Abstract
Aim
A decrease in proteinuria has been considered protective from renal damage in lupus nephritis (LN), but a cut-off point has yet to be established. The aim of this study was to identify the predictors of renal damage in patients with LN and to determine the best cut-off point for a decrease in proteinuria.
Methods
We included patients with LN defined clinically or histologically. Possible predictors of renal damage at the time of LN diagnosis were examined: proteinuria, low complement, anti-double-stranded DNA antibodies, red cell casts, creatinine level, hypertension, renal activity (assessed by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)), prednisone dose, immunosuppressive drugs and antimalarial use. Sociodemographic variables were included at baseline. Proteinuria was assessed at baseline and at 12 months, to determine if early response (proteinuria <0.8 g/day within 12 months since LN diagnosis) is protective of renal damage occurrence. Renal damage was defined as an increase of one or more points in the renal domain of The Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI). Cox regression models using a backward selection method were performed.
Results
Five hundred and two patients with systemic lupus erythematosus patients were included; 120 patients (23.9%) accrued renal damage during their follow-up. Early response to treatment (HR=0.58), antimalarial use (HR=0.54) and a high SES (HR=0.25) were protective of renal damage occurrence, whereas male gender (HR=1.83), hypertension (HR=1.86) and the renal component of the SLEDAI (HR=2.02) were risk factors for its occurrence.
Conclusions
Early response, antimalarial use and high SES were protective of renal damage, while male gender, hypertension and higher renal activity were risk factors for its occurrence in patients with LN.
Keywords: Lupus Erythematosus, Systemic, Lupus Nephritis, Autoimmune Diseases
Key messages.
What is already known about this subject?
Most of the response criteria for lupus nephritis include proteinuria <0.5 g/day at 12 months.
However, proteinuria <0.8 g/day at 12 months has proven to be a good predictor of long-term renal damage.
What does this study add?
The proteinuria cut-off point of <0.8 g/day at 12 months was protective of the occurrence of early renal damage.
Antimalarial use and high SES were also protective of renal damage, while male gender, hypertension and higher renal activity were risk factors for its occurrence.
How might this impact on clinical practice?
The proteinuria cut-off point at 12 months needs to be re-evaluated.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease of unknown aetiology. Lupus nephritis (LN) is one of its most common and serious manifestations. A recent, large multiethnic, multinational, inception cohort of 1827 patients reported that LN occurred in 700 (38.3%) of their patients with SLE and was more frequent in those of African, Asian or Hispanic ancestry. Despite standard of care, the estimated 10-year incidence of end-stage renal disease (ESRD) in these patients with LN was 4.3%.1 Additionally, LN significantly reduces the survival of patients with SLE, in particular in the proliferative types.2 3
Treatment of LN should aim at inducing remission of kidney inflammation and maintaining remission of kidney inflammation so as to preserve renal function and improve survival.4 In fact, the induction of clinical remission in LN is predictive of long-term patient prognosis as well as of renal survival.5 There is no universal agreement on the definition of nephritis remission or low disease activity state, as well as the time points for changing therapies in these patients.4 It has been identified that even a partial remission has significantly better outcomes in those patients compared with those who did not achieve it.6
Although there are markers of LN such as proteinuria and haematuria, these findings are not sufficient to predict the extent and severity of kidney damage. This reinforces the need to identify predictors of renal damage accrual in patients with SLE and act upon them.
Previous studies have shown that the prognosis of LN depends on several factors including sociodemographic, clinical, serological and genetic features.7 It has also been demonstrated that higher uric acid levels contribute to the development of new renal damage in Peruvian patients with SLE.8 In addition, renal markers such as low creatinine clearance, proteinuria, hypertension and nephrotic syndrome are associated with poor prognosis among patients with LN.9
In a Caucasian population, proteinuria <0.8 g/24 hours at 12 months has proven to be a predictor of good outcome in patients of the Euro-Lupus Nephritis Trial; the outcome was defined as a serum creatinine ≤1 mg/dl 7 years after entry into the trial (sensitivity=81% and specificity=78%).10 In the MAINTAIN Nephritis Trial, proteinuria <0.7 g/day was the best predictor of long-term renal outcome (sensitivity=71% and specificity=75%).11 In a Brazilian lupus population with severe nephritis from a tertiary centre (baseline mean creatinine of 1.73±1.34 mg/dL), in which only 40% of the patients were non-Caucasian, demonstrated that proteinuria <0.8 g/24 hours at 12 months of follow-up was the best predictor of long-term renal outcome.12 These three studies, however, evaluated only long-term renal damage, and therefore, they excluded patients with early development of ESRD, that is, within the first year after the nephritis episode.
There is limited information about predictors of kidney damage particularly in multiethnic lupus patients. Due to this, the objective of this study was to identify factors predictive of short-term renal damage in our Latin American population, a large, mixed race cohort with a standard follow-up.
METHODS
As previously described, GLADEL (Grupo Latino-Americano De Estudio del Lupus) is a multiethnic, multinational, multicenter cohort, including 34 centres from nine Latin American countries participated by randomly incorporating patients with SLE within 2 years of diagnosis.13 Recruitment started in 1997 and finished in 2004. Patients were included in the cohort based on the physician’s diagnosis; however, the large majority fulfilled four or more of the American College of Rheumatology (ACR) classification criteria for SLE.14
Data included socioeconomic–demographic and clinical characteristics, treatment features and laboratory tests. The general characteristics and composition of the entire GLADEL cohort have been described in detail elsewhere.13
For this study, we included patients with LN defined clinically (proteinuria greater than 0.5 g/day on two or more occasions or the presence of red cell casts) or histologically (renal biopsy compatible with LN histopathology classes II, III, IV, V according to the WHO).15 For these analyses, the follow-up started at the time LN was defined. Patients with ESRD (regardless of dialysis or transplantation) were excluded.
The following variables were considered as possible predictors of renal damage: proteinuria, low complement, anti-double-stranded DNA, red cell casts, creatinine level at the time LN was defined, hypertension, the renal component of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), prednisone dose, use of immunosuppressive drugs and antimalarials. The following baseline variables were also included: gender, age at nephritis diagnosis, residence: rural or urban, ethnic group and socioeconomic status (SES).
Proteinuria was assessed at LN diagnosis and after 12 months, to determine if early response defined as proteinuria <0.8 g/day within 12 months from the diagnosis of LN is protective of renal damage occurrence in patients with SLE. Previous reports studying long-term prognosis included patients with at least 7 years of follow-up; we defined short-term as a follow-up of less than 7 years. Renal damage was defined as an increase of at least one point in the renal domain of the SLICC/ACR damage index.
STATISTICAL ANALYSES
Categorical variables were summarised as frequencies and percentages, whereas continuous variables were presented as medians and their interquartile range (IQRs).
In order to evaluate the predictors of renal damage, univariable and multivariable Cox regression models were performed using a backward selection method with an alpha level to stay in the model set at 0.05. We performed a model using the definition for early response as proteinuria <0.8 g/day. We also made a receiver operating characteristic (ROC) curve to determine the best cut-off point for proteinuria.
Three additional models were also carried out; one in which the renal component of the SLEDAI was omitted, a second one in which the components of renal damage were examined separately, and a third one in which only patients with biopsy-proven LN were included. Finally, a comparison between patients with proliferative and non-proliferative nephritis was also carried out.
Statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute, Cary, NC, USA).
RESULTS
A total of 502 patients who fulfilled the inclusion criteria were included. Demographic and disease-related features are shown in table 1. Most of the patients were females (451; 89.8%), with a median age at SLE diagnosis of 26.0 IQR (19.0–35.0) years and median age at nephritis diagnosis of 27.0 (20.3–36.2) with a median follow-up after nephritis diagnosis of 3.6 (1.6–5.2) years. Two hundred and one (40.2%) were Caucasian, 224 (44.8%) were Mestizo, 58 (11.6%) were African-Latin American. At baseline, the median serum creatinine was 1.0 mg/dl IQR (0.8–1.2).
Table 1.
Baseline features of patients with SLE with lupus nephritis defined clinically or histologically (N=502) and defined histologically (N=241) from the GLADEL cohort
| Variable | N=502 | N=241 |
|---|---|---|
| Female gender, n (%) | 451 (89.8) | 211 (87.6) |
| Age at diagnosis, years, median (IQR) | 26.0 (19.0–35.0) | 26.0 (20.0–34.0) |
| Age at nephritis diagnosis, years, median (IQR) | 27.0 (20.3–36.2) | 26.8 (20.3–34.7) |
| Follow-up after nephritis diagnosis, years, median (IQR) | 3.6 (1.6–5.2) | 2.9 (0.9–4.9) |
| Creatinine at baseline, mg/dl, median (IQR) | 1.0 (0.8–1.2) | 1.0 (0.8–1.4) |
| Proteinuria at baseline, mg/day, median (IQR) | 1154 (500.0–2690.0) | 1600 (700–3210) |
| SDI at baseline, median (IQR) | 1.0 (0.0–2.0) | 1 (0–2) |
| Ethnic group | ||
| Caucasian, n (%) | 201/500 (40.2) | 88 (36.7) |
| Mestizo, n (%) | 224/500 (44.8) | 117 (48.7) |
| Afro Latin-American, n (%) | 58/500 (11.6) | 25 (10.4) |
| Other, n (%) | 17/500 (3.4) | 10 (4.2) |
| Socioeconomic status | ||
| High, n (%) | 31/499 (6.2) | 13 (5.4) |
| Medium, n (%) | 150/499 (30.1) | 83 (34.4) |
| Low, n (%) | 318/499 (63.7) | 145 (60.2) |
| Residency | ||
| Urban, n (%) | 456/500 (91.2) | 221 (92.1) |
| Rural, n (%) | 44/500 (8.8) | 19 (7.9) |
IQR, Interquartile range; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
One hundred and twenty (23.9%) patients achieved early response defined as proteinuria <0.8 g/day.
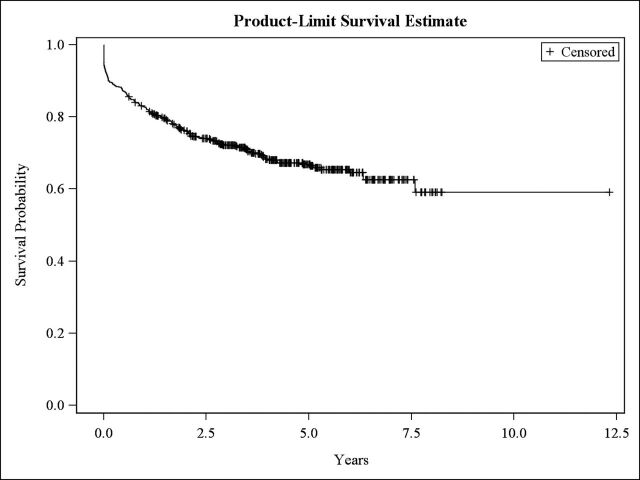
One hundred and sixty-one patients (32.1%) accrued renal damage during their follow-up (figure 1).
Figure 1.
Kaplan-Meier curve: Renal damage-free survival curve. Patients with LN (clinically or biopsy-proven). n=502.
Univariable and multivariable models are depicted in table 2. Early response to treatment defined as proteinuria <0.8 g/day within 12 months from the diagnosis of LN (HR=0.58) and antimalarial use (HR=0.54) and a high SES (HR=0.25) were protective of the occurrence of renal damage, whereas male gender (HR=1.83), hypertension (HR=1.86) and the renal component of the SLEDAI (HR=2.02) were risk factors for renal damage occurrence.
Table 2.
Predictors of renal damage in patients with lupus nephritis*
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age at nephritis diagnosis | 0.99 (0.97–1.00) | 0.049 | ||
| Gender (male) | 1.60 (1.04–2.48) | 0.033 | 1.83 (1.17–2.86) | 0.008 |
| Residence (rural) | 0.87 (0.49–1.53) | 0.620 | ||
| Ethnicity | ||||
| Caucasian | Ref. | |||
| Mestizo | 1.51 (1.07–2.13) | 0.191 | ||
| African Latin-American | 1.41 (0.84–2.35) | 0.195 | ||
| Others | 1.04 (0.38–2.89) | 0.933 | ||
| SES | ||||
| High | 0.27 (0.10–0.74) | 0.011 | 0.25 (0.09–0.69) | 0.007 |
| Medium | 0.62 (0.43–0.89) | 0.001 | 0.70 (0.49–1.01) | 0.058 |
| Low | Ref. | Ref. | ||
| Early treatment response | 0.49 (0.32–0.76) | 0.002 | 0.57 (0.37–0.90) | 0.014 |
| Proteinuria | 1.39 (0.72–2.59) | 0.338 | ||
| Low complement | 1.42 (1.03–1.96) | 0.032 | ||
| Anti-dsDNA | 1.28 (0.94–1.75) | 0.118 | ||
| Red cell casts | 2.10 (1.54–2.87) | <0.001 | ||
| Creatinine level | 1.18 (1.09–1.26) | <0.001 | ||
| Hypertension | 2.37 (1.70–3.30) | <0.001 | 1.86 (1.31–2.64) | <0.001 |
| Renal component of SLEDAI | 2.26 (1.62–3.16) | <0.001 | 2.02 (1.43–2.84) | <0.001 |
| Prednisone dose | 1.01 (1.00–1.01) | 0.008 | ||
| Immunosuppressive drugs use | 1.50 (1.10–2.05) | 0.011 | ||
| Antimalarial use | 0.454 (0.37–0.79) | <0.001 | 0.54 (0.37–0.79) | 0.002 |
*Clinically or histologically.
Anti-dsDNA, Anti-double-stranded DNA; SES, Socioeconomic status; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
In the ROC curve, we found that the cut-off point of 0.8 g/day had good sensitivity and specificity, with area under curve of 0.57, better than 0.5 g/day and 0.7 g/day with 0.53 and 0.56, respectively.
In the alternative analysis in which the renal component of the SLEDAI was omitted, the results were consistent with those of the main analysis. Early response defined as proteinuria <0.8 g/day within 12 months from the diagnosis of LN (HR=0.57) and antimalarial use (HR=0.55) where protective of the occurrence of renal damage, whereas male gender (HR=1.74) and hypertension (HR=2.20) were risk factors for renal damage occurrence.
The components of renal damage: glomerular filtration rate (GFR) <50%, proteinuria >3.5 g/day and ESRD have been examined as three distinct endpoints.
Eighty-nine patients (89/502) reached GFR <50%. In a multivariable analysis, antimalarial use (OR 0.51, p=0.024) and azathioprine (OR 0.08, p=0.026) use were protective, while hypertension (OR 3.55, p<0.001) and cyclophosphamide use (OR 1.73, p=0.0405) were predictors of damage.
Twenty-four patients (24/502) reached ESRD, but we were not able to identify any predictor in the analyses performed (data nor shown).
Ninety-five patients (95/502) reached proteinuria ≥3.5 g/day for at least 6 months. In the multivariable analysis, early response defined as proteinuria <0.8 g/day was protective of nephrotic damage (OR 0.31, p<0.001), whereas the presence of red cellular cast (OR 3.34, p<0.001) and male gender (OR 3.80, p<0.001) were associated with damage manifested as proteinuria ≥3.5 g/day.
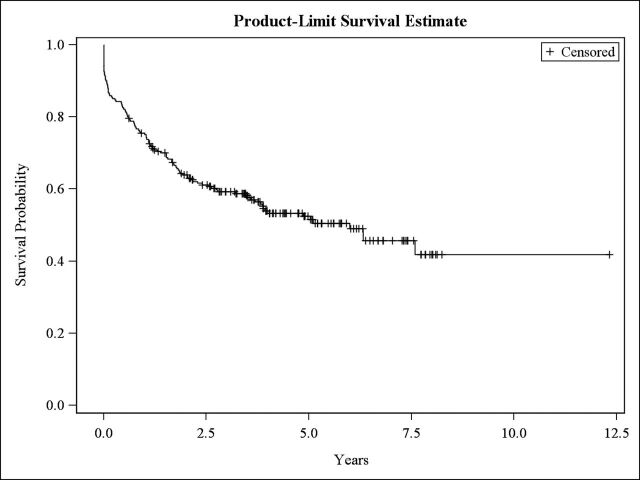
Additional analyses were performed in those patients with biopsy-proven LN (n=241) and in those with proliferative nephritis (n=162); as per the renal biopsy data, 58 patients were classified as (24.1%) Class II, 24 (9.9%) as Class III, 138 (57.3%) as Class IV and 21 (8.7%) as Class V LN. Of them, 113 (46.9%) accrued renal damage during the follow-up (figure 2). We observed differences in terms of creatinine levels (p=0.009) between both groups, and difference in terms of proteinuria values being higher in those undergoing a renal biopsy (p=<0.001). High (HR=0.24) SES was protective from damage, whereas hypertension (HR=1.75) was predictive of damage in the multivariable analyses, as depicted in table 3.
Figure 2.
Kaplan-Meier curve: Renal damage-free survival curve. Patients with LN (biopsy-proven). n=241.
Table 3.
Predictors of renal damage. Patients with biopsy-proven lupus nephritis (n=241)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at nephritis diagnosis | 0.99 (0.97–1.01) | 0.275 | ||
| Gender (male) | 1.11 (0.66–1.86) | 0.687 | ||
| Residence (rural) | 0.64 (0.30–1.38) | 0.253 | ||
| Ethnicity | ||||
| Caucasian | Ref. | |||
| Mestizo | 1.02 (0.68–1.53) | 0.930 | ||
| Afro Latin-American | 1.53 (0.85–2.770) | 0.159 | ||
| Others | 0.46 (0.11–1.92) | 0.288 | ||
| SES | ||||
| High | 0.21 (0.05–0.87) | 0.032 | 0.24 (0.06–0.897) | 0.045 |
| Medium | 0.69 (0.46–1.03) | 0.072 | 0.71 (0.48–1.07) | 0.101 |
| Low | Ref. | Ref. | ||
| Early treatment response | 0.54 (0.32–0.94) | 0.028 | 0.67 (0.38–1.16) | 0.151 |
| Proteinuria | 2.01 (1.0564–3.85) | 0.036 | ||
| Low complement | 1.28 (0.88–1.86) | 0.204 | ||
| Anti-dsDNA | 0.91 (0.63–1.32) | 0.228 | ||
| Creatinine level | 1.21 (1.05–1.38) | 0.007 | ||
| Hypertension | 1.91 (1.29–2.83) | 0.001 | 1.75 (1.18–2.61) | 0.006 |
| Renal component of SLEDAI | 1.41 (0.95–2.09) | 0.086 | ||
| Prednisone dose | 1.00 (0.99–1.66) | 1.019 | ||
| Immunosuppressive drugs use | 1.01 (0.69–1.47) | 0.958 | ||
| Antimalarial use | 0.67 (0.426–1.08) | 0.101 | ||
| Non-proliferative biopsy | 0.748 (0.49–1.14) | 0.178 |
Anti-dsDNA, anti-double-stranded DNA; SES, Socioeconomic status; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
We compared proliferative (Class III and IV) vs non-proliferative nephritis (Class II and V). We found that non-proliferative nephritis accrued less damage (HR 0.75), however this finding was not statistically significant (p=0.178)
DISCUSSION
Organ damage accrual is a predictor of further damage, morbidity and early mortality.16 Treat-to-target approach aims to improve management of SLE through target-based goals17; however, the treatment goal in LN has not been clearly defined. This study, performed in a Latin American population, demonstrated that early response defined as proteinuria <0.8 g/day within 12 months from the diagnosis of LN, antimalarial use and a higher SES are protective of renal damage occurrence. On the other hand, male gender, hypertension and renal activity were predictive of renal damage.
Regarding sociodemographic factors, male gender was a risk factor, and high SES was protective of renal damage occurrence in patients with SLE. Similarly, Cheng et al found that the phenotypic pattern and disease activity varied between Chinese patients with SLE by regional economic factors as educational level, availability of medical personnel and area of residency.18 In the Mestizo population, it has been demonstrated that rural residency is associated with high levels of disease activity and renal disease occurrence.19 We therefore do not have a good explanation for the fact that urban residence was predictive of renal damage, although this was only evident in the subgroup of patients with biopsy-proven LN.
We hypothesise that rural patients who require a renal biopsy need to be referred to an urban medical facility for the procedure; the ability to do so may reflect a higher SES in these patients than in those who ended up not having a biopsy.
To the best of our knowledge, there are no reports about SES and its relationship with renal damage in patients with SLE.
A high score in the renal domain of the SLEDAI was predictive of damage; similar results were reported by Kandane et al; patients with active LN were more likely to accrue any organ damage compared with those without active LN [HR = 1.52 95% CI: (1.16–1.97), p=0.02].20
The impact of proteinuria on renal damage was previously described in a Japanese population, in which achieving complete renal response (CR) was based on the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for LN, which were: a urine protein-to-creatinine ratio 50 mg/mmol and normal or near-normal renal function; at 3 or 6 months was associated with maintaining CR at 3 years (p=0.029 and p=0.009, respectively).21
Likewise, the impact of proteinuria at 12 months on renal damage is consistent with the long-term data from the Euro-Lupus Nephritis Trial, which included only Caucasian patients; the absolute level of proteinuria of <0.8 g/day at 12 months was the best individual predictor of good long-term renal outcome at 7 years in these patients.10 Likewise, in another Caucasian cohort, a very similar proteinuria cut-off (<0.7 g/day at 12 months) to predict good long-term outcome11 was found. Importantly, in both studies, the addition of creatinine to the analyses did not improve the performance of proteinuria alone. And, in a population of mixed ethnic background, Ugolini et al reported in a real-life clinical setting, that proteinuria <0.8 g/24 hours at 12 months was the best single predictor of being free from dialysis at 7 years, in patients with severe biopsy-proven LN.12
These studies, taking together, support the notion that a cut-off point different from proteinuria ≤0.5 g/day is needed; based on our current study and those of others, we suggest that the cut-off point of 0.8 g/day 12 months after LN onset could be a useful target. In fact, the 2019 update of the EULAR recommendations include proteinuria ≤0.8 g/day as a predictor of a favourable long-term outcome.22 In the analysis in which the individual components of the renal damage rather than overall renal damage were considered as the outcome, we found that the use of azathioprine was protective, whereas the use of cyclophosphamide was a predictor of a decrease in GFR <50%; we think that this is because cyclophosphamide could be used to treat patients with more severe manifestations of nephritis. We did not find the urine red blood cell count as a predictor in our analysis; we did so based on previous results in which the addition of urine red blood cells ≤5/hpf to proteinuria at 12 months decreased the sensitivity to predict good long-term renal disease.10 11
Importantly, the present study confirms that antimalarial use protects from the development of renal damage. Previously, Pons-Estel et al have shown evidence that antimalarials are negatively associated with the occurrence of SLE renal disease23 24; taken together, our data reinforces the importance of the use of antimalarials in the treatment of SLE.
Additionally, we found hypertension to be a predictor of damage; similar information has been reported in Chinese patients with LN in which hypertension was an independent risk factor for chronic kidney disease [HR=2.432, 95% CI: (1.575–3.754), p<0.001]25; hypertension at nephritis onset and uncontrolled hypertension have been associated with adverse outcomes in patients with SLE.26
Our study has some limitations. First, the relatively short time of follow-up precludes us to evaluate the long-term impact on damage accrual; second, not all patients had a renal biopsy. However, in the analyses of the subset with biopsy, the results were quite similar. Adherence was not assessed in the GLADEL cohort and that precluded us to make more confident conclusions about its impact on treatment. Another limitation of our study is the time period when patients were recruited, that was between 1997 and 2004; back then the knowledge about the disease, diagnostic strategies, availability and access to treatments and the use of the WHO classification rather than The 2003 International Society of Nephrology (ISN)/Renal Pathology Society (RPS) classification of lupus nephritis currently being used calls for caution in the interpretation of the results presented. Finally, the distinction between damage and flares was based on the physician’s opinion rather than on histopathological information.
An advantage of the present study, however, is the multiethnic nature of the population studied, with the large number of patients included. Additionally, it is important to consider that previous long-term studies have excluded patients who developed ESRD in the first year after LN onset, and the fact that we made a sub-analysis including the components of renal damage as an outcome rather than just the creatinine values.10–12
In conclusion, data from this large, multiethnic Latin American SLE cohort show that early response to treatment, antimalarial use and high SES were protective of renal damage occurrence. On the other hand, male gender, the presence of hypertension and a higher score in the renal domain of the SLEDAI were risk factors for its occurrence/progression. This emphasises the need to control these risk factors, particularly hypertension and initiate aggressive treatment of nephritis as early as possible. Taken together with the available literature, our data strongly support changing the proteinuria cut-off point at 12 months from 0.5 g/day to 0.8 g/day.
Footnotes
Twitter: Guillermo Pons-Estel @gponsestel and Manuel F Ugarte-Gil @mugartegil.
Acknowledgements: We are grateful to Daniel Villalba and Leonardo Grasso for providing expert assistance with the ARTHROS (version 6.0) software. All authors were involved in drafting or revising this article critically for important intellectual content, and all authors approved the final version to be published. Dr Cristina Reategui-Sokolova, Manuel F. Ugarte-Gil, Graciela S. Alarcón, and Bernardo A. Pons-Estel have full access to all the data from the study and take responsibility for their integrity and the accuracy of the analyses performed. We hank our GLADEL patients and staff who made this cohort possible. Preliminary results were presented at the 2019 ACR Congress. https://acrabstracts.org/abstract/predictors-of-renal-damage-in-systemic-lupus-erythematosus-patients-from-latin-america/?msg=fail&shared=email
Collaborators: On behalf of GLADEL, María Flavia Ceballos Recalde, Edson Velozo, Jorge A Manni, Sebastián Grimaudo, Judith Sarano, Emilce Schneeberger, María S Arriola, Graciela Gómez, Ana Inés Marcos, Juan Carlos Marcos†, Hugo R Scherbarth†, Jorge A López, Estela L Motta, Cristina Drenkard, Susana Gamron, Laura Onetti, Sandra Buliubasich, Silvana Gentiletti†, Norberto Quagliatto†, Alberto A Gentiletti, Daniel Machado†, Marcelo Abdala, Simón Palatnik†, Guillermo A Berbotto, Carlos A Battagliotti†, Alexandre Wagner S. Souza, Lilian T Lavras Costallat, Manoel Barros Bertolo, Ibsen Bellini Coimbra, Joao C Tavares Brenol, Ricardo Xavier, Tamara Micênic, Ângela Luzia Branco Duarte, Cláudia Diniz Lopes Marques, Tatiana Ferracine Pacheco, José Fernando Molina-Restrepo, Javier Molina-López, Luis A Ramírez, Oscar Uribe, Antonio Iglesias-Rodríguez, Eduardo Egea-Bermejo, Renato A Guzmán-Moreno, José F Restrepo-Suarez, Alfredo Hernández-Martínez, Sergio Iacobelli, Leonardo R Guzmán, Abraham García-Kutzbach, Claudia Castellanos, Erwin Cajas, Donato Alarcón-Segovia†, Antonio R Villa, Gerardo Orozco-Barocio, Magali L Estrada-Contreras, María Josefina Sauza del Pozo, Laura E Aranda Baca, Adelfia Urenda Quezada, Guillermo F Huerta-Yañez, José Luis Alfaro-Lozano, Jorge M Cucho-Venegas, Cecilia P Chung, Magaly Alva-Linares, Isaac Abadi, Neriza Rangel, Jorge Vivas.
†Deceased.
Contributors: DW, GJP-E, RQ, RMS, MPS, LJC, ERS, MAG, AA, FC, GAB, EIS, EFBN, EB, ACdOeSM, NADS, FC, GV, MG-T, GAR-L, LM, OJN, MHC, LAB-F, M-CA, LHS, MP-H, IGdlT, MIS, RC-D and MHE-S contributed to the conception and drafting of the article. CR-S, MFU-G, GBH, BAP-E and GA participated in every phase of the conception and drafting of the article. All listed authors provided critical revision for important intellectual content and final approval.
Funding: The GLADEL cohort received no specific funding from agencies in the public, commercial, or not-for-profit sectors for the execution of this work.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability statement: Data are available in a public, open-access repository. All data relevant to the study are included in the article or uploaded as supplemental information.
REFERENCES
- 1. Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 2016;55:252–62. 10.1093/rheumatology/kev311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danila MI, Pons-Estel GJ, Zhang J, et al. Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology 2008;48:542–5. 10.1093/rheumatology/kep012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mok CC, Kwok RCL, Yip PSF. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:2154–60. 10.1002/art.38006 [DOI] [PubMed] [Google Scholar]
- 4. Mok CC Is treat-to-target in lupus nephritis realistic in clinical practice? Curr Rheumatol Rev 2018;15:2–6. 10.2174/1573397114666180406100857 [DOI] [PubMed] [Google Scholar]
- 5. Korbet SM, Lewis EJ, Schwartz MM, et al. Factors predictive of outcome in severe lupus nephritis. Lupus nephritis collaborative study group. Am J Kidney Dis 2000;35:904–14. 10.1016/S0272-6386(00)70262-9 [DOI] [PubMed] [Google Scholar]
- 6. Chen YE, Korbert SM, Katz RS, et al. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 2008;3:46–53. 10.2215/CJN.03280807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alarcón GS, McGwin G, Petri M, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med 2006;3:e396 10.1371/journal.pmed.0030396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reátegui-Sokolova C, Ugarte-Gil MF, Gamboa-Cárdenas RV, et al. Serum uric acid levels contribute to new renal damage in systemic lupus erythematosus patients. Clin Rheumatol 2017;36:845–52. 10.1007/s10067-017-3538-4 [DOI] [PubMed] [Google Scholar]
- 9. Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 1851;2006:1846 10.1038/sj.ki.5000243 [DOI] [PubMed] [Google Scholar]
- 10. Dall’Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-lupus nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. 10.1002/art.39026 [DOI] [PubMed] [Google Scholar]
- 11. Tamirou F, Lauwerys BR, Dall’Era M, et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN nephritis trial. Lupus Sci Med 2015;2 10.1136/lupus-2015-000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ugolini-Lopes MR, Seguro LPC, Castro MXF, et al. Early proteinuria response: a valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci Med 2017;4:e000213 10.1136/lupus-2017-000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among ‘hispanics’. Medicine (Baltimore) 2004;83:1–17. 10.1097/01.md.0000104742.42401.e2 [DOI] [PubMed] [Google Scholar]
- 14. Hochberg MC Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 15. Churg J LHS. Renal disease: classification and atlas of glomerular disease. Tokyo: Igaku-Shoin, 1982. [Google Scholar]
- 16. Nived O, Jönsen A, Bengtsson AA, et al. High predictive value of the systemic lupus international collaborating clinics/American college of rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–400. [PubMed] [Google Scholar]
- 17. van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. 10.1136/annrheumdis-2013-205139 [DOI] [PubMed] [Google Scholar]
- 18. Cheng Y, Li M, Zhao J, et al. Chinese SLE treatment and research group (CSTAR) registry:VIII. Influence of socioeconomic and geographical variables on disease phenotype and activity in Chinese patients with SLE. Int J Rheum Dis 2018;21:716–24. 10.1111/1756-185X.13057 [DOI] [PubMed] [Google Scholar]
- 19. Pons-Estel GJ, Saurit V, Alarcón GS, et al. The impact of rural residency on the expression and outcome of systemic lupus erythematosus: data from a multiethnic Latin American cohort. Lupus 2012;21:1397–404. 10.1177/0961203312458465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandane-Rathnayake R, Kent JR, Louthrenoo W, et al. Longitudinal associations of active renal disease with irreversible organ damage accrual in systemic lupus erythematosus. Lupus 2019;28:1669–77. 10.1177/0961203319887799 [DOI] [PubMed] [Google Scholar]
- 21. Hanaoka H, Kaneko Y, Kuwana M, et al. Early achievement of complete renal response predicts good long-term renal outcome and low systemic damage in newly diagnosed lupus nephritis class III or IV. Mod Rheumatol 2015;25:714–8. 10.3109/14397595.2014.1003172 [DOI] [PubMed] [Google Scholar]
- 22. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 23. Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Anti-malarials exert a protective effect while Mestizo patients are at increased risk of developing SLE renal disease: data from a Latin-American cohort. Rheumatology (Oxford) 2012;51:1293–8. 10.1093/rheumatology/ker514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pons-Estel GJ, Alarcón GS, Burgos PI, et al. Mestizos with systemic lupus erythematosus develop renal disease early while antimalarials retard its appearance: data from a Latin American cohort. Lupus 2013;22:899–907. 10.1177/0961203313496339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sui M, Ye X, Ma J, et al. Epidemiology and risk factors for chronic kidney disease in Chinese patients with biopsy-proven lupus nephritis. Intern Med J 2015;45:1167–72. 10.1111/imj.12840 [DOI] [PubMed] [Google Scholar]
- 26. Mahmoud GA, Zayed HS, Ghoniem SA. Renal outcomes among Egyptian lupus nephritis patients: a retrospective analysis of 135 cases from a single centre. Lupus 2015;24:331–8. 10.1177/0961203314567751 [DOI] [PubMed] [Google Scholar]