Abstract
Background
Many modified lateral lumbar interbody fusion techniques for lumbar degenerative diseases have been described by different authors. However, relatively high rates of vascular injury, peritoneal laceration, and even ureteral injury have been reported.
Purpose
The objectives of this study were firstly to present the detailed, standardized technical notes and describe the required standard characteristics of the designed surgical system of LaLIF and secondly to evaluate clinical outcomes and highlight the approach-related complications.
Methods
The mini-open LaLIF is described in a step-wise manner. The outcome measures were operative parameters, self-report measures, radiographic measures, and complications within 1 month of surgery. Operative parameters measured included operative time, intraoperative blood loss, and length of hospital stay. The self-report measures include Visual Analogue Scale (VAS), Oswestry disability index (ODI), and Short Form 36 Health Survey (SF-36) score. The radiographic measures including the intervertebral foraminal height (FH), intervertebral disc height (DH), and intervertebral foraminal area (FA) were assessed with plain radiography. The complication profiles were classified into intraoperative and postoperative (up to 1 month). Intraoperative complications were subcategorized into neurologic, vascular, ureteral, peritoneal, and vertebral injuries. Postoperative complications were subcategorized into infection, cage migration, and subsidence.
Results
A total of 126 patients who underwent LaLIF between April 2016 and December 2018 by a senior author were retrospectively reviewed. There were 54 males and 72 females (range 42–89 years old, average 65 ± 11 years old). The mean follow-up was 20 ± 11 months (range 6–38 months). The LaLIF was conducted at 188 levels in 126 patients, with 1 level in 75 cases, 2 levels in 42, 3 levels in 7, and 4 levels in 2 cases. There were 114 patients who underwent stand-alone LaLIF and 12 patients required secondary posterior fixation. The mean operative time, intraoperative blood loss, and length of hospital stay were recorded. The patient-reported outcome scores (VAS, ODI, and SF-36) and radiographic parameters (FH, DH, and FA) demonstrated a significant improvement after surgery and at the last follow-up. There were 25 (19.8%) complications in the 126 patients. The intraoperative complications accounted for 19 cases (15.1%) and postoperative accounted for 6 cases (4.8%). The most frequent complications were neurological injury (6.3%) and temporary psoas injury (6.3%).
Conclusions
The mini-open LaLIF, as a reproducible novel technique, can be performed safely at L2-L5. It is associated with reliable mid-term clinical outcomes and an acceptable complication profile when compared to traditional LLIF due to the advancements in the modified incision site, direct visualization, and usage of strictly vertical trajectory in multiple steps with the specially designed LaLIF system.
Translational potential statement
To make the lateral lumbar fusion process repeatable and also maintain a shallow learning curve, especially for surgeons in the early stages of learning, by using instruments with the required standard characteristics, the standardized surgical steps, modified incision site, vertical trajectory, and the direct visualization during the entire procedure.
Keywords: Lateral-anterior lumbar interbody fusion (LaLIF), Surgical system, Retroperitoneal techniques, Pre-psoas techniques, Mid-term follow-up, Strictly vertical trajectory
1. Introduction
The utilization of the retroperitoneal lateral lumbar interbody fusion (LLIF) for degenerative spinal disorders has increased due to its minimally invasive advantage and powerful indirect decompression [1]. Since the LLIF was first described by McAfee in 1998 [2], an immense amount of variation in the surgical approach has been developed. Currently, LLIF is mainly performed using two approaches relative to the psoas muscle: transpsoas and antepsoas.
The transpsoas approach, known as the extreme lateral interbody fusion, was developed in 2006 to decrease the inherent complications, such as major vascular injury and visceral injury [3] associated with the anterior retroperitoneal approach. The transpsoas approach, however, poses inherent risks to neural structures due to the anatomical course of the lumbar plexus through the psoas muscle [1].
The antepsoas, pre-psoas, or oblique lumbar interbody fusion was described first by Mayer in 1997. This approach implemented a smaller incision combined with an abdominal muscle-splitting to avoid dissection of the great vessels that the anterior lumbar interbody fusion (ALIF) requires [4]. Clinical outcomes and perioperative complications were reported by Kaiser in 2002 [5]. With the increasing number of spinal fusions performed through this retroperitoneal oblique approach, the term oblique lumbar interbody fusion (OLIF) was coined by Silvestre in 2012 [6]. Because accessing the intervertebral discs from a lateral approach is still challenging due to the potential risk to neural structures, Davis described an anatomic oblique corridor in a cadaveric study to further define access via the oblique retroperitoneal approach [7]. While several studies have investigated the outcomes and complication profiles of the OLIF, the reports of perioperative complications in the literature vary significantly. Ultimately, Woods [8] and Molloy [9] standardized the technical description of the antepsoas approach to improving the reproducibility of the OLIF. In light of the complications that have been reported, the OLIF tends to have fewer vascular complications than ALIF and fewer neurologic complications than the transpsoas approach [10]. However, relatively high rates of vascular injury, peritoneal laceration, ureteral injury and implant subsidence, and intraoperative endplate damage, have been reported for OLIF [8,11]. Thus, many studies have focused on improving both the transpsoas and antepsoas techniques [12].
We previously published a study reporting a novel classification to provide guiding information for case selection of a lateral anterior lumbar interbody fusion (LaLIF) technique. In that study, we evaluated the potential risks of LaLIF by analyzing correlations between the surgical difficulty of LaLIF and anatomic characteristics in radiographic images [13]. LaLIF is an antepsoas approach by using a modified incision site, direct visualization, and strictly vertical trajectory in multiple steps with a specially designed instrument system, this technique hopes to: 1) minimize the risk of intraoperative complications associated with OLIF, 2) make LaLIF repeatable with a shallow learning curve. The objectives of this study were firstly to present the detailed, standardized technical notes and describe the required standard characteristics of the designed surgical system of LaLIF and secondly to evaluate clinical outcomes and highlight the approach-related complications.
2. Materials and methods
2.1. Patient selection and surgical indications
Patients who presented with axial low back pain with or without severe leg pain were considered candidates for this surgery if they failed conservative, traditional nonoperative management for at least 3–6 months. Patients had the diagnosis of lumbar disc herniation, degenerative stenosis, degenerative spondylolisthesis, and degenerative scoliosis. Contraindications included disc sequestration; severe bony stenosis; severe degenerative scoliosis >40°; high-grade spondylolisthesis; severe lumbar osteoporosis; or previous abdominal surgery.
Procedures were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Human Experimentation of the First Affiliated Hospital of Sun Yat-sen University. All patients and their relatives had been informed prior to the commencement of this study and corresponding informed consent had been signed as well.
2.2. Surgical techniques
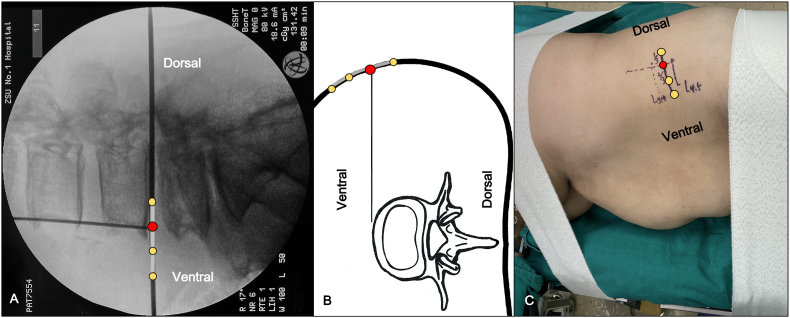
Under general anesthesia, muscle relaxants can be used because neuromonitoring is not necessary. The patient is placed in the right lateral decubitus position with an axillary roll. A bump may be used under the patient’s waist to open the gap between the T12 rib and the iliac crest. The chest and skin over the greater trochanter are secured with Urgostrapping (Urgo, France) to maintain the patient position. The hip and knee are slightly flexed to decrease abdominal muscle tension. The bony protrusions are cushioned. After the patient has been securely positioned in the right lateral decubitus position, the c-arm is rotated to 0° (Fig. 1A) and slightly tilt the surgical table forward and backward in the axial plane (Fig. 1B) to achieve a parallel projection of the vertebral endplates at the level of interest (Fig. 1C). This projection is also confirmed by the symmetry of lumbar pedicles, and this is recorded as the ‘0 position’ (Fig. 1D). At this point, the c-arm is rotated 90° (Fig. 1E and F) to obtain a ‘true lateral radiograph’ at the level of interest (Fig. 1G), recording this position as the ‘the angle of strictly vertical trajectory’ to perform the annulus release, templating, and placement of the implant (Fig. 1H). Two surgical table-mounted armboards help to keep the bilateral upper extremities in the proper anterior position for this procedure.
Fig. 1.
Illustration of getting a ‘true lateral radiograph’ for the level of interest. Fig. 1A. The diagram shows that the c-arm is rotated to 0°. Fig. 1B. The diagram shows that the surgical table is slightly tilted forward and backward in the axial plane to achieve a parallel projection of the vertebral endplates at the level of interest. Fig. 1C. The AP radiograph shows that the ‘true AP radiograph’ is achieved in ‘0 position’. Fig. 1D. This photo shows the ‘0 position’. Fig. 1E. The graph shows that the c-arm is rotated 90°. Fig. 1F. The diagram shows the projection of the target level. Fig. 1G. The lateral radiograph shows that A ′true lateral radiograph’ at the level of interest is obtained. Fig. 1H. The photo shows the ‘angle of strictly vertical trajectory’ position.
Two Kirschner-wires (K-wires) and a true lateral radiograph are used to verify the level of interest, identify the direction parallel to the target disc space and the anterior margin of the vertebral body (Fig. 2A). Lines are then drawn to delineate the incision site. After wide aseptic skin preparation, an incision approximal 4–6 cm is made starting from the anterior one-third of the disc space in a direction parallel to the target disc space (Fig. 2B and C). Then, the three muscular layers of the abdominal wall are split using blunt finger dissection parallel to their fibers of the external oblique, internal oblique, and transversus abdominis. Electrocautery may be used to open the fascia. With muscle relaxants, up to three disc spaces can be accessed through one single skin incision using a “sliding window” technique without making two separate paths through the abdominal wall. However, these sliding movements are limited to a small range of distances even with retractors and strong retraction force. It is necessary to gently feel the ‘hard’ spine obliquely and posteriorly, then push the moveable soft tissue anteriorly before opening the transversalis fascia to prevent accidental entry into the peritoneal cavity. The retroperitoneal fat normally protrudes into visualization after opening the transversalis fascia.
Fig. 2.
Illustration of making a LaLIF incision. Fig. 2A. The lateral radiograph shows that the incision is made starting from the anterior one-third of the disc space in a direction parallel to the target disc space. The red dot indicates the anterior margin of the vertebral body on a ‘true lateral radiograph’. Fig. 2B. The diagram shows that the 4–6 cm incision is divided into three equal parts. The red dot indicates the anterior margin of the disc space on a ‘true lateral radiograph’. Fig. 2C. The photo shows that lines are then drawn to delineate the anterior and posterior aspects of the disc space and the anterior margin of the spinal column. (For interpretation of the references to colour/colour in this figure legend, the reader is referred to the Web version of this article.)
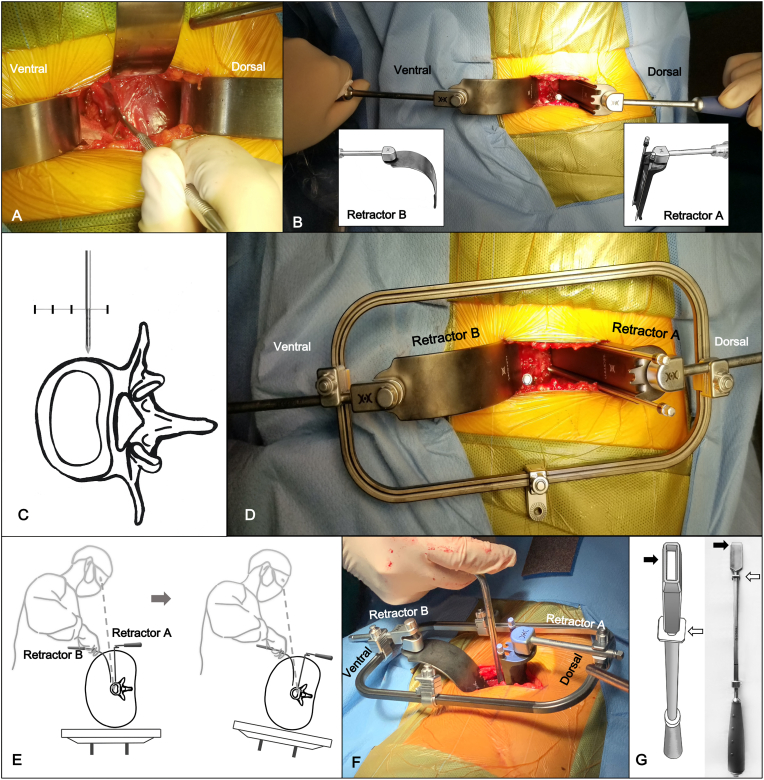
After opening the transversalis fascia, retroperitoneal fat is normally on top of the psoas muscle in the surgical field which can be exposed by inserting a traditional S-shaped retractor (or a LaLIF system “C” shaped retractor B, WEIGO, Inc. Weihai City, China). After direct visualization of retroperitoneal fat, gently push the retroperitoneal fat posteriorly towards the vertebra to roughly palpate the psoas major and its anterior border. Then slight retraction of the peritoneal contents and retroperitoneal fat can be done anteriorly. When the peritoneal contents are retracted anteriorly, a large gauze pad under the S-shaped retractor is used to protect the peritoneum, peritoneal contents, and major vascular which may be injured by the sharp edge of the retractor and excessive force. Once the belly of the psoas muscle is visualized after the peritoneum is swept anteriorly (Fig. 3A), fingertip palpation is performed to locate the intervertebral disc beneath the psoas major and its anterior border. A Cobb elevator is used to dissect the partial attachment of the psoas muscle off the spine so that the belly of the psoas can be gently retracted posteriorly with a traditional S-shaped retractor (or a LaLIF system straight “L” shaped retractor A, WEIGO, Inc. Weihai City, China). Once the intervertebral disc and the anterior border of the psoas muscle are visualized, a Steinmann pin is inserted into the disc space to verify the disc level and the anterior-posterior location with a ‘true lateral radiograph’.
Fig. 3.
Illustration of LaLIF retractor system setup and discectomy. Fig. 3A. The photo shows that the belly of the psoas is visualized after the peritoneum is swept anteriorly. Fig. 3B. The photo shows that the straight “L” shaped retractor A is used to retract the psoas muscle posteriorly, and the “C” shaped retractor B is used to retract the peritoneal contents slightly anteriorly. Fig. 3C. The diagram shows that one Steinmann pin is drilled in a strictly vertical direction to anchor into the cortex of the cephalad vertebral body (at the junction of the anterior three-fourths and posterior one-fourth of the disc space near the border of the disc space, and parallel to the vertebral). Fig. 3D. The photo shows that retractor A and retractor B is connected to the outer frame with an adjustable locking device. Fig. 3E. The diagram shows that the surgical table is tilted 20–30° backward in the axial plane to provide better visualization of the posterior discectomy. Fig. 3F. The photo shows that discectomy and decompression are performed as standard protocol. Fig. 3G. The photo and diagram show a specially designed hollow annulus cutter with a depth limit (The black arrow shows the hollow annulus cutter. The white arrow shows the depth limit.).
Two specially designed retractors, straight “L” shaped retractor A and “C” shaped retractor B (LaLIF Retractor System, Patent No. CN209678575U, WEIGO, Inc. Weihai City, China), are introduced to replace these two S-shaped retractors that were used before. The straight “L” shaped retractor A with two nail slots is used to retract the psoas muscle posteriorly, and the “C” shaped retractor B is used to retract the peritoneal contents slightly anteriorly (Fig. 3B). Meanwhile, the design of the “C” shaped retractor B can help the surgeon who is standing on the ventral side of the patient perform the surgery under direct visualization much easier. Besides, a headlight is used for improved vision. Once direct visualization of the intervertebral disc is obtained by proper retraction of retractors A and B, an annulotomy of 1.5–2 cm 1/3 anterior-medial of the disc space is performed to identify the borders of the disc space. One Steinmann pin is drilled in a strictly vertical direction to anchor into the cortex of the cephalad vertebral body (at the junction of the anterior three fourths and posterior one-fourth of the disc space near the border of the disc space, parallel to the vertebra) (Fig. 3C), the straight “L” shaped retractor A is fixed to the Steinmann pin by keeping it in its nail slot. Then, the other Steinmann pin is placed to the caudal vertebral body through the slot on retractor A. Both Steinmann pins should be placed adjacent to the disc space to avoid injury to the segmental vessels.
For hands-free retracting, an outer frame is attached to the fixed straight “L” shaped retractor A with a locking joint. Then the retractor B is connected to the outer frame with an adjustable locking device (Fig. 3D). Adjust the relative distance between the outer frame and retractor B to provide proper anterior traction, keeping retractor B hands-free.
Once the surgical portal is created between retractors, the surgical table is tilted 20–30° backward in the axial plane to provide better visualization of the posterior discectomy (Fig. 3E). By performing the discectomy and decompression using a specially designed “Z" shaped instrument (LaLIF System, WEIGO, Inc. Weihai City, China) as standard protocol (Fig. 3F), the posterior annulus can be left intact during disc removal to avoid sharp instruments from perforating into the epidural space or neural foramina.
At this point, the surgical table is tilted back to 0° position to allow annulus release, templating, and final placement of the implant in a strictly vertical trajectory. A specially designed hollow annulus cutter with depth limit (Fig. 3G) (LaLIF System, WEIGO, Inc. Weihai City, China) to avoid injuring the contralateral nerve root while performing the contralateral annulus release is used to release the contralateral annulus.
After templating, confirmed by the true AP and true lateral radiographs (Fig. 4A), a specially designed implant which has an olive-shaped ventral (anterior) portion to match the curved shape of the anterior annulus (length 40–60 mm, width 20 mm, and height 8–16 mm, LaLIF System, Patent No. CN209019061U, WEIGO, Inc. Weihai City, China) packed with allograft (Fig. 4B), is placed into the disc space in a strictly vertical trajectory. At this point, an orthogonal maneuver is not performed. True AP and lateral radiographs are used to confirm the implant position in the disc space (Fig. 4C). The retractor is removed delicately, the fascial layer is closed with 0 Vicryl and the subcuticular layer is closed with 2.0 Vicryl sutures. Skin staples are used for skin closure. Patients are allowed to do ambulate using a back brace 3 days after surgery.
Fig. 4.
Illustration of implant placement. Fig. 4A. The radiographs show that templating is confirmed by the true AP and lateral radiographs. Fig. 4B. The photo shows that a specially designed implant which has an olive-shaped ventral (anterior) part is packed with allograft. Fig. 4C. The radiograph shows that the implant position in the disc space is confirmed by the true AP and lateral radiographs.
2.3. Outcomes assessment
All patients were followed up postoperatively using a predesigned protocol. In this study, Visual Analogue Scale (VAS), the Oswestry Disability Index (ODI), and the Short Form 36 Health Survey (SF-36) scores were used to assess the clinical outcomes. VAS scores were recorded preoperatively, on postoperative day #3, and at final follow-up. ODI scores and SF-36 scores were measured preoperatively and at the final follow-up. Operative parameters measured included operative time, intraoperative blood loss, and length of hospital stay. Plain radiographs were obtained preoperatively, on postoperative day #3, and at final follow-up. Radiographic outcome measures including the intervertebral foraminal height (FH), intervertebral disc height (DH), and intervertebral foraminal area (FA). Averages of FH, DH, and FA in multiple levels were calculated and recorded. FH, DH, and FA were measured on neutral lateral radiographs. ImageJ Fiji (2.0 macOS) was used to measure these data.
The recorded data were classified into intraoperative and postoperative complications. Intraoperative complications were subcategorized into the type of damage (neurologic, vascular, ureteral, peritoneal, and vertebral). Postoperative complications were subcategorized into infection, cage migration, subsidence, and other complications. Perioperative complications up to 1 month postoperative were reviewed for the present study.
2.4. Statistical analysis
All statistical analyses in this study were performed using SPSS 17.0 statistical software (IBM Inc., USA) and Prism 7.0 statistical software (Graphpad, USA). Continuous variables were reported as means ± standard deviations and were compared using the unpaired t-test; categorical variables were compared using the Chi-squared test. For all analyses, P < .05 was regarded as statistical significance.
3. Results
The demographic and clinical characteristics of the 126 patients, including 54 males and 72 females, are summarized in Table 1. The average age was 65 ± 11 years (range 42–89 years old). The variables included mean age, gender, follow-up period, and levels fused. All the patients were followed up with a mean time of 20 months (ranging from 6 to 37 months). The mini-open LaLIF procedure was performed at 188 levels in 126 patients, with 1 level in 75 cases, 2 levels in 42, 3 levels in 7, and 4 levels in 2 cases. There were 114 cases who underwent stand-alone LaLIF, and 12 cases underwent secondary posterior fixation.
Table 1.
The demographic and clinical characteristics.
| Content | Value | Proportion | ||
|---|---|---|---|---|
| Demographic | ||||
| Follow-up | 2016/04 ~ 2018/12 | — | ||
| Case | 126 | — | ||
| Male | 54 | 42.9% | ||
| Female | 72 | 57.1% | ||
| Age | 42 - 89 (65 ± 11) | — | ||
| Follow-up (Month) | 6 - 37 (20 ± 11) | — | ||
| Diagnosis | ||||
| Spinal stenosis | 94 | 74.6% | ||
| Degenerative lumbar spondylolisthesis | 21 | 16.7% | ||
| lumbar disc herniation | 20 | 15.9% | ||
| Degenerative scoliosis | 10 | 7.9% | ||
| Level | ||||
| 1 level | 75 | 59.5% | ||
| 2 levels | 42 | 33.3% | ||
| 3 levels | 7 | 5.6% | ||
| 4 levels | 2 | 1.6% | ||
| L2/3 | 17 | 13.5% | ||
| L3/4 | 65 | 51.6% | ||
| L4/5 | 103 | 81.7% | ||
| stand-alone | 114 | 90.5% | ||
| required secondary posterior fixation | 12 | 9.5% | ||
The mean operative time, intraoperative blood loss, and length of hospital stay were recorded. As shown in Table 2, the mean operative results are reported here. The mean operative time was 99.6 ± 34.4 min for 1 level, 126.3 ± 33.8 min for 2 levels, 169.2 ± 68.5 min for 3 levels, and 205 ± 21.2 min for 4 levels. The intraoperative blood loss was 35.2 ± 24.3 mL for 1 level, 58.1 ± 50 mL for 2 levels, 216 ± 239.9 mL for 3 levels, and 142.5 ± 152 mL for 4 levels. The mean length of hospital stay was 8 ± 3 days for 1 level, 9.6 ± 5.5 days for 2 levels, 17 ± 9.6 days for 3 levels, and 16 ± 5.7 days for 4 levels.
Table 2.
No. of Levels with respective operative time, Intraoperative blood loss and length of hospital stay.
| Operative Parameter | Value |
|---|---|
| Operative time (min) | |
| 1 level | 99.6 ± 34.4 |
| 2 levels | 126.3 ± 33.8 |
| 3 levels | 169.2 ± 68.5 |
| 4 levels | 205 ± 21.2 |
| Intraoperative blood loss (mL) | |
| 1 level | 35.2 ± 24.3 |
| 2 levels | 58.1 ± 50 |
| 3 levels | 216 ± 239.9 |
| 4 levels | 142.5 ± 152 |
| Length of hospital stay (days) | |
| 1 level | 8.0 ± 3.0 |
| 2 levels | 9.6 ± 5.5 |
| 3 levels | 17 ± 9.6 |
| 4 levels | 16 ± 5.7 |
The postoperative patient-reported outcomes (VAS scores, ODI, SF-36 scores) had significant improvement after surgery and was maintained until the last follow-up. Table 3 shows the results of patient-reported outcomes in this study, including VAS, ODI, and SF-36 scores. The average preoperative VAS was 7.2 ± 1.4. The average postoperative VAS at three days was 3.1 ± 0.9. The average final follow-up VAS was 1.9 ± 0.8. The difference between the preoperative and postoperative average VAS was significantly different (all P < .05). This included a significant decrease after surgery and was maintained until the last follow-up. The average preoperative ODI was 64.5 ± 4. The average final follow-up ODI was 7.3 ± 2.6. The difference between the preoperative and postoperative average ODI was significantly different (all P < .05). This included a significant drop after surgery and was maintained until the last follow-up. The SF-36 scores included a significant increase after surgery and were maintained until the last follow-up.
Table 3.
The patient reported outcomes.
| Outcome Measure | Preoperative | Postoperative day #3 | Last follow-up | pa Value |
|---|---|---|---|---|
| VAS | 7.2 ± 1.4 | 3.1 ± 0.9 | 1.9 ± 0.8 | <.05 |
| ODI (%) | 64.5 ± 4 | — | 7.3 ± 2.6 | <.05 |
| SF-36 (Physiological function) | 37.8 ± 9.7 | — | 74.2 ± 10.8 | <.05 |
Unpaired t test
Compared to the preoperative measurements, the postoperative radiologic parameters (FH, DH, and FA) showed a significant improvement after surgery and was maintained until the last follow-up. As described in Table 4, the average preoperative FH, DH, FA was 15.3 ± 3.5 mm, 7.8 ± 2.3 mm, 85.7 ± 18.5 mm2. The average postoperative day #3 FH, DH, FA was 18.8 ± 3.4 mm, 12.4 ± 2.3 mm, 119.4 ± 16.7 mm2. The average final follow-up FH, DH, FA was 16.5 ± 3.4 mm, 12 ± 2.2 mm, 112.1 ± 18.7 mm2. The increase in disc height between the preoperative and last follow-up was 13.3%, 69.2%, 37.5%. This included an increase after surgery and was maintained until the last follow-up. The difference between the preoperative and postoperative FH, DH, FA was significantly different (all P < 0.05).
Table 4.
Radiologic parameters.
| Radiologic Parameter | Preoperative | Postoperative day #3 | Last follow-up | Increasing Rate | Pa Value |
|---|---|---|---|---|---|
| FH/mm | 15.3 ± 3.5 | 18.8 ± 3.4 | 16.5 ± 3.4 | 13.3 ± 34.1% | <.05 |
| DH/mm | 7.8 ± 2.3 | 12.4 ± 2.3 | 12.0 ± 2.2 | 69.2 ± 63.9% | <.05 |
| FA/mm2 | 85.7 ± 18.5 | 119.4 ± 16.7 | 112.1 ± 18.7 | 37.5 ± 39.9% | <.05 |
Unpaired t test
Table 5 shows the perioperative complications. A total of 25 complications (19.8%) were recorded. Intraoperative complications accounted for 19 cases (15.1%) in this series. Eight cases (6.3%) had the neurological injury, seven (5.6%) of these cases had sympathetic chain symptoms, one case (0.8%) had nerve root symptom (numbness). Of these patients, seven sympathetic chain symptoms resolved within 4 weeks after treatment, one case had residual neurological symptoms (numbness) at last follow-up. Two cases (1.6%) had the vascular injury during surgery with one case of iliolumbar vein injury was detected with a psoas major hematoma. The patient was placed on bed rest for 1 week and was treated with hemostatic agents and blood transfusions. One case had peritoneal laceration which was repaired successfully during surgery. Eight cases had temporary psoas dysfunction and complained about transient hip flexion weakness. No spinal nerve, cauda equina, and ureteral injury were observed in these series. Postoperative complications up to 1 month were reviewed in this series which accounted for six cases (4.8%). Two cases (1.6%) had deep wound infections, among which one patient had a psoas major abscess. The patients were treated with surgical debridement and antibiotics and no residual complications at final follow-up. Four cases (3.2%) had cage migration postoperatively among which two cases had no symptoms or further implant migration. For the other two cases, the patients were treated with a second stage posterior fixation due to persistent neurological symptoms. However, at the final follow-up, there was no further cage migration or subsidence.
Table 5.
Perioperative complications.
| Perioperative complications | No. of Pts (%) |
|---|---|
| Intraoperative (19 Cases, 15.1%) | |
| Neurological injury | 8 (6.3) |
| Nerve root symptom | 1 (0.8) |
| Sympathetic chain symptom | 7 (5.6) |
| Vascular injury | 2 (1.6) |
| Segmental artery | 1 (0.8) |
| Other vessels (iliolumbar vein) | 1 (0.8) |
| Peritoneum laceration | 1 (0.8) |
| Temporary psoas injury (transient thigh pain and hip flexion weakness) | 8 (6.3) |
| Postoperative (within 1 months after surgery, 6 cases, 4.8%) | |
| Surgical site infection | 2 (1.6) |
| Cage migration | 4 (3.2) |
| Total | 25 (19.8) |
4. Discussion
The minimally invasive retroperitoneal lateral lumbar interbody fusion (LLIF) has been used for 20 years since its first introduction [4]. In this period, many modifications with lateral or oblique approaches have been described by different authors [3,[5], [6], [7], [8], [9],14]. However, these techniques have been associated with complications such as neurologic, vascular, ureteral, peritoneal, and vertebral injury, infection, cage migration & subsidence [6,10,12,[15], [16], [17], [18]]. Among these techniques, the antepsoas approach is highly promising which uses a natural anatomic corridor approaching the interest level to avoid neural, vascular, and visceral injury [8]. Thus, this procedure has gained more attention recently, and it continues to evolve for purpose of reducing the risk of perioperative complications.
Without consideration of the different terms used to describe different antepsoas techniques (minimally invasive extraperitoneal approach [19], mini-open antero-lateral [20], OLIF [6], lateral retroperitoneal approach [21], extensile anterolateral [9], antero-oblique approach [22]), this type of antepsoas techniques includes the following key elements: (1) the patient is in the lateral decubitus position; (2) there is a blunt dissection of the external oblique, the internal oblique, and the transversus abdominis sequentially to reach the retroperitoneal space; and (3) the usage of the natural corridor between the left lateral border of the aorta and the anterior medial border of the psoas. In light of the complications, the OLIF has fewer vascular complications than ALIF and lower neurologic complications than transpsoas approaches [10]. However, the relatively high rates of vascular injury, peritoneal laceration, ureteral injury, and implant subsidence still occur with OLIF [8,10,11]. Thus, the focus has been placed on improving both techniques since subtle differences affect the complication profiles [12].
Based on the key elements of antepsoas techniques, a novel procedure which is performed via a mini-open lateral-anterior approach to the anterior annulus is presented. We suggest using the term LaLIF (Lateral-anterior Lumbar Interbody Fusion), just as ALIF and PLIF are used. The detailed technical description of mini-open LaLIF as follows: (1) The mini-open incision, approximately 4–6 cm, is made in an oblique direction parallel to the target disc space starting from the anterior one-third of the disc space under true lateral fluoroscopy. The incision site is located posteriorly to an OLIF incision and anteriorly to a transpsoas incision which is more suitable to get direct visualization and use a strictly vertical trajectory in multiple steps. (2) The specially designed LaLIF system (straight “L” shaped retractor A with two nail slots, “C” shaped retractor B, Implant with an olive-shaped ventral (anterior) part, and hollow annulus cutter with depth limit) provides direct visualization during the entire procedure; thus, neuromonitoring is not necessary during access, and muscle relaxants can be used. Furthermore, the standardized system makes LaLIF repeatable and also has a shallow learning curve; (3) The annulus release, templating, and final placement of the implant are performed in a strictly vertical trajectory. The Orthogonal maneuver which rotates the obliquely inserted instrument to direct lateral is not performed; (4) Specially designed implants have an olive-shaped ventral (anterior) part and especially suitable for the curve shape of the anterior annulus, it can provide more space with anterior curve shape for loading more graft material because of its relatively larger size (length 40–60 mm, width 20 mm, and height 8–16 mm). A specially designed hollow annulus cutter with a depth limit (LaLIF System, WEIGO, Inc. Weihai City, China) is used to avoid injuring the contralateral nerve root while performing the contralateral annulus release. (5) The surgical table is tilted forward and backward in the axial plane according to different surgical steps including incision (0° or 20° backward), dissection (20–30° backward), discectomy (20–30° backward), and decompression (20–40° backward), annulus release (0°), templating (0°), implant placement (0°). In addition, this technique is adaptable without the LaLIF system which avoids potential confusion between the naming of techniques and particular commercial products (such as the OLIF and OLIF25 implant, Medtronic, US). Before the specially designed instruments were introduced, our team used a traditional S-shaped retractor, which has been forcefully bent for adapting multiple surgical purposes and as exposure tools during the entire procedure.
The primary objective in LaLIF surgery is to minimize the risk of intraoperative complications associated with OLIF, such as vascular injury, peritoneal laceration, ureteral injury. Three factors in the LaLIF technique contribute to achieving this goal: the location of the incision, direct visualization during the entire procedure, and using a strictly vertical trajectory in multiple steps. The first factor is the incision site. The modified incision is approximately 4–6 cm starting from the anterior one-third of the disc space. This is in distinction to OLIF where the incision is approximately 3–5 cm from the midportion of the AP line [8]. Thus, the LaLIF incision site is placed dorsally compared to OLIF. This potentially places less stretch on the peritoneal contents which may lessen the risk of major vascular injury, peritoneal laceration, and ureteral injury. Ultimately, there was only one patient in this series with peritoneal laceration, and no patient had a major vascular or ureteral injury. The risk of peritoneal and ureteral complications after LaLIF seems to be lower than what has been reported with the OLIF approach (peritoneal complication rates of 1.9% and ureteral complication rates of 0.3%) [16,17]. Furthermore, the ‘strictly vertical trajectory’ can be used easily due to the dorsally placed incision site. However, LaLIF cannot be used to gain access to the L5–S1 disc, similar to the transpsoas approach, the corridor is limited by the superior edge of the iliac crest because of the dorsally located incision cite. The second factor is direct visualization during the entire procedure with LaLIF retractors. Due to the advantage of specially designed LaLIF retractors, ideal direct visualization can be obtained during the entire procedure. This is in distinction to OLIF where direct visualization is not obtained since sequential dilation is performed to displace the surrounding tissues [8]. Therefore, neuromonitoring was not used during access in this series, and the muscle relaxants can be sufficiently used which significantly minimizes the psoas retraction forces compared to OLIF. Not the same as the tube retractor in OLIF which is attached to the flexible arm, the specially designed LaLIF retractor is able to separately holding by hands. Thus, the surgeon and his/her assistant can flexibly adjust the retraction force, distance, and duration time of the retracting process. Thus, postoperative psoas weakness was 6.3% with the use of LaLIF which is lower than 13.5% with the OLIF approach [16]. Furthermore, all 8 cases reported with transient thigh pain and hip flexion weakness are reported in the early phase after the introduction of LaLIF surgery. When the surgeon’s skills and experience on the LaLIF become sophisticated, the processing time of each level is limited to 20 min and there is no sign of transient thigh pain and hip flexion weakness with short-term retraction of the psoas muscle. In addition, Because of direct visualization, there was only one patient in this series who had segmental vessel injury, which is lower than 2.8% in OLIF [11]. The third factor is using a strictly vertical trajectory in multiple steps. Particular attention should be paid to the orientation and force while performing the templating, contralateral annulus release, and ultimate placement of the implant in OLIF. It may be possible to perforate the posterior annulus towards the epidural space or contralateral neural foramina with sharp instruments with excessive force in the oblique trajectory [8,21]. Some surgeons, however, report using stereotactic navigation to avoid injuring the nerves, especially the contralateral nerve root [23]. Besides, placing the cage in an oblique direction may not be able to restore coronal deformity as it would be done [21]. Therefore, by using a ‘strictly vertical trajectory’, the LaLIF procedure provides easier identification and direct access of posterior and contralateral annulus to avoid the risks of neurological injury associated with the oblique corridor. Additionally, the coronal deformity is restored by placing the implant in a strictly vertical direction. Thus, there is only 1 patient with nerve root symptom with the use of LaLIF, which is lower than 13.7% [24] in the transpsoas approach and 13.5% [16] in OLIF. In summary, by using the dorsally placed incision site and specially designed LaLIF retractors, direct visualization can be obtained during the entire procedure. Thus, neuromonitoring is not necessary and muscle relaxants can be sufficiently used so that the psoas can be gently and easily retracted posteriorly. Ultimately, the strictly vertical trajectory can be used in multiple steps to minimize the risk of intraoperative complications and restore lumbar coronal deformity.
The secondary objective in LaLIF surgery is to make the process repeatable and also maintain a shallow learning curve. For achieving that goal, a series of specially designed LaLIF instruments are introduced to standardize the mini-open LaLIF technique (straight “L” shaped retractor A with two nail slots, “C” shaped retractor B, Implant with olive-shaped ventral (anterior) part; hollow annulus cutter with a depth limit.). In addition, the surgical table is tilted forward and backward in the axial plane for adapting multiple surgical purposes, such as tilting 20–30° backward to provide better visualization for a thoroughly posterior discectomy and tilting back to the 0° position to perform implant placement in a strictly vertical trajectory. Furthermore, we found that using a strictly vertical trajectory is significantly important because one of the inherent drawbacks to the OLIF is that the oblique trajectory can be disorienting. Whereas the ALIF and transpsoas approaches allow a perpendicular angle to the spinal column, the OLIF approach does not [23,25]. Reports have shown that lateral insertion of the implant provides ideal placement because the kinematic center of rotation is located posteriorly within the device [26]; therefore, the annulus release, templating, and final placement of the implant in mini-open LaLIF technique is performed in a strictly vertical trajectory guided by ‘true lateral radiograph’ to avoid disorientation which is common in the early stages of learning. In addition, LaLIF surgery places a specially designed implant in disc space which has an olive-shaped ventral (anterior) part that matches the curved shape of the anterior annulus. Reports have shown that anteriorly placed implants allowed for high fusion rates and indirect neural decompression [8]. Because of its relatively larger size (length 40–60 mm, width 20 mm, and height 8–16 mm) and the anterior curved shape, the LaLIF implant can provide more space for various types of graft material. Therefore, the LaLIF implant may achieve better fusion rates compared to ALIF, LLIF, and OLIF.
Limitations do exist with the mini-open LaLIF technique and this LaLIF study. For the LaLIF technique, even temporary posterior retraction of the psoas muscle during annulus release, templating, and final placement of the implant may post the risk of psoas muscle injury and other related neurological complications. Meanwhile, the risk of sympathetic trunk injury exists. Furthermore, just as in other lateral approaches, potential exposure sites can be limited by the inferior edge of the 12th rib and the superior edge of the iliac crest. For this LaLIF study, endplate damage cases have not been recorded because patients who had severe lumbar osteoporosis were excluded from the study. Furthermore, fusion rate analysis and detailed discussion of complications have not been reported thus far, we will provide these in future studies.
5. Conclusion
Based on perioperative complications and mid-term follow-up data, the mini-open LaLIF is a reproducible novel technique that can be performed safely at L2-L5 for degenerative lumbar conditions. Due to the advancements in its modified incision site, direct visualization, and the usage of a strictly vertical trajectory in multiple steps with the specially designed retractors, the mini-open LaLIF provides an alternative approach that obtains reliable mid-term clinical outcomes, minimizes the risks of perioperative complications, and makes the retroperitoneal approach repeatable with a shallow learning curve. Longer follow-up is required, but mid-term follow-up outcomes are encouraging.
Translational potentials
The translational potential of this study is to make the lumbar fusion process repeatable and also maintain a shallow learning curve, especially for surgeons in the early stages of learning, by using instruments with the required standard characteristics, the standardized surgical steps, modified incision site, vertical trajectory, and the direct visualization during the entire procedure.
Declaration of competing interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
The authors thank Dean Chou from UCSF for his assistance with paper review.
References
- 1.Taba HA, Williams SK. Lateral lumbar interbody fusion. Neurosurg Clin N Am. 2020;31(1):33–42. doi: 10.1016/j.nec.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 2.McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976) 1998;23(13):1476–1484. doi: 10.1097/00007632-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22(6):691–699. doi: 10.1097/00007632-199703150-00023. discussion 700. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser MG, Haid RW, Jr., Subach BR, Miller JS, Smith CD, Rodts GE., Jr. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51(1):97–103. doi: 10.1097/00006123-200207000-00015. discussion -5. [DOI] [PubMed] [Google Scholar]
- 6.Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: Oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6(2):89–97. doi: 10.4184/asj.2012.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis TT, Hynes RA, Fung DA, Spann SW, MacMillan M, Kwon B. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine. 2014;21(5):785–793. doi: 10.3171/2014.7.SPINE13564. [DOI] [PubMed] [Google Scholar]
- 8.Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J. 2017;17(4):545–553. doi: 10.1016/j.spinee.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Molloy S, Butler JS, Benton A, Malhotra K, Selvadurai S, Agu O. A new extensile anterolateral retroperitoneal approach for lumbar interbody fusion from L1 to S1: a prospective series with clinical outcomes. Spine J. 2016;16(6):786–791. doi: 10.1016/j.spinee.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Mehren C, Mayer HM, Zandanell C, Siepe CJ, Korge A. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complications. Clin. Orthopaedics Related Res. 2016;474(9):2020–2027. doi: 10.1007/s11999-016-4883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu DS, Walker CT, Godzik J, Turner JD, Smith W, Uribe JS. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: a literature review. Ann Transl Med. 2018;6(6):7. doi: 10.21037/atm.2018.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker CT, Farber SH, Cole TS, Xu DS, Godzik J, Whiting AC. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine. 2019:1–15. doi: 10.3171/2018.9.SPINE18800. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Cui HW, Li ZH, Wang JR, Li ZM, Upadhyay AM. Correlation study of radiographic characteristics and operative difficulty in lateral-anterior lumbar interbody fusion (LaLIF) at the L4-5 level: a novel classification for case selection. Eur Spine J. 2020:1–11. doi: 10.1007/s00586-020-06570-w. [DOI] [PubMed] [Google Scholar]
- 14.Ohtori S, Mannoji C, Orita S, Yamauchi K, Eguchi Y, Ochiai N. Mini-open anterior retroperitoneal lumbar interbody fusion: Oblique lateral interbody fusion for degenerated lumbar spinal kyphoscoliosis. Asian Spine J. 2015;9(4):565–572. doi: 10.4184/asj.2015.9.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAfee PC. Vascular injury during anterior lumbar spine surgery. Spine J. 2005;5(1):118. doi: 10.1016/j.spinee.2004.09.008. author reply -9. [DOI] [PubMed] [Google Scholar]
- 16.Abe K, Orita S, Mannoji C, Motegi H, Aramomi M, Ishikawa T. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: Perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017;42(1):55–62. doi: 10.1097/BRS.0000000000001650. [DOI] [PubMed] [Google Scholar]
- 17.Fujibayashi S, Kawakami N, Asazuma T, Ito M, Mizutani J, Nagashima H. Complications associated with lateral interbody fusion: Nationwide survey of 2998 cases during the first 2 years of its use in Japan. Spine (Phila Pa 1976) 2017;42(19):1478–1484. doi: 10.1097/BRS.0000000000002139. [DOI] [PubMed] [Google Scholar]
- 18.Tannoury T, Kempegowda H, Haddadi K, Tannoury C. Complications associated with minimally invasive anterior to the psoas (ATP) fusion of the lumbosacral spine. Spine (Phila Pa 1976) 2019;44(19):E1122–E1129. doi: 10.1097/BRS.0000000000003071. [DOI] [PubMed] [Google Scholar]
- 19.Saraph V, Lerch C, Walochnik N, Bach CM, Krismer M, Wimmer C. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13(5):425–431. doi: 10.1007/s00586-004-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel NP, Birch BD, Dement SE, Elbert GA. The mini-open anterolateral approach for degenerative thoracolumbar disease. Clin Neurol Neurosurg. 2010;112(10):853–857. doi: 10.1016/j.clineuro.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Aghayev K, Vrionis FD. Mini-open lateral retroperitoneal lumbar spine approach using psoas muscle retraction technique. Technical report and initial results on six patients. Eur Spine J. 2013;22(9):2113–2119. doi: 10.1007/s00586-013-2931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan K, Maharaj M, Assem Y, Mobbs RJ. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF) J Clin Neurosci. 2016;31:23–29. doi: 10.1016/j.jocn.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 23.DiGiorgio AM, Edwards CS, Virk MS, Mummaneni PV, Chou D. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurg Focus. 2017;43(2):E14. doi: 10.3171/2017.5.FOCUS17168. [DOI] [PubMed] [Google Scholar]
- 24.Joseph JR, Smith BW, La Marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus. 2015;39(4):E4. doi: 10.3171/2015.7.FOCUS15278. [DOI] [PubMed] [Google Scholar]
- 25.DiGiorgio AM, Edwards CS, Virk MS, Chou D. Lateral prepsoas (Oblique) approach nuances. Neurosurg Clin N Am. 2018;29(3):419–426. doi: 10.1016/j.nec.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L. Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine. 2011;14(1):38–45. doi: 10.3171/2010.9.SPINE09865. [DOI] [PubMed] [Google Scholar]