Abstract
Neutral lipid storage disease with myopathy (NLSDM) is a rare autosomal recessive disorder, due to an enzymatic error of lipid metabolism. Patients present always with skeletal muscle myopathy and variable cardiac and hepatic involvement. NLSDM is caused by mutations in the PNPLA2 gene, which encodes the adipose triglyceride lipase (ATGL). Here we report the molecular characterization and clinical findings of two NLSDM siblings carrying the novel c.187+1G > C homozygous PNPLA2 mutation, localized in the splice site of intron 2. Molecular analyses revealed that neither aberrant PNPLA2 mRNA isoforms, nor ATGL mutated protein were detectable in patient's cells.
Clinically, both patients presented early onset muscle weakness, in particular of proximal upper limb muscles. In almost 15 years, muscle damage affected also distal upper limbs.
This is a NLSDM family, displaying a severe PNPLA2 mutation in two siblings with clinical presentation characterized by an early onset, but a slowly evolution of severe myopathy.
Keywords: Cardiomyopathy, Lipid metabolism, Neutral lipid storage disease with myopathy, PNPLA2, Splicing mutation, Triglyceride lipase
Introduction
Lipid storage myopathies (LSMs) are a group of metabolic disorders caused by different errors of fats metabolism.1 Impairment of ability to metabolize fats induces an abnormal lipid storage in muscle fiber, as well as in other tissues.
Loss or decrease of adipose triglyceride lipase (ATGL) function is the biochemical defect associated with the onset of a particular LSM, called neutral lipid storage disease with myopathy (NLSDM; MIM 610717).2 ATGL is a lipid droplet-associated enzyme which mobilizes the first fatty acid from triacylglycerols (TAGs), stored within lipid droplets (LDs).3 ATGL protein is composed of 504 amino acids and shows two functional regions: a patatin domain (amino acids I10-L178), containing the catalytic dyad (amino acids S47 and D166) and also three LC3-interacting region (LIR) motifs (amino acids C15–V20; P86–V91; S145–V150), and a hydrophobic domain (amino acids P315–P360), involved in LD binding.4, 5
Until now, forty different PNPLA2 mutations have been identified.2, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The majority of them determine either no protein production or the synthesis of truncated proteins. These mutants show loss of function or a severe decrease of enzymatic activity. The remaining variants are missense mutations, which usually maintain a residual lipolytic activity.11, 16, 17
NLSDM is an autosomal recessive inborn error of neutral lipid metabolism, reported in 56 subjects worldwide.2, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 In this LSM, TAGs are accumulated in cytoplasmic LDs of most tissues, including muscles, heart, liver and peripheral blood. Histological and electron microscopy findings reveal a markedly increase in the number and dimension of LDs located in the muscle fibers, particularly in type I fibers.13, 14, 17, 19 The abnormal storage of neutral lipid primarily determines a progressive skeletal muscle myopathy which involves both proximal and distal muscles. In advances cases, muscular atrophy can also be observed. Other clinical symptoms are cardiomyopathy, hepatomegaly, diabetes, chronic pancreatitis and short stature.
In this study we clinically and genetically characterized two siblings affected by NLSDM. In these patients a novel splice site mutation of PNPLA2 gene was identified. This mutation completely abolishes PNPLA2 mRNA and ATGL protein production, causing an early onset of severe progressive skeletal muscle myopathy but with slow evolution.
Materials and methods
An Italian NLSDM patient was recruited. This study was conducted in full accordance with the World Medical Association Declaration of Helsinki (version 2008). Informed consent for genetic analysis was obtained from patient and healthy subjects.
Muscle histopathology
Routine standard histological techniques, including haematoxylin & eosin (H&E), and Oil-Red-O (ORO), were applied to transverse sections (8 μm thick) of the quadriceps muscle biopsy.
For electron microscopy, a small fragment of muscle tissue was fixed in 4% glutaraldehyde in phosphate buffer, postfixed in osmium tetroxide, dehydrated, and embedded in epon resin. Thin sections were examined with an electron microscope (FEI Tecnai G2).
Myoblasts cell culture
NLSDM and control myoblasts were obtained from muscle biopsies and cultured in DMEM supplemented with 20% FBS, 1% penicillin−streptomycin, 2 mM l-glutamine, 10 μg/ml insulin (Sigma-Aldrich), 2.5 ng/ml basic fibroblast growth factor (Gibco), and 10 ng/ml epidermal growth factor (Gibco).
Myoblasts were seeded on coverslips, stained with Nile Red (NR) and DAPI, and observed with a Leica MB5000B microscope. Fluorescence images were captured using a Leica DFC480 R2 digital camera and a Leica Application Suite (LAS) software.
Genomic DNA investigation
Peripheral blood samples were obtained from male patient and healthy subjects. Genomic DNA was extracted using Puregene DNA Isolation kit (Qiagen, Venlo, Netherlands). Genetic analysis of PNPLA2 exons sequence and their flanking regions was performed as previously reported (Tavian et al, 2012). All PCR products were purified using NucleoSpin Extract II (Macherey-Nagel, Germany) and sequenced on 3730 DNA analyser by the BigDyew Terminator V1.1 Cycle sequencing kit (Applied Biosystems, Foster City, CA, USA).
RT-PCR analysis of PNPLA2 gene expression in myoblasts
RNA was isolated from myoblasts using TRIzol (Invitrogen, Carslbad, CA, USA), treated with DNase I (ThermoFosher Scientific, Waltham, MA, USA) and converted to cDNA as previously described (Tavian et al, 2012). 50 ng of cDNA were amplified using the following primers: ATGL-1F (GCGGCCCCAGTCAGACGCAG) and ATGL-2R (CTCGCGGGGAAACATCGCGG); ATGL-2F (TTCTTCGCCTCCGCCAGCGG) and ATGL-3R (CTCGCGGGGAAACATCGCGG); ATGL-3F (TCTAAAGAGGCCCGGAAGCG) and ATGL-7R (GTAATCCTCCGCTTGGGCGC); ATGL-7F (CTGAACCGGCCCAACCCCTT) and ATGL-10R (GGTCCGCGGGGGCGGGAG). GAPDH expression was used as the internal control.21
Results
Case presentation
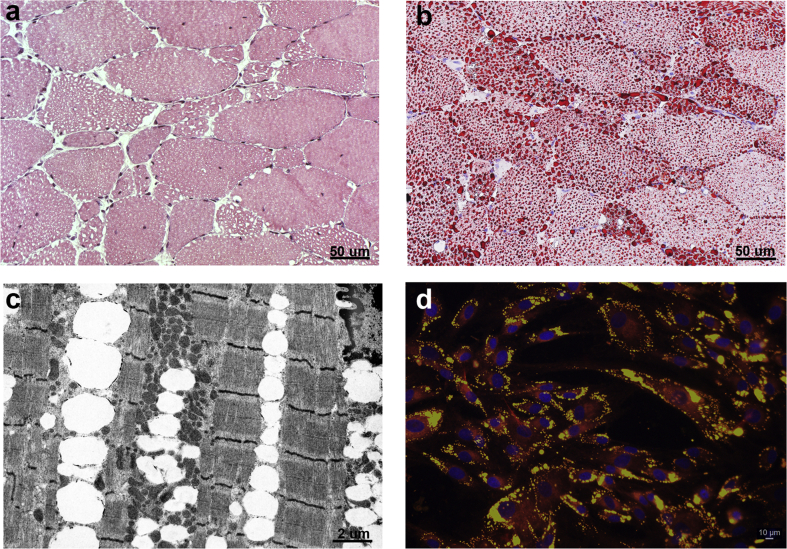
The proband presented around the age of 30 years with proximal upper limb muscle weakness, followed by involvement of lower limbs. His previous medical history had been unremarkable. The disease slowly progressed over the years and the patient was wheelchair-bound at the age of 46 years. At the same age, distal weakness of upper limbs was noticed. He has never reported bulbar or ocular symptoms or myalgia. Electromyography performed at the age of 35 years showed a myopathic pattern. At the age of 48 years a heart echo scan showed apical and lateral hypokinesis. Creatine kinase ranged between 1126 and 1574 U/L (normal value < 190 U/L). The patient was first admitted in our outpatient clinic at the age of 49 years. Neurological examination showed no antigravitary movements of proximal and distal upper limb muscles, slightly worse on wrist and finger extensors than in flexors, foot dorsal flexion and, limited to the right side, thigh and knee extension. In addition, neck extensor muscles were moderately (MRC = 3) weak and mild (MRC = 4) weakness of thigh flexion on the right and knee extension on the left side was observed. The patient was able to get up from the wheelchair with the help of upper limbs and able to stand with support, but not able to walk. Cranial nerves were normal. Tendon retractions or scapular winging were absent. Forced vital capacity was reduced to the 60% of normal value and the patient had no indication for non-invasive ventilation (NIV). A muscle biopsy, taken at age 50, revealed vacuolated fibers on H&E (Fig. 1a) and lipid droplets accumulation on O.R.O staining (Fig. 1b). Moreover, electron microscopy showed numerous LDs located between myofibrils in most fibers (Fig. 1c). Finally, myoblasts, stained with NR, exhibited an excessive neutral lipids storage in the LDs (Fig. 1d). The patient was then lost to follow-up and died at the age of around 54 years, probably due to heart problems.
Figure 1.
Histochemical characterization of NLSDM male patient. Muscle biopsy cryosections, stained with haematoxylin & eosin (a) and Oil-Red-O (b), reveal microvacuoles and abnormal storage of lipids. (c) Electron microscopy displays massive line-up of lipid droplets without signs of mitochondrial alteration. (d) Immunofluorescent image of cultured myoblasts shows increase of lipid droplet accumulation inside the cells. Myoblasts are stained with Nile Red and nuclei are counterstained with DAPI (blue).
Parents were not consanguineous, but born in the same small town. The proband had a healthy brother and a sister displaying similar pathological features, although presenting earlier, at the age of 18 years. The affected sister was wheelchair-bound since the age of 33 years, required NIV since the age of 46 years, whereas tracheostomy for respiratory failure and pace-maker implantation were performed at the age of 47 years. Unfortunately, she was never investigated in our institute.
Molecular analysis
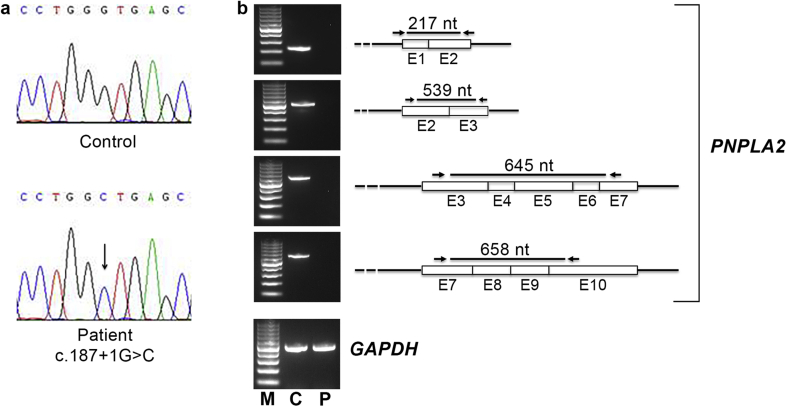
PNPLA2 gene investigation detected a novel homozygous splice site mutation (c.187+1G > C), which was submitted to GenBank (accession number MK645897) (Fig. 2a). This mutation was not found in 100 healthy controls nor was present in dbSNP, 1000 genomes and Exome Variant Server data banks. The variation is localized in the splice site of intron 2 and is predicted to cause the retention of intron 2, producing an aberrant mRNA and a truncated ATGL protein. However, a comparative PNPLA2 RT-PCR analysis, performed from myoblasts of male patient and a control subject, revealed that in NLSDM sample no PNPLA2 mRNA could be detected (Fig. 2b). Moreover, Western blotting investigation showed the lack of ATGL protein production in NLSDM cells (data not shown).
Figure 2.
Identification and molecular characterization of PNPLA2 mutation. (a) Electropherogram of PNPLA2 exon 2-intron 2 showing a splice-site mutation (c.187+1G > C) in homozygous status. (b) RT-PCR analysis performed with four different pairs of primers reveals complete lack of PNPLA2 RNA in patient sample. From upper image: first pair of primers encompassing exons 1 and 2 (217 nt); second pair encompassing exons 2 and 3 (539 nt); third pair encompassing exons 3, 4, 5, 6 and 7 (645 nt); fourth pair encompassing exons 7, 8, 9 and 10 (645 nt). GAPDH has been used as control for RNA normalization (512 nt). M: marker; C: control; P: patient.
Discussion
ATGL deficiency causes defects of neutral lipids metabolism and an excessive storage of TAGs in cytoplasmic LDs. The impairment of TAGs hydrolysis determines NLSDM onset. In this disorder the genotype–phenotype correlation is not easily established. In general, the majority of patients, carrying mutations which lead a dramatic decrease of ATGL function, have a severe outcome. These subjects, in addition to an early onset of skeletal muscle myopathy, often develop a cardiac dysfunction (40% of patients) with clinical manifestations ranging from minimal symptoms to severe conditions.6, 12, 13, 20 On the contrary, NLSDM patients with ATGL variations that retain a residual lipolytic activity may show a slow progressive myopathy, without hearth failure.16, 17
In this study we report two siblings presenting early muscle damage which slowly progressed over the years. This clinical phenotype is related to a PNPLA2 mutation that results in complete loss of gene expression. Usually, a splice site mutation does not determine the lack of mRNA.22 Sometimes, splice site mutations can activate cryptic sites determining the creation of novel donor or acceptor points.23 In the muscle of our patient we failed to detect PNPLA2 mRNA. Similarly, no PNPLA2 transcript was detected in two NLSDM Chinese patients, characterized by a mutation in the donor site of intron 6 (IVS6 + 2T).10 In rare cases, it could be hypothesized that some mutations in the splicing canonical points cause the creation of cryptic splice sites and the production of very unstable mRNAs which are immediately degraded. Further studies are needed to find which mechanisms are involved in the full lack of PNPLA2 transcript.
A different homozygous PNPLA2 mutation affecting the same invariant nucleotide of the donor splice-site of intron 2 (c.187 + 1G > A) has previously been described in two Chinese families.8, 24 In the first family, there were 2 siblings who manifested muscle weakness since the age of 35 (female patient) and 45 (male patient). In addition, male subject displayed dilated cardiomyopathy. In the second family, two brothers, who developed muscle weakness in the middle age and presented cardiac defects, were described. Despite similar PNPLA2 mutation, in our patients muscle weakness presented earlier than in Chinese subjects, but myopathy progression was slow. Moreover, our female patient manifested late onset of respiratory damage, and only 30 years after the onset of muscle weakness, she needed pacemaker implantation. These clinical differences might be related to many factors: genetic modifiers which can change disease development,25 epigenetic influences, as well as different lifestyle, diet and gender.
Indeed, the analysis of NLSDM patients described worldwide might support the hypothesis that the phenotype variability of this LSM might explained not only by different PNPLA2 mutations but also to others causes. Generally, in the presence of PNPLA2 mutations with similar effects on ATGL activity, Far East patients showed a more severe cardiac involvement, compared to NLSDM subjects from Italy.15 Moreover, some authors reported clinical variability in siblings with the same PNPLA2 mutations. We described an Italian family in which the daughter manifested leg myalgia and cramps at the age of 25, while her brother, who was an athlete, had the first symptoms (leg cramps) at 40 years of age.11 In a Japanese family, two affected members were identified. The female presented at the age of 21 muscle weakness that evolved in slowly progressive skeletal myopathy with cardiac involvement. She died at the age of 66 for hearth failure. Her brother displayed very early skeletal muscle myopathy onset (5 years old), with mild evolution, and a dramatic hearth failure that caused patient death at the age of 59.20 Finally, another paper by our group described an Italian family in which NLSDM was diagnosed in three members. Two brothers had late onset myopathy, while their sister did not present muscle involvement but hepatomegaly and diabetes.17 In these cases, as well as in our present patients, the manifestation of clinical symptoms with variable degree of severity might be associated both with gender of patients and lifestyle. Indeed, it has been reported that in pre-menopausal women endogenous estrogens play a protective role against cardiovascular disorders. These hormones regulate lipid metabolism, in particular in liver and adipose tissue but they can exert an effect also in other tissues.26 Moreover, estrogens prevent lipid accumulation and inflammation in skeletal muscle.27 Finally, the estrogens participate to regulation of expression of peroxisome proliferator activated receptors (PPARs), a group of receptors involved in fatty acids and glucose utilization in skeletal and cardiac cells.28 The effect of diet and exercise on number and size of skeletal muscle LDs29 has also been investigated. Caloric restriction, as well as acute exercise, decrease lipid accumulation in muscle cells, while long-term exercises act on improving insulin sensitivity and then alter LD morphology, cytoplasmic localization and their interaction with mitochondria. Additional clinical studies are required to clarify whether ATGL mutations combined with different lifestyle or gender can play a key role in development of various NLSDM phenotype.
In conclusion, a novel pathogenic mutation of PNPLA2 gene has been identified in two siblings affected by NLSDM. This mutation completely abolishes gene expression and determines early onset of muscle weakness which evolves in slowly progressive myopathy. These data expand the spectrum of PNPLA2 mutations onset and provide further evidence of phenotypic heterogeneity in NLSDM.
Author contributions
DT performed the molecular analysis, cell culture and critically revised the manuscript; LM and LM carried out the clinical characterization of patients; MM and CB performed the histopathological characterization; SM conceived the study, supervised it and wrote the manuscript. All authors read and approved the final manuscript.
Conflict of Interests
The authors have no conflicts of interest to declare.
Funding
This work was supported by grant GGP14066 from Telethon Foundation.
Acknowledgements
The authors are grateful to the patient for their kind cooperation, to the EuroBioBank and the Telethon Network of Genetic Biobanks (GTB12001F) for providing biological samples and to professor Francesco Mauri for his scientific assistance.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Angelini C., Pennisi E., Missaglia S. Metabolic lipid muscle disorders: biomarkers and treatment. Ther Adv Neurol Disord. 2019;12 doi: 10.1177/1756286419843359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer J., Lefevre C., Morava E. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39(1):28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 3.Missaglia S., Coleman R.A., Mordente A. Neutral lipid storage diseases as cellular model to study lipid droplet function. Cells. 2019;8(2) doi: 10.3390/cells8020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweiger M., Lass A., Zimmermann R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297(2) doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Lopez N., Garcia-Macia M., Sahu S. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the Brown adipose tissue and liver. Cell Metab. 2016;23(1):113–127. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campagna F., Nanni L., Quagliarini F. Novel mutations in the adipose triglyceride lipase gene causing neutral lipid storage disease with myopathy. Biochem Biophys Res Commun. 2008;377(3):843–846. doi: 10.1016/j.bbrc.2008.10.081. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K., Inoguchi T., Maeda Y. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93(7):2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Hong D., Wang Z. A novel PNPLA2 mutation causes neutral lipid storage disease with myopathy (NLSDM) presenting muscular dystrophic features with lipid storage and rimmed vacuoles. Clin Neuropathol. 2010;29(6):351–356. doi: 10.5414/npp29351. [DOI] [PubMed] [Google Scholar]
- 9.Akman H.O., Davidzon G., Tanji K. Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul Disord. 2010;20(6):397–402. doi: 10.1016/j.nmd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin P., Li W., Wen B. Novel PNPLA2 gene mutations in Chinese Han patients causing neutral lipid storage disease with myopathy. J Hum Genet. 2012;57(10):679–681. doi: 10.1038/jhg.2012.84. [DOI] [PubMed] [Google Scholar]
- 11.Tavian D., Missaglia S., Redaelli C. Contribution of novel ATGL missense mutations to the clinical phenotype of NLSD-M: a strikingly low amount of lipase activity may preserve cardiac function. Hum Mol Genet. 2012;21(24):5318–5328. doi: 10.1093/hmg/dds388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano K., Tanaka T., Ikeda Y. Genetic mutations in the adipose triglyceride lipase and myocardial overexpression of peroxisome proliferated activated receptor- in patients with triglyceride deposit cardiomyovasculopathy. Biochem Biophys Res Commun. 2014;443(2):574–579. doi: 10.1016/j.bbrc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Pasanisi M.B., Missaglia S., Cassandrini D. Severe cardiomyopathy in a young patient with complete deficiency of adipose triglyceride lipase due to a novel mutation in PNPLA2 gene. Int J Cardiol. 2016;207:165–167. doi: 10.1016/j.ijcard.2016.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missaglia S., Maggi L., Mora M. Late onset of neutral lipid storage disease due to novel PNPLA2 mutations causing a total loss of lipase activity in a patient with myopathy and slight cardiac involvement. Neuromuscul Disord. 2017;27(5):481–486. doi: 10.1016/j.nmd.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennisi E.M., Arca M., Bertini E. Neutral Lipid Storage Diseases: clinical/genetic features and natural history in a large cohort of Italian patients. Orphanet J Rare Dis. 2017;12(1) doi: 10.1186/s13023-017-0646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennisi E.M., Missaglia S., Dimauro S. A myopathy with unusual features caused by PNPLA2 gene mutations. Muscle Nerve. 2015;51(4):609–613. doi: 10.1002/mus.24477. [DOI] [PubMed] [Google Scholar]
- 17.Missaglia S., Tasca E., Angelini C. Novel missense mutations in PNPLA2 causing late onset and clinical heterogeneity of neutral lipid storage disease with myopathy in three siblings. Mol Genet Metab. 2015;115(2-3):110–117. doi: 10.1016/j.ymgme.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Coassin S., Schweiger M., Kloss-Brandstätter A. Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genet. 2010;6(12) doi: 10.1371/journal.pgen.1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilich P., Horvath R., Krause S. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol. 2011;258(11):1987–1997. doi: 10.1007/s00415-011-6055-4. [DOI] [PubMed] [Google Scholar]
- 20.Higashi M., Hirano K., Kobayashi K. Distinct cardiac phenotype between two homozygotes born in a village with accumulation of a genetic deficiency of adipose triglyceride lipase. Int J Cardiol. 2015;192:30–32. doi: 10.1016/j.ijcard.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Missaglia S., Valadares E.R., Moro L. Early onset of Chanarin-Dorfman syndrome with severe liver involvement in a patient with a complex rearrangement of ABHD5 promoter. BMC Med Genet. 2014;15 doi: 10.1186/1471-2350-15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramowicz A., Gos M. Splicing mutations in human genetic disorders: examples, detection, and confirmation. J Appl Genet. 2018;59(3):253–268. doi: 10.1007/s13353-018-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaz-Drago R., Custódio N., Carmo-Fonseca M. Deep intronic mutations and human disease. Hum Genet. 2017;136(9):1093–1111. doi: 10.1007/s00439-017-1809-4. [DOI] [PubMed] [Google Scholar]
- 24.Xu C., Zhao Y., Liu J. Muscle MRI in neutral lipid storage disease with myopathy carrying mutationc 187+1G>A. Musc Nerve. 2015;51(6):922–927. doi: 10.1002/mus.24507. [DOI] [PubMed] [Google Scholar]
- 25.Riordan J.D., Nadeau J.H. Modifier genes, Network resilience, and the genetics of health. Am J Hum Genet. 2017;101(2):177–191. doi: 10.1016/j.ajhg.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmisano B.T., Zhu L., Eckel R.H. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018;15:45–55. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauvais-Jarvis M., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aseer K.J., Kim S.W., Lee D.G. Gender-dimorphic regulation of muscular proteins in response to high fat diet and sex steroid hormones. Biotechnol Bioproc Eng. 2014;19:811–828. doi: 10.3109/10715762.2014.896003. [DOI] [PubMed] [Google Scholar]
- 29.Daemen S., van Polanen N., Hesselink K.C. The effect of diet and exercise on lipid droplet dynamics in human muscle tissue. J Exp Biol. 2018;221(Pt Suppl 1) doi: 10.1242/jeb.167015. [DOI] [PubMed] [Google Scholar]