Abstract
Inhibitory checkpoint molecules include programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), cytotoxic T lymphocyte antigen-4 (CTLA-4), human endogenous retrovirus-H Long terminal repeat-associating 2 (HHLA2), B7 homolog 4 protein (B7-H4), T cell membrane protein-3 (TIM-3) and Lymphocyte-activation gene 3 (LAG-3), which are up-regulated during tumorigenesis. These pathways are essential to down-regulate the immune system by blocking the activation of T cells. In recent years, immune checkpoint blockers (ICBs) against PD-1, PD-L1, CTLA-4 or TIM-3 has made remarkable progress in the clinical application, revolutionizing the treatment of malignant tumors and improving patients' overall survival. However, the efficacy of ICBs in some patients does not seem to be good enough, and more immune-related adverse events (irAEs) will inevitably occur. Therefore, biomarkers research provides practical guidance for clinicians to identify patients who are most likely to benefit from or exhibit resistance to particular types of immune checkpoint therapy. There are two points in general. On the one hand, given the spatial and temporal differential expression of immune checkpoint molecules during immunosuppression process, it is essential to understand their mechanisms to design the most effective individualized therapy. On the other hand, due to the lack of potent immune checkpoints, it is necessary to combine them with novel biomarkers (such as exosomes and ctDNA) and other anticancer modalities (such as chemotherapy and radiotherapy).
Keywords: Circulating tumor DNA (ctDNA), Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Exosome, Immune checkpoint, Lymphocyte-activation gene 3 (LAG-3), Programmed cell death protein ligand 1 (PD-L1), Programmed death-1 receptor (PD-1), T cell immunoglobulin domain and mucin domain 3 (TIM-3)
Abbreviation
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- TIM-3
T cell immunoglobulin domain and mucin domain 3
- LAG-3
lymphocyte-activation gene 3
- BTLA-4
B- and T-lymphocyte attenuator 4
- PD-1
programmed death-1 receptor
- PD-L1
programmed cell death protein ligand 1
- HHLA2
human endogenous retrovirus-H long terminal repeat-associating 2
- ICBs
immune checkpoint blockers
- mAbs
monoclonal antibody blockers
- irAEs
immune-related adverse events
- ctDNA
circulating tumor DNA
- mMEL
metastatic melanoma
- PMBCL
primary mediastinal large B-cell lymphoma
- AEG
adenocarcinoma of the esophagogastric junction
- WT
wild-type
- mMCC
metastatic Merkel cell carcinoma
- NSCLC
non-small cell lung cancer
- ns-NSCLC
non-squamous non-small cell lung cancer
- M/UR
unresectable or metastatic
- RCC
renal cell carcinoma
- UC
urothelial carcinoma
- cHL
classic Hodgkin lymphoma
- GC
gastric cancer
- HCC
hepatocellular carcinoma
- CC
cervical cancer
- LA/M
locally advanced or metastatic
Introduction
Tumor immunotherapies are considered to be one of the most important progress in tumor treatment, mainly including cancer vaccines, therapeutic antibody, immune system modulators, adoptive T cell transfer (ACT), and immune checkpoints blockade.1 A significant advance in cancer immunotherapy is the discovery of immune checkpoint proteins, which are a series of molecules involved in co-stimulation pathways and coinhibitory pathways in immune responses.2 These molecules include the B7-CD28 family of ligands and receptors, such as CTLA-4, B7-H4, HHLA2, PD-1/PD-L, B7-H3,3, 4 and the other immunoglobulin superfamily members such as LAG-3 and TIM-3.5, 6 Inhibitory checkpoint signals can maintain tolerance and immune homeostasis and protect against immune-mediated tissue damage.7, 8 Furthermore, these inhibitory checkpoints are key mediators of T cell dysfunction during cancer progression, preventing effective anti-tumor immunity.9, 10 Given the vital role of immune checkpoints in the immune system, the research of targeted drugs is now attracting much more attention. Immune checkpoint blockers (ICBs) have become the main drugs for the treatment of melanoma, and these drugs could also be used as treatments for people with renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), urinary maltose carcinoma, head and neck cancer, ovarian cancer and various lymphomas. The first approved immunotherapy drug is Ipilimumab (Yervoy), a cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) drug. Ipilimumab (Yervoy) is approved by the US Food and Drug Administration (FDA) in 2011 for the treatment of advanced melanoma patients.11 Programmed cell death-1 (PD-1) monoclonal antibodies such as Nivolumab (Opdivo) and Pembrolizumab (Keytruda) have been approved by the FDA for the treatment of melanoma, Hodgkin Disease, head and neck cancer, bladder cancer and non-small cell lung cancer.12, 13 Programmed cell death ligand-1 (PD-L1), T cell membrane protein-3 (TIM-3), B7-H3 (CD276), B7-H4 (B7x), Human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2) and Lymphocyte activation-gene-3 (LAG-3) have recently shown great promise in cancer immunotherapy.14 However, there are still many important issues to be addressed. First, the coinhibitory checkpoint could be co-expressed on T cells. Therefore, combined blockade of the inhibitory receptors PD-1 and CTLA-4,15 LAG-3 and PD-15 or PD-1 and Tim-36 shows better tumor clearance than blocking alone. A better understanding of the synergistic mechanism between molecular pathways can effectively predict the resistance caused by the increase of another immune checkpoint during the treatment of inhibitors. Second, given the function of inhibitory checkpoint molecules in the immune system, immune checkpoint blocking therapy is at risk of immune-related adverse events (irAEs).16, 17 Therefore, it is necessary to identify and prevent the unique irAEs of these drugs in time to improve the safety of tumor immunotherapy. This review focuses on the latest knowledge of immune checkpoints and prospects of potential combinatorial therapeutic strategies in tumor immunotherapy.
Immune checkpoint
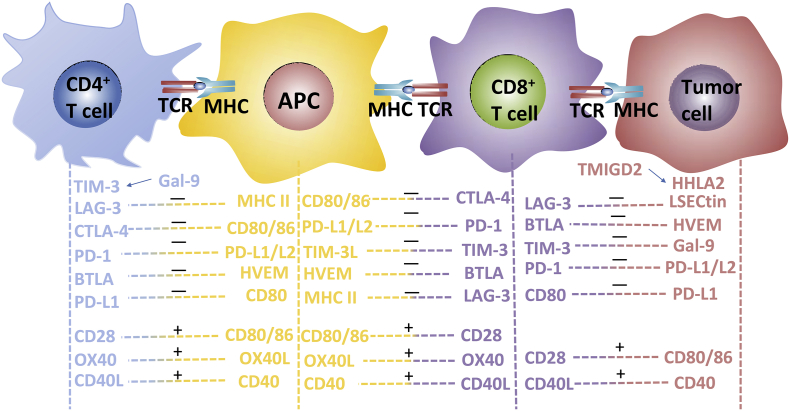
The inhibitory checkpoint is similar to a brake system that provides an inhibitory signal to the immune system, blocking activation signals from the T cell costimulatory receptors and co-stimulators.18 It is expressed after T cell activation so that the immune system is not overactivated and remains self-tolerant in the case of infection and inflammation.19, 20 T cell receptor (TCR) interacts with the major histocompatibility complex (MHC) to transmit the first signal. The second antigen-independent coinhibitory signal that protects against excessive immune response includes PD-1/PD-L1(PD-L2), CTLA-4/CD80(CD86), CD80/PD-L1, TIM-3/CEACAM (GAL-9), BTLA-4/HVEM and LAG-3/MHC. The second antigen-independent costimulatory signal for T lymphocyte activation includes CD40/CD40L, CD28/CD80(CD86), OX40/OX40L and CD27/CD70 (Fig. 1). To achieve an effective anti-tumor immune response, it is necessary to activate T-cells with tumor-killing effect, then the activated T-cells to immerse the tumor microenvironment, and finally the activated T-cells to kill tumor cells.21 However, tumors use a variety of strategies to attenuate T-cell-mediated attacks. An important part of this tumor escape strategy is the up-regulation of immune inhibitory mechanisms.22 The expression of immune checkpoint molecules increased in various cancers and related to the prognosis of patients.23, 24 Subsequently, we will discuss the details of various immune checkpoints.
Figure 1.
T-cell activation and inactivation are multi-signal processes. The immune system needs to maintain an optimal balance between maintaining self-tolerance and clearing tumor cells. This state is regulated by a range of receptors and ligands. T cell receptor (TCR) interacts with the major histocompatibility complex (MHC) to transmit the first signal. The second antigen-independent coinhibitory signal that protects against excessive immune response includes PD-1/PD-L1(PD-L2), CTLA-4/CD80 (CD86), CD80/PD-L1, TIM-3/CEACAM (GAL-9), BTLA-4/HVEM, LAG-3/MHCll (LSECtin) and HHLA2 (TMIGD2). The second antigen-independent costimulatory signal for T lymphocyte activation includes CD40/CD40L, CD28/CD80(CD86), OX40/OX40L and CD27/CD70.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)
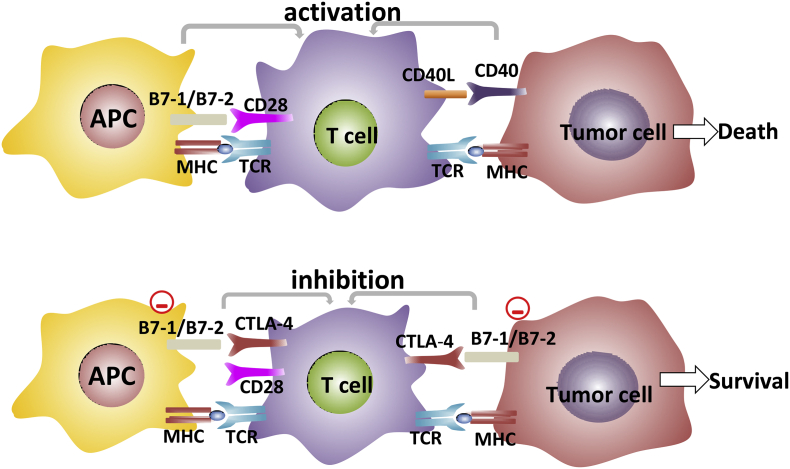
T cell receptor (TCR) interacts with the antigen peptide MHC complex to transmit the first signal of T cell activation. T cell activation also requires the pairing of co-stimulant molecules. The interaction of CD28 on T cells with either B7-1 (CD80) or B7-2 (CD86) on antigen-presenting cells (APCs) delivers the second signal of T cell activation.25 CTLA-4 is a member of CD28-B7 immunoglobulin superfamily and shares about 30% amino acid identity with CD28.26 In the early stages of T cell activation, CTLA-4 is upregulated and its expression occurs almost entirely in CD4+ and CD8+ T cells. CTLA-4 competes with the CD28 receptor for binding to CD80/CD86 ligands and has a stronger affinity than CD28.27, 28 The binding of CTLA-4 to CD80/CD86 plays a critical role in downregulating T cell activation and maintaining T cell tolerance (Fig. 2). CTLA-4 signal leads to inhibition of T cell proliferation and reduction of cytokine secretion.29, 30 Besides, CTLA-4 stimulates the production of TGF-β through B7 and inhibits the process of antigen presentation by APCs cells. Based on these facts, CTLA-4-blocking antibodies were developed. Moreover, preclinical and clinical evidence suggests that the combination of CTLA-4 with other therapeutic agents can significantly prolong patient survival with controllable safety.31, 32
Figure 2.
The TCR interacts with the antigen peptide MHC complex to transmit the first signal of T cell activation. T cells activation also requires paired stimulatory molecules. The interaction of CD28 on T cells with either B7-1 (CD80) or B7-2 (CD86) on APC has been shown to transmit the second signal of T cell activation. The interaction of CD40L on T cells with CD40 on tumor cells has also been shown to transmit the second signal of T cell activation. With the continuous stimulation of tumor antigen on T cells, CTLA-4 is expressed on the surface of T cells. It competes with the CD28 receptor for binding to CD80/CD86 ligands and has a stronger affinity than CD28. CTLA-4 binding to CD80/CD86 inhibits the activation and proliferation of T cells, induces T cell apoptosis, and leads to the immune escape of cancer cells.
The soluble form of CTLA-4 is encoded by a spliced mRNA transcript of the CTLA-4 gene lacking the transmembrane domain.33 Healthy individuals' serum contains soluble CTLA-4 (sCTLA-4), but it is higher in autoimmune diseases and cancer patients. Studies show that sCTLA-4 can bind to CD80/CD86 ligands on APC, which may play a role in regulating immune homeostasis.34, 35 Some data suggest that a better overall survival is associated with high CTLA-4 levels, and CTLA-4 antibodies have proven clinically effective in metastatic melanoma.36 In different tumor types treated with radiotherapy or chemotherapy, better OS was also associated with high serum sCTLA-4 levels.37 Maria Pia Pistillo's study showed that serum sCTLA-4 overexpression was positively correlated with favorable clinical outcomes in metastatic melanoma patients under the treatment of ipilimumab. sCTLA-4 enhances the effect of ipilimumab, perhaps sCTLA-4 prevents CTLA-4 from binding to its ligand and promotes tumor immune tolerance.38 Study evidenced sCTLA-4 might be used as a valuable biomarker for predicting the efficacy of immune checkpoint blockers (ICBs).
Programmed death-1 receptor (PD-1) and its ligands (PD-L1/PD-L2)
In 1992, Ishida et al. isolated cDNA of PD-1 and suggested that the activation of the PD-1 gene may be involved in classical programmed cell death (apoptosis).39 The study found that PD-1 is a negative regulator of adaptive immune responses, expressed on activated T and B lymphocytes, splenic dendritic cells (DCs), and natural killer (NK) cells.40, 41 PD-1 has two ligands, PD-L1 and PD-L2, also known as B7-H1 and B7-DC, both of which are type 1 transmembrane glycoproteins. The different expression patterns of PD-L1 and PD-L2 specifies their different functions. PD-L1 is up-regulated on the surface of activated T cells, B cells, DCs, macrophages, monocytes, keratinocytes, endothelial cells and myoblasts, and also at low levels in non-lymphoid organs such as the heart, placenta, Skeletal muscle, lung, liver, spleen and thymus. A few reports reveal that PD-L1 is also expressed and distributed in tumor stromal cells.42, 43 PD-L2 is only expressed in dendritic cells, macrophages, monocytes and up-regulated on the surface of activated T cells, B cells and other tissue-derived immune cells.44 Therefore, PD-L1 plays a role in maintaining peripheral tolerance, and PD-L2 plays a role in the immune response of lymph nodes. Activation of the PD-1/PD-L1 signaling pathway can inhibit the activity of effector T cells, thereby preventing the occurrence of autoimmunity, while in the tumor microenvironment, the decrease of cellular immunological function can mediate tumor immune escape. In this way, a variety of malignant tumors, such as lung cancer, melanoma, gastric cancer, pancreatic cancer, breast cancer, renal cell carcinoma, can induce the formation of the immunosuppressive tumor microenvironment, thereby escaping the body's anti-tumor immune response.45, 46, 47, 48
In recent years, tumor immunotherapy targeting PD-1: PD-L1/PD-L2 has made promising progress, but we still need to conduct in-depth studies on the combined application of multiple biomarkers. Studies have shown that high expression of PD-L1 is associated with a better outcome in non-small cell lung cancer (NSCLC) patients treated with anti-PD-1 antibodies.49 In clinical trials of melanoma anti-PD-1 antibodies, tumor patients with PD-L1-positive expression also had a better prognosis.50, 51 Another clinical trial showed that nivolumab combined with ipilimumab had better survival results than nivolumab monotherapy, while in patients with PD-L1 ≥1%, the effect of the two groups was similar, suggesting that the efficacy of anti-PD-1 antibodies were largely dependent on the expression of PD-L1.52 Preclinical studies using mouse models have demonstrated that immune checkpoints such as TIM-3 are upregulated when treated with anti-PD-1 antibody, and it may result in resistance to anti-PD-1 antibody therapy.53 In this way, patients evaluation with PD-L1 or TIM-3 as predictors will achieve better-individualized treatment than direct application of inhibitor therapy.
The detectable soluble PD-L1 (sPD-L1) in the blood is derived from the alternative variants of the PD-L1 transcripts and may be related to ICBs-mediated anti-tumor response cytokines such as IFN-α, IFN-γ, or TNF-α.54, 55 Studies have shown that elevated plasma sPD-L1 levels are associated with less clinical benefit in NSCLC patients treated with nivolumab. The results showed that patients with low plasma sPD-L1 levels achieved complete or partial response in a larger proportion than those with high plasma sPD-L1 levels. Besides, patients with low plasma sPD-L1 levels developed the progressive disease in a lower proportion than those with high plasma sPD-L1 levels.56 Wang L's study showed that plasma sPD-L1 levels may predict the treatment response and survival outcome in multiple myeloma (MM) patients, and the overall response rate of patients with low sPD-L1 was higher than that of patients with high sPD-L1 level.57 In patients with clear cell renal cell carcinoma (ccRCC), higher sPD-L1 levels are associated with increased risk of death, larger tumors, and tumor grade.58 It is speculated that sPD-L1 may damage the host's immune system, thereby promoting cancer progression and poor clinical outcomes. Therefore, plasma sPD-L1 levels may be a valuable biomarker for predicting the ICBs treatment response.
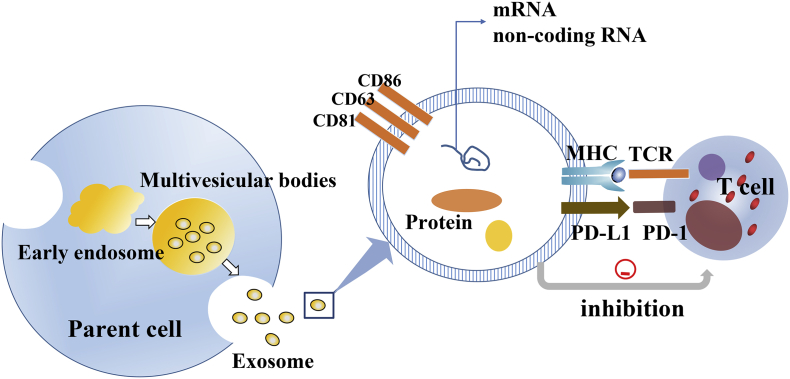
The tumor-derived fragment DNA in the blood is called circulating tumor DNA (ctDNA), and it is speculated to be associated with the passive release of dead cells and the active release of living cells.59, 60 Some studies suggested that ctDNA could be a useful marker of ICBs treatment progress. It has been reported that melanoma patients with continuously elevated ctDNA during the treatment of anti-PD-1 antibodies have worse responses. The level of ctDNA can be used as a valuable biomarker for identifying pseudoprogression and actual progression during the treatment of ICBs.61, 62 Tumor mutational burden is related to the efficacy of checkpoint inhibitor therapy. The hypermutated state assessed by ctDNA correlates with better outcomes, which may be an increased immunogenic neoantigen produced by a hypermutated genome, thereby enhancing the chance of response.63 In recent years, exosomes have been used as biomarkers for predicting clinical response. Exosomes are vesicles of 40–100 nm in diameter released by various cells, which contain proteins, RNA and DNA.64 Exosomes not only transfer membrane components but also have intercellular communication (Fig. 3). PD-L1 in exosomes released by cancer cells inhibits T cell activity by signaling via PD-1, and its level is up-regulated by IFN-γ.65 Exosomes PD-L1 are associated with the response of melanoma patients to pembrolizumab and are significantly elevated in patients who are not responding to anti-PD-1 therapy.66 Tucci et al. investigated the levels of exosome from both T-cells and the relative expression of PD-1 in metastatic melanoma patients treated with ipilimumab. They demonstrated that higher levels of PD-1 by exosome were significantly associated with improved overall survival (OS) in metastatic melanoma population.67 Studies show that exosomes containing various immune-related proteins, including PD-1, PD-L1, and CTLA-4, which may reflect potential T cell activity and serve as biomarkers for ICBs therapeutic responses.
Figure 3.
The exosome is a membranous vesicle with a diameter of about 60–100 nm, which is released into the extracellular matrix by the fusion of multiple intracellular vesicles with the cell membrane. Exosomes not only transfer membrane components but also have intercellular communication. The exosome is produced by all types of cells under physiological conditions, but tumor cells are generous producers of the exosome. Exosomes can carry DNA, mRNA, lncRNAs, immune checkpoint molecules, and microRNAs. These molecules can regulate cells in a variety of ways after being transferred to target cells.
T cell immunoglobulin domain and mucin domain 3 (TIM-3)
TIM-3, discovered by Kuchroo and colleagues in 2002, plays an important role in the induction of autoimmune diseases.68 In humans, Tim-3 is expressed on activated CD4+ and CD8+ T cells, peripheral blood monocytes, macrophages, NK cells, and some APCs. The ligands for TIM-3 include galectin-9, ceacam-1, high mobility group box-1 (HMGB-1) and phosphatidylserine.69 Inna M. Yasinska et al. demonstrated that the Tim-3/galectin-9 pathway may have an inhibitory effect on host anti-cancer immune surveillance in breast cancer and other types of cancer.70 TIM-3 levels increased during ipilimumab treatment and were associated with overall survival (OS) in melanoma patients. Melanoma patients with lower expression of inhibitory receptor TIM-3 had higher survival rates.71 Gulidanna Shayan et al. have confirmed that Tim-3 is up-regulated from persistently growing tumors in TIL with partial response to PD-1 therapy. This provides further support for a dual-targeted blockade of immunological checkpoints, such as rationally combined with blockade of Tim-3 and anti-PD-1 antibody therapy to achieve more effective cancer immunotherapy.72 Soluble Tim-3 (sTIM-3) is derived from the product of Tim-3 shed by the ADAM 10/17 proteolytic enzymes. Isabel Gonçalves Silva's study showed that sTim-3 could bind to target proteins and attenuate IL-2 production, thereby reducing the anti-cancer activity of T lymphocytes.73
Lymphocyte-activation gene 3 (LAG-3)
Lymphocyte-activation gene 3 (LAG-3) is a novel transmembrane protein discovered in the 1990s, which has a similar domain structure and 20% sequence homology with an affinity 100 times higher than CD4.74, 75 LAG-3 expressing cell mainly was natural killer cells (NK), activated T cells, peripheral Tregs, and tumor-infiltrating lymphocytes.76, 77 LAG-3 interacts with major histocompatibility complex 2 (MHC class II) expressed on APC and sinusoidal endothelial cell lectin (LSECtin) expressed in several tumor subtypes, which may result in down-regulation of immune response.78, 79, 80 Studies have shown that LAG-3 and PD-1 inhibition pathways have synergistic effects to promote immune tolerance, T cell dysfunction, and T cell exhaustion.81, 82 Hence, a combination of anti-LAG3 antibodies with anti-PD-1 antibodies is a promising cancer treatment strategy compared with single immunological checkpoint treatment.83 It has been reported that anti-LAG-3 (BMS986016) plus anti-PD-1 (nivolumab) combination improves the therapeutic effect of the melanoma population resistant to PD-1 therapy.84 In particular, soluble LAG-3 (sLAG-3), unlike LAG-3, can cause DCs to mature and attack tumor cells. Frédéric Triebel's findings suggest that high levels of serum Th1 activity marker sLAG-3 may be associated with increased survival in certain subpopulations of breast cancer patients.85
Human endogenous retrovirus-H long terminal repeat-associating 2 (HHLA2)
As a member of the B7 family, HHLA2 plays an important role in T cell proliferation and cytokine production. Ruihua Zhao's research found that HHLA2 reduced the production of IFN-γ, TNF-α, IL-5, IL-10, IL-13, IL-17A, and IL-22.86 In humans, HHLA2 is expressed on monocytes and induced on B cells after stimulation with IFN-γ.86 HHLA2 interacts with transmembrane and immunoglobulin domain containing 2 (TMIGD2) (expressed on naïve T cells as well as dendritic cells, monocytes, and B cells), which may result in down-regulation of immune response.87 HHLA2 and TMIGD2 are both absent in mice but present in humans and primates. HHLA2 expression is limited in normal tissues and is only expressed in epithelial cells in some tissues. However, HHLA2 is highly expressed in most malignant tumor tissues. Koirala P et al. have confirmed that HHLA2 is expressed in the majority of osteosarcoma. Osteosarcoma patients with higher expression of T cell co-inhibitory HHLA2 are associated with metastatic disease and poorer survival.88 Studies have shown that the HHLA2 may play an important role in lung cancer, especially in tumors that are PD-L1-negative or escape PD-1/PD-L1 blockade.89 HHLA2 may become a new target for cancer therapy, but its expression and receptor recognition need to be further studied.
B7 homolog 3 protein (B7-H3)
B7 homolog 3 protein (B7-H3) is a novel transmembrane protein discovered by Lieping Chen in 2001.90 However, the B7-H3 binding partner is still unknown. One study has found that B7-H3 activates tumor-specific cytotoxic T lymphocytes (CTL), thereby enhancing anti-tumor immunity.91 Another group argues that B7-H3 inhibits the function of T cells and NK cells, and reduces IL-2, IL-12, and IFN-γ, thereby causing tumor immune escape.92 Hence, B7-H3 expressed on T cells, NK cells and APC has a dual role, it is a negative regulator of adaptive immune responses with a partial co-stimulation.92 Xingxing Zang's study showed that strong B7-H3 expression on prostate cancer tumor cells is associated with an increased risk of clinical cancer recurrence and cancer-specific death.93 In patients with non-small cell lung cancer (NSCLC), higher B7-H3 protein levels are also associated with poor survival.94 Most studies suggest that B7-H3 expression is associated with poor prognosis although it has a partial costimulatory function. The detectable soluble B7-H3 (sB7-H3) in serum/plasma is derived from the release of monocytes, DCs, activated T cells, and various mB7-H3+ carcinoma cells. It is speculated that sB7-H3 may as an active form affect cell–-cell interactions by binding to the B7-H3 receptor (B7-H3R) on activated T cells.95 However, whether sB7-H3 can be used as a valuable predictive biomarker needs further research. Currently, a phase I study of enoblituzumab (a mAb reactive to B7-H3) is underway. It enhances the antitumor function in patients whose tumors overexpress B7-H3 through effective antibody-dependent cell-mediated cytotoxicity (ADCC).
B7 homolog 4 protein (B7-H4)
B7-H4 (also known as B7S1, B7x) is a negative checkpoint ligand, and its putative receptor may be expressed on activated human T cells. Study finds that B7-H3 inhibits T cell proliferation and cytokine production by arresting cell cycle progression of T cells at a relatively early stage.96 Although B7-H4 mRNA is expressed in most tissues, B7-H4 protein is predominantly detected in tumor cells of human cancer tissues (such as renal cell carcinoma, melanoma, and kidney cancer).97, 98 Studies have shown that high expression of B7-H4 is correlated with poor prognosis in patients with renal cell carcinoma and prostate cancer, and may become a valuable prognostic marker against them.93, 99 Lei Wu et al. demonstrate that in patients with oral squamous cell carcinoma, higher B7-H4 expression is associated with poor patient outcome and pathological features. Their research data also indicate that B7-H4 may be a novel target for molecular targeted therapy.100 In patients with renal cell carcinoma and ovarian cancer, soluble B7-H4 (sB7-H4) can be detected in serum/plasma, which can partially inhibit the production of allogeneic cytolytic T cells (CTL) in vitro.100, 101, 102 Several studies showed that serum sB7-H4 overexpression was correlated with poor clinical outcomes in non-small cell lung cancer, hepatocellular carcinoma and osteosarcoma.103, 104, 105 Serum sB7-H4 has proven to be a valuable prognostic marker for patients with non-metastatic clear cell renal cell carcinoma.106 Currently, phase 1a/1b clinical trial study of FPA150 (first-in-class B7-H4 antibody) is underway. It enhances the antitumor function in patients whose tumors overexpress B7-H4 through effective antibody-dependent cell-mediated cytotoxicity (ADCC).
Immune checkpoint blockers(ICBs)
Cytotoxic T-lymphocyte-associated antigen (CTLA)-4 blockade
Ipilimumab (Yervoy), a fully humanized IgG1kappa monoclonal antibody, has a high-affinity binding to the extracellular domain of CTLA-4. Different from ipilimumab, tremelimumab is an IgG2kappa monoclonal antibody. Ipilimumab and tremelimumab bind to the same region on CTLA-4 with similar binding affinity, and there is no substantial difference in preclinical data between the two drugs.107 They blockade the interaction between CTLA-4 and B7-1 (or B7-2), thereby restoring conventional T cell reactivity toward the malignant cell (Fig. 4). On October 28, 2015, ipilimumab is approved by the FDA for the treatment of resected stage III melanoma and advanced unresectable melanoma (Fig. 5).108 Multiple studies have shown that the clinical activity of ipilimumab in combination therapy is encouraging. James Larkin's study found that combinations of nivolumab and ipilimumab for melanoma had longer progression-free survival and higher objective response rates than ipilimumab alone. Results: the median progression-free survival was 11.5 months for nivolumab combined with ipilimumab, 2.9 months for ipilimumab alone, and 6.9 months for nivolumab alone.109 Besides, clinical trials of ipilimumab for renal cell carcinoma, prostate cancer, urothelial carcinoma, and ovarian cancer are underway. In the ongoing phase 3, nivolumab combined with ipilimumab is more effective than sunitinib in treating advanced renal cell carcinoma, with higher overall survival and lower incidence of immune-related adverse events.110, 111 On April 16, 2018, the FDA approved the combination of nivolumab and ipilimumab for the treatment of advanced renal cell carcinoma (Fig. 5).111 H-J J Lenz's research results suggested that low-dose ipilimumab plus nivolumab had promising clinical efficacy and tolerance as first-line therapy for patients with microsatellite instable-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC).112 On July 11, 2018, the combination of nivolumab and ipilimumab were approved by FDA for the treatment of MSI-H/dMMR mCRC which would progress after chemotherapy (Fig. 5).
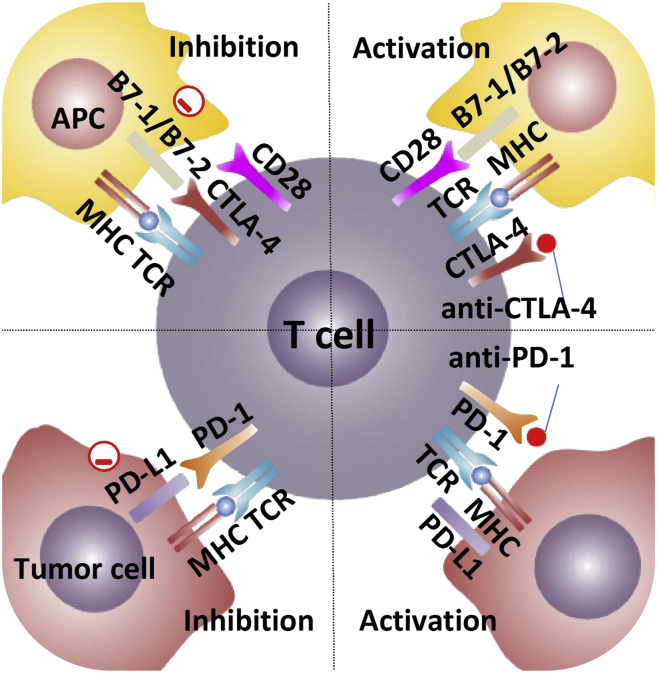
Figure 4.
Anti-CTLA-4 monoclonal antibodies have a very high affinity for CTLA-4, which reduce T cell exhaustion and reinvigorate the anti-tumor response by blocking the interaction between inhibitory receptors CTLA-4 on effector T cells and its ligands B7-1 (CD80) and B7-2 (CD86) on APC. Anti-PD-1 monoclonal antibodies bind to PD-1 with high affinity, blocking its interactions with PD-L1(B7-H1) and PD-L2 (B7-DC).
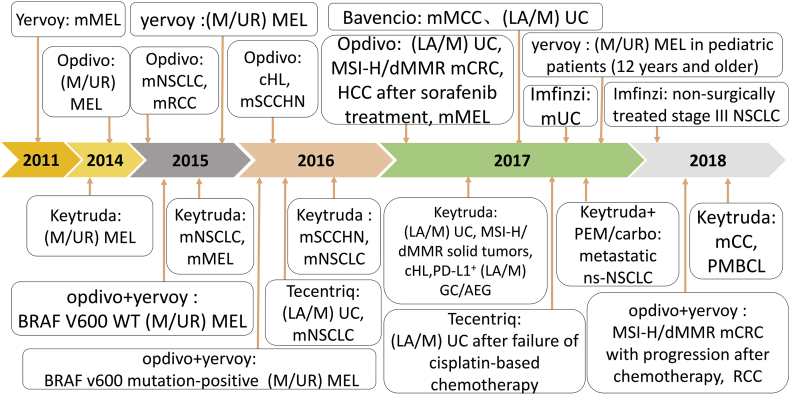
Figure 5.
Timeline diagram of FDA-approved immune checkpoint drugs. Abbreviations: renal cell carcinoma (RCC); gastric cancer (GC); non-small Cell Lung Cancer (NSCLC); metastatic Merkel cell carcinoma (mMCC); locally advanced or metastatic (LA/M); non-squamous non-small cell lung cancer (ns-NSCLC); adenocarcinoma of the esophagogastric junction (AEG); cervical cancer (CC); primary mediastinal large B-cell lymphoma (PMBCL); urothelial carcinoma (UC); classic Hodgkin lymphoma (cHL); hepatocellular carcinoma (HCC); metastatic melanoma (mMEL); squamous cell carcinoma of the head and neck (SCCHN); wild-type (WT); unresectable or metastatic (M/UR).
Programmed death (PD-1) blockade
Nivolumab (Opdivo), a completely human immunoglobulin (Ig) G4 monoclonal antibody that binds PD-1 with high affinity and blocks its interactions with both PD-L1(B7-H1) and PD-L2 (B7-DC)113(Fig. 4). Nivolumab has good anti-tumor activity and a favorable safety profile. Caroline Robert's study shows that nivolumab has a promising effect in treating patients with metastatic melanoma who have no BRAF mutations. The results showed that compared with BRAF inhibitors (dacarbazine), the median progression-free survival of the nivolumab group was 5.1 months and the objective response rate was 40.0%, while the median progression-free survival of the dacarbazine group was 2.2 months and the objective response rate was 13.9%.114, 115 On December 22, 2014, Nivolumab was approved by the FDA for the treatment of unretractable or metastatic advanced melanoma (Fig. 5).113 However, BRAF mutations are widely present in metastatic melanoma and have a low response rate in the treatment of BRAF inhibitor monotherapy. Currently, there is clinical evidence that BRAF inhibitors, such as dacarbazine, can be combined with checkpoint blockade.116 In the preclinical model, significant synergistic effects in inhibiting melanoma growth were observed not only in the triple combination of dabrafenib, trametinib, and anti-PD-1 treatment but also in the combination of dabrafenib or trametinib plus anti-PD1 therapy.117 On January 13, 2016, the FDA approved the combination of nivolumab and ipilimumab for the treatment of BRAFV600 wild-type and BRAFV600 mutation-positive unresectable or metastatic melanoma (Fig. 5). Nivolumab was approved by the Food and Drug Administration (FDA) for patients with melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), classical Hodgkin's lymphoma, head and neck squamous cell carcinoma (HNSCC), metastatic urothelial carcinoma (UC) and MSI-H/dMMR mCRC118, 119, 120, 121 (Table 1). Among these indications, nivolumab and ipilimumab are combined in patients with MSI-H/dMMR mCRC who have progressed after chemotherapy, medium-high risk advanced renal cell carcinoma and BRAFV600 mutation-positive melanoma. On June 15, 2018, Opdivo was launched in China, becoming the first PD-1 therapy on the market in China.
Table 1.
Overview of the included studies and immune checkpoint blockers.
| Target | ICBs | Trade name | FDA approved indications |
|---|---|---|---|
| CTLA-4 | Ipilimumab | Yervoy | Resected stage III melanoma and advanced unresectable melanoma. |
| PD-1 | Nivolumab | Opdivo | Melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), classical Hodgkin's lymphoma, head and neck squamous cell carcinoma (HNSCC), metastatic urothelial carcinoma (UC) and microsatellite instable-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC). |
| Pembrolizumab | Keytruda | Metastatic advanced melanoma, metastatic NSCLC, metastatic HNSCC, locally advanced or metastatic UC, advanced cervical cancer, metastatic gastric cancer with PD-L1 positive, primary mediastinal B-cell lymphoma (PMBCL) and MSI-H/dMMR solid tumors. | |
| PD-L1 | Atezolizumab | Tecentriq | Locally advanced or metastatic UC and metastatic NSCLC with progression on or after platinum-based chemotherapy. |
| Durvalumab | Imfinzi | Locally advanced or metastatic UC and stage III NSCLC who did not have disease progression after a platinum-based chemotherapy. | |
| Avelumab | Bavencio | Adults and pediatric patients 12 years and older with metastatic Merkel-cell carcinoma (MCC) and locally advanced or metastatic UC. |
Pembrolizumab (Keytruda) is a potent, highly selective, fully humanized IgG4 isotype antibody against PD-1 with excellent safety, tolerance, and effectiveness.122 The anti-PD-1 antibodies not only has significant antitumor activity in patients with advanced melanoma, lung carcinoma, and hematologic malignancies but also has an acceptable side-effect profile.123 At present, PD-1 combined with chemotherapy has a promising performance in the treatment of many types of tumors. In the phase 2 trial, adding pembrolizumab to chemotherapy significantly increased the response rate and prolonged progression-free survival compared with chemotherapy alone.49 Pembrolizumab was approved by FDA for patients with metastatic advanced melanoma, metastatic NSCLC, metastatic HNSCC, metastatic urothelial carcinoma (UC), advanced cervical cancer, metastatic gastric cancer with PD-L1 positive, primary mediastinal B-cell lymphoma (PMBCL) and MSI-H/dMMR solid tumors33, 124, 125, 126, 127 (Table 1). Pembrolizumab is the first anti-PD-1 inhibitors approved in the United States and is eligible for the FDA's “breakthrough therapeutic”.
Programmed death-ligand 1 (PD-L1) blockade
Atezolizumab (Tecentriq) is a high-affinity, fully human IgG1 monoclonal antibody that inhibits the interaction of PD-L1 with both PD-1 and CD80 receptors on the surface of tumor cells and tumor-infiltrating myeloid cells.128 By blocking the PD-L1/PD-1 pathway, atezolizumab can reduce T cell exhaustion and downstream inhibition of cytokines, thereby reinvigorating the anti-tumor response. In vitro studies have shown that treatment with atezolizumab may cause cytokine changes, including a transient increase in IL-18, IFNγ, and CXCL11, and a transient decrease in IL-6. Atezolizumab is the first PD-L1 inhibitor approved by the FDA for metastatic UC and metastatic NSCLC with progression on or after platinum-based chemotherapy (Table 1).129, 130 Atezolizumab is generally well tolerated and the possible irAEs are fatigue, decreased appetite, dyspnea, cough, nausea. Serious irAEs are rare, including pneumonitis, hepatitis, endocrinopathies, and colitis. Based on the severity of these events, determine whether atezolizumab should be withheld or discontinued permanently.131 To maximize the potential of atezolizumab, research should focus on rational combinatorial therapeutic strategies and potent biomarkers. Nowadays, many trials of PD-L1 inhibitors in combination with chemotherapy, radiotherapy, and immune checkpoint inhibitors are underway. Studies have shown that the addition of PD-L1 inhibitor atezolizumab to MEK inhibitors cobimetinib and BRAF inhibitor vemurafenib results in a manageable safety profile and promising anti-tumor activity in BRAF mutant melanoma patients.132, 133 MEK inhibitors and Atezolizumab were combined in the Ib phase study of patients with microsatellite-stabilized colorectal cancer, and the results supported the continued evaluation of the combination.134
Durvalumab (Imfinzi) is a fully-humanized IgG1kappa monoclonal antibody that blocks the binding of PD-L1 to PD-1 and CD80 receptors, thereby reducing immunosuppression signals in the tumor microenvironment and overcoming the inhibition of T cell activation.135 Durvalumab does not block the interaction of PD-L2 with PD-1.136 Scott J. Antonia's study shows that durvalumab treatment has a longer progression-free survival than placebo treatment in stage III non-small cell lung cancer.137 On February 16, 2018, durvalumab was certified by the FDA as a breakthrough therapy for the treatment of stage III NSCLC who did not have disease progression after a platinum-based chemotherapy (Fig. 5). However, durvalumab's response rate was limited in PD-L1− tumors. David Planchard's research data suggest that combined durvalumab and the anti-CTLA-4 antibody tremelimumab may be beneficial to PD-L1− tumor patients.138
Avelumab (Bavencio) is a whole monoclonal antibody of IgG1 that blocking the formation of PD-1/PDL1 ligand pairs. Unlike most PD-1/PD-L1 antibodies, avelumab has strong antibody-dependent cell-mediated cytotoxicity (ADCC) activity. Due to its powerful ADCC activity, avelumab not only prevents the immune escape of cancer cells but also mediates NK cells to kill cancer cells. Through ADCC's killing mechanism, avelumab is less affected by TIM-3 upwards, theoretically avoiding the switch to other immune checkpoint inhibitors due to drug resistance.139 The ongoing prospective study assessed whether the combination of two ADCC-induced monoclonal antibodies cetuximab and avelumab could generate beneficial immune effects on metastatic colorectal cancer (CRC) and metastatic squamous cell carcinoma of the head and neck (SCCHN).140 In 2017, Avelumab was approved by FDA for patients with Merkel-cell carcinoma (MCC) and locally advanced or metastatic urothelial carcinoma (Fig. 5).141, 142
Conclusion
The immune checkpoint is the immune system's regulator, which enables the body to maintain self-tolerance while responding effectively to protects the body against foreign materials. Many types of tumor cells can escape the elimination of the immune system with the help of inhibitory checkpoints. Therefore, one of the crucial methods for treating a wide range of tumors is immunotherapy with immune checkpoint blockers (ICBs). Immune checkpoint blockers can “release the brakes” of the immune system, activate T cell-mediated immune responses, thereby restoring immune system function. FDA-approved drugs have shown positive effects on melanoma, non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCCA), and renal cell carcinoma (RCC). In recent years, many preclinical or clinical focuses on cognitive combination therapy to improve the response rate of ICBs and reduce immune-related adverse reactions (irAEs). Studies have shown that these immune checkpoint pathways may be relatively unique and non-redundant, which provides a clinical basis for blocking multiple checkpoints to enhance anti-tumor immunity. However, given the limitations of tumor heterogeneity and the lack of potent antibodies, biomarkers such as ctDNA and exosome are crucial to predict the effectiveness of patients in ICBs treatment. So far, the relevant mechanisms and developed drugs are only a small part of immunotherapy and need further research.
Conflict of Interests
The authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (81872200, 31900558), the Natural Science Foundation of Hubei Province (2018CFB510), the Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (CXPY2017029), and the Fundamental Research Funds for the Central Universities (2042018kf0091).
Author contributions
H.Jiang drafted the manuscript. G. Liu and Y. Li reviewed and edited the manuscript. Y. Pan conceived of the study, and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yirong Li, Email: liyirong201705@163.com.
Yunbao Pan, Email: panyunbao@outlook.com.
References
- 1.Lizée G., Overwijk W.W., Radvanyi L., Gao J., Sharma P., Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2013;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- 2.Kean L.S., Turka L.A., Blazar B.R. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy. Immunol Rev. 2017;276(1):192–212. doi: 10.1111/imr.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janakiram M., Shah U.A., Liu W., Zhao A., Schoenberg M.P., Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276(1):26–39. doi: 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni L., Dong C. New checkpoints in cancer immunotherapy. Immunol Rev. 2017;276(1):52–65. doi: 10.1111/imr.12524. [DOI] [PubMed] [Google Scholar]
- 5.Andrews L.P., Marciscano A.E., Drake C.G., Vignali D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M., Zhu C., Kuchroo V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 8.Nurieva R., Thomas S., Nguyen T. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25(11):2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai H.-F., Hsu P.-N. Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci. 2017;24(1):e35. doi: 10.1186/s12929-017-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn S.D., Shin H., Haining W.N. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A., Puzanov I., Dummer R. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucca L.E., Hafler D.A. Co-inhibitory blockade while preserving tolerance: checkpoint inhibitors for glioblastoma. Immunol Rev. 2017;276(1):9–25. doi: 10.1111/imr.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tison A., Quéré G., Misery L. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a Nationwide Multicenter Cohort study. Arthritis Rheumatol. 2019;71(12):2100–2111. doi: 10.1002/art.41068. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J.C., Chen W.D., Alvarez J.B. Cancer immune checkpoint blockade therapy and its associated autoimmune cardiotoxicity. Acta Pharmacol Sin. 2018;39(11):1693–1698. doi: 10.1038/s41401-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabie N., Lichtman A.H., Padera R. T cell checkpoint regulators in the heart. Cardiovasc Res. 2019;115(5):869–877. doi: 10.1093/cvr/cvz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasagi S., Kawano S., Kumagai S. PD-1 and autoimmunity. Crit Rev Immunol. 2011;31(4):265–295. doi: 10.1615/critrevimmunol.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Vignali D.A. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity. 2016;44(5):1034–1051. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28(8):383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruse Y., Kawano S., Jinno T. Significant association of increased PD-L1 and PD-1 expression with nodal metastasis and a poor prognosis in oral squamous cell carcinoma. Int J Oral. 2018;47(7):836–845. doi: 10.1016/j.ijom.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Nakano M., Ito M., Tanaka R. PD-1+ TIM-3+ T cells in malignant ascites predict prognosis of gastrointestinal cancer. Cancer Sci. 2018;109(9):2986–2992. doi: 10.1111/cas.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linsley P.S., Clark E.A., Ledbetter J.A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walunas T.L., Lenschow D.J., Bakker C.Y. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 27.Callahan M.K., Postow M.A., Wolchok J.D. Antibodies to stimulate host immunity: lessons from ipilimumab. In: Cancer Immunotherapy. ed. Elsevier; 2013:287–307. [Google Scholar]
- 28.Soskic B., Qureshi O.S., Hou T., Sansom D.M. A transendocytosis perspective on the CD28/CTLA-4 pathway. Adv Immunol. 2014;124:95–136. doi: 10.1016/B978-0-12-800147-9.00004-2. [DOI] [PubMed] [Google Scholar]
- 29.Azuma M., Ito D., Yagita H. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 30.Allemani C., Weir H.K., Carreira H. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grosso J.F., Jure-Kunkel M.N. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immunity Arch. 2013;13(1):e5. [PMC free article] [PubMed] [Google Scholar]
- 32.Gubens M.A., Sequist L.V., Stevenson J. Phase I/II study of pembrolizumab (pembro) plus ipilimumab (Ipi) as second-line therapy for NSCLC: KEYNOTE-021 cohorts D and H. Am Soc Clin Oncol. May 20, 2016;34(15_suppl) 9027–9027. [Google Scholar]
- 33.Prasad V., Kaestner V., Mailankody S. Cancer drugs approved based on biomarkers and not tumor type—FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol. 2018;4(2):157–158. doi: 10.1001/jamaoncol.2017.4182. [DOI] [PubMed] [Google Scholar]
- 34.Magistrelli G., Jeannin P., Herbault N. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29(11):3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Sato S., Fujimoto M., Hasegawa M. Serum soluble CTLA-4 levels are increased in diffuse cutaneous systemic sclerosis. Rheumatology. 2004;43(10):1261–1266. doi: 10.1093/rheumatology/keh303. [DOI] [PubMed] [Google Scholar]
- 36.Liu H., Pan Y., Meng S., Zhang W., Zhou F. Current treatment options of T cell-associated immunotherapy in multiple myeloma. Clin Exp Med. 2017;17(4):431–439. doi: 10.1007/s10238-017-0450-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q., Hu P., Deng G. Soluble cytotoxic T-lymphocyte antigen 4: a favorable predictor in malignant tumors after therapy. OncoTargets Ther. 2017;10:2147–2154. doi: 10.2147/OTT.S128451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pistillo M.P., Fontana V., Morabito A. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother. 2019;68(1):97–107. doi: 10.1007/s00262-018-2258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao S., Wang S., Zhu Y. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113(23):5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan S.S., Akram M., King E.C., Dockrell H.M., Cliff J.M. PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS One. 2015;10(9):e0137646. doi: 10.1371/journal.pone.0137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu K., Kryczek I., Chen L., Zou W., Welling T.H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma W., Luo D.Z., Chen Y., Dang Y. Expression and clinical significance of PD-L1 and PD-1 in non-small cell lung cancer. J Pract Med. 2011;27(9):1551–1554. [Google Scholar]
- 44.Dzik S. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin 10 secretion. Transfus Med Rev. 2000;3(14):285. [Google Scholar]
- 45.Ji M., Liu Y., Li Q. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015;13(1):e5. doi: 10.1186/s12967-014-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bigelow E., Bever K.M., Xu H. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013;(71):e4059. doi: 10.3791/4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J.W., Nam K.H., Ahn S.-H. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19(1):42–52. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 48.Mittendorf E.A., Philips A.V., Meric-Bernstam F. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi L., Rodríguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 50.Weber J.S., D'Angelo S.P., Minor D. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 51.Daud A.I., Wolchok J.D., Robert C. Programmed death-ligand 1 expression and response to the anti–programmed death 1 antibody Pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolchok J.D., Chiarion-Sileni V., Gonzalez R. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koyama S., Akbay E.A., Li Y.Y. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:e10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J., Mahoney K.M., Giobbie-Hurder A. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossille D., Gressier M., Damotte D. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 56.Okuma Y., Wakui H., Utsumi H. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):410–417. doi: 10.1016/j.cllc.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Wang L., Wang H., Chen H. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6(38):41228–41236. doi: 10.18632/oncotarget.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frigola X., Inman B.A., Lohse C.M. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17(7):1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 60.Stroun M., Lyautey J., Lederrey C., Olson-Sand A., Anker P. About the possible origin and mechanism of circulating DNA: apoptosis and active DNA release. Clin Chim Acta. 2001;313(1–2):139–142. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 61.Lee J.H., Long G.V., Menzies A.M. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti–programmed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717–721. doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J., Long G., Boyd S. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol. 2017;28(5):1130–1136. doi: 10.1093/annonc/mdx026. [DOI] [PubMed] [Google Scholar]
- 63.Khagi Y., Goodman A.M., Daniels G.A. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor–based immunotherapy. Clin Cancer Res. 2017;23(19):5729–5736. doi: 10.1158/1078-0432.CCR-17-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simons M., Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Del Re M., Marconcini R., Pasquini G. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–824. doi: 10.1038/bjc.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G., Huang A.C., Zhang W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tucci M., Passarelli A., Mannavola F. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. OncoImmunology. 2018;7(2):e1387706. doi: 10.1080/2162402X.2017.1387706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monney L., Sabatos C.A., Gaglia J.L. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 69.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasinska I.M., Sakhnevych S.S., Pavlova L. The Tim-3-galectin-9 pathway and its regulatory mechanisms in human breast cancer. Front Immunol. 2019;10:e1594. doi: 10.3389/fimmu.2019.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tallerico R., Cristiani C.M., Staaf E. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. OncoImmunology. 2017;6(2):e1261242. doi: 10.1080/2162402X.2016.1261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shayan G., Srivastava R., Li J., Schmitt N., Kane L.P., Ferris R.L.J.O. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. OncoImmunology. 2017;6(1):e1261779. doi: 10.1080/2162402X.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva I.G., Yasinska I.M., Sakhnevych S.S. The Tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Triebel F., Jitsukawa S., Baixeras E. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber S., Karjalainen K. Mouse CD4 binds MHC class II with extremely low affinity. Int Immunol. 1993;5(6):695–698. doi: 10.1093/intimm/5.6.695. [DOI] [PubMed] [Google Scholar]
- 76.Baixeras E., Huard B., Miossec C. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176(2):327–337. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Workman C.J., Rice D.S., Dugger K.J., Kurschner C., Vignali D.A. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32(8):2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 78.Huard B., Prigent P., Pagès F., Bruniquel D., Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26(5):1180–1186. doi: 10.1002/eji.1830260533. [DOI] [PubMed] [Google Scholar]
- 79.Xu F., Liu J., Liu D. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74(13):3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- 80.Yang J., Wang H., Wang M. Involvement of LSECtin in the hepatic natural killer cell response. Biochem Biophys Res Commun. 2016;476(1):49–55. doi: 10.1016/j.bbrc.2016.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P. Tumor-infiltrating NY-ESO-1–specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woo S.-R., Turnis M.E., Goldberg M.V. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ascierto P., Bono P., Bhatia S. LBA18Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann Oncol. 2017;28(suppl l_5) [Google Scholar]
- 84.Ascierto P.A., Melero I., Bhatia S. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (Nivo) in Pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. Am Soc Clin Oncol. May 20, 2017;35(15_suppl) 9520–9520. [Google Scholar]
- 85.Triebel F., Hacene K., Pichon M.F. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 2006;235(1):147–153. doi: 10.1016/j.canlet.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 86.Zhao R., Chinai J.M., Buhl S. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janakiram M., Chinai J.M., Fineberg S. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(10):2359–2366. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koirala P., Roth M.E., Gill J. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:srep31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schalper K.A., Carvajal-Hausdorf D., McLaughlin J. Differential expression and significance of PD-L1, Ido-1, and B7-H4 in human lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(2):370–378. doi: 10.1158/1078-0432.CCR-16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chapoval A.I., Ni J., Lau J.S. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 91.Hofmeyer K.A., Ray A., Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105(30):10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suh W.-K., Gajewska B.U., Okada H. The B7 family member B7-H3 preferentially down-regulates T helper type 1–mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 93.Zang X., Thompson R.H., Al-Ahmadie H.A. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Altan M., Pelekanou V., Schalper K.A. B7-H3 expression in NSCLC and its association with B7-H4, PD-L1 and tumor-infiltrating lymphocytes. Clin Cancer Res. 2017;23(17):5202–5209. doi: 10.1158/1078-0432.CCR-16-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang G., Hou J., Shi J., Yu G., Lu B., Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Y., Cui C., Su M. Skint8, a novel B7 family-related molecule, negatively regulates T cell responses. J Immunol. 2019;203(2):400–407. doi: 10.4049/jimmunol.1800639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zang X., Allison J.P. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13(18):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 98.Quandt D., Fiedler E., Boettcher D., Marsch W.C., Seliger B. B7-h4 expression in human melanoma: its association with patients' survival and antitumor immune response. Clin Cancer Res. 2011;17(10):3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 99.Krambeck A.E., Thompson R.H., Dong H. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu L., Deng W.-W., Yu G.-T. B7-H4 expression indicates poor prognosis of oral squamous cell carcinoma. Cancer Immunol Immunother. 2016;65(9):1035–1045. doi: 10.1007/s00262-016-1867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson R.H., Zang X., Lohse C.M. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68(15):6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simon I., Zhuo S., Corral L. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66(3):1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Xu C., Wang Y., Yu L., Zhang X. Prognostic values of B7-H4 in non-small cell lung cancer. Biomarkers. 2016:1–16. doi: 10.1080/1354750X.2016.1203997. [DOI] [PubMed] [Google Scholar]
- 104.Zhang C., Li Y., Wang Y. Diagnostic value of serum B7-H4 for hepatocellular carcinoma. J Surg Res. 2015;197(2):301–306. doi: 10.1016/j.jss.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 105.Dong Q., Ma X. B7-H4 expression is associated with tumor progression and prognosis in patients with osteosarcoma. BioMed Res Int. 2015;2015:156432. doi: 10.1155/2015/156432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Azuma T., Sato Y., Ohno T., Azuma M., Kume H. Serum soluble B7-H4 is a prognostic marker for patients with non-metastatic clear cell renal cell carcinoma. PLoS One. 2018;13(7):e0199719. doi: 10.1371/journal.pone.0199719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He M., Chai Y., Qi J. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8(40):67129–67139. doi: 10.18632/oncotarget.18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Padda A., Schiopu E., Sovich J., Ma V., Alva A., Fecher L. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6(1):e12. doi: 10.1186/s40425-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Motzer R.J., Tannir N.M., McDermott D.F. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cella D., Grünwald V., Escudier B. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20(2):297–310. doi: 10.1016/S1470-2045(18)30778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lenz H.J., Van Cutsem E., Limon M. LBA18_PR Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) Ann Oncol. 2018;29(suppl l_8) mdy424. [Google Scholar]
- 113.Force J., Salama A.K. First-line treatment of metastatic melanoma: role of nivolumab. ImmunoTargets Ther. 2017;6:1–10. doi: 10.2147/ITT.S110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robert C., Long G.V., Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 115.Long G.V., Atkinson V., Ascierto P.A. Nivolumab Improved Survival vs Dacarbazine in Patients with Untreated Advanced Melanoma. J Transl Med. 2015;13(Suppl 1):06. [Google Scholar]
- 116.Griffin M., Scotto D., Josephs D.H. BRAF inhibitors: resistance and the promise of combination treatments for melanoma. Oncotarget. 2017;8(44):78174–78192. doi: 10.18632/oncotarget.19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu-Lieskovan S., Mok S., Moreno B.H. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAFV600E melanoma. Sci Transl Med. 2015;7(279) doi: 10.1126/scitranslmed.aaa4691. 279ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferris R.L., Blumenschein G., Jr., Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. J N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McDermott J., Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today. 2015;51(1):7–20. doi: 10.1358/dot.2015.51.1.2250387. [DOI] [PubMed] [Google Scholar]
- 123.Martin-Liberal J., Kordbacheh T., Larkin J. Safety of pembrolizumab for the treatment of melanoma. Expert Opin Drug Saf. 2015;14(6):957–964. doi: 10.1517/14740338.2015.1021774. [DOI] [PubMed] [Google Scholar]
- 124.Antonia S., Goldberg S.B., Balmanoukian A. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barone A., Hazarika M., Theoret M.R. FDA approval summary: pembrolizumab for the treatment of patients with unresectable or metastatic melanoma. Clin Cancer Res. 2017;23(19):5661–5665. doi: 10.1158/1078-0432.CCR-16-0664. [DOI] [PubMed] [Google Scholar]
- 126.Fashoyin-Aje L., Donoghue M., Chen H. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103–109. doi: 10.1634/theoncologist.2018-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lim S.H., Sun J-M, Lee S-H, Ahn J.S., Park K., Ahn M-J. 2016. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Opin Biol Ther. 16(3):397–406. [DOI] [PubMed] [Google Scholar]
- 128.Syn N.L., Teng M.W., Mok T.S., Soo R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 129.Redman J.M., Madan R.A. 18th ed. Taylor & Francis; 2018. Spotlight on Atezolizumab and Its Potential as an Oncology Agent; pp. 719–722. Vol. 18. [DOI] [PubMed] [Google Scholar]
- 130.Liu E., Guha A., Jia K. Cardiogenic shock in a patient being treated with atezolizumab for metastatic non-small cell lung cancer. Lung Cancer. 2017;114:106–107. doi: 10.1016/j.lungcan.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 131.Riudavets M., Barba A., Maroto P. Correlation between Immune-Related Adverse Events (irAEs) and Efficacy in Patients with Solid Tumors Treated with Immune-checkpoints Inhibitors (ICIs) American Society of Clinical Oncology; 2018. 36, no. 15_suppl 3064–3064. [Google Scholar]
- 132.Sullivan R.J., Hamid O., Gonzalez R. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. 2019;25(6):929–935. doi: 10.1038/s41591-019-0474-7. [DOI] [PubMed] [Google Scholar]
- 133.Hwu P., Hamid O., Gonzalez R. Preliminary safety and clinical activity of atezolizumab combined with cobimetinib and vemurafenib in BRAF V600-mutant metastatic melanoma. Eur Soc Med Oncol. 1 October 2016;27(suppl_6) 1109PD. [Google Scholar]
- 134.Bendell J.C., Kim T.W., Goh B.C. Clinical activity and safety of cobimetinib (Cobi) and atezolizumab in colorectal cancer (CRC) Am Soc Clin Oncol. May 20, 2016;34(15_suppl) 3502–3502. [Google Scholar]
- 135.Syed Y.Y. Durvalumab: first global approval. Drugs. 2017;77(12):1369–1376. doi: 10.1007/s40265-017-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stewart R., Morrow M., Hammond S.A. Identification and characterization of MEDI4736, an antagonistic anti–PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 137.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 138.Planchard D., Yokoi T., McCleod M.J. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: Rationale and Protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232–236. doi: 10.1016/j.cllc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Kim E.S. Avelumab: first global approval. Drugs. 2017;77(8):929–937. doi: 10.1007/s40265-017-0749-6. [DOI] [PubMed] [Google Scholar]
- 140.Ferris R.L., Lenz H.-J., Trotta A.M. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. 2018;63:48–60. doi: 10.1016/j.ctrv.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larroquette M., Gross-Goupil M., Daste A., Robert G., Ravaud A., Domblides C. Which place for avelumab in the management of urothelial carcinoma? Expert Opin Biol Ther. 2019;(9):863–870. doi: 10.1080/14712598.2019.1637412. [DOI] [PubMed] [Google Scholar]
- 142.Baker M., Cordes L., Brownell I. Avelumab: a new standard for treating metastatic Merkel cell carcinoma. Expert Rev Anticancer Ther. 2018;18(4):319–326. doi: 10.1080/14737140.2018.1445528. [DOI] [PubMed] [Google Scholar]