar]
- 60.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Hear Lung Transplant. 2009;28:1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Niebler RA, Ghanayem NS, Shah TK, et al. Use of a HeartWare ventricular assist device in a patient with failed Fontan circulation. Ann Thorac Surg. 2014;97:e115–e116. doi: 10.1016/j.athoracsur.2013.11.075. [DOI] [PubMed] [Google Scholar]
- 62.Fraser CD, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367:532–541. doi: 10.1056/NEJMoa1014164. [DOI] [PubMed] [Google Scholar]
- 63.Bittle GJ, Morales D, Deatrick KB, et al. Stem cell therapy for hypoplastic left heart syndrome mechanism, clinical application, and future directions. Circ Res. 2018;123:288–300. doi: 10.1161/CIRCRESAHA.117.311206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burkhart HM, Qureshi MY, Rossano JW, et al. Autologous stem cell therapy for hypoplastic left heart syndrome: Safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg. 2019;158:1614–1623. doi: 10.1016/j.jtcvs.2019.06.001. [DOI] [PubMed] [Google Scholar]