Abstract
Background
COVID-19 (severe acute respiratory syndrome coronavirus 2) infected patients have increased risk for thrombotic events, which initially may have been under recognized. The existence of cardiovascular emboli can be directly life threatening when obstructing the blood flow to vital organs such as the brain or other parts of the body. The exact mechanism for this hypercoagulable state in COVID-19 patients yet remains to be elucidated.
Case summary
A 72-year-old man critically ill with COVID-19 was diagnosed with a free-floating and mural thrombus in the thoracic aorta. Subsequent distal embolization to the limbs led to ischaemia and necrosis of the right foot. Treatment with heparin and anticoagulants reduced thrombus load in the ascending and thoracic aorta.
Discussion
One-third of COVID-19 patients show major thrombotic events, mostly pulmonary emboli. The endothelial expression of angiotensin-converting enzyme-2 receptors makes it feasible that in patients with viraemia direct viral-toxicity to the endothelium of also the large arteries results in local thrombus formation. Up to date, prophylactic anticoagulants are recommended in all patients that are hospitalized with COVID-19 infections to prevent venous and arterial thrombotic complications.
Keywords: COVID-19, SARS-CoV2, Aortic thrombus, Ascending aorta, Thoracic aorta, Case report , Anticoagulation, Heparin
Learning points
Prophylactic anticoagulants are recommended in all patients that are hospitalized with COVID-19 infections to prevent venous and arterial thrombotic complications.
Elevated D-dimer levels upon hospital admission of COVID-19 patients may accordingly guide the choice of therapeutic anticoagulants.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic unprecedentedly pressurized the healthcare systems in many countries, in part due to a novel spectrum of associated clinical symptoms. While most individuals infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) only experience mild to moderate symptoms, some (<2%) suffer from more a critical or fatal course of infection.1 In COVID-19 patients, a striking amount of coagulation abnormalities and is found, despite prophylactic anticoagulation treatment.1,2 Here, we present a case with a critical course of COVID-19 infection with major thrombotic events in the ascending and descending thoracic aorta.
Timeline
| Day 1 | A 72-year-old patient with a critical course of COVID-19 (severe acute respiratory syndrome coronavirus 2) infection admitted to the intensive care unit (ICU) for intubation, inotropes, and continuous veno-venous hemofiltration |
| Day 21 | Extubation |
| Day 23 | Re-intubation due to progressive respiratory insufficiency
|
| Day 24 | Physical examination: blue toe syndrome |
| Day 25 | Computed tomography angiography vasculature lower extremities: occlusion of the right posterior tibial artery |
| Day 30 | Dark blue toes/signs of toe necrosis |
| Day 30 | Follow-up CT angiography: decrease of the aortic thrombus load |
| Day 35 |
Weaning from mechanical ventilation Stop heparin therapy, start oral anticoagulants |
| Day 45 | Intensive care unit acquired weakness and ongoing delirium |
| Day 90 | Normal cognitive functions, substantial physical rehabilitation in progress |
| Day 150 | Surgical surveillance and amputation of necrotic toes, general daily life activities independently |
Case presentation
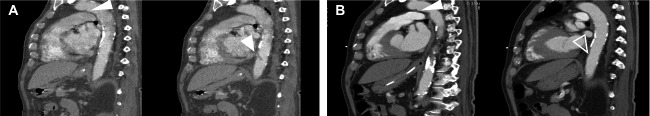
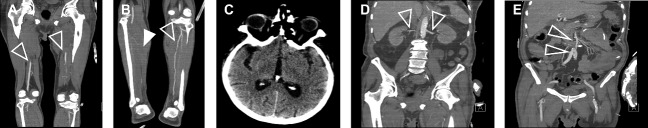
A 72-year-old Caucasian male, no relevant medical history or medication use, was admitted to the intensive care unit (ICU) with respiratory failure, shock, and multi-organ failure (Creatinine > 250 µmol/L, GFR < 20 mL/min) from a critical SARS-CoV-2 infection for which he received mechanical ventilation, inotropes, antibiotics, and continuous veno-venous haemofiltration (5000 IU heparin/L 0.9% saline). Upon admission, the physical examination did not show abnormalities of the extremities and coagulation parameters were normal (aPTT 24–34 s, INR 1.05). Three weeks later the patient was extubated and free of inotropes and dialysis, however, due to progressive respiratory insufficiency, a re-intubation was required two days later. Electrocardiogram showed a sinus rhythm with signs of ischaemia but not acute myocardial infarction, Aspirin was therefore started. Supplementary diagnostic CT thorax angiography showed a severe COVID-19 related bilateral ground-glass pattern of the lungs without signs of pulmonary embolism (D-dimer was not determined because of a high acute phase response (e.g. CRP 172 mg/L) and adequate imaging for detection of pulmonary embolism). Moreover, CT angiography revealed a mural thrombus (4 mm × 6 mm) in the arch of the ascending aorta (Figure 1A, left panel) and a free-floating thrombus (3.5 mm × 25 mm) in the distal thoracic aorta (Figure 1A, right panel). Besides, minor age-compatible atherosclerotic plaques were seen, mostly in the descending aorta and not at the site of the aortic thrombi. Treatment with unfractionated heparin was started with a therapeutic target range (aPTT 48–68 s, 1.5–2.5 × patient baseline). Physical examination the following day revealed a blue toe syndrome, more profound on the right foot when compared to the left. Additional CT angiography of the peripheral vasculature of the legs revealed an occlusion of the right posterior tibial artery without signs of significant atherosclerosis (Figure 2A,B). Computed tomography brain did not show signs of cerebrovascular ischaemia (Figure 2C). Computed tomography angiography of the abdomen revealed intact renal and mesenteric perfusion (Figure 2D,E). Follow-up CT aorta 1 week later showed stabilization of the mural thrombus (4 mm × 5 mm) and resolution of the free-floating thrombus (Figure 1B).
Figure 1.
COVID-19 related thrombi in the aorta. (A) Sagittal views of aortic computed tomography angiography displaying a mural thrombus in the aortic arch (left side, indicated by white arrow) and a free-floating thrombus in the distal thoracic aorta (right side, indicated by white arrow). (B) Computed tomography images displaying the situation after 7 days of Heparin treatment. Note: The free-floating thrombus in the thoracic aorta has decreased in size (right side, indicated by transparent arrow), while the thrombus in the arch appears similar in size. White arrow indicates a thrombus. Transparent arrow indicates (partial) thrombus resolution.
Figure 2.
Distal embolization in the legs but no other parts of the body. Computed tomography images from other organs and extremities showing (A) absence of thrombi in the upper leg vasculature (transparent arrows), (B) presence of a stop in the right posterior tibial artery (white arrow) but not in the left posterior tibial artery (transparent arrow), (C) no cerebral vascular involvement, (D) adequate perfusion of the renal arteries (transparent arrows), and (E) good blood flow in the mesenteric and femoral arteries (transparent arrows). Transparent arrow indicates perfusion. White arrow indicates a stop.
Despite reduction of thrombus load in the aorta and prevention of new thrombi by heparin treatment for a week, the digits 1–3 of the right foot became necrotic (Figure 3A,B), while the left lower limb recovered. Presumably, this compromised blood supply to the right foot was caused by a distal embolization of a large part of the free-floating thrombus in the thoracic aorta. During the course of these events, the patient persistently tested positive for SARS-CoV-2 (direct PCR on nasopharyngeal swab). Two weeks after admission the kidney function improved (Creatinine 157 µmol/L, GFR 38 mL/min) and eventually returned to normal for age (Creatinine 102 µmol/L, GFR 61 mL/min). Moreover, one blood culture was positive for an Enterococcus faecium that was sensitive to Vancomycin treatment. Blood cultures taken one day later showed no growth of microbes. Additional echocardiography showed no signs of endocarditis.
Figure 3.
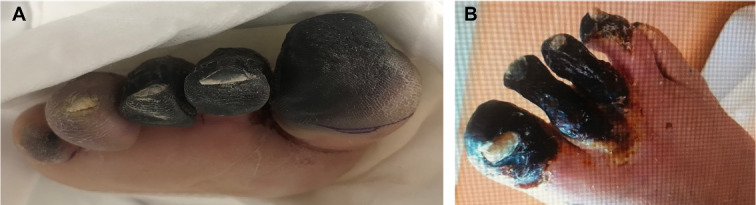
Distal embolization of the right leg resulted in necrosis of the foot. (A) Image of necrotic digit 1–3 right as observed in the second week post-ICU admission. (B) Image of the necrotic right foot with partial necrosis of also digit 4 as observed in the outpatient setting 10 weeks after initial diagnosis.
After second time weaning from mechanical ventilation 5 days later, and during rehabilitation, the patient switched to anticoagulant treatment with coumarins (acenocoumarol) (target INR 2.0–3.0) for a scheduled duration of at least 3 months and until follow-up CT aorta was scheduled. Upon arrival at the COVID unit, the patient was still suffering from an ICU acquired weakness and a delirium. In the weeks after, the patient’s cognitive functions improved and he was subsequently transferred to a rehabilitation facility expected to make a full cognitive and substantial physical recovery. Amputation of the necrotic digits 1–3 of the right foot was performed 3 months after discharge.
Discussion
A free-floating thrombus in the ascending aorta is a rare finding.3 Pathophysiologically, thrombus formation in large vessels only occurs in case of altered intravascular flow (e.g. atrial fibrillation, aneurysm), inflammation of the vessel wall (e.g. vasculitis), or damage of the endothelium in combination with inflammation (e.g. rheumatic or bacterial endocarditis, ulcerated plaques).3 In COVID-19 patients, coagulation abnormalities and thrombosis are frequently found, despite prophylactic anticoagulation treatment. Over 30% of patients in ICU suffer from pulmonary embolism or other thrombosis, including two other cases of aortic thrombi.2,4,5 The triggers for the hypercoagulable state in COVID-19 patients remain to be elucidated. Suspected triggers are hyperinflammation- and hypoxia-related damage to endothelium, and subsequent thrombus formation in small- to medium-size vessels.1 However, massive thrombus formation in the aorta in absence of vessel wall damage or massive atherosclerosis is unexpected in viral infections. Initial reports on SARS-CoV-2 have shown a high specificity for virus binding to the angiotensin-converting enzyme-2 (ACE-2) receptor as a mechanism to invade host cells. The ACE-2 receptor is expressed widely in the human body, including on the vascular endothelium.6 A small postmortem study in 12 patients who died from COVID-19 found that viraemia was present in about 60% of cases. High viral load in other organs than the lungs was observed in the liver, kidney, and heart,7 suggesting a haematogenic spread of the virus through the body. Therefore, it is imaginable that the aortic thromboembolism in these patients may be caused by ACE-2 mediated SARS-CoV-2 infection of the large vessel wall, resulting in local injury and intravascular activation of the clotting cascade. The superinfection in this patient may have been an additional contributing factor to the hypercoagulable state, however, generally, E. faecium infections are not directly associated with arterial emboli, and therefore it seems unlikely to be the main substrate.8
In the case we presented here, the patient suffered from a critical course of SARS-CoV-2 infection with thrombotic complications of the aorta. The peripheral ischaemia/necrosis most likely is explained by distal embolization of the free-floating thrombus, especially since upper extremities and other organs in this case were not involved. Clinical awareness should be in place for recognition of distal embolization to the brain, spleen, kidneys and/or other organs. In this case, treatment with heparin was effective for reducing thrombus load in the aorta and for preventing new thrombi formation, however, did not prevent necrosis of the lower right extremity. Other treatment options can include thrombolysis, interventional, or surgical removal of thrombi. In light of the severity of COVID-19 in this patient, these advanced options for blood flow restoration to the right foot were considered too high risk for adverse events. The existence of venous and/or arterial thromboembolic events, especially early on in the pandemic, has been under-recognized in patients suffering from a severe course COVID-19 infection and D-dimer level measurement at admission will facilitate early recognition.9–11
Conclusion
In summary, in this case, a 72-year-old Caucasian male with severe COVID-19 experienced complications resulting in multiple thrombi at the luminal side of the aorta. Heparin treatment in this case decreased thrombi size and possibly prevented the involvement of other organs, but could not resolve toe ischaemia. In retrospect, D-dimer levels upon hospitalization could have led to an earlier recognition of the hypercoagulable state in this patient and hence earlier start of anticoagulants. Recent retrospective studies indicate that an elevated D-dimer is associated with catastrophic outcomes in COVID-19 patients. Currently, a number of randomized clinical trials (NCT04372589, NCT04367831, NCT04345848, and NCT04366960) are investigating the efficacy of dose-specific thrombo-prophylaxis. Therefore, it should be considered to measure D-dimer levels upon hospital admission and to treat hospitalized COVID-19 patients accordingly with prophylactic or therapeutic anticoagulants.9–11
Lead author biography

Dr. Jan W. Buikema is a fellow in cardiology with a focus on heart failure at the University Medical Center Utrecht. In 2014, he obtained his PhD degree in regenerative medicine from Utrecht University. As part of his PhD, he did a 3 years research fellowship at Harvard Medical School, studying the roles of Wnt signaling and ventricular growth during development. Afterwards, he continued his residency and cardiology fellowships in Utrecht. Between 2017 and 2019, Jan worked as a postdoctoral fellow at Stanford University which allowed him to evolve his research on the role of Wnt signaling in massive proliferation of human iPSC-derived cardiomyocytes and patient specific disease models.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidelines.
Conflict of interest: none declared.
Funding: Folkert W. Asselbergs is supported by UCL Hospitals NIHR Biomedical Research Centre. Jan W. Buikema is supported by Netherlands Heart Institute fellowship.
Supplementary Material
References
- 1. Bikdeli BMadhavan MVJimenez DChuich TDreyfus IDriggin Eet al. . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FAKruip MVan Der Meer NArbous MSGommers DKant KMet al. . Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalangos A, Baldovinos A, Vuille C, Montessuit M, Faidutti B.. Floating thrombus in the ascending aorta: a rare cause of peripheral emboli. J Vasc Surg 1997;26:150–154. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez-Pinto T, Luna-Rodriguez A, Moreno-Estebanez A, Agirre-Beitia G, Rodriguez-Antiguedad A. et al. Emergency room neurology in times of COVID-19: malignant ischemic stroke and SARS-COV2 infection. Eur J Neurol 2020;27:e35–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Berre A, Marteau V, Emmerich J, Zins M.. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Intervent Imaging 2020;101:321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J, Turner A. et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wichmann D, Sperhake J, Lutgehetmann M, Steurer S, Edler C, Heinemann A. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Internal Med 2020;173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kajihara T, Nakamura S, Iwanaga N, Oshima K, Takazono T, Miyazaki T. et al. Clinical characteristics and risk factors of enterococcal infections in Nagasaki, Japan: a retrospective study. BMC Infect Dis 2015;15:426. 10.1186/s12879-015-1175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020;7:e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.