Abstract
Purpose:
To evaluate the additive intraocular pressure–lowering effect of twice-daily brinzolamide 1%/brimonidine 0.2% fixed-dose combination (BBFC) as an adjunct to a prostaglandin analog (PGA) in patients with open-angle glaucoma or ocular hypertension insufficiently controlled with PGA monotherapy.
Methods:
In this Phase 4, double-masked trial, patients aged ⩾18 years, with a mean intraocular pressure of ⩾19 and <32 mm Hg in at least one eye were randomized (1:1) to receive BBFC + PGA (n = 96) or vehicle + PGA (n = 92) for 6 weeks. The primary endpoint was the mean change in diurnal intraocular pressure from baseline (averaged over 09:00 and 11:00 h) at Week 6.
Results:
The mean diurnal intraocular pressure at baseline was similar in the BBFC + PGA (22.8 mm Hg) and vehicle + PGA (22.9 mm Hg) groups. The least squares mean change in diurnal intraocular pressure from baseline at Week 6 was greater with BBFC + PGA (−5.59 mm Hg (95% confidence interval: −6.2 to −5.0)) than with vehicle + PGA (−2.15 mm Hg (95% confidence interval: −2.7 to −1.6)); the treatment difference was statistically significant in favor of BBFC + PGA (−3.44 mm Hg, (95% confidence interval: −4.2 to −2.7); p < 0.001). Ocular adverse events were reported in 21.1% and 8.7% of patients in the BBFC + PGA and vehicle + PGA groups, respectively. The most frequent ocular adverse event was ocular hyperemia (5.3%) in the BBFC + PGA group and blurred vision (2.2%) in the vehicle + PGA group.
Conclusion:
BBFC + PGA significantly reduced mean diurnal intraocular pressure than PGA alone in patients with open-angle glaucoma or ocular hypertension. The safety findings with BBFC + PGA were consistent with the known safety profile of the individual medications.
Keywords: Brinzolamide/brimonidine fixed-dose combination, open-angle glaucoma, ocular hypertension, prostaglandin analogs, intraocular pressure reduction
Introduction
Elevated intraocular pressure (IOP) is the primary risk factor for development and progression of open-angle glaucoma or for conversion of ocular hypertension to glaucoma that may lead to visual field deterioration if left untreated.1–4 Reduction of IOP is the mainstay of treatment of open-angle glaucoma and ocular hypertension.
European Glaucoma Society guidelines suggest that treatment can be initiated with a monotherapy with prostaglandin analogs (PGAs) considered as effective first-line ocular hypotensives.5,6 However, monotherapy may be insufficient to achieve and maintain target IOP in the long term: ~40%–75% of patients require two or more medications for sufficient IOP reduction after 2–5 years of treatment.7,8 Fifty percent of patients require a change of initial monotherapy during the first 2 years of treatment with insufficient IOP-lowering accounting for 80% of these cases.9
Use of multiple medications or frequent dosing can decrease patient adherence to and persistence with therapy,10,11 which may contribute to insufficient IOP reduction. Use of fixed-dose combinations provides the convenience of two or more medications in a single formulation and a reduction of dosing frequency and exposure to preservatives. Hence, use of fixed-dose combinations potentially improves patient comfort, and adherence to and persistence with treatment.12,13 While the majority of fixed-dose combination therapies for glaucoma treatment include a topical β-blocker, many patients with glaucoma have contraindications to this compound.5
Brinzolamide 10 mg/mL / brimonidine 2 mg/mL is the only fixed-dose combination ophthalmic suspension (BBFC; SIMBRINZA®, Novartis Pharma AG) that does not contain a β-blocker. BBFC is indicated twice daily in the European Union and thrice daily in the United States for the treatment of patients with open-angle glaucoma or ocular hypertension. BBFC dosed thrice daily (approved dosing in the United States) has been shown to effectively lower IOP in patients with open-angle glaucoma or ocular hypertension inadequately controlled with PGA monotherapy.14,15 However, data on a twice-daily regimen of BBFC (approved dosing in most countries) as an adjunct to PGA are not available. In this trial, the additive IOP-lowering effect of twice-daily BBFC in patients with open-angle glaucoma or ocular hypertension who were insufficiently controlled on a PGA were evaluated.
Methods
Study design
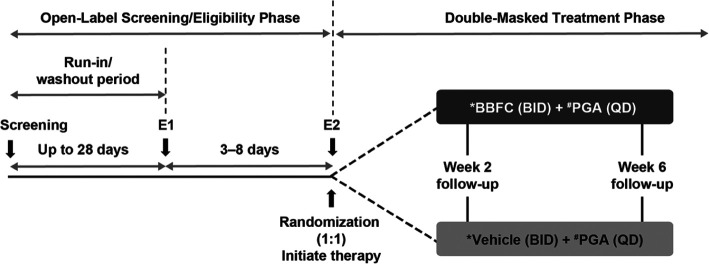
This was a 6-week, Phase 4, randomized (1:1), double-masked, parallel group trial conducted across 30 sites in 10 countries (Argentina, Australia, Canada, Chile, France, Germany, Greece, Israel, Spain, and the United Kingdom) from 7 August 2015 to 27 February 2018 (NCT02419508).The study had two sequential phases with five visits (Figure 1). Following screening, eligible patients not on a study-specific branded PGA (Travatan® (Travoprost 0.004%, Novartis Pharma AG), Lumigan® (Bimatoprost 0.01%, Allergan), or Xalatan® (Latanoprost 0.005%, Pfizer)) were assigned a study-specific branded PGA by the trial investigator for a minimum of 28 days prior to screening. Simultaneously, patients on multiple IOP-lowering medications started the appropriate washout period based on the types of additional ocular hypotensive medications (miotics and carbonic anhydrase inhibitors 5 ± 1 days, α and α/β agonists 14 ± 1 days, β-antagonists and PGAs 28 ± 1 days, combination drugs up to 28 ± 1 days). Following the run-in/washout period, eligible patients were randomized 1:1 using interactive response technology to receive twice-daily BBFC or vehicle (09:00 and 21:00 h) as an adjunct to once-daily PGA (given at bedtime), for 6 weeks (Figure 1).
Figure 1.
Study design.
BBFC: brinzolamide 1%/brimonidine 0.2% fixed-dose combination; BID: twice daily; E1: eligibility visit 1; E2: eligibility visit 2; IOP: intraocular pressure; PGA: prostaglandin analog; QD: once daily.
*One drop instilled at 09:00 and 21:00 h.
#One drop instilled at bedtime.
Randomization was stratified according to region and type of PGA. To avoid potential selection bias, investigators, patients, the trial sponsor, investigational center staff, and clinical monitors were masked to treatment assignments throughout the trial. BBFC and vehicle were provided to the trial investigators in identical opaque bottles with masked labels, identified with a kit and protocol number, while PGA medications were unmasked. One eye per patient was chosen as the study eye and only the study eye was used for analysis (details on study eye selection are given in Supplementary Information, Appendix 1).
The trial was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with the International Conference on Harmonization (ICH), Good Clinical Practice (GCP) Consolidated Guideline, and other regulations as applicable. The trial protocol and all its amendments were approved by an Independent Ethics Committee/Institutional Review Board. All patients provided written informed consent before trial initiation.
Patients
Eligible patients were aged ⩾18 years, diagnosed with either open-angle glaucoma (including exfoliation or pigment dispersion syndromes) or ocular hypertension, with mean IOP at 09:00 h ⩾19 and <32 mm Hg in at least one (and the same) eye at both eligibility visits (after washout and simultaneous PGA run-in). The mean IOP was ⩾21 and <32 mm Hg at the start of the study, but was amended to ⩾19 mm Hg during the study, due to recruitment challenges. Key exclusion criteria are given in Supplementary Information, Appendix 2.
Study endpoints
The primary efficacy endpoint was the mean change from baseline in diurnal IOP at Week 6 (averaged over the 09:00-h and 11:00-h time points; a 12-h trough and a 2-h peak, respectively).
Secondary endpoints were (1) mean diurnal IOP (averaged over the 09:00-h and 11:00-h time points) at Week 6; (2) mean percent change from baseline in diurnal IOP (IOP at baseline averaged over the 09:00-h and 11:00-h time points) at Week 6; (3) mean and percentage mean change from baseline in IOP at 11:00 h at Week 6; and (4) mean and percentage mean change from baseline in IOP at 09:00 h at Week 6. A 16:00-h time point for IOP assessment was also planned, but was removed during the study, owing to recruitment challenges.
Key exploratory endpoints were (1) mean diurnal IOP at Week 2; (2) percentage of patients achieving IOP target (⩽12, ⩽13, ⩽14, . . ., ⩽18 mm Hg) at Week 6; (3) mean change from baseline in ocular perfusion pressure (calculated as 2/3 (diastolic blood pressure + 1/3 (systolic blood pressure – diastolic blood pressure) – IOP)) at Week 6 (diurnal and individual time points).
Safety endpoints were occurrence and characteristics of adverse events (AEs), change in best-corrected visual acuity (BCVA; BCVA scoring was calculated as the number of Early Treatment Diabetic Retinopathy Study (ETDRS) letters correctly read + 30 at a distance of 3 or 4 m), perimetry and dilated fundus examination, slit lamp examination, and vital signs (blood pressure and pulse rates).
Assessments
IOP was measured using Goldmann applanation tonometry at screening and at the 09:00-h and 11:00-h time points during the two eligibility visits and at the Weeks 2 and 6 follow-up visits. Details on assessment of IOP, BCVA, achromatic automated perimetry, dilated fundus examination, and slit-lamp biomicroscopy examination are given in Supplementary Information, Appendix 3.
Statistics
Sample size
Eighty-one evaluable patients per treatment group were required to yield at least 90% power to detect a 2.0 mm Hg difference in mean change from baseline in diurnal IOP at Week 6 (primary efficacy analysis) between the treatment groups. This calculation assumed a common standard deviation (SD) for mean change from baseline in diurnal IOP as small as 3.5 mm Hg and as large as 3.9 mm Hg and the use of a two-sample two-sided t-test performed at the α = 0.05 level of significance. Assuming a dropout rate of 10%, over 90 patients per treatment group were randomized to ensure the required number of evaluable patients in the primary efficacy analysis.
Statistical method
The full analysis set, which included all randomized subjects with a baseline assessment and who completed at least one scheduled on-therapy study visit, was used for all efficacy analyses. Treatment differences in mean diurnal IOP change from baseline were examined with a pair-wise test at each scheduled on-therapy visit with Week 6 as the primary endpoint. Pair-wise tests were based on the least squares means derived from using a mixed-model repeated measures analysis. This model included factors for PGA, region, treatment, visit, and treatment by visit interaction. Baseline diurnal IOP was included in the model as a covariate. To ensure type I error was controlled over the set of study hypotheses at the 5% level of significance (two-sided), a fixed sequence testing strategy was employed.
The testing order (all efficacy endpoints at Week 6) was as follows: (1) difference between treatments in mean change from baseline in diurnal IOP; (2) difference between treatments in mean diurnal IOP; (3) difference between treatments in mean percentage diurnal IOP change from baseline; (4) difference between treatments in IOP change from baseline at 11:00 h; (5) difference between treatments in percentage IOP change from baseline at 11:00 h; (6) difference between treatments in IOP change from baseline at 09:00 h; and (7) difference between treatments in percentage IOP change from baseline at 09:00 h. Significance for a comparison was claimed only if the null hypothesis was rejected (p < 0.05) for the previous endpoint in the series. Analysis of treatment differences of all secondary endpoints used the same methods as those for the primary endpoint. Exploratory analyses were descriptive in nature.
Safety results were summarized descriptively for the safety set, which included all patients who received at least one dose of masked investigational drug.
Results
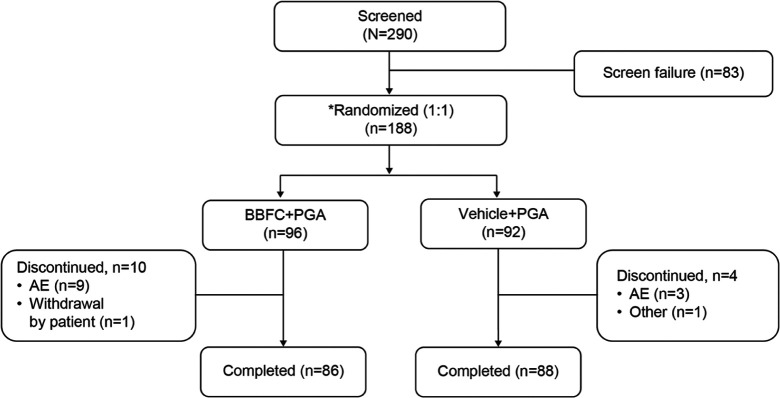
In total, 188 patients were randomized, BBFC + PGA (n = 96) and vehicle + PGA (n = 92); of whom, 174 (92.6%) completed the study; AEs were the most common reason for study discontinuation in both groups (Figure 2). The full analysis set and safety set included 187 (99.5%) patients (one patient in the BBFC + PGA group did not receive the investigational drug and was excluded).
Figure 2.
Patient disposition.
AE: adverse event; BBFC: brinzolamide 1%/brimonidine 0.2% fixed-dose combination; N: total number of patients; n: number of patients; PGA: prostaglandin analog.
*Nineteen patients were screened but not randomized due to an AE (n = 1), withdrawal by patient (n = 16), and other (n = 2).
The mean (SD) age of patients in the full analysis set was 67.2 (11.17) years, 52.4% were female and 92% were White. The proportion of patients diagnosed with open-angle glaucoma and ocular hypertension in the study eye was 81.3% and 18.2%, respectively. Patient demographics and baseline characteristics were similar between the treatment groups, except for numerically more female patients in the BBFC + PGA versus vehicle + PGA group (Table 1). The mean (SD) diurnal IOP at baseline was comparable in the BBFC + PGA (22.8 (2.39) mm Hg) and vehicle + PGA (22.9 (2.32) mm Hg) groups.
Table 1.
Demographics and baseline characteristics (full analysis set).
| Characteristics | BBFC + PGA N = 95 |
Vehicle + PGA N = 92 |
|---|---|---|
| Age, years (mean (±SD)) | 66.5 (10.70) | 67.9 (11.65) |
| Gender, female, n (%) | 55 (57.9) | 43 (46.7) |
| Race, n (%) | ||
| White | 87 (91.6) | 85 (92.4) |
| Black or African American | 5 (5.3) | 7 (7.6) |
| Asian | 3 (3.2) | 0 (0.0) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 23 (24.2) | 29 (31.5) |
| Not Hispanic or Latino | 72 (75.8) | 62 (67.4) |
| Unknown | 0 (0.0) | 1 (1.1) |
| Baseline diurnal IOP (mean (±SD), mm Hg) | 22.8 (2.39) | 22.9 (2.32) |
| Baseline IOP category, n (%) | ||
| 19–26 mm Hg | 89 (93.7) | 84 (91.3) |
| 27–32 mm Hg | 6 (6.3) | 7 (7.6) |
| PGA monotherapy, n (%) | ||
| Bimatoprost 0.01% | 32 (33.7) | 30 (32.6) |
| Latanoprost 0.005% | 38 (40.0) | 37 (40.2) |
| Travoprost 0.004% | 25 (26.3) | 25 (27.2) |
| Corneal thickness (mean (± SD) μm) | 539.1 (34.43) | 545.5 (33.73) |
| Corneal thickness categories | ||
| ⩽0.55 μm | 57 (60.0) | 45 (48.9) |
| >0.55–0.60 μm | 35 (36.8) | 43 (46.7) |
| >0.60 μm | 3 (3.2) | 4 (4.3) |
| Diagnosis, n (%) | ||
| Open-angle glaucoma | 78 (82.1) | 74 (80.4) |
| Ocular hypertension | 17 (17.9) | 17 (18.5) |
BBFC: brinzolamide 1%/brimonidine 0.2% fixed-dose combination; N: total number of patients; PGA: prostaglandin analog; SD: standard deviation; n: number of patients; IOP: intraocular pressure.
One patient with an IOP level < 19 mm Hg was randomized in error from the site and received treatment. This patient was included in the full analysis set. Baseline IOP is expressed as mean (SD) and defined as the average of 09:00 a.m. and 11:00 a.m. values.
Efficacy outcomes
The least squares mean change in diurnal IOP from baseline at Week 6 was greater with BBFC + PGA (−5.59 mm Hg (95% confidence interval (CI): −6.2 to −5.0)) than with vehicle + PGA (−2.15 mm Hg (95% CI: −2.7 to −1.6)); the treatment difference was statistically significant in favor of BBFC + PGA (−3.44 mm Hg, (95% CI: −4.2 to −2.7); P < 0.001). The study met its primary objective (Figure 3). Results of the primary endpoint were similar in the subset of patients with 16:00 h data (BBFC + PGA, n = 58; vehicle + PGA, n = 63) at baseline and at Weeks 2 and 6 (Supplementary Table 1).
Figure 3.
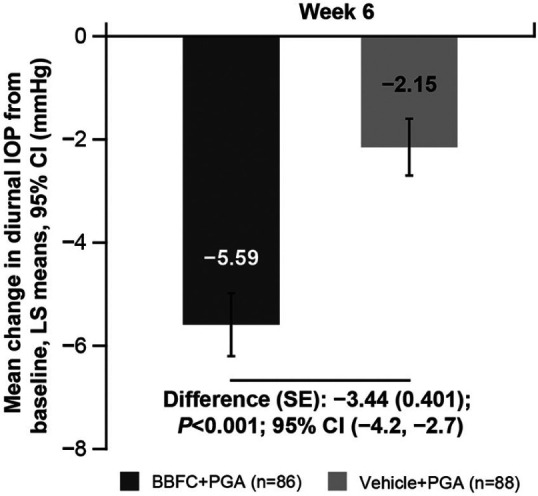
Mean change in diurnal IOP from baseline at Week 6 (full analysis set).
n: number of patients with non-missing values of diurnal IOP change and any of the other co-variates in the model at the corresponding time point of interest; BBFC: brinzolamide 1% and brimonidine 0.2% fixed-dose combination; CI: confidence interval; IOP: intraocular pressure; LS: least squares; SE: standard error; PGA: prostaglandin analog.
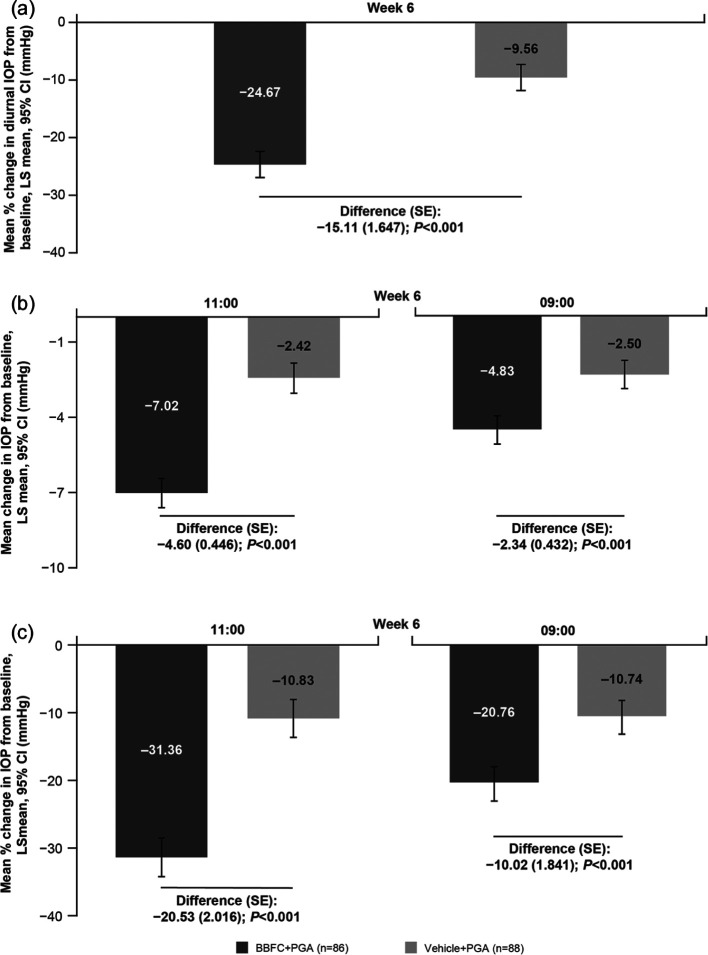
The least squares mean diurnal IOP at Week 6 was 17.3 (95% CI: 16.7 to 17.9) mm Hg with BBFC + PGA and 20.75 (95% CI: 20.2 to 21.3) mm Hg with vehicle + PGA; the treatment difference was statistically significant −3.44 mm Hg (95% CI: −4.2 to −2.7; p < 0.001). The mean percentage change in diurnal IOP at Week 6 was higher with BBFC + PGA versus vehicle + PGA (treatment difference: −15.1%; p < 0.001, Figure 4(a)). Similarly, the mean change and mean percentage change from baseline at the peak (11:00 h), and trough (09:00 h) time points at Week 6 were also greater with BBFC + PGA versus vehicle + PGA (all P < 0.001, Figure 4(b) and (c)). There was a notable change in mean (SD) diurnal IOP from baseline at Week 2 with BBFC + PGA (−5.2 (2.59) mm Hg) versus vehicle + PGA (−1.3 (2.14) mm Hg); the mean percentage reduction was 22.8% and 5.9%, respectively.
Figure 4.
(a) Mean percentage change in diurnal IOP from baseline at Week 6 (full analysis set), (b) mean change in IOP from baseline at 11:00 (peak) and 09:00 (trough) time points at Week 6 (full analysis set), and (c) mean percentage change in IOP from baseline at 11:00 (peak) and 09:00 (trough) time points at Week 6 (full analysis set).
n: number of patients with non-missing values of diurnal IOP change and any of the other covariates in the model at the corresponding time point of interest; BBFC: brinzolamide 1% and brimonidine 0.2% fixed-dose combination; CI: confidence interval; IOP: intraocular pressure; LS: least squares; SE: standard error; PGA: prostaglandin analog.
Target IOP ⩽ 18 mm Hg at Week 6 was achieved by 60.0% of patients with BBFC + PGA versus 20.7% patients with vehicle + PGA. The proportion of patients achieving IOP targets of ⩽12 to ⩽18 mm Hg at Week 6 was higher in the BBFC + PGA group than the vehicle group (Supplementary Figure 1). The ocular perfusion pressure at baseline was comparable between the two groups (Supplementary Table 2). The mean (SD) change from baseline at Week 6 in ocular perfusion pressure was 2.4 (4.30) mm Hg with BBFC + PGA and 0.6 (3.74) mm Hg with vehicle + PGA. The mean change at the 09:00-h and 11:00-h time points at Week 6 were also higher with BBFC + PGA versus vehicle + PGA (Supplementary Table 2).
Safety outcomes
The median time of exposure was 43 days (minimum: 2 days, maximum: 71 days) in both groups. Overall, 37.9% and 14.1% of patients in the BBFC + PGA and vehicle + PGA groups experienced at least one AE, respectively. The ocular AE with the highest incidence was ocular hyperemia (5.3%) in the BBFC + PGA group and blurred vision (2.2%) in the vehicle + PGA group (Table 2). The non-ocular AE with the highest incidence was dry mouth (5.3%) in the BBFC + PGA group (Table 2). One serious AE was reported during the study; an event of cardiac failure (of moderate severity) in the BBFC + PGA group. It was considered by the investigator to not be treatment related. No deaths were reported during this study. Treatment-related AEs occurred in 22 (23.2%) patients in the BBFC + PGA group and 4 (4.3%) patients in the vehicle + PGA group (Supplementary Table 3). Treatment discontinuations owing to AEs were reported for eight (8.4%) patients in the BBFC + PGA group (ocular discomfort in two patients, and arrhythmia, cardiac failure, allergic conjunctivitis, ocular hyperemia, and dizziness in one patient each) and three (3.3%) patients in the vehicle + PGA group (allergic conjunctivitis, eye allergy, and anxiety in one patient each).
Table 2.
Adverse events (safety set).
| System organ class; preferred term | BBFC + PGA N = 95 |
Vehicle + PGA N = 92 |
|---|---|---|
| Any event, n (%) | 36 (37.9) | 13 (14.1) |
| Ocular AEs (any event) | 20 (21.1) | 8 (8.7) |
| Ocular AEs (⩾2%) | ||
| Ocular hyperemiaa | 5 (5.3) | 1 (1.1) |
| Conjunctival hyperemiaa | 4 (4.2) | 1 (1.1) |
| Vision blurred | 2 (2.1) | 2 (2.2) |
| Dry eye | 3 (3.2) | 0 (0.0) |
| Eye irritation | 3 (3.2) | 0 (0.0) |
| Ocular discomfort | 3 (3.2) | 0 (0.0) |
| Non-ocular AEs (any event) | 19 (20.0) | 6 (6.5) |
| Non-ocular AEs (>2%) | ||
| Dry mouth | 5 (5.3) | 0 (0.0) |
| Fatigue | 2 (2.1) | 0 (0.0) |
| Nasopharyngitis | 4 (4.2) | 0 (0.0) |
| Dizziness | 2 (2.1) | 0 (0.0) |
| Hypertension | 2 (2.1) | 0 (0.0) |
BBFC: brinzolamide 1%/brimonidine 0.2% fixed-dose combination; PGA: prostaglandin analog; IOP, intraocular pressure; N: total number of patients; n: number of patients; AE: adverse event; MedDRA: Medical Dictionary for Regulatory Activities
A patient with multiple occurrences of an AE under one treatment was counted only once in this AE category for that treatment. MedDRA version 17.0 used for reporting of AEs.
Based on investigator’s judgment.
Results for BCVA, perimetry and dilated fundus examination, slit lamp examination, and vital signs (blood pressure and pulse rates) are given in Supplementary Information, Appendix 4.
Discussion
In this study, twice-daily BBFC as an adjunct to PGA showed an additive IOP-lowering effect in patients with open-angle glaucoma or ocular hypertension. Clinically meaningful IOP reductions were observed as early as Week 2, which were maintained up to Week 6. The study met its primary objective; a greater mean reduction in diurnal IOP from baseline at Week 6 was achieved with the BBFC + PGA compared with PGA + vehicle (5.6 mm Hg vs 2.1 mm Hg, p < 0.001). These results, taken together with previous studies showing clinically meaningful diurnal IOP reductions with thrice-daily BBFC as an adjunct to a PGA versus vehicle + PGA (Fechner et al. 5.7 mm Hg vs 1.9 mm Hg; Feldman et al. 5.1 mm Hg vs 2.2 mm Hg),14,15 demonstrate that twice-daily dosing of BBFC is also effective in patients with open-angle glaucoma/ocular hypertension who were not sufficiently controlled with PGA treatment alone. This finding is especially relevant considering that high frequency of dosing negatively affects adherence; a simpler dosing regimen may help to maintain or improve patient adherence.16
Results of the Early Manifest Glaucoma Trial demonstrated that the risk of disease progression is reduced by 10% for each 1 mm Hg decrease in mean IOP.17 Thus, the 5.6 mm Hg reduction in IOP with twice-daily BBFC is clinically relevant. A meta-analysis of data from 822 patients with open-angle glaucoma or exfoliative glaucoma from 5 randomized clinical trials demonstrated that maintaining the mean pressure of ⩽18 mm Hg allowed 78% of patients to remain stable over 5 years.1 In our study, at Week 6, 60% of the patients on BBFC + PGA achieved a target IOP of ⩽18 mm Hg providing further evidence that twice-daily BBFC + PGA has the potential to reduce the risk of disease progression and visual field loss with long-term use.
Significant reductions in IOP with twice-daily BBFC + PGA were observed at both the 2-h peak and 12-h trough time points, with a higher mean reduction at 11:00 h (11.00: 7.02 mm Hg and 9:00: 4.83 mm Hg, both P < 0.001). The peak and trough efficacy observed in this study was comparable with results observed in studies of thrice-daily BBFC as an adjunct to PGA in patients with open-angle glaucoma or ocular hypertension.14,15 The peak and trough effects of BBFC were seen at 11:00 h and 09:00 h, respectively, and were critical time points of this study. Assessment of IOP control later in the day would give a superior diurnal understanding of control. In the subset of patients in this study with 16:00-h IOP data, IOP reductions were observed at each time point (09:00, 11:00, and 16:00) at Weeks 2 and 6 in both BBFC + PGA and vehicle + PGA groups.
Studies have shown that low ocular perfusion pressure is associated with increased risk of glaucoma and glaucoma progression.18–20 In this study, twice-daily BBFC + PGA had a minimal effect on ocular perfusion pressure compared with baseline after 6 weeks of treatment. Indeed, there was a small increase in ocular perfusion pressure related to change in IOP and small changes in systolic and diastolic blood pressure.
The vehicle effect observed in this study at peak and trough time points (−2.42 mm Hg (−10.8%) and −2.5 mm Hg (−10.7%)) was consistent with observations in studies with thrice-daily dosing of BBFC.14,15 This may reflect regression to the mean or improved adherence with active medication(s) when participating in a clinical trial. However, observations of IOP reduction with vehicle alone suggest that one or more components of the vehicle formulations may slightly lower IOP. A meta-analysis of clinical trials that studied ocular hypotensive drugs has also demonstrated that treatment with vehicle results in a small but measurable decrease in IOP.1
As anticipated when a patient is exposed to three active medications (compared with only one in the vehicle + PGA group), AEs were more common in patients receiving BBFC + PGA. However, the overall safety profile of BBFC + PGA was consistent with the safety profile of all three individual components. No new safety concerns were identified from the addition of BBFC to a PGA.
Overall, the frequency of AEs reported with twice-daily dosing of BBFC + PGA in this study were lower than those reported with thrice-daily dosing of BBFC in earlier studies (Fechner et al. (BBFC + PGA): AEs 35.5%, serious AEs (SAEs) 1.1%, ocular hyperemia 5.4%, and blurred vision 9.7%; Feldman et al. (BBFC + travoprost): AEs 37.6%, SAEs nil, ocular (conjunctival) hyperemia 12.8%, blurred vision 6%).14,15 However, this is not unexpected given the twice-daily versus thrice-daily dosing regimens of BBFC.
An added advantage of BBFC in patients with open-angle glaucoma/ocular hypertension is that it is the only available fixed-dose combination without a β-blocker. Many patients with glaucoma have co-morbidities such as chronic pulmonary obstructive disease, asthma, or bradycardia. β-blockers are known to cause systemic adverse reactions, including bradycardia, irregular pulse, and asthma, especially in the elderly.21,22 For example, the use of timolol maleate has increased the need for bronchodilator therapy in 47% of patients with glaucoma.23 BBFC may be a suitable treatment option for elderly patients with glaucoma and patients in whom β-blockers are contraindicated. This is evident from the present study as well as from earlier studies in which BBFC has been safe and effective in patients with glaucoma or hypertension (mean age > 65 years).14,15 However, indication for use depends on the ever-present balance between benefit versus risk. Use of BBFC in elderly patients with the risk of glaucoma, but without a definitive diagnosis of measurable damage, needs to be justified. One strength of this study is that it included patients from multiple countries; one weakness was the relatively short duration of follow-up, another was the lack of 24-h IOP monitoring.
Conclusion
The study results suggest twice-daily BBFC as an adjunct to PGA is a suitable treatment option for patients with open-angle glaucoma or ocular hypertension for whom PGA monotherapy provides insufficient IOP reduction. The safety profile of BBFC + PGA was consistent with the known safety profiles of brinzolamide, brimonidine, and PGAs.
Supplemental Material
Supplemental material, Supplementary_information for Brinzolamide/brimonidine fixed-dose combination bid as an adjunct to a prostaglandin analog for open-angle glaucoma/ocular hypertension by Fotis Topouzis, Ivan Goldberg, Katharina Bell, Andrew J Tatham, Antonia Ridolfi, Douglas Hubatsch, Marcelo Nicolela, Phillipe Denis and S Fabian Lerner in European Journal of Ophthalmology
Acknowledgments
The medical writing support and editorial assistance during the development of the manuscript was provided by Swati Bhandari (Novartis Healthcare Pvt Ltd, Hyderabad, India).
Footnotes
Declaration of conflicting interests: The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F.T. received grants/research support from Alcon, Pfizer, Novartis, and Thea; consultation fees from Alcon, Bayer, Allergan, Pfizer, and Thea; company sponsored speaker’s bureau: Novartis, Thea, Alcon, Allergan, and Santen. M.N. received research grant from Allergan, Novartis/Alcon, and Aerie; and advisory boards for Allergan and Novartis/Alcon. S.F.L. received grants/research support/speaker from Allergan, Glaukos, Iridex, and Novartis/Alcon. A.J.T. received grants/research support from Novartis, Heidelberg Engineering, Allergan, Inc., Sensimed, and Thea; is a consultant for Allergan, Inc. P.D. is a consultant for Thea, Allergan, Novartis. I.G. is a Advisory Board Member for Novartis, Allergan, and Mundipharma; speaker at supported Symposia for Novartis, Allergan, Mundipharma, and Pfizer. A.R. and D.H. are employees of Novartis. K.B. had nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Novartis Pharma AG, Basel, Switzerland.
Trial registration: This trial was registered with ClinicalTrials.gov (NCT02419508).
Supplemental material: Supplemental material for this article is available online.
References
- 1. Stewart WC, Kolker AE, Sharpe ED, et al. Long-term progression at individual mean intraocular pressure levels in primary open-angle and exfoliative glaucoma. Eur J Ophthalmol 2008; 18(5): 765–770. [DOI] [PubMed] [Google Scholar]
- 2. Wilson MR, Kosoko O, Cowan CL, Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol 2002; 134: 399–405. [DOI] [PubMed] [Google Scholar]
- 3. Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol 2008; 53(Suppl. 1): S3–S10. [DOI] [PubMed] [Google Scholar]
- 4. Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol 2014; 92(5): 421–425. [DOI] [PubMed] [Google Scholar]
- 5. European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed., https://www.eugs.org/eng/egs_guidelines_reg.asp?l=1 (accessed 11 August 2018). [DOI] [PubMed]
- 6. Stewart WC, Konstas AG, Nelson LA, et al. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology 2008; 115(7): 1117–1122.e1. [DOI] [PubMed] [Google Scholar]
- 7. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120(6): 701–713. [DOI] [PubMed] [Google Scholar]
- 8. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001; 108: 1943–1953. [DOI] [PubMed] [Google Scholar]
- 9. Oostenbrink JB, Rutten-van Molken MP, Opdenoordt TS. The treatment of newly diagnosed patients with glaucoma or with ocular hypertension in The Netherlands: an observational study of costs and initial treatment success based on retrospective chart review. Doc Ophthalmol 1999; 98: 285–299. [DOI] [PubMed] [Google Scholar]
- 10. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy. Ophthalmology 2005; 112(5): 863–868. [DOI] [PubMed] [Google Scholar]
- 11. Robin AL, Novack GD, Covert DW. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 2007; 144(4): 533–540. [DOI] [PubMed] [Google Scholar]
- 12. Higginbotham EJ, Hansen J, Davis EJ, et al. Glaucoma medication persistence with a fixed combination versus multiple bottles. Curr Med Res Opin 2009; 25(10): 2543–2547. [DOI] [PubMed] [Google Scholar]
- 13. Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007; 120(8): 713–719. [DOI] [PubMed] [Google Scholar]
- 14. Fechtner RD, Myers JS, Hubatsch DA, et al. Ocular hypotensive effect of fixed-combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial. Eye 2016; 30(10): 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldman RM, Katz G, McMenemy M, et al. A randomized trial of fixed-dose combination brinzolamide 1%/brimonidine 0.2% as adjunctive therapy to travoprost 0.004. Am J Ophthalmol 2016; 165: 188–197. [DOI] [PubMed] [Google Scholar]
- 16. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008; 31(3): 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120(10): 1268–1279. [DOI] [PubMed] [Google Scholar]
- 18. Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 1995; 113: 918–924. [DOI] [PubMed] [Google Scholar]
- 19. Leske MC, Wu SY, Hennis A, et al. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 2008; 115(1): 85–93. [DOI] [PubMed] [Google Scholar]
- 20. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007; 114(11): 1965–1972. [DOI] [PubMed] [Google Scholar]
- 21. Nygaard HA, Hovding G. Adverse effects of local use of beta-blockaders in glaucoma. A literature review and a survey of reports to the adverse drug reaction authority 1986-95. Tidsskr Nor Laegeforen 1997; 117(14): 2019–2021. [PubMed] [Google Scholar]
- 22. Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol 2014; 8: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avorn J, Glynn RJ, Gurwitz JH, et al. Adverse pulmonary effects of topical beta blockers used in the treatment of glaucoma. J Glaucoma 1993; 2(3): 158–165. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_information for Brinzolamide/brimonidine fixed-dose combination bid as an adjunct to a prostaglandin analog for open-angle glaucoma/ocular hypertension by Fotis Topouzis, Ivan Goldberg, Katharina Bell, Andrew J Tatham, Antonia Ridolfi, Douglas Hubatsch, Marcelo Nicolela, Phillipe Denis and S Fabian Lerner in European Journal of Ophthalmology