Abstract
Abstract
Objective: The robotic-assisted approach to simple prostatectomy (RASP) was conceived, essentially reproducing the fundaments of open simple prostatectomy. Since the first report, RASP underwent several technical modifications. The study aims to identify and describe the current robotic surgery techniques to approach benign prostatic hyperplasia (BPH).
Methods
The paper performed a non-systematic literature review accessing PubMed and Embase databases for all full-text articles published from 2008 to May 2020, assessing robot-assisted surgical techniques for BPH treatment using the terms “robot-assisted simple prostatectomy” OR “robotic simple prostatectomy” OR “RASP” AND “surgical technique”.
Results
After careful review of 180 studies in PubMed and 198 in Embase, 16 papers reporting different RASP techniques. After the first procedure described by Sotelo et al. [9], several authors contributed to the development of the RASP technique. John et al. [24] proposed the extraperitoneal access, and Yuh et al. [23] first reported the adenoma transcapsular dissection. Some modifications were proposed by Coelho et al. [31] on trigonization, posterior reconstruction, and urethro-vesical anastomosis. Other groups focused on urethral-preserving procedures. Moschovas et al. [28] and Clavijo et al. [32] recently described an intrafascial RASP with the removal of the entire prostatic tissue. Finally, Kaouk et al. [29] reported the feasibility and safety of the da Vinci Single Port approach.
Conclusion
In the last eighteen years, the robotic-assisted approach to BPH disease has been evolved, and different techniques have been described. This review details all the technical developments on RASP that distinctive groups have proposed since the multiport robotic platforms until the new da Vinci Single Port.
Keywords: Simple prostatectomy, Benign prostatic hyperplasia, Robotic-assisted, Minimally invasive
1. Introduction
Benign prostatic hyperplasia (BPH) is a prevalent diagnosis among older men. This non-malignant prostatic tissue growth is the leading cause of lower urinary tract symptoms (LUTS) with symptoms such as urgency, frequency, nocturia, weak urinary stream, and incomplete bladder emptying [1]. In the United States, an estimate of 15 million men over 30 years old are affected by LUTS and about 50%–75% of all men over 50 years old experience BPH/LUTS [2]. Given the increased incidence of BPH/LUTS and the significant concern and impact on the quality of life (QoL), an increasing number of patients under urological care meet the treatment criteria [3].
Despite the different pharmacological drugs available to treat BPH/LUTS, the surgical approach is often required [4,5]. The first surgical reports for BPH disease were described in the early 1900s when the digital enucleation of the adenoma was initially proposed by Freyer [6] in a transvesical approach, and years later by Millin [7] describing a transcapsular technique. Since then, the technological improvements evolved the BPH surgical approach with the creation of several techniques in the last decades until the robotic surgery to access large size prostates. Also, some other methods are described in the literature, such as the laser enucleation of the prostate (HoLEP®), thulium laser enucleation of the prostate (ThuLEP®), 532 nm laser enucleation of the prostate (Greenlight®), Diode laser treatment of the prostate, prostatic urethral lift (UroLift®), intraprostatic injections, image-guided robotic waterjet ablation of the prostate (AquaBeam®), water vapor energy ablation (Rezum System®) and prostatic artery embolization (PAE) [[8], [9], [10], [11], [12], [13], [14], [15], [16]].
In the current literature, simple prostatectomy and HoLEP are the preferred techniques for prostates larger than 80 mL [5]. Although open simple prostatectomy (OSP) has excellent functional results with significant improvements in uroflowmetry parameters, this procedure has considerable complications, remarkably blood loss, and transfusion rates [17,18]. The peri-operative blood transfusion rates after OSP are significant, ranging from 7% to 24%, with reoperation rates of 3.7% [19]. In this scenario, to decrease the surgical morbidity, minimally invasive surgical techniques for simple prostatectomy have been introduced in 2002 by Mariano et al.[20] describing a pure laparoscopic simple prostatectomy (LSP). Two years later, Sotelo et al. [9] reported the first robot-assisted simple prostatectomy (RASP) as a feasible procedure related to reasonable complication rates reduced length of stay (LOS). Currently, the RASP has become one of the standards minimally invasive techniques for large prostates with studies showing improvement of peri-operative outcomes without compromising functional outcomes [21,22]. With the widespread adoption of robotic platforms worldwide, this study aims to perform a comprehensive review of the current robotic surgery techniques to approach the BPH disease.
2. Materials and methods
2.1. Evidence acquisition
A non-systematic review of the literature was performed in PubMed® and Embase® databases for all full-text articles published from 2008 to May 2020, assessing robot-assisted surgical techniques for BPH treatment using the terms “robot-assisted simple prostatectomy” OR “robotic simple prostatectomy” OR “RASP” AND “surgical technique”. We only considered studies in the English language and excluded review studies. Only human studies were considered, and studies involving animals were excluded from the review. When finding papers from the same institution, with overlapping populations, only the latest published articles were considered.
Two investigators (F.T. and M.M.) independently screened all articles titles and abstracts, focusing on different RASP surgical techniques. Any discrepancies about eligibility were solved by investigators (L.L, and S.B.). References were manually reviewed (O.C), and a senior investigator (V.P) was consulted to identify supplementary studies of interest.
2.2. Evidence synthesis
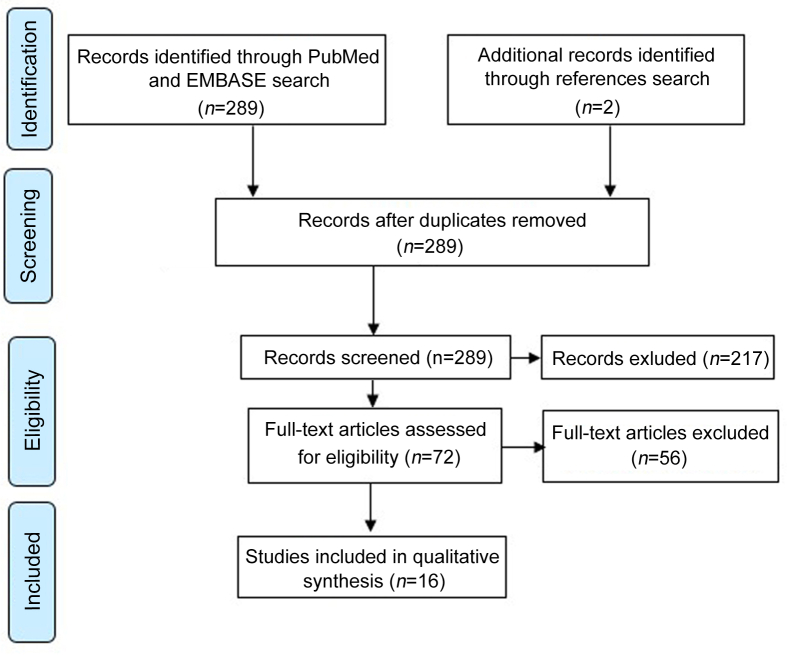
Our electronic search identified a total of 180 studies in PubMed® and 198 in Embase®. After duplicates removal, a total of 282 studies were identified. Seventy-two papers were eligible for detailed review, which ultimately yielded 16 studies reporting different surgical techniques to approach BPH disease (Fig. 1). All data retrieved from the reviewed studies were recorded in a database and summarized in Table 1. Along with the technical contributions, the following characteristics were recorded: Number of patients (n), mean age, operative time (OT), estimated blood loss (EBL), prostate size, length of stay (LOS), catheter time, transfusion rates, initial prostate-specific antigen (PSA) and pre- and post-operative International Prostatic Symptoms Score (IPSS).
Figure 1.
Evidence synthesis.
Table 1.
Peri-operative outcomes from selected studies.
| Reference | n | Age, year | OT, min | EBL, mL | Prostate volume, mL | LOS, day | Catheter time, day | Transfusion rate (%) | PSA, ng/mL | IPSS pre-operative | IPSS post-operative |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sotelo et al., 2008 [9] | 7 | 64.7 | 195 | 382 | 77.6 | 1.3 | 7.5 | 14.3 | 12.5 | 22 | 7.2 |
| Yuh et al., 2008 [23] | 3 | 76.7 | 211 | 558 | 301 | 1.3 | NR | 33 | 25.1 | 17.7 | NR |
| John et al., 2009 [24] | 13 | 70 | 210 | 500 | 82 | 6 | 6 | 0 | NR | NR | NR |
| Fareed et al., 2012 [30] | 8 | 67.2 | 228 | 584 | 78 | 6 | 15 | 0 | 15.9 | 18.2 | 4.83 |
| Coelho et al., 2012 [31] | 6 | 69 | 90 | 208 | 145 | 1 | 4.8 | 0 | 6.9 | 19.8 | 5.5 |
| Clavijo et al., 2013 [32] | 10 | 71.1 | 106 | 375 | 81 | 1 | 8.9 | NR | 5.81 | 18.8 | 1.67 |
| Elsamra et al., 2014 [33] | 15 | 68.7 | 189 | 290 | 110 | 8.4 | 2.6 | 0 | 10.8 | 16.2 | 4.5 |
| Leslie et al., 2014 [34] | 25 | 72.9 | 241 | 143 | NR | 4 | 9 | 4 | 9.4 | 23.9 | 3.5 |
| Stolzemburg et al., 2018 [35] | 10 | 63.1 | 122 | 228 | 102 | 8.4 | 7.4 | 0 | 7.3 | 21 | 3.4 |
| Castillo et al., 2016 [36] | 34 | 68 | 96 | 200 | 76 | 2.2 | 4.6 | 5.8 | 7.3 | 23.5 | 7.1 |
| Falavolti et al., 2017 [26] | 18 | 74.3 | 205 | 200 | 100 | 3.2 | 5.6 | 0 | NR | 25.2 | 8 |
| Simone et al., 2019 [37] | 12 | 63 | 150 | 250 | 78 | 3 | 7 | 8 | 5.6 | 33 | 6 |
| Cacciamani et al., 2018 [25] | 23 | 69.4 | 160 | 98.6 | 63.1 | 2.1 | NR | NR | 7.45 | 23.1 | NR |
| Wang et al., 2018 [27] | 27 | 64 | 169 | 235 | 47.5 | 3 | 1 | 0 | 4.2 | 25 | NR |
| Moschovas et al., 2020 [28] | 34 | 71 | 126 | 160 | 145 | 2 | NR | 0 | NR | 21 | 8 |
| Kaouk et al., 2020 [29] | 10 | 74 | 190 | 100 | 84.3 | 0.8 | 7 to 12 | 0 | NR | NR | NR |
EBL, estimated blood loss; IPSS, International Prostatic Symptoms Score; LOS, length of stay; NR, not report; OT, operative time; PSA, prostate-specific antigen.
3. Results
A total of 16 original full-text articles from various institutions met the eligibility criteria and were found to add novel technical contributions to the surgery of RASP [9,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]]. Table 1 summarizes the articles included in the analysis, highlighting the technical contribution along with peri-operative and functional outcomes. Table 2 summarizes all technical contributions to RASP. In this section, we described the different surgical techniques reported during the last 12 years using robotic technology to treat BPH. Also, we added some illustrations of challenging BPH surgeries performed in our center [Figure 2, Figure 3, Figure 4].
Table 2.
RASP technical modifications.
| Reference | Technique highlight |
|---|---|
| Sotelo et al., 2008 [9] | First RASP description; transperitoneal; horizontal cystostomy. |
| Yuh et al., 2008 [23] | Introduction of retropubic RASP (transcapsular). |
| John et al., 2009 [24] | Extraperitoneal approach; balloon disection; vertical cystostomy. |
| Fareed et al., 2012 [30] | Early reports on SP RASP with DaVinci S™. |
| Coelho et al., 2012 [31] | Capsule approximation, posterior reconstruction, urethral anastomosis. |
| Clavijo et al., 2013 [32] | Intrafascial technique; puboprostatic attachments preservation. |
| Elsamra et al., 2014 [33] | Use of tenaculum by assistant surgeon in a 2nd console. |
| Leslie et al., 2014 [34] | Transperitoneal RASP, vertical cystostomy, stay sutures. |
| Stolzemburg et al., 2018 [35] | Extraperitoneal approach, 5- and 7-o'clock hemostatic stitches. |
| Castillo et al., 2016 [36] | 180° posterior urethro-vesical anastomosis. |
| Falavolti et al., 2017 [26] | Internal iliac artery clamping. |
| Simone et al., 2019 [37] | Urethral-sparing RASP; use of NIFI (indocyanine green). |
| Cacciamani et al., 2018 [25] | 360° vesico-urethral anastomosis; no posterior reconstruction. |
| Wang et al., 2018 [27] | Urethral-sparing RASP; ejaculation preserving. |
| Moschovas et al., 2020 [28] | Modified RASP; intrafascial; total excision of prostate tissue. |
| Kaouk et al., 2020 [29] | First SP RASP series with DaVinci SP™. |
NIFI, near-infrared fluorescence imaging; RASP, robot-assisted simple prostatectomy; SP, single-port.
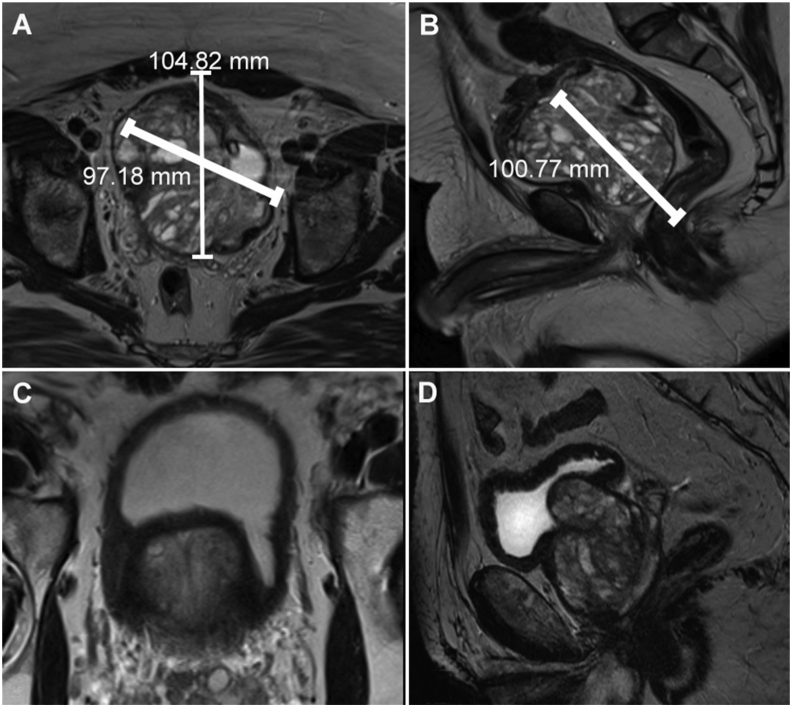
Figure 2.
Magnetic resonance imaging. Figs. 2A and 2B illustrate a 550 g prostate 60 days after arterial embolization; Figs. 2C and 2D illustrate prostates with big median lobes.
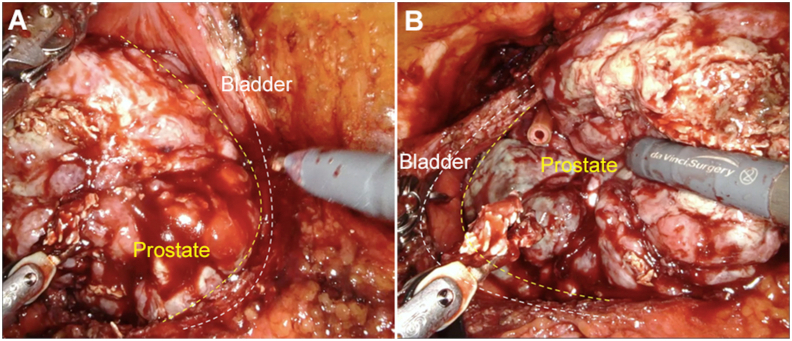
Figure 3.
Intraoperative aspect of a big prostate (550 g) after arterial embolization. (A) Right prostate side and bladder wall; (B) Left prostate side and bladder wall.
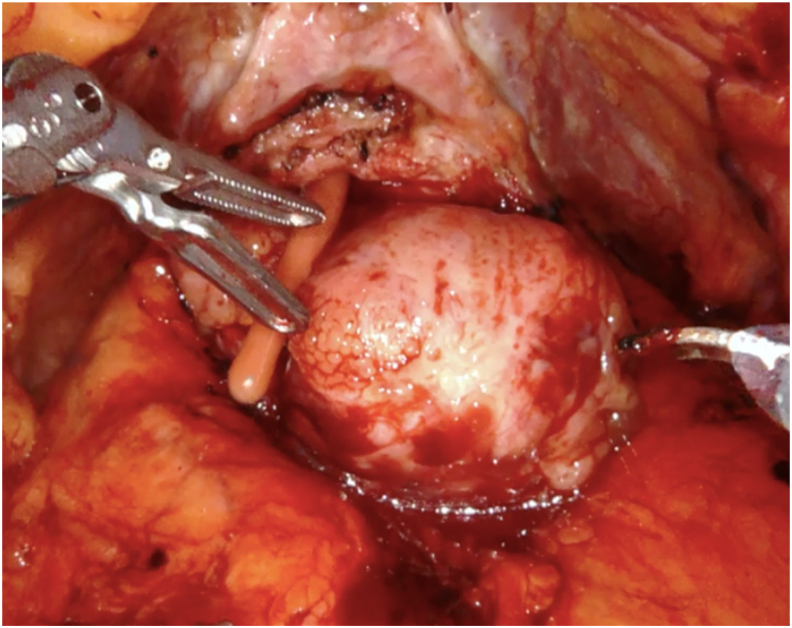
Figure 4.
Intraoperative view of a prostate with big median lobe.
3.1. Anesthesia, patient positioning and trocar placement
The surgical technique of RASP (extra or transperitoneal) essentially reproduces the fundaments of the open simple prostatectomy (OSP) with adaptations for the DaVinci® robotic platform. The procedure is performed under general anesthesia, and the patient is positioned in the Trendelenburg position after carefully secure with belts and protection pads at all articulations. We usually place four robotic trocars and two assistant ports, similar to the radical prostatectomy [9,28,31]. A Foley catheter is placed after sterile draping.
3.2. The first RASP technique in the literature
In a pioneer study, Sotelo et al. [9] first described a transperitoneal Freyer-like RASP, reporting seven consecutive cases, and set the stage for the robotic-assisted treatment of BPH in large prostates. In this technique, after entering the Retzius space and clearing the prostate's anterior surface from the fatty tissue, a horizontal cystostomy incision is performed proximally to the bladder neck. It is crucial to identify the ureteral orifices before the incision in the bladder mucosa and adenoma enucleation. The adenoma enucleation is carried bluntly using the electrocautery. Retraction stitches are placed on the lateral lobes to create traction and improve exposure. Additional care is taken at the prostatic apex when transecting the urethra to avoid the external sphincter injury. Finally, the trigonization is performed by approximation sutures of the bladder neck mucosa to the prostatic fossa floor and sometimes to the posterior urethral edge. After placing a 24 Fr Foley catheter, the cystostomy is carefully closed with running absorbable sutures. In this technique, the author described a pelvic drain placement after the adenoma removal with EndoCatch® [9].
Years later, a group from the University of Southern California reported a cohort of patients robotically operated for BPH using Sotelo's technique with a modification on the cystostomy incision. Instead of opening the bladder in a horizontal fashion, a vertical cystostomy was made and stay sutures were also placed along the edges to provide adequate exposure [34].
3.3. Transcapsular technique
Later in 2008, Yuh et al. [23] reported the first three cases of RASP with a transcapsular approach for large prostates, demonstrating the feasibility of this Millin-like technique with potential benefits regarding EBL [23].
3.4. Extraperitoneal access
John et al. [24] published a cohort of 13 patients who underwent surgery through preperitoneal access. A balloon dilation creates the extraperitoneal space, and five to six trocars are positioned according to the robotic RP technique. After granting the extraperitoneal space, the bladder is filled with 200 mL of saline. The author described a vertical bladder incision at the prostatic vesical junction to access the adenoma. Hemostatic sutures were placed in the prostatic fossa prior to Couvelaire catheter placement. Then, the transvesical-capsular incision was closed with a running suture [24].
In 2018, Stolzemburg et al. [35] published a cohort using the same extraperitoneal approach and a prolonged vertical cystostomy at the prostatic capsule. This group routinely placed hemostatic sutures at 5- and 7-o'clock positions of the prostatic capsule, similarly to classical OSP techniques.
3.5. Urethro-vesical anastomosis (UVA)
Coelho et al. [31] described a transperitoneal RASP in which a UVA was performed. The endopelvic fascia is opened, and the dorsal vein complex (DVC) is ligated. After the adenoma enucleation through a horizontal cystostomy, the posterior bladder neck is sutured to the posterior urethra with two Monocryl 3-0 stitches and the proximal edge of the prostatic capsule is approximated to the distal capsule. With another pair of Monocryl 3-0 sutures tied together with 10 knots, a complete van Velthoven vesical-urethral anastomosis is performed. The operation was carried out without other particularities with an 18 Fr Foley catheter placement and cystostomy closure [31].
Two other groups described modifications of this UVA approach [25,36]. Cacciamani et al. [25] performed a 360° reconstruction, circumferentially suturing the bladder neck to the urethra carefully avoiding the ureteral orifices, without granting the posterior reconstructions proposed by Coelho et al. [31]. Afterward, to allow future endoscopic access to the prostatic bed without compromising the hemostasis, a Chilean group reported a halfway UVA, approximating 180° of the posterior aspect of the bladder neck mucosa to the posterior urethra [36].
3.6. Tenaculum forceps application
In 2018, Elsamra et al. [33] reported the use of Tenaculum forceps at the robotic arms #1 or #2 instead of stay sutures to lift the adenoma in a transperitoneal RASP. This modification granted technical advantages as the stay sutures often rip through the adenoma, requiring multiple placements, increasing blood loss, and OT. In this technique, the author described the robotic tenaculum controlled by an assistant surgeon on a second robotic console applying dynamic traction and reducing the time necessary to switch control to the fourth arm to apply traction [33].
3.7. Internal iliac artery clamping
In a transperitoneal RASP cohort of 18 patients, Falavolti et al. [26] described a RASP technique with temporary internal iliac artery clamping. After accessing the Retzius space, the internal iliac arteries are isolated with vessel loops. Before horizontal cystostomy, the isolated arteries are occluded by bulldog clamps, and the enucleation is carried without particularities with the adenoma removal en bloc. Before trigonization and two-layer bladder closure, the bulldog clamps are removed [26].
3.8. Urethra-sparing simple prostatectomy
Wang et al. [27] developed the first urethra-sparing RASP in 2018. After granting extraperitoneal access, the bladder neck junction is exposed, and the plane between the adenoma and the prostatic urethra develops under the anterior commissure. The dissection is carefully carried in an antegrade fashion until the apex avoiding urethral lesions. Once the apex is dissected, the prostatic lobe is removed to facilitate the contralateral lobe's exposure, which is now dissected and removed. The urethral integrity is tested by filling the bladder with 100–150 mL of saline while inspecting the urethra for perforations. Immediate urethral repair is performed with a 4-0 absorbable suture in case of any leakage. Continuous bladder irrigation (CBI) is not necessary [27].
Later in 2018, Simone et al. [37] described this technique in a transperitoneal approach using near-infrared fluorescence imaging (NIFI). Once the bladder neck is isolated and the proximal urethra exposed, 50 mL of indocyanine green (IG) is injected into the bladder through an 18 Fr Foley catheter placed in the navicular fossa. The adenoma dissection adenoma from the capsule and prostatic urethra was carried from the prostatic base to the apex, continuously switching from conventional light to NIFI to ensure the integrity of the prostatic urethra [37].
3.9. Modified simple prostatectomy
In a cohort of 34 patients with BPH/LUTS and prostates over 100 mL, Moschovas et al. [28] proposed a modified RASP by resecting not only the adenoma but the entire prostate. The technique describes an intrafascial dissection of the prostate, minimal apical dissection, and seminal vesicles preservation. The anterior bladder neck is opened, the posterior bladder wall is incised, and the dissection extends until the seminal vesicles are preserved. In sequence, the Denonvillier's fascia is dissected, and the scope toggled to 30° up to perform the neurovascular bundle (NVB) and prostatic fascia antegrade dissection. The endopelvic fascia is opened with careful preservation of lateral prostatic fascia, and the retrograde dissection of lateral NVB was carried through an intrafascial plane. In sequence, the prostatic lateral pedicles are clipped with Hem-o-loks, and the apex is carefully dissected. Finally, the urethra is divided, and the posterior reconstruction and UVA are performed [28,38].
In a smaller cohort of patients, Clavijo et al. [32] also have reported an intrafascial technique for RASP with the preservation of puboprostatic ligaments, periprostatic fascia, and seminal vesicles. After controlling the lateral prostatic pedicles with a 2-0 polyglactin hemostatic suture, a back-bleeding suture is placed to control the anterior prostatic veins. Dissection is carried in a retrograde fashion, starting with endopelvic fascia opening and DVC ligation and division until the NVB dissection and release. Then, the bladder neck is dissected and opened. The procedure continues without particularities with seminal vesicles preservation and UVA [32].
3.10. Single-port approach to simple prostatectomy
The first single-port approach to RASP was described by Fareed et al. [30] in 2012. Using a DaVinci S™ Surgical System (Intuitive Surgical, Sunnyvale, CA, US) and a Gelport™ device (Applied Medical, Santa Margarita, CA, US), a transvesical single-port access was guaranteed. This procedure required that the prostatic urethra was incised endoscopically with a Collin's knife before the robot docking. After granting the pneumovesicum, the adenoma enucleation was carried similarly to the previously described suprapubic techniques [30].
3.11. da Vinci single-port technique
Recently, Kaouk et al. [29] described a single-port RASP technique in a series to evaluate the safety and feasibility of this approach to BPH treatment. A suprapubic 3 cm skin incision is made as cranially as possible and the bladder dome dissected and opened. After placing four stay sutures to lift the bladder wall, a GelPoint™ (Applied Medical, Santa Margarita, CA, US) and an assistant port are placed. After creating the pneumovesicum, the bladder mucosa is incised above the adenoma, and the enucleation is performed. With this technique, the adenomas are excised in pieces, and in very large prostates, the robot is undocked briefly to remove the fragments. Trigonization is then performed, and the robot is undocked to allow the bladder closure [29].
4. Discussion
RASP represents a natural minimally invasive surgical treatment of BPH/LUTS. With the widespread adoption of robotic surgery over the world, the number of this type of surgery performed per year increased substantially. In a recent analysis, data from over 1 300 cases show a significant increase in the proportion of patients being operated for LUTS/BPH through a robotic platform. While in 2008 LSP represented nearly 90% of laparoscopic minimally invasive surgeries (i.e., LSP and RSP) for BPH, in the following years there has been a continuous inversion in this scenario, with RASP being responsible for about 75% the surgeries performed [22]. Some aspects that might have contributed to this increase are the increasing number of robotic platforms installed worldwide and the obvious RASP ergonomic advantage over LSP. Additionally, the learning curve for RASP is considerably shorter than LSP [39].
Although some guidelines consider this robotic approach as a technique under investigation, mainly due to the lack of randomized-controlled trials on the subject, there are sufficient data on both LSP and RASP feasibility and safety for men with LUTS/BPH and larger prostates (>80 mL) [5]. A meta-analysis performed with 27 observational studies, comprising 764 patients with a mean prostate volume of 113.5 mL, showed that minimally invasive simple prostatectomy provided functional improvements similar to OSP with no difference in IPSS improvement and Qmax, with lower EBL, shorter LOS and shorter time to catheter removal [21]. Autorino et al. [22] reported similar results in a recent multi-institutional analysis of 1 330 consecutive patients (36.6% RASP and 63.4% LSP) with a median prostate volume of 100 mL (range: 89–128 mL). The reported median EBL was 200 mL (range: 150–300 mL), and the transfusion rate was 3.5%, with 2.2% of intraoperative complications and a 10.6% overall complication rate. Regarding functional outcomes, at 12 months patients who have undergone RASP showed an IPSS of 7 (pre-operative IPSS 23; p<0.001) and a Qmax of 25 mL/s (pre-operative Qmax 8 mL/s) [22].
Since the first procedure described by Sotelo et al. [9], the use of robot-assisted platforms to treat BPH added some advantages to the simple prostatectomy outcomes, especially when considering peri-operative outcomes. By reviewing all the RASP techniques published to date, some aspects need to be addressed.
First, from the bleeding control standpoint, the pneumoperitoneum results in a tamponade of open vessels during blunt dissection within the prostatic fossa and positively impacts on bleeding. The combination of the 3-dimensional (3D) stereoscopic vision to the robotic instrument articulation allows accurate visualization and control of the bleeding vessels. While the series of OSP presented transfusion rates of 7%–24% [19], the robotic series of this review had no reports of massive blood loss. The lowest reported EBL was 98.6 mL in Cacciamani et al. [25] series, followed by Kaouk et al. (100 mL) [29] and Leslie et al. (143 mL) [34]. Early vascular control also was described to improve surgery safety and reduce bleeding with transfusion rates lower than other published series [26,40]. The UVA technique following the adenoma enucleation proposed by Coelho et al. [31] has emerged under the rationale that covering the crude prostatic fossa area would primarily eliminate the need for continuous bladder irrigation and therefore decrease EBL, and shorten LOS. In the studies on which UVA was performed, EBL ranged from 98.6 mL to 208 mL and LOS ranged form 1 to 2.2 days. [25,31,32].
Second, the extraperitoneal approach proposed by John et al. [24] and Stolzemburg et al. [35] aimed to aggregate the benefits that have been previously reported for this type of access in RP [41]. In these two series, however, the lower post-operative pain and ileus did not translate to shorter LOS (6 days and 8.4 days, respectively) [24,35]. The lowest LOS was reported in the single-port RASP series from Kaouk et al. [29], where patients were discharged 19 h after surgery. The highest LOS was reported by Elsamra et al. [33] and Stolzemburg et al.[35], being 8.4 days on both studies.
Third, as prostate cancer and BPH affect the male population from the same age group, finding prostate cancer at a pathological exam after surgeries for BPH is not rare. In a large multi-institutional analysis pathology report described prostate cancer in 4% of the specimens [22]. Autopsy studies reported that 83% of prostate cancer arises in prostates with concomitant BPH [42,43]. Based on this premise, Moschovas et al. [28] and Clavijo et al. [32] proposed modified RASP techniques that extract the entire prostatic tissue with an intrafascial fashion, preserving all apical attachments along with periprostatic fascia and seminal vesicles. Surprisingly, half of the patients on Moschovas et al. [28] series presented prostate cancer at pathology report, despite having pre-operative biopsy conforming BPH. None of the patients in Clavijo et al. [32] series presented with prostate cancer.
Finally, the evolving technology has led to the development of the single-port RASP. Since 2012, different authors reported this technique, but due to the lack of triangulation and instrument collision, this approach had an increased risk of complications [30]. After the DaVinci SP™ clearance by the Food and Drug Administration (FDA) in 2018, the single-port approach to BPH treatment gained another minimally invasive surgical alternative. Despite the low number of reports describing this new technology in the literature, the platform is safe and reproduces the intra- and post-operative outcomes of the previous robotic series [29].
Our study is not devoid of limitations. As the review aimed to identify and describe the different robotic-assisted surgical techniques for BPH treatment, any comparisons between studies should be performed with extreme caution. The global quality of the studies is low due to the retrospective design of the series with a reduced number of cases, non-standardized criteria to select the surgical treatment, lack of randomization, and no control group for comparisons. Also, the ways of reporting the functional outcomes and complications are different between the studies. However, this is the first review in the literature reporting the details and nuances of different robotic approaches to treat patients with BPH.
5. Conclusion
In the last 18 years, the robotic-assisted approach to BPH disease has been evolved, and different techniques have been described. This review details all the technical developments on RASP, highlighting and locating over time all landmarks and different anatomic access that distinctive groups have proposed since the multiport robotic platforms until the new da Vinci Single-Port.
Author contributions
Study design: Marcio Covas Moschovas, Frederico Timóteo.
Data acquisition: Kulthe Ramesh Seetharam Bhat, Leonardo Lins, Oseas de Castro Neves.
Data analysis: Leonardo Lins, Oseas de Castro Neves.
Drafting of the manuscript: Marcio Covas Moschovas, Frederico Timóteo.
Critical revision of the manuscript: Vipul R. Patel.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Irwin D.E., Kopp Z.S., Agatep B., Milsom I., Abrams P. Tract S, Overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–1139. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 2.Egan K.B. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43:289–297. doi: 10.1016/j.ucl.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Wei J.T., Calhoun E., Jacobsen S.J. Urologic diseases in American Project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 4.Brandon Van Asseldonk B.V., Barkin J., Elterman D.S. Medical therapy for benign prostatic hyperplasia: a review. Curr J Oncol. 2015;22(Suppl 1):7–17. [PubMed] [Google Scholar]
- 5.Gratzke C., Bachmann A., Descazeaud A., Drake M.J., Madersbacher S., Mamoulakis C. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–1109. doi: 10.1016/j.eururo.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Freyer P.J. A new method of performing perineal prostatectomy. BMJ. 1900;1:698–699. doi: 10.1136/bmj.1.2047.698-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millin T. The surgery of prostatic obstructions. Ir J Med Sci. 1947;22:185–189. doi: 10.1007/BF02937798. [DOI] [PubMed] [Google Scholar]
- 8.Jean-Nicolas Cornu J.N., Ahyai S., Bachmann A., de la Rosette J., Gilling P., Gratzke C. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol. 2015;67:1066–1096. doi: 10.1016/j.eururo.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Sotelo R., Clavijo R., Carmona O., Garcia A., Banda E., Miranda M. Robotic simple prostatectomy. J Urol. 2008;179:513–515. doi: 10.1016/j.juro.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Tan A., Liao C., Mo Z., Cao Y. Meta-analysis of holmium laser enucleation versus transurethral resection of the prostate for symptomatic prostatic obstruction. Br J Surg. 2007;94:1201–1208. doi: 10.1002/bjs.5916. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y., Xue B., Mohammad N.A., Chen D., Sun X., Yang J. Greenlight high-performance system (HPS) 120-W laser vaporization versus transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a meta-analysis of the published results of randomized controlled trials. Lasers Med Sci. 2016;31:485–495. doi: 10.1007/s10103-016-1895-x. [DOI] [PubMed] [Google Scholar]
- 12.Cetinkaya M., Onem K., Rifaioglu M.M., Yalcin V. 980-Nm diode laser vaporization versus transurethral resection of the prostate for benign prostatic hyperplasia: randomized controlled study. J Urol. 2015;12:2355–2361. [PubMed] [Google Scholar]
- 13.Sønksen J., Barber N.J., Speakman M.J., Berges R., Wetterauer U., Greene D. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68:643–652. doi: 10.1016/j.eururo.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Gilling P., Barber N., Bidair M., Anderson P., Sutton M., Aho T. Water: a double-blind, randomized, controlled trial of aquablation® vs. transurethral resection of the prostate in benign prostatic hyperplasia. J Urol. 2018;199:1252–1261. doi: 10.1016/j.juro.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 15.McVary K.T., Gange S.N., Gittelman M.C., Goldberg K.A., Patel K., Shore N.D. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med. 2016;13:924–933. doi: 10.1016/j.jsxm.2016.03.372. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale F.C., Iscaife A., Yoshinaga E.M., Moreira A.M., Antunes A.A., Srougi M. Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol. 2016;39:44–52. doi: 10.1007/s00270-015-1202-4. [DOI] [PubMed] [Google Scholar]
- 17.Suer E., Gokce I., Yaman O., Anafarta K., Göğüş O. Open prostatectomy is still a valid option for large prostates: a high-volume, single-center experience. Urology. 2008;72:90–94. doi: 10.1016/j.urology.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Pokorny M., Novara G., Geurts N., Dovey Z., de Groote R., Ploumidis A. Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Eur Urol. 2015;68:451–457. doi: 10.1016/j.eururo.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Gratzke C., Schlenker B., Seitz M., Karl A., Hermanek P., Lack N. Complications and early postoperative outcome after open prostatectomy in patients with benign prostatic enlargement: results of a prospective multicenter study. J Urol. 2007;177:1419–1422. doi: 10.1016/j.juro.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 20.Mariano M.B., Graziottin T.M., Tefilli M.V. Laparoscopic prostatectomy with vascular control for benign prostatic hyperplasia. J Urol. 2002;167:2528–2529. [PubMed] [Google Scholar]
- 21.Lucca I., Shariat S.F., Hofbauer S.L., Klatte T. Outcomes of minimally invasive simple prostatectomy for benign prostatic hyperplasia: a systematic review and meta-analysis. World J Urol. 2015;33:563–570. doi: 10.1007/s00345-014-1324-3. [DOI] [PubMed] [Google Scholar]
- 22.Autorino R., Zargar H., Mariano M.B., Sanchez-Salas R., Sotelo R.J., Chlosta P.L. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American multi-institutional analysis. Eur Urol. 2015;68:86–94. doi: 10.1016/j.eururo.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Yuh B., Laungani R., Perlmutter A., Eun D., Peabody J., Mohler J.L. Robot-assisted Millin's retropubic prostatectomy: case series. Can J Urol. 2008;15:4101–4105. [PubMed] [Google Scholar]
- 24.John H., Bucher C., Engel N., Fischer B., Fehr J.L. Preperitoneal robotic prostate adenomectomy. Urology. 2009;73:811–815. doi: 10.1016/j.urology.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Cacciamani G., Medina L., Ashrafi A., Landsberger H., Winter M., Mekhail P. Transvesical robot-assisted simple prostatectomy with 360° circumferential reconstruction: step-by-step technique. BJU Int. 2018;122:344–348. doi: 10.1111/bju.14203. [DOI] [PubMed] [Google Scholar]
- 26.Falavolti C., Petitti T., Buscarini M. Robot-assisted simple prostatectomy with temporary internal iliac arteries clamping: our preliminary results. Mini-invasive Surg. 2017;1:35–40. [Google Scholar]
- 27.Wang P., Xia D., Ye S., Kong D., Qin J., Jing T. Robotic-assisted urethra-sparing simple prostatectomy via an extraperitoneal approach. Urology. 2018;119:85–90. doi: 10.1016/j.urology.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Moschovas M.C., Bhat S., Fikret O., Travis R., Vipul P. Modified simple prostatectomy: an approach to address large volume BPH and associated prostate cancers. J Robot Surg. 2020;14:543–548. doi: 10.1007/s11701-019-01038-6. [DOI] [PubMed] [Google Scholar]
- 29.Kaouk J., Sawczyn G., Wilson C., Aminsharifi A., Fareed K., Garisto J. Single-port percutaneous transvesical simple prostatectomy using the SP robotic system: initial clinical experience. Urology. 2020;141:171–173. doi: 10.1016/j.urology.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Fareed K., Zaytoun O.M., Autorino R., White W.M., Crouzet S., Yakoubi R. Robotic single port suprapubic transvesical enucleation of the prostate (R-STEP): initial experience. BJU Int. 2012;110:732–737. doi: 10.1111/j.1464-410X.2012.10954.x. [DOI] [PubMed] [Google Scholar]
- 31.Coelho R.F., Chauhan S., Sivaraman A., Palmer K.J., Orvieto M.A., Rocco B. Modified technique of robotic-assisted simple prostatectomy: advantages of a vesico-urethral anastomosis. BJU Int. 2012;109:426–433. doi: 10.1111/j.1464-410X.2011.010401.x. [DOI] [PubMed] [Google Scholar]
- 32.Clavijo R., Carmona O., de Andrade R., Garza R., Fernandez G., Sotelo R. Robot-assisted intrafascial simple prostatectomy: novel technique. J Endourol. 2013;27:328–332. doi: 10.1089/end.2012.0212. [DOI] [PubMed] [Google Scholar]
- 33.Elsamra S.E., Gupta N., Ahmed H., Leavitt D., Kreshover J., Kavoussi L. Robotic assisted laparoscopic simple suprapubic prostatectomy—the Smith Institute for Urology experience with an evolving technique. Asian J Urol. 2014;1:55–59. doi: 10.1016/j.ajur.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie S., Abreu A.L., Chopra S., Ramos P., Park D., Berger A.K. Transvesical robotic simple prostatectomy: initial clinical experience. Eur Urol. 2014;66:321–329. doi: 10.1016/j.eururo.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Stolzenburg J.U., Kallidonis P., Kyriazis I., Kotsiris D., Ntasiotis P., Liatsikos E.N. Robot-assisted simple prostatectomy by an extraperitoneal approach. J Endourol. 2018;32:S39–S43. doi: 10.1089/end.2017.0714. [DOI] [PubMed] [Google Scholar]
- 36.Castillo O., Vidal-Mora I., Rodriguez-Carlin A., Silva A., Schatloff O., Borgna V. Modified urethrovesical anastomosis during robot-assisted simple prostatectomy: technique and results. Prostate Int. 2016;4:61–64. doi: 10.1016/j.prnil.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simone G., Misuraca L., Anceschi U., Minisola F., Ferriero M., Guaglianone S. Urethra and ejaculation preserving robot-assisted simple prostatectomy: near-infrared fluorescence imaging-guided madigan technique. Eur Urol. 2019;75:492–497. doi: 10.1016/j.eururo.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 38.Rocco B., Cozzi G., Spinelli M.G., Coelho R.F., Patel V.R., Tewari A. Posterior musculofascial reconstruction after radical prostatectomy: a systematic review of the literature. Eur Urol. 2012;62:779–790. doi: 10.1016/j.eururo.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Johnson B., Sorokin I., Singla N., Roehrborn C., Gahan J.C. Determining the learning curve for robot-assisted simple prostatectomy in surgeons familiar with robotic surgery. J Endourol. 2018;32:865–870. doi: 10.1089/end.2018.0377. [DOI] [PubMed] [Google Scholar]
- 40.Shaheen A., Quinlan D. Feasibility of open simple prostatectomy with early vascular control. BJU Int. 2004;93:349–352. doi: 10.1111/j.1464-410x.2003.04614.x. [DOI] [PubMed] [Google Scholar]
- 41.Remzi M., Klingler H.C., Tinzl M.V., Fong Y.K., Lodde M., Kiss B. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy verus open retropubic radical prostatectomy. Eur Urol. 2005;48:83–89. doi: 10.1016/j.eururo.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Bostwick D.G., Cooner W.H., Denis L., Jones G.W., Scardino P.T., Murphy G.P. The association of benign prostatic hyperplasia and cancer of the prostate. Cancer. 1992;70:291–301. doi: 10.1002/1097-0142(19920701)70:1+<291::aid-cncr2820701317>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Hammarsten J., Högstedt B. Calculated fast-growing benign prostatic hyperplasia. Scand J Urol Nephrol. 2002;36:330–338. doi: 10.1080/003655902320783827. [DOI] [PubMed] [Google Scholar]