Abstract
Phototherapy is universally recognized as the first option for treating neonatal jaundice due to its unparalleled efficiency and safety in reducing the high serum free bilirubin levels and limiting its neurotoxic effects. However, several studies have suggested that phototherapy may elicit a series of short- and long-term adverse reactions associated with pediatric diseases, including hemolysis, allergic diseases, DNA damage or even cancer. The aim of the present review was to summarize the etiology, mechanism, associated risks and therapeutic strategies for reducing high neonatal serum bilirubin levels. In order to shed light on the negative effects of phototherapy and to encourage implementation of a reasonable and standardized phototherapy scheme in the clinic, the present review sought to highlight the current understanding of the adverse reactions of phototherapy, as it is necessary to further study the mechanism underlying the development of the adverse effects of phototherapy in infants in order to explore novel therapeutic alternatives.
Keywords: neonatal, hyperbilirubinemia, phototherapy, jaundice, adverse reaction
1. Introduction
Neonatal jaundice is present in >50% of full-term newborns and it is more serious in late-preterm infants (1,2). Neonatal jaundice may be classed as physiological or pathological (3,4) and is principally caused by an increase in serum bilirubin during the neonatal period, which causes yellow discoloration of the skin, mucous membranes and sclerae (5). Neonatal hyperbilirubinemia is caused by an increase in blood bilirubin levels above the normal range. An increase in the levels of unconjugated bilirubin is the most common manifestation of neonatal jaundice.
Although there are several treatment schemes available in the clinical setting, blue light is generally the preferred choice for preventing the development of bilirubin encephalopathy or nuclear jaundice caused by excessive accumulation of unconjugated bilirubin (6,7). A high serum level of free bilirubin may cause neurotoxicity, but mildly elevated bilirubin concentrations are beneficial due to their protective antioxidant role in cell membranes, while markedly low concentrations may also be harmful (8,9). However, during the treatment of neonatal hyperbilirubinemia, the level of free bilirubin in the serum is decreased, which may weaken the protective effect of bilirubin on the cell membrane, thus making the cell membrane susceptible to injury and causing cell apoptosis. This injury may be associated with the oxidative stress induced by phototherapy, or with the upregulation of the BAX gene, which promotes apoptosis induced by phototherapy (10).
Moreover, accumulating evidence in recent years has indicated that phototherapy may elicit a series of adverse reactions, including DNA damage (11), cancer risk (12) and mortality (13). Since the mechanism underlying certain phototherapy-induced adverse effects remains unclear, additional in-depth studies are required. Furthermore, novel treatments must be designed to prevent serious harm to newborns.
2. Neonatal hyperbilirubinemia
Etiology
During the neonatal period, several factors, including preterm birth, exclusive breastfeeding, infection (such as pulmonary and skin infections), hemolysis (due to blood type incompatibility), internal bleeding (such as cranial hematoma), hypoxia, acidosis, hypoglycemia and genetic factors (4), represent common causes of elevated plasma bilirubin levels (14,15).
Pathogenesis
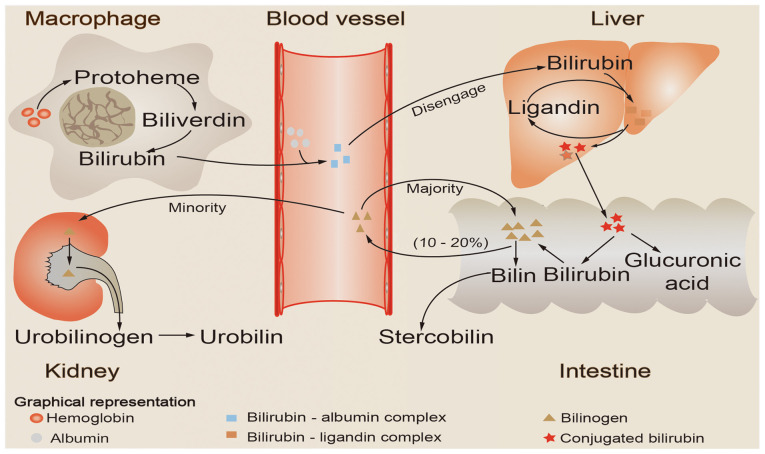
Aging red blood cells are recognized and phagocytosed by mononuclear macrophages in the circulation, which then release hemoglobin. The released hemoglobin is decomposed into two major components, globin and heme; heme is catalyzed by heme oxygenase to create biliverdin, which is then reduced to bilirubin by biliverdin reductase (16). The bilirubin produced during this process is referred to as unconjugated bilirubin, and the majority of the bilirubin produced daily in healthy individuals is derived from this pathway (17). Free bilirubin is first bound to plasma albumin and then transported to the liver as a bilirubin-albumin complex. Subsequently, bilirubin is first separated from albumin and is then taken up by hepatocytes. It is then combined with ligandins (Y and Z proteins), thus forming the bilirubin-ligand complex, and is then transported to the smooth endoplasmic reticulum of the hepatocyte, where it is conjugated with glucuronic acid to form conjugated bilirubin (1). Conjugated bilirubin, which is characterized by strong water solubility, is then secreted by hepatocytes into the bile and drained into the small intestinal lumen. Under the action of intestinal flora, conjugated bilirubin is hydrolyzed and reduced to generate bilinogen, the majority of which is excreted in the feces, while a small quantity of bilinogen is reabsorbed into the circulation by intestinal mucosal cells. The majority (~90%) of the reabsorbed bilinogen is discharged into the intestinal cavity with bile, forming the enterohepatic circulation of bilinogen (18), whereas only a small amount (~10%) enters the systemic circulation, passes through the kidneys and is excreted in the urine (Fig. 1).
Figure 1.
Schematic illustration of bilirubin metabolism. Aging red blood cells are recognized and phagocytosed by mononuclear macrophages in the circulation, which then release hemoglobin. The released hemoglobin is catabolized to produce heme, which is then reduced and oxidized to bilirubin. Bilirubin formed by this process first binds to plasma albumin and is then transported to the liver as a bilirubin-albumin complex. Next, bilirubin is first separated from albumin and then taken up by hepatocytes. Subsequently, it is combined with ligandins (Y and Z proteins) to form a bilirubin-ligand complex, and is then transported to the smooth endoplasmic reticulum of the hepatocyte, where is conjugated with glucuronic acid to form conjugated bilirubin. Conjugated bilirubin is then released into the intestine with bile, hydrolyzed and reduced to generate bilinogen, the majority of which is excreted with the feces, while a small quantity of bilinogen is reabsorbed into the circulation by intestinal mucosal cells. The majority (~90%) of the reabsorbed bilinogen is discharged into the intestinal cavity with bile, forming the enterohepatic circulation of bilinogen, whereas only a small amount (~10%) enters the systemic circulation, passes through the kidneys and is excreted in the urine.
The number of bacteria in the infantile gut is very low, and the bilirubin that enters the small intestine is not metabolized by bacteria, but is rather excreted directly in the stool; however, disruption in bilirubin liver uptake and/or binding ability, or obstruction of bilirubin excretion (for example, due to hepatitis or biliary atresia) during the neonatal period lead to an excess of plasma bilirubin (19,20). Therefore, increased bilirubin levels caused by abnormal processing by the liver and/or blocked excretion disrupt the balance between the production and elimination of bilirubin, which may be key factors causing neonatal hyperbilirubinemia (21). Furthermore, the pathogenesis of neonatal hyperbilirubinemia may be associated with infection, hypoxia leading to decreased glucuronic transferase activity, congenital deficiency of glucose-6-phosphate dehydrogenase, mutation of the uridine diphosphate glucuronic acid transferase 1A gene and perinatal factors, such as preterm birth and/or the use of oxytocin (22).
Neurotoxicity
As free bilirubin is lipid-soluble, it can cross the blood-brain barrier (23). However, the neonatal ability to take up, conjugate and excrete bilirubin is poor, particularly in premature infants with an immature blood-brain barrier. When excessive amounts of free bilirubin cross the blood-brain barrier, they may form deposits in the globus pallidus, cerebellum, thalamus, hippocampus and other parts of the brain, thereby causing severe neurotoxicity (24,25), acute bilirubin encephalopathy (26) or kernicterus, which may lead to chronic and permanent damage of the nervous system (27-30). Children with nuclear jaundice often suffer from permanent neurological sequelae, such as paralysis, epilepsy, deafness, speech and motor dysfunction (31-34), potentially even leading to death (35,36).
Treatment
To prevent the serious damage to the nervous system caused by free serum bilirubin, early detection and timely intervention are crucial. In clinical practice, liver enzyme inducers, albumin, intravenous immunoglobulin (37), phototherapy (38) and blood exchange therapy (39) are the treatments mainly used for neonatal hyperbilirubinemia. Phototherapy is widely used, and blue light therapy is currently employed to treat neonatal jaundice in the majority of countries, including China. Blue light is employed mainly because bilirubin absorbs light, particularly on the blue part of the spectrum, near the main wavelength peak of 460 nm (40,41); in addition, compared with green and ultraviolet (UV) light, bilirubin molecules absorb blue light more readily (3). Furthermore, although UV light accounts for a small proportion (~0.3%) of the traditional blue-green phototherapy, when the skin is exposed to UV light, the pro-inflammatory pathway of the skin's immune system is activated, which increases the production of inflammatory factors and triggers an autoimmune response. In addition, genes can easily mutate upon exposure to UV light, which may increase the risk of cancer (42,43). Phototherapy is a simple, convenient, non-invasive and effective method for scavenging unconjugated plasma bilirubin. Exchange transfusion therapy, which is generally performed after failure of phototherapy (44), may lead to severe complications, such as embolism, sepsis, necrotizing enterocolitis or even death. Therefore, phototherapy may alleviate the need for blood exchange therapy, and is often the preferred treatment for neonatal hyperbilirubinemia, which is mainly caused by increased levels of unconjugated bilirubin (45).
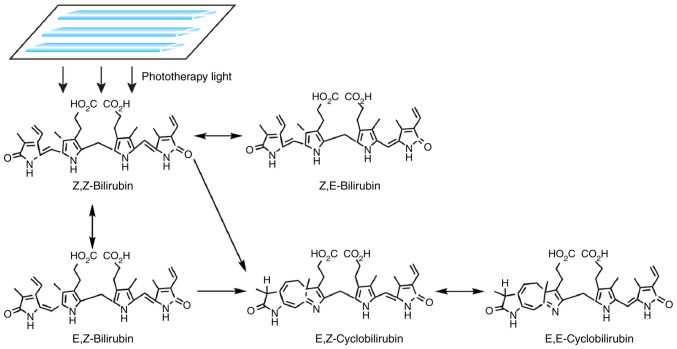
Upon exposure to light, non-polar unconjugated bilirubin (Z,Z-bilirubin) in the skin is converted to water-soluble bilirubin isomers (Fig. 2), including Z,E-bilirubin, E,Z-bilirubin, E,Z-cyclobilirubin and E,E-cyclobilirubin; the former two are configurational isomers, while the latter two are structural isomers (46,47). Furthermore, certain photooxidation reactions occur (48), which convert free bilirubin into colorless polar molecules. These water-soluble substances can be directly excreted from the body in the bile or urine (47,49), thereby attenuating the unconjugated plasma bilirubin to prevent the development of bilirubin-induced encephalopathy (48).
Figure 2.
Mechanism of action of phototherapy for neonatal hyperbilirubinemia. Upon exposure to light, non-polar unconjugated bilirubin (Z,Z-bilirubin) in the skin is converted into water-soluble bilirubin isomers, including Z,E-bilirubin, E,Z-bilirubin, E,E-bilirubin, E,Z-cyclobilirubin and E,E-cyclobilirubin.
However, phototherapy used for neonatal hyperbilirubinemia may compromise the protective barrier of the skin. A series of studies have demonstrated that phototherapy also evokes numerous serious adverse reactions in newborns (13,42,50,51). To implement a reasonable and standard phototherapy strategy in the clinical setting, the negative effects of phototherapy are summarized in Tables I and II.
Table I.
Acute adverse reactions to phototherapy in neonatal hyperbilirubinemia.
| Acute adverse reactions | Underlying mechanisms | Signal molecules | Targets | Preventive measures | (Refs.) |
|---|---|---|---|---|---|
| Interference with mother-infant interaction | - | - | - | More contact between the infant and the mother should be encouraged during the phototherapy interval | (52-54) |
| Alteration of circadian rhythm | Abnormal expression of circadian rhythm genes; decreased plasma melatonin | Decreased Bmal1 expression; significantly increased Cry1 expression | - | The time of phototherapy should be adjusted according to the individual physiological characteristics of each patient | (55-58) |
| Dehydration | Increased bilirubin decomposition products; alterations in intestinal transmembrane potential | - | - | Water and electrolytes should be replenished when necessary | (18,48,59,60) |
| Hypocalcemia | Increased urinary calcium excretion; decreased plasma melatonin, enhanced bone calcium absorption, reduce blood calcium levels | - | - | Blood calcium must be closely monitored and supplementation administered if necessary | (61-66) |
| Rash | - | - | - | Adjustment of light exposure time, intensity and distance to avoid skin damage | (67-69) |
| Bronze baby syndrome | May be associated with the deposition of bilirubin photoisomers in vivo | - | - | No need for preventive measures | (47,70,71) |
| Hemolysis | May be associated with the oxidative stress of phototherapy | - | Erythrocyte membrane | Intermittent phototherapy can be used to reduce oxidative stress in non-severe cases | (41,72-74) |
| Altered hemodynamics | Increased NO production and relaxed vascular smooth muscle relaxation through the cGMP-protein kinase A pathway; plasma ET and NO levels increase with the prolongation of phototherapy time, and the increase in NO levels may cause vasodilation | The ratio of NO to ET goes up. | Blood vessels | Blood pressure should be closely monitored | (75-79) |
| Patent ductus arteriosus | Ca2+-dependent K+ channels are activated to relax the smooth muscle in the wall of the great vessels | - | Smooth muscle of the cardiovascular system | Adequate coverage of the chest during phototherapy | (76,80-85) |
| Retinal injury | During phototherapy, the absorption of photons by the retina is more significant. | - | - | Protective blindfold and appropriate Eye care | (86-89) |
Bmal1, brain and muscle ARNT-like1; Cry1, cryptochrome 1; cGMP, cyclic guanosine monophosphate; ET, endothelin; NO, nitric oxide.
Table II.
Late adverse reactions to phototherapy in neonatal hyperbilirubinemia.
| Late adverse reactions | Underlying mechanisms | Signal molecules | Targets | Preventive measures | (Refs.) |
|---|---|---|---|---|---|
| Phototherapy and allergic diseases | Oxidative stress induced by phototherapy damages the relevant regulatory genes of Th2 to Th1 conversion, resulting in disrupted Th2 to Th1 conversion | - | Correlation regulatory genes converted from Th2 to Th1 | Intermittent phototherapy applied when possible | (8,42,90-100) |
| DNA damage by phototherapy | May be associated with production of oxygen free radicals, BCL2 downregulation and BAX upregulation | BCL2 gene, BAX gene | DNA of the mitochondria and nucleus | Intermittent phototherapy applied when possible to reduce oxidative stress | (10,11,41,50,100-106) |
| Phototherapy and tumor | May be associated with oxidative stress | - | - | Intermittent phototherapy applied when possible to reduce oxidative stress associated with tumorigenesis | (12,51,107-112) |
| Phototherapy and infant mortality | May be associated with oxidative stress | - | - | Intermittent phototherapy applied when possible to shorten the duration of light exposure | (13,113-115) |
3. Adverse reactions of phototherapy for neonatal hyperbilirubinemia
Acute adverse reactions Interference with mother-infant interaction
It was previously reported that mothers caressing infants is a major factor contributing to their psychosomatic development (52). Current treatments for jaundice in the newborn usually require separating the newborn from the mother. Apart from cases with severe jaundice, phototherapy may generally be interrupted to allow breastfeeding or parent visitations, so as to enable skin contact and mother-infant interaction and reduce the anxiety of the parents (53). In addition, phototherapy may temporarily affect the vision, hearing and alertness of the newborn (54).
Alteration of circadian rhythm
It has been reported that brain and muscle ARNT-like1 (Bmal1) and cryptochrome 1 (Cry1) are two major circadian rhythm genes expressed in neonatal peripheral blood monocytes (55). Chen et al (56) reported that, after phototherapy, the expression of Bmal1 decreased in peripheral monocytes of neonates, whereas the expression of Cry1 was significantly enhanced, whereas the plasma melatonin, which is involved in the regulation of the circadian rhythm (57,58), was downregulated. In addition, it has been reported that newborns receiving phototherapy have more frequent crying episodes compared to those receiving no therapy for clinical jaundice, which may be associated with changes in the circadian rhythm during neonatal phototherapy (56).
Dehydration
Dehydration may occur during phototherapy, particularly in premature infants. By measuring the skin moisture content of premature infants before and after phototherapy, Maayan-Metzger et al (59) found that the mean skin moisture loss increased by 26.4% during phototherapy, with the most significant loss observed in the elbow socket, groin and back. The warming effect of conventional phototherapy increases water loss from the body surface, while light-emitting diode (LED) phototherapy, which is currently widely used, causes less water loss. Additionally, phototherapy may cause excessive bilirubin decomposition, which is excreted through the intestine, thus stimulating the intestinal wall and altering the transmembrane potential difference across the epithelium (48), thereby causing diarrhea with consequent loss of water, sodium and potassium (18,60).
Hypocalcemia
Following neonatal phototherapy, the serum level of total free calcium is often diminished, leading to hypocalcemia, the incidence of which is higher among premature infants compared with that among full-term infants (61). The mechanism underlying the development of hypocalcemia driven by phototherapy remains unclear, but it may be associated with increased excretion of calcium in the urine during phototherapy. It may also be caused by light passing through the skull, which exerts an inhibitory effect on the pineal gland, thus leading to decreased melatonin secretion, which in turn leads to a decrease in cortisol secretion, thus enhancing the absorption of calcium by bone tissue and causing a decrease in blood calcium levels (62,63). Therefore, it has been hypothesized that hypocalcemia may be alleviated by wearing protective headgear during phototherapy (64,65). Moreover, to avoid severe complications caused by hypocalcemia, such as convulsions, laryngeal spasm or apnea (66), blood calcium levels should be closely monitored during phototherapy, and calcium supplements should be administered when necessary.
Rash
Certain newborns develop petechiae and skin rashes from phototherapy, which gradually fade when phototherapy is discontinued. Petechiae may be associated with light-induced thrombocytopenia (67); thus, the platelet count should be closely monitored during phototherapy. A small number of infants with cholestatic jaundice develop purpuric rash and bullous eruptions after phototherapy, which may increase the total circulating porphyrin levels (68). Since bilirubin may act as a photosensitizer, children with congenital porphyria or a family history of porphyria may develop severe blisters due to enhanced photosensitivity; thus, phototherapy is an absolute contraindication in these patients (69).
Bronze baby syndrome
The bronze baby syndrome is an irregular pigmentation resulting from phototherapy in newborn infants with neonatal jaundice that is mainly noticeable in the skin, mucous membranes and urine, and generally occurs in neonates with elevated serum conjugated bilirubin levels (70). However, the reason for this phenomenon remains unclear; it may be associated with the accumulation of photoisomers of bilirubin in the body (47) and pigmentation may be caused by the deposition of biliverdin (71). Obstructive jaundice and hepatic insufficiency are more likely to predispose to this syndrome (47).
Hemolysis
The occurrence and aggravation of hemolysis may also be associated with phototherapy. Since the neonatal antioxidant capacity is weak and phototherapy may increase oxidative stress in infants with neonatal jaundice, this may reduce the levels of antioxidants, such as reduced glutathione and ascorbic acid (72), leading to an imbalance of the oxidative and antioxidant defense system in the neonatal body (73,74), with alterations of the erythrocyte membrane structure and ensuing hemolysis (41). Phototherapy leads to an increase in lipid peroxidation of erythrocyte membranes, which can aggravate hemolysis. Furthermore, the loss of riboflavin resulting from phototherapy may reduce the activity of erythrocyte glutathione reductase and induce hemolysis.
Effect of phototherapy on hemodynamics
Phototherapy may also cause hemodynamic changes (75). In response to phototherapy, photoreceptors in blood vessels may promote the generation of nitric oxide (NO), which can cause vascular smooth muscle relaxation (76). NO can activate guanylate cyclase (GC); when GC is activated, its catalytic intracellular domain can catalyze the decomposition of guanosine triphosphate, which leads to an increase in cyclic guanosine monophosphate (cGMP) levels in the cytoplasm and causes vascular smooth muscle relaxation through the cGMP-protein kinase A pathway (77). Liu et al (78) reported that phototherapy may affect the levels of endothelin (ET) and NO in the blood, thus altering the hemodynamics of premature infants. However, hemodynamic homeostasis is normally maintained by two important vasoactive substances, ET and NO. When the NO:ET ratio increases, it causes vasodilation and low blood pressure; when the arterial blood pressure decreases, the body adjusts through the baroreceptor reflex negative feedback loop, which weakens the reflex, thereby increasing heart rate and blood pressure (79).
Patent ductus arteriosus
Functional closure of the ductus arteriosus usually occurs immediately after birth. However, the ductus arteriosus may fail to close in infants who receive phototherapy. A prospective study investigating the association between phototherapy and patent ductus arteriosus (80) reported that phototherapy greatly increased the incidence of patent ductus arteriosus among children with markedly low birth weight. In a study by Benders et al (76), >50% of premature infants receiving phototherapy were diagnosed with patent ductus arteriosus, the re-opening of the ductus arteriosus may be evoked by blue light penetrating the chest wall of the premature infant and causing relaxation of the smooth muscle of the cardiovascular system (such as the ascending aorta, left pulmonary artery and ductus arteriosus) by activating the Ca2+-dependent K+ channel (81). The patent ductus arteriosus can shunt blood from the aorta to the pulmonary artery, significantly increasing the blood volume in the pulmonary circulation and increasing the load on the left heart, thus leading to complications such as congestive heart failure or even death (82,83). It has been reported in the literature that appropriate shielding of the chest during phototherapy may reduce the incidence of patent ductus arteriosus induced by phototherapy (84). However, some researchers disagree with this preventive measure (85).
Retinal injury
Retinal damage represents another challenge associated with phototherapy for neonatal jaundice (86). The light-sensitive retinas absorb photons more readily when exposed to blue light, which is the most effective at degrading bilirubin. Following continuous or stronger blue light irradiation, the retinal function degenerates due to a significant increase in retinal cell death rate (87,88). However, a previous study reported no significant association between phototherapy and permanent eye damage in children (89). The association between phototherapy and retinal injury requires further investigation in the future.
Late adverse reactions Phototherapy and allergic diseases
Eosinophilic cationic protein (ECP) is the basic protein released by eosinophilic granulocytes (90) and serves as an indicator of the activation of eosinophilic granulocytes, which play a crucial role in allergic and immune reactions. By measuring the serum ECP level of neonates with hyperbilirubinemia before and after phototherapy, a previous study revealed that the ECP level after receiving LED phototherapy was significantly higher compared with that before phototherapy [median, 37.5 vs. 16.3 ng/ml (range, 5.4-192.0 vs. 3.6-106.0 ng/ml), respectively; P=0.006], which suggested that children with hyperbilirubinemia who had received phototherapy were more likely to develop allergic conditions in the future (91). However, allergic diseases are often associated with an abnormal immune system function, in which helper T (Th) cells play a crucial role. Th cells are generally divided into Th1 and Th2 cells. Th1 cells are mainly involved in delayed hypersensitivity reactions and cellular immunity, while Th2 cells are primarily involved in humoral immunity and facilitation of antibody production by B cells. The immune system normally switches from a Th2 to a Th1 immune response during the neonatal period (92).
Glutathione is a known antioxidant that is hydrophilic and prevents oxidation of water-soluble proteins, thereby protecting substances in the cytoplasm (93). It was previously reported that bilirubin has physiological properties that complement those of glutathione in cellular defense by protecting the cell membrane (94). By contrast, bilirubin is lipophilic. Under physiological conditions, bilirubin is a natural antioxidant and has cytoprotective properties (95). It can prevent lipid peroxidation of cell membranes caused by hydroxyl radicals, thus maintaining cell membrane stability and preventing cell apoptosis. It can also remove reactive oxygen species (ROS) and reactive nitrogen species, as well as prevent oxidative damage and diseases associated with oxidative stress. In addition, it can promote the transformation of a Th2 immune response into a Th1 immune response (96). A previous in vitro study demonstrated that bilirubin also plays an important role in inhibiting tumor cell proliferation and promoting tumor cell apoptosis, which may be associated with the extracellular signal-regulated kinase pathway (97). When the bilirubin concentration is excessive, it acts as a pro-oxidant, causing irreversible nerve damage (98). It was previously demonstrated that unbound bilirubin may stimulate the production of endogenous ROS in platelets, thereby inducing platelet apoptosis, which is a process mediated mainly by the p38 mitogen activated protein kinase pathway; in addition, bilirubin may damage mitochondria, inhibiting the energy metabolic process of the cell and causing cell apoptosis (99). Low levels of bilirubin may also be harmful (8). Phototherapy may disrupt the protective role of the cell membrane by lowering serum bilirubin, causing production of free oxygen radicals; it may also damage lymphocyte DNA and cause a disturbance of the skin cytokine environment, which disrupts the Th2 to Th1 conversion, thus leading to allergic diseases such as asthma and allergic rhinitis (42,100).
DNA damage by phototherapy
Whether phototherapy can damage DNA remains a controversial topic. Although it has been suggested that phototherapy cannot trigger DNA damage (101), other studies have suggested that phototherapy may damage the DNA of neonatal peripheral blood lymphocytes (11,50). Under the influence of visible light, cellular DNA may be mutated and the DNA chain may be broken, with consequent sister chromatid exchange. Previous studies have proposed that phototherapy can damage the DNA of neonates and induce apoptosis of peripheral blood lymphocytes, while neonatal hyperbilirubinemia per se does not cause DNA damage. Aycicek et al (100) concluded that endogenous mononuclear leukocyte DNA was damaged in infants with jaundice treated with traditional and intensive phototherapy. Tatli et al (102) also confirmed that phototherapy was associated with DNA damage in neonates. The main mechanism through which phototherapy removes unconjugated bilirubin from the body is by isomerization. As the newborn's antioxidant mechanisms are immature and the skin has low antioxidant capacity, blue light directly induces the generation of free oxygen radicals, which may cause DNA damage in the mitochondria and nucleus of the cell (41,103).
p53 is a tumor suppressor gene that is mainly involved in maintaining normal cell growth and inhibiting malignant proliferation, and participates in the process of DNA replication and repair (104). If the repair mechanism fails, p53 induces cell apoptosis to prevent malignant transformation (105). Under normal conditions, the content of p53 in cells is markedly low and has a short half-life, but its content can significantly increase during cell proliferation. After exploring the genotoxicity and apoptosis induced by phototherapy in peripheral blood lymphocytes of full-term infants, Yahia et al (106) reported that the DNA damage markers (tail DNA% and tail moment) and the p53 levels of newborns with hyperbilirubinemia subjected to phototherapy were significantly higher compared with those prior to phototherapy (all P<0.0001). Furthermore, it has been proposed that the DNA damage and cell apoptosis caused by bilirubin and phototherapy in newborns with hyperbilirubinemia may be associated with the downregulation of the BCL-2 gene (which can inhibit apoptosis) and the upregulation of the BAX gene (which can promote apoptosis) (10).
Phototherapy and tumors
Phototherapy may increase the risk of cancer in children (107). A large epidemiological study found that newborns treated with phototherapy may be at a higher risk of developing cancer later in life (51). During an 11-year follow-up of ~800,000 infants after birth, the study suggested that children treated with phototherapy were more than twice as likely to develop solid tumors after 4 years of age compared with those who did not receive phototherapy, but the potential cancer risk of bilirubin could not be ruled out (108). Another large retrospective cohort study reported that children who received phototherapy had a higher incidence of cancer compared with those who did not receive phototherapy, with a ratio of ~1.4 (P=0.01); in particular, the incidence of non-lymphocytic leukemia increased significantly (12). However, the association was weakened due to the large and uncontrollable confounders during the study period. A previous retrospective cohort study on the risk of skin cancer from neonatal jaundice phototherapy did not confirm that phototherapy significantly increases the risk of skin cancer (109). However, due to the confounding variables and limited statistical power (follow-up was interrupted for some members of the cohort as they emigrated), this possibility cannot be excluded. In addition, a previous study demonstrated that phototherapy can increase the incidence of neonatal nevi, and melanocytic nevi are the most important risk factor for the occurrence of skin melanoma (110). Therefore, when children receive phototherapy, the nevi should be closely monitored to prevent the development of melanoma. It has also been suggested that neonatal phototherapy may increase the risk of hemangioma in infants, which may be associated with oxidative stress caused by phototherapy, which can damage vascular endothelial cells and stimulate the formation of new blood vessels (111). The increased risk of cancer in children treated with phototherapy may be associated with hyperbilirubinemia per se, phototherapy or a combination of the two (112).
Phototherapy and infant mortality
Phototherapy is widely used in the treatment of neonatal jaundice, and it can indeed prevent the neurotoxicity caused by hyperbilirubinemia (113). In an attempt to elucidate the effect of phototherapy on infant mortality, Morris et al (13) conducted a large multicenter randomized controlled trial, and the results demonstrated that infant mortality did not differ significantly between infants with jaundice who received aggressive or conservative phototherapy [24 vs. 23%, respectively; 95% confidence interval (CI): 0.90-1.22], and the possibility of neural development disorders was reduced by 26 and 30%, respectively (95% CI, 0.74-0.99). However, for infants whose birth weight ranged between 501 and 750 g, the mortality rate was higher in the aggressive phototherapy group compared with that in the conservative group (39 vs. 34%, respectively). Therefore, attention should be paid to the increasing mortality rate possibly associated with phototherapy. Prolonged duration of phototherapy (114) and increased oxidative stress (115) may be relevant factors associated with increased mortality.
Other adverse reactions
When the jaundiced newborn is treated with blue phototherapy, apart from the areas protected by the black blindfold and the diaper, all other areas are exposed to illumination. As a result, neonates with jaundice treated with blue light often experience alterations in body temperature (60). Since the wavelength of absorption of blue light by riboflavin is similar to that of bilirubin, both riboflavin and bilirubin will decompose at the same time when a newborn with jaundice receives blue light therapy, leading to the loss of riboflavin in the body. The riboflavin deficiency will reduce the synthesis of active riboflavin adenine dinucleotide, impair the hydrogen delivery of erythrocytes, reduce glutathione reductase and weaken the activity of erythrocyte glutathione reductase (116), thus aggravating hemolysis. In addition, the risk of secondary intestinal obstruction may increase after phototherapy. The velocity of blood flow in the upper mesenteric artery at the end of the diastolic period is accelerated post-phototherapy, indicating that the mesenteric vascular smooth muscle may undergo diastolic changes during phototherapy, leading to mesenteric ischemia, which may be one of the causes of intestinal obstruction in premature infants (117). A retrospective study reported that phototherapy is associated with the incidence of intestinal obstruction in infants with markedly low birth weight (118). In addition, blue light treatment during the neonatal period may also be associated with subsequent diseases, such as diabetes, autism and epilepsy (119,120).
4. Preventive measures for adverse reactions caused by phototherapy of neonatal hyperbilirubinemia
Intermittent phototherapy may be used in non-severe cases, which may increase the contact time between the infant and the mother. The efficacy of intermittent phototherapy is comparable to that of continuous phototherapy, in addition to increasing the interaction time of the mother and the infant, intermittent phototherapy may also reduce the adverse reactions caused by continuous phototherapy (3), including oxidative stress, hemolysis, allergic reactions, DNA damage, cancer and increased mortality. Due to the effect of phototherapy on the circadian rhythm of children, the time of light exposure can be adjusted according to the physiological characteristics of the infants. The intensity and distance of illumination should also be adjusted, and the duration of illumination should be controlled. Close monitoring of the temperature of both the infant and the incubator is also important. To prevent the loss of water and electrolytes (such as Na+, K+ and Ca2+) caused by phototherapy, water and electrolytes must be replenished when necessary. To address the loss of riboflavin caused by phototherapy, vitamin B2 supplementation should be routine practice. Appropriate light exposure time, intensity and distance should be used to avoid skin damage. As regards the rash caused by phototherapy, no treatment is generally required. If the skin becomes cracked or infected, active skin care, such as disinfection with iodophor, should be administered. Blood pressure should be closely monitored to prevent hemodynamic changes caused by phototherapy. Adequate coverage of the chest during phototherapy can reduce the incidence of patent ductus arteriosus. To prevent direct exposure of the eye to blue light and minimize the risk of damage to the retina, an appropriate blindfold should be applied and secured in place during phototherapy. However, as the incidence of conjunctivitis is increased among children receiving phototherapy who wear eye masks over prolonged periods of time, thorough eye care, such as cleaning eye secretions and surrounding skin with normal saline cotton balls, must be applied.
5. Conclusions
It is generally acknowledged that blue phototherapy is a simple, effective and safe treatment for neonatal hyperbilirubinemia. However, the possible adverse reactions of phototherapy, including hemolysis, allergic disease, DNA damage and cancer, must be taken into consideration. To avoid serious harm to the infant's health, it is necessary to standardize, rationalize and normalize phototherapy in the clinical setting. More in-depth studies are also required to shed light on the mechanism underlying adverse reactions to phototherapy in infants, and to explore and optimize novel therapeutic schemes in the future. Due to the potentially toxic effects of blue light therapy, green LED light may also be used to reduce total serum bilirubin levels (121), as it may cause fewer adverse reactions compared with blue light. Therefore, green light therapy must be more extensively investigated in the future.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by grants from the National Natural Science Foundation of China (grant nos. 31700736 and 81872412), the Hubei Province Scientific and Technological Research Project (grant no. D20201306), the Hubei Province Health Research Project (WJ2019-01), the Leading Talent Program of Yangtze Talent Project, Hubei Medical Youth Tip-Top Talent, Leading Talent Program of Yangtze Talent Project, and the College Students Innovative Entrepreneurial Training Program in Yangtze University (grant nos. 2018184, 2019372 and Yz2020334).
Availability of data and materials
Not applicable.
Authors' contributions
JW, GG, AL and XW wrote the manuscript. JW and WQC performed the literature search and produced the figures and tables. XW conceived and reviewed the original manuscript. JW and XW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mitra S, Rennie J. Neonatal jaundice: Aetiology, diagnosis and treatment. Br J Hosp Med (Lond) 2017;78:699–704. doi: 10.12968/hmed.2017.78.12.699. [DOI] [PubMed] [Google Scholar]
- 2.Greco C, Arnolda G, Boo NY, Iskander IF, Okolo AA, Rohsiswatmo R, Shapiro SM, Watchko J, Wennberg RP, Tiribelli C, Coda Zabetta CD. Neonatal jaundice in low- and middle-income countries: Lessons and future directions from the 2015 don ostrow trieste yellow retreat. Neonatology. 2016;110:172–180. doi: 10.1159/000445708. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Wu X, Ma A, Zhang M, Liu Y. Analysis of therapeutic effect of intermittent and continuous phototherapy on neonatal hemolytic jaundice. Exp Ther Med. 2019;17:4007–4012. doi: 10.3892/etm.2019.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshmukh J, Deshmukh M, Patole S. Probiotics for the management of neonatal hyperbilirubinemia: A systematic review of randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:154–163. doi: 10.1080/14767058.2017.1369520. [DOI] [PubMed] [Google Scholar]
- 5.Mojtahedi SY, Izadi A, Seirafi G, Khedmat L, Tavakolizadeh R. Risk factors associated with neonatal jaundice: A cross-sectional study from Iran. Open Access Maced J Med Sci. 2018;6:1387–1393. doi: 10.3889/oamjms.2018.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Y, Jin Y, Meng H, Wen M. An analysis on treatment effect of blue light phototherapy combined with Bifico in treating neonatal hemolytic jaundice. Exp Ther Med. 2018;16:1360–1364. doi: 10.3892/etm.2018.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebbesen F, Hansen TWR, Maisels MJ. Update on phototherapy in jaundiced neonates. Curr Pediatr Rev. 2017;13:176–180. doi: 10.2174/1573396313666170718150056. [DOI] [PubMed] [Google Scholar]
- 8.Arnold C, Tyson JE, Pedroza C, Carlo WA, Stevenson DK, Wong R, Dempsey A, Khan A, Fonseca R, Wyckoff M, et al. Cycled phototherapy dose-finding study for extremely low-birth-weight infants: A randomized clinical trial. JAMA Pediatr. 2020;174:649–656. doi: 10.1001/jamapediatrics.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roll EB, Christensen T, Gederaas OA. Effects of bilirubin and phototherapy on osmotic fragility and haematoporphyrin-induced photohaemolysis of normal erythrocytes and spherocytes. Acta Paediatr. 2005;94:1443–1447. doi: 10.1111/j.1651-2227.2005.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 10.El-Abdin MYZ, El-Salam MA, Ibrhim MY, Koraa SSM, Mahmoud E. Phototherapy and DNA changes in full term neonates with hyperbilirubinemia. Egypt J Med Hum Genet. 2012;13:29–35. [Google Scholar]
- 11.Mesbah-Namin SA, Shahidi M, Nakhshab M. An increased genotoxic risk in lymphocytes from phototherapy-treated hyperbilirubinemic neonates. Iran Biomed J. 2017;21:182–189. doi: 10.18869/acadpub.ibj.21.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective cohort study of phototherapy and childhood cancer in northern california. Pediatrics. 2016;137(e20151354) doi: 10.1542/peds.2015-1354. [DOI] [PubMed] [Google Scholar]
- 13.Morris BH, Oh W, Tyson JE, Stevenson DK, Phelps DL, O'Shea TM, McDavid GE, Perritt RL, Van Meurs KP, Vohr BR, et al. Aggressive vs conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885–1896. doi: 10.1056/NEJMoa0803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das S, van Landeghem FKH. Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics (Basel) 2019;9(24) doi: 10.3390/diagnostics9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alizadeh Taheri P, Sadeghi M, Sajjadian N. Severe neonatal hyperbilirubinemia leading to exchange transfusion. Med J Islam Repub Iran. 2014;28(64) [PMC free article] [PubMed] [Google Scholar]
- 16.Ansong-Assoku B, Ankola PA. Neonatal jaundice. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL), 2020. PMID: 30422525. [PubMed] [Google Scholar]
- 17.Kalakonda A, Jenkins BA, John S. Physiology, bilirubin. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL), 2020. [PubMed] [Google Scholar]
- 18.Stokowski LA. Fundamentals of phototherapy for neonatal jaundice. Adv Neonatal Care. 2006;6:303–312. doi: 10.1016/j.adnc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Tabrizi SO, Mirghafourvand M, Dost AJ, Mohammad-Alizadeh-Charandabi S, Javadzadeh Y, Seyedi R. Effect of metoclopramide administration to mothers on neonatal bilirubin and maternal prolactin: A randomized, controlled, clinical trial. World J Pediatr. 2019;15:135–142. doi: 10.1007/s12519-018-0217-8. [DOI] [PubMed] [Google Scholar]
- 20.Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: A global perspective. Lancet Child Adolesc Health. 2018;2:610–620. doi: 10.1016/S2352-4642(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev. 2020;100:1291–1346. doi: 10.1152/physrev.00004.2019. [DOI] [PubMed] [Google Scholar]
- 22.Zhou YY, Lee LY, Ng SY, Hia CP, Low KT, Chong YS, Goh DL. UGT1A1 haplotype mutation among Asians in Singapore. Neonatology. 2009;96:150–155. doi: 10.1159/000209851. [DOI] [PubMed] [Google Scholar]
- 23.Allen D. Neonatal jaundice. Nurs Child Young People. 2016;28(11) doi: 10.7748/ncyp.28.6.11.s15. [DOI] [PubMed] [Google Scholar]
- 24.Amin SB, Wang H. Unbound unconjugated hyperbilirubinemia is associated with central apnea in premature infants. J Pediatr. 2015;166:571–575. doi: 10.1016/j.jpeds.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spoorthi SM, Dandinavar SF, Ratageri VH, Wari PK. Prediction of neonatal hyperbilirubinemia using 1st day serum bilirubin levels. Indian J Pediatr. 2019;86:174–176. doi: 10.1007/s12098-018-2633-0. [DOI] [PubMed] [Google Scholar]
- 26.Mir SE, van der Geest BAM, Been JV. Management of neonatal jaundice in low- and lower-middle-income countries. BMJ Paediatr Open. 2019;3(e000408) doi: 10.1080/20469047.2019.1707397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkén J, Håkansson S, Ekéus C, Gustafson P, Norman M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw Open. 2019;2(e190858) doi: 10.1001/jamanetworkopen.2019.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aprillia Z, Gayatri D, Waluyanti FT. Sensitivity, specificity, and accuracy of kramer examination of neonatal jaundice: Comparison with total bilirubin serum. Compr Child Adolesc Nurs. 2017;40:88–94. doi: 10.1080/24694193.2017.1386975. [DOI] [PubMed] [Google Scholar]
- 29.van der Schoor LWE, van Faassen M, Kema I, Baptist DH, Olthuis AJ, Jonker JW, Verkade HJ, Groen H, Hulzebos CV. Blue LED phototherapy in preterm infants: Effects on an oxidative marker of DNA damage. Arch Dis Child Fetal Neonatal Ed. 2020;105:628–633. doi: 10.1136/archdischild-2019-317024. [DOI] [PubMed] [Google Scholar]
- 30.Karimzadeh P, Fallahi M, Kazemian M, Taslimi Taleghani N, Nouripour S, Radfar M. Bilirubin induced encephalopathy. Iran J Child Neurol. 2020;14:7–19. [PMC free article] [PubMed] [Google Scholar]
- 31.Rennie JM, Beer J, Upton M. Learning from claims: Hyperbilirubinaemia and kernicterus. Arch Dis Child Fetal Neonatal Ed. 2019;104:F202–F204. doi: 10.1136/archdischild-2017-314622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The Neurological sequelae of neonatal hyperbilirubinemia: Definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs) Curr Pediatr Rev. 2017;13:199–209. doi: 10.2174/1573396313666170815100214. [DOI] [PubMed] [Google Scholar]
- 33.Lee BK, Le Ray I, Sun JY, Wikman A, Reilly M, Johansson S. Haemolytic and nonhaemolytic neonatal jaundice have different risk factor profiles. Acta Paediatr. 2016;105:1444–1450. doi: 10.1111/apa.13470. [DOI] [PubMed] [Google Scholar]
- 34.Dean E. Neonatal jaundice. Nurs Stand. 2016;30(15) doi: 10.7748/ns.30.44.15.s17. [DOI] [PubMed] [Google Scholar]
- 35.Slusher TM, Zamora TG, Appiah D, Stanke JU, Strand MA, Lee BW, Richardson SB, Keating EM, Siddappa AM, Olusanya BO. Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr Open. 2017;1(e000105) doi: 10.1136/bmjpo-2017-000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahr TM, Christensen RD, Agarwal AM, George TI, Bhutani VK. The neonatal acute bilirubin encephalopathy registry (NABER): Background, aims, and protocol. Neonatology. 2019;115:242–246. doi: 10.1159/000495518. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J, Wei C, Zhao M, Zhao D. Phototherapy is associated with the decrease in serum globulin levels in neonatal hyperbilirubinemia. Biomed Rep. 2019;10:63–69. doi: 10.3892/br.2018.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodgate P, Jardine LA. Neonatal jaundice: Phototherapy. BMJ Clin Evid. 2015;2015(0319) [PMC free article] [PubMed] [Google Scholar]
- 39.Slusher TM, Zipursky A, Bhutani VK. A global need for affordable neonatal jaundice technologies. Semin Perinatol. 2011;35:185–191. doi: 10.1053/j.semperi.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Cai A, Qi S, Su Z, Shen H, Yang Y, Cai W, Dai Y. A pilot metabolic profiling study of patients with neonatal jaundice and response to phototherapy. Clin Transl Sci. 2016;9:216–220. doi: 10.1111/cts.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kale Y, Aydemir O, Celik Ü, Kavurt S, Isikoglu S, Bas AY, Demirel N. Effects of phototherapy using different light sources on oxidant and antioxidant status of neonates with jaundice. Early Hum Dev. 2013;89:957–960. doi: 10.1016/j.earlhumdev.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Tham EH, Loo EXL, Goh A, Teoh OH, Yap F, Tan KH, Godfrey KM, Van Bever H, Lee BW, Chong YS, Shek LP. Phototherapy for neonatal hyperbilirubinemia and childhood eczema, rhinitis and wheeze. Pediatr Neonatol. 2019;60:28–34. doi: 10.1016/j.pedneo.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159:1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterham M, Bhatia R, Donath S, Molesworth C, Tan K, Stewart M. Phototherapy in transport for neonates with unconjugated hyperbilirubinaemia. J Paediatr Child Health. 2016;52:67–71. doi: 10.1111/jpc.12984. [DOI] [PubMed] [Google Scholar]
- 45.Donneborg ML, Vandborg PK, Hansen BM, Rodrigo-Domingo M, Ebbesen F. Double versus single intensive phototherapy with LEDs in treatment of neonatal hyperbilirubinemia. J Perinatol. 2018;38:154–158. doi: 10.1038/jp.2017.167. [DOI] [PubMed] [Google Scholar]
- 46.Ebbesen F, Madsen PH, Vandborg PK, Jakobsen LH, Trydal T, Vreman HJ. Bilirubin isomer distribution in jaundiced neonates during phototherapy with LED light centered at 497 nm (turquoise) vs 459 nm (blue) Pediatr Res. 2016;80:511–515. doi: 10.1038/pr.2016.115. [DOI] [PubMed] [Google Scholar]
- 47.Itoh S, Okada H, Kuboi T, Kusaka T. Phototherapy for neonatal hyperbilirubinemia. Pediatr Int. 2017;59:959–966. doi: 10.1111/ped.13332. [DOI] [PubMed] [Google Scholar]
- 48.Faulhaber FRS, Procianoy RS, Silveira RC. Side effects of phototherapy on neonates. Am J Perinatol. 2019;36:252–257. doi: 10.1055/s-0038-1667379. [DOI] [PubMed] [Google Scholar]
- 49.Altuntas N, Dogan OC, Kislal FM. Effect of phototherapy on neutrophil VCS parameters and white blood cells. J Coll Physicians Surg Pak. 2019;29:453–455. doi: 10.29271/jcpsp.2019.05.453. [DOI] [PubMed] [Google Scholar]
- 50.Ramy N, Ghany EA, Alsharany W, Nada A, Darwish RK, Rabie WA, Aly H. Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatol. 2016;36:132–136. doi: 10.1038/jp.2015.166. [DOI] [PubMed] [Google Scholar]
- 51.Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal phototherapy and infantile cancer. Pediatrics. 2016;137(e20151353) doi: 10.1542/peds.2015-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abedi F, Mirbagher Ajorpaz N, Esalatmanesh S, Rahemi Z, Gilasi HR, Kafaei Atrian M, Hosseinian M. The effect of tactile-kinesthetic stimulation on growth indices of healthy neonates. J Bodyw Mov Ther. 2018;22:308–312. doi: 10.1016/j.jbmt.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Dalili H, Sheikhi S, Shariat M, Haghnazarian E. Effects of baby massage on neonatal jaundice in healthy Iranian infants: A pilot study. Infant Behav Dev. 2016;42:22–26. doi: 10.1016/j.infbeh.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Ju SH, Lin CH. The effect of moderate non-hemolytic jaundice and phototherapy on newborn behavior. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1991;32:31–41. [PubMed] [Google Scholar]
- 55.Kavcic P, Rojc B, Dolenc-Groselj L, Claustrat B, Fujs K, Poljak M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat Med J. 2011;52:594–603. doi: 10.3325/cmj.2011.52.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen A, Du L, Xu Y, Chen L, Wu Y. The effect of blue light exposure on the expression of circadian genes: Bmal1 and cryptochrome 1 in peripheral blood mononuclear cells of jaundiced neonates. Pediatr Res. 2005;58:1180–1184. doi: 10.1203/01.pdr.0000183663.98446.05. [DOI] [PubMed] [Google Scholar]
- 57.Yeganeh Salehpour M, Mollica A, Momtaz S, Sanadgol N, Farzaei MH. Melatonin and multiple sclerosis: From plausible neuropharmacological mechanisms of action to experimental and clinical evidence. Clin Drug Investig. 2019;39:607–624. doi: 10.1007/s40261-019-00793-6. [DOI] [PubMed] [Google Scholar]
- 58.Tarocco A, Caroccia N, Morciano G, Wieckowski MR, Ancora G, Garani G, Pinton P. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019;10(317) doi: 10.1038/s41419-019-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maayan-Metzger A, Yosipovitch G, Hadad E, Sirota L. Transepidermal water loss and skin hydration in preterm infants during phototherapy. Am J Perinatol. 2001;18:393–396. doi: 10.1055/s-2001-18698. [DOI] [PubMed] [Google Scholar]
- 60.Kumar P, Murki S, Malik GK, Chawla D, Deorari AK, Karthi N, Subramanian S, Sravanthi J, Gaddam P, Singh SN. Light emitting diodes versus compact fluorescent tubes for phototherapy in neonatal jaundice: A multi center randomized controlled trial. Indian Pediatr. 2010;47:131–137. doi: 10.1007/s13312-010-0020-7. [DOI] [PubMed] [Google Scholar]
- 61.Asghar I, Khan IA, Hassan F. doi: 10.3233/NPM-200442. Effect of head covering on phototherapy induced hypocalcemia in term neonates with hyperbilirubinemia: A randomised controlled study. J Neonatal Perinatal Med: October 10, 2020 (Online ahead of print). [DOI] [PubMed] [Google Scholar]
- 62.Khan M, Malik KA, Bai R. Hypocalcemia in jaundiced neonates receiving phototherapy. Pak J Med Sci. 2016;32:1449–1452. doi: 10.12669/pjms.326.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gheshmi AN, Naderi S, Homayrani E, Safari B. Prevalence of hypocalcemia after phototherapy among neonates who underwent phototherapy in Koodakan Hospital in Bandar Abbas in 2013. Electron Physician. 2015;7:1387–1390. doi: 10.14661/1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barekatain B, Badiea Z, Hoseini N. The effect of head covering in prevention of phototherapy-induced hypocalcemia in icterus newborns with gestational age less than 35 weeks. Adv Biomed Res. 2016;5(176) doi: 10.4103/2277-9175.190992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kargar M, Jamshidi Z, Beheshtipour N, Pishva N, Jamali M. Effect of head covering on phototherapy-induced hypocalcaemia in icterus newborns; a randomized controlled trial. Int J Community Based Nurs Midwifery. 2014;2:121–126. [PMC free article] [PubMed] [Google Scholar]
- 66.Shahriarpanah S, Haji Ebrahim Tehrani F, Davati A, Ansari I. Effect of phototherapy on serum level of calcium, magnesium and vitamin D in infants with hyperbilirubinemia. Iran J Pathol. 2018;13:357–362. [PMC free article] [PubMed] [Google Scholar]
- 67.Khera S, Gupta R. Incidence of thrombocytopenia following phototherapy in hyperbilirubinemic neonates. Med J Armed Forces India. 2011;67:329–332. doi: 10.1016/S0377-1237(11)60078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaRusso J, Wilson J, Ceilley R. Phototherapy-induced purpuric eruption in a neonate. J Clin Aesthet Dermatol. 2015;8:46–48. [PMC free article] [PubMed] [Google Scholar]
- 69.Jeffrey Maisels M. Phototherapy and skin rashes. Pediatr Dermatol. 2013;30:636–637. doi: 10.1111/pde.12083. [DOI] [PubMed] [Google Scholar]
- 70.Le TN, Reese J. Bronze baby syndrome. J Pediatr. 2017;188:301–301.e1. doi: 10.1016/j.jpeds.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Kar S, Mohankar A, Krishnan A. Bronze baby syndrome. Indian Pediatr. 2013;50(624) doi: 10.1007/s13312-013-0161-6. [DOI] [PubMed] [Google Scholar]
- 72.Ayyappan S, Philip S, Bharathy N, Ramesh V, Kumar CN, Swathi S, Kumar AA. Antioxidant status in neonatal jaundice before and after phototherapy. J Pharm Bioallied Sci. 2015;7 (Suppl 1):S16–S21. doi: 10.4103/0975-7406.155766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demirel G, Uras N, Celik IH, Aksoy HT, Oguz SS, Erdeve O, Erel O, Dilmen U. Comparison of total oxidant/antioxidant status in unconjugated hyperbilirubinemia of newborn before and after conventional and LED phototherapy: A prospective randomized controlled trial. Clin Invest Med. 2010;33:335–341. doi: 10.25011/cim.v33i5.14359. [DOI] [PubMed] [Google Scholar]
- 74.Suzen S, Gurer-Orhan H, Saso L. Detection of reactive oxygen and nitrogen species by electron paramagnetic resonance (EPR) technique. Molecules. 2017;22(181) doi: 10.3390/molecules22010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uhrikova Z, Zibolen M, Javorka K, Chladekova L, Javorka M. Hyperbilirubinemia and phototherapy in newborns: Effects on cardiac autonomic control. Early Hum Dev. 2015;91:351–356. doi: 10.1016/j.earlhumdev.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Benders MJ, Van Bel F, Van de Bor M. Cardiac output and ductal reopening during phototherapy in preterm infants. Acta Paediatr. 1999;88:1014–1019. doi: 10.1080/08035259950168540. [DOI] [PubMed] [Google Scholar]
- 77.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 78.Liu GS, Wu H, Wu BQ, Huang RZ, Zhao LH, Wen Y. Effect of phototherapy on blood endothelin and nitric oxide levels: Clinical significance in preterm infants. World J Pediatr. 2008;4:31–35. doi: 10.1007/s12519-008-0006-x. [DOI] [PubMed] [Google Scholar]
- 79.Benders MJ, van Bel F, van de Bor M. Haemodynamic consequences of phototherapy in term infants. Eur J Pediatr. 1999;158:323–328. doi: 10.1007/s004310051082. [DOI] [PubMed] [Google Scholar]
- 80.Barefield ES, Dwyer MD, Cassady G. Association of patent ductus arteriosus and phototherapy in infants weighing less than 1000 g. J Perinatol. 1993;13:376–380. [PubMed] [Google Scholar]
- 81.Batenburg WW, Kappers MH, Eikmann MJ, Ramzan SN, de Vries R, Danser AH. Light-induced vs bradykinin-induced relaxation of coronary arteries: Do S-nitrosothiols act as endothelium-derived hyperpolarizing factors? J Hypertens. 2009;27:1631–1640. doi: 10.1097/HJH.0b013e32832bff54. [DOI] [PubMed] [Google Scholar]
- 82.Bhola K, Foster JP, Osborn DA. Chest shielding for prevention of a haemodynamically significant patent ductus arteriosus in preterm infants receiving phototherapy. Cochrane Database Syst Rev. 2015;(CD009816) doi: 10.1002/14651858.CD009816.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–1030. doi: 10.1542/peds.2009-3506. [DOI] [PubMed] [Google Scholar]
- 84.Mannan J, Amin SB. Meta-analysis of the effect of chest shielding on preventing patent ductus arteriosus in premature infants. Am J Perinatol. 2017;34:359–363. doi: 10.1055/s-0036-1592079. [DOI] [PubMed] [Google Scholar]
- 85.Travadi J, Simmer K, Ramsay J, Doherty D, Hagan R. Patent ductus arteriosus in extremely preterm infants receiving phototherapy: Does shielding the chest make a difference? A randomized, controlled trial. Acta Paediatr. 2006;95:1418–1423. doi: 10.1080/08035250600771458. [DOI] [PubMed] [Google Scholar]
- 86.Nakanishi-Ueda T, Majima HJ, Watanabe K, Ueda T, Indo HP, Suenaga S, Hisamitsu T, Ozawa T, Yasuhara H, Koide R. Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells. Free Radic Res. 2013;47:774–780. doi: 10.3109/10715762.2013.829570. [DOI] [PubMed] [Google Scholar]
- 87.Grimm C, Wenzel A, Williams T, Rol P, Hafezi F, Remé C. Rhodopsin-mediated blue-light damage to the rat retina: Effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci. 2001;42:497–505. [PubMed] [Google Scholar]
- 88.Chen P, Lai Z, Wu Y, Xu L, Cai X, Qiu J, Yang P, Yang M, Zhou P, Zhuang J, et al. Retinal neuron is more sensitive to blue light-induced damage than glia cell due to DNA double-strand breaks. Cells. 2019;8(68) doi: 10.3390/cells8010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kara S, Yalniz-Akkaya Z, Yeniaras A, Örnek F, Bilge YD. Ocular findings on follow-up in children who received phototherapy for neonatal jaundice. J Chin Med Assoc. 2017;80:729–732. doi: 10.1016/j.jcma.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Lin L, Chen Z, Tang X, Dai F, Wei J, Sun G. 5-Oxo-ETE from nasal epithelial cells upregulates eosinophil cation protein by eosinophils in nasal polyps in vitro. Int Arch Allergy Immunol. 2018;177:107–115. doi: 10.1159/000489819. [DOI] [PubMed] [Google Scholar]
- 91.Beken S, Aydin B, Zenciroğğlu A, Dilli D, Özkan E, Dursun A, Okumus N. The effects of phototherapy on eosinophil and eosinophilic cationic protein in newborns with hyperbilirubinemia. Fetal Pediatr Pathol. 2014;33:151–156. doi: 10.3109/15513815.2014.883456. [DOI] [PubMed] [Google Scholar]
- 92.Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21:e733–e739. doi: 10.1111/j.1399-3038.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 93.Magi S, Piccirillo S, Amoroso S, Lariccia V. Excitatory amino acid transporters (EAATs): Glutamate transport and beyond. Int J Mol Sci. 2019;20(5674) doi: 10.3390/ijms20225674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karadag F, Sengul CB, Enli Y, Karakulah K, Alacam H, Kaptanoglu B, Kalkanci O, Herken H. Relationship between serum bilirubin levels and metabolic syndrome in patients with schizophrenia spectrum disorders. Clin Psychopharmacol Neurosci. 2017;15:153–162. doi: 10.9758/cpn.2017.15.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gloria-Bottini F, Bottini E. Is there a role of early neonatal events in susceptibility to allergy? Int J Biomed Sci. 2010;6:8–12. [PMC free article] [PubMed] [Google Scholar]
- 97.Ollinger R, Kogler P, Troppmair J, Hermann M, Wurm M, Drasche A, Königsrainer I, Amberger A, Weiss H, Ofner D, et al. Bilirubin inhibits tumor cell growth via activation of ERK. Cell Cycle. 2007;6:3078–3085. doi: 10.4161/cc.6.24.5022. [DOI] [PubMed] [Google Scholar]
- 98.Rawat V, Bortolussi G, Gazzin S, Tiribelli C, Muro AF. Bilirubin-induced oxidative stress leads to DNA damage in the cerebellum of hyperbilirubinemic neonatal mice and activates DNA double-strand break repair pathways in human cells. Oxid Med Cell Longev. 2018;2018(1801243) doi: 10.1155/2018/1801243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.NaveenKumar SK, Thushara RM, Sundaram MS, Hemshekhar M, Paul M, Thirunavukkarasu C, Basappa Nagaraju G, Raghavan SC, Girish KS, et al. Unconjugated bilirubin exerts pro-apoptotic effect on platelets via p38-MAPK activation. Sci Rep. 2015;5(15045) doi: 10.1038/srep15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aycicek A, Kocyigit A, Erel O, Senturk H. Phototherapy causes DNA damage in peripheral mononuclear leukocytes in term infants. J Pediatr (Rio J) 2008;84:141–146. doi: 10.2223/JPED.1765. [DOI] [PubMed] [Google Scholar]
- 101.Gómez-Meda BC, Barros-Hernández A, Guzmán-Bárcenas J, Lemus-Varela Mde L, Zamora-Perez AL, Torres-Mendoza BM, Gallegos-Arreola MP, Armendáriz-Borunda J, Zúñiga-González GM. Effects of blue light phototherapy on DNA integrity in preterm newborns. J Photochem Photobiol B. 2014;141:283–287. doi: 10.1016/j.jphotobiol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 102.Tatli MM, Minnet C, Kocyigit A, Karadag A. Phototherapy increases DNA damage in lymphocytes of hyperbilirubinemic neonates. Mutat Res. 2008;654:93–95. doi: 10.1016/j.mrgentox.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 103.Bulut O, Erek A, Duruyen S. Effects of hyperbilirubinemia on markers of genotoxicity and total oxidant and antioxidant status in newborns. Drug Chem Toxicol. 2020:1–5. doi: 10.1080/01480545.2019.1710182. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 104.Hong B, van den Heuvel AP, Prabhu VV, Zhang S, El-Deiry WS. Targeting tumor suppressor p53 for cancer therapy: Strategies, challenges and opportunities. Curr Drug Targets. 2014;15:80–89. doi: 10.2174/1389450114666140106101412. [DOI] [PubMed] [Google Scholar]
- 105.Kanapathipillai M. Treating p53 mutant aggregation-associated cancer. Cancers (Basel) 2018;10(154) doi: 10.3390/cancers10060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yahia S, Shabaan A, Gouida M, El-Ghanam D, Eldegla H, El-Bakary A, Abdel-Hady H. Influence of hyperbilirubinemia and phototherapy on markers of genotoxicity and apoptosis in full-term infants. Eur J Pediatr. 2015;174:459–464. doi: 10.1007/s00431-014-2418-z. [DOI] [PubMed] [Google Scholar]
- 107.Tyson JE, Miller CC. Whether neonatal phototherapy increases the risk of cancer in children is a disturbing unresolved issue. Evid Based Med. 2017;22:39–40. doi: 10.1136/ebmed-2016-110528. [DOI] [PubMed] [Google Scholar]
- 108.Auger N, Laverdiere C, Ayoub A, Lo E, Luu TM. Neonatal phototherapy and future risk of childhood cancer. Int J Cancer. 2019;145:2061–2069. doi: 10.1002/ijc.32158. [DOI] [PubMed] [Google Scholar]
- 109.Brewster DH, Tucker JS, Fleming M, Morris C, Stockton DL, Lloyd DJ, Bhattacharya S, Chalmers JW. Risk of skin cancer after neonatal phototherapy: Retrospective cohort study. Arch Dis Child. 2010;95:826–831. doi: 10.1136/adc.2009.179275. [DOI] [PubMed] [Google Scholar]
- 110.Matichard E, Le Hénanff A, Sanders A, Leguyadec J, Crickx B, Descamps V. Effect of neonatal phototherapy on melanocytic nevus count in children. Arch Dermatol. 2006;142:1599–1604. doi: 10.1001/archderm.142.12.1599. [DOI] [PubMed] [Google Scholar]
- 111.Auger N, Ayoub A, Lo E, Luu TM. Increased risk of hemangioma after exposure to neonatal phototherapy in infants with predisposing risk factors. Acta Paediatr. 2019;108:1447–1452. doi: 10.1111/apa.14727. [DOI] [PubMed] [Google Scholar]
- 112.Kanmaz HG, Okur N, Dilli D, Yeşilyurt A, Oğuz ŞS. The effect of phototherapy on sister chromatid exchange with different light density in newborn hyperbilirubinemia. Turk Pediatri Ars. 2017;52:202–207. doi: 10.5152/TurkPediatriArs.2017.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arnold C, Pedroza C, Tyson JE. Phototherapy in ELBW newborns: Does it work? Is it safe? The evidence from randomized clinical trials. Semin Perinatol. 2014;38:452–464. doi: 10.1053/j.semperi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Hansen TW. Let there be light-but should there be less? J Perinatol. 2012;32:649–651. doi: 10.1038/jp.2012.80. [DOI] [PubMed] [Google Scholar]
- 115.Lamola AA. A pharmacologic view of phototherapy. Clin Perinatol. 2016;43:259–276. doi: 10.1016/j.clp.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 116.Sisson TR. Photodegradation of riboflavin in neonates. Fed Proc. 1987;46:1883–1885. [PubMed] [Google Scholar]
- 117.Kadalraja R, Patole SK, Muller R, Whitehall JS. Is mesenteric blood flow compromised during phototherapy in preterm neonates? Arch Dis Child Fetal Neonatal Ed. 2004;89(F564) doi: 10.1136/adc.2004.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raghavan K, Thomas E, Patole S, Muller R. Is phototherapy a risk factor for ileus in high-risk neonates? J Matern Fetal Neonatal Med. 2005;18:129–131. doi: 10.1080/14767050500233076. [DOI] [PubMed] [Google Scholar]
- 119.Rosenberg K, Mechcatie E. Increased seizure risk after phototherapy for jaundice. Am J Nurs. 2019;119:50–51. doi: 10.1097/01.NAJ.0000552612.04276.7c. [DOI] [PubMed] [Google Scholar]
- 120.Newman TB, Wu YW, Kuzniewicz MW, Grimes BA, McCulloch CE. Childhood seizures after phototherapy. Pediatrics. 2018;142(e20180648) doi: 10.1542/peds.2018-0648. [DOI] [PubMed] [Google Scholar]
- 121.Kuboi T, Kusaka T, Okada H, Arioka M, Nii K, Takahashi M, Yamato S, Sadamura T, Jinnai W, Nakano A, Itoh S. Green light-emitting diode phototherapy for neonatal hyperbilirubinemia: Randomized controlled trial. Pediatr Int. 2019;61:465–470. doi: 10.1111/ped.13821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.