Background:
Graduate medical education (GME) programs are vital to developing future plastic surgeons. However, their cost-efficiency has yet to be contextualized. This cohort quality improvement (QI) project aimed to measure the indirect costs an institution assumes in training surgical residents, by comparing the differences in operative time and procedural charges between a resident and a physician assistant (PA) first-assisting during adolescent reduction mammaplasty.
Methods:
From 2013 to 2019, adolescent bilateral reduction mammaplasty procedures first-assisted by either a resident or physician assistant were considered for analysis. Financial data, including all hospital and physician expenditures and operation duration, patient demographics, and outcomes data were retrospectively collected.
Results:
A total of 49 reduction mammaplasty cases were included for analysis. Residents had an average of 5.9 ± 1.5 years of post-graduate surgical training, whereas the PA had 2 years of surgical experience. Procedures first-assisted by a surgical resident took a mean/median of 34 minutes longer and were $3750 more expensive, respectively, than cases first-assisted by a PA (P < 0.01, both).
Conclusions:
Reduction mammaplasty procedures were longer and accrued higher charges when first-assisted by a surgical resident than by a PA. Although Graduate Medical Education programs are necessary to train the next generation of surgeons, they may result in unintended opportunity costs for teaching hospitals. Federal support to academic medical centers aims to cushion the cost of residential training, but is insufficient to compensate for resident inefficiency. Hospitals may consider incorporating PAs into the Graduate Medical Education paradigm to alleviate administrative burden, lower operational charges, and enhance resident training curricula.
INTRODUCTION
Graduate medical education (GME) programs enable newly graduated medical students to apply their technical knowledge in the clinical setting. They produce an inherent return on investment for government and healthcare centers to maintain a steady supply of qualified healthcare providers. However, the costs of GME on medical institutions and individual departments have not been fully contextualized. In the United States, funding allocated to GME programs covers (1) direct and (2) indirect costs, amounting to more than $16 billion annually.1,2 Medicare, which is the single largest program providing explicit support for GME, contributes about $10 billion each year for direct and indirect GME expenses, with other programs like the VA, Department of Defense, and Public Health Service contributing modest support3 (Fig. 1).
Fig. 1.
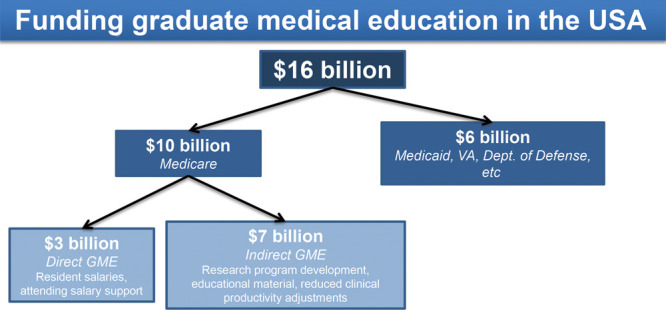
Cost breakdown of DGME payments.
Of the $10 billion Medicare pays annually, almost $3 billion supports direct graduate medical education (DGME) costs such as salaries of residents and their supervising physicians. However, these DGME payments do not completely cover a teaching hospital’s actual costs incurred by the residency program; rather, they pay for only Medicare’s share of DGME costs, rendering teaching hospitals responsible for footing the rest of the bill.4 The remaining $7 billion of Medicare’s contributions go toward indirect graduate medical education (IGME) costs like research program development, educational material preparation, and compensation for reduced clinical productivity due to residential training, yet is similarly insufficient to cover all actual indirect costs.2,3,5 For indirect cost compensation, teaching hospitals can submit claims to receive additional payments for services rendered to Medicare payments, calculated as a percentage add-on to the basic price per case.6
Although Medicare subsidizes the higher costs hospitals incur with training programs, these adjustments are mainly reflected in the hospitals’ increased patient care costs associated with treating more complex patients, not resident training costs.3 In an attempt to bridge this financial gap, teaching hospitals usually demand higher rates than nonteaching community hospitals, but despite increased prices for services, teaching hospitals must still devote significant resources to support GME programming. One study found that roughly 60% of the differences in charges between teaching and nonteaching hospitals were due to indirect expenses of GME, an expensive consequence of an outdated funding structure.7 The GME funding structure was first outlined in the 1997 Balanced Budget Act, yet these policies have rarely been revisited in recent decades, and no longer properly reflect adequate compensation for an increasingly complex and exponentially expanding healthcare system.8
In addition to increasing financial burden upon teaching hospitals sponsoring resident training programs, the implementation of the 80-hour resident workweek has shifted the administrative and clinical burden of GME onto attending physicians.9–11 In the past, attending physicians could delegate some of these responsibilities to other providers, such as residents, to focus on training curricula, but in an age of increasing accountability and QI, attendings are expected to be more involved in clinical and administrative activities. With swelling program costs, growing obligations of healthcare providers, resident duty-hour restrictions, and increased involvement of attending physicians, the benefits and costs of GME programs as currently designed are in question.
To address the gaps generated by the 80-hour resident workweek and growing non-clinical demands, many hospitals turn to advanced providers such as physician assistants (PAs), particularly in surgical specialties. Incorporating more advanced providers relieves pressure on both physicians and their practices, granting physicians more time to devote to their other clinical and administrative responsibilities, and has been shown to significantly reduce resident workload and enhance patient care.12–15 The growth of PAs in the United States is staggering—31% growth projected to 2028, compared with a steady 7% growth for physicians and residents (Fig. 2).
Fig. 2.
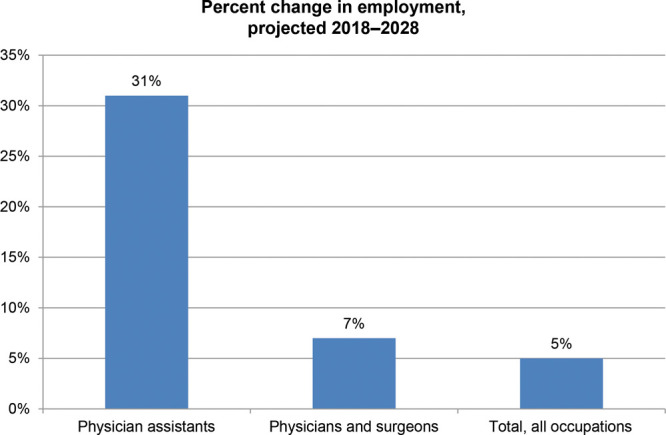
Job outlook of PAs and physicians/surgeons, 2018–2028. Reprinted with permission from U.S. Bureau of Labor Statistics, Employment Projections Program.16,17
Historically, residents have first-assisted attending surgeons in most operations. However, with the increase in skilled PAs, the debate for first assists turns thorny. Although PAs may be more clinically skilled than residents, they are more expensive for a department to support, given the average salary for a PA is $110,000.18 Meanwhile, the average salary for a PGY 6 plastic surgical resident in our geographic area is $82,000, which is partially paid for by Medicare as a DGME expense.19 Given these factors, surgeons and academic hospitals at large find themselves at a crossroads: Do they employ residents, who are less expensive given federal funding subsidies but are not as experienced, or PAs, who are more expensive but potentially more stable in their tenure and proficient in clinical skill?
To contextualize this dilemma, we designed this QI project to exemplify the unexpected financial burden of training surgical residents by comparing operation duration and procedural charges in adolescent reduction mammaplasty procedures first-assisted by either surgical residents or PAs. We hypothesize that there will be a disparity in operating time and charges associated with training surgical residents, suggesting that GME programs may need to consider a shift in the current reimbursement structure to accommodate the costs teaching hospitals absorb by supporting residency programs.
METHODS
Breast Reduction Mammaplasty Participants
This project was exempt from IRB review and approval due to its QI designation. Consent was not required for retrospective chart review. Bilateral reduction mammaplasty was chosen as the procedure of interest, given its prevalence, consistent approach and duration, low complication rate, and requirement of moderate skill level.20 Adolescent patients undergoing bilateral breast reduction mammaplasty between 2013 and 2019 with no other significant past or current medical history were considered for inclusion in case-analysis. All procedures were conducted under the supervision of a plastic surgery attending.
Financial and Perioperative Data
Financial data, including all hospital and physician expenditures and operation duration, were retrospectively collected from the Department of Plastic and Oral Surgery at Boston Children’s Hospital. Perioperative variables were collected from the electronic medical record. Procedural charges and final receipt figures after reimbursement were queried from billing records and included operative, departmental, and ancillary patient charges. We recognize that charge data without reimbursement adjustments are not ideal. However, due to hospital confidentiality stipulations, we are unable to publicly publish the true cost of each procedure after reimbursement, and present charge data by proxy.
For the purposes of this project, we define an “indirect cost” as an expense incurred from any performance differential observed among first-assistants, quantified by operation duration. We hypothesize that duration (corresponding to higher operating room, ancillary, and anesthesia charges) is the largest driving variable in differences in expenditures and that this variable encompasses other contributing factors (ie, expertise level and technique).
Resident and PA Cohort Criteria
The resident cohort consisted of surgeries first-assisted by 1 surgical resident with at least 3 years of surgical training (PGY 3). The PA cohort consisted of surgeries first-assisted by a PA with at least 2 years of training related to the plastic surgery subspecialty.
Data Management and Statistical Methods
A total cost analysis was employed using a fee-for-service approach with charges from the Department of Plastic and Oral Surgery, alongside related services such as anesthesia, pathology, and other hospital-related services offered in relation to the procedure. Statistical analyses were conducted using SPSS, version 23.0 (IBM Corp., Armonk, N.Y.) Independent two-sample t tests and chi-square tests were used to compare demographics, clinical information, and operation duration. Shapiro-Wilk tests were used to determine normality and skew. Mann-Whitney tests were used to assess differences in total charge between cohorts. A maximum threshold of 20% missing data was used for all analyses and P < 0.05 was considered statistically significant for all analyses.
RESULTS
Sample Demographics
A total of 49 reduction mammaplasty procedures were included in analyses: PA first-assist (n = 25) and resident first-assist (n = 24). All patients underwent bilateral reduction mammaplasty using an inferior pedicle technique with Wise-pattern technique. All procedures were performed in the hospital setting either at a major urban center or at a satellite facility. Patients and their families decided whether they wished to be discharged the day of surgery or stay the night in the inpatient unit. In this sample, 64% of all patients spent 1 night in the inpatient unit, whereas the remaining patients were discharged the same day of surgery. The majority of patients in both the PA and resident cohorts (84%) had commercial insurance, with no significant difference between first-assistant type (P = 0.56, Table 1). Patients in both cohorts were of comparable age, ethnicity, BMI category, and had similar amounts of breast tissue resected during reduction (P > 0.05, all). The mean patient age at the time of surgery was 18.3 ± 2.2 years, and most patients were overweight or obese (76%). The median [interquartile range (IQR)] amount of breast tissue resected was 1586 g (631 g). All patients exhibited similar rates of post-operative complications, regardless of first-assistant type during their reduction (P = 0.53).
Table 1.
Sample Characteristics for Reduction Mammaplasty Procedures
| PA First-assist (n = 25) | Resident First-assist (n = 24) | Total (n = 49) | P | |
|---|---|---|---|---|
| Mean (SD) age, y | 18.6 (2.6) | 18.1 (1.8) | 18.3 (2.2) | 0.42* |
| Race, n (%) | 0.57† | |||
| White, non-Hispanic | 11 (44%) | 13 (52%) | 24 (48%) | |
| Minority | 14 (56%) | 12 (48%) | 26 (52%) | |
| Insurance type (%) | 0.56† | |||
| Public | 4 (16%) | 3 (13%) | 7 (14%) | |
| Commercial | 20 (80%) | 21 (88%) | 41 (84%) | |
| Self-pay | 1 (4%) | 0 (0%) | 1 (2%) | |
| BMI Category, n (%) | 0.88† | |||
| Healthy | 6 (24%) | 6 (25%) | ||
| Overweight | 9 (36%) | 10 (42%) | ||
| Obese | 10 (40%) | 8 (33%) | ||
| Median total breast tissue resected (IQR), g | 1501 (600) | 1431 (773) | 1501 (631) | 0.88‡ |
| Complications, any | 4 (16%) | 3 (13%) | 7 (14%) | 0.53† |
*Independent samples t-test.
†Chi-square test.
‡Samples median test.
A total of 15 residents and 1 PA qualified for inclusion. The vast majority of the resident cohort was on the independent plastic surgery track and had previously completed a general surgery residency (n = 11, 73%), whereas the remaining residents were part of an integrated plastic surgery training program (n = 4, 27%). The average postgraduate year (PGY) for the resident cohort was 5.9 ± 1.5 years. The PA had 2 years of experience. All procedures were supervised by one of the three attendings.
Procedural Charges and Operation Duration
Cases performed with a resident as first-assist were a median of $3750 more expensive and took an average of 34 minutes longer than those performed with a PA (P < 0.01, both) (Table 2) (Figs. 3–4).
Table 2.
Procedural Charge and Operation Duration
| PA First-assist (n = 25) | Resident First-assist (n = 24) | Total (n = 49) | P | |
|---|---|---|---|---|
| Charge (USD) | <0.01* | |||
| Median | $28,996.70 | $32,747.47 | $29,766.02 | |
| IQR | $3,393.52 | $7,277.26 | $6,615.25 | |
| Minimum | $24,766.73 | $25,120.73 | $24,766.73 | |
| Maximum | $39,775.02 | $43,752.67 | $43,752.67 | |
| Operation Duration (min) | <0.001† | |||
| Mean | 158 | 192 | 174 | |
| SD (SD) | 20 | 28 | 49 | |
| Minimum | 125 | 152 | 125 | |
| Maximum | 208 | 261 | 261 |
*Mann-Whitney test.
†Independent samples t-test.
Fig. 3.
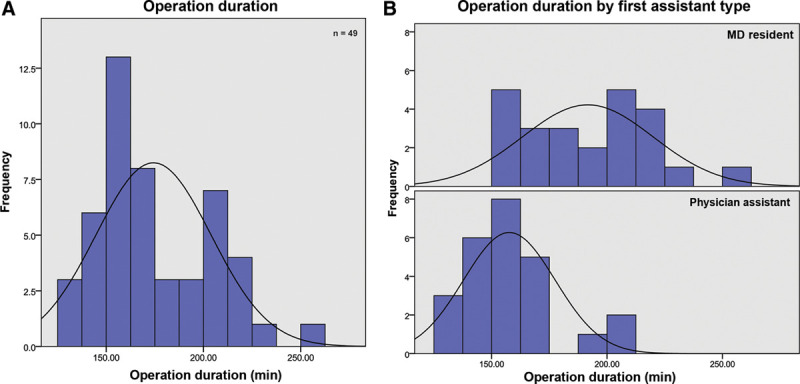
Operation duration analysis. (A) All cases. (B) By first assistant type.
Fig. 4.
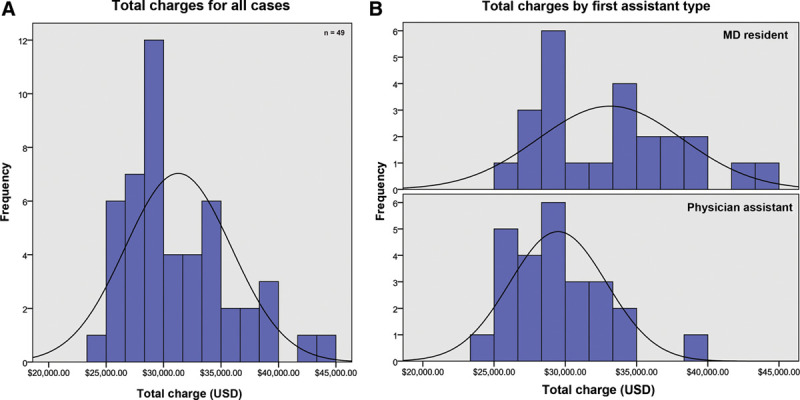
Total charge analysis. (A) All cases. (B) By first assistant type.
DISCUSSION
This QI project is the first of its kind to quantify the indirect cost of GME programming on an academic medical center by comparing the performance of a PA and a surgical resident as first-assists during adolescent bilateral reduction mammaplasty, a common surgical procedure. We found that operations first-assisted by residents were significantly longer and accrued higher charges than those assisted by PAs. Although previous studies have focused primarily on comparing resident and advanced practitioner performance on patient outcomes like length of stay and direct patient care cost, none have contextualized the financial implications of these 2 groups of providers on teaching institutions, or how these differences may relate to graduate medical education programming.14,21–23
We recognize that charge data alone are not sufficient without accounting for reimbursement. However, the percent receipt for both cohorts was similar. Although the charges values are inflated without reimbursement applied, the charge data reflect a similar proportional difference between the true PA and surgical resident case receipts.
Despite using charge data as a proxy, we found overall that charge and receipt data become less useful once greater context of the payment structure for PAs and residents is taken into account. Instead, we found that the observed time differential between PA and surgical residents is a clearer, more valuable measure of indirect cost of surgical resident training during reduction mammaplasty procedures.
Though residents and PAs play distinct roles in medicine at large, during a reduction mammaplasty, they both serve the same role as first assistants to an attending surgeon, rendering a direct performance comparison appropriate. Given their tenure in training, the resident cohort might be expected to exhibit equivalent or superior levels of surgical skill as a well-trained PA. However, the PA outperformed the resident cohort in operation duration. These differences suggest that teaching hospitals and subsequent surgical departments may assume potentially significant opportunity costs due to training surgical residents, even those with extensive experience, in comparison with skilled non-physicians.
It is interesting to note that although almost 75% of our resident cohort completed an additional general surgery residency before pursuing an independent plastic surgery training program (amounting to an average PGY of 6 years), they still underperformed compared with a PA who had only been operating for 2 or lesser years. Are there factors in graduate medical education programming delaying a surgical resident’s ability to develop technical expertise on par with advanced providers?
We hypothesize that the main factors contributing to differences in procedural charge and length are due to (1) resident instruction, which increases the length of procedure and in turn drives the charge of each procedure higher, and (2) graduate medical education programming restrictions. Other studies have observed similar increases in operation duration and subsequent cost when a resident is present in the OR, driven largely by resident instruction.24 The 80-hour workweek regulations combined with post-call restrictions reduce residents’ time spent in the operating room, limiting their ability to develop expertise. In addition to the 80-hour workweek limitation, another potential explanation for the deficit between resident and PA performance is the rotational design of residency programs. Although rotating services every 2 months exposes plastic surgical residents to a wide variety of procedures, it makes developing surgical proficiency in any one operation more difficult. Conversely, PAs tend to focus on the operations performed by a single-provider for many months or years, enabling them to gain tenure and hone their surgical skill set.
The 34-minute deficit observed between PA and resident performance in our analysis has drastic implications for overall clinical productivity. Our department performs, on average, 100 adolescent breast reductions per year; if every reduction were first-assisted by a PA rather than by a resident, the department could potentially recoup up to 60 hours, or 2.5 days, per year, to devote to either additional procedures or other tasks. This is just one operation. Our department, along all other entities which train residents, faces a substantial economic opportunity cost due to the observed resident/PA performance differential.
Although some direct GME costs are subsidized by the government, a large portion of indirect costs such as those found in the present QI project are not reimbursed, leaving teaching hospitals to assume the deficit.1,2 Additionally, if AMCs train more residents than the approved “resident cap” determined in 1997, the hospital must find sources apart from Medicare to cover training costs.8 Even for residents under the cap, hospitals must secure other sources of funding because federal support is not sufficient to pay for all GME expenses. Therefore, for AMCs with robust GME programs, such as our institution, these differences in resident and advanced practitioner performance could create substantial financial burden.
Our recommendation is not to do away with graduate medical education programs; they are vital to training the future generation of surgeons. Rather, we suggest first increasing federal compensation to teaching hospitals to assist with GME expenses. AMCs are currently responsible for 80% of GME expenses.3 Coupled with increasing procedural charges and reduced reimbursements, GME programs may not be financially sustainable in the future as currently designed. Increased Medicare support would ensure that teaching hospitals can train sufficient number of surgeons to meet increasing patient demands, while reducing financial pressure on institutions.
Another potential solution is to restructure the GME paradigm by incorporating PAs into the educational structure. Other studies have demonstrated that incorporating advanced providers can optimize the resident clinical service-to-education balance, reduce clinical burden, and be financially feasible for some departments.25,26 By reducing clinical and administrative workload, PAs increase the teaching time between attendings and residents. Previous studies have shown that PAs augment graduate medical education programs by teaching residents common procedures and surgical skills.12 With PAs reinforcing basic technique, attendings can reserve time and energy for instructing residents about complex and exceptional cases. Although PAs greatly contribute to resident education, their current role in GME programs is not formally defined.27,28 By formally including PAs in GME program design, AMCs could potentially evade the opportunity costs associated with residents first-assisting rather than PAs, and could also relieve administrative burden on attending surgeons and enhance the quality of their resident instruction. It would also expand the roles and responsibilities of PAs, and increase educational exposure for residents.
However, we recognize that not all teaching hospitals have the financial bandwidth to support PAs. For hospitals with fewer resources, the cost of adding PAs may be too great despite the difference in efficiency because residents are often the only option to fill clinician positions. Federal programs like Medicare may consider bridging this fiscal gap by reimbursing these institutions for the indirect costs of GME, in particular for surgical residency programs that may experience differences in clinical efficiency. Further research is needed to determine the cost-efficiency of residents and PAs in a surgical context.
This QI project is limited in scope because only 1 type of surgical procedure performed at 1 large tertiary care facility was considered for analysis, which may not be generalizable across all procedures and departments. However, as costs increase with limited reimbursement, these analyses will become increasingly important when considering the future feasibility of plastic surgery training programs. Data from the resident cohort may also be variable, given the heterogeneity of the resident physician cohort compared with the consistency of the singular PA. However, few PAs in our department qualified for inclusion, given that only a handful regularly perform reduction mammaplasties. In contrast, many surgical residents rotate through our program every year. The compensation difference between residents and PAs deserves further cost–benefit analysis, and a future study should utilize a prospective design to observe differences between first-assistant performance. Please note that financial values reflect the total amount charged without accounting for reimbursement (due to hospital confidentiality), and do not reflect the out-of-pocket price patients may pay for this procedure.
CONCLUSIONS
In this QI project, reduction mammaplasty procedures first-assisted by a resident took a longer time and accrued higher charges than cases first-assisted by a PA. Though teaching hospitals play an integral role in shaping future surgeons, this analysis exemplifies the indirect financial costs they may assume while training surgical residents. Although we are unable to disclose confidential charge data, this project suggests that institutions should be additionally reimbursed for these indirect costs, and may consider incorporating PAs into surgical training program design to enhance both cost-efficiency and surgical resident instruction.
Footnotes
Published online 25 January 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Grover A, Slavin PL, Willson P. The economics of academic medical centers. N Engl J Med. 2014;370:2360–2362. [DOI] [PubMed] [Google Scholar]
- 2.Chandra A, Khullar D, Wilensky GR. The economics of graduate medical education. N Engl J Med. 2014;370:2357–2360. [DOI] [PubMed] [Google Scholar]
- 3.Association of American Medical Colleges. Medicare payments for graduate medical education: what every medical student, resident, and advisor needs to know. Available at https://www.aamc.org/data-reports/faculty-institutions/report/medicare-payments-graduate-medical-education-what-every-medical-student-resident-and-advisor-needs. Published April 2019. Accessed March 17, 2020.
- 4.Congressional Research Service. Medicare graduate medical education payments: an overview. Available at https://fas.org/sgp/crs/misc/IF10960.pdf. Published February 19, 2019. Accessed September 1, 2020.
- 5.Congressional Research Service. Federal support for graduate medical education: an overview. Available at https://fas.org/sgp/crs/misc/R44376.pdf. Published December 27, 2018. Accessed September 1, 2020.
- 6.CGS Medicare. Medical education reimbursement. 2020. Available at https://www.cgsmedicare.com/parta/audit/mepr/reimbursement.html. Accessed October 1, 2020.
- 7.Koenig L, Dobson A, Ho S, et al. Estimating the mission-related costs of teaching hospitals. Health Aff (Millwood). 2003;22:112–122. [DOI] [PubMed] [Google Scholar]
- 8.Balanced Budget Act of 1997 § 105, U.S.C. § 33 (1997).
- 9.Hopkins TJ. United States limits resident physicians to 80 hour working week. BMJ. 2003;326:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis FR., Jr. Should we limit resident work hours? Ann Surg. 2003;237:458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulmer C, Miller D, M Wolman, et al. (Eds) Institute of Medicine (US) Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work schedule to Improve Patient Safety. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. 2009Washington, D.C.: National Academies Press; [PubMed] [Google Scholar]
- 12.Dies N, Rashid S, Shandling M, et al. Physician assistants reduce resident workload and improve care in an academic surgical setting. JAAPA. 2016;29:41–46. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SA, Davis SA, Banes CT, et al. Impact of advanced practice providers (nurse practitioners and physician assistants) on surgical residents’ critical care experience. J Surg Res. 2015;199:7–12. [DOI] [PubMed] [Google Scholar]
- 14.Iannuzzi MC, Iannuzzi JC, Holtsbery A, et al. Comparing hospitalist-resident to hospitalist-midlevel practitioner team performance on length of stay and direct patient care cost. J Grad Med Educ. 2015;7:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buch KE, Genovese MY, Conigliaro JL, et al. Non-physician practitioners’ overall enhancement to a surgical resident’s experience. J Surg Educ. 2008;65:50–53. [DOI] [PubMed] [Google Scholar]
- 16.Occupational Outlook Handbook: Physician Assistants. U.S. Bureau of Labor Statistics, Employment Projections Program. https://www.bls.gov/ooh/healthcare/physician-assistants.htm#tab-6. Accessed February 19, 2020.
- 17.Occupational Outlook Handbook: Physicians and Surgeons. U.S. Bureau of Labor Statistics, Employment Projections Program. https://www.bls.gov/ooh/healthcare/physicians-and-surgeons.htm#tab-6. Accessed February 19, 2020.
- 18.American Academy of PAs. AAPA salary report: nationwide PA compensation and benefits data. 2019. Available at https://www.aapa.org/shop/salary-report/. Accessed March 17, 2020.
- 19.Boston Medical Center. Intern, resident, and fellow benefits. 2020. Available at https://www.bmc.org/medical-professionals/graduate-medical-education-gme/house-staff/intern-resident-and-fellow-benefits. Accessed August 28, 2020.
- 20.Nuzzi LC, Firriolo JM, Pike CM, et al. Complications and quality of life following reduction mammaplasty in adolescents and young women. Plast Reconstr Surg. 2019;144:572–581. [DOI] [PubMed] [Google Scholar]
- 21.Johal J, Dodd A. Physician extenders on surgical services: a systematic review. J Can Surg. 2017;60:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang R, Columbo JA, Kunkel ST, et al. Residents’ impressions of the impact of advanced practice providers on surgical training. J Am Coll Surg. 2018;226:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreeftenberg HG, Aarts JT, de Bie A, et al. An alternative ICU staffing model: implementation of the non-physician provider. Neth J Med. 2018;76:176–183. [PubMed] [Google Scholar]
- 24.Allen RW, Pruitt M, Taaffe KM. Effect of resident involvement on operative time and operating room staffing costs. J Surg Educ. 2016;73:979–985. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, D’Souza M, Singman EL, et al. Integration of a physician assistant into an ophthalmology consult service in an academic setting. Am J Ophthalmol. 2018;190:125–133. [DOI] [PubMed] [Google Scholar]
- 26.Russell JC, Kaplowe J, Heinrich J. One hospital’s successful 20-year experience with physician assistants in graduate medical education. Acad Med. 1999;74:641–645. [DOI] [PubMed] [Google Scholar]
- 27.Resnick AS, Todd BA, Mullen JL, et al. How do surgical residents and non-physician practitioners play together in the sandbox? Curr Surg. 2006;63:155–164. [DOI] [PubMed] [Google Scholar]
- 28.Clark CJ, Hildreth A, Migaly J, et al. Supervisor, colleague, or assistant: general surgery resident perceptions of advanced practitioners. Am Surg. 2018;84:294–299. [PubMed] [Google Scholar]


