Background:
The purpose of mastectomy for the female-to-male transgender patient is to produce a masculine appearance of the chest. A number of algorithms have been proposed for selecting the surgical technique; these have generally been based on the degree of breast ptosis and the quality and elasticity of the skin. We present a series of subcutaneous mastectomies operated on by 1 surgeon during the last 2 decades. Based on our experience, we suggest a classification system for selecting surgical technique.
Methods:
Data were collected from the files of female-to-male transgender persons who underwent surgery during 2003–2019. The data included background and surgery information. Pictures from the clinic’s archive of the patients before, during, and after surgery were collected and analyzed.
Results:
In total, 220 mastectomies were performed on 110 patients aged 13.5–50 years (mean 22.5 ±6.1). The excision averaged 443 g per breast (range: 85–2550). A periareolar approach was performed in 14 (12.7%), omega-shaped resection (nipple–areola complex on scar) in 2 (1.8%), spindle-shaped mastectomy with a dermal nipple–areola complex flap approach in 38 (34.5%), and a complete mastectomy with a free nipple–areola complex graft in 56 (50.9%). Complications included 2 hypertrophic scars, 6 hematomas requiring revision surgery, 3 wound dehiscences, and 3 cases of partial nipple necrosis.
Conclusions:
Analysis of the data led to a proposed classification for female-to-male transgender mastectomy (Wolf’s classification), based on skin excess and the distance between the original and the planned position of the nipple–areola complex.
INTRODUCTION
Transgender refers to people whose gender identity and gender expression are different from the gender to which they were born. The rate of transgender individuals in the US population is estimated as 0.6%.1 The first gender identity clinic in the United States was established at John Hopkins Hospital in 1966.2 The number of persons seeking gender-affirming procedures (previously called “sex reassignment surgery”) has increased dramatically since.3 Transgender men have been reported to be twice as likely to seek gender-affirming surgery as transgender women, and subcutaneous mastectomy is generally the first surgery opted by transgender men.3,4 Transmasculine chest reconstruction is a safe procedure; the rate of complications was reported as similar to that of mastectomy, which is indicated for cancer risk reduction and gynecomastia treatment.5
According to the World Professional Association for Transgender Health (WPATH), the criteria for mastectomy and the creation of a male chest in female-to-male transgender persons includes “persistent, well-documented gender dysphoria.”6 Notably, hormone therapy is not a prerequisite to surgery.
The objectives of subcutaneous mastectomy include defining an esthetic contour of the thorax by removing breast tissue and excess skin, reducing and repositioning the nipple–areola complex, releasing the inframammary fold, and minimizing scars on the chest wall. Transgender breast surgery is an esthetic challenge due to breast volume, breast ptosis, the size and location of the nipple–areola complex, excess skin, and loss of skin elasticity, namely due to breast binding.
Several surgical techniques are implemented to meet the esthetic and safety goals of subcutaneous mastectomy. The selection of technique depends on the surgeon’s preference and on the particular breast characteristics of the patient. A number of algorithms have been proposed7–11 to help in the selection. These mainly relate to the breast cup, the degree of breast ptosis, and the quality and elasticity of the skin. Less commonly, measured distances have been included in proposed algorithms.11 A recently published systematic review concluded that more research is needed to improve the selection of candidates for surgery, selection of the optimal chest-contouring techniques, and the patient-reported outcomes for these techniques.12
In this report, we present the surgical techniques, the complications, and the corrections following 220 subcutaneous mastectomies in 110 transgender men. All the operations were performed by the senior author during the last 2 decades. We discuss the learning curve and changes in technique selection over the period. We also present a classification system aimed to contribute to the selection of surgical technique. Unique to this system is the inclusion of a measurement that is related to the desired outcome of the surgery.
TECHNIQUES AND METHODS
Surgical Techniques
Four surgical approaches were performed: the periareolar approach, omega-shaped resection (nipple–areola complex on scar), spindle-shaped mastectomy with nipple–areola complex flap, and complete mastectomy with a free nipple–areola complex graft. (See Video 1 [online], which displays animation of the periareolar technique.) (See Video 2 [online], which displays animation of the omega mastectomy with NAC on scar technique.) (See Video 3 [online], which displays animation of the nipple–areola complex flap technique.) (See Video 4 [online], which displays the operation of the nipple–areola complex flap technique.) (See Video 5, which displays animation of the free nipple–areola graft technique.)
Video 1. Video 1 - Periareolar Cut Final.
Video 2. Video 2 - Animation of the omega mastectomy with NAC on scar technique.
Video 3. Video 3 - Nipple Areola complex flap technique: Animation.
Video 4. Video 4- Nipple Areola complex flap technique: Operation video.
Video 5. Video 5 - Animation of the free Nipple Areola graft technique.
The periareolar approach involves cutting around the areola and then reducing it and creating a dermal flap on which the nipple–areola complex is based. Next, the breast tissue is resected through this incision, and finally, circular stitching of the areola shrinks the chest skin around it. A similar approach is the omega-shaped resection, which extends the incision medially and laterally. At the end of the operation, a horizontal stitch line is made at the nipple height. In the third approach, a spindle-shaped mastectomy leaves a lower 2-mm-thick dermal flap, on which the nipple–areola complex was based, and inferior and superior skin flaps. A round skin excision is made in the superior skin flap at the location of the new nipple. The nipple–areola complex is delivered through this round opening and inset in place, with a horizontal stitch line about 2 cm below the inferior border of the new nipple–areola complex. The final surgical approach entails complete spindle-shaped mastectomy of the breast, with a free nipple–areola graft implanted at the site designated for the new nipple, after de-epithelialization. This technique is similar to that described by Conway, for reduction mammoplasty breast amputation.13 In the last 2 approaches, the location of the nipple was determined together with the patient during preoperative planning, by affixing a nipple-shaped plaster to the new location of the NAC.
Patients and Data Collection
The study population was comprised of all the patients who underwent subcutaneous mastectomy for chest masculinization by the senior author between 2003 and 2019. A total of 220 mastectomies were performed in 110 consecutive surgeries, along with chest wall and nipple–areola complex contouring. Data were collected from the medical records and from the clinical pictures of the patients. These included a number of preoperative measurements, namely the following distances: between the lowest point of the breast contour and the designated nipple position, between the current nipple position and its planned position, between the current nipple position and the midline, between the suprasternal notch and the current nipple position, and between the suprasternal notch and the position designated for the nipple.
Data were collected regarding risk factors, background diseases, medications, smoking, and hormone therapy (namely testosterone). Data were also collected regarding previous surgical procedures, including plastic, orthopedic, bariatric, and gynecological surgeries. Data collected from the surgical reports included length of surgery, surgical technique, excision weight, liposuction aspirate volume, use of drains, and abnormal surgical events. Complications and the steps that were taken to correct them were also recorded. To assess the learning curve, we stratified the cases by the first 10, next 50 (cases 11–60), and the last 50. According to these categories, we assessed time of operation, total complications, hematomas, and revision surgeries.
Wolf’s Classification Details
Over the course of the study period, the senior author formulated a classification system to aid in determining the minimal operation that should be performed for optimal cosmetic results and safety. We determined 4 categories of breasts according to 2 criteria (Table 1). The first criterion (skin excess) established 3 classifications (I, II, and III): none or minimal, moderate, and substantial, respectively. These classifications usually corresponded with the expected breast tissue size: small, medium, and large, respectively. The second criterion (the distance of the nipple–areola complex to the planned position) entailed 3 possibilities: small (0–2 cm), medium (2–5 cm), and large (>5 cm). Classification II (moderate skin excess) was subdivided into 2 categories according to the distance of the nipple–areola complex to the planned position: small (classification IIa) and medium (classification IIb). According to the proposed system, for each of the four classifications (I, IIa, IIb, III), a different minimal operation is possible: periareolar, nipple–areola complex on scar [Omega], spindle-shaped mastectomy with nipple–areola complex dermal flap, and complete mastectomy with free nipple–areola complex graft (Figs. 1–4). We suggest that when the expected breast tissue size does not correspond with the excess of skin, the surgeon should rethink the type of operation and consider the given dimensions. Thus, the expected breast tissue size may facilitate the surgeon’s decision-making in atypical cases.
Table 1.
Wolf’s Female-to-Male Transgender Breast Classification with the Proposed Minimal Surgical Technique
| Minimal Operation | Expected Tissue Size | Nipple–Areola Complex Distance to Planned Position | Skin Excess | Class |
|---|---|---|---|---|
| Periareolar | Small | In place | None or minimal | I |
| 0–2 cm | ||||
| Nipple–areola complex on scar, Omega | Medium | In place | Moderate | IIa |
| 0–2 cm | ||||
| Vest over pants, nipple–areola complex flap | Medium | Medium distance | Moderate | IIb |
| 2–5 cm | ||||
| Breast amputation, free nipple–areola complex graft | Large | High distance | Substantial | III |
| More than 5 cm |
Fig. 1.

A patient with class I breasts who underwent periareolar subcutaneous mastectomy. A, Before surgery. B, After surgery.
Fig. 4.

A patient with class III breast who underwent spindle-shaped subcutaneous mastectomy with free nipple–areola complex graft approach. A, Before surgery. B, After surgery.
Fig. 2.
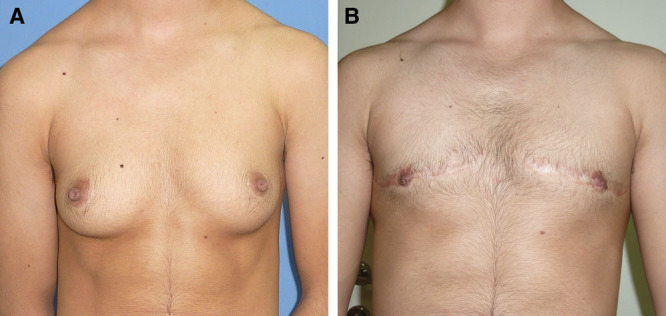
A patient with class IIa breasts who underwent omega subcutaneous mastectomy. A, Before surgery. B, After surgery.
Fig. 3.
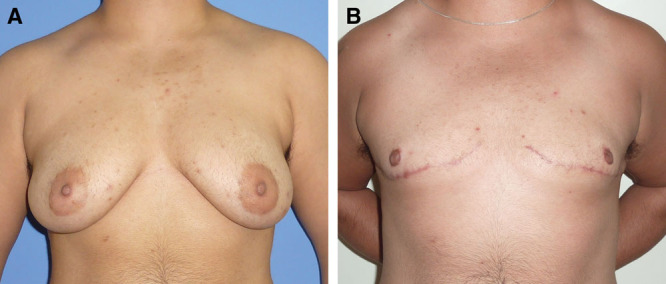
A patient with class IIb breasts who underwent spindle-shaped subcutaneous mastectomy with vest over pants approach. A, Before surgery. B, After surgery.
The administrative manager of the Plastic Surgery Unit, a medical intern (without experience in plastic surgery), and a resident of plastic surgery evaluated the results of each surgery according to preoperative and postoperative photographs, on a 1–5 Likert scale. For patients who underwent revisional surgeries, the evaluations were recorded after each procedure. The means of the 3 evaluators were calculated.
Statistical Analysis
Descriptive statistics were presented as means, SDs, and percentiles. Normal distributions of the quantitative parameters were tested by the Kolmogorov-Smirnov test. Accordingly, parametric (ANOVA) and non-parametric (Kruskal Wallis) tests were used to assess the differences between 3 groups. The relationship between classification levels and types of surgery was tested with Somers’ d tests. A multivariate general linear model was assessed for predicting the esthetic result by the type of surgery, classification, and the interaction between surgery versus classification. P < 0.05 was considered significant. SPSS (version 25) was used for all the statistical analyses.
RESULTS
Overall Characteristics and Outcomes
A total of 220 mastectomies were performed in 110 patients. Their mean age was 22.5±6.1 years; the range was 13.5–50. Twenty-nine (26.4%) of the patients had various background conditions such as asthma, migraine, diabetes, fibromyalgia, hypertension, hypothyroidism, epilepsy, inflammatory bowel disease, and mental illness. Seventy (63.6%) were taking hormone-testosterone therapy regularly before the surgery.
The mean resection weight of each breast was 443 g (range 85–2550). Twenty-five (22.9%) of the patients underwent liposuction, with an average volume of 416 cm3 (range 50–1200 cm3). Ninety-eight (89.1%) of the patients were left with drains at the end of the operation. The mean duration of surgery was 2 hours and 2 minutes (range: 1:10–3:10). The mean operation time for the first 20 cases was 2:28 (1:55–3:10), and for the last 20 cases: 1:26 (1:10–2:10). The mean score for esthetics was 4.07 + 1.00.
Major complications included 2 cases of hypertrophic scars, 6 cases of bleeding requiring revision several hours after surgery (5.4%), 4 (3.6%) cases of seroma, 3 (2.8%) cases of dehiscence, and 2 (1.8%) of partial unilateral nipple necrosis. In total, 18 (16.6%) underwent revision surgery due to complications and to improve esthetic results. Revision surgeries were performed for the following reasons: 6 due to insufficient esthetic results and a change in the approach (in 2 of them the surgical approach was changed to a different type of surgery), 6 scar revisions with liposuction (one of them included areola reduction), and 6 immediate revision surgeries due to bleeding. The mean aesthetic score after revision surgeries was 4.11 ± 0.65 (P = 0.007), compared with a mean 1.88 for the first surgeries of the same individuals.
Postoperative hematoma that required revision surgical intervention occurred a few hours after the first operation in 5 (7.0%) of the patients who were taking testosterone and in 1 (2.6%) of those who were not taking testosterone. This difference was not statistically significant (P > 0.05, Fisher’s exact test P value = 0.66). Although testosterone treatment and postoperative hemorrhage have been linked, the bleeding cannot be statistically attributed to testosterone therapy.
Analysis of the learning curve demonstrated the following trends for the first 10 cases, next 50 (cases 11–60), and the last 50 cases; decreasing complications: 8 (80%), 9 (16%), and 1 (1.8%), respectively; and decreasing revision surgeries, 7 (70%), 4 (8%), and 1 (2%), respectively. The respective rates for hematomas were: 0 (0%), 4 (8%), and 2 (4%).
The mean distance between the lowest point of the breast contour and the designated nipple position was 10.3 ± 3.0 cm; between the current nipple position and its planned position, 7.2 ± 3.9 cm; between the current nipple position and midline, 10.9 ± 1.8 cm; between the suprasternal notch and the current nipple position, 23.1 ± 4.0 cm; and between the supra sternal notch and the planned nipple position, 17.3 ± 1.3 cm.
Characteristics and Outcomes according to the Type of Surgery
Fourteen (12.7%) patients underwent the periareolar approach, 2 (1.8%) underwent omega-shaped resection, 38 (34.5%) underwent spindle-shaped mastectomy, and 56 (50.9%) underwent complete mastectomy with a free nipple–areola graft. Of the last 30 cases, 1 nipple–areola complex flap and 29 free nipple–areola complex grafts were performed. Table 2 presents characteristics of the patients and of the surgeries, and outcomes, according to the type of surgery. Esthetic scores were lower for the periareolar and nipple–areola complex on scar approaches (2.50 ± 1.55 and 4.00 ± 0.71, respectively) than for the nipple–areola complex flap and nipple–areola complex graft approaches (4.19 ± 0.75 and 4.37 ± 0.54, respectively). We found statistical significant difference between periareolar versus NAC flap techniques (P = 0.003) and between periareolar versus NAC graft techniques (P < 0.0001) (Table 2).
Table 2.
Patient Characteristics and Outcomes of Subcutaneous Mastectomy, according to Surgical Technique
| Periareolar | Nipple–Areola Complex on Scar | Nipple–Areola Complex Flap | Nipple–Areola Complex Free Graft | Total | P | |
|---|---|---|---|---|---|---|
| N | 14 | 2 | 38 | 56 | 110 | |
| Age (y) | 24.6 ± 6.8 | 26.5 ± 6.3 | 22.3 ± 6.3 | 22.0 ± 5.9 | 22.5 ± 6.1 | NS |
| Total resection weight (g) | 485 ± 362 | 310 ± 56 | 681 ± 394 | 1157 ± 853 | 887 ± 711 | P2 = 0.007 |
| P3 = 0.004 | ||||||
| Treated with testosterone | 5 (35.7%) | 2 (100%) | 26 (68.4%) | 37 (66.1%) | 70 (63.6%) | P1 = 0.055 |
| P2 = 0.065 | ||||||
| Mean operating time | 1:58 ± 0:20 | 2:24 ± 0:27 | 2:21 ± 0:18 | 1:49 ± 0:21 | 2:02 ± 0:25 | P3 < 0.0001 |
| Hematoma | 0 | 0 | 3 (7.9%) | 3 (5.3%) | 6 (5.4%) | NS |
| Other complications requiring intervention | 7 (50%) | 1 (50%) | 4 (10.5%) | 0 (0%) | 12 (10.9%) | |
| Revisions | 7 (50%) | 1 (50%) | 7 (18.4%) | 3 (5.3%) | 18 (16.6%) | NS |
| Aesthetic score | 2.5 ± 1.55 | 4.00 ± 0.71 | 4.19 ± 0.75 | 4.37 ± 0.54 | 4.07 ± 1.00 |
P1 = 0.003 P2 < 0.0001 |
The data are presented as means ± SDs or as numbers (%).
P1, periareolar versus nipple–areola complex flap; P2, periareolar versus nipple–areola complex graft; P3, nipple–areola complex graft versus nipple–areola complex flap.
Surgeries Performed according to the Proposed Classification
According to our proposed system: 5 (5%) of our patients were classified as category I, 11 (10%) as category IIa, 46 (42%) as IIb, and 48 (43%) as III (Table 3). A positive correlation was observed between the classification of breasts and the procedures performed (P = 0.001, r = 0.31 by Somers’ d test).
Table 3.
Surgical Techniques Performed according to the Proposed Classification
| Proposed Classification | Technique Performed | ||||
|---|---|---|---|---|---|
| Periareolar | Nipple–Areola Complex on Scar | Nipple–Areola Complex Flap | Nipple–areola Complex Graft | Total | |
| I | 4 (80%) | 1 (20%) | 0 | 0 | 5 |
| IIa | 1 (9%) | 1 (9%) | 5 (46%) | 4 (36%) | 11 |
| IIb | 3 (7%) | 0 | 24 (52%) | 19 (41%) | 46 |
| III | 6 (13%) | 0 | 9 (19 %) | 33 (68%) | 48 |
| Total | 14 | 2 | 38 | 56 | 110 |
A multivariate general linear model was calculated to predict the level of the esthetic score by classification and type of surgery. This model demonstrated high statistically significant matches between Wolf’s classification and the type of surgery, which yielded high esthetic scores (Table 4).
Table 4.
Multivariate General Linear Model for Predicting the Esthetic Result by Correlating between Types of Surgery and Wolf’s Classification
| Parameter | Estimate | P |
|---|---|---|
| Intercept | 4.288 | <0.0001 |
| [Classification I] * [periareolar] | 0.087 | 0.792 |
| [Classification IIa] * [periareolar] | −0.788 | 0.216 |
| [Classification IIa] * [NAC flap] | 0.312 | 0.299 |
| [Classification IIa] * [NAC graft] | 0.337 | 0.310 |
| [Classification IIb] * [periareolar] | −1.788 | <0.0001 |
| [Classification IIb] * [NAC flap] | −0.17 | 0.919 |
| [Classification IIb] * [NAC graft] | 0.186 | 0.303 |
| [Classification III] * [periareolar] | −3.205 | <0.0001 |
| [Classification III] * [NAC flap] | −0.510 | 0.032 |
| [Classification III] * [NAC graft] | 0 |
Values in boldface indicate the statistically significant (P < 0.05) results.
Adjusted R2 = 0.618. Dependent variable: aesthetic score. NAC: nipple–areola complex. The model’s equation is: Dependent variable: aesthetic score = 4.288+ (−3.205*periareolar) OR (−0.510*NAC flap) + (3.292*classification I) OR (0.280* periareolar* classification IIa) OR (1.231* periareolar* classification IIb).
DISCUSSION
This report of 220 subcutaneous mastectomies in 110 patients demonstrated a surgery requiring complication rate of 16.4%, of which 33% were hematomas. The overall mean esthetic score was assessed as 4.07 of 5. The mean scores were better for the 2 procedures that entail nipple–areola complex major repositioning. For the 12 (11%) patients who underwent revisions, the esthetic score increased from 1.88 following the first operation to 4.11 following the second operation.
Before developing our classification system, we selected the type of surgery for each patient according to our intuition and accumulating experience. After devising the classification, we analyzed the procedures performed and our decision-making process. We found a positive correlation of breast classification and procedure type with esthetic score. This supports the utility of our classification system for selecting the surgical type of subcutaneous mastectomy for female-to-male transgender.
Similar to other proposals for the selection of surgical technique, the amount of excess skin was central to our classification system. However, in contrast to other proposals, we included a preoperative measurement of the skin excess. The postoperative position of the nipple was determined preoperatively according to the patient’s preference and the measurements taken. Accordingly, for patients for whom the distance of the nipple–areola complex to the planned position exceeded 2 cm, nipple–areola complex major repositioning was favored. For breasts with moderate excess skin, 2 techniques of repositioning were offered: the nipple–areola complex flap technique and complete breast amputation with free nipple–areola complex graft. However, the latter technique was preferred for breasts with large expected tissue size. For breasts with substantial skin excess and a large distance between the nipple–areola complex and the planned position, breast amputation with free nipple–areola complex graft was the preferred option.
Other classification systems for the selection of mastectomy technique focused on breast contour and did not include quantitative measurements. Monstrey et al7 and Wolter et al8 developed algorithms based on breast cup, ptosis, and elasticity. Their complication rates were similar to ours. Yet, free-nipple grafting was performed in <20% of the patients in each of these cohorts, compared with 50.9% in our cohort. The rate of revisions for esthetic reasons, 32%, in Monstrey et al’s study was considerably higher than our rate (10.9%). Top et al9 proposed an algorithm based on skin excess and elasticity. In addition, they considered the size of the nipple–areola complex and the patient’s willingness to accept a vertical scar. Free nipple–areola complex grafting was performed in 31% of the patients. The overall complication rate of 13% was similar to ours. Remarkably, the authors stated that none of the patients needed a revision.
From the data of their large cohort, Bluebond-Langer et al10 developed a grading scale based on 3 parameters: the amount of glandular tissue (minimal, moderate, or significant), skin laxity (little or increased), and the relation of the nipple–areola complex to the inframammary fold (above, at, or below). Accordingly, this scale incorporated a qualitative assessment of the preoperative nipple–areola complex position. For patients with greater skin laxity, the application of their scale demonstrated better esthetic outcomes, and less frequent need for revision, following free nipple grafting compared with circumareolar incision.
Using regression tree analysis, Knox et al11 created a patient-selection algorithm for selection of surgical technique. Unlike the previously mentioned algorithms, this algorithm included a quantitative measure, namely the nipple to inframammary fold distance. The concentric circular procedure was considered the default procedure due to the reduced scar burden, the more natural nipple–areola complex, and the possibility of preserving nipple–areola complex sensation.
Compared with the proposals for selecting surgery described above, the novelty of our classification system is its incorporation of a quantitative measure, namely the distance from the nipple to its planned position. This position is decided by candidates for surgery by eyeball selection, ie, viewing the position with the aid of a nipple-like plaster, and subsequent measure.
Forty-two percent of the patients in our cohort were classified with the highest category (class III) of our proposed system. Yet, an even higher proportion (50%) underwent the technique with the largest incision. Moreover, we performed major nipple repositioning procedures in 85% of our patients, either by a dermal flap or using a free nipple–areola complex graft. Our esthetic results were favorable, despite the large incisions involved in such procedures. These findings concur with those of the others who concluded that performing techniques with smaller incisions in individuals with larger ptosis or less elasticity risks poorer esthetic results due to puckering and wrinkling of skin.14–16 Specifically, grafting enables resizing and repositioning of the nipple–areola complex; and elimination of the nipple–areola complex pedicle facilitates flattening the chest to a masculine contour. Notably, our trend of increasing performance of major nipple repositioning is consistent with the growing preference among our patients for a more masculine contour, over a shorter scar. This concurs with opinions reported by other patients, and the consequent increase in free nipple grafting techniques in other centers.15 In contrast, earlier algorithms7 focused on the likelihood that patients would be unwilling to accept free nipple grafting. Accordingly, some designated nipple sensitivity as one of the goals of surgery.8 The preservation of sensation in many of the grafted nipple–areola complexes was unexpected; further research of this phenomenon is needed.
With the growing demand for subcutaneous mastectomies in female-to-male transgender persons, identifying the best surgery for each patient is expected to become increasingly important.17 Selection of technique should consider the surgeon’s experience, preoperative characteristics, complication risks, and esthetic aspects. The latter may include patients’ opinions on postoperative outcomes, as in the classification system proposed herein. Our findings, together with other studies that proposed algorithms or classifications for improving selection, suggest that, when uncertainty arises, free nipple grafting may provide the safest solution, with the greatest patient satisfaction. Despite the changes in attitudes and techniques over a number of decades, the multidisciplinary team approach remains a core element of treatment.
ACKNOWLEDGMENT
Ethical approval for this study was obtained from Hillel Yaffe Medical Center Institutional Review Board (approval no.: 0085-18-hymc).
Footnotes
Published online 25 January 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Safer JD, Tangpricha V. Care of transgender persons. N Engl J Med. 2019;381:2451–2460. [DOI] [PubMed] [Google Scholar]
- 2.Siotos C, Neira PM, Lau BD, et al. Origins of gender affirmation surgery: the history of the first gender identity clinic in the United States at Johns Hopkins. Ann Plast Surg. 2019;83:132–136. [DOI] [PubMed] [Google Scholar]
- 3.Lane M, Ives GC, Sluiter EC, et al. Trends in gender-affirming surgery in insured patients in the United States. Plast Reconstr Surg Glob Open. 2018;6:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kailas M, Lu HMS, Rothman EF, et al. Prevalence and types of gender-affirming surgery among a sample of transgender endocrinology patients prior to state expansion of insurance coverage. Endocr Pract. 2017;23:780–786. [DOI] [PubMed] [Google Scholar]
- 5.Cuccolo NG, Kang CO, Boskey ER, et al. Mastectomy in transgender and cisgender patients: a comparative analysis of epidemiology and postoperative outcomes. Plast Reconstr Surg Glob Open. 2019;7:e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend. 2012;13:165–232. [Google Scholar]
- 7.Monstrey S, Selvaggi G, Ceulemans P, et al. Chest-wall contouring surgery in female-to-male transsexuals: a new algorithm. Plast Reconstr Surg. 2008;121:849–859. [DOI] [PubMed] [Google Scholar]
- 8.Wolter A, Diedrichson J, Scholz T, et al. Sexual reassignment surgery in female-to-male transsexuals: an algorithm for subcutaneous mastectomy. J Plast Reconstr Aesthet Surg. 2015;68:184–191. [DOI] [PubMed] [Google Scholar]
- 9.Top H, Balta S. Transsexual mastectomy: selection of appropriate technique according to breast characteristics. Balkan Med J. 2017;34:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluebond-Langner R, Berli JU, Sabino J, et al. Top surgery in transgender men: how far can you push the envelope? Plast Reconstr Surg. 2017;139:873e–882e. [DOI] [PubMed] [Google Scholar]
- 11.Knox ADC, Ho AL, Leung L, et al. A review of 101 consecutive subcutaneous mastectomies and male chest contouring using the concentric circular and free nipple graft techniques in female-to-male transgender patients. Plast Reconstr Surg. 2017;139:1260e–1272e. [DOI] [PubMed] [Google Scholar]
- 12.Cohen WA, Shah NR, Iwanicki M, et al. Female-to-male transgender chest contouring: a systematic review of outcomes and knowledge gaps. Ann Plast Surg. 2019;83:589–593. [DOI] [PubMed] [Google Scholar]
- 13.Conway H. Mammaplasty; analysis of 110 consecutive cases with end-results. Plast Reconstr Surg (1946). 1952;10:303–315. [PubMed] [Google Scholar]
- 14.van de Grift TC, Elfering L, Bouman MB, et al. Surgical indications and outcomes of mastectomy in transmen: a prospective study of technical and self-reported measures. Plast Reconstr Surg. 2017;140:415e–424e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claes KEY, D’Arpa S, Monstrey SJ. Chest surgery for transgender and gender nonconforming individuals. Clin Plast Surg. 2018;45:369–380. [DOI] [PubMed] [Google Scholar]
- 16.Kühn S, Keval S, Sader R, et al. Mastectomy in female-to-male transgender patients: a single-center 24-year retrospective analysis. Arch Plast Surg. 2019;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammari T, Sluiter EC, Gast K, et al. Female-to-male gender-affirming chest reconstruction surgery. Aesthet Surg J. 2019;39:150–163. [DOI] [PubMed] [Google Scholar]


