Abstract
Introduction
Treat-to-target strategies are used in several chronic diseases to improve outcomes. Treatment goals have also been suggested for psoriasis, but there is currently no consensus on targets, and guidance is needed to implement this strategy in clinical practice. The project ‘Treat to Target Italia’ was launched by a scientific board (SB) of 10 psoriasis experts to generate expert consensus recommendations.
Methods
On the basis of the published literature, their clinical experience, and the results of a survey among Italian dermatologists, the SB identified four relevant topics: (1) clinical remission; (2) quality of life; (3) abrogation of systemic inflammation; (4) safety. They drafted 20 statements addressing these four topics and submitted them to a panel of 28 dermatologists, in a Delphi process, to achieve consensus (greater than 80% agreement).
Results
Consensus was reached on all statements. Treatment goals defining clinical remission should include a 90% improvement from baseline in the Psoriasis Area and Severity Index (PASI90 response) or an absolute PASI score of less than or equal to 3. Patient’s quality of life and satisfaction are important targets. If PASI targets are achieved, there should be no or very low impact of psoriasis on quality of life [Dermatology Life Quality Index (DLQI) score less than or equal to 3]. If PASI or DLQI goals are not achieved within 3–4 months, treatment should be changed. Abrogation of systemic inflammation may be crucial for preventing or delaying inflammatory comorbidities. Safety is an equally important target as efficacy.
Conclusion
These 20 consensus statements define the parameters of a treat-to-target strategy for psoriasis in Italy. It is hoped that use of these in the management of patients with psoriasis will improve treatment outcomes and patient health-related quality of life.
Keywords: Consensus, Plaque psoriasis, Quality of life, Systemic inflammation, Treat-to-target
Key Summary Points
| Why carry out this study? |
| Patients with moderate-to-severe psoriasis suffer from negative impacts on their health-related quality of life (HRQoL) and significant psychosocial disability. |
| Despite the availability of effective systemic therapy for these patients, many are undertreated, with a global study indicating that nearly 60% of patients fail to reach treatment goals. |
| A consensus-based treat-to-target approach in psoriasis may better guide clinicians, leading to improved treatment outcomes and patient HRQoL. |
| The ‘Treat to Target Italia’ project was undertaken by 10 psoriasis experts who developed 20 statements based on a literature review and results of a survey of Italian dermatologists; these statements were then reviewed by a panel of 28 dermatologists using the Delphi process to achieve consensus. |
| What was learned from the study? |
| Consensus was reached on all statements, including those on treatment goals defining remission: a 90% improvement from baseline in the Psoriasis Area and Severity Index (PASI90 response) or an absolute PASI score of less than or equal to 3. |
| Dermatologists easily agreed on the treat-to-target strategy for patients with psoriasis that was patient-centred with emphasis on objective measures of disease severity and patient HRQoL, and on treatment safety. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13317611.
Introduction
Psoriasis is a chronic, immune-mediated inflammatory disease of the skin frequently encountered in clinical practice, with plaque-type psoriasis being the most prevalent clinical form [1–3]. The type and severity of clinical manifestations are highly variable, but it is now widely recognised that the cutaneous manifestations represent one part of a complex disease phenotype [4, 5]. Furthermore, chronic plaque psoriasis is often associated with comorbidities that are typically characterised by systemic inflammation, such as psoriatic arthritis [6], atherosclerosis [7], metabolic syndrome [8] and obesity [8], which are known to increase the risk of myocardial infarction [9] and stroke [10].
Moderate-to-severe psoriasis causes significant psychosocial disability and negatively impacts patient health-related quality of life (HRQoL) [11, 12], increasing the risk of psychiatric comorbidities, such as depression and anxiety [13].
Patients with moderate-to-severe psoriasis are eligible for systemic therapies [14], including conventional systemic therapies and biologicals. The prescription of biological therapy is restricted to hospital-based dermatologists in Italy. Biological therapies selectively targeting mediators of psoriasis pathogenesis (including tumour necrosis factor α [TNFα], both interleukin [IL]-12 and IL-23, IL-17, and IL-23 alone) have proven to be effective and well tolerated [15–24]. Clinical trials with these drugs have shown that a significant proportion of patients can achieve a 90% or 100% decrease of their baselines Psoriasis Area and Severity Index (PASI) scores (PASI90 or PASI100 response, respectively) [15–24]. These findings, along with a recognition of the need to manage the heterogeneous manifestations of psoriasis, have recently led to ambitious goals of treatment, such as the achievement of PASI90 or PASI100 responses, or an absolute physician’s global assessment (PGA) score of 0–1 (clear/almost clear skin). These targets are now considered feasible for patients receiving treatment for moderate-to-severe plaque psoriasis in clinical practice [25–30]. In parallel, the possibility of implementing a treat-to-target approach to the management of psoriasis has raised considerable interest among dermatologists [25–27, 30–33].
Various treatment targets have also been suggested for the management of psoriasis [25, 27, 30–34]. For example, according to current Italian guidelines on the systemic treatment of moderate-to-severe plaque psoriasis, clear or almost clear skin is the ultimate goal of treatment and a PASI90 response is regarded as the most relevant treatment outcome [32]. Achieving an absolute PASI score of 1–2 may also be relevant according to these guidelines [26, 32].
Despite these efforts, a treat-to-target approach is being inconsistently applied in dermatological clinical practice. Data from several studies indicate that the treatment of psoriasis continues to be suboptimal, with substantial proportions of patients with moderate-to-severe psoriasis not receiving any therapy or receiving topical treatment only [35–37].
The treat-to-target approach to the management of psoriatic disease is still evolving, and requires clear guidance for physicians on the treatment goal, for both the cutaneous and other manifestations of psoriasis. The strategy also needs to be patient-centric, and not just the pursuit of clear skin at any cost. Patient’s HRQoL needs to be considered, along with their comorbidities, the adverse effects of treatment and treatment preferences [38]. The project ‘Treat to Target Italia’ was launched by a group of psoriasis experts and was prompted by the need to develop recommendations for guiding dermatologists in the treatment-to-target of psoriasis in clinical practice in Italy. In particular, the project addressed the following four topics: (1) clinical remission of psoriasis; (2) patient HRQoL; (3) abrogation of systemic inflammation; and (4) safety of treatment. We present the results of the project and a set of 20 consensus statements addressing issues related to the four domains.
Methods
Design
The ‘Treat to Target Italia’ project was launched in 2019 by a group of 10 Italian experts in psoriasis, who acted as the scientific board of the project. The aim of the project was to define the therapeutic objectives in the management of patients with psoriasis in clinical practice. More detailed objectives included identifying a therapeutic target and assessment of this target over time; establishing the time to the achievement of the target; identification of practice-oriented efficacy measures to improve disease staging and follow-up; understanding the correlation between disease state and HRQoL; defining personalised therapeutic targets; and describing the optimal timing of reassessments to ensure long-term maintenance of the results achieved. The scientific board drafted a set of evidence- and consensus-based statements regarding therapeutic targets in psoriasis treatment and chose the Delphi method for consensus methodology [39, 40].
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Development of Consensus Statements
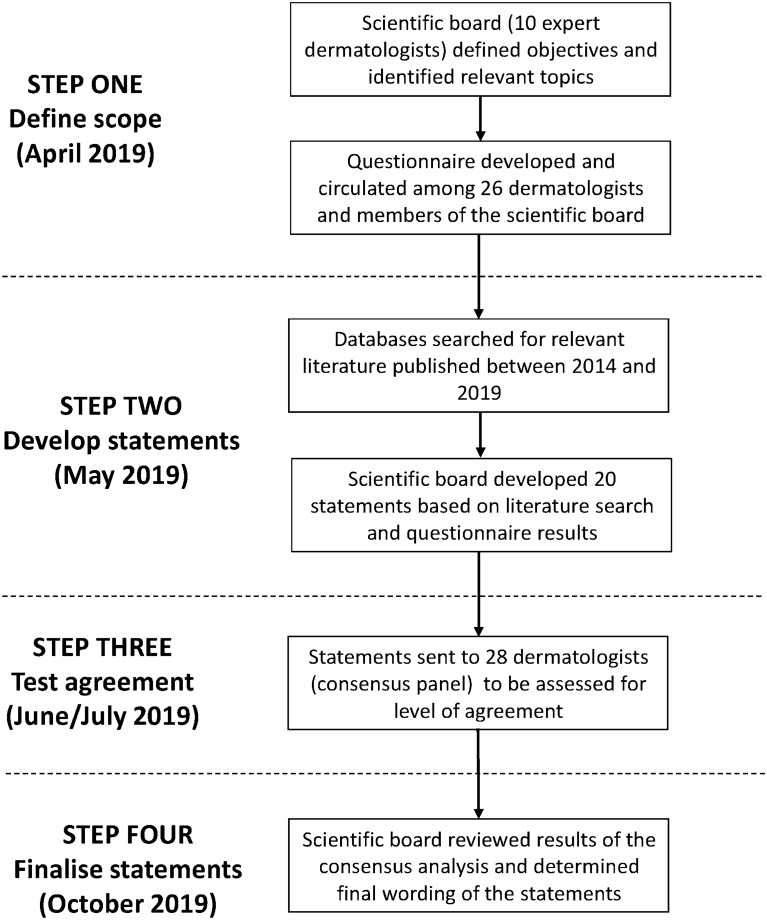
The consensus methodology is shown in Fig. 1. It consisted of a four-step process conducted between April 2019 and October 2019. The first step was to define the scope of the project. The scientific board met first in April 2019 in Rome to define the objectives of the ‘Treat to Target Italia’ project and identified topics relevant to the targeted treatment of psoriasis, based on published evidence and their expertise, namely (1) clinical remission of psoriasis; (2) patient HRQoL; (3) abrogation of systemic inflammation; and (4) safety of treatment. The scientific board also designed the strategy for searching the literature related to these topics (see below) and developed a survey to gauge the opinion of Italian dermatologists about the targets of psoriasis treatment. A 25-item questionnaire was developed and sent via e-mail to a panel of 26 dermatologists, as well as to each member of the scientific board (April–May 2019). The surveyed dermatologists were selected on the basis of their recognised expertise in the management of patients with moderate-to-severe psoriasis..
Fig. 1.
Illustration of the consensus methodology consisting of a four-step process and including a Delphi exercise
Step 2 of the consensus development process was to make statements based on the survey results and literature review. Literature was identified by searching the EBM Reviews, Cochrane Database of Systematic Reviews, Embase and MEDLINE databases for articles published in English between January 2014 and April 2019. The search involved various combinations of terms related to “inflammation”, “clinical remission”, “patient satisfaction/quality of life”, “safety” and “psoriasis”. A second meeting of the scientific board was held at the end of May 2019 in Milan to develop a set of statements covering the four topics, which had been previously identified as relevant for the treat-to-target approach. To draft the statements, the scientific board relied on their expertise, the evidence from the published literature, and the results of the preliminary survey among Italian dermatologists. Each statement was extensively discussed during the meeting. A total of 20 statements (nine for the topic “Clinical remission”, three for “Patient quality of life”, five for “Abrogation of inflammation”, three for “Safety”) were developed by the scientific board.
The third step in the process was to obtain feedback on these statements from a wider group of dermatologists testing the consensus (two additional experts were included in the panel to better represent the whole Italian territory). The 20 statements were circulated via an online survey to 28 dermatologists (consensus panel), most of whom had participated in the preliminary survey. These dermatologists were asked to complete the survey in June/July 2019. The survey asked them to express their level of agreement/disagreement with each statement using a 5-point scale (1 = total disagreement, 2 = disagreement, 3 = agreement, 4 = strong agreement, 5 = total agreement). Consensus was defined by more than 80% agreement (scores of 3–5) or disagreement (scores of 1 or 2). The voting process was performed online and was anonymous.
In step 4, the scientific board analysed the results of the first round of voting. As consensus was reached on all statements, there was no need for a second round of voting. The results were discussed at a plenary meeting attended by the scientific board and the consensus panel of dermatologists in October 2019 in Rome. During this meeting, the statements underwent minor editing and were finalised to the present version.
Results and Discussion
Thirty-five of the 36 dermatologists (97%) invited to participate in the preliminary survey provided their opinion about various aspects and practicalities of the treat-to-target approach to psoriasis management by answering all questions in the 25-item questionnaire.
During the Delphi method, 28 dermatologists on the consensus panel expressed their agreement or disagreement on the 20 statements produced by the scientific board (100% response rate). Positive consensus was reached on all statements. The statements and results of the first and only round of voting are shown in Tables 1, 2, 3 and 4. The background of all the dermatologists included in the study was similar: they were university hospital doctors with a specific clinical expertise in managing patients with psoriasis with biological therapy.
Table 1.
Level of consensus on statements about clinical remission targets
| Statements | Scores applied, n | Level of consensus, % | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | ||
| 1.1 An adaptable and personalised strategy aimed at achieving the therapeutic objectives (i.e. treat to target) can be useful in psoriasis clinical practice | 0 | 0 | 0 | 4 | 24 | 28 | 100 |
| 1.2 Several factors should be considered when choosing a systemic treatment in patients with moderate-to-severe psoriasis. They include disease severity and localisation (i.e. sensitive areas), coexistence of psoriatic arthritis or other comorbidities, impact of the disease on the patient’s quality of life, patient’s preference and treatment risk–benefit ratio | 0 | 0 | 0 | 4 | 24 | 28 | 100 |
| 1.3 Dermatologists should use PASI or PGA or BSA to objectively assess psoriasis in daily practice | 0 | 1 | 6 | 6 | 15 | 28 | 96 |
| 1.4 The PASI90 response best defines the therapeutic objective | 0 | 2 | 3 | 11 | 12 | 28 | 93 |
| 1.5 The absolute PASI value that defines the optimal therapeutic objective should be less than or equal to 3 | 0 | 3 | 5 | 6 | 14 | 28 | 89 |
| 1.6 PASI90 or absolute PASI less than or equal to 3 could not be adequate treatment goals in the case of involvement of sensitive areas | 0 | 3 | 3 | 10 | 12 | 28 | 89 |
| 1.7 If the target of PASI90 or absolute PASI score less than or equal to 3 is not reached after 3–4 months of therapy, a change in treatment should be considered | 0 | 5 | 5 | 12 | 6 | 28 | 82 |
| 1.8 PASI90 or absolute PASI less than or equal to 3 should be maintained over time | 0 | 2 | 3 | 9 | 14 | 28 | 93 |
| 1.9 The impact of psoriasis on patient’s quality of life should be taken into consideration when considering treatment goals | 0 | 0 | 1 | 6 | 21 | 28 | 100 |
BSA body surface area, PASI Psoriasis Area Severity Index, PASI90 90% decrease in PASI score, PGA physician’s global assessment
Table 2.
Level of consensus on statements about patient health-related quality of life targets
| Statements | Scores applied, n | Level of consensus, % | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | ||
| 2.1 Quality of life is an important outcome from the patient and physician perspective and should be included in the therapeutic targets. Achievement of treatment goal implies no impact or minimal impact of the disease on quality of life, e.g. DLQI less than or equal to 3 | 0 | 1 | 2 | 10 | 15 | 28 | 96 |
| 2.2 Treat to target in psoriasis should include patient-centric targets, such as patient satisfaction | 0 | 0 | 2 | 15 | 11 | 28 | 100 |
| 2.3 If the target of disease-related quality of life is not reached after 3–4 months of therapy, a change in treatment should be considered | 1 | 1 | 6 | 12 | 8 | 28 | 93 |
DLQI Dermatology Life Quality Index
Table 3.
Level of consensus on statements related to abrogation of systemic inflammation
| Statements | Scores applied, n | Level of consensus, % | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | ||
| 3.1 Psoriasis-related systemic inflammation can affect joints, liver, nervous system and cardiovascular system | 0 | 0 | 5 | 6 | 17 | 28 | 100 |
| 3.2 Attention should be paid to early recognition of psoriatic arthritis | 0 | 0 | 0 | 6 | 22 | 28 | 100 |
| 3.3 Moderate-to-severe psoriasis can be associated with various comorbidities that can benefit from, or be worsened by, anti-psoriatic therapy | 0 | 0 | 5 | 5 | 18 | 28 | 100 |
| 3.4 Biological drugs showing a high selectivity in inhibiting inflammatory signals can improve comorbidities that share pathogenic pathways with psoriasis | 0 | 2 | 3 | 8 | 15 | 28 | 93 |
| 3.5 In obese patients, body weight reduction may positively impact on overall response to anti-psoriatic therapy | 0 | 0 | 2 | 8 | 18 | 28 | 100 |
Table 4.
Level of consensus on statements related to treatment safety
| Statements | Scores applied, n | Level of consensus, % | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | ||
| 4.1 Safety should be considered as important as efficacy | 0 | 0 | 3 | 3 | 22 | 28 | 100 |
| 4.2 Targeted therapies show a very favourable safety profile | 0 | 0 | 2 | 13 | 13 | 28 | 100 |
| 4.3 Safety should be assessed periodically, according to the patient’s and drug’s characteristics | 0 | 0 | 2 | 7 | 19 | 28 | 100 |
In the following sections, the consensus statements from each topic will be discussed along with the most relevant results from the preliminary survey and the supporting scientific evidence when available.
Clinical Remission of Psoriasis
There was full agreement among the members of the consensus panel regarding targets for clinical remission (Table 1). The choice of systemic therapy should consider several factors, including disease severity and localisation (i.e. sensitive areas), comorbidities (including psoriatic arthritis), impact on quality of life and patient preferences (statements 1.1 and 1.2). According to the consensus, treatment goals that define clinical remission of psoriasis include a PASI90 response or an absolute PASI score less than or equal to 3 (statements 1.3, 1.4 and 1.5). Such goals may, however, need to be reconsidered in patients with psoriasis affecting sensitive body areas, such as face, scalp, palms, soles, nails and genitalia (statement 1.6). Evidence shows that the involvement of these areas has a negative psychological impact [41], which translates into worse disease severity compared with disease severity assessed by objective measures (such as BSA or PASI) only [42, 43]. The treatment goal (PASI90 response or absolute PASI score less than or equal to 3) should be maintained over time, which implies a tight control of disease course (statement 1.8). If the treatment goal is not achieved within 3–4 months of treatment, therapy should be changed (statement 1.7). Finally, there was a strong consensus about the role of HRQoL when defining or adjusting treatment goals (statement 1.9).
The preliminary survey highlighted that around two-thirds of dermatologists considered a patient-centred approach as very important to the definition of treatment goals (63%) and their assessment (60%). PASI change from baseline and absolute PASI values were considered to be very effective measures of disease severity improvements by 39% and 48% of respondents, respectively.
PASI90 has been suggested by several authors as the new target of psoriasis treatment because, compared with other measures of psoriasis improvement, PASI90 appears to be associated with greater improvements in DLQI values and higher rates of absolute DLQI values of 0–1, corresponding to no impact of psoriasis on HRQoL [29, 44]. It also takes into account baseline disease severity, which as noted above, was considered a very effective measure of treatment response by 39% of respondents. The clinical relevance and feasibility of PASI90 and PASI100 responses are also reflected in the increasing use of these measures as primary and secondary endpoints in clinical trials [15, 18, 19, 22, 24]. The first phase 3 trial to use PASI90 as a primary endpoint was the CLEAR trial, which compared secukinumab with ustekinumab in patients with moderate-to-severe psoriasis [23]. At week 16, PASI90 response rate was achieved in 79% of patients treated with secukinumab compared with 58% treated with ustekinumab (P < 0.0001). PASI100 responses at 16 weeks were 44% and 28% in secukinumab and ustekinumab patients, respectively (P < 0.0001). A systematic review and network meta-analysis of interleukin inhibitors in moderate-to-severe plaque psoriasis found that 12–16 weeks’ treatment with IL-17, IL-12/23 and IL-23 inhibitors was associated with high efficiency in achieving PASI75, PASI100 and sPGA 0/1 or IGA 0/1 or PGA 0/1. The IL-23 inhibitor risankizumab was considered to have the greatest efficacy and lowest safety risk [45].
The Spanish Psoriasis Group recently redefined the targets of psoriasis treatment with biological therapy [25]. According to the consensus achieved by that group, absolute PASI values are useful measures in clinical practice and correlate better with DLQI than relative PASI improvements. Absolute PASI values less than or equal to 3 define the achievement of treatment goals. A reduction in the dose of biological therapy is possible in patients with complete or near complete response (PGA 0/1; PASI90; absolute PASI from less than 2 to 3). Criteria for returning to full-dose biological therapy include absolute PASI values of at least 5 or loss of PASI75 response. The consensus statements issued by the Spanish group also provided detailed indications about the timing of response assessment, which varies according to the biological drug used: at week 12 for adalimumab, 14 for infliximab and 16 for ustekinumab and secukinumab (no consensus on etanercept or apremilast) [25].
An absolute PASI value less than or equal to 3 is also the criterion to continue current treatment recommended by the recent French expert-opinion guidelines on the use of systemic treatments for moderate-to-severe psoriasis [34]. According to these guidelines, absolute PASI values are easier to calculate than relative PASI values, are independent of baseline severity assessments and correlate more precisely with a clear/almost clear status (i.e. PGA score of 0–1). The relevance of absolute PASI scores has also been highlighted by a recent analysis of real-world data based on the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) [28]. This analysis found 90% concordance between an absolute PASI score of less than or equal to 2 and PASI90 response, and 88% concordance between absolute PASI less than or equal to 4 and PASI75 response. A 90% concordance was also reported for PGA clear/almost clear and PASI less than or equal to 2. The ‘Treat to Target Italia’ panel considered that an absolute PASI less than or equal to 2 and PGA of clear/almost clear was too restrictive, and that the PASI less than or equal to 3 goal recommended in the French and Spanish guidelines was more acceptable when applying the treat-to-target approach to psoriasis management in clinical practice. Indeed, the utility of absolute PASI scores has been illustrated in a recent post hoc analysis of pooled phase 3 study results. The authors found that compared with percentage PASI improvement, absolute PASI score was more reliable in determining disease activity in patients with moderate-to-severe plaque psoriasis [46].
Patient Health-Related Quality of Life
As described above (relating to statement 1.9), patient HRQoL is an important target of treatment. If treatment targets are achieved, there should be no residual impact of psoriasis on HRQoL or the impact should be very low. A common measure of HRQoL in patients with psoriasis is the DLQI, and a study investigated the relationship between such scores and patients’ perception of the impairment of their skin-related quality of life. The following DLQI scores defined the degree of psoriasis interference: scores 0–1, no effect; 2–5, small effect; 6–10, moderate effect; 11–20, very large effect; 21–30, extremely large effect [47]. On the basis of these data, the consensus statement defines a DLQI goal of less than or equal to 3 (statement 2.1). Similar to the timing recommended for the assessment of treatment response and for treatment adjustments (statement 1.7), if the HRQoL target of DLQI less than or equal to 3 is not reached after 3–4 months of treatment, therapy should be changed (statement 2.3).
The preliminary questionnaire highlighted an elevated level of awareness among the surveyed dermatologists about the importance of HRQoL in the treat-to-target management of psoriasis (85% considered HRQoL as a very important component of the treatment goals). According to the Delphi survey, 80% of dermatologists assess HRQoL of patients with psoriasis in their routine practice by calculating the DLQI score (74%), based on an overall assessment of patient satisfaction (89%).
The relevance of HRQoL in the treat-to-target management of psoriasis is supported by an increasing body of evidence suggesting that effective treatment correlates with improvement of DLQI scores [48–50]. A US survey involving dermatologists and patients investigated the relationship between psoriasis severity and quality of life (DLQI and EuroQoL 5-Dimension Health questionnaire) and work productivity (Work Productivity and Activity Impairment questionnaire) [48]. More severe psoriasis correlated with increased symptoms (itching, pain and scaling), reduced quality of life, and impaired work productivity [48]. A real-world observational study in patients treated with adalimumab found that the improvements in patient HRQoL and psychological functioning reported at 16 weeks were paralleled by improvements in skin disease [49].
Abrogation of Systemic Inflammation
Chronic systemic inflammation associated with psoriasis can affect a number of tissues and organs leading to the development or worsening of comorbidities, including psoriatic arthritis, cardiovascular disease and depression (statement 3.1) [51–53]. Early recognition of psoriatic arthritis is crucial (statement 3.2), particularly given the prevalence of this comorbidity in patients with psoriasis [6, 54]. Systemic therapies for psoriasis can improve or worsen comorbidities (statement 3.3). As biological drugs target inflammatory pathways that are also likely to be involved in the pathogenesis of comorbidities, their use may be beneficial for these comorbidities as well as psoriasis (statement 3.4). For example, there is emerging evidence that biological therapies have favourable effects on reversing the underlying pathogenic processes in cardiovascular disease such as endothelial dysfunction and atherosclerotic plaque progression [55, 56]. Also, early aggressive control of systemic inflammation may prevent or delay the damage associated with comorbidities, including psoriatic arthritis [52].
The preliminary survey showed that comorbidities associated with psoriasis, including psoriatic arthritis, metabolic syndrome, obesity, diabetes mellitus, cardiovascular disease, inflammatory bowel disease and depression, play a central role in therapeutic decisions.
Safety
There was full agreement that safety of treatment is equally as important as efficacy when defining treatment targets (statement 4.1). The safety of the selected therapy should be monitored according to medication and patient characteristics (statement 4.3). There was also full consensus about the more favourable safety profile of biologicals compared with traditional systemic treatments for psoriasis, especially for long-term therapy (statement 4.2).
The safety and tolerability of systemic therapy is a major issue in the management of moderate-to-severe psoriasis. Concern about the safety of systemic therapies is one of the main reasons why patients with moderate-to-severe psoriasis are often inadequately treated. However, a large body of evidence from clinical trials and post-marketing pharmacovigilance registries supports the safety of biologicals for the treatment of psoriasis [57–64]. Biologicals are better tolerated than conventional systemic therapies, particularly for long-term treatment. It should be noted that each class of biological therapy has a specific safety profile. Overall biologicals are associated with an increased risk of infection, including upper respiratory tract infections for TNFα inhibitors and candida infection for IL-17 inhibitors.
Drug retention rates are a useful measure of treatment effectiveness and safety [58]. Evidence shows that retention rates of traditional systemic treatments for psoriasis are shorter than retention rates of biologicals, mainly due to poor tolerability [58]. The most common reason for discontinuation of biologicals is loss of efficacy [58]. A real-world study using data from the BADBIR pharmacovigilance registry to evaluate the persistence of biologicals (adalimumab, etanercept, infliximab and ustekinumab) in biological-naïve patients with psoriasis found that treatment discontinuation was generally due to loss of response to treatment, rather than to safety issues [65]. Similar findings were provided by an analysis of data from the prospective, international Psoriasis Longitudinal Assessment and Registry (PSOLAR), in which the most common cause of treatment discontinuation was loss of efficacy [66].
Limitations
We acknowledge the inherent bias in the non-random selection of 10 expert dermatologists, most of whom are from university hospitals. However, we believe this may be offset somewhat by the extensive range of clinical experience held by the scientific board, and their level of involvement in producing these high-quality guidelines, which might not have been possible if 10 dermatologists had been randomly selected. Another possible limitation is the lack of a patient perspective during consensus development; however, this was indirectly mitigated by an assessment of patient HRQoL data. Moreover, we acknowledge the limited number of dermatologists (N = 28) answering Delphi as a limitation of the study. However, they were hospital-based specialists with a specific clinical expertise in managing patients with psoriasis with biological therapy.
Conclusions
Defining treatment targets enables physicians and patients to closely follow treatment progress, to modify treatment when the goals are not met, and to optimise therapeutic interventions. Here, we provide 20 consensus statements to guide dermatologists in the adoption of the treat-to-target strategy for the management of psoriasis in clinical practice.
This is the first initiative to define the parameters of a treat-to-target strategy for psoriasis in Italy. It was somewhat surprising that complete consensus was reached on all statements after the first round of voting in the Delphi method. This may be explained by the fact that the dermatologists on the consensus panel had comparable expertise, were from specialised dermatology centres, and were fully acquainted with the latest treatment strategies for psoriasis. A consensus panel composed of general dermatologists with less expertise in managing psoriasis might have provided different results. On the other hand, it was encouraging to note that consensus exists on treatment goals among Italian psoriasis experts.
The treat-to-target strategy proposed here is strongly patient-centred with an emphasis on both objective measures of disease severity and patient HRQoL. Recommended targets are PASI90 response or alternatively absolute PASI less than or equal to 3, although these targets may be adjusted in patients with involvement of sensitive body areas. With regard to HRQoL, the proposed target is DLQI less than or equal to 3 (very low to no impact). If PASI and DLQI targets are not reached within 3–4 months, treatment should be modified. The present statements also stress the importance of early recognition of psoriatic arthritis and selecting agents that abrogate systemic inflammation. Abrogation of systemic inflammation is aimed at improving psoriasis and preventing or postponing the development of inflammatory comorbidities. Safety is a target that is as important as efficacy, and treatment with biologicals requires regular monitoring of adverse events.
As the treatment options for psoriasis continue to evolve, therapeutic targets will need to be updated. Currently, no general international consensus exists about treatment targets in psoriasis. This may be a consequence of the lack of clear correlations between suggested target scores and patient-reported outcomes. Further investigations on the impact of the treat-to-target strategy on patient HRQoL will contribute to refining the approach and identifying generally accepted targets.
Acknowledgements
Funding
Novartis Farma is the sole sponsor for this project and provided funding to invited participants, including honoraria for the development of the survey and attendance at the meetings and reimbursement for travel to the meetings. Novartis Farma provided funding to a third-party vendor (Springer Healthcare Italia) for logistical support at the meetings, survey development and preparation of a basic report from the survey data, and medical writing assistance for the manuscript. Novartis personnel attended the meetings to provide logistical support but did not give input to the survey development. Novartis was not involved in the development of the manuscript but did have an opportunity to review the final manuscript draft. No payments were made to the authors for the development of this manuscript. The journal's Rapid Service Fee was funded by Novartis Farma (Italy).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial and Other Assistance
We would like to thank Catherine Rees of Springer Healthcare Communications who edited later drafts of this manuscript. This medical writing assistance was funded by Novartis Farma.
Disclosures
Paolo Gisondi has been a consultant and/or speaker for Abbvie, Almirall, Celgene, Ducray, Janssen, LEO Pharma, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, Sandoz, UCB. Marina Talamonti has served as a scientific adviser and/or clinical study investigator for Abbvie, Amgen, Janssen, LEO Pharma, Lilly, Novartis, Sanofi Genzyme, and as paid speaker for Janssen, Novartis, and Sanofi Genzyme. Andrea Chiricozzi has served as a scientific adviser and/or clinical study investigator for Abbvie, Almirall, Fresenius Kabi, Janssen, LEO Pharma, Lilly, Novartis, Sanofi Genzyme, and UCB-pharma, and as paid speaker for Abbvie, Almirall, Janssen, LEO Pharma, Lilly, Novartis, and Sanofi Genzyme. Stefano Piaserico has been a consultant and/or speaker for Abbvie, Almirall, Celgene, Difa Cooper, Galderma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sandoz, UCB. Paolo Amerio has served as a consultant or Speaker for Abbvie, Celgene, Pfizer, Sanofi-Genzyme, Eli Lilly, and Jannsen, Galderma. Anna Balato has received honoraria as speaker and consultant for Sanofi, Abbvie, Eli Lilly. Federico Bardazzi has been consultant, adviser and clinical study investigator for Eli Lilly, Abbvie, Novartis, LEO Pharma, Sandoz, Bristol Myers, Abiogen-Pharma, Celgene, and Janssen-Cilag. Piergiacomo Calzavara Pinton has been a consultant and/or speaker for Abbvie, Almirall, Celgene, Pierre Fabre, Galderma, LEO Pharma, Sanofi, Novartis, Cantabria, Mylan. Anna Campanati has received honoraria as speaker, advisory member and consultant for Abbvie, Almirall, Celgene, Eli Lilly, Galderma, LEO Pharma, Mylan, MSD, Pfizer, Pierre Fabre, Sanofi, UCB Pharma. Angelo Cattaneo has participated in clinical trials and has served as an advisory board member for Abbvie, Pfizer, Celgene, Novartis, Galderma, Lilly, LEO pharma, Almirall. Paolo Dapavo has been a scientific consultant/speaker/clinical study investigator for AbbVie, Celgene, Novartis, Eli Lilly, Novartis, Almirall, LEO Pharma, Sandoz, Janssen-Cilag. Clara De Simone has received honoraria as speaker and consultant for Abbvie, Almirall, Biogen, Eli Lilly, LEO Pharma, Novartis, Janssen, Sanofi, Pfizer, and UCB Pharma. Maria C. Fargnoli has served as a speaker, board member and/or received research grants from Almirall, Abbvie, Galderma, LEO Pharma, Mylan, Medac Pharma, Celgene, Pierre Fabre, UCB, Lilly, Pfizer, Janssen, Novartis, Sanofi-Genzyme, Roche, Sunpharma, and MSD. Claudio Guarneri has been a scientific consultant/speaker/clinical study investigator for Abbvie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Almirall, LEO Pharma. Claudia Lasagni declares a conflict of interest with Abbvie, Novartis, Lilly and Almiral. Francesco Loconsole served on advisory boards and/or received honoraria for lectures from Abbvie, Jansen-Cilag, Novartis, Lilly, Sanofi. Ada Lo Schiavo has received honoraria as Speaker and consultant for Novartis, Celgene Janssen, Eli Lilly, Genzyme, and Abbvie. Giovanna Malara has received honoraria as Speaker and consultant for Janssen, Sanofi, Abbvie, Eli Lilly, Novartis, and Celgene. Maria L. Musumeci has been consultant, adviser and clinical study investigator for Eli Lilly, Abbvie, Novartis, LEO Pharma, Celgene, Janssen-Cilag, Almirall, Biogen. Luigi Naldi has acted as a consultant/participant in advisory boards for Abbvie, Amgen, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius, Galderma, IDI-Farmaceutici, Janssen-Cilag, LEO Foundation, L’Oreal, Lilly, Mylan, Menarini, Novartis, Pfizer, Sanofi Pasteur, Sanofi Genzyme, UCB. Manuela Papini has served as a scientific adviser and/or clinical study investigator for Janssen-Cilag, LEO Pharma, Novartis, and Sanofi Genzyme. Aurora Parodi has participated in clinical trials and has served as an advisory board member for Abbvie, Pfizer, Celgene, Novartis, Galderma, Lilly, LEO pharma, Almirall. Concetta Potenza has been a scientific consultant/speaker/clinical study investigator for Abbvie, Almirall, Alfasigma, Celgene, Janssen, Eli Lilly, Novartis, Parexel, Pfizer, Sanofi, LEO Pharma. Franco Rongioletti has received honoraria as a consultant or Speaker for Abbvie, Lilly, Janssen, Novartis, Almirall, and Sanofi Genzyme. Luca Stingeni has been a consultant and/or speaker for Abbvie, Almirall, Celgene, Janssen, Eli Lilly, Novartis, Sanofi Genzyme. Rossana Tiberio has received honoraria as a consultant or Speaker for Abbvie, Lilly, Janssen, Novartis, and Sanofi Genzyme. Luca Bianchi has received honoraria as speaker or consultant for Abbvie, Janssen, Almirall, Eli Lilly, LEO Pharma, Novartis, Sanofi, Pfizer, and UCB Pharma. Antonio Costanzo is supported by grants from the Ministry of Health (grant no. CO-2013–02356463), has served as an advisory board member and consultant, and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. Francesco Cusano has been scientific adviser, speaker and/or clinical study investigator for Abbvie, Almirall, Celgene, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sanofi Genzyme. Giampiero Girolomoni served as consultant for AbbVie, Abiogen, Almirall, Amgen, Biogen, Bristol-Meyers Squibb, Celgene, Celltrion, Eli Lilly, Genzyme, LEO Pharma, Menlo therapeutics, Novartis, Pfizer, Regeneron, Samsung, Sandoz and Sanofi. Anna M. Offidani has been a consultant and/or speaker for Abbvie, Galderma, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, UCB. Ketty Peris reports personal fees from AbbVie, Almirall, Biogen, Celgene, Eli Lilly, Gal derma, LEO Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, Sun Pharma and Janssen, outside the submitted work. Valentina Dini, Maria Laura Flori, Marco Galluzzo, Piergiorgio Malagoli, Santo R. Mercuri, Francesca Prignano and Marina Venturini have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Footnotes
Luca Bianchi, Antonio Costanzo, Francesco Cusano, Giampiero Girolomoni, Anna M. Offidani and Ketty Peris jointly directed this work.
Paolo Gisondi, Marina Talamonti, Andrea Chiricozzi, and Stefano Piaserico contributed equally to this work.
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) project team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 3.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 4.Reich K. The concept of psoriasis as a systemic inflammation: implications for disease management. J Eur Acad Dermatol Venereol. 2012;26(Suppl 2):3–11. doi: 10.1111/j.1468-3083.2011.04410.x. [DOI] [PubMed] [Google Scholar]
- 5.Villasenor-Park J, Wheeler D, Grandinetti L. Psoriasis: evolving treatment for a complex disease. Cleve Clin J Med. 2012;79(6):413–423. doi: 10.3949/ccjm.79a.11133. [DOI] [PubMed] [Google Scholar]
- 6.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisondi P, Girolomoni G. Psoriasis and atherothrombotic diseases: disease-specific and non-disease-specific risk factors. Semin Thromb Hemost. 2009;35(3):313–324. doi: 10.1055/s-0029-1222610. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AD, Gilutz H, Henkin Y, et al. Psoriasis and the metabolic syndrome. Acta Derm Venereol. 2007;87(6):506–509. doi: 10.2340/00015555-0297. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–284. [PubMed] [Google Scholar]
- 12.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–ii23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo PA, Ilchef R, Cooper AJ. Psychiatric morbidity in psoriasis: a review. Australas J Dermatol. 2004;45(3):155–159. doi: 10.1111/j.1440-0960.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 14.Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris–update 2015–Short version–EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. doi: 10.1111/jdv.13354. [DOI] [PubMed] [Google Scholar]
- 15.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 16.Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60–9e9. 10.1016/j.jaad.2016.08.008. [DOI] [PubMed]
- 17.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 18.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 19.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 21.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 22.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 23.Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Thaci D, Humeniuk J, Frambach Y, et al. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE) Br J Dermatol. 2015;173(3):777–787. doi: 10.1111/bjd.13814. [DOI] [PubMed] [Google Scholar]
- 25.Carretero G, Puig L, Carrascosa JM, et al. Redefining the therapeutic objective in psoriatic patients candidates for biological therapy. J Dermatolog Treat. 2018;29(4):334–346. doi: 10.1080/09546634.2017.1395794. [DOI] [PubMed] [Google Scholar]
- 26.Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774–790. doi: 10.1111/jdv.14114. [DOI] [PubMed] [Google Scholar]
- 27.Gulliver W, Lynde C, Dutz JP, et al. Think beyond the skin: 2014 Canadian expert opinion paper on treating to target in plaque psoriasis. J Cutan Med Surg. 2015;19(1):22–27. doi: 10.2310/7750.2014.13151. [DOI] [PubMed] [Google Scholar]
- 28.Mahil SK, Wilson N, Dand N, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR) Br J Dermatol. 2019 doi: 10.1111/bjd.18333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648. doi: 10.1111/jdv.12817. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298. doi: 10.1016/j.jaad.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Baker C, Mack A, Cooper A, et al. Treatment goals for moderate to severe psoriasis: an Australian consensus. Australas J Dermatol. 2013;54(2):148–154. doi: 10.1111/ajd.12014. [DOI] [PubMed] [Google Scholar]
- 32.Gladman DD, Poulin Y, Adams K, et al. Treating psoriasis and psoriatic arthritis: position paper on applying the treat-to-target concept to Canadian daily practice. J Rheumatol. 2017;44(4):519–534. doi: 10.3899/jrheum.161473. [DOI] [PubMed] [Google Scholar]
- 33.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. doi: 10.1007/s00403-010-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amatore F, Villani AP, Tauber M, Viguier M, Guillot B, Psoriasis Research Group of the French Society of Dermatology. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33(3):464–83. 10.1111/jdv.15340. [DOI] [PMC free article] [PubMed]
- 35.Armstrong A, Jarvis S, Boehncke WH, et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: results of the clear about psoriasis worldwide survey. J Eur Acad Dermatol Venereol. 2018;32(12):2200–2207. doi: 10.1111/jdv.15065. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong AW, Koning JW, Rowse S, Tan H, Mamolo C, Kaur M. Under-treatment of patients with moderate to severe psoriasis in the United States: analysis of medication usage with health plan data. Dermatol Ther (Heidelb) 2017;7(1):97–109. doi: 10.1007/s13555-016-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97. doi: 10.1007/s40257-015-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynde CW, Beecker J, Dutz J, et al. Treating to target(s) with interleukin-17 inhibitors. J Cutan Med Surg. 2019;23(2_suppl):3S-34S. 10.1177/1203475418824565. [DOI] [PubMed]
- 39.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015. [PubMed] [Google Scholar]
- 40.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezinska-Wcislo L, Slomian A. Associations between site of skin lesions and depression, social anxiety, body-related emotions and feelings of stigmatization in psoriasis patients. Postepy Dermatol Alergol. 2018;35(1):60–66. doi: 10.5114/pdia.2016.62287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardazzi F, Starace M, Bruni F, Magnano M, Piraccini BM, Alessandrini A. Nail psoriasis: an updated review and expert opinion on available treatments, including biologics. Acta Derm Venereol. 2019;99(6):516–523. doi: 10.2340/00015555-3098. [DOI] [PubMed] [Google Scholar]
- 43.Dopytalska K, Sobolewski P, Blaszczak A, Szymanska E, Walecka I. Psoriasis in special localizations. Reumatologia. 2018;56(6):392–398. doi: 10.5114/reum.2018.80718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77–82e7. 10.1016/j.jaad.2016.03.026. [DOI] [PubMed]
- 45.Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161. doi: 10.1155/2019/2546161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon KB, Reich K, Crowley JJ, et al. Disease activity and treatment efficacy using patient-level Psoriasis Area and Severity Index scores from tildrakizumab phase 3 clinical trials. J Dermatolog Treat. 2020:1–10. 10.1080/09546634.2020.1747590. [DOI] [PubMed]
- 47.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 48.Korman NJ, Zhao Y, Pike J, Roberts J. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41(5):514–521. doi: 10.1111/ced.12841. [DOI] [PubMed] [Google Scholar]
- 49.Leman J, Walton S, Layton AM, et al. The real world impact of adalimumab on quality of life and the physical and psychological effects of moderate-to-severe psoriasis: a UK prospective, multicenter, observational study. J Dermatolog Treat. 2019:1–9. 10.1080/09546634.2019.1592096. [DOI] [PubMed]
- 50.Puig L, Augustin M, Blauvelt A, et al. Effect of secukinumab on quality of life and psoriasis-related symptoms: a comparative analysis versus ustekinumab from the CLEAR 52-week study. J Am Acad Dermatol. 2018;78(4):741–748. doi: 10.1016/j.jaad.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L. Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis. Br J Dermatol. 2017;176(3):732–740. doi: 10.1111/bjd.15149. [DOI] [PubMed] [Google Scholar]
- 52.Kerdel F, Don F. The importance of early treatment in psoriasis and management of disease progression. J Drugs Dermatol. 2018;17(7):737–742. [PubMed] [Google Scholar]
- 53.Pietrzak D, Pietrzak A, Grywalska E, et al. Serum concentrations of interleukin 18 and 25-hydroxyvitamin D3 correlate with depression severity in men with psoriasis. PLoS One. 2018;13(8):e0201589. doi: 10.1371/journal.pone.0201589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 55.Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–728. doi: 10.1093/cvr/cvz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Stebut E, Reich K, Thaci D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054–1062. doi: 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 57.Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111. doi: 10.1186/s13075-019-1882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gisondi P, Tessari G, di Mercurio M, Del Giglio M, Girolomoni G. Retention rate of systemic drugs in patients with chronic plaque psoriasis. Clin Dermatol. 2013;1(1):8–14. [Google Scholar]
- 59.Howell ST, Cardwell LA, Feldman SR. Treating moderate-to-severe plaque psoriasis with guselkumab: a review of phase II and phase III trials. Ann Pharmacother. 2018;52(4):380–387. doi: 10.1177/1060028017743268. [DOI] [PubMed] [Google Scholar]
- 60.Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72(1):115–122. doi: 10.1016/j.jaad.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langley RG, Kimball AB, Nak H, et al. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019;33(2):333–339. doi: 10.1111/jdv.15242. [DOI] [PubMed] [Google Scholar]
- 62.Strober B, Crowley J, Langley RG, et al. Systematic review of the real-world evidence of adalimumab safety in psoriasis registries. J Eur Acad Dermatol Venereol. 2018;32(12):2126–2133. doi: 10.1111/jdv.15203. [DOI] [PubMed] [Google Scholar]
- 63.Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13(12):1441–1448. [PubMed] [Google Scholar]
- 64.Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatol. 2015;14(7):706–714. [PubMed] [Google Scholar]
- 65.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 66.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Eur Acad Dermatol Venereol. 2016;30(7):1148–1158. doi: 10.1111/jdv.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.