Abstract
Objective
To conduct a meta-analysis assessing the perioperative, functional and oncological outcomes of partial nephrectomy (PN) and radical nephrectomy (RN) for T1b tumours. The primary endpoints were the oncological outcomes. The secondary endpoints were the perioperative and functional outcomes.
Methods
A systematic literature review was performed by searching multiple databases through February 2019 to identify eligible comparative studies according to the Preferred Reporting Items for Systematic Review and Meta-analysis statement. Identified reports were assessed according to the Newcastle-Ottawa Scale for nonrandomized controlled trials.
Results
Overall, 13 retrospective cohort studies were included in the analysis. Patients undergoing PN were younger (weighted mean difference [WMD] −3.49 years, 95% confidence interval [CI] −5.16 to −1.82; p<0.0001) and had smaller masses (WMD −0.45 cm, 95% CI −0.59 to −0.31; p<0.0001). There were no differences in the oncological outcome, which was demonstrated by progression-free survival (hazard ratio [HR] 0.70; p=0.22), cancer-specific mortality (HR 0.91; p=0.57) and all-cause mortality (HR 1.01; p=0.96). The two procedures were similar in estimated blood loss (WMD −16.47 mL; p=0.53) and postoperative complications (risk ratio [RR] 1.32; p=0.10), and PN provided better renal function preservation and was related to a lower likelihood of chronic kidney disease onset (RR 0.38; p=0.006).
Conclusion
PN is an effective treatment for T1b tumours because it offers similar surgical morbidity, equivalent cancer control, and better renal preservation compared to RN.
Keywords: Kidney cancer, Partial nephrectomy, Radical nephrectomy, Renal cancer, Survival, Renal function
1. Introduction
Partial nephrectomy (PN) is recommended for patients with T1a (<4 cm) tumours in the European Association of Urology (EAU) guidelines [1]. For larger renal tumours, radical nephrectomy (RN) is still the standard treatment, but in selected cases, PN could be more beneficial than RN [2].
PN can provide better renal function preservation [3], which may lower the risk of severe cardiovascular events and improve overall survival [4]. However, this benefit was not observed in the prospective randomized controlled EORTC 30904 trial [5,6]. The difference in the clinical impact of medically versus surgically induced chronic kidney disease could be a possible reason [7]. Additionally, a multi-centre retrospective study suggested that poorer renal function was related to shorter cancer-specific survival (CSS) in patients undergoing surgery for renal tumours, which could support the use of PN [8].
The role of PN still needs to be confirmed for T1b (4–7 cm) renal masses. Several analyses of the Surveillance, Epidemiology, and End Results (SEER) database have suggested that PN provides equivalent cancer control to RN for both T1b and T2 renal masses [[9], [10], [11], [12], [13]], but PN use still remains limited in both Europe [14] and the USA [11].
One previous systematic review and meta-analysis performed by Kim et al. [15] compared all-cause mortality, cancer-specific mortality and the rate of severe chronic kidney disease (CKD) between PN and RN for localized renal tumours. However, most of the studies included in this review focused on T1a tumours. Therefore, Mir et al. [16] conducted a systematic review and meta-analysis to define the role of PN in larger tumours (T1b and above). Mir et al. [16] compared the perioperative complications, renal function changes and oncological outcomes of PN and RN for T1b and T2 tumours and suggested that PN could provide acceptable cancer control and better renal function preservation with a higher risk of complications. However, the patients undergoing PN were an average of ∼2.5 years younger and presented with smaller tumours (weighted mean difference [WMD] −0.65 cm, 95% confidence interval [CI] −0.81 to −0.49; p<0.001), and the authors did not conduct a separate subgroup analysis of the outcomes for T1b.
With the aim of minimizing the selection bias, we designed the present study as a meta-analysis comparing the perioperative, functional and oncological outcomes for PN versus RN for T1b renal tumours. By extracting adjusted hazard ratios from the included studies, we evaluated oncological outcomes and made the conclusions more convincing.
2. Methods
2.1. Search strategy
The evidence search was performed in multiple databases (PubMed, Cochrane Library, and Web of Science) from their inceptions to February 2019 to identify studies comparing PN to RN for T1b renal tumours.
Separate searches were carried out using both diagnosis (renal mass, kidney cancer, renal tumour, 4 cm, and T1b) and intervention (partial nephrectomy, radical nephrectomy, and nephron-sparing surgery) terms.
We also conducted a reference list search and cited reference search from full-text articles that met the study selection criteria.
2.2. Inclusion criteria, study eligibility, and data extraction
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria were used for article selection (Fig. 1), which was performed by two investigators (G.L. and Y.Z.). Studies comparing PN to RN for renal masses were included regardless of the technique. All studies were ascertained to contain oncological, perioperative or functional outcomes and were written in English. First, the titles and abstracts were screened to determine whether studies might potentially fit the inclusion criteria. Second, full texts were assessed to ascertain whether studies should be included. Studies without primary data (i.e., reviews, commentaries, and letters) were excluded, but the reference lists were examined to identify additional studies of interest. References from the included studies were also reviewed to ensure that relevant studies were included. Discrepancies were resolved by discussion with a third reviewer (W.O.).
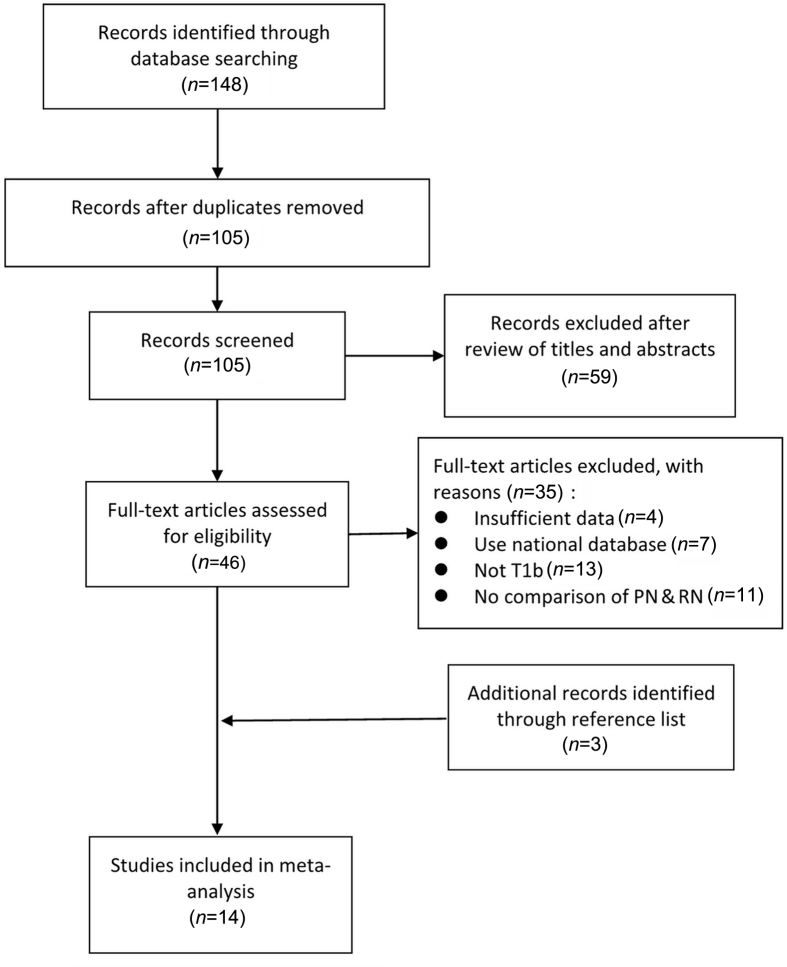
Figure 1.
PRISMA flow chart of the study identification process. Of the 14 studies ultimately included, in one case, the same group reported two separate analyses for different endpoints (Roos et al. [19,20]; see the text). PN, partial nephrectomy; RN, radical nephrectomy; PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-analysis.
For studies containing oncological outcomes (progression-free survival, cancer-specific mortality, and all-cause mortality), we extracted the hazard ratios (HRs) to evaluate the effect of different procedures. When univariate regression and multivariate regression were both performed, we chose the HRs from multivariate regression, as these HRs were adjusted for confounders (i.e., age, tumour size, and stage). For studies including perioperative outcomes (operative time, estimated blood loss, complications, and hospital stay) or functional outcomes (postoperative renal function, postoperative CKD, and decline in estimated glomerular filtration rate [eGFR]), the baseline data of the patients (age, tumour size, baseline renal function, and baseline CKD) were extracted to evaluate selection bias.
2.3. Assessment of study quality
The quality of each study was determined using the Newcastle–Ottawa Scale (NOS) for nonrandomized controlled trials (www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The maximum score of the scale is 9. A total score of 5 or lower is considered low quality, 6–7 is considered intermediate quality, and 8–9 is considered high quality.
2.4. Data analysis
A formal meta-analysis of studies comparing PN to RN for cT1b tumours was conducted. In addition, a sensitivity analysis was performed by excluding the lowest-weighted or lowest-scored studies.
For continuous outcomes, WMD was used to measure differences, whereas the risk ratio (RR) with 95% CI was calculated for binary variables. For studies reporting medians and ranges (or interquartile ranges [IQR]), validated mathematical models were used to convert the median (range or IQR) to the mean (standard deviation) [17,18].
When two publications were reported by the same group and it was clear that the same dataset was used for different study endpoints, relevant parameters were counted only once for the scope of the present analysis. This was the case for the series reported by Roos et al. [19,20]. When two publications were reported by the same group and the same dataset was used for the same endpoint, the data with more recent outcomes and longer follow-up times were extracted. For the two publications reported by Weight et al. [21,22], considering the potential overlap of the dataset, we extracted the perioperative and functional data from the study [21], as the baseline characteristics of the two arms were more comparable in the study. As the oncological outcomes had been adjusted for confounders, we extracted oncological outcomes from the study [22] for its larger sample size. There were several studies based on data accessed from a national database [[9], [10], [11], [12], [13],23,24], and we excluded all of these because of the potential data duplication and inner heterogeneity of medical institutions.
A fixed-effects model was used to calculate the pooled estimates if no significant heterogeneity was identified (I2<50%); otherwise, a random-effects model was used. Egger's linear regression and funnel plot were examined to evaluate the publication bias. All statistical analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK).
3. Results
Overall, 13 retrospective cohort studies were included in the analysis (Table 1) [[19], [20], [21], [22],[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. Of these studies, two separate analyses for two endpoints in the same study were reported by the same group [19,20].
Table 1.
Studies included in the meta-analysis for assessment of outcomes for PN versus RN for cT1b and higher renal tumours.
| Study | Study period | Study design | Study origin | T stage | PN/RN cases (n) | PN/RN median FU (month) | Surgical technique | SQ |
|---|---|---|---|---|---|---|---|---|
| Lee et al., 2017 [32] | 1994–2014 | RTP, MI, PM | South Korea | T1b and T2 | 317/841 | 46 | NS | 8 |
| Roos et al., 2011 and 2012 [19,20]a | 1988–2007 | RTP, SC, PM | Germany | T1b and T2 | 101/146 | 52.8/79.2 | Open | 7 |
| Antonelli et al., 2012 [25] | 1995–2007 | RTP, MI, | Italy | T1b | 198/1426 | 47 | Open | 6 |
| Cai et al., 2018 [26] | 2005–2012 | RTP, SC | China | T1b | 39/160 | 67/70 | Lap | 8 |
| Dash et al., 2006 [27] | 1998–2004 | RTP, SC | USA | T1b | 45/151 | 21 | Open | 7 |
| Jang et al., 2016 [30] | 1999–2011 | RTP, MI, PM | Korea | T1b | 100/100 | 42.6/48.1 | Open | 8 |
| Milonas et al., 2013 [33] | 1998–2009 | RTP, SC | Lithuania | T1b | 34/317 | 74.76/77.28b | Open | 7 |
| Thompson et al., 2009 [34] | 1989–2006 | RTP, MI | USA | T1b | 286/873 | 57.6 | Open/lap | 6 |
| Weight et al., 2010 [22] | 1999–2006 | RTP, SC | USA | T1b | 524/480 | 50/46 | Open/lap | 6 |
| Deklaj et al., 2010 [28] | 2002–2008 | RTP, SC | USA | T1b | 33/52 | 15/21 | Lap | 7 |
| Weight et al., 2010 [21] | 1999–2006 | RTP, SC | USA | T1b | 212/298 | 49/41 | Open/lap | 6 |
| Iizuka et al., 2012 [29] | 1979–2011 | RTP, SC | Japan | T1b | 67/195 | 22.4/71.1 | Open | 7 |
| Kim et al., 2010 [31] | 1995–2004 | RTP, SC | Korea | T1b | 18/52 | 78.2/66.5b | Open | 7 |
PN, partial nephrectomy; RN, radical nephrectomy; FU, follow-up; SQ, study quality according to the Newcastle–Ottawa Scale; RTP, retrospective; SC, single-centre; MI, multi-institutional; PM, pair-matched; NS, not specified; lap, laparoscopic.
These groups reported two separate analyses for the same dataset.
Mean.
The characteristics of the included studies are presented in Table 1. All included studies were retrospective in nature and compared the relevant outcomes of PN and RN. Two studies focused on the outcomes of T1b and T2 tumours and conducted subgroup analyses for T1b tumours [19,20,32]. Four studies were conducted based on multi-institutional data [25,30,32,34], while the data in other studies were collected from a single centre [[19], [20], [21], [22],[26], [27], [28], [29],31,33]. Most of these studies focused partially or totally on open surgery, and laparoscopic techniques were used for all surgeries in only two studies [26,28].
Patients undergoing PN were younger (WMD −3.49 years, 95% CI −5.16 to −1.82; p<0.0001) with smaller masses (WMD −0.45 cm, 95% CI −0.59 to −0.31; p<0.0001). Notably, in most analyses of different outcomes, the differences in age and tumour size were not significant (Table 2).
Table 2.
Summary of baseline characteristics and outcomes of different analyses.
| Outcomes | Stage | Included studies | Baseline (WMD and 95% CI) |
Effect and 95% CI | |
|---|---|---|---|---|---|
| Age (year) | Tumour size (cm) | ||||
| Operative time | T1b | [29,30,33] | −0.61 (−2.36, 1.15) | −0.31 (−0.65, 0.04) | WMD –3.98 (−14.99, 7.02) |
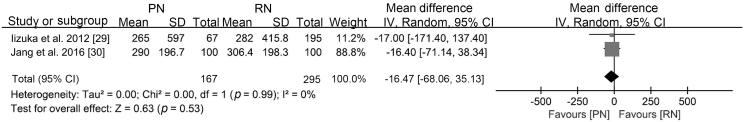
| Estimated blood loss | T1b | [29,30] | −0.43 (−2.43, 1.57) | −0.19 (−0.56, 0.19) | WMD –16.47 (−68.06, 35.13) |
| Postoperative complications | T1b | [20,28,30,33] | −2.21 (−4.34, −0.08) | −0.33 (−0.66, 0.01) | RR 1.32 (0.95, 1.84) |
| Transfusion | T1b | [20,30] | −2.19 (−5.72, 1.34) | −0.13 (−0.51, 0.24) | RR 1.10 (0.69, 1.78) |
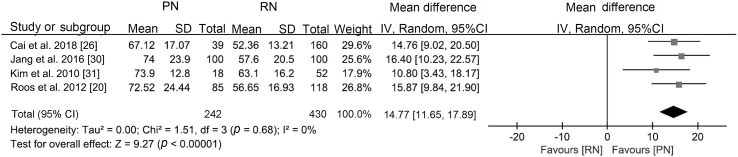
| Postoperative renal function | T1b | [20,26,30,31] | −3.24 (−6.43, −0.06) | −0.25 (−0.51, 0.01) | WMD 14.77 (11.65, 17.89) |
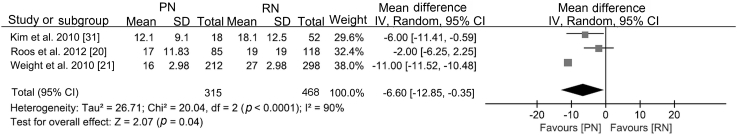
| Decline in renal function | T1b | [20,21,31] | −5.71 (−8.20, −3.21) | −0.65 (−0.80, −0.50) | WMD −6.60 (−12.85, −0.35) |
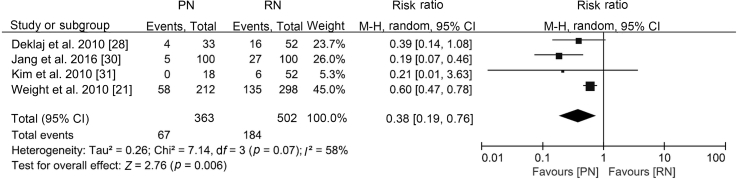
| Onset of CKD | T1b | [21,28,30,31] | −4.84 (−8.87, −0.82) | −0.39 (−0.81, 0.03) | RR 0.38 (0.19, 0.76) |
| Progression | T1b | [27,32] | NAa | NAa | HR 0.70 (0.40, 1.24) |
| Cancer-specific mortality | T1b | [22,25,26,30,[32], [33], [34]] | NAa | NAa | HR 0.91 (0.66, 1.26) |
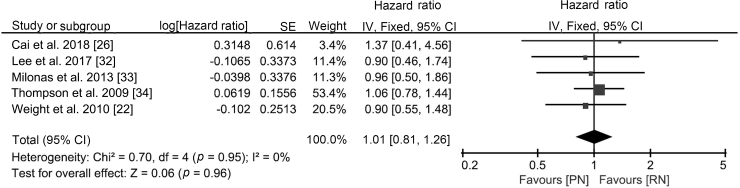
| All-cause mortality | T1b | [22,26,[32], [33], [34]] | NAa | NAa | HR 1.01 (0.81, 1.26) |
WMD, weighted mean difference; CI, confidence interval; RR, risk ratio; HR, Hazard ratio; NA, not applicable; CKD, chronic kidney disease; RR, risk ratio.
Baseline differences for oncological outcomes were not showed in this table, as HRs were adjusted with ages and tumour sizes in included studies.
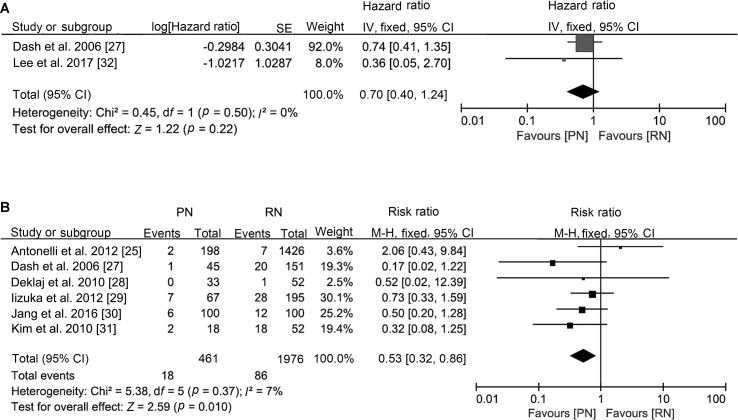
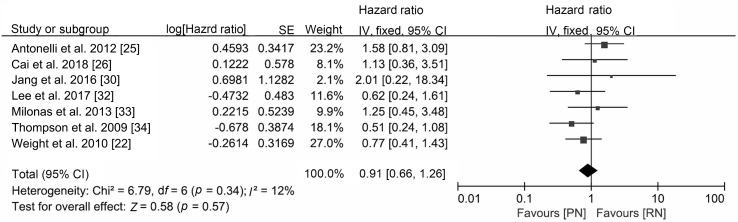
The oncological outcomes showed no difference in progression-free survival (HR 0.70, 95% CI 0.40 to 1.24; p=0.22; Fig. 2), cancer-specific mortality (HR 0.91, 95% CI 0.66 to 1.26; p=0.57; Fig. 3) or all-cause mortality (HR 1.01, 95% CI 0.81 to 1.26; p=0.96; Supplement Fig. 1). Only two studies were included in the progression-free survival analysis; therefore, an analysis of recurrence rate was performed and concluded that the incidence of recurrence was lower after PN (RR 0.53, 95% CI 0.32 to 0.86; p=0.010; Fig. 2).
Figure 2.
Forest plots of cancer progression after PN versus RN for T1b tumours. (A) Forest plot of progression-free survival for PN versus RN for T1b tumours; (B) Forest plot of recurrence rate for PN versus RN for T1b tumours. PN, partial nephrectomy; RN, radical nephrectomy; SE, standard error; CI, confidence interval.
Figure 3.
Forest plot of cancer-specific survival for PN versus RN for T1b tumours. PN, partial nephrectomy; RN, radical nephrectomy; SE, standard error; CI, confidence interval.
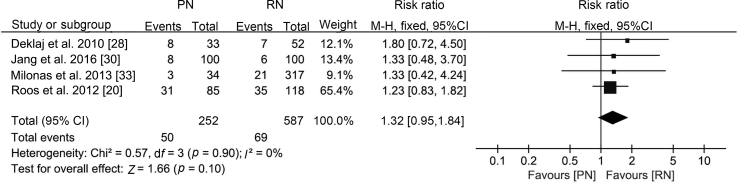
Regarding the perioperative outcomes, there was no difference in estimated blood loss (WMD −16.47 mL, 95% CI −68.06 to 35.13; p=0.53; Supplement Fig. 2) or postoperative complications (RR 1.32, 95% CI 0.95 to 1.84; p=0.10; Fig. 4).
Figure 4.
Forest plot of postoperative complications for PN versus RN for T1b tumours. PN, partial nephrectomy; RN, radical nephrectomy; CI, confidence interval.
With similar baseline renal function (WMD 0.27 mL/min, 95% CI −2.47 to 3.02; p=0.85), PN was associated with better postoperative renal function (WMD 14.77 mL/min, 95% CI 11.65 to 17.89; p<0.0001; Supplement Fig. 3), a smaller decline in eGFR (WMD −6.60 mL/min, 95% CI −12.85 to −0.35; p<0.0001; Supplement Fig. 4), and a lower likelihood of postoperative CKD onset (RR 0.38, 95% CI 0.19 to 0.76; p=0.006; Fig. 5).
Figure 5.
Forest plot of onset of CKD for PN versus RN for T1b tumours. CKD, chronic kidney disease; PN, partial nephrectomy; RN, radical nephrectomy; CI, confidence interval.
Sensitivity analyses were performed by excluding the lowest-weighted or lowest-scored studies. Most outcomes remained consistent with the previous outcomes, except for the decline in renal function. After excluding the study by Weight et al. [21], the difference in the decline in renal function became negative. Moreover, there were only three studies included in the analysis, and the study by Roos et al. [20] was the only study with negative results. In this paper, the decline in function was presented as the median and range, and the specific statistics were 17 (1–72) for PN and 19 (4–118) for RN. We estimated the SD of the two arms based on the range, but the distribution was unknown, and the negative result should be considered with caution. Additionally, in this study, the baseline eGFR was similar for PN and RN, and the postoperative renal function was significantly different.
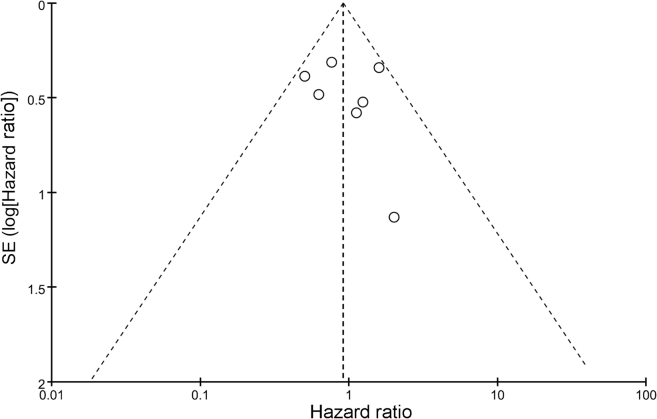
A funnel plot of the studies included in the meta-analysis reporting CSS is shown in Fig. 6. Publication bias was not significant for any of the results.
Figure 6.
Funnel plot of the studies included in the meta-analysis reporting cancer-specific survival. SE, standard error.
4. Discussion
To our knowledge, this is the first meta-analysis comparing PN to RN for T1b renal masses. Mir et al. [16] conducted a systematic review and meta-analysis to compare the perioperative, functional and oncological outcomes; however, they did not conduct a subgroup analysis for T1b tumours, and the selection bias in their meta-analysis was significant. We further analysed the baseline characteristics according to tumour stage and evaluated the selection bias to obtain more convincing conclusions (Table 2). For T1b patients, PN is as effective as RN in cancer control but has better renal function preservation under a similar risk of postoperative complications.
To synthesize the oncological outcomes, we used the HRs that had been adjusted for potential confounders (Table 3). The oncological equivalence between PN and RN demonstrated by our study was consistent with the findings of EORTC 30904, the only prospective randomized trial comparing PN to RN for kidney cancer, although patients were limited to masses <5 cm [6]. Although many confounders were adjusted, reliable parameters assessing patient surgical conditions, such as the American Society of Anesthesiologists score, Charlson comorbidity index, Eastern Cooperative Oncology Group score, and tumour complexity scores, such as RENAL and PADUA, were used only in partial studies, and it was technically impossible to perform a pooled analysis.
Table 3.
Adjusted factors of oncological outcomes.
| Study | Adjusted factors |
|---|---|
| Lee et al. [32] | Age, BMI, sex, tumour size, cellular grade, and pathologic stage |
| Antonelli et al. [25] | Age, sex, tumour size, pathologic stage, and surgical margin status |
| Cai et al. [26] | Age, ASA score, and pathologic stage |
| Dash et al. [27] | Age, grade, stage, tumour size, and vascular invasion |
| Jang et al. [30] | Age, sex, comorbidities, BMI, tumour size and depth, histologic type, and preoperative eGFR |
| Milonas et al. [33] | Age, tumour size, pathologic stage, ASA score, and tumour grade |
| Thompson et al. [34] | Age, CCI, pathologic stage, tumour size, histological subtype, and chronic kidney disease |
| Weight et al. [22] | Age, tumour size, presence of contralateral disease, solitary kidney status, and CCI |
| Roos et al. [19] | Age, symptoms at presentation, type of surgery, tumour size, and ASA score |
ASA score, American Society of Anesthesiologists score; CCI, Charlson comorbidity index; ECOG score, Eastern Cooperative Oncology Group score; BMI, body mass index; eGFR, estimated glomerular filtration rate.
The consideration of surgical morbidity is essential when comparing high-risk surgical procedures. The estimated blood loss during surgery did not differ among T1b patients, which is different from the conclusion of Mir et al. [16]. PN was also related to a higher likelihood of postoperative complications for T2 patients in the study by Mir et al. [16], while in our analysis, the difference was not significant; however, considering the selection bias (Table 2), the equivalence in surgical morbidity should be confirmed with caution. In the EORTC 30904 trial, there was a slightly higher risk for PN, which alerted us to be more conservative to the equal risk of complications in T1b. However, the increase in risk was still acceptable.
The better preservation of renal function is the motivation to conduct nephron-sparing surgery (NSS). CKD is associated with a higher risk of severe cardiovascular disease, cancer-specific mortality, and all-cause mortality, and RN increases the risk of CKD [3,4,8,35]. In our analysis, PN showed better preservation of renal function than RN and resulted in a lower rate of CKD, although all-cause mortality did not improve. Functional outcomes of EORTC 30904 suggested that PN reduced the incidence of at least moderate renal dysfunction (eGFR<60), but the beneficial impact of NSS on eGFR did not improve survival [5,6], which is consistent with our study. Regarding cancer-specific mortality, although no difference was observed in the two groups, according to a previous multi-centre analysis [8], PN might be a better option for patients with abnormal renal function because the preservation of renal function may result in longer CSS.
Recurrence is an essential topic when we discuss the adoption of PN. Our analysis of progression-free survival reported similar outcomes for PN and RN but a lower recurrence rate for PN. Notably, the tumour sizes for PN were smaller than those for RN in most included studies, and selection bias could be a potential reason. However, in the pair-matched cohort study, the recurrence rate with PN (6/100) was still lower than that with RN (12/100) [30]. Another explanation is that the loss of renal function can lead to an imbalance in inflammation activation and suppression, ultimately resulting in uraemia-related immune deficiency [36]. The progression-free survival analysis also suggested that PN might be related to better survival, and the difference might become significant if more studies were included. Our analysis suggested that the progression-free survival of PN was not inferior to that of RN.
Several studies have compared the oncological outcomes of PN and RN for T1b-only tumours. Badalato et al. [12] and Meskawi et al. [11] suggested that PN and RN were comparable in all-cause mortality and cancer-specific mortality in their analysis of the SEER database. There were also many outcomes reported for T1b-2 patients. Ristau et al. [24] described the improved overall survival of PN, which was not observed in patients aged ≥65 years, in their analysis of the National Cancer Database from 2004 to 2014 that included 212 016 patients. In the analysis of the SEER database reported by Zhang et al. [9], an advantage of PN in overall survival was not observed, but PN was related to better CSS. Compared to our outcomes in the meta-analysis, these previous studies were consistent with our conclusions for T1b tumours, although they were more optimistic about PN for T1b-2 tumours. One possible explanation is the lack of measurable parameters assessing tumour complexity in these databases. Surgical procedures are more likely to be complex for larger masses. In these cases, with larger tumours, patients with more surgically resectable tumours tend to undergo NSS. Particularly for these studies analysing national databases, selection bias could not be ignored because of the heterogeneity of medical institutions and large sample size. In conclusion, PN may provide equivalent oncological outcomes for T1b patients when feasible.
The complexity of the tumour may inform decisions in surgical treatment, but it could not be measured in a standard manner until the RENAL score was created in 2009 [37]. In our included studies, only one study used the RENAL score to assess the complexity of tumours [29]. The nephrometry scores of PN and RN were matched in this study, but the study did not investigate the oncological outcomes. Jang et al. [30] used propensity scores, including tumour size and depth, to assess oncological and functional outcomes and concluded that compared with RN, PN showed similar progression-free survival (p=0.66) and CSS (p=0.52) and better overall survival (p=0.003). Both Deklaj et al. [28] and Kim et al. [31] matched tumour locations in their studies, and no significant difference in oncological outcomes was observed. A study using the RENAL score compared the oncological outcomes of PN and RN for renal masses no less than 7 cm and found no significant difference [38]. These studies indicated that PN could provide similar cancer control to RN given similar tumour complexity.
Our study had several limitations. First, as a meta-analysis of retrospective studies, several biases were inevitable because of the retrospective nature. Exclusion of patients with missing data, a lack of detailed clinical information and a central histopathological review were potential sources of bias. Second, as an important factor that affects both clinical decision-making and prognosis, the complexity of the tumours was not appropriately measured in most included studies, which is a problem that could be solved by designing a better prospective randomized trial using a proper scoring system.
5. Conclusion
Our systematic review and meta-analysis suggested that PN is superior to RN with similar surgical morbidity, oncological outcomes and better renal function preservation for T1b tumours. Our findings could be helpful in evidence-based clinical decision-making but should be critically interpreted based on an assessment of the complexity of the tumour. Nevertheless, a properly designed, prospective randomized clinical trial is still warranted to confirm the role of PN in the treatment of larger renal tumours.
Author contributions
Study concept and design: Yucong Zhang, Heng Li.
Data acquisition: Gongwei Long, Yucong Zhang, Wei Ouyang.
Data analysis: Haojie Shang, Beichen Ding, Guoliang Sun, Man Liu.
Drafting of manuscript: Yucong Zhang, Gongwei Long.
Critical revision of the manuscript: Heng Li, Yuan Chen, Hua Xu, Zhangqun Ye.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2019.11.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplement Fig. 1.
Forest plot of overall survival for partial nephrectomy (PN) versus radical nephrectomy (RN) for T1b tumors. SE, standard error; CI, confidence interval.
Supplement Fig. 2.
Forest plot of estimated blood loss for partial nephrectomy (PN) versus radical nephrectomy (RN) for T1b tumors. SD, standard deviation; CI, confidence interval.
Supplement Fig. 3.
Forest plot of postoperative eGFR for partial nephrectomy (PN) versus radical nephrectomy (RN) for T1b tumors. eGFR, estimated glomerular filtration. SD, standard deviation; CI, confidence interval.
Supplement Fig. 4.
Forest plot of decline in eGFR for partial nephrectomy (PN) versus radical nephrectomy (RN) for T1b tumors. eGFR, estimated glomerular filtration. SD, standard deviation; CI, confidence interval.
References
- 1.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.J., Liss M.A., Derweesh I.H. Outcomes of partial nephrectomy for clinical T1b and T2 renal tumors. Curr Opin Urol. 2014;24:448–452. doi: 10.1097/MOU.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 3.Huang W.C., Levey A.S., Serio A.M., Snyder M., Vickers A.J., Raj G.V. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Scosyrev E., Messing E.M., Sylvester R., Campbell S., Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65:372–377. doi: 10.1016/j.eururo.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Van Poppel H., Da Pozzo L., Albrecht W., Matveev V., Bono A., Borkowski A. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Lane B.R., Campbell S.C., Demirjian S., Fergany A.F. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649–1655. doi: 10.1016/j.juro.2012.11.121. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A., Minervini A., Sandri M., Bertini R., Bertolo R., Carini M. Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer-specific mortality in renal cell carcinoma. Eur Urol. 2018;74:661–667. doi: 10.1016/j.eururo.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Zhao Z., Duan X., Deng T., Cai C., Wu W. Partial versus radical nephrectomy for T1b-2N0M0 renal tumors: a propensity score matching study based on the SEER database. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193530. doi: 10.1371/journal.pone.0193530. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shum C.F., Bahler C.D., Sundaram C.P. Matched comparison between partial nephrectomy and radical nephrectomy for T2 N0 M0 tumors, a study based on the national cancer database. J Endourol. 2017;31:800–805. doi: 10.1089/end.2017.0190. [DOI] [PubMed] [Google Scholar]
- 11.Meskawi M., Becker A., Bianchi M., Trinh Q.D., Roghmann F., Tian Z. Partial and radical nephrectomy provide comparable long-term cancer control for T1b renal cell carcinoma. Int J Urol. 2014;21:122–128. doi: 10.1111/iju.12204. [DOI] [PubMed] [Google Scholar]
- 12.Badalato G.M., Kates M., Wisnivesky J.P., Choudhury A.R., McKiernan J.M. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: a propensity scoring approach. BJU Int. 2012;109:1457–1462. doi: 10.1111/j.1464-410X.2011.10597.x. [DOI] [PubMed] [Google Scholar]
- 13.Alanee S., Nutt M., Moore A., Holland B., Dynda D., Wilber A. Partial nephrectomy for T2 renal masses: contemporary trends and oncologic efficacy. Int Urol Nephrol. 2015;47:945–950. doi: 10.1007/s11255-015-0975-3. [DOI] [PubMed] [Google Scholar]
- 14.Hadjipavlou M., Khan F., Fowler S., Joyce A., Keeley F.X., Sriprasad S. Partial vs. radical nephrectomy for T1 renal tumours: an analysis from the British association of urological surgeons nephrectomy audit. BJU Int. 2016;117:62–71. doi: 10.1111/bju.13114. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.P., Murad M.H., Thompson R.H., Boorjian S.A., Weight C.J., Han L.C. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012 doi: 10.1016/j.juro.2012.10.026. pii: S0022-5347(12)05254-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Mir M.C., Derweesh I., Porpiglia F., Zargar H., Mottrie A., Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71:606–617. doi: 10.1016/j.eururo.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 17.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos F.C., Brenner W., Muller M., Schubert C., Jager W.J., Thuroff J.W. Oncologic long-term outcome of elective nephron-sparing surgery versus radical nephrectomy in patients with renal cell carcinoma stage pT1b or greater in a matched-pair cohort. Urology. 2011;77:803–808. doi: 10.1016/j.urology.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Roos F.C., Brenner W., Thomas C., Jager W., Thuroff J.W., Hampel C. Functional analysis of elective nephron-sparing surgery vs. radical nephrectomy for renal tumors larger than 4 cm. Urology. 2012;79:607–613. doi: 10.1016/j.urology.2011.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Weight C.J., Larson B.T., Gao T., Campbell S.C., Lane B.R., Kaouk J.H. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology. 2010;76:631–637. doi: 10.1016/j.urology.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 22.Weight C.J., Larson B.T., Fergany A.F., Gao T., Lane B.R., Campbell S.C. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Huang W.C., Elkin E.B., Levey A.S., Jang T.L., Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–62. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ristau B.T., Handorf E.A., Cahn D.B., Kutikov A., Uzzo R.G., Smaldone M.C. Partial nephrectomy is not associated with an overall survival advantage over radical nephrectomy in elderly patients with stage Ib-II renal masses: an analysis of the national cancer data base. Cancer. 2018;124:3839–3848. doi: 10.1002/cncr.31582. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A., Ficarra V., Bertini R., Carini M., Carmignani G., Corti S. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: results of a retrospective, comparative, multi-institutional study. BJU Int. 2012;109:1013–1018. doi: 10.1111/j.1464-410X.2011.10431.x. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y., Li H.Z., Zhang Y.S. Comparison of partial and radical laparascopic nephrectomy: long-term outcomes for clinical T1b renal cell carcinoma. Urol J. 2018;15:16–20. doi: 10.22037/uj.v0i0.3913. [DOI] [PubMed] [Google Scholar]
- 27.Dash A., Vickers A.J., Schachter L.R., Bach A.M., Snyder M.E., Russo P. Comparison of outcomes in elective partial vs. radical nephrectomy for clear cell renal cell carcinoma of 4–7 cm. BJU Int. 2006;97:939–945. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 28.Deklaj T., Lifshitz D.A., Shikanov S.A., Katz M.H., Zorn K.C., Shalhav A.L. Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1bN0M0 renal tumors: comparison of perioperative, pathological, and functional outcomes. J Endourol. 2010;24:1603–1607. doi: 10.1089/end.2009.0312. [DOI] [PubMed] [Google Scholar]
- 29.Iizuka J., Kondo T., Hashimoto Y., Kobayashi H., Ikezawa E., Takagi T. Similar functional outcomes after partial nephrectomy for clinical T1b and T1a renal cell carcinoma. Int J Urol. 2012;19:980–986. doi: 10.1111/j.1442-2042.2012.03085.x. [DOI] [PubMed] [Google Scholar]
- 30.Jang H.A., Kim J.W., Byun S.S., Hong S.H., Kim Y.J., Park Y.H. Oncologic and functional outcomes after partial nephrectomy versus radical nephrectomy in T1b renal cell carcinoma: a multicenter, matched case-control study in Korean patients. Cancer Res Treat. 2016;48:612–620. doi: 10.4143/crt.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J.M., Song P.H., Kim H.T., Park T.C. Comparison of partial and radical nephrectomy for pT1b renal cell carcinoma. Korean J Urol. 2010;51:596–600. doi: 10.4111/kju.2010.51.9.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H., Oh J.J., Byun S.S., Jeong C.W., Kwak C., Jeong B.C. Can partial nephrectomy provide equal oncological efficiency and safety compared with radical nephrectomy in patients with renal cell carcinoma (≥4 cm)? A propensity score-matched study. Urol Oncol. 2017;35:379–385. doi: 10.1016/j.urolonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Milonas D., Skulcius G., Baltrimavicius R., Auskalnis S., Kincius M., Matjosaitis A. Comparison of long-term results after nephron-sparing surgery and radical nephrectomy in treating 4- to 7-cm renal cell carcinoma. Medicina (Kaunas) 2013;49:223–228. [PubMed] [Google Scholar]
- 34.Thompson R.H., Siddiqui S., Lohse C.M., Leibovich B.C., Russo P., Blute M.L. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601–2606. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKiernan J., Simmons R., Katz J., Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816–820. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 36.Kato S., Chmielewski M., Honda H., Pecoits-Filho R., Matsuo S., Yuzawa Y. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutikov A., Uzzo R.G. The RENAL. Nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Kopp R.P., Mehrazin R., Palazzi K.L., Liss M.A., Jabaji R., Mirheydar H.S. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114:708–718. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]