Abstract
Objective
To regulate food intake, our brain constantly integrates external cues, such as the incentive value of a potential food reward, with internal state signals, such as hunger feelings. Incentive motivation refers to the processes that translate an expected reward into the effort spent to obtain the reward; the magnitude and probability of a reward involved in prompting motivated behaviour are encoded by the dopaminergic (DA) midbrain and its mesoaccumbens DA projections. This type of reward circuity is particularly sensitive to the metabolic state signalled by peripheral mediators, such as insulin or glucagon-like peptide 1 (GLP-1). While in rodents the modulatory effect of metabolic state signals on motivated behaviour is well documented, evidence of state-dependent modulation and the role of incentive motivation underlying overeating in humans is lacking.
Methods
In a randomised, placebo-controlled, crossover design, 21 lean (body mass index [BMI] < 25 kg/m2) and 16 obese (BMI³ 30 kg/m2) volunteer participants received either liraglutide as a GLP-1 analogue or placebo on two separate testing days. Incentive motivation was measured using a behavioural task in which participants were required to exert physical effort using a handgrip to win different amounts of food and monetary rewards. Hunger levels were measured using visual analogue scales; insulin, glucose, and systemic insulin resistance as assessed by the homeostasis model assessment of insulin resistance (HOMA-IR) were quantified at baseline.
Results
In this report, we demonstrate that incentive motivation increases with hunger in lean humans (F(1,42) = 5.31, p = 0.026, β = 0.19) independently of incentive type (food and non-food reward). This effect of hunger is not evident in obese humans (F(1,62) = 1.93, p = 0.17, β = −0.12). Motivational drive related to hunger is affected by peripheral insulin sensitivity (two-way interaction, F(1, 35) = 6.23, p = 0.017, β = −0.281). In humans with higher insulin sensitivity, hunger increases motivation, while poorer insulin sensitivity dampens the motivational effect of hunger. The GLP-1 analogue application blunts the interaction effect of hunger on motivation depending on insulin sensitivity (three-way interaction, F(1, 127) = 5.11, p = 0.026); no difference in motivated behaviour could be found between humans with normal or impaired insulin sensitivity under GLP-1 administration.
Conclusion
We report a differential effect of hunger on motivation depending on insulin sensitivity. We further revealed the modulatory role of GLP-1 in adaptive, motivated behaviour in humans and its interaction with peripheral insulin sensitivity and hunger. Our results suggest that GLP-1 might restore dysregulated processes of midbrain DA function and hence motivational behaviour in insulin-resistant humans.
Keywords: Glucagon-like peptide-1, Insulin sensitivity, Regulation of motivational behaviour, Obesity, Hunger
Highlights
-
•
Hunger increases incentive motivation in lean but not obese humans.
-
•
Impaired peripheral insulin sensitivity reduces the motivational effect of hunger.
-
•
GLP-1 modulates incentive motivation independently of incentive type (food and non-food reward).
-
•
GLP-1 analogue application normalises the motivational effect of hunger depending on insulin sensitivity.
1. Introduction
The growing obesity epidemic represents one of the greatest health challenges of the 21st century, leading to increased risk of severe comorbidities such as cardiovascular disease or cancer [1,2]. While continuous excessive food intake has long been identified as one of the leading causes promoting obesity, the physiological mechanisms driving food intake behaviour and overeating remain poorly understood, especially in humans.
Our daily behaviours are driven by basic needs often without us noticing. Hunger is one of these basic behavioural drivers, as food serves as the energetic foundation for all biological processes. To regulate the body's need for food and the subsequent internal sensation of hunger, the brain has developed precise physiological and behavioural mechanisms to keep the body operating at optimal levels; to ensure this physiological homeostasis and adapt behavioural responses, our brain constantly integrates information about the metabolic state with external environmental cues [[3], [4], [5]]. Although there has been much investigation on the basic homeostatic mechanisms of hunger and the behavioural consequences thereof [6,7], significant gaps remain in understanding how metabolic signals prompt and external cues incentivise the behavioural aspect of the hunger response.
External cues comprise strong motivational signals, such as the incentive value of an expected reward, but also the effort required to obtain the reward [[8], [9], [10]]. Hence, everyday decisions in favour of or against food intake are based on cost-benefit analyses weighing the potential food reward against the cost of spending effort to obtain it. The incentive theory of motivation [11,12] suggests that behaviour is primarily motivated by anticipated rewards and reinforcement. Thus, behavioural drive and hence incentivised motivation refers to the processes that translate expected reward into effort spent [13]. These processes include forming a subjective representation of the potential reward magnitude, which determines effort exertion [14] and is critical for initiating motivated behaviour [9,15]. Notably, the subjective reward magnitude depends markedly on the internal state of the organism [10]; a food reward is regarded as more valuable in a hungry than in a sated state [16].
The magnitude and probability of a reward [9,17] is encoded by dopamine (DA) neurons in the ventral tegmental area (VTA) and its mesoaccumbens DA projections. By promoting the formation of cue–reward associations, VTA DA neurons play a central role in mediating motivated behaviours [18]. DA terminals in the nucleus accumbens (NAc) specifically respond to reward-predictive cues [19,20]. In fact, the activity of VTA DA neurons and NAc DA levels have the capacity to prompt reward-directed action initiation and effort exertion [21,22]. In human pharmacological intervention studies, a lower dopaminergic tone was shown to result in lower effort spending and motivation [8,23,24].
Related to overeating, alterations in the mesoaccumbens DA pathway have been consistently linked to obesity in animal studies [[25], [26], [27]]. In obese humans, alterations in the fronto-mesostriatal DA circuitry have been generally related to an impaired reward system [28,29]. Reduced binding potential of striatal dopamine receptors has been hypothesised to be associated with a heightened striatal dopaminergic tone, leading to an imbalance between anticipation and consumption of food reward [[30], [31], [32], [33], [34], [35]]. While it was reproducibly shown that obese vs lean humans show greater neural activation in reward-related regions anticipating rewarding stimuli, neural activation in response to obtained food rewards decreases [[36], [37], [38], [39]]; however, findings of the reinforcing capacity and its link to incentive motivation and effort spending underlying obesity portray a heterogeneous picture [40,41].
These studies, however, may rest on an incomplete assumption about modulatory aspects of midbrain DA function and body mass index (BMI) as decisive variables to nuanced facets of motivated behaviour. While being strongly implicated in incentive motivation, VTA DA neuron firing and mesoaccumbens DA pathways are also particularly sensitive to nutritional value [16,27], post-ingestive effects of food [42,43], and metabolic state signalled by neuropeptides and peripheral peptidergic mediators [[44], [45], [46], [47]]. These bodily signals may bias our food intake behaviour more than just external cues per se; in fact, they may also shape the incentive cue value, mediating its reinforcement efficiency depending on the metabolic state.
Notably, VTA DA neurons express many receptors that respond to peripheral peptides that signal metabolic status [48]; the glucagon-like peptide 1 (GLP-1) receptor is a particularly prominent example [49,50] that has also been used as target for the development of drug therapies aimed at curbing overeating [51,52]. While GLP-1 acts primarily on pancreatic islets to enhance glucose-induced insulin secretion, it can induce metabolic actions to maintain glucose homeostasis by interacting with its receptors expressed on neurons and cells in the enteric and central nervous system [53,54].
Related to the mesoaccumbens DA pathway, rodent studies demonstrated that activation of GLP-1 receptors in the VTA by endogenous GLP-1 specifically reduces the excitatory synaptic strength of DA neurons that project to the NAc [55], decreasing the reinforcing efficiency of appetitive cues and adapting motivated behaviour [51,[56], [57], [58]].
In line with the work in rodents, GLP-1 analogues were reliably shown to lead to reduced food intake and to induce weight loss in obese humans [[59], [60], [61]]. However, while in rodents the modulatory effect of GLP-1 on DA neurocircuitry and motivational behaviour is well documented [56,62], evidence of a modulatory role of GLP-1 affecting motivational behaviour in humans is lacking.
To this end, the present randomised, placebo-controlled, and crossover study assessed the modulatory role of GLP-1 in motivated behaviour of lean (BMI < 25 kg/m2) in comparison to obese (BMI³ 30 kg/m2) humans. To account for the hunger state, subjective hunger ratings were assessed. To consider the metabolic state and particularly the physiological role of GLP-1 in maintenance of glucose homeostasis, fasted insulin and glucose levels were acquired. As a readout for incentivised motivated behaviour, we adapted a classic behavioural paradigm of effort spending that was first suggested by Pessiglione et al. [63] and further refined by Le Bouc et al. [23]. In this task, different amounts of possible reward are used as incentives. Volunteer participants were required to exert physical effort (force) on a handgrip to win different amounts of food and non-food rewards (money).
Interestingly, although we detected a differential effect of hunger on incentive motivation between lean and obese humans (F(1, 137) = 3.98, p = 0.048), we could not find a GLP-1-interaction with BMI. Based on the physiological role of GLP-1 in the regulation of insulin secretion and prior evidence that insulin sensitivity modulates excitatory input of VTA DA neurons [64] as well as mesostriatal functional connectivity [65], we predicted that GLP-1 interactions with motivational functions of DA might change with BMI depending on peripheral insulin sensitivity. Hence, we analysed the effect of insulin sensitivity on incentive motivation with peripheral insulin sensitivity being assessed by the homeostasis model assessment of insulin resistance (HOMA-IR) [66,67]. It is important to note that none of the studies’ participants suffered from diabetes.
2. Materials and methods
2.1. Participants
Twenty-five subjects with normal weight (BMI: 22.42 ± 0.22 kg/m2) and 25 obese subjects (BMI: 35.61 ± 0.87 kg/m2) were recruited from the pre-existing database of volunteers maintained at the Max Planck Institute of Metabolism Research based on a power analysis assuming an a error = 0.05, power = 0.95, and a small-to medium-effect size of Cohen's d = 0.35 (equivalent to f = 0.175). This power analysis was performed for a mixed-effects model targeting the three-way interaction of group (lean vs obese), intervention (GLP-1 vs placebo), and hunger level, yielding a total sample size of N = 46.
All the participants were non-smokers between the ages of 20–40 years with no history of neurological, psychiatric, metabolic, or eating disorders. In the course of the analysis, 13 subjects had to be excluded due to low engagement in the task: 7 subjects were excluded as they reported wanting of the monetary or food reward lower than 3 (of 10) points, 3 participants had not invested sufficient effort in the calibration so they engaged constantly to press with >75% of their maximum force for every single trial, and for 3 participants their preferred amount of food reward was not clearly identifiable. Hence, 21 lean (BMI: 22.56 ± 0.38 kg/m2, age: 26.87 ± 1.4 years, 9 female) and 16 obese (BMI: 35.32 ± 1.36 kg/m2, age: 27.20 ± 1.3 years, 9 female) subjects were included for further data analysis (Table 1).
Table 1.
Participants’ characteristics.
| Parameter | Mean ± SEM |
|---|---|
| N (lean) | 37 (21) |
| Age (years) | 26.49 ± 0.9 |
| lean | 26.87 ± 1.4 |
| obese | 27.20 ± 1.3 |
| BMI (kg/m2) | 27.68 ± 1.22 |
| lean | 22.56 ± 0.19 |
| obese | 35.32 ± 0.66 |
Note: BMI = body mass index, SEM = standard error of the mean.
After analysing the effects of groups stratified by BMI (normal vs obese), we examined how incentive motivation may change with systemic insulin resistance as assessed by the homeostatic model of insulin resistance (HOMA-IR) [ 66; 67]. For this purpose, the HOMA-IR of each participant was calculated as (fasting serum glucose in mg/dl × fasting serum insulin in mU/l)/405, with lower values indicating a higher degree of insulin sensitivity. Only the HOMA-IR of the placebo day was calculated, as GLP-1 analogues may increase insulin secretion and alter the HOMA-IR. The HOMA-IR was used as a continuous variable later in the analysis. The final subject selection (N = 37) allowed for a power of 0.91 for the three-way interaction of the HOMA-IR, hunger, and intervention within the used mixed-effects model, with an effect size of partial h2 = 0.03 (equivalent to f = 0.176) and an a error = 0.05.
All the subjects provided written informed consent to participate in the experiment, which was approved by the local ethics committee of the Medical Faculty of the University of Cologne (Cologne, Germany; No. 16–251).
2.2. Study design
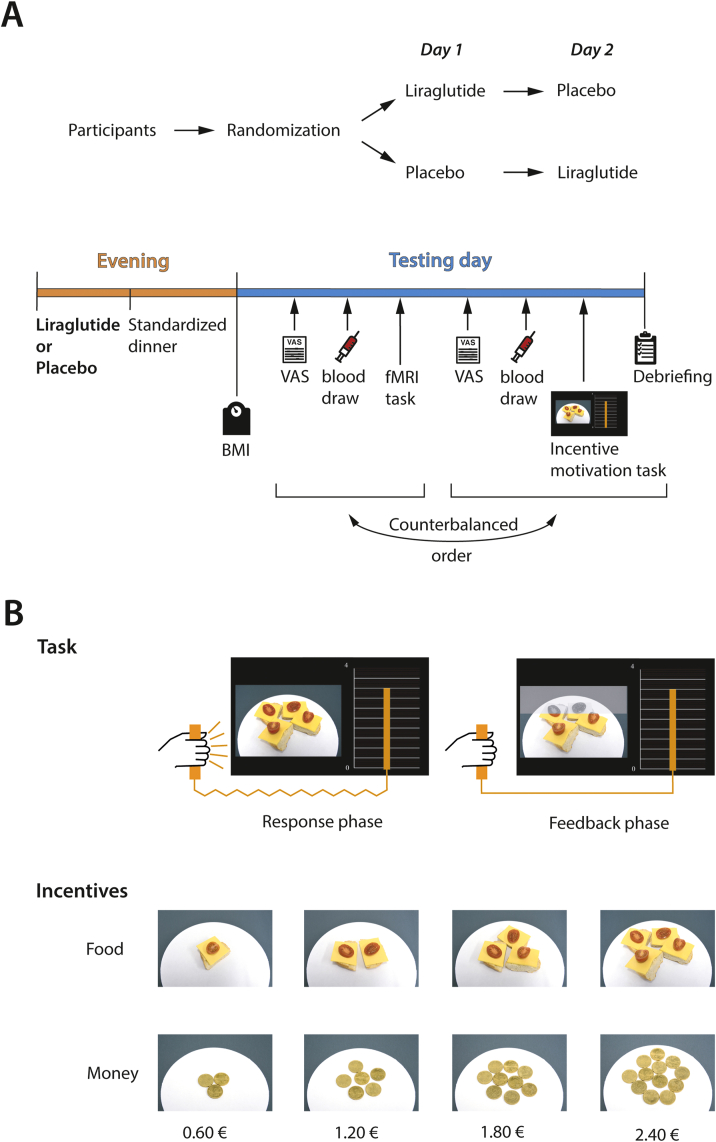
The study was conducted in a single blinded, placebo-controlled, randomised, cross-over design. Each volunteer participated on two testing days lasting a maximum of 2 h each. Both testing days were separated by at least one week to allow for a sufficient wash-out period [68]. The order of the intervention (GLP-1 vs placebo) was counter-balanced (Figure 1A).
Figure 1.
Study design and behavioural task. A. In this within-subject design, the subjects received either placebo or liraglutide and a standardised dinner the evening prior to each testing day. After fasting overnight, BMI, hunger rating, and a blood draw (glucose and insulin levels) were assessed the next morning followed by the behavioural task with a short debriefing. B. After a fixation cross, the subjects were shown either a monetary cue (0.6 €, 1.2 €, 1.8 €, or 2.4 €) or a food cue (1/4, 2/4, 3/4, or 4/4 of a bread roll with cheese) presented on a white plate, for which the participants exerted force to win the presented cues. Online feedback on the force produced was provided by an orange bar ascending with increasing force exertion. During the following feedback phase, the participants could relax their arm, and direct feedback about the amount of the presented reward that the subjects would have won was displayed by a colour change in the cue image. The different levels of incentives (food and money) are also depicted in detail. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The evening prior to each testing day, the participants received an agonistic GLP-1 analogue (see as follows) or an equal volume of saline solution and a standardised dinner with equal kcal amounts per individual (see Supplemental Material). The next morning, the participants arrived fasted at the institute at 8 a.m. and their BMI was measured using a Seca mBCA 515 (medical Body Composition Analyser). As this study was part of a larger experiment, all the participants not only underwent the behavioural task detailed as follows but also an fMRI task that was related to a different study question (sensory learning) and that is reported elsewhere. The order of the behavioural task and fMRI task was counterbalanced.
Before each task, hunger levels were assessed via visual analogue scales and blood drawn to measure insulin and glucose. After the behavioural task, individual liking and wanting of the reward types (money and food) and the participants’ compliance were evaluated in a short debriefing.
2.3. GLP-1 analogue
A subcutaneous injection of 0.6 mg of liraglutide (Novo Nordisk) was used as an agonistic GLP-1 analogue. As the maximum plasma concentration of liraglutide is reached approximately 11–13 h after injection [68], liraglutide was administered the evening prior to the testing day between 7 and 8 p.m. to ensure sufficient blood plasma levels at the start of the testing day. As a placebo condition, an equal volume of saline solution was injected subcutaneously.
2.4. Incentive motivation task
Incentive motivation was assessed by measuring effort spending for external cues in a behavioural paradigm (Figure 1B). Two incentive types (food and money) could be earned by squeezing a handgrip device (hand dynamometer HD-BTA, Vernier). The task was programmed in MATLAB (version 2014b, MathWorks) using the Psychophysics Toolbox (version 3.0.11) [69,70] and a toolbox dedicated to enabling communication between MATLAB and the device (https://github.com/lionel-rigoux/vernier-toolbox).
Prior to the task, the participants performed a calibration in which they were instructed to squeeze the handgrip three times in a sequence as hard as possible. For each participant, the highest force reached, , was scaled to define the subject's individual maximum voluntary contraction
Each participant's individual maximum contraction was used as a reference point related to the force of each trial in the task. Note, was scaled up to ensure that was always higher than the maximum force in each trial during the task. However, if the subjects' maximum force in one trial during the incentive motivation task exceeded the initially calibrated , the maximum force exerted during this trial was used to recalculate the maximum voluntary contraction in subsequent analyses.
Following the calibration, printed instructions were provided to each participant. The subjects were informed that the percentage of exerted maximum force related to how much of the presented stimulus they could win (e.g. if a subject squeezed with 80% of their maximum voluntary contraction, they could win 80% of the displayed food or monetary stimulus). Before starting the task, a short training session of 4 test trials (2 food and 2 money trials) with randomly assigned stimulus amounts was provided.
In total, the task comprised 128 trials with a total duration of 25 min. The trials were divided into 4 blocks displaying monetary stimuli and 4 blocks with food stimuli. Hence, any block consisted of 16 trials in which varying stimulus amounts were presented. Food and monetary blocks alternated. Each trial consisted of two phases: a response phase and feedback phase.
During the response phase, one of four monetary amounts (0.6 €, 1.2 €, 1.8 €, or 2.4 €) or one of four food amounts (1/4, 2/4, 3/4, or 4/4 of a bread roll with cheese) was displayed as an incentive on the left-hand side of the screen. While the food or monetary stimulus was displayed, the participants exerted force on the handgrip. Feedback of the performance was directly provided by an orange bar ascending in height in proportion to the exerted force. Each stimulus amount was displayed 4 times per block. The response phase lasted for 3 s.
During the following feedback phase, the participants could relax their arm, and direct feedback on the amount of the presented reward the participant would have won was displayed (for example, if the participant exerted 80% of his/her maximum voluntary contraction, 80% of the displayed stimulus changed in colour representing the amount won). The feedback phase lasted for 3.5 s.
The subjects were informed that one food trial and one monetary trial were chosen at random at the end of the experiment and that they were granted the reward won in this trial. To provide a supplementary stimulus to motivate the subjects for greater effort exertion, one plate with the food stimulus and one with the monetary stimulus were placed within viewing range of each subject during the task.
2.5. Hunger and liking ratings
To control for differences in hunger states between testing days, we instructed the participants to rate their hunger prior to the task on each testing day using a visual analogue scale as previously described [71]. In brief, on a 100 mm visual analogue scale (0 mm = “sehr hungrig (very hungry)” and 100 mm = “gar nicht hungrig (not hungry at all)”), the subjects were asked to mark the point that most accurately represented their perception of their current hunger state.
Likewise, to explore the individual incentive value of cues, liking of the items (separately for money and food) was rated on a 100 mm vertical scale with “Mag ich gar nicht (not liking the item at all)” at the lower anchor point and “Mag ich sehr (liking the item a lot)” at the upper anchor point [72].
2.6. Insulin levels
As GLP-1 analogues are reported to increase insulin secretion [73], we monitored insulin levels to control for insulin effects at the onset of the behavioural task. This monitoring was achieved by a blood draw directly before starting the task and measuring the insulin level within. Glucose levels were assessed from the same blood draw.
2.7. Statistical analysis
Statistical analysis was performed in MATLAB (version 2014b, MathWorks) and R (version 4.0.0) [74] using the ImerTest package (version 3.1–2) [75]. GraphPad Prism (version 8.0) was used to visualise the results. Statistical significance was reported at a level of p < .05.
The analysis of the acquired data followed a two-level approach. On a first (subject) level, a general linear model was used to assess the interaction of incentive and force exerted for food or monetary reward separately. Note, as not all the participants experienced the same amount of food as rewarding, incentive levels were recalibrated based on the amount the subjects individually preferred (for details, see Supplemental Material). Both incentive types were analysed separately to include the type of incentive for the subsequent second-level analysis. Hence, the following statistical models were applied to the data:
for money as an incentive type and
for food as an incentive type, where the coefficients indexed with related to monetary and those indexed with related to food incentives. The regression coefficients represent the individual motivation to spend physical effort for incentives. That is, a low indicates that a participant did not increase their effort spending much with increasing incentive and thus revealed a low incentive motivation.
A further analysis was performed to test for differences in force exertion between the two types of incentives. In this study, a mixed-effects model was established including “type of incentive” (money/food), “level of incentive,” and their interaction as independent variables (fixed effects) and subject as the random effect. Post hoc comparisons were calculated using Tukey's procedure facilitated by the lsmeans package (version 2.30-0; [76]) in R.
On the second (group) level, we assessed the effect of the intervention (GLP-1 vs placebo) on incentive motivation ( and ) with a mixed-effects model considering the independent variables “type of incentive” (money vs food), intervention (GLP-1 vs placebo), hunger, and group (lean vs obese) as fixed effects with the subject as a random effect. This design lent structure to examining the effects of the intervention and hunger while considering the BMI as a group-differentiating criterion. Thus, hunger was used as a continuous variable while group, intervention, and “type of incentive” were used as factorial variables. We also controlled for the individual baseline insulin level, liking of the respective incentive, and order of the testing days.
To consider an effect of peripheral insulin sensitivity on motivation, we established a further mixed-effects model with the independent variable HOMA-IR instead of group (lean vs obese). Thus, HOMA-IR and hunger were used as continuous variables and intervention (GLP-1 vs placebo) as well as “type of incentive” (money vs food) as factorial variables:
Given the complexity of our three-way interaction of intervention, hunger, and HOMA-IR (F(1, 127) = 5.11, p = 0.026), we also analysed the effect of the intervention (GLP-1 or placebo) separately with mixed-effects models testing the effect of incentive, hunger, and HOMA-IR while controlling for insulin levels, liking of incentive, and measuring day.
3. Results
To evaluate the modulatory role of GLP-1 in motivated behaviour, we conducted a placebo-controlled behavioural study employing an established effort spending task in lean and obese human participants. In this task, individuals were required to exert physical effort using a handgrip to win different amounts of reward and adapt their behaviour to changing external cues with different reward types. To examine implications of reinforcement, we incentivised the task with either a food or non-food reward; to consider the impact of internal state modulation, we assessed hunger levels; and we tested for the modulatory role of GLP-1 and its interaction with peripheral insulin sensitivity, hunger, and incentive on task outcome.
3.1. Effort exertion increased with increasing incentive value and liking
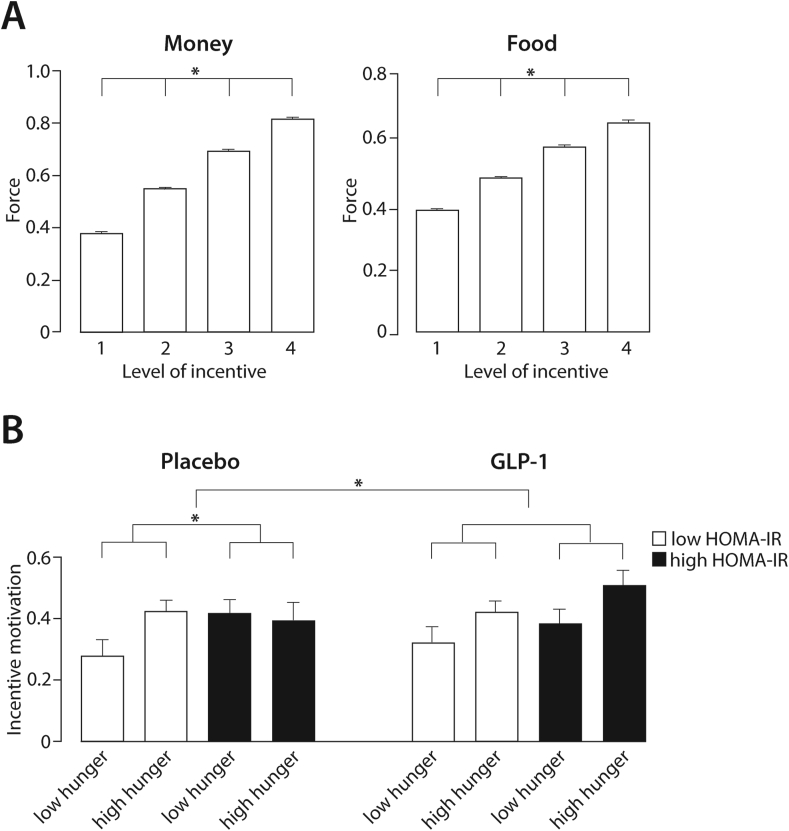
In addition to the suggestion that behaviour is motivated by internal drives (hunger), incentive theory suggests that external cues leverage reinforcement (incentives). Thus, in comparing behavioural performance on the task between different types of incentives (food vs money), we examined force exertion for the varying levels of food and monetary amounts (1/4 up to 4/4 of a bread roll with cheese or 0.60 € up to 2.4 €). In congruence with Pessiglione et al. (2007) revealing that individuals spend higher effort for higher reward value per se (the main effect of level of incentive on force: F(3,9435) = 1886.73, p < 0.0001; see Supplemental Material), we also found a significant two-way interaction revealing that force exertion increased more for increasing monetary rewards than for increasing food rewards (F(3,9435) = 65.42, p < 0.0001, Figure 2A; for further details, see Supplemental Material).
Figure 2.
A. Force exertion for different levels of incentive. The force exerted by all of the participants for monetary and food rewards is shown as mean ± SEM. The exerted force increased with higher amounts of incentives. B. GLP-1 modulated incentive motivation depending on insulin sensitivity and hunger. The modulatory effect of GLP-1 was tested in a three-way interaction of hunger HOMA-IR intervention (F(1, 120) = 5.11, p = 0.026). In the placebo condition, in the individuals with a low HOMA-IR, increasing hunger levels promoted incentive motivation; in turn, in those with a high HOMA-IR, higher hunger levels did not lead to an increase in incentive motivation (F(1, 35) = 6.23, p = 0.017, β = −0.281). Under the GLP-1 intervention, no significant difference between the participants with low HOMA-IR and high HOMA-IR could be detected (two-way interaction, F(1, 34) = 0.13, p = 0.72). In the analysis, hunger and HOMA-IR were used as continuous variables. For illustration purposes, HOMA-IR is depicted as a categorical variable distinguishing between the participants with a low HOMA-IR and high HOMA-IR using a median split (median = 1.68) shown as mean with SEM.
Furthermore, as liking of a reward is essential for its incentive value, we controlled for the effect of liking on effort exertion, revealing that higher liking of the incentive linked to stronger motivational drive (β = 0.016, t = 2.35, p = 0.02; for further details, see Supplemental Material).
3.2. In lean humans, incentive motivation increased with increasing hunger levels
We assessed the modulation of effort exertion under GLP-1 depending on hunger level in lean and obese humans (lean BMI < 25 kg/m2, obese BMI ³ 30 kg/m2). While the three-way interaction of intervention, hunger, and group was borderline to significant (F(1, 116) = 3.68, p = 0.057; Supplemental Table 4), a significant two-way interaction of group and hunger (F(1, 137) = 3.98, p = 0.048) was detected. Given the complexity of such an interaction, however, and to ascertain how hunger affects incentive motivation differently in lean and obese humans, we analysed both groups separately showing that in the lean subjects, incentive motivation increased with increasing hunger levels (F(1, 42) = 5.31, p = 0.026, β = 0.19; Supplemental Table 5). In the obese group, however, no significant effect of hunger on incentive motivation was detectable (F(1, 62) = 1.93, p = 0.17, β = −0.12; Supplemental Table 6).
3.3. Modulation of incentive motivation by GLP-1 differed depending on insulin sensitivity and hunger level
Based on the physiological role of GLP-1 in regulating insulin secretion [53] and evidence linking insulin signalling to motivational behaviour (see Introduction), we hypothesised that GLP-1 interactions with motivational functions might change depending on peripheral insulin sensitivity. Hence, we tested the three-way interaction of GLP-1, HOMA-IR, and hunger level (F(1, 127) = 5.11, p = 0.026; Supplemental Table 7), which clearly indicated a modulation of incentive motivation by GLP-1 differing between the participants depending on their peripheral insulin sensitivity and hunger level. To ascertain this complex interaction, we analysed the GLP-1 and placebo condition separately (Figure 2B).
For the placebo condition, we found a significant interaction between hunger and insulin resistance (F(1, 35) = 6.23, p = 0.017, β = −0.281), revealing that with increasing HOMA-IR, the positive effect of hunger on incentive motivation was reduced. Hence, in the subjects with a high peripheral insulin sensitivity, increasing hunger levels promoted incentive motivation; in turn, in those with poorer insulin sensitivity, higher hunger levels did not lead to an increase in incentive motivation (see Supplemental Table 8). In the GLP-1 intervention, no significant difference between the participants with good insulin sensitivity and poor insulin sensitivity could be detected (two-way interaction hunger: insulin sensitivity, F(1, 34) = 0.13, p = 0.72; see Supplemental Table 9).
4. Discussion
To regulate food intake, our brain constantly integrates internal state signals such as hunger with external cues, such as the incentive value of a potential food reward. To adapt behavioural responses, metabolic modulators from the periphery impact on brain circuitry to ensure physiological homeostasis. This study provides an analysis of the modulatory effect of hunger on motivated behaviour in humans by considering GLP-1 and insulin sensitivity as metabolic modulators as well as the role of the incentive value reflected by different external cues (money and food).
Regarding the role of external cues, we demonstrated that the level of liking of the presented cues determined their incentive value as higher liking rendered higher motivation. To this end, it is important to note that our findings suggest a role of metabolic modulation of motivated behaviour independent of incentive type (food or money). This finding underscores evidence from psychological literature suggesting that biologically based motivation can affect behaviours in unrelated domains that are irrelevant to the biological motive [77]. From a more neurobiologically centred perspective, modulation of motivational drive independent of incentive type might indicate neural encoding by basal subcortical circuits, as “cognitive” cortical representations of motivationally relevant cues would influence action selection by weighing the current physiological needs against the predicted consequences of responding to certain cues [78].
Addressing the role of the internal state and metabolic signals as modulators of motivation, we demonstrated that incentive motivation depends on the internal state in lean humans, as it increases with increasing hunger. In obese humans, however, hunger does not affect incentive motivation. This effect of hunger on motivational drive is modulated by the insulin sensitivity of the individual, as the degree of insulin resistance determines the magnitude of the effect of hunger on incentive motivation under placebo conditions. The higher the HOMA-IR index, the lower is the positive effect of hunger on incentive motivation (see Figure 2B). This effect of hunger revealed in our work was also observed in animal (rodent) studies showing that neurons in the lateral hypothalamus (LH) sense fasting (or sated) states and regulate motivation [45,79,80]; low glucose levels that occur in the fasting state activate glutamate and orexin co-expressing neurons in the LH, which project to and excite VTA DA neurons [81]. GABAergic LH-neurons project to the VTA [82], and their activation increases motivation for food [79]. As previously mentioned, our findings significantly extend the effect of hunger on incentive motivation and non-food rewards in humans, as the influence of hunger and insulin sensitivity applied for both types of incentives (food and money). This is in line with animal studies deciphering GABAergic LH inputs in the VTA that contribute to motivational salience in multiple contexts [83].
Our results also reveal that the effect of hunger on incentive motivation is modulated by the peripheral insulin sensitivity of the individual. In previous studies, we showed that systemic insulin sensitivity may have an impact on DA projections of the midbrain [65], which is in line with other recent human studies emphasising that peripheral insulin sensitivity is a better predictor of altered DA signalling than BMI [[84], [85], [86]]. Animal studies revealed altered DA clearance and synthesis in the VTA and DA terminals in the NAc due to insulin resistance [87,88], and insulin resistance has been associated with maladaptive eating and motivational behaviour [87,89,90]. However, the detailed neuronal mechanisms on how insulin sensitivity affects DA signalling within the midbrain and hence incentive motivation remain to be elucidated.
We further show that upon GLP-1 receptor agonist (liraglutide) treatment, the effects of hunger and insulin resistance on incentive motivation are blunted as no differential effect of hunger on motivation depending on insulin sensitivity could be detected. Thus, no difference between insulin-resistant and insulin-sensitive humans could be identified under GLP-1 treatment, indicating that GLP-1 normalises the effect of hunger and insulin sensitivity on motivation. While Figure 2B suggests that GLP-1 restores motivational drive in insulin-resistant humans to a non-insulin-resistant levels, it cannot be statistically differentiated if GLP-1 reinstated the effect of hunger on motivation in the insulin-resistant participants or blunted the effect of hunger on motivation in the insulin-sensitive subjects (or both; see Supplemental Tables 10 and 11). Considering that GLP-1 application has a stronger effect on peripheral insulin secretion in insulin-resistant humans than insulin-sensitive individuals [91], it seems reasonable to assume that the central effect of GLP-1 is equally stronger in insulin-resistant humans than insulin-sensitive individuals, indicating that improvement of insulin-resistant humans is likely.
Related to treatment with GLP-1 receptor agonists, while the peripherally administered agonist exendin-4 was revealed to bind to both astrocytes and neurons in the VTA in rodents [50], peripherally administered liraglutide has not yet been shown to enter the VTA or NAc but could be detected in the circumventricular organs, hypothalamus (the paraventricular nucleus, supraoptic nucleus, and supraoptic decussation but not the lateral hypothalamus) [92,93], and solitary nucleus [NTS, 93]. Hence, although not demonstrated, it can be hypothesised that peripheral liraglutide may also bind directly to GLP-1 receptors within the VTA and thus affects motivational behaviour. In rodent studies with normal-weight animals, GLP-1 receptor activation in the VTA was reported to reduce motivational behaviour [56,57]. In detail, phasic DA responses in the VTA to food-predictive cues could be suppressed by the central administration of the GLP-1 receptor agonist exendin-4 [62]. However, it needs to be considered that these murine results are not fully comparable to our human results as hunger/fasting times were not included in the analysis.
An alternative and more likely access route might be via vagal afferents in the NTS, as peripheral liraglutide was shown to enter the NTS [93]. Peripherally administered liraglutide might bind to GLP-1 receptor-expressing glutamatergic and GABAergic neurons [93] as well as astrocytes [94,95] within the NTS, which regulate the GLP-1 producing neurons in the NTS. These GLP-1 producing neurons project to the VTA, suppressing activity of DA neurons in the mesoaccumbens pathway [55]. Comparing the influence of GLP-1 on incentive motivation in our human study with animal reports, similar behavioural results were found in normal-weight rodents with activation of GLP-1 receptors in the NTS reducing food and drug reward behaviour by targeting VTA DA neurons [96,97]. However, data on the effect of GLP-1 on motivational behaviour in insulin-resistant/obese rodents and the effect of hunger/fasting time are lacking.
Collectively, one reasonable mechanism underlying our behavioural findings is that GLP-1 receptor activation possibly in the NTS modulates incentive motivation through its action on midbrain DA neurons, which are regulated by hunger and insulin sensitivity.
Methodological caveats worth pointing out are first, that although it was controlled for peripheral insulin levels in our analyses, it needs to be considered that the effect attributed to GLP-1 on motivation could also be an overlapping effect of GLP-1 and insulin, as insulin is secreted in a GLP-1 and insulin sensitivity-dependent manner [91]. Second, although our behavioural results are greatly compatible with the aforementioned animal data on the underlying neuronal processes, the proposed molecular mechanisms explaining the observed behaviour remain speculative. Our approach will thus require further validation on a neural level.
In sum, we provide an assessment of the regulation of incentive motivation in humans by internal and metabolic state parameters as reflected by hunger, GLP-1, and insulin sensitivity, respectively. We propose an explanatory approach addressing the underlying neural mechanisms of the observed behaviour. Moreover, our results suggest a role of GLP-1 to restore dysregulated processes underlying motivational behaviour in obesity.
Author contributions
Ruth Hanssen: Conceptualisation, methodology, software, formal analysis, investigation, and writing.
Alina Chloé Kretschmer: Software, investigation, and formal analysis.
Lionel Rigoux: Methodology, software, and validation.
Kerstin Albus: Investigation and project administration.
Sharmili Edwin Thanarajah: Software and validation.
Tamara Sitnikow: Investigation.
Corina Melzer: Software and validation.
Oliver A. Cornely: Project administration and funding acquisition.
Jens C. Brüning: Funding acquisition, conceptualisation, and supervision.
Marc Tittgemeyer: Funding acquisition, conceptualisation, supervision, and writing.
All of the authors agreed on the final version of the manuscript.
Data availability statement
All the data will be made available upon request. The request necessitates that the purpose of data re-analysis is in line with the study's aims as approved by the ethics review board (see text) and the participants' consent. Furthermore, consent to data privacy must be assured by signing an agreement form accordingly.
Acknowledgements
The authors thank Patrick Weyer and Elke Bannemer for their outstanding support in data acquisition, Bertram Huth for his fantastic help with figure layouts, and student assistant Marcel Scharge for providing great help in programming the task design.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101163.
Conflict of interest
The authors declare no competing financial interests. J.C.B and M.T. are supported by funding from the German Center for Diabetes Research (DZD) – Project-ID 82DZD00502 & 82DZD03C2G. M.T. furthermore receives funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 431549029 – SFB 1451.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D.A., Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Craig A.D. An interoceptive neuroanatomical perspective on feelings, energy, and effort. Behavioral and Brain Sciences. 2013;36(6):685–686. doi: 10.1017/S0140525X13001489. discussion 707-626. [DOI] [PubMed] [Google Scholar]
- 4.Sutton A.K., Krashes M.J. Integrating hunger with rival motivations. Trends in Endocrinology and Metabolism. 2020;31(7):495–507. doi: 10.1016/j.tem.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Williams D.L. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiology & Behavior. 2014;136:194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler L.R., Knight Z.A. A spotlight on appetite. Neuron. 2018;97(4):739–741. doi: 10.1016/j.neuron.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett C.J., Li C., Webber E., Tsaousidou E., Xue S.Y., Bruning J.C. Hunger-driven motivational state competition. Neuron. 2016;92(1):187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salamone J.D., Correa M., Yang J.H., Rotolo R., Presby R. Dopamine, effort-based choice, and behavioral economics: basic and translational Research. Frontiers in Behavioral Neuroscience. 2018;12:52. doi: 10.3389/fnbeh.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobler P.N., Fiorillo C.D., Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 10.van Swieten M.M.H., Bogacz R. Modeling the effects of motivation on choice and learning in the basal ganglia. PLoS Computational Biology. 2020;16(5) doi: 10.1371/journal.pcbi.1007465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killeen P.R. Incentive theory. Nebraska Symposium on Motivation. 1981;29:169–216. [PubMed] [Google Scholar]
- 12.De Houwer J., Hughes S. MPI Press; 2020. The psychology of learning. An introduction from a functional-cognitive perspective. [Google Scholar]
- 13.Berridge K.C. Motivation concepts in behavioral neuroscience. Physiology & Behavior. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt L., Lebreton M., Clery-Melin M.L., Daunizeau J., Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology. 2012;10(2) doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minamimoto T., La Camera G., Richmond B.J. Measuring and modeling the interaction among reward size, delay to reward, and satiation level on motivation in monkeys. Journal of Neurophysiology. 2009;101(1):437–447. doi: 10.1152/jn.90959.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Araujo I.E., Schatzker M., Small D.M. Rethinking food reward. Annual Review of Psychology. 2020;71(1):139–164. doi: 10.1146/annurev-psych-122216-011643. [DOI] [PubMed] [Google Scholar]
- 17.Fiorillo C.D., Tobler P.N., Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 18.Kremer Y., Flakowski J., Rohner C., Luscher C. Context-dependent multiplexing by individual VTA dopamine neurons. Journal of Neuroscience. 2020;40(39):7489–7509. doi: 10.1523/JNEUROSCI.0502-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong J.W., Afjei S.A., Pollak Dorocic I., Peck J.R., Liu C., Kim C.K. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101(1):133–151. doi: 10.1016/j.neuron.2018.11.005. e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge K.C., Kringelbach M.L. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton M.E., Bouret S. What is the relationship between dopamine and effort? Trends in Neurosciences. 2019;42(2):79–91. doi: 10.1016/j.tins.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syed E.C., Grima L.L., Magill P.J., Bogacz R., Brown P., Walton M.E. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nature Neuroscience. 2016;19(1):34–36. doi: 10.1038/nn.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bouc R., Rigoux L., Schmidt L., Degos B., Welter M.L., Vidailhet M. Computational dissection of dopamine motor and motivational functions in humans. Journal of Neuroscience. 2016;36(25):6623–6633. doi: 10.1523/JNEUROSCI.3078-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong T.T., Bonnelle V., Manohar S., Veromann K.R., Muhammed K., Tofaris G.K. Dopamine enhances willingness to exert effort for reward in Parkinson's disease. Cortex. 2015;69:40–46. doi: 10.1016/j.cortex.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenny P.J. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boekhoudt L., Roelofs T.J.M., de Jong J.W., de Leeuw A.E., Luijendijk M.C.M., Wolterink-Donselaar I.G. Does activation of midbrain dopamine neurons promote or reduce feeding? International Journal of Obesity. 2017;41(7):1131–1140. doi: 10.1038/ijo.2017.74. [DOI] [PubMed] [Google Scholar]
- 27.Mazzone C.M., Liang-Guallpa J., Li C., Wolcott N.S., Boone M.H., Southern M. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nature Neuroscience. 2020;23(10):1253–1266. doi: 10.1038/s41593-020-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow N.D., Wang G.J., Baler R.D. Reward, dopamine and the control of food intake: implications for obesity. Trends in Cognitive Sciences. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow N.D., Wise R.A., Baler R. The dopamine motive system: implications for drug and food addiction. Nature Reviews Neuroscience. 2017;18(12):741–752. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 30.Stice E., Spoor S., Bohon C., Veldhuizen M.G., Small D.M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Weijer B.A., van de Giessen E., van Amelsvoort T.A., Boot E., Braak B., Janssen I.M. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Research. 2011;1(1):37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horstmann A., Fenske W.K., Hankir M.K. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obesity Reviews. 2015;16(10):821–830. doi: 10.1111/obr.12303. [DOI] [PubMed] [Google Scholar]
- 33.Wang G.J., Volkow N.D., Logan J., Pappas N.R., Wong C.T., Zhu W. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 34.van Bloemendaal L., Veltman D.J., Ten Kulve J.S., Groot P.F., Ruhe H.G., Barkhof F. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes, Obesity and Metabolism. 2015;17(9):878–886. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 35.Edwin Thanarajah S., Tittgemeyer M. Food reward and gut-brain signalling. Neuroforum. 2020;26(1):1–9. [Google Scholar]
- 36.Nummenmaa L., Hirvonen J., Hannukainen J.C., Immonen H., Lindroos M.M., Salminen P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce A.S., Holsen L.M., Chambers R.J., Martin L.E., Brooks W.M., Zarcone J.R. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity. 2010;34(10):1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stice E., Yokum S., Bohon C., Marti N., Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothemund Y., Preuschhof C., Bohner G., Bauknecht H.C., Klingebiel R., Flor H. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Giesen J.C., Havermans R.C., Douven A., Tekelenburg M., Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity. 2010;18(5):966–970. doi: 10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- 41.Mathar D., Horstmann A., Pleger B., Villringer A., Neumann J. Is it worth the effort? Novel insights into obesity-associated alterations in cost-benefit decision-making. Frontiers in Behavioral Neuroscience. 2015;9(360):360. doi: 10.3389/fnbeh.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes A.B., Alves da Silva J., Almeida J., Cui G., Gerfen C.R., Costa R.M. Postingestive modulation of food seeking depends on vagus-mediated dopamine neuron activity. Neuron. 2020;106(5):778–788. doi: 10.1016/j.neuron.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwin Thanarajah S., Backes H., DiFeliceantonio A.G., Albus K., Cremer A.L., Hanssen R. Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metabolism. 2019;29(3):695–706. doi: 10.1016/j.cmet.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Aitken T.J., Greenfield V.Y., Wassum K.M. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. Journal of Neurochemistry. 2016;136(5):1026–1036. doi: 10.1111/jnc.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrario C.R., Labouebe G., Liu S., Nieh E.H., Routh V.H., Xu S. Homeostasis meets motivation in the battle to control food intake. Journal of Neuroscience. 2016;36(45):11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naef L., Pitman K.A., Borgland S.L. Mesolimbic dopamine and its neuromodulators in obesity and binge eating. CNS Spectrums. 2015;20(6):574–583. doi: 10.1017/S1092852915000693. [DOI] [PubMed] [Google Scholar]
- 47.Fulton S., Pissios P., Manchon R.P., Stiles L., Frank L., Pothos E.N. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Liu S., Borgland S.L. Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience. 2015;289:19–42. doi: 10.1016/j.neuroscience.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 49.Alhadeff A.L., Rupprecht L.E., Hayes M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez N.S., Ige K.Y., Mietlicki-Baase E.G., Molina-Castro G.C., Turner C.A., Hayes M.R. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018;43(10):2000–2008. doi: 10.1038/s41386-018-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies M.J., Bergenstal R., Bode B., Kushner R.F., Lewin A., Skjoth T.V. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Journal of the American Medical Association. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 52.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 53.Cabou C., Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. The Review of Diabetic Studies. 2011;8(3):418–431. doi: 10.1900/RDS.2011.8.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timper K., Del Rio-Martin A., Cremer A.L., Bremser S., Alber J., Giavalisco P. GLP-1 receptor signaling in astrocytes regulates fatty acid oxidation, mitochondrial integrity, and function. Cell Metabolism. 2020;31(6):1189–1205. doi: 10.1016/j.cmet.2020.05.001. e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X.F., Liu J.J., Xia J., Liu J., Mirabella V., Pang Z.P. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Reports. 2015;12(5):726–733. doi: 10.1016/j.celrep.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. Journal of Neuroscience. 2012;32(14):4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mietlicki-Baase E.G., Ortinski P.I., Rupprecht L.E., Olivos D.R., Alhadeff A.L., Pierce R.C. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. American Journal of Physiology. Endocrinology and Metabolism. 2013;305(11):E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt H.D., Mietlicki-Baase E.G., Ige K.Y., Maurer J.J., Reiner D.J., Zimmer D.J. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2016;41(7):1917–1928. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blundell J., Finlayson G., Axelsen M., Flint A., Gibbons C., Kvist T. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obesity and Metabolism. 2017;19(9):1242–1251. doi: 10.1111/dom.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 61.O'Neil P.M., Birkenfeld A.L., McGowan B., Mosenzon O., Pedersen S.D., Wharton S. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 62.Konanur V.R., Hsu T.M., Kanoski S.E., Hayes M.R., Roitman M.F. Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiology & Behavior. 2020;215:112771. doi: 10.1016/j.physbeh.2019.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pessiglione M., Schmidt L., Draganski B., Kalisch R., Lau H., Dolan R.J. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316(5826):904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Könner A.C., Hess S., Tovar S., Mesaros A., Sanchez-Lasheras C., Evers N. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metabolism. 2011;13(6):720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Edwin Thanarajah S., Iglesias S., Kuzmanovic B., Rigoux L., Stephan K.E., Bruning J.C. Modulation of midbrain neurocircuitry by intranasal insulin. NeuroImage. 2019;194:120–127. doi: 10.1016/j.neuroimage.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 66.McAuley K.A., Mann J.I., Chase J.G., Lotz T.F., Shaw G.M. Point: HOMA--satisfactory for the time being: HOMA: the best bet for the simple determination of insulin sensitivity, until something better comes along. Diabetes Care. 2007;30(9):2411–2413. doi: 10.2337/dc07-1067. [DOI] [PubMed] [Google Scholar]
- 67.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 68.Agerso H., Jensen L.B., Elbrond B., Rolan P., Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45(2):195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 69.Brainard D.H. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 70.Pelli D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- 71.Ahearn E.P. The use of visual analog scales in mood disorders: a critical review. Journal of Psychiatric Research. 1997;31(5):569–579. doi: 10.1016/s0022-3956(97)00029-0. [DOI] [PubMed] [Google Scholar]
- 72.Lim J., Wood A., Green B.G. Derivation and evaluation of a labeled hedonic scale. Chemical Senses. 2009;34(9):739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croom K.F., McCormack P.L. Liraglutide: a review of its use in type 2 diabetes mellitus. Drugs. 2009;69(14):1985–2004. doi: 10.2165/11201060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.R Core Team . R Foundation for Statistical Computing; 2017. R: a language and environment for statistical computing. [Google Scholar]
- 75.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. [Google Scholar]
- 76.Lenth R.V. Least-squares means: TheRPackagelsmeans. Journal of Statistical Software. 2016;69(1):1–33. [Google Scholar]
- 77.Xu A.J., Schwarz N., Wyer R.S., Jr. Hunger promotes acquisition of nonfood objects. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2688–2692. doi: 10.1073/pnas.1417712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Livneh Y., Ramesh R.N., Burgess C.R., Levandowski K.M., Madara J.C., Fenselau H. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546(7660):611–616. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schiffino F.L., Siemian J.N., Petrella M., Laing B.T., Sarsfield S., Borja C.B. Activation of a lateral hypothalamic-ventral tegmental circuit gates motivation. PloS One. 2019;14(7) doi: 10.1371/journal.pone.0219522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nieh E.H., Matthews G.A., Allsop S.A., Presbrey K.N., Leppla C.A., Wichmann R. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheng Z., Santiago A.M., Thomas M.P., Routh V.H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Molecular and Cellular Neuroscience. 2014;62:30–41. doi: 10.1016/j.mcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Godfrey N., Borgland S.L. Diversity in the lateral hypothalamic input to the ventral tegmental area. Neuropharmacology. 2019;154:4–12. doi: 10.1016/j.neuropharm.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Nieh E.H., Vander Weele C.M., Matthews G.A., Presbrey K.N., Wichmann R., Leppla C.A. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90(6):1286–1298. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eckstrand K.L., Mummareddy N., Kang H., Cowan R., Zhou M., Zald D. An insulin resistance associated neural correlate of impulsivity in type 2 diabetes mellitus. PloS One. 2017;12(12) doi: 10.1371/journal.pone.0189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tiedemann L.J., Schmid S.M., Hettel J., Giesen K., Francke P., Buchel C. Central insulin modulates food valuation via mesolimbic pathways. Nature Communications. 2017;8(1):16052. doi: 10.1038/ncomms16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisenstein S.A., Gredysa D.M., Antenor-Dorsey J.A., Green L., Arbelaez A.M., Koller J.M. Insulin, central dopamine D2 receptors, and monetary reward discounting in obesity. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0133621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kleinridders A., Cai W., Cappellucci L., Ghazarian A., Collins W.R., Vienberg S.G. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(11):3463–3468. doi: 10.1073/pnas.1500877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fordahl S.C., Jones S.R. High-Fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chemical Neuroscience. 2017;8(2):290–299. doi: 10.1021/acschemneuro.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heni M., Kullmann S., Preissl H., Fritsche A., Haring H.U. Impaired insulin action in the human brain: causes and metabolic consequences. Nature Reviews Endocrinology. 2015;11(12):701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 90.Liu S., Labouebe G., Karunakaran S., Clee S.M., Borgland S.L. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutrition & Diabetes. 2013;3(12):e97. doi: 10.1038/nutd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Ronne J., Alanentalo T., Baquero A.F. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fortin S.M., Lipsky R.K., Lhamo R., Chen J., Kim E., Borner T. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Science Translational Medicine. 2020;12(533) doi: 10.1126/scitranslmed.aay8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Timper K., Brüning J.C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Disease models & mechanisms. 2017;10(6):679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richard J.E., Anderberg R.H., Goteson A., Gribble F.M., Reimann F., Skibicka K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vallöf D., Vestlund J., Jerlhag E. Glucagon-like peptide-1 receptors within the nucleus of the solitary tract regulate alcohol-mediated behaviors in rodents. Neuropharmacology. 2019;149:124–132. doi: 10.1016/j.neuropharm.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 97.Aulinger B.A., Vahl T.P., Wilson-Perez H.E., Prigeon R.L., D'Alessio D.A. Beta-Cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. Journal of Clinical Endocrinology & Metabolism. 2015;100(6):2489–2496. doi: 10.1210/jc.2014-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data will be made available upon request. The request necessitates that the purpose of data re-analysis is in line with the study's aims as approved by the ethics review board (see text) and the participants' consent. Furthermore, consent to data privacy must be assured by signing an agreement form accordingly.