Abstract
This phase 2, randomised, double-blind, placebo-controlled trial evaluated the efficacy and safety of lebrikizumab, an interleukin (IL)-13 monoclonal antibody, alone or with background pirfenidone therapy, in patients with idiopathic pulmonary fibrosis (IPF).
Patients with IPF aged ≥40 years with forced vital capacity (FVC) of 40%–100% predicted and diffusing capacity for carbon monoxide of 25%–90% predicted and who were treatment-naïve (cohort A) or receiving pirfenidone (2403 mg·day−1; cohort B) were randomised 1:1 to receive lebrikizumab 250 mg or placebo subcutaneously every 4 weeks. The primary endpoint was annualised rate of FVC % predicted decline over 52 weeks.
In cohort A, 154 patients were randomised to receive lebrikizumab (n=78) or placebo (n=76). In cohort B, 351 patients receiving pirfenidone were randomised to receive lebrikizumab (n=174) or placebo (n=177). Baseline demographics were balanced across treatment arms in both cohorts. The primary endpoint (annualised rate of FVC % predicted decline) was not met in cohort A (lebrikizumab versus placebo, −5.2% versus −6.2%; p=0.456) or cohort B (lebrikizumab versus placebo, −5.5% versus −6.0%; p=0.557). In cohort B, a non-statistically significant imbalance in mortality favouring combination therapy was observed (hazard ratio 0.42 (95% CI 0.17–1.04)). Pharmacodynamic biomarkers indicated lebrikizumab activity. The safety profile was consistent with that in previous studies of lebrikizumab and pirfenidone as monotherapies.
Lebrikizumab alone or with pirfenidone was not associated with reduced FVC % predicted decline over 52 weeks despite evidence of pharmacodynamic activity. Lebrikizumab was well tolerated with a favourable safety profile. These findings suggest that blocking IL-13 may not be sufficient to achieve a lung function benefit in patients with IPF.
Short abstract
This phase 2 RCT found no benefit in FVC decline over 52 weeks in IPF patients for lebrikizumab versus placebo as monotherapy (n=78 versus 76) or in combination with pirfenidone (n=174 versus 177); pirfenidone therapy was consistent with previous results https://bit.ly/313NVR8
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible, fibrosing lung disease with an unpredictable rate of decline, a poor prognosis and a 10-year survival rate of ≤15% [1–3]. Pirfenidone is one of two approved antifibrotic therapies for IPF. Pirfenidone slows lung function decline as measured by % predicted forced vital capacity (FVC), improves progression-free survival (PFS) and reduces all-cause mortality [4–6]. However, little benefit has been shown for dyspnoea, quality of life or other clinically meaningful outcomes in the pivotal trials [4, 5]. As a result, there remains an unmet need for identifying new treatments that may offer additional clinical benefit to patients with IPF.
Interleukin (IL)-13 is a potent activator of fibroblasts, promoting extracellular matrix synthesis with potential pathogenic roles in fibrosis [7–10]. In mouse models, IL-13 deficiency or defective IL-13 signalling reduced lung fibrosis, whereas overexpression of IL-13 increased lung fibrosis [11–15]. In lung biopsy samples from patients with IPF, expression levels of IL-13, IL-13 receptors and IL-13 target genes were increased compared with normal controls [16, 17]. In bronchoalveolar lavage fluid from patients with IPF, IL-13 levels were elevated compared with normal controls, and IL-13 levels were negatively correlated with key measures of lung function, such as % predicted FVC and % predicted diffusing capacity for carbon monoxide (DLCO), suggesting pathogenic functions of IL-13 in patients with IPF [18]. C–C motif ligand 18 (CCL18) and periostin are IL-13 pathway biomarkers with levels that are elevated in IPF and are associated with lung function decline or death [19].
Lebrikizumab is a humanised monoclonal antibody that specifically binds soluble IL-13 to neutralise its activity and inhibit subsequent downstream signalling [20]. The RIFF study was designed initially to evaluate lebrikizumab as monotherapy and subsequent to the approval of pirfenidone, a second cohort study was added to evaluate lebrikizumab combination with pirfenidone for the treatment of patients with IPF.
Methods
Study design
RIFF (NCT01872689) was a randomised, multicentre, double-blind, placebo-controlled study of lebrikizumab versus placebo in patients with IPF. RIFF was initially designed as a time-to-event trial to assess the benefit of lebrikizumab on PFS. Sample size calculations, randomisation, blinding and dosing administration can be found in the supplementary material.
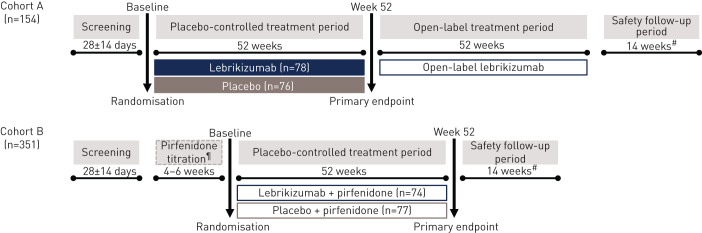
After the US Food and Drug Administration approved pirfenidone in October 2014, the RIFF protocol was amended in January 2015 to limit the number of patients (total 150 patients) and duration of blinded monotherapy assessment (52 weeks), designated as cohort A. Cohort B was added to assess the benefit of lebrikizumab versus placebo in patients receiving background pirfenidone therapy. The two cohorts were independent and enrolled sequentially. Patients entered a 28-day screening period after providing written informed consent.
In cohort A, patients were randomised 1:1 to receive lebrikizumab 250 mg monotherapy or placebo every 4 weeks for ≥52 weeks (figure 1). Study treatment was administered via subcutaneous injection, with the first injection occurring at randomisation (day 1, visit 2). After the placebo-controlled period, patients who did not discontinue received open-label lebrikizumab treatment for 52 weeks. All patients were followed for 18 weeks after last dose of study treatment (safety follow-up).
FIGURE 1.
Study design. #: Safety follow-up ended 18 weeks after the last dose of study drug; ¶: titration period allowed for patients who were pirfenidone-naïve at the time of enrolment.
In cohort B, patients were randomised 1:1 to either lebrikizumab 250 mg or placebo every 4 weeks in combination with pirfenidone (≤2403 mg·day−1) for 52 weeks (figure 1). Pirfenidone-naïve patients initiated a run-in period (4–6 weeks) to allow pirfenidone titration to 2403 mg·day−1 (per prescribing information), the highest dose tolerated or recommended dose per country-specific guidelines [21]. Study treatment and safety follow-up matched those of cohort A.
This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Approval was obtained from all institutional review boards prior to study initiation. Ethics approvals can be found in the supplementary material.
Patients
Key eligibility criteria included age ≥40 years, diagnosis of IPF per the 2011 international guidelines ≤5 years before screening (confirmed by central review of high-resolution computed tomography during screening period or ≤12 months prior to screening, with multidisciplinary evaluation if needed), stable (<10% difference in FVC (L) between screening and randomisation), % predicted FVC ≥40% and ≤100%, % predicted DLCO ≥25% and ≤90% and 6-min walk distance (6MWD) ≥100 m [3]. In cohort A, patients received no background IPF therapy for ≥4 weeks prior to randomisation and throughout the placebo-controlled period. In cohort B, patients received pirfenidone ≤2403 mg·day−1 for ≥4 weeks prior to randomisation and throughout the placebo-controlled period. Key exclusion criteria can be found in the supplementary material.
Assessments
Serum chemokine CCL13, CCL18 and periostin, biomarkers of the IL-13 pathway, were measured at baseline and weeks 4, 24 and 52. In the prior development programme of lebrikizumab in asthma, antibodies against phospholipase B-like protein 2 (PLBL2), a process-related protein impurity, were detected in some patients; no association between immunogenicity and safety was observed [22]. Blood samples for detecting and characterising anti-drug antibodies (ADAs) to lebrikizumab and PLBL2 were collected at baseline and regular intervals to assess immunogenicity. Additional biomarker assessment and immunogenicity details can be found in the supplementary material.
Adverse events, serious adverse events (SAEs) and adverse events of special interest (AESIs) were assessed during the 52-week placebo-controlled period in both cohorts and during the lebrikizumab exposure period (from first dose of lebrikizumab until the end of safety follow-up) in cohort A. Adverse events were reported by the investigator and coded per Medical Dictionary for Regulatory Activities terms (version 20.1); no adjudication was performed. AESIs included anaphylactic reactions, local injection-site reactions, infections and malignancies. AESIs related to pirfenidone as identified in the phase 3 clinical trials included photosensitivity or rash, gastrointestinal-related adverse events (e.g. nausea, diarrhoea, vomiting) and elevated liver enzyme levels [4, 5].
Endpoints and analyses
The primary endpoint was the annualised rate of decline in % predicted FVC through week 52 in the ITT population, which was compared across treatment arms with use of a random slope model at a 0.05 two-sided significance level. From the model, least squares means for the annualised rate of decline in each treatment arm included all FVC measurements collected at baseline, weeks 1, 4, 12, 24, 36, 44 and 52, and the difference between the two treatment arms was provided with 95% confidence intervals. No imputation for missing results because of death or other reasons were implemented. Missing data were handled by the model under the missing-at-random assumption (i.e. assuming that % predicted FVC decreases linearly over time).
Secondary efficacy endpoints in cohort A and cohort B included PFS, annualised rates of decline in % predicted DLCO and 6MWD at week 52, annualised rate of change from baseline in A Tool to Assess the Quality of Life in IPF (ATAQ-IPF) total score at week 52 and time from randomisation to death from any cause [23]. The Borg Category Ratio 10 Scale (Borg scale) was collected as an exploratory endpoint. In cohort A, the Saint George's Respiratory Questionnaire was assessed. In cohort B, time-to-event endpoints were also evaluated, including time to respiratory-related hospitalisation, acute exacerbation or death, ≥10% absolute decline in % predicted FVC or death and ≥15% absolute decline in DLCO or death. Kaplan–Meier estimates, log-rank tests stratified by baseline lung function for p values and Cox regression models to estimate hazard ratios and 95% confidence intervals were used to compare time-to-event endpoints.
All patients who received ≥1 dose of study drug and had ≥1 non-missing pharmacokinetic observation were included in the pharmacokinetic-evaluable population. Changes from baseline levels for pharmacodynamic biomarkers were evaluated in the ITT population.
Results
Patients
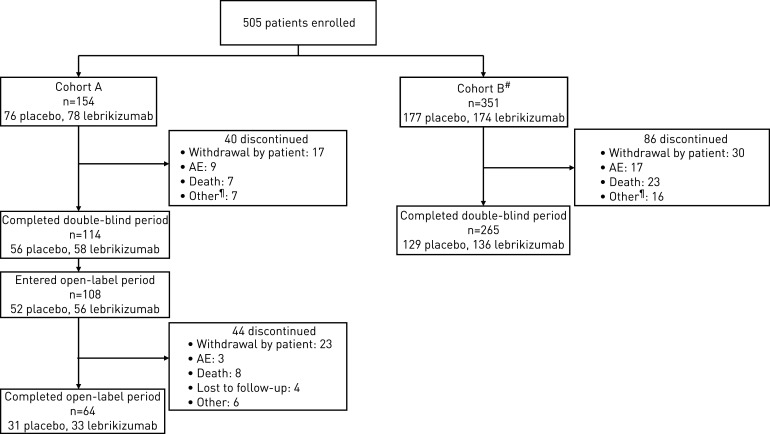
In cohort A, 325 patients were screened at 156 sites in 13 countries (figure 2) between October 2012 and April 2015. Of 154 patients randomised (screen fail rate, 53%), 76 patients received placebo and 78 received lebrikizumab; 114 patients (74%) completed the placebo-controlled period. Of patients who completed the placebo-controlled period, 108 continued and received open-label lebrikizumab, and 64 of these patients (59%) completed the open-label period. In cohort B, 623 patients were screened between May 2015 and August 2016. Of 351 randomised receiving background pirfenidone therapy (screen fail rate, 44%), 174 patients received lebrikizumab and 177 received placebo; 265 patients (75%) completed the placebo-controlled period. The most common reasons for discontinuation in both cohorts were withdrawal by patient, death and adverse events.
FIGURE 2.
Patient disposition. AE: adverse event. #: With background pirfenidone therapy; ¶: other reasons for discontinuation during the placebo-controlled period included lack of efficacy, lost to follow-up, physician decision and protocol violation.
The demographic profiles of the patients enrolled in the two treatment arms of cohort A and cohort B were comparable (table 1; supplementary table S1). The majority of patients were male (83% and 81% in cohorts A and B, respectively) and white (82% and 86%). The median (range) age was 70 (51–88) years in cohort A and 69 (50–86) years in cohort B.
TABLE 1.
Baseline demographics and clinical characteristics
| Characteristic | Cohort A | Cohort B | ||||
| Lebrikizumab (n=78) | Placebo (n=76) | All patients (N=154) | Lebrikizumab+pirfenidone (n=174) | Placebo+pirfenidone (n=177) | All patients (N=351) | |
| Age years | 70 (51–88) | 69 (52–84) | 70 (51–88) | 69 (52–85) | 69 (50–86) | 69 (50–86) |
| Male n (%) | 65 (83.3) | 63 (82.9) | 128 (83.1) | 137 (78.7) | 147 (83.1) | 284 (80.9) |
| White n (%) | 66 (84.6) | 60 (78.9) | 126 (81.8) | 151 (86.8) | 149 (84.2) | 300 (85.5) |
| Time since diagnosis years | 0.92 (0.1–5.0) | 1.53 (0.1–4.5) | 1.07 (0.1–5.0) | 1.51 (0.1–4.9) | 1.54 (0.1–5.1) | 1.54 (0.1–5.1) |
| FEV1/FVC ratio | 0.80 (0.6–0.9) | 0.81 (0.6–1.0) | 0.81 (0.6–1.0) | 0.81 (0.7–1.0) | 0.82 (0.7–1.0) | 0.82 (0.7–1.0) |
| FVC % predicted | 73.0 (38.0–98.8) | 72.8 (39.0–99.4) | 73.0 (38.0–99.4) | 71.5 (42.8–101.2) | 73.4 (39.7–98.3) | 72 (39.7–101.2) |
| DLCO % predicted | 41.7 (22.3–73.6) | 40.9 (25.0–67.9) | 41.1 (22.3–73.6) | 43.0 (12.9–71.3) | 43.0 (19.5–84.0) | 43.0 (12.9–84.0) |
| ATAQ-IPF total score# | 70.5 (34–114) | 69.0 (34–119) | 70.0 (34–119) | 71.0 (36–117) | 70.0 (34–118) | 70.0 (34–118) |
| HRCT UIP diagnosis n (%) | ||||||
| n | 78 | 75 | 153 | 174 | 177 | 348 |
| Definite UIP | 71 (91.0) | 70 (93.3) | 141 (92.6) | 142 (81.6) | 148 (83.6) | 287 (82.6) |
| Possible UIP | 7 (9.0) | 5 (6.7) | 12 (7.8) | 28 (16.1) | 28 (15.8) | 56 (16.0) |
| Inconsistent with UIP | 0 | 0 | 0 | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| Surgical biopsy n (%) | ||||||
| n | 78 | 76 | 154 | 172 | 176 | 348 |
| Definite UIP | 31 (39.7) | 24 (31.6) | 55 (35.7) | 61 (35.5) | 54 (30.7) | 115 (33.0) |
| Probable UIP | 5 (6.4) | 11 (14.5) | 16 (10.4) | 17 (9.9) | 8 (4.5) | 25 (7.2) |
| Possible UIP | 0 | 0 | 0 | 0 | 0 | 0 |
| Not UIP | 0 | 0 | 0 | 0 | 0 | 0 |
| NA | 42 (53.8) | 41 (53.9) | 83 (53.9) | 94 (54.7) | 114 (64.8) | 208 (59.8) |
Data are presented as median (range), unless otherwise stated. All values are from measurements at baseline visit (day of randomisation); eligibility was based on measurements at screening visit. #: range is 31–124. ATAQ-IPF: A Tool to Assess Quality of Life in IPF; DLCO: diffusing capacity of the lungs for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; HRCT: high-resolution computed tomography; UIP: usual interstitial pneumonia.
Baseline disease characteristics, including % predicted FVC and % predicted DLCO, were comparable between treatment arms in both cohorts. Most patients (≥93%) had ≥1 medical history finding, including both active comorbid conditions and inactive past conditions. The most common targeted medical history conditions (≥20% in either arm) in cohort A were gastro-oesophageal reflux disease (GORD; lebrikizumab, 39.7% versus placebo, 42.1%), arthritis (19.2% versus 26.3%), coronary artery disease (29.5% versus 15.8%), type 2 diabetes mellitus (19.2% versus 25.0%) and pneumonia (20.5% versus 11.8%). In cohort B, the most common conditions were GORD (lebrikizumab, 52.3% versus placebo, 54.8%), coronary artery disease (24.1% versus 20.9%) and arthritis (19.5% versus 24.9%). Most patients (≥98%) in both treatment arms of both cohorts received ≥1 concomitant medication during the placebo-controlled period (supplementary table S2). The most common (≥50%) concomitant medications in any treatment arm were proton-pump inhibitors, statins, steroids and salicylates.
Efficacy
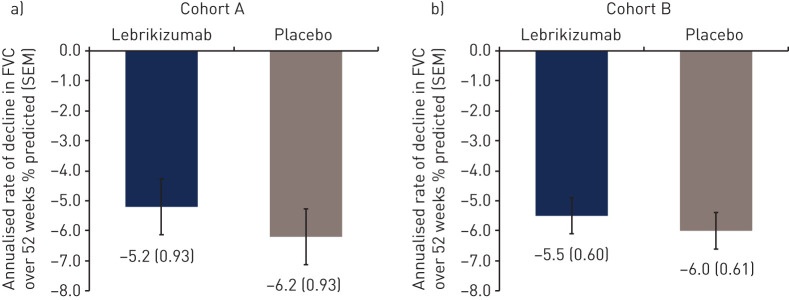
The primary endpoint was not met for either cohort (figure 3). No difference was observed in the annualised rate±sem of decline in % predicted FVC over 52 weeks in either cohort A (lebrikizumab, −5.2±0.93% versus placebo, −6.2±0.93%; difference, 0.98±1.31%; p=0.456) or cohort B (lebrikizumab, −5.5±0.60% versus placebo, −6.0±0.61%; difference, 0.50±0.85%; p=0.557).
FIGURE 3.
Annualised rate of decline in % predicted forced vital capacity (FVC) over 52 weeks. Cohort B was receiving background pirfenidone therapy.
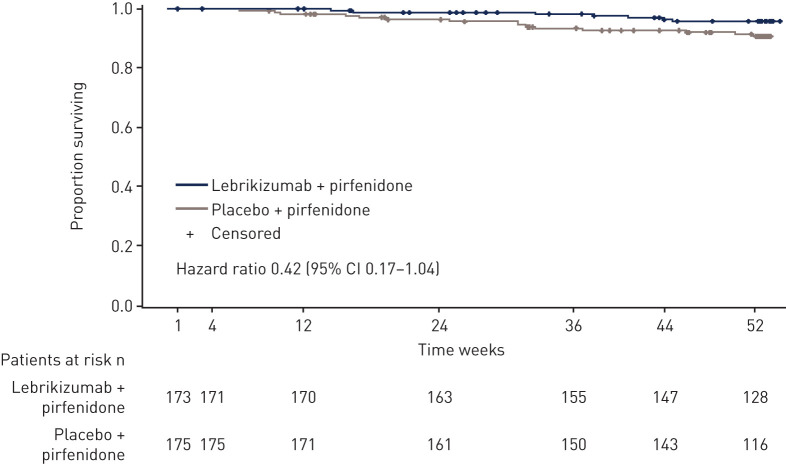
In cohort A, no effect on mortality was observed, with other secondary endpoints showing numerical trends that favoured lebrikizumab (table 2). A numerical imbalance in mortality favouring lebrikizumab plus pirfenidone treatment was observed in cohort B (HR 0.42 (95% CI 0.17–1.04); p=0.053) (figure 4). The small difference in event rate (seven deaths for lebrikizumab plus pirfenidone; 15 deaths for placebo plus pirfenidone) was driven by more deaths due to acute exacerbations in the placebo plus pirfenidone arm of cohort B. A similar trend was observed in favour of lebrikizumab for time to first acute exacerbation or death in cohort B (HR 0.61 (95% CI 0.29–1.25)) (supplementary figure S1). No treatment benefit was observed for other secondary endpoints in cohort B (table 2).
TABLE 2.
Summary of secondary endpoints
| Cohort A | Cohort B | |||||
| Lebrikizumab (n=78) | Placebo (n=76) | p-value | Lebrikizumab+pirfenidone (n=174) | Placebo+pirfenidone (n=177) | p-value | |
| Time-to-event analyses HR (95% CI) | ||||||
| PFS# | 0.65 (0.39–1.09) | 0.097¶ | 1.01 (0.72–1.42) | 0.93¶ | ||
| Time to first ≥10% absolute decline in % predicted FVC or all-cause mortality | 0.79 (0.44–1.41) | 0.42¶ | 0.84 (0.56–1.24) | 0.37¶ | ||
| Time to first ≥10% absolute decline in % predicted DLCO or all-cause mortality | 0.72 (0.23–2.26) | 0.56¶ | 0.68 (0.37–1.23) | 0.19¶ | ||
| Time to first respiratory hospitalisation | NA | NA | 0.89 (0.52–1.54) | 0.68¶ | ||
| Time to first acute IPF exacerbation or death from any cause | 1.21 (0.41–3.61) | 0.73¶ | 0.61 (0.29–1.25) | 0.17¶ | ||
| Time to first SGRQ worsening ≥7 or all-cause mortality | 0.84 (0.54–1.31) | 0.44¶ | NA | NA | ||
| Time to all-cause mortality through week 52 | 1.01 (0.25–4.02) | 0.99¶ | 0.42 (0.17–1.04) | 0.053¶ | ||
| Annualised rate of decrease in % predicted DLCO over 52 weeks | ||||||
| Slope | −4.24 | −4.78 | 0.607 | −5.57 | −5.75 | 0.780 |
| Absolute difference | 0.54 | 0.18 | ||||
| Relative difference % | 11.3 | 3.2 | ||||
| Decline ≥15% or death n (%) | 1 (2) | 3 (6) | 3 (2.5) | 5 (4.5) | ||
| Relative difference % | −67.3 | −44.9 | ||||
| Annualised rate of decline in 6MWD | ||||||
| Slope | −22.7 | −44.6 | 0.312 | −46.9 | −25.6 | 0.203 |
| Absolute difference | 21.9 | −21.4 | ||||
| Relative difference % | 49.1 | −83.7 | ||||
| Annualised rate of decline in ATAQ-IPF total score over 52 weeks | ||||||
| Slope | 4.78 | 6.89 | 0.385 | 5.45 | 5.61 | 0.905 |
| Absolute difference | −2.10 | −0.16 | ||||
| Relative difference % | −30.5 | −2.9 | ||||
| Proportion of patients with ≥10% decline in % predicted FVC or death from any cause | ||||||
| Decline ≥10% or death n (%) | 9 (16.4) | 12 (22.6) | 0.89 | 22 (16.4) | 19 (15.8) | 0.67 |
| Relative difference % | −27.7 | 3.7 | ||||
ATAQ-IPF: A Tool to Assess Quality of Life in IPF; DLCO: diffusing capacity of the lungs for carbon monoxide; FVC: forced vital capacity; HR: hazard ratio; IPF: idiopathic pulmonary fibrosis; PFS: progression-free survival; SGRQ: St. George's Respiratory Questionnaire; 6MWD: 6-min walk distance. #: PFS was defined as time from randomisation to the first occurrence of: death from any cause, non-elective hospitalisation for any cause, relative decline in FVC ≥10%; ¶: log rank.
FIGURE 4.
Time from randomisation to death from any cause in cohort B.
Exploratory biomarker analyses
Baseline levels of serum CCL13, CCL18 and periostin were comparable between the arms within each cohort (supplementary table S3). Decreases in all three biomarkers were observed in the lebrikizumab-treated patients for both cohorts compared with their respective placebo or pirfenidone-treated controls, suggesting that lebrikizumab is active to block a component of these circulating biomarkers that is downstream of IL-13 (supplementary figure S2). The level of biomarker modulation between patients treated with lebrikizumab was consistent regardless of background pirfenidone treatment.
Pharmacokinetics
Following subcutaneous administration of lebrikizumab 250 mg every 4 weeks with or without pirfenidone, the observed mean trough concentrations were similar at week 4 (supplementary table S4). The observed mean trough concentrations increased ≈2-fold from week 4 to week 52 for both cohorts. The mean±sd half-life estimates of lebrikizumab were similar across both cohorts (23.5±5.36 days for cohort A and 21.9±4.79 days for cohort B). Assessment of the exposure relationship to FVC change across 52 weeks in cohort B suggested the response was not strongly associated with exposure (supplementary figure S3). No exposure relationships were identified in patients that were hospitalised for a respiratory cause compared with those who were not, at weeks 24, 36 and 52 or for patients that later died.
Safety
The duration of lebrikizumab treatment was similar between cohort A and cohort B (supplementary table S5). The total number of adverse events was comparable between treatment arms in both cohorts (table 3). During the placebo-controlled period, adverse events were reported in 75 (96.2%) and 71 (93.4%) patients in the lebrikizumab and placebo arms of cohort A, respectively, and 158 (90.8%) and 171 (96.6%) in the lebrikizumab and placebo arms of cohort B, respectively. The frequencies of common adverse events were generally similar between treatment arms in cohort A and were similar or lower in the lebrikizumab arm than in the placebo arm of cohort B (supplementary table S6). Photosensitivity reactions were less frequent in the lebrikizumab arm than in the placebo arm of cohort B (3.4% versus 12.4%, respectively). During the lebrikizumab exposure period, 96.9% of patients in cohort A experienced ≥1 adverse event (supplementary table S7).
TABLE 3.
Overview of safety during the placebo-controlled period
| Event | Cohort A¶ | Cohort B | ||||
| Lebrikizumab (n=78) | Placebo (n=76) | All patients (N=154) | Lebrikizumab+pirfenidone (n=174) | Placebo+pirfenidone (n=177) | All patients (N=351) | |
| Patients with ≥1 AE | 75 (96.2) | 71 (93.4) | 146 (94.8) | 158 (90.8) | 171 (96.6) | 329 (93.7) |
| Total AEs | 531 | 446 | 977 | 1013 | 1052 | 2065 |
| Deaths | 3 (3.8) | 3 (3.9) | 6 (3.9) | 5 (2.9) | 10 (5.6) | 15 (4.3) |
| Patients withdrawn from study due to AE | 5 (6.4) | 9 (11.8) | 14 (9.1) | 16 (9.2) | 27 (15.3) | 43 (12.3) |
| Patients with ≥1 AE with fatal outcome | 3 (3.8) | 3 (3.9) | 6 (3.9) | 9 (5.2) | 13 (7.3) | 22 (6.3) |
| Patients with ≥1 SAE | 23 (29.5) | 19 (25.0) | 42 (27.3) | 56 (32.2) | 47 (26.6) | 103 (29.3) |
| Patients with ≥1 SAE leading to withdrawal from treatment | 6 (7.7) | 6 (7.9) | 12 (7.8) | 12 (6.9) | 14 (7.9) | 26 (7.4) |
| Patients with ≥1 SAE leading to dose modification/interruption | 5 (6.4) | 3 (3.9) | 8 (5.2) | 6 (3.4) | 4 (2.3) | 10 (2.8) |
| Patients with ≥1 treatment-related SAE | 2 (2.6) | 1 (1.3) | 3 (1.9) | 3 (1.7) | 6 (3.4) | 9 (2.6) |
| Patients with ≥1 AE leading to withdrawal from treatment | 8 (10.3) | 9 (11.8) | 17 (11.0) | 20 (11.5) | 26 (14.7) | 46 (13.1) |
| Patients with ≥1 AE leading to dose modification/interruption | 7 (9.0) | 5 (6.6) | 12 (7.8) | 11 (6.3) | 11 (6.2) | 22 (6.3) |
| Patients with ≥1 treatment-related AE | 25 (32.1) | 19 (25.0) | 44 (28.6) | 30 (17.2) | 30 (16.9) | 60 (17.1) |
| Patients with ≥1 treatment-related AE leading to withdrawal from treatment | 0 | 2 (2.6) | 2 (1.3) | 3 (1.7) | 8 (4.5) | 11 (3.1) |
| Patients with ≥1 treatment-related AE leading to dose modification/interruption | 2 (2.6) | 0 | 2 (1.3) | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| AESIs | ||||||
| Injection-site reactions | 13 (16.7) | 6 (7.9) | 19 (12.3) | 5 (2.9) | 5 (2.8) | 10 (2.8) |
| Infections (broad) | 51 (65.4) | 41 (53.9) | 92 (59.7) | 106 (60.9) | 114 (64.4) | 220 (62.7) |
| Infections (narrow) | 0 | 0 | 0 | 0 | 1 (0.6) | 1 (0.3) |
| Malignancies# | 5 (6.4) | 2 (2.6) | 7 (4.5) | 13 (7.5) | 12 (6.8) | 25 (7.1) |
| AEs related to pirfenidone | 5 (6.4) | 3 (3.9) | 8 (5.2) | 64 (36.8) | 70 (39.5) | 134 (38.2) |
| AEs leading to withdrawal from pirfenidone | 1 (1.3) | 1 (1.3) | 2 (1.3) | 9 (5.2) | 12 (6.8) | 21 (6.0) |
Data are presented as n (%) or n. AE: adverse event; AESI: adverse event of special interest; NA: not available; SAE: serious adverse event. #: Identified with Standardised Medical Dictionary for Regulatory Activities (MedDRA) Queries “narrow”; ¶: patients were not required to stop study drug to be treated with pirfenidone.
In the placebo-controlled period, 23 (29.5%) and 19 (25.0%) patients reported SAEs in the lebrikizumab and placebo arms of cohort A, respectively; 56 (32.2%) and 47 (26.6%) reported SAEs in the lebrikizumab and placebo arms of cohort B, respectively (table 3). Treatment-related SAEs (investigator assessment) were reported in two patients who received lebrikizumab and one who received placebo in cohort A and three who received lebrikizumab and six who received placebo in cohort B with background pirfenidone. The most common SAEs (≥2% of patients in either arm in either cohort) were IPF (worsening or exacerbation as reported by the investigator), respiratory tract infection and pneumonia. In cohort A, pneumothorax, pulmonary embolism and cardiac failure SAEs also occurred in ≥2% of patients (in either arm). During the lebrikizumab exposure period, 57 patients (43.8%) in cohort A experienced a total of 92 SAEs, of which three had treatment-related SAEs as assessed by the investigator. Incidences of AESIs were comparable between treatment arms in both cohorts (table 3). No adverse events met Sampson's criteria for anaphylaxis.
In cohort A, 19 patients died during the study; 15 deaths occurred during the lebrikizumab exposure period (supplementary table S8). During the placebo-controlled period, three patients (3.8%) in the lebrikizumab arm and three (3.9%) in the placebo arm died. Nine deaths occurred during open-label lebrikizumab treatment and four during safety-follow-up. The most common cause of death in cohort A was IPF (10 patients). Three deaths in cohort A were considered related to study drug by the investigator: IPF (during safety follow-up following double-blind placebo treatment), acute respiratory failure (5 days after receiving open-label lebrikizumab following double-blind placebo treatment) and pulmonary embolism (during the placebo-controlled period following lebrikizumab treatment).
In cohort B, 29 patients died; 22 deaths occurred during the placebo-controlled period: nine (5.2%) and 13 (7.3%) in the lebrikizumab and placebo arms, respectively (supplementary table S8). Seven deaths in cohort B occurred during safety-follow-up. IPF was the most common cause of death in both lebrikizumab and placebo arms (five and eight patients, respectively). Of 29 deaths in cohort B, one in the placebo arm with background pirfenidone (due to IPF, during the placebo-controlled period) was considered related to study drug by the investigator.
Lebrikizumab ADAs and PLBL2 antibodies were detected in 5.7% (14 of 247) and 24.7% (42 of 170), respectively, of lebrikizumab-treated patients in both cohorts. The median time to onset of lebrikizumab ADAs was ∼12 weeks. There was no evidence to suggest an adverse impact the presence of positive anti-lebrikizumab antibodies or anti-PLB2 antibodies on safety profiles of patients who received lebrikizumab treatment (data not shown).
Discussion
RIFF failed to meet the primary endpoint in patients with IPF. Lebrikizumab monotherapy (cohort A) was not associated with a treatment benefit on lung function, mortality or other patient-relevant outcomes. Addition of lebrikizumab to background pirfenidone therapy (cohort B) was also not associated with a treatment benefit on lung function. The overall rates of FVC decline observed in cohorts A and B were similar despite the use of background pirfenidone in cohort B. However, direct comparisons cannot be made as they were conducted as separate studies in different trial eras under a single protocol. In cohort B, a non-statistically significant imbalance in mortality favouring combination therapy was observed (HR 0.42 (95% CI 0.17–1.04)). The difference in event rate was driven by more deaths due to acute exacerbations in patients who received placebo with background pirfenidone. A similar trend favouring lebrikizumab was observed for time to acute exacerbation or death in cohort B (HR 0.61 (95% CI 0.29–1.25)). Reduced mortality is an important outcome in IPF. In the phase 3 trials of pirfenidone and nintedanib, which led to their approval to treat IPF, the effect on mortality was reported across individual studies and in pooled analyses combining phase 3 data for each therapy [4–6, 24, 25]. A mortality benefit was not observed for nintedanib in either individual trial nor in the pooled analysis [24, 25]. In the pirfenidone phase 3 studies, a mortality benefit was observed in one out of three phase 3 trials and in the pooled analysis [4–6]. Thus, the observed limited reduction in the rate of acute exacerbations and subsequent death of patients treated with combination therapy may warrant further investigation, as this study was powered to show only lung function changes.
Other clinical trials in IPF that have assessed IL-13 antibodies have also demonstrated a lack of efficacy in this patient population, suggesting that IL-13 may not be an appropriate therapeutic target in IPF. A phase 2 randomised, double-blind, placebo-controlled trial that assessed the safety, tolerability and change in FVC at 52 weeks of the anti–IL-13 QAX576 in 60 patients with IPF was terminated [26]. A phase 2, randomised, double-blind, placebo-controlled trial that assessed change in % predicted FVC at 72 weeks of the anti–IL-13 tralokinumab in 302 patients with IPF was terminated due to lack of efficacy after the interim analysis [27]. A phase 2, randomised, double-blind, placebo-controlled trial that assessed change in % predicted FVC at 52 weeks of the anti–IL-13/IL-4 SAR156597 in 325 patients with IPF showed no significant difference in the primary efficacy endpoint [28]. However, a similar trend towards a decrease in acute exacerbations was reported in cohort B. The reported rates of acute exacerbations in the IPF clinical trial setting is variable (4–28%) likely due to differences in study population, acute exacerbation definition and statistical methods [29]. In cohort B, the event rate in the lebrikizumab plus pirfenidone arm was 2.9% (five acute exacerbations) versus 6.2% (11) in placebo plus pirfenidone arm (HR 0.45 (0.16–1.31); p=0.1346). Although the overall number of acute exacerbations was low and the difference in the rate of acute exacerbations did not reach statistical significance, it is interesting to note that there were no deaths associated with acute exacerbations in the lebrikizumab plus pirfenidone arm, while seven of 11 patients in the placebo plus pirfenidone arm died, consistent with reported rates of mortality post-acute exacerbation ≥50%. While these findings are similar to results reported in the SAR15697 trial targeting IL-4 and IL-13, there was no evidence of a treatment difference between lebrikizumab and placebo in cohort A (HR 1.21 (0.41–3.61); p=0.73).
The pharmacokinetics (e.g. half-life estimates) and pharmacodynamics of lebrikizumab were similar in both cohorts, suggesting that background pirfenidone therapy did not impact lebrikizumab concentrations or activity. Pharmacodynamic measures supported lebrikizumab biological activity, both alone and in the presence of background pirfenidone therapy. Furthermore, efficacy/response relationships were generally flat across endpoints and did not suggest that increased dosing would have led to greater improvements in efficacy endpoints.
This study has several limitations. Each independent cohort was powered to detect differences in lung function between lebrikizumab and placebo treatment arms, but not differences in other outcomes. The approval of two antifibrotic therapies in late 2014 contributed to early patient discontinuation in cohort A, which limited the amount of data captured to evaluate lebrikizumab as monotherapy. During the recruitment phase of cohort A, 168 patients (51.6%) screened did not meet eligibility criteria similar to other anti-IL-13 trials executed between 2012 and 2016. It has been suggested that many of the patients referred to the anti-IL-13 trials may have been screening failures from the large phase 3 trials completing at that time and thus may not reflect the real-world population or populations studied in the prior phase 3 trials. In contrast, FVC and DLCO measurements used standardised equipment, training and efforts with central over-read; thus, standardisation issues did not likely contribute to the lack of observed effect.
Lebrikizumab as monotherapy and in combination with pirfenidone was well tolerated, with a favourable safety profile, consistent with those reported for lebrikizumab and pirfenidone individually in phase 2 and 3 clinical trials [4, 5, 30, 31]. The incidence of lebrikizumab or PLBL2 antibodies in both lebrikizumab- and placebo-treated patients did not appear to affect pharmacokinetics or safety.
In conclusion, lebrikizumab monotherapy or in combination with background pirfenidone therapy did not reduce lung function decline or provide other clinically significant benefits in patients with IPF.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02442-2019.SUPPLEMENT (675.4KB, pdf)
Shareable PDF
Acknowledgments
We thank the patients and their families who participated in this study. The RIFF study was funded by F. Hoffmann-La Roche Ltd and Genentech, Inc. We thank Becca Sheng of Genentech, Inc., for contributions to the pharmacodynamics analysis. Support for third-party writing assistance, furnished by Christine Gould of Health Interactions, Inc., was provided by F. Hoffmann-La Roche Ltd.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This study is registered at clinicaltrials.gov as NCT01872689. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's global policy on the sharing of clinical information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Author contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; all authors contributed substantially to the study design, the data analysis and interpretation and the writing of the manuscript.
Conflict of interest: T.M. Maher reports grants from GlaxoSmithKline and University of California, Berkeley (UCB), during the conduct of the study; and he served as a consultant and speaker for Apellis, AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, GlaxoSmithKline, Indalo, Novartis, Pliant, ProMetic, Respivnat, Roche, Samumed and UCB.
Conflict of interest: U. Costabel was a member of a study adjudication committee for Gilead; has served as a consultant and speaker for Bayer, Boehringer Ingelheim and InterMune/Roche; has received grants from Boehringer Ingelheim and InterMune and has served as a consultant for Centocor, Fibrogen, Gilead, GlaxoSmithKline, UCB Celltech and Biogen.
Conflict of interest: M.K. Glassberg reports grants from Genentech/Roche during the conduct of the study; was a member of the ASCEND study steering committee; and has served as a consultant for Bellerophon, Boehringer Ingelheim, Bristol Myers Squibb, InterMune, RedX Pharma and Roche.
Conflict of interest: Y. Kondoh reports grants from Ministry of Health, Labour and Welfare, Japan, during the conduct of the study and has served as a consultant for Genentech and has served as a consultant and speaker for Asahi Kasei, Boehringer Ingelheim, Eisai, Janssen, Kyorin, Mitsubishi Tanabe Pharma, Novartis and Shionogi.
Conflict of interest: T. Ogura reports grants from Boehringer Ingelheim and the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study and has served as a consultant or speaker for Asahi Kasei, Boehringer Ingelheim, Eisai, Nitto Denko, Shionogi and Toray.
Conflict of interest: M.B. Scholand has served on advisory boards for Boehringer Ingelheim and InterMune/Roche/Genentech.
Conflict of interest: D. Kardatzke is an employee of Genentech, Inc.
Conflict of interest: M. Howard is an employee of Genentech, Inc.
Conflict of interest: J. Olsson is an employee of Genentech, Inc.
Conflict of interest: M. Neighbors is an employee of Genentech, Inc.
Conflict of interest: P. Belloni is an employee of Genentech, Inc.
Conflict of interest: J.J. Swigris reports grants from InterMune, personal fees from Genentech, Inc., during the conduct of the study; was a member of the ASCEND study steering committee; served on a scientific advisory board for InterMune and served as a consultant to Boehringer Ingelheim and Roche.
Support statement: This study and manuscript were sponsored by F. Hoffmann-La Roche Ltd. and Genentech, Inc. The sponsor contributed to study design, data collection, data analysis, data interpretation, and writing of the report. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ley B, Collard HR, King TE, Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 431–440. doi: 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 2.Lynch JP, Belperio JA. Idiopathic pulmonary fibrosis In: Baughman RP, du Bois RM, eds. Diffuse lung disease: a practical approach. Switzerland, Springer Nature, 2012; pp. 171–194. [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 5.King TE J, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 6.Nathan SD, Albera C, Bradford WZ, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med 2017; 5: 33–41. doi: 10.1016/S2213-2600(16)30326-5 [DOI] [PubMed] [Google Scholar]
- 7.Chiaramonte MG, Donaldson DD, Cheever AW, et al. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999; 104: 777–785. doi: 10.1172/JCI7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, et al. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 1998; 101: 2129–2139. doi: 10.1172/JCI741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallon PG, Richardson EJ, McKenzie GJ, et al. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 2000; 164: 2585–2591. doi: 10.4049/jimmunol.164.5.2585 [DOI] [PubMed] [Google Scholar]
- 10.Oriente A, Fedarko NS, Pacocha SE, et al. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 2000; 292: 988–994. [PubMed] [Google Scholar]
- 11.Chung SI, Horton JA, Ramalingam TR, et al. IL-13 is a therapeutic target in radiation lung injury. Sci Rep 2016; 6: 39714. doi: 10.1038/srep39714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias JA, Zheng T, Lee CG, et al. Transgenic modeling of interleukin-13 in the lung. Chest 2003; 123: Suppl. 3, 339S–345S. doi: 10.1378/chest.123.3_suppl.339S [DOI] [PubMed] [Google Scholar]
- 13.Fichtner-Feigl S, Strober W, Kawakami K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 2006; 12: 99–106. doi: 10.1038/nm1332 [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Jin H, Ullenbruch M, et al. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol 2004; 173: 3425–3431. doi: 10.4049/jimmunol.173.5.3425 [DOI] [PubMed] [Google Scholar]
- 15.Kolodsick JE, Toews GB, Jakubzick C, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 2004; 172: 4068–4076. doi: 10.4049/jimmunol.172.7.4068 [DOI] [PubMed] [Google Scholar]
- 16.Chandriani S, DePianto DJ, N'Diaye EN, et al. Endogenously expressed IL-13Ralpha2 attenuates IL-13-mediated responses but does not activate signaling in human lung fibroblasts. J Immunol 2014; 193: 111–119. doi: 10.4049/jimmunol.1301761 [DOI] [PubMed] [Google Scholar]
- 17.Jakubzick C, Kunkel SL, Puri RK, et al. Therapeutic targeting of IL-4- and IL-13-responsive cells in pulmonary fibrosis. Immunol Res 2004; 30: 339–349. doi: 10.1385/IR:30:3:339 [DOI] [PubMed] [Google Scholar]
- 18.Park SW, Ahn MH, Jang HK, et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci 2009; 24: 614–620. doi: 10.3346/jkms.2009.24.4.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neighbors M, Cabanski CR, Ramalingam TR, et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir Med 2018; 6: 615–626. doi: 10.1016/S2213-2600(18)30185-1 [DOI] [PubMed] [Google Scholar]
- 20.Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol 2013; 425: 1330–1339. doi: 10.1016/j.jmb.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 21.Genentech I Esbriet (pirfenidone) capsules and film-coated tablets, for oral use [package insert]. South San Francisco, CA, 2017. [Google Scholar]
- 22.Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 2015; 70: 748–756. doi: 10.1136/thoraxjnl-2014-206719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swigris JJ, Wilson SR, Green KE, et al. Development of the ATAQ-IPF: a tool to assess quality of life in IPF. Health Qual Life Outcomes 2010; 8: 77. doi: 10.1186/1477-7525-8-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 25.Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS® trials. Respir Med 2016; 113: 74–79. doi: 10.1016/j.rmed.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med 2014; 190: 867–878. doi: 10.1164/rccm.201403-0509PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker JM, Glaspole IN, Lancaster LH, et al. A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2018; 197: 94–103. doi: 10.1164/rccm.201704-0784OC [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Richeldi L, Crestani B, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772). Eur Respir J 2018; 52: 1801130. doi: 10.1183/13993003.01130-2018 [DOI] [PubMed] [Google Scholar]
- 29.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 30.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011; 365: 1088–1098. doi: 10.1056/NEJMoa1106469 [DOI] [PubMed] [Google Scholar]
- 31.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016; 4: 781–796. doi: 10.1016/S2213-2600(16)30265-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02442-2019.SUPPLEMENT (675.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02442-2019.Shareable (380.2KB, pdf)