Abstract
Microcephaly and macrocephaly can be considered both cranial growth defects and clinical symptoms. There are two assessment criteria: one applied in dysmorphology and another conventionally used in clinical practice. The determination of which definition or under which paradigm the terminology should be applied can vary on a daily basis and from case to case as necessity dictates, as can defining the relationship between microcephaly or macrocephaly and syndromes or diseases associated with neurodysfunction. Thus, there is a need for standardization of the definition of microcephaly and macrocephaly. This study was designed to investigate associations between abnormal cranial development (head size) and diseases or syndromes linked to neurodysfunction based on essential data collected upon admission of patients to the Neurological Rehabilitation Ward for Children and Adolescents in Poland. The retrospective analysis involved 327 children and adolescents with medical conditions associated with neurodysfunction. Two assessment criteria were applied to identify subgroups of patients with microcephaly, normal head size, and macrocephaly: one system commonly used in clinical practice and another applied in dysmorphology. Based on the results, children and adolescents with syndromes or diseases associated with neurodysfunction present abnormal cranial development (head size), and microcephaly rarely co-occurs with neuromuscular disease. Macrocephaly frequently co-occurs with neural tube defects or neuromuscular diseases and rarely with cerebral palsy (p < 0.05); microcephaly frequently co-occurs with epilepsy and hypothyroidism (p < 0.001). Traditional classification facilitates the identification of a greater number of relationships and is therefore recommended for use in daily practice. There is a need to standardize the definition of microcephaly and macrocephaly and to include them in ‘Human Phenotype Ontology’ terms.
Subject terms: Development of the nervous system, Diseases of the nervous system
Introduction
Microcephaly and macrocephaly are cranial growth defects as well as clinical symptoms. It is commonly known that head size is an indicator of brain size. The size of the cranium is assessed by measuring head circumference (HC), or more precisely occipitofrontal circumference (OFC), and by comparing the result to a biological reference system. Subsequently, it must be determined whether the head circumference measured is normal or abnormal, with the latter reflecting microcephaly or macrocephaly. There are two assessment criteria: one applied in dysmorphology and another traditionally used in clinical practice1–6. According to the first classification, microcephaly occurs when head circumference is smaller than the value corresponding to the difference between the mean and 3 standard deviations (x − 3SD), whereas macrocephaly occurs when the head circumference is larger than the value corresponding to the total of the mean and 3 standard deviations (x + 3SD). According to the second classification, microcephaly occurs when head circumference is smaller than the value corresponding to the difference between the mean and 2 standard deviations (x − 2SD) and macrocephaly when the head circumference is larger than the value corresponding to the total of the mean and 2 standard deviations (x + 2SD)1–6. As these definitions are not equivalent, an attempt has been made to identify which system should be used on a daily basis and the relationship between microcephaly or macrocephaly as well as the syndromes or diseases associated with neurodysfunction. Accordingly, there is a need for standardization of the definition of microcephaly and macrocephaly and for their inclusion in Human Phenotype Ontology (HPO) terms. Our own clinical observations have revealed that some of the children admitted to Neurological Rehabilitation Wards for Children and Adolescents in Poland are not diagnosed. We have sought to identify a relationship between symptoms and diagnosis in a group differentiated in terms of diagnosis: children and adolescents with congenital nervous system disorders or neurological syndromes with one or more neurodysfunctions visible since infancy. If a clear relationship between relative head size and diagnosis cannot be established, then knowledge of the relationship between microcephaly/macrocephaly and the group or subgroup of diseases with which it coexists may be a useful indicator in the differential diagnostic process.
This study was designed to investigate relationships between abnormal cranial development (head size) and diseases or syndromes linked to neurodysfunction based on essential data collected upon admission of patients to the Neurological Rehabilitation Ward for Children and Adolescents.
Materials and methods
Participants and setting
A retrospective study examined relevant information related to 327 patients receiving treatment during 2012–2016 at the Neurological Rehabilitation Ward for Children and Adolescents in St. Queen Jadwiga Regional Hospital No. 2 in Rzeszów, at the Clinical Regional Rehabilitation and Education Centre (KRORE), Poland. The following eligibility criteria were applied: age between 4 and 18 years; congenital nervous system disorder diagnosis or a neurological syndrome accompanied by at least one neurodysfunction manifesting from early childhood; availability of head circumference measurements; hospitalization in the period from 2012 to 2016; a single selected patient admission procedure; complete data (diagnosis, as well as anthropometric measurements—head circumference); and informed consent obtained from both the patient and parents/legal guardians. The following exclusion criteria were used: age under 4 or above 18 years (there was no biological frame of reference available); no diagnosis of a congenital nervous system disorder or neurological syndrome accompanied by at least one neurodysfunction manifesting in early childhood; more than one congenital nervous system disorder or neurological syndrome (for example: cerebral palsy (CP) co-occurring with Down syndrome; CP co-occurring with neural tube defect or with phenylketonuria); no head circumference measurements available; hospitalization before 2012 or after 2016; more than one selected patient admission procedure; lack of complete data (diagnosis, as well as anthropometric measurements); and lack of informed consent from both the patient and parents/legal guardians.
This study focuses only on classical definitions, taking into account the disturbance of the growth process (head circumference enlargement); the differentiation of body proportions (head size vs. body size) was not analyzed. Similar criteria were also adopted by Arroyo4, Sarno et al.5, and Jayaraman et al.6.
During the relevant period, 2637 children and adolescents were hospitalized at KRORE. An initial review of the medical records showed that 327 patients met the above inclusion criteria and that 2310 children did not meet the eligibility criteria. Ultimately, the retrospective analysis considered data related to 327 children (143 girls—43.7%, 184 boys—56.3%), with a mean age of 9.7 ± 4.3 years (median—9.0 years, minimum—4 years, maximum—18 years). Although there was a disproportionate ratio of boys to girls in our cohort, many similar studies enrolling more boys than girls have been conducted in the field of neurodysfunction. It was therefore concluded that conducting such a research analysis of this type would be acceptable given the data available7,8.
Study protocol
The study was reviewed and approved by the Bioethics Commission of the Medical Faculty at the University of Rzeszow (Approval Ref. no. 10/04/2019). All procedures were carried out in full compliance with the Declaration of Helsinki. Informed consent was given by the children and their parents/legal guardians and by the director of the hospital. Letters of consent were received before the study protocol was filed with the bioethics committee.
Procedures and data analyses
Basic data, acquired upon the patients’ admission, i.e., age, sex, principal and additional diagnosis, and head circumference (HC), were considered in the retrospective analysis. Anthropometric measurements were performed by KRORE staff in accordance with the principles adopted in the hospital; principal and additional diagnoses, as determined prior to hospitalization at KRORE, were performed by relevant specialists (geneticists, neurologists, endocrinologists, etc.). The patients involved in the study presented various diseases or syndromes linked with nervous system damage; more specifically, they were all affected by congenital disorders and/or conditions, with or without encephalopathy, in association with motor defects (neurodysfunctions) manifesting from infancy. The patients were divided into subgroups according to the suspected presence or lack of encephalopathy, etiopathogenesis and nature, following the criteria proposed in the literature1. The intention of the authors was to indicate the relationship with the disease or syndrome, and if impossible, based on different subgroups. This would allow for an effective differential diagnostic process with a given subgroup of diseases and/or syndromes1,3.
To identify subgroups of patients with microcephaly, normal head size, and macrocephaly, two assessment criteria were applied: one system commonly used in clinical practice and another applied in dysmorphology2,4–6. For analyses of all of the above criteria, the z-score for head circumference (z-score HC) was computed for each patient. Normative values published earlier9 were applied as references. For years, one of the authors has been participating in research on the secular trend in the development of children and youth from Rzeszow (the capital of Podkarpackie Province). Research on the somatic development of children and adolescents from Rzeszow began in 1978/1979 and was repeated in 1993/1994, 2003/2004 and 2013/20149–15, which enabled ascertainment of the rules of growth in children and adolescents. At the Clinical Regional Rehabilitation and Education Centre (Rzeszow, Poland), the daily clinical work of this author involves children and adolescents with neurodysfunctions, and she often observes changes in their physical development. To define disorders such as microcephaly/macrocephaly, an additional anthropometric parameter (head circumference) was introduced during the research conducted in 2013/20149. It was the first time that tables of norms containing mean values and standard deviation of the head circumference, taking into account age and sex, were presented9. Both studied groups of school children (Rzeszow, Poland) and the Clinical Regional Rehabilitation and Education Centre (Rzeszow, Poland) are ethnically homogeneous. A similar methodology based on the z-score and reference system was used earlier in research16. When calculating the z-score, the value of the anthropometric feature in children and adolescents affected by syndromes or diseases associated with neurodysfunction was used, as was the value of the mean and standard deviation of the anthropometric feature from the reference system, taking into account sex and age16. The selected anthropometric parameter used was head circumference9.
Further analyses focused on possible correlations between co-occurring abnormalities of cranial development, i.e., microcephaly or macrocephaly, and medical conditions linked to neurodysfunction; assessment relative to the subgroups identified and within these subgroups was also carried out. Furthermore, additional diagnoses, i.e., hypothyroidism, symptomatic epilepsy and genetically conditioned epilepsy syndrome were considered. Adjusted standardized residuals (ASRs) are shown in crosstabs, in addition to percentages. Values below and above the threshold of 1.96 were assumed to reflect a smaller and a greater number, respectively, than in the case of a random distribution. Methods of statistical inference were used to assess to what degree intergroup differences corresponded to certain regularities found in the target population and to what extent this could be due to some random differences. A chi-square test of independence was applied due to the nominal nature of the characteristics subjected to comparative analyses. Relationships between the dependent qualitative and independent quantitative variables were examined using nominal regression. Statistical significance was assumed at p < 0.05.
Results
Seven subgroups were identified (Table 1A). Six of these comprised children with medical conditions usually presenting with encephalopathy, as follows: progressive metabolic disorders (2.1%), progressive epilepsy—genetically determined epileptic syndromes (0.3%), nonprogressive cerebral palsy (73.1%), nonprogressive neural tube defects (7.3%), nonprogressive genetic disorders (chromosomal aberrations, monogenic disorders excluding neuromuscular diseases) (7%), and nonprogressive toxicity (0.3%). There was also one subgroup comprising patients with neuromuscular disorders (9.8%), which commonly occur without encephalopathy. Based on the expected presence and characteristics of encephalopathy1,3, the six small subgroups were combined into two larger subgroups corresponding to progressive encephalopathy (PE) (2.4%) and nonprogressive encephalopathy (NPE) (88.1%); the third group consisted of patients with neuromuscular diseases (NMDs) (9.8%). Given that neural tube defects vary greatly17 and that surgical treatment is of key importance for development18, the children in this subgroup were divided based on the cause of the surgery (myelomeningocele and hydrocephalus versus myelomeningocele alone). The remaining cases, for which no surgical treatment was applied, are also presented. Both chromosomal aberrations and genetic mutations were included in the subgroup with genetic disorders (GD), as in other studies19,20. The characteristics of the study group are presented in Table 1.
Table 1.
Characteristics of the study group (A) and abnormal cranial development (B–D).
| (A) Characteristics of 327 patients with neurodysfunction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diseases and syndromes associated with neurodysfunction (principal diagnosis) | Classification with regard to the etiopathogenesis, presence and character of encephalopathy | Classification with regard to the presence and character of encephalopathy | ||||||
| N | %* | N | %* | N | % | |||
| NBIA-MPAN, Neurodegeneration with Brain Iron Accumulation—Mitochondrial Protein Associated Neurodegeneration | 2 | 0.6 | MD, encephalopathy in metabolic disorder | 7 | 2.1 | PE, progressive encephalopathy | 8 | 2.4 |
| GSD II, Pompe disease | 1 | 0.3 | ||||||
| LCHAD, long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency | 1 | 0.3 | ||||||
| SLO, Smith–Lemli–Opitz syndrome | 1 | 0.3 | ||||||
| GLUT1d, glucose transporter 1 deficiency | 1 | 0.3 | ||||||
| NKH, nonketotic hyperglycinemia | 1 | 0.3 | ||||||
| SMEI, Dravet syndrome | 1 | 0.3 | EE, epileptic encephalopathy | 1 | 0.3 | |||
| sasMMC&HCP, state following surgery due to lumbar myelomeningocele and hydrocephalus | 17 | 5.2 | NTDs, encephalopathy in neural tube defects | 24 | 7.3 | NPE, nonprogressive encephalopathy | 287 | 88.1 |
| sasMMC, state following surgery due to lumbar myelomeningocele | 3 | 0.9 | ||||||
| sasMM, state following surgery due to parieto-occipital meningocele | 1 | 0.3 | ||||||
| ACM, Arnold–Chiari malformation | 2 | 0.6 | ||||||
| HCP, isolated hydrocephalus | 1 | 0.3 | ||||||
| DS, Down syndrome | 11 | 3.4 | GD, encephalopathy in genetic disorders | 23 | 7.0 | |||
| ES, Edwards syndrome | 1 | 0.3 | ||||||
| PMS, Phelan–McDermid syndrome | 2 | 0.6 | ||||||
| MWS, Mowat–Wilson syndrome | 1 | 0.3 | ||||||
| AS, Angelman syndrome | 1 | 0.3 | ||||||
| DGS, Di George syndrome | 1 | 0.3 | ||||||
| 46,XY,del(X)(q24) | 1 | 0.3 | ||||||
| CdLS, Cornelia de Lange syndrome | 1 | 0.3 | ||||||
| SDS, Schwachman–Diamond syndrome | 1 | 0.3 | ||||||
| PWS, Prader–Willi syndrome | 1 | 0.3 | ||||||
| 46 XX, add(2)(q25) | 1 | 0.3 | ||||||
| 46XX, del (12) (q24.21q24.23) | 1 | 0.3 | ||||||
| FAS, fetal alcohol syndrome | 1 | 0.3 | TE, toxic encephalopathy | 1 | 0.3 | |||
| CP, cerebral palsy | 239 | 73.1 | CP encephalopathy in cerebral palsy | 239 | 73.1 | |||
| HMSN, hereditary motor and sensory polyneuropathy | 8 | 2.4 | NMD, neuromuscular disorders | 32 | 9.8 | NMD, neuromuscular disorders | 32 | 9.8 |
| LGMD, muscular dystrophy limb-girdle | 7 | 2.1 | ||||||
| BMD, Becker muscular dystrophy | 3 | 0.9 | ||||||
| DMD, Duchenne muscular dystrophy | 7 | 2.1 | ||||||
| TD, Thomsen disease | 1 | 0.3 | ||||||
| AMC&N arthrogryposis multiplex congenita with neuropathy | 3 | 0.9 | ||||||
| CM, congenital myopathy | 1 | 0.3 | ||||||
| SMA, spinal muscular atrophy | 2 | 0.6 | ||||||
| Total | 327 | 99,7 | Total | 327 | 99,9 | Total | 327 | 100 |
| (B) Numerical characteristics of z-score HC | ||||||||
|---|---|---|---|---|---|---|---|---|
| z-score | N | Me | SD | c25 | c75 | Min | Max | |
| z-score HC | 327 | − 0.53 | − 0.54 | 2.14 | − 1.71 | 0.86 | − 7.36 | 8.29 |
| (C) Cranial growth abnormalities (the size of the head)—criteria | ||||||||
|---|---|---|---|---|---|---|---|---|
| The size of the head | Dysmorphology classification (HC) | Traditional classification (HC) | ||||||
| Normal | − 3 ≥ z-score HC ≤ 3 | − 2 ≥ z-score HC ≤ 2 | ||||||
| Microcephaly | z-score HC < − 3 | z-score HC < − 2 | ||||||
| Macrocephaly | z-score HC > 3 | z-score HC > 2 | ||||||
| (D) Prevalence of cranial growth abnormalities (the size of the head) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dysmorphology classification (HC) | The size of the head | Traditional classification (HC) | ||||||
| N | N% | N | N% | |||||
| 273 | 83.5 | Normal | 224 | 68.5 | ||||
| 41 | 12.5 | Microcephaly | 72 | 22 | ||||
| 13 | 4.0 | Macrocephaly | 31 | 9.5 | ||||
N numbers of patients, % percent, z-score HC z-score for head circumference, SD standard deviation, Min minimum value, Max maximum value.
aThe percentages have been rounded to one digit after the decimal point, so the total value is 99.7% and 99.9% instead of 100%
Patients with CP constituted a significant majority (73.1%). Based on the diagnoses, the cases involved spastic (93.7%; N = 223), mixed (5%; N = 12), and ataxic (1.7%; N = 4) types of CP, as based on Hagberg’s classification1,3; no cases involved a dyskinetic type. In the group of children with spastic CP, 40.4% were found to have diplegia (N = 90), 34.1% (N = 76) to have tetraplegia, and 25.6% to have hemiplegia (N = 57). The primary diagnoses were accompanied by additional diagnoses of symptomatic epilepsy 26.3% (N = 86) and hypothyroidism 4.3% (N = 14). Selected numerical characteristics of z-score HC, i.e., the arithmetic mean (), median (Me), standard deviation (SD), minimum (Min), maximum value (Max), 25th centile (c25) and 75th centile (c75), are shown in Table 1B. The mean and median z-score HC in the study group was lower than 0 and higher than − 1, respectively.
Taking into account dysmorphology criteria (Table 1C), normal head size was found in 83.5% of the patients, as compared to 16.5% of the patients presenting with abnormalities, namely, microcephaly 12.5% and macrocephaly 4.0% (Table 1D). In accordance with traditional criteria (Table 1C), normal head size was found in 68.5%, and abnormalities were identified in 31.5% of the patients (microcephaly 22% and macrocephaly 9.5%) (Table 1D).
Subsequent analyses focused on relationships between co-occurring abnormalities of cranial growth (head size) and diseases/syndromes associated with neurodysfunction, also relative to (and between) the identified subgroups. Additional diagnoses were also considered.
The findings showed more/less common co-occurrence of as well as statistically significant relationships between the size of the head—dysmorphology classification (HC) and the following:
entities/syndromes associated with neurodysfunction (Table 2A). Normal head size rarely co-occurred with isolated hydrocephalus (HCP), Edwards syndrome (ES), or Di George syndrome (DGS). Microcephaly frequently co-occurred with ES, DGS, and CP. Macrocephaly was rarely found to co-occur with CP and frequently with ACM (Arnold–Chiari malformation), HCP, Phelan–McDermid syndrome (PMS), and Becker’s muscular dystrophy (BMD). The relationship was statistically significant (p = 0.026),
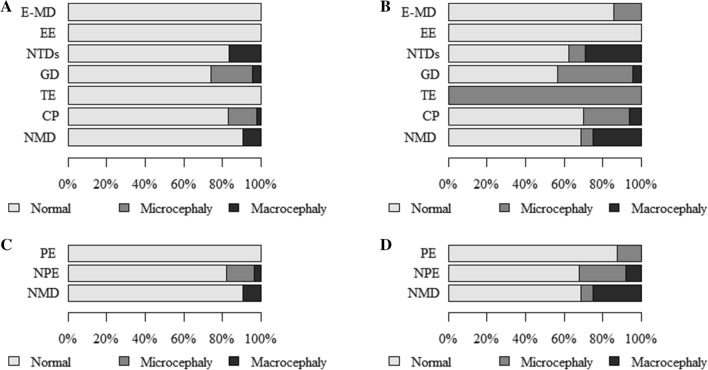
classification with regard to the etiopathogenesis, presence and character of encephalopathy (Table 2B and Fig. 1A). Microcephaly was found to co-occur more frequently in children with CP but rarely in those with NMD. Macrocephaly frequently co-occurred with neural tube defects (NTDs) but rarely with CP. The relationship was statistically significant (p = 0.010),
classification with regard to the presence and character of encephalopathy (Table 4A and Fig. 1C). Microcephaly frequently occurred in NPE and rarely in the subgroup with NMD. The relationship was statistically significant (p = 0.060, Cp = 0.146),
epilepsy (Table 4F and Fig. 2D). Normal head size rarely co-occurred with epilepsy and frequently with a lack of epilepsy. Microcephaly and epilepsy were often found to co-occur. Microcephaly and lack of epilepsy co-occurred rarely. The relationship was statistically significant (p < 0.001),
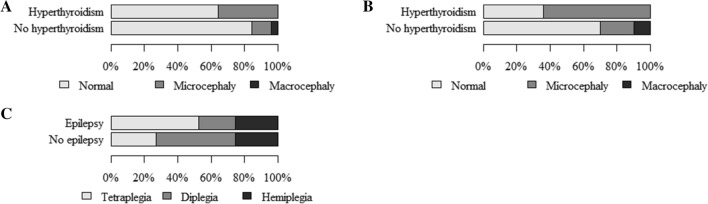
hypothyroidism (Table 5A and Fig. 3A). Normal head circumference often co-occurred with a lack of hypothyroidism and was rarely found in patients with hypothyroidism. Microcephaly frequently co-occurred with hypothyroidism and rarely in the absence of this condition. The relationship was statistically significant (p = 0.024, Cp = 0.105).
Table 2.
The size of the head—dysmorphological classification (HC) and (A) units and syndromes associated with neurodysfunction, (B) classification with regard to the etiopathogenesis, presence and character of encephalopathy.
| (A) The size of the head—dysmorphology classification (HC) and entities/syndromes associated with neurodysfunction | |||||||
|---|---|---|---|---|---|---|---|
| Entities and syndromes associated with neurodysfunction | The size of the head—dysmorphology classification (HC) by z-score HC (p = 0.026; Cp = 0.465) | Total | |||||
| Normal | Microcephaly | Macrocephaly | |||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| NBIA-MPAN | 2 | 0.6 | 0 | − 0.5 | 0 | − 0.3 | 2 |
| GSD II | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| LCHAD | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| SLO | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| GLUT1d | 1 | 0,4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| NKH | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| SMEI | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| sasMMC&HCP | 15 (88%) | 0.5 | 0 | − 1.6 | 2 (12%) | 1.7 | 17 |
| sasMMC | 3 | 0.8 | 0 | − 0.7 | 0 | − 0.4 | 3 |
| sasMM | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| ACM | 1 (50%) | − 1.3 | 0 | − 0.5 | 1 (50%) | 3.3 | 2 |
| HCP | 0 | − 2.3 | 0 | − 0.4 | 1 | 4.9 | 1 |
| DS | 8 (73%) | − 1.0 | 3 (27%) | 1.5 | 0 | − 0.7 | 11 |
| ES | 0 | − 2.3 | 1 | 2.6 | 0 | − 0.2 | 1 |
| PMS | 1 (50%) | − 1.3 | 0 | − 0.5 | 1 (50%) | 3.3 | 2 |
| MWS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| AS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| DGS | 0 | − 2.3 | 1 | 2.6 | 0 | − 0.2 | 1 |
| 46,XY,del(X)(q24) | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| CdLS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| SDS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| PWS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| 46 XX, add(2)(q25) | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| 46XX, del (12) (q24.21q24.23) | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| FAS | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| CP | 198 (83%) | − 0.5 | 36 (15%) | 2.3 | 5 (2%) | − 2.9 | 239 |
| HMSN | 7 (87.5%) | 0.3 | 0 | − 1.1 | 1 (12.5%) | 1.2 | 8 |
| LGMD | 7 | 1.2 | 0 | − 1.0 | 0 | − 0.5 | 7 |
| BMD | 2 (67%) | − 0.8 | 0 | − 0.7 | 1 (33%) | 2.6 | 3 |
| DMD | 6 (86%) | 0.2 | 0 | − 0.4 | 1 (14%) | 1.4 | 7 |
| TD | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| AMC&N | 3 | 0.8 | 0 | − 0.7 | 0 | − 0.4 | 3 |
| CM | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| SMA | 2 | 0.6 | 0 | − 0.5 | 0 | − 0.3 | 2 |
| Total | 273 (83.5%) | 41 (12.5%) | 13 (4%) | 327 | |||
| (B) The size of the head—dysmorphological classification (HC) and classification with regard to etiopathogenesis, presence and character of encephalopathy | |||||||
|---|---|---|---|---|---|---|---|
| Classification with regard to the etiopathogenesis, presence and character of encephalopathy | The size of the head—dysmorphology classification (HC) by z-score HC (p = 0.010; Cp = 0.273) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| E-MD | 7 | 1.2 | 0 | − 1.0 | 0 | − 0.5 | 7 |
| EE | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| NTDs | 20 (83%) | 0.0 | 0 | − 1.9 | 4 (17%) | 3.3 | 24 |
| GD | 17 (74%) | − 1.3 | 5 (22%) | 1.4 | 1 (4%) | 0.1 | 23 |
| TE | 1 | 0.4 | 0 | − 0.4 | 0 | − 0.2 | 1 |
| CP | 198 (83%) | − 0.5 | 36 (15%) | 2.3 | 5 (2%) | − 2.9 | 239 |
| NMD | 29 (91%) | 1.1 | 0 | − 2.3 | 3 (9%) | 1.6 | 32 |
| Total | 273 (83.5%) | 41 (12.5%) | 13 (4%) | 327 | |||
N numbers of patients; % percent; p probability value calculated by chi-square test of independence; Cp Pearson’s Contingency Coefficient C, Cp ≥ 0, values distant from 0 reflect some relationship, values approaching 1 correspond to a perfect association; ASR adjusted standardized residuals, values > 1.96 reflect a greater number, and those below < − 1.96 correspond to a smaller number than a random distribution.
Figure 1.
The size of the head: (A)—dysmorphology classification and classification based on the etiopathogenesis, presence and character of encephalopathy; (B)—traditional classification and classification based on the etiopathogenesis, presence and character of encephalopathy; (C)—dysmorphology classification and classification based on the presence and character of encephalopathy; (D)—traditional classification and classification based on the presence and character of encephalopathy.
Table 4.
The size of the head and classification with regard to the presence and character of encephalopathy (A, B), neural tube defects (C), type of spasticity (D, E), and epilepsy (F, G).
| (A) The size of the head—dysmorphology classification (HC) and classification with regard to the presence and character of encephalopathy | |||||||
|---|---|---|---|---|---|---|---|
| Classification with regard to the presence and character of encephalopathy | The size of the head—dysmorphology classification (HC) by z-score HC (p = 0.060; Cp = 0.164) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| PE | 8 | 1.3 | 0 | − 1.1 | 0 | − 0.6 | 8 |
| NPE | 236 (82%) | − 1.6 | 41 (14%) | 2.6 | 10 (4%) | − 1.2 | 287 |
| NMD | 29 (91%) | 1.1 | 0 | − 2.3 | 3 (9%) | 1.6 | 32 |
| Total | 273 (83.5%) | 41 (12.5%) | 13 (4%) | 327 | |||
| (B) The size of the head—traditional classification (HC) and classification with regard to the presence and character of encephalopathy | |||||||
|---|---|---|---|---|---|---|---|
| Classification with regard to the presence and character of encephalopathy | The size of the head—traditional classification (HC) by z-score HC (p = 0.006; Cp = 0.206) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| PE | 7 (87.5%) | 1.2 | 1 (12.5%) | − 0.7 | 0 | − 0.9 | 8 |
| NPE | 195 (68%) | − 0.6 | 69 (24%) | 2.4 | 23 (8%) | − 2.4 | 287 |
| NMD | 22 (69%) | 0.0 | 2 (6%) | − 2.3 | 8 (25%) | 3.2 | 24 |
| Total | 224 (68.5%) | 72 (22%) | 31 (9.5%) | 327 | |||
| (C) Relationship between the size of the head—traditional classification (HC) and encephalopathy in neural tube defects | |||||||
|---|---|---|---|---|---|---|---|
| Encephalopathy in neural tube defects | The size of the head—traditional classification (HC) by z-score HC (p = 0.004; Cp = 0.564) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| sasMMC&HCP | 14 (82%) | 3.1 | 0 | − 2.3 | 3 (18%) | − 1.9 | 17 |
| sasMMC, sasMM, ACM, HCP | 1 (14%) | − 3.1 | 2 (29%) | 2.3 | 4 (57%) | 1.9 | 7 |
| Total | 15 (63%) | 2 (8%) | 7 (29%) | 24 | |||
| (D) The size of the head—traditional classification (HC) and type of spasticity | |||||||
|---|---|---|---|---|---|---|---|
| Type of spasticity | The size of the head—traditional classification (HC) by z-score HC (p = 0.034; Cp = 0.211) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Diplegia | 62 (69%) | − 0.4 | 19 (21%) | − 0.6 | 9 (10%) | 1.9 | 90 |
| Hemiplegia | 47 (82.5%) | 2.3 | 8 (14%) | − 1.9 | 2 (3.5%) | − 1.0 | 57 |
| Tetraplegia | 48 (63%) | − 1.7 | 25 (33%) | 2.4 | 3 (4%) | − 1.0 | 76 |
| Total | 157 (71%) | 52 (23%) | 14 (6%) | 223 | |||
| (E) Relationship between the size of the head—traditional classification (HC) and type of spasticity | |||||||
|---|---|---|---|---|---|---|---|
| Type of spasticity | The size of the head—traditional classification (HC) by z-score HC (p = 0.041; Cp = 0.167) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Tetraplegic | 48 (63%) | − 1.7 | 25 (33%) | 2.4 | 3 (4%) | − 1.0 | 76 |
| Other: diplegic, hemiplegic | 109 (74%) | 1.7 | 27 (18%) | − 2.4 | 11 (8%) | 1.0 | 147 |
| Total | 157 (70%) | 52 (23%) | 14 (7%) | 223 | |||
| (F) The size of the head—dysmorphology classification (HC) and epilepsy | |||||||
|---|---|---|---|---|---|---|---|
| Additional diagnosis epilepsy | The size of the head—dysmorphology classification (HC) by z-score HC (p = 0.000; Cp = 0.251) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Present | 59 (69%) | − 4.3 | 23 (27%) | 4.6 | 4 (4%) | 0.4 | 86 |
| Absent | 214 (89%) | 4.3 | 18 (7%) | − 4.6 | 9 (4%) | − 0.4 | 241 |
| Total | 273 (83.5%) | 41 (12.5%) | 13 (4%) | 327 | |||
| (G) The size of the head—traditional classification (HC) and epilepsy | |||||||
|---|---|---|---|---|---|---|---|
| Additional diagnosis epilepsy | The size of the head—traditional classification (HC) by z-score HC (p < 0.001; Cp = 0.245) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Present | 45 (52%) | − 3.8 | 34 (40%) | 4.6 | 7 (8%) | − 0.5 | 86 |
| Absent | 179 (74%) | 3.8 | 38 (16%) | − 4.6 | 24 (10%) | 0.5 | 241 |
| Total | 224 (68.5%) | 72 (22%) | 31 (9.5%) | 327 | |||
N numbers of patients; % percent; p probability value calculated by chi-square test of independence; Cp Pearson’s Contingency Coefficient C, Cp ≥ 0, values distant from 0 reflect some relationship; values approaching 1 correspond to a perfect association; ASR adjusted standardized residuals, values > 1.96 reflect a greater number, and those below < − 1.96 correspond to a smaller number than a random distribution.
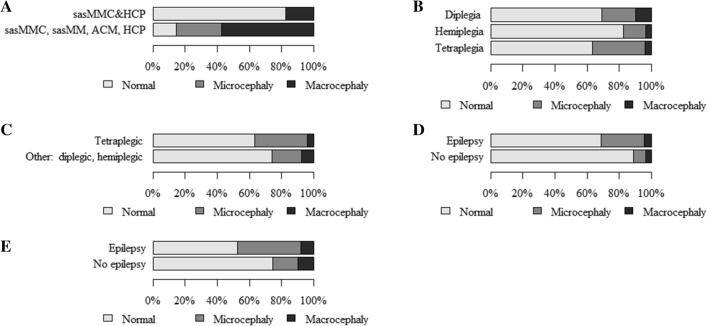
Figure 2.
The size of the head: (A)—traditional classification and encephalopathy in neural tube defects; (B)—traditional classification and type of spasticity (Diplegia, Hemiplegia, Tetraplegia); (C)—traditional classification and type of spasticity (Tetraplegic, Other: diplegic and hemiplegic together); (D)—dysmorphology classification and epilepsy; (E)—traditional classification and epilepsy.
Table 5.
The size of the head, hypothyroidism, epilepsy, type of spasticity.
| (A) The size of the head—dysmorphology classification (HC) and hypothyroidism | |||||||
|---|---|---|---|---|---|---|---|
| Additional diagnosis hypothyroidism | The size of the head—dysmorphology classification (HC) by z-score HC (p = 0.024; Cp = 0.150) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Present | 9 (64%) | − 2.0 | 5 (36%) | 2.7 | 0 | − 0.8 | 14 |
| Absent | 264 (84.5%) | 2.0 | 36 (11.5%) | − 2.7 | 13 (4%) | 0.8 | 313 |
| Total | 273 (83.5%) | 41 (12.5%) | 13 (4%) | 327 | |||
| (B) The size of the head—traditional classification (HC) and hypothyroidism | |||||||
|---|---|---|---|---|---|---|---|
| Additional diagnosis hypothyroidism | The size of the head—traditional classification (HC) by z-score HC (p < 0.001; Cp = 0.213) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Present | 5 (36%) | − 2.7 | 9 (64%) | 3.9 | 0 | − 1.2 | 14 |
| Absent | 219 (70%) | 2.7 | 63 (20%) | − 3.9 | 31 (10%) | 1.2 | 313 |
| Total | 224 (68.5%) | 72 (22%) | 31 (9.5%) | 327 | |||
| (C) Relationship between epilepsy and type of spasticity | |||||||
|---|---|---|---|---|---|---|---|
| Additional diagnosis epilepsy | Type of spasticity (p < 0.001; Cp = 0.257) | Total | |||||
| Tetraplegia | Diplegia | Hemiplegia | |||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| Present | 33 (52%) | 3.6 | 14 (22%) | − 3.5 | 16 (26%) | 0.0 | 63 |
| Absent | 43 (27%) | − 3.6 | 76 (47%) | 3.5 | 41 (26%) | 0.0 | 160 |
| Total | 76 (34%) | 90 (40%) | 57 (26%) | 223 | |||
| (D) Relationship between type of spasticity and z-score HC | |||||||
|---|---|---|---|---|---|---|---|
| Nominal regression | Quantitative dependent variable z-score HC | ||||||
| Qualitative dependent variable type of spasticity | |||||||
| Tetraplegia (34%) | < 0.001 | p | |||||
|
0.782 0.677–0.904 |
OR | ||||||
| Other: diplegia, hemiplegia (66%) | Reference group | ||||||
| (E) Relationship between hypothyroidism and z-score HC | |||||||
|---|---|---|---|---|---|---|---|
| Nominal regression | Quantitative dependent variable z-score HC | ||||||
| Qualitative dependent variable additional diagnosis | |||||||
| Presence of hypothyroidism (4.5%) | < 0.001 | p | |||||
|
0.599 0.457–0.785 |
OR | ||||||
| Lack of hypothyroidism (95.7%) | Reference group | ||||||
| (F) Relationship between epilepsy and z-score HC | |||||||
|---|---|---|---|---|---|---|---|
| Nominal regression | Quantitative dependent variable z-score HC | ||||||
| Qualitative dependent variable additional diagnosis | |||||||
| Presence of epilepsy (26%) | < 0.001 | p | |||||
|
0.804 0.711–0.909 |
OR | ||||||
| Lack of epilepsy (74%) | Reference group | ||||||
N numbers of patients; % percent; p probability value calculated by chi-square test of independence; Cp Pearson’s Contingency Coefficient C, Cp ≥ 0, values distant from 0 reflect some relationship; values approaching 1 correspond to a perfect association; ASR Adjusted standardized residuals, values > 1.96 reflect a greater number, and those below < − 1.96 correspond to a smaller number than a random distribution; OR odds ratio (95% confidence interval).
Figure 3.
The size of the head: (A)—dysmorphology classification and hypothyroidism; (B)—traditional classification and hypothyroidism; (C)—relationship between epilepsy and type of spasticity.
In addition, the findings showed more/less common co-occurrence of as well as statistically significant relationships between the size of the head—traditional classification (HC), as follows:
entities/syndromes associated with neurodysfunction (Table 3A). Normal head size rarely co-occurred with ACM and muscular dystrophy limb girdle (LGMD). Microcephaly was rarely found to co-occur with the state following surgery due to lumbar myelomeningocele and hydrocephalus (sasMMC&HCP) and frequently with Down syndrome (DS). Macrocephaly rarely found to co-occur with CP and frequently with the state following surgery due to lumbar myelomeningocele (sasMMC), ACM, HCP, PMS, and LGMD. The relationship was statistically significant (p < 0.001),
classification with regard to etiopathogenesis, presence and character of encephalopathy (Table 3B and Fig. 1B). Microcephaly frequently co-occurred in children with GD and rarely in those with NMD. Macrocephaly frequently co-occurred with NTDs or NMD and rarely with CP. The relationship was statistically significant (p < 0.001),
classification with regard to the presence and character of encephalopathy (Table 4B and Fig. 1D). Microcephaly frequently occurred in NPE and rarely in the subgroup with NMD. Macrocephaly was found more often in the group with NMD and less frequently in the subgroup with NPE. The relationship was statistically significant (p = 0.006, Cp = 0.206),
encephalopathy in neural tube defects (Table 4C and Fig. 2A). Normal head size was often found in sasMMC&HCP and rarely in sasMMC, a state following surgery due to parieto-occipital meningocele (sasMM), ACM, and HCP. Microcephaly co-occurred frequently with sasMMC, sasMM, ACM, HCP but rarely with sasMMC&HCP. The relationship was statistically significant (p = 0.004, Cp = 0.564),
type of spasticity (Table 4D and Fig. 2B). Normal head size was more frequent in children with hemiplegia. Microcephaly was more often associated with tetraplegia. The relationship was statistically significant (p = 0.034, Cp = 0.211),
type of spasticity (Table 4E and Fig. 2C). Normal head size was rarely found to co-occur with tetraplegia. Microcephaly more often accompanied tetraplegia. The relationship was statistically significant (p = 0.034; Cp = 0.211),
epilepsy (Table 4G and Fig. 2E). Normal head circumference rarely co-occurred with epilepsy and frequently with a lack of epilepsy. Microcephaly often co-occurred with epilepsy and rarely when epilepsy was not present. The relationship was statistically significant (p < 0.001),
hypothyroidism (Table 5B and Fig. 3B). Normal head circumference frequently co-occurred in the absence of hypothyroidism and was rarely found in patients with hypothyroidism. Microcephaly frequently co-occurred with hypothyroidism, and in the absence of this condition, it rarely was found. The relationship was statistically significant (p < 0.001).
Table 3.
The size of the head—traditional classification (HC) and (A) units and syndromes associated with neurodysfunction, (B) classification with regard to the etiopathogenesis, presence and character of encephalopathy.
| (A) The size of the head—traditional classification (HC) and entities and syndromes associated with neurodysfunction | |||||||
|---|---|---|---|---|---|---|---|
| Entities and syndromes associated with neurodysfunction | The size of the head—traditional classification (HC) by z-score HC (p < 0.001; Cp = 0.509) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| NBIA-MPAN | 2 | 1.0 | 0 | − 0.8 | 0 | − 0.5 | 2 (100.0%) |
| GSD II | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| LCHAD | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| SLO | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| GLUT1d | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| NKH | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| SMEI | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| sasMMC&HCP | 14 (82%) | 1.3 | 0 | − 2.3 | 3 (18%) | 1.2 | 17 |
| sasMMC | 1 (33%) | − 1.3 | 0 | − 0.9 | 2 (67%) | 3.4 | 3 |
| sasMM | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| ACM | 0 | − 2.1 | 1 (50%) | 1.0 | 1 (50%) | 2.0 | 2 |
| HCP | 0 | − 1.5 | 0 | − 0.5 | 1 | 3.1 | 1 |
| DS | 5 (45.5%) | − 1.7 | 6 (54.5%) | 2.6 | 0 | − 1.1 | 11 |
| ES | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| PMS | 1 (50%) | − 0.6 | 0 | − 0.8 | 1 (50%) | 2.0 | 2 |
| MWS | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| AS | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| DGS | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| 46,XY,del(X)(q24) | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| CdLS | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| SDS | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| PWS | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| 46 XX, add(2)(q25) | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| 46XX, del (12) (q24.21q24.23) | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| FAS | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| CP | 167 (70%) | 0.9 | 57 (24%) | 1.3 | 15 (6%) | − 3.3 | 239 |
| HMSN | 7 (87.5%) | 1.2 | 0 | − 1.5 | 1 (12.5%) | 0.3 | 8 |
| LGMD | 2 (29%) | − 2.3 | 0 | − 1.4 | 5 (71%) | 5.7 | 7 |
| BMD | 2 (67%) | − 0.1 | 0 | − 0.9 | 1 (33%) | 1.4 | 3 |
| DMD | 6 (86%) | 1.0 | 0 | − 1.4 | 1 (14%) | 0.4 | 7 |
| TD | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| AMC&N | 2 (67%) | − 0.1 | 1 (33%) | 0.5 | 0 | − 0.6 | 3 |
| CM | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| SMA | 1 (50%) | − 0.6 | 1 (50%) | 1.0 | 0 | − 0.5 | 2 |
| Total | 224 (68.5%) | 72 (22.0%) | 31 (9.5%) | 327 | |||
| (B) The size of the head—traditional classification (HC) and classification with regard to the etiopathogenesis, presence and character of encephalopathy | |||||||
|---|---|---|---|---|---|---|---|
| Classification with regard to the etiopathogenesis, presence and character of encephalopathy | The size of the head—traditional classification (HC) by z-score HC (p < 0.001; Cp = 0.315) | ||||||
| Normal | Microcephaly | Macrocephaly | Total | ||||
| N (%) | ASR | N (%) | ASR | N (%) | ASR | N (%) | |
| E-MD | 6 (86%) | 1.0 | 1 (14%) | − 0.5 | 0 | − 0.9 | 7 |
| EE | 1 | 0.7 | 0 | − 0.5 | 0 | − 0.3 | 1 |
| NTDs | 15 (62.5%) | − 0.7 | 2 (8.5%) | − 1.7 | 7 (29%) | − 3.4 | 24 |
| GD | 13 (56.5%) | − 1.3 | 9 (39%) | 2.1 | 1 (4.5%) | − 0.9 | 23 |
| TE | 0 | − 1.5 | 1 | 1.9 | 0 | − 0.3 | 1 |
| CP | 167 (70%) | 0.9 | 57 (24%) | 1.3 | 15 (6%) | − 3.3 | 239 |
| NMD | 22 (69%) | 0.0 | 2 (6%) | − 2.3 | 8 (25%) | 3.2 | 32 |
| Total | 224 (68.5%) | 72 (22%) | 31 (9.5%) | 327 | |||
N numbers of patients; % percent; p probability value calculated by chi-square test of independence, Cp Pearson’s Contingency Coefficient C, Cp ≥ 0, values distant from 0 reflect some relationship, values approaching 1 correspond to a perfect association; ASR adjusted standardized residuals, values > 1.96 reflect a greater number, and those below < − 1.96 correspond to a smaller number than a random distribution.
Cp is higher for the traditional classification:
amounting to 0.509 compared to the dysmorphology criterion of 0.465 (Tables 2A, 3A). The traditional criterion in this case more effectively differentiated the relationship between head size and specific medical conditions,
amounting to 0.315 compared to the dysmorphology criterion of 0.273 (Tables 2B, 3B). The traditional classification in this case more effectively differentiated the relationship between head size and the seven subgroups distinguished based on classification considering etiopathogenesis, as well as the occurrence and character of encephalopathy,
amounting to 0.206 compared to the dysmorphology criterion of 0.146 (Table 4A,B). The traditional classification in this case more effectively differentiated the relationship between head size and the three subgroups distinguished based on classification taking into account the occurrence and character of encephalopathy,
amounting to 0.213 compared to the dysmorphology criterion of 0.150 (Table 5A,B). The criterion of ± 2SD in this case more effectively differentiated the relationship between head size and hypothyroidism.
Cp was higher for dysmorphology than for traditional classification, at 0.251 and 0.245, respectively (Table 4F,G). The criterion of ± 3 SD in this case more effectively differentiated the relationship between head size and epilepsy.
Further analyses focused on the relationships between the quantitative independent variable, i.e., z-score HC, and a dependent qualitative variables such as divisions within subgroups or the presence of an additional diagnosis. Significant effects were found in the following cases: intragroup differences related to the spastic type, tetraplegia versus other, i.e., diplegia, hemiplegia (Table 5D); intragroup differences related to additional diagnoses, i.e., presence/absence of hypothyroidism (Table 5E); and presence/absence of epilepsy (Table 5F). In the first case, (Table 5D), higher z-score HC values correspond to a lower probability of co-occurring tetraplegia (p < 0.001). The likelihood of tetraplegia decreased with higher z-score HC (OR = 0.782). In the second case (Table 5E), higher z-score HC values corresponded to a lower probability of co-occurring hypothyroidism (p < 0.001). With a higher z-score HC, the likelihood of hypothyroidism decreased (OR = 0.599). In the third case (Table 5F), greater values of z-score HC reflect a lower likelihood of cooccurring epilepsy (p < 0.001); i.e., the higher the z-score HC value was, the lower the likelihood of cooccurring epilepsy was (OR = 0.801).
Relationships between additional diagnoses and tetraplegia were also examined, and epilepsy frequently co-occurred with tetraplegia (p < 0.001) (Table 5C and Fig. 3C).
Discussion
Developmental defects of the cranium and the brain may be clinically isolated or occur as part of complex syndromes associated with other neurodevelopmental challenges, brain defects and abnormal body growth21. Research has shown that macrocephaly and microcephaly remain poorly defined and that a uniform diagnostic approach is urgently needed21–29. For the purposes of this article, the definitions of microcephaly and macrocephaly, including growth disorders, were used4–6. As disturbances in the differentiation of body proportions were not taken into account, relative or absolute microcephaly/macrocephaly was not distinguished. There is a relationship between the size of the brain and the size of the skull30, the body size31, and the neurological capacity of organisms31. Previous studies have shown that tetraplegia is accompanied by short stature3, though current studies have shown that tetraplegia is accompanied by microcephaly, which confirms the theory of encephalysis. Therefore, tetraplegia was not excluded from the analysis of relative microcephaly/macrocephaly; indeed, a proportional reduction in body size may be associated with a reduced brain size31. Definitive etiological diagnosis is crucial for predicting outcomes and for designing treatments administered to affected patients21–29, and this observation provided motivation for the current study. Another reason was the fact that, to our knowledge, no studies have investigated relationships between co-occurring abnormal cranium development and diseases or syndromes linked to neurodysfunction, also grouped according to the presented classification1,3. Hence, this study is the first to offer scientific evidence related to this issue.
According to Pirozzi et al.21, neurodevelopmental impairments linked to abnormal growth of the brain, with or without cortical defects, significantly contribute to morbidity and mortality and are associated with numerous consequences of a neurodevelopmental nature that manifest in infancy or early childhood21. The majority of children experiencing the diseases and syndromes discussed also had encephalopathy, indicating its prevalence. Encephalopathies are all conditions affecting brain structure. It is possible to distinguish various types of encephalopathy depending on the patient’s age and causes of the condition32,33. By taking into account the characteristics and expected presence of encephalopathy, the current study identified patients with progressive and nonprogressive encephalopathy. Less than 10% of the children in the study group were affected by neuromuscular disorders. Given that encephalopathies comprise disorders of hereditary or nonhereditary nature, i.e., congenital or acquired conditions that are primarily characterized by brain damage32,33 and may affect head size, it seems justified that the present findings show significant correlations of head size in both assessment systems (dysmorphology versus traditional) to patient classification based on the etiopathogenesis and presence and character of encephalopathy.
The use of a classification system differentiating the size of the head based on dysmorphological criteria allowed us to uncover the common cooccurrence of (as well as statistically significant relationships between) microcephaly and NPE (classification based on the presence and character of encephalopathy), CP (classification based on the etiopathogenesis, presence and character of encephalopathy), ES, DGS, and CP (entities/syndromes associated with neurodysfunction). Macrocephaly is frequently found with NTDs (classification based on the etiopathogenesis, presence and character of encephalopathy), ACM, HCP, PMS, and BMD (entities/syndromes associated with neurodysfunction). Microcephaly rarely cooccurs in children with NMD and macrocephaly with CP. The definition of microcephaly6,34 and macrocephaly35 based on dysmorphological criteria is used by geneticists6,34,35, and it has been used for studies on the etiopathogenesis of microcephaly primary hereditary. Genes responsible for microcephaly primary hereditary implicate a wide variety of molecular and cellular mechanisms involved in the regulation of cerebral cortical size during development. Studying these syndromes can also reveal molecular mechanisms that are critical for the regulation of brain size and human brain evolution6. Primary (of prenatal onset) or secondary (of postnatal onset) microcephaly results from an imbalance between progenitor cell production and cell death that leads to a reduced number of neuronal and glial cells within the brain, resulting in reduced brain growth. Secondary microcephaly is associated with increased neuronal death and can be associated with serine deficiency or thiamine pyrophosphate transporter deficiency and metabolic diseases associated with progressive encephalopathy34. For example, macrocephaly (criterion > 3 SD) occurs in megalencephaly-polymicrogyria-polydactyly hydrocephalus syndrome35.
The use of a classification system that differentiates the size of the head based on traditional criteria allowed us to show that microcephaly frequently occurs in NPE (classification based on the presence and character of encephalopathy), in children and adolescents with GD (classification based on the etiopathogenesis, presence and character of encephalopathy), and in DS (entities/syndromes associated with neurodysfunction). Macrocephaly was found more often in the group with NMD (classification with regard to the presence and character of encephalopathy), NTDs, or NMD (classification with regard to the etiopathogenesis, presence and character of encephalopathy) and was frequently found to co-occur with sasMMC, ACM, HCP, PMS, and LGMD (entities/syndromes associated with neurodysfunction). Conversely, microcephaly rarely co-occurred in children with NMD and macrocephaly with CP, results that were significant. The definition of microcephaly4,5 and macrocephaly36–38 based on traditional criteria is used by clinicians, who often use magnetic resonance imaging4,5,36–38, microbiological5,38 and genetic tests4,5,36,37 in the differential diagnostic process. Our research confirms the utility of genetic tests for diagnostic and differential processes.
The advantage of the present study is the fact that its findings provide an unambiguous answer to the question formulated in the purpose of the study, namely, which of the two existing criteria used in assessing cranial development defects—the system applied in dysmorphology and the one traditionally used in clinical practice—should be employed in daily work. The presented evidence suggests greater effectiveness of the traditional classification, as it enabled the identification of more relationships. Moreover, the traditional classification more effectively differentiated relationships between head size and all the subgroups distinguished based on the system taking into account the etiopathogenesis as well as the presence and character of encephalopathy.
Different definitions of microcephaly are used in studies examining the relationship between microcephaly and epilepsy: HC < 3percentile22, HC < 2SD23,24, HC < 3SD25. The current study shows that in children and adolescents with diseases or syndromes linked to neurodysfunction, microcephaly co-occurred with epilepsy as well as with hypothyroidism. Von der Hagen et al.22 carried out a retrospective study in a group of 680 children with microcephaly. The causes of this condition were identified in 59% of the children, whereas no definite diagnosis was formulated for the remaining 41%. In the former group, genetic causes were identified in approximately half of the patients; perinatal and postnatal brain damage accounted for 45% and 3% of the cases, respectively. In the study by Von der Hagen et al., epilepsy was diagnosed in 43% of children with microcephaly22. Similar results were reported by Abdel-Salam et al.23. Although their study involved a much smaller group, i.e., only 66 children with microcephaly, the authors also identified epilepsy in 40.9% of them23. Furthermore, it has been reported that epilepsy is more common in children with secondary microcephaly than in those with primary microcephaly23,24. Similarly, Ashwal et al.25 pointed out that microcephaly is associated with comorbidities such as epilepsy25. In our study, the criterion of ± 3 SD more effectively differentiated the relationship between head size and epilepsy.
Another finding of the current study is related to the co-occurrence of microcephaly and hypothyroidism in children and adolescents with diseases or syndromes associated with neurodysfunction. Research has shown that normal thyroid metabolism is necessary for human development, including the formation and functioning of the brain. However, abnormal thyroid metabolism is increasingly diagnosed in the spectrum of pediatric neurological disorders39. Moreover, in their literature review investigating neurological symptoms of impaired thyroid metabolism, Kurian and Jungbluth39 also point out the correlation between hypothyroidism and microcephaly39. A similar relationship was reported by Carré et al.40. Nonetheless, the criteria for the diagnosis of microcephaly are often not included In reviews of the literature considering the relationship between microcephaly, cerebral abnormalities and hypothyroidism39,40. In our study, the criterion of ± 2 SD effectively differentiated the relationship between head size and hypothyroidism.
The current study also shows that microcephaly more commonly co-occurs with tetraplegia linked to CP and that the former is often found to exist simultaneously with epilepsy. The occurrence of microcephaly (criterion: HC < 3 SD) in patients with spastic quadriplegia was reported by Cavallin et al.41, and Singhi and Sain42 reported microcephaly (criterion: HC < 2SD) in 64.27% of children with CP in North India42, and microcephaly is the most frequent type being spastic quadriplegia42,43. Hadjipanayis et al.44 carried out a study involving 323 patients with CP palsy and observed that epilepsy occurred in this group at a rate of 41.8% and in nearly one in two patients with spastic tetraplegia44. Reports that emphasize the fact that subjects with epilepsy are more at risk of accidental injuries than are individuals who do not experience seizures can be found in the literature. The most common injuries include head contusions, which consequently may lead to spastic quadriplegia45.
Based on our retrospective analyses, we were able to systematize certain data, correlations and definitions46. Human developmental abnormalities such as microcephaly and macrocephaly are phenotypic abnormalities, and their definitions should be standardized and included in HPO terms47,48. We hope that the dependencies presented herein will contribute to the differential diagnostic process4,10 and to standardization of the definitions of microcephaly and macrocephaly.
Limitations
The limitations of this study are as follows. (a) This study was retrospective in nature. However, to conduct such a retrospective analysis, studies on the secular development trend of children and adolescents in Rzeszow were carried out in 2013 and 2014 with the measurement of head circumference, and without this, the present analysis would not be possible. Another limitation is (b) the dominance of the male sex in the study group. Further prospective studies using a larger group of subjects are necessary.
Conclusions
Children and adolescents with syndromes or diseases associated with neurodysfunction present abnormal cranial development (head size). Microcephaly rarely co-occurs in children with NMD; macrocephaly frequently co-occurs with NTDs or NMD but rarely with CP. Children and adolescents with syndromes or diseases associated with neurodysfunction have microcephaly co-occurring with epilepsy and hypothyroidism. It was shown that traditional classification enabled the identification of a greater number of relationships; therefore, it should be used in daily practice. Overall, there is a need for standardization of the definition of microcephaly and macrocephaly and for their inclusion in HPO terms.
Abbreviations
- HC
Head circumference
- OFC
Occipitofrontal circumference
- x − 3SD
Difference between the mean and 3 standard deviations
- x + 3SD
Total of the mean and 3 standard deviations
- x − 2SD
Difference between the mean and 2 standard deviations
- x + 2SD
Total of the mean and 2 standard deviations
- SD
Standard deviation
- KRORE
Clinical Regional Rehabilitation and Education Centre
- CP
Cerebral palsy
- z-score HC
Z-Score for head circumference
- ASR
Adjusted standardized residual
- PE
Progressive encephalopathy
- NPE
Nonprogressive encephalopathy
- NMD
Neuromuscular disease
- GD
Genetic disorder
Arithmetic mean
- Me
Median
- Min
Minimum value
- Max
Maximum value
- c25
25Th centile
- c75
75Th centile
- HCP
Isolated hydrocephalus
- ES
Edwards syndrome
- DGS
Di George syndrome
- ACM
Arnold–Chiari malformation
- PMS
Phelan–McDermid syndrome
- BMD
Becker’s muscular dystrophy
- NTD
Neural tube defect
- LGMD
Muscular dystrophy limb-girdle
- sasMMC&HCP
State following surgery due to lumbar myelomeningocele and hydrocephalus
- DS
Down syndrome
- sasMMC
State following surgery due to lumbar myelomeningocele
- sasMM
State following surgery due to parieto-occipital meningocele
- HPO
Human Phenotype Ontology
Author contributions
A.G. wrote the main manuscript. L.P. wrote the main manuscript and performed the research study. M.D. and J.P.-B. performed the statistical analysis and prepared Tables 1, 2, 3, 4 and 5. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaciński, M. Diseases of the nervous system in Pediatrics 2. Warsaw (ed. Kawalec, W., Grenda R., & Ziółkowska H.) 741–787 (PZWL Medical Publishing, 2012).
- 2.Szczałuba K, Obersztyn E, Mazurczak T. Microcephaly as a frequent symptom in clinical practice—Differential diagnosis taking into account its etiopathegenesis. Child Neurol. 2006;30:41–50. [Google Scholar]
- 3.Perenc L, Guzik A, Podgórska-Bednarz J, Drużbicki M. Growth disorders in children and adolescent affected by syndromes or diseases associated with neurodysfunction. Sci. Rep. 2019;9:16436. doi: 10.1038/s41598-019-52918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo HA. Microcefalia [Microcephaly] Medicina (B Aires). 2018;78:94–100. [PubMed] [Google Scholar]
- 5.Sarno M, et al. Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome. Ultrasound. Obstet. Gynecol. 2017;50:717–722. doi: 10.1002/uog.17303. [DOI] [PubMed] [Google Scholar]
- 6.Jayaraman D, Bae BI, Walsh CA. The genetics of primary microcephaly. Annu. Rev. Genom. Hum. Genet. 2018;31:177–200. doi: 10.1146/annurev-genom-083117-021441. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell-Luria AH, et al. Heterozygous variants in KMT2E cause a spectrum of neurodevelopmental disorders and epilepsy. Am. J. Hum. Genet. 2019;104:1210–1222. doi: 10.1016/j.ajhg.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu GK, Ahmed S, Akhter S, Islam S. Neuro-imaging changes in cerebral palsy: A cross sectional study. Mymensingh. Med. J. 2020;29:121–128. [PubMed] [Google Scholar]
- 9.Perenc L, Radochońska A, Błajda J. Changeableness of selected characteristics of the head in the Rzeszów children and adolescents aged 4–18 in during a 35-year period. Eur. J. Clin. Exp. Med. 2017;15:217–232. doi: 10.15584/ejcem.2017.3.5. [DOI] [Google Scholar]
- 10.Radochońska A, Perenc L. Changeableness of selected morphological characteristics of head in the Rzeszow children and adolescents aged 3–18 in 25-year period. Prz. Med. Uniw. Rz. 2008;2:142–155. [Google Scholar]
- 11.Radochońska A, Perenc L. Trends in physical development of children and adolescents from Rzeszów in 25-year period of 1978–2004. Prz. Med. Uniw. Rz. 2009;3:239–250. [Google Scholar]
- 12.Radochońska A, Perenc L. Changes in proportions of the body build in the Rzeszow children and youth aged 3–18 years during the period of 25 years (1978–2004) Prz. Med. Uniw. Rz. 2010;1:30–48. [Google Scholar]
- 13.Perenc L, Radochońska A, Błajda J. Development of body adiposity in children and adolescents from Rzeszow, and its variability over 35 years. Med. Rev. 2016;14:27–47. doi: 10.15584/medrev.2016.1.3. [DOI] [Google Scholar]
- 14.Perenc L, Radochońska A, Błajda J. Somatic growth in children and adolescents from Rzeszow, aged 4–18, and its variability over the thirty-five year period from 1978/79 to 2013/14. Med. Rev. 2016;3:244–265. doi: 10.15584/medrev.2016.3.1. [DOI] [Google Scholar]
- 15.Perenc L, Radochońska A, Błajda J. Changes in body proportions of children and adolescents from Rzeszów during a 35-year period from 1978/79–2013/14. Eur. J. Clin. Exp. Med. 2018;4:267–282. [Google Scholar]
- 16.Perenc L, et al. Assessment of body adiposity preterm children at the beginning of school age. Sci. Rep. 2019;9:6207. doi: 10.1038/s41598-019-42715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perenc L, Kwolek A. Studies on the co-occurrence of meningomyelocele and other developmental anomalies of cerebrum and skull. Med. Rev. 2005;1:23–25. [Google Scholar]
- 18.Perenc L. Evaluation of somatic development of children operated because of meningomyelocele in virtue of the frequency analysis of occurrence of anthropometric measures in intervals of the mean and pathological values. Przeg. Med. Uniw. Rzeszow. 2005;2:125–139. [Google Scholar]
- 19.Pasternak-Pietrzak K, Kądziela K, Pyrżak B. Diagnostic difficulties in a girl with short stature. Pediatr. Dypl. 2017;21:27–33. [Google Scholar]
- 20.Perliczko E, Horodnicka-Józwa A, Walczak M. Preliminary tests before diagnosing growth hormone deficiency (exclusion criteria) Endokrynol. Pediatr. 2009;9:14–22. [Google Scholar]
- 21.Pirozzi F, Nelson B, Mirzaa G. From microcephaly to megalencephaly: Determinants of brain size. Dialog. Clin. Neurosci. 2018;20:267–282. doi: 10.31887/DCNS.2018.20.4/gmirzaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von der Hagen M, et al. Diagnostic approach to microcephaly in childhood: A two-center study and review of the literature. Dev. Med. Child. Neurol. 2014;56:732–741. doi: 10.1111/dmcn.12425. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Salam GM, Halasz AA, Czeizel AE. Association of epilepsy with different groups of microcephaly. Dev. Med. Child. Neurol. 2000;42:760–767. doi: 10.1017/S0012162200001419. [DOI] [PubMed] [Google Scholar]
- 24.Qazi QH, Reed TE. A problem in diagnosis of primary versus secondary microcephaly. Clin. Genet. 1973;4:46–52. doi: 10.1111/j.1399-0004.1973.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 25.Ashwal S, Michelson D, Plawner L, Dobyns WB. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73:887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vertinsky AT, Barnes PD. Macrocephaly, increased intracranial pressure, and hydrocephalus in the infant and young child. Top. Magn. Reson. Imaging. 2007;18:31–51. doi: 10.1097/RMR.0b013e3180d0a753. [DOI] [PubMed] [Google Scholar]
- 27.Olney AH. Macrocephaly syndromes. Semin. Pediatr. Neurol. 2007;14:128–135. doi: 10.1016/j.spen.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Williams CA, Dagli A, Battaglia A. Genetic disorders associated with macrocephaly. Am. J. Med. Genet A. 2008;146A:2023–2037. doi: 10.1002/ajmg.a.32434. [DOI] [PubMed] [Google Scholar]
- 29.Winden KD, Yuskaitis CJ, Poduri A. Megalencephaly and macrocephaly. Semin. Neurol. 2015;35:277–287. doi: 10.1055/s-0035-1552622. [DOI] [PubMed] [Google Scholar]
- 30.Lesciotto KM, Richtsmeier JT. Craniofacial skeletal response to encephalization: How do we know what we think we know? Am. J. Phys. Anthropol. 2019;168:27–46. doi: 10.1002/ajpa.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marugán-Lobón J, Watanabe A, Kawabe S. Studying avian encephalization with geometric morphometrics. J. Anat. 2016;229:191–203. doi: 10.1111/joa.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies E, Connolly DJ, Mordekar SR. Encephalopathy in children: An approach to assessment and management. Arch. Dis. Child. 2012;97:452–458. doi: 10.1136/adc.2011.300998. [DOI] [PubMed] [Google Scholar]
- 33.Abeyakoon O, Batty R, Mordekar S. The encephalopathic child. NRJ. 2011;1:577–596. doi: 10.1177/197140091102400403. [DOI] [PubMed] [Google Scholar]
- 34.Passemard S, Kaindl AM, Verloes A. Microcephaly. Handb. Clin. Neurol. 2013;111:129–141. doi: 10.1016/B978-0-444-52891-9.00013-0. [DOI] [PubMed] [Google Scholar]
- 35.Mirzaa, G. MPPH syndrome in GeneReviews [Internet]. Seattle (WA) (ed. Adam, M.P., Ardinger, H.H. & Pagon, R.A.) 1–17 (University of Washington, Seattle, 1993-2021).
- 36.Tan AP, Mankad K, Gonçalves FG, Talenti G, Alexia E. Macrocephaly: Solving the diagnostic dilemma. Top. Magn. Reson Imaging. 2018;27:197–217. doi: 10.1097/RMR.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 37.Gaona VA. Macrocefalia en la infancia [Macrocephaly in childhood] Medicina (B Aires). 2018;78:101–107. [PubMed] [Google Scholar]
- 38.Jones, S. & Samanta, D. Macrocephaly in StatPearls [Internet]. Treasure Island (FL) 1–8 (StarPearls, 2020).
- 39.Kurian MA, Jungbluth H. Genetic disorders of thyroid metabolism and brain development. Dev. Med. Child. Neurol. 2014;56:627–634. doi: 10.1111/dmcn.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carré A, et al. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum. Mol. Genet. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 41.Cavallin M, et al. TLE1, a key player in neurogenesis, a new candidate gene for autosomal recessive postnatal microcephaly. Eur. J. Med. Genet. 2018;61:729–732. doi: 10.1016/j.ejmg.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Singhi P, Saini AG. Changes in the clinical spectrum of cerebral palsy over two decades in North India—An analysis of 1212 cases. J. Trop. Pediatr. 2013;59:434–440. doi: 10.1093/tropej/fmt035. [DOI] [PubMed] [Google Scholar]
- 43.Singhi PD, Ray M, Suri G. Clinical spectrum of cerebral palsy in north India—An analysis of 1000 cases. J. Trop. Pediatr. 2002;48:162–166. doi: 10.1093/tropej/48.3.162. [DOI] [PubMed] [Google Scholar]
- 44.Hadjipanayis A, Hadjichristodoulou C, Youroukos S. Epilepsy in patients with cerebral palsy. Dev. Med. Child. Neurol. 1997;39:659–663. doi: 10.1111/j.1469-8749.1997.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 45.Kozak HH, Yeşilbudak Z, Şişman L, Uca AU. Quadriplegia following epileptic seizure: Things to keep in mind. J. Korean Neurosurg. Soc. 2016;59:319–321. doi: 10.3340/jkns.2016.59.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crevier-Sorbo G, et al. Assessment and treatment of childhood epilepsy in Haiti. Epilepsia Open. 2020;5:190–197. doi: 10.1002/epi4.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Notaro M, Schubach M, Robinson PN, Valentini G. Prediction of Human Phenotype Ontology terms by means of hierarchical ensemble methods. BMC Bioinform. 2017;18:449. doi: 10.1186/s12859-017-1854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasteiger LM, et al. Registry Working Party of the European Society for Immunodeficiencies (ESID). Supplementation of the ESID registry working definitions for the clinical diagnosis of inborn errors of immunity with encoded human phenotype ontology (HPO) terms. J. Allergy. Clin. Immunol. Pract. 2020;8:1778. doi: 10.1016/j.jaip.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]