Abstract
The discovery of the rapid antidepressant effects of the dissociative anaesthetic ketamine, an uncompetitive N-Methyl-D-Aspartate receptor antagonist, is arguably the most important breakthrough in depression research in the last 50 years. Ketamine remains an off-label treatment for treatment-resistant depression with factors that limit widespread use including its dissociative effects and abuse potential. Ketamine is a racemic mixture, composed of equal amounts of (S)-ketamine and (R)-ketamine. An (S)-ketamine nasal spray has been developed and approved for use in treatment-resistant depression in the United States and Europe; however, some concerns regarding efficacy and side effects remain. Although (R)-ketamine is a less potent N-Methyl-D-Aspartate receptor antagonist than (S)-ketamine, increasing preclinical evidence suggests (R)-ketamine may have more potent and longer lasting antidepressant effects than (S)-ketamine, alongside fewer side effects. Furthermore, a recent pilot trial of (R)-ketamine has demonstrated rapid-acting and sustained antidepressant effects in individuals with treatment-resistant depression. Research is ongoing to determine the specific cellular and molecular mechanisms underlying the antidepressant actions of ketamine and its component enantiomers in an effort to develop future rapid-acting antidepressants that lack undesirable effects. Here, we briefly review findings regarding the antidepressant effects of ketamine and its enantiomers before considering underlying mechanisms including N-Methyl-D-Aspartate receptor antagonism, γ-aminobutyric acid-ergic interneuron inhibition, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor activation, brain-derived neurotrophic factor and tropomyosin kinase B signalling, mammalian target of rapamycin complex 1 and extracellular signal-regulated kinase signalling, inhibition of glycogen synthase kinase-3 and inhibition of lateral habenula bursting, alongside potential roles of the monoaminergic and opioid receptor systems.
Keywords: Ketamine, (S)-ketamine, (R)-ketamine, depression, NMDA receptor, AMPA receptor, BDNF, TrkB, mTORC1, ERK, GSK-3, 5-HT, dopamine, opioid receptor
Introduction
There are significant limitations to current widely prescribed antidepressant treatments. These include a significant delay in the onset of therapeutic action (weeks to months) and approximately one-third of patients with major depressive disorder (MDD) failing to demonstrate an adequate response (Al-Harbi, 2012). For individuals with depression, particularly if suffering from suicidal ideation, these time lags and resistance to standard treatments can be extremely harmful (Hantouche et al., 2010).
Increasing evidence has revealed that the dissociative anaesthetic ketamine, an uncompetitive N-Methyl-D-Aspartate (NMDA) receptor antagonist, has the potential to overcome such limitations, demonstrating rapid antidepressant and anti-suicidal effects, even in treatment-resistant patients (Coyle and Laws, 2015; Kishimoto et al., 2016). It has been proposed that ketamine’s antidepressant effects are primarily mediated through NMDA receptor antagonism, resulting in disinhibition of pyramidal cells and an acute cortical glutamate surge, with downstream effects on synaptogenesis and neuroplastic pathways (Lener et al., 2017). However, the precise molecular and cellular processes underlying ketamine’s antidepressant effects are still not clear and evidence suggests that mechanisms other than NMDA receptor inhibition play a more crucial role in the antidepressant effects of ketamine, its component enantiomers and metabolites (Jelen et al., 2018; Zanos et al., 2016).
In this review, we summarise findings on the antidepressant effects of ketamine and its enantiomers. We then discuss underlying therapeutic mechanisms, exploring the case that ketamine’s enantiomers and metabolites may produce complementary antidepressant effects via distinct mechanisms, before considering future directions of enquiry.
Ketamine enantiomers and metabolites
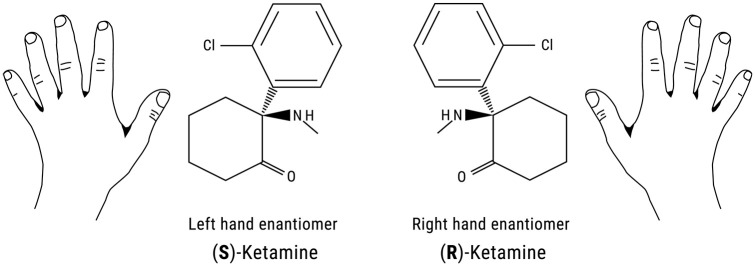
Ketamine is a racemic mixture that consists of equal amounts of two enantiomers, (S)-ketamine and (R)-ketamine (or esketamine and arketamine) (Figure 1). (S)-ketamine has a three to fourfold greater binding affinity for the NMDA receptor than (R)-ketamine (Ki = 0.30 μM and Ki = 1.4 μM respectively) (Ebert et al., 1997). In humans, (S)-ketamine is more potent than (R)-ketamine both as an anaesthetic and as an analgesic, which is putatively explained by its higher affinity for the NMDA receptor (White et al., 1980, 1985). It was argued that because of its increased potency, lower doses of (S)-ketamine could be used in anaesthesia/analgesia with faster recovery times and therefore potentially some diminution in dissociative and psychotomimetic side effects (Kohrs and Durieux, 1998). However, direct comparative studies of (S)- and (R)-ketamine have suggested otherwise. In one study, higher rates of psychotomimetic side effects were seen in an (S)-ketamine treated group, despite the dose of (S)-ketamine being lower than (R)-ketamine (0.45 mg/kg and 1.8 mg/kg respectively) (Mathisen et al., 1995). Furthermore, a healthy volunteer study from Vollenweider et al. (1997) found that although (S)-ketamine administration produced acute psychosis-like reactions (ego-dissolution, illusions and hallucinations, thought disorders, paranoid ideations), in the same individuals (R)-ketamine did not produce any psychotic symptoms but instead led to a state of relaxation and a feeling of wellbeing.
Figure 1.
Chemical structure of ketamine enantiomers. (S)-ketamine and (R)-ketamine are a pair of stereoisomers that are non-superimposable mirror images of each other. An example of familiar objects that are related in such a way are the left and right hand.
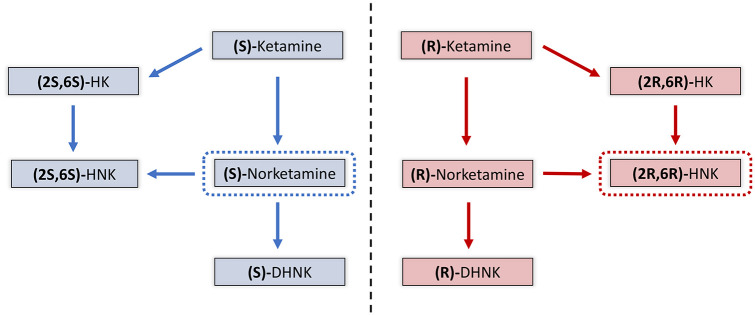
(S)-ketamine and (R)-ketamine both undergo extensive metabolism by cytochrome P450 enzymes to corresponding forms of norketamine, dehydronorketamine (DHNK), hydroxyketamine (HK) and hydroxynorketamines (HNKs) (Zanos et al., 2018; Zarate et al., 2012a) (Figure 2). (S)-ketamine or (R)-ketamine is first demethylated by either CYP3A4 or CYP2B6 to (S)-norketamine or (R)-norketamine. (S)-norketamine or (R)-norketamine are subsequently metabolised to (S)-DHNK or (R)-DHNK or HNKs. Hydroxylation of (S)-norketamine or (R)-norketamine by CYP2A6 at the six position results in (2S,6S)-HNK and (2R,6R)-HNK respectively, which are the major HNK metabolites found in plasma following ketamine infusion (Moaddel et al., 2010; Zarate et al., 2012a). CYP2A6 can also directly hydroxylate (S)-ketamine or (R)-ketamine to form (2S,6S)-HK and (2R,6R)-HK, which are further transformed to (2S,6S)-HNK or (2R,6R)-HNK (Desta et al., 2012). Of these metabolites, (2R,6R)-HNK and (S)-norketamine have attracted particular interest as candidate antidepressants in their own right (Yang et al., 2018a; Zanos et al., 2016).
Figure 2.
Major metabolites of (S)-ketamine and (R)-ketamine. (S)-ketamine or (R)-ketamine are initially metabolised to (S)-norketamine or (R)-norketamine via CYP3A4 or CYP2B6. (S)-norketamine or (R)-norketamine are further metabolised to (S)-dehydronorketamine (DHNK) or (R)-DHNK. (S)-norketamine or (R)-norketamine are metabolised to (2S,6S)-hydroxynorketamine (HNK) or (2R,6R)-HNK via CYP2A6. (S)-ketamine or (R)-ketamine may also be metabolised to (2S,6S)-HK or (2R,6R)-hydroxyketamine (HK) via CYP2A6 before transformation to (2S,6S)-HNK or (2R,6R)-HNK. Metabolites identified as candidate antidepressants are highlighted in dashed boxes.
Ketamine as an antidepressant
(R,S)-ketamine: In the first double-blind, placebo-controlled study of racemic ketamine in MDD, it was demonstrated that a single sub-anaesthetic intravenous (IV) infusion (0.5 mg/kg over 40 mins) resulted in rapid antidepressant effects, within hours of administration (Berman et al., 2000). A number of subsequent studies have also demonstrated the rapid-acting antidepressant effects of ketamine in treatment-resistant unipolar and bipolar depression (Price et al., 2009; Zarate et al., 2006, 2012b). Findings have been reviewed in several meta-analyses, which report robust antidepressant and anti-suicidal effects, lasting up to 1 week, in treatment-resistant MDD and bipolar depression (Kishimoto et al., 2016; Wilkinson et al., 2018), with acute dissociative symptoms being the most commonly reported side effect (Kishimoto et al., 2016).
Unfortunately, the antidepressant effect of a single dose of ketamine is not generally sustained beyond 1 week (Kishimoto et al., 2016). In the first randomised controlled trial (RCT) of repeated ketamine administration, it was shown that twice- or thrice-weekly administration of IV ketamine (0.5 mg/kg over 40 mins) was sufficient to maintain antidepressant efficacy over 15 days in individuals with treatment-resistant depression (TRD) (Singh et al., 2016b). Similar findings have also been reported in open-label repeated infusion studies (Rasmussen et al., 2013; Shiroma et al., 2014; Zheng et al., 2018). In contrast, a recent RCT investigating the effects of six ketamine infusions (0.5 mg/kg over 45 mins) or saline placebo over 3 weeks in severe TRD with current, chronic suicidal ideation failed to demonstrate a significant difference in depression severity or suicidality at the 3 week endpoint (Ionescu et al., 2019). However, this study was limited by a small sample size (out of 26 randomised patients, n=13 per group, only 14 completed the entire study) and therefore may have been underpowered to detect a true difference between treatment groups. In addition, all patients were maintained on their medication regimes throughout the infusion phase and the impact that concomitant medications may have had on ketamine’s effects cannot be ruled out.
Although there are specialist centres around the world and an increasing number of ketamine clinics in the United States offering ketamine infusions for depression (Ketamine-Clinics-Directory, 2020), the use of repeated infusions may not be the most practical due to the resources required. Other routes of administration (oral, sublingual, intranasal, intramuscular or subcutaneous) could prove to be simpler and more feasible alternatives for repeated administration but few studies have evaluated these options (Andrade, 2017).
(S)-ketamine: As NMDA receptor antagonism was understood to play a key role in ketamine’s antidepressant mechanism, (S)-ketamine was investigated as a novel antidepressant candidate by Janssen Research & Development due to its higher affinity for the NMDA receptor. In a first proof-of-concept trial, IV (S)-ketamine at doses of 0.2 mg/kg and 0.4 mg/kg led to rapid and robust antidepressant effects in individuals with TRD (Singh et al., 2016a). Side effects included headache, nausea and dissociation. It was suggested that as improvements in depressive symptoms were not significantly different between the two tested doses that a lower dose of (S)-ketamine may allow for better tolerability while maintaining efficacy (Singh et al., 2016a).
A fixed-dose (S)-ketamine nasal spray has subsequently been developed and tested in TRD. A number of Phase II and III trials have shown that intranasal (S)-ketamine plus an existing or newly initiated oral antidepressant outperforms placebo plus an oral antidepressant for individuals with TRD (Canuso et al., 2018; Daly et al., 2018, 2019; Popova et al., 2019), although others failed to demonstrate positive results (Fedgchin et al., 2019; Ochs-Ross et al., 2020). In a large discontinuation study, 297 individuals with TRD who met response or remission criteria following 16 weeks of treatment with intranasal (S)-ketamine (56 mg or 84 mg twice weekly) plus an oral antidepressant were entered into a randomised withdrawal phase (to continue with (S)-ketamine or switch to placebo) (Daly et al., 2019). Those randomised to continue treatment with intermittently administered (S)-ketamine nasal spray plus an oral antidepressant had a significantly delayed time to relapse compared to those treated with placebo nasal spray and oral antidepressants. A subsequent open-label study has examined the long-term safety of (S)-ketamine nasal spray plus a new oral antidepressant in patients with TRD (Wajs et al., 2020). Common treatment-emergent adverse events included dizziness, dissociation, nausea and headache, which mostly occurred on dosing days, were mild to moderate in severity and resolved on the same day. Longitudinal analysis showed dissociative symptoms declined over subsequent administrations and cognitive performance was generally found to either improve or remained stable compared with baseline. Similar long-term maintenance or safety evidence of this level are not available for (R,S)- or (R)-ketamine at this time.
Considering the available evidence, the United States Food and Drug Administration and European Medicines Agency have approved the (S)-ketamine nasal spray, SpravatoTM, for adults with TRD in combination with an oral antidepressant. However, some questions remain regarding uncertainty of efficacy, safety, potential for abuse and need for careful monitoring, which currently limit wider use (Kryst et al., 2020; Turner, 2019).
(R)-ketamine: Preclinical findings have suggested (R)-ketamine has the potential for more potent and longer-lasting antidepressant effects than both ketamine and (S)-ketamine and it appears to have less behavioural side-effects and abuse liability (Chang et al., 2019; Fukumoto et al., 2017; Yang et al., 2015; Zanos et al., 2016). Given initial findings from Vollenweider et al. (1997) in healthy subjects, where (R)-ketamine did not produce psychosis-like symptoms as seen with (S)-ketamine, but instead feelings of relaxation and wellbeing, researchers have now begun to explore the antidepressant potential of (R)-ketamine in humans (Leal et al., 2020).
In the first open-label pilot study of (R)-ketamine, seven subjects with TRD received a single IV infusion of (R)-ketamine at a dose of 0.5 mg/kg over 40 mins (Leal et al., 2020). Mean Montgomery-Åsberg Depression Rating Scale scores dropped significantly from 30.7 at baseline to 10.4 at day 1 after the infusion, with 71% of subjects showing an antidepressant response at day 1 and 57% at day 7. Interestingly, dissociation was nearly absent with minimal haemodynamic effects. However, it should be noted that five out of the seven patients in this study were taking antipsychotic medications (quetiapine (three), risperidone (one), aripiprazole (one)) that might have resulted in lower blood pressure or less dissociation. Naturally, the results of this small open-label study must be interpreted with caution. A clinical trial is underway by Perception Neuroscience to further investigate safety and tolerability of differing doses of (R)-ketamine in healthy volunteers before exploring its potential in depression (Universal Trial Number: U1111-1241-1005). In addition, a large trial comparing the efficacy and safety of (R)-ketamine with (S)-ketamine and (R,S)-ketamine in TRD is already underway in China (ChiCTR1800015879).
Mechanistic considerations
NMDA receptor antagonism and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation
It has been widely acknowledged that the rapid antidepressant effects of ketamine are mediated through blockade of NMDA receptors located on γ-aminobutyric acid (GABA)-ergic inhibitory interneurons (Krystal et al., 2019a). This in turn leads to a disinhibition of pyramidal cells and an acute cortical glutamate surge. Subsequent activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors appears to play a key role in the antidepressant effects as demonstrated by preclinical work that has shown pre-treatment with an AMPA receptor antagonist blocks the antidepressant effects of ketamine and its enantiomers in rodent models (Autry et al., 2011; Maeng et al., 2008; Yang et al., 2015).
It has been reported that the metabolism of (R,S)-ketamine to HNK, is required for ketamine’s antidepressant-like effects in rodents (Zanos et al., 2016). Administration of the (R)-enantiomer (2R,6R)-HNK was associated with greater and longer-lasting antidepressant effects than (2S,6S)-HNK and MK-801, a more potent NMDA receptor antagonist. Importantly, (2R,6R)-HNK lacked ketamine-related side-effects and abuse potential in this model. Furthermore, the antidepressant effects were independent of any action on NMDA receptors but instead required AMPA receptor activation (Zanos et al., 2016). Subsequent work has also suggested an important role of presynaptic group II metabotropic glutamate (mGlu2) autoreceptor inhibition in the antidepressant actions of (2R,6R)-HNK (Zanos et al., 2019). Although findings with regards to antidepressant-like effects of (2R,6R)-HNK in rodents have been replicated by other independent laboratories (Fukumoto et al., 2019; Pham et al., 2018), other studies from one laboratory were unable to reproduce this and instead suggest unmetabolised (R)-ketamine itself may be responsible for the antidepressant actions (Shirayama and Hashimoto, 2018; Yamaguchi et al., 2018; Yang et al., 2017a). Clinical trials have not yet examined the utility of (2R,6R)-HNK as a rapid-acting antidepressant. However, clinical studies investigating ketamine metabolite plasma levels as biomarkers have found that higher (2R,6R)-HNK levels were associated with less improvement in depressive symptoms (Farmer et al., 2020; Grunebaum et al., 2019), which is counterintuitive considering preclinical findings. Regardless, (2R,6R)-HNK, with its potential to modulate mGlu2 and AMPA receptor function, remains a promising candidate antidepressant and work to validate this compound for clinical use is ongoing at the United States National Institute for Mental Health (Kraus et al., 2019).
(S)-ketamine is primarily metabolised to (S)-norketamine and it has been shown in an animal model that although the antidepressant actions of the metabolite are similarly potent to the parent compound, (S)-norketamine appeared to lack the associated side effects (Yang et al., 2018a). Importantly, the findings of this study suggest that AMPA receptor activation is not necessary for the antidepressant actions of (S)-nortketamine as AMPA receptor antagonists did not block its antidepressant effects, instead highlighting a role for brain-derived neurotrophic factor (BDNF), tropomyosin kinase B (TrkB) and mechanistic target of rapamycin complex (mTORC) signalling (Yang et al., 2018a). There is, however, some recent clinical evidence that found no relationship between norketamine concentration (neither (S)-norketamine nor (R)-norketamine) and antidepressant response, following administration of (R,S)-ketamine to individuals with TRD (Farmer et al., 2020).
GABAergic interneuron inhibition
The preferential action of (R,S)-ketamine at GABAergic interneurons is supported by findings that the NMDA receptor antagonist MK-801 initially inhibits the firing of fast-spiking GABAergic interneurons and, at a delayed rate, increases the firing rate of pyramidal neurons (Homayoun and Moghaddam, 2007). Widman et al. (2018) have since demonstrated that perfusion of hippocampal rat brain slices with (R,S)-ketamine enhances the excitability of pyramidal cells indirectly by reducing synaptic GABAergic inhibition, thus causing disinhibition. A recent study found that knockdown of a key NMDA receptor subunit, GluN2B, on GABAergic interneurons resulted in a significant increase (disinhibition) of spontaneous excitatory postsynaptic currents on layer V pyramidal cells in mouse brain slices (Gerhard et al., 2020). Moreover, knockdown of GluN2B on GABAergic interneurons but not pyramidal cells of the medial prefrontal cortex (mPFC) had antidepressant-like effects and occluded or blocked the antidepressant behavioural effects of (R,S)-ketamine. Further supporting this disinhibition hypothesis, administration of negative allosteric modulators of GABAA receptors (GABA-NAMs) exert rapid antidepressant actions similar to (R,S)-ketamine in animal models (Fischell et al., 2015; Zanos et al., 2017), likely through disinhibition of excitatory glutamatergic neurotransmission (Towers et al., 2004). Although GABAergic interneuron inhibition via NMDA receptors appears to serve as an important initial target of (R,S)-ketamine, further work is needed to determine if this mechanism is as relevant for each of ketamine’s enantiomers and metabolites.
BDNF-TrkB signalling
BDNF and its receptor TrkB have been consistently implicated in the aetiology of depression and mechanism of action of current antidepressants (Duman and Monteggia, 2006; Dwivedi, 2009; Hashimoto et al., 2004). BDNF serves a key a role in processes including neuronal maturation, synapse formation and synaptic plasticity (Park and Poo, 2013). Findings from preclinical work suggest BDNF-TrkB signalling in the hippocampus and prefrontal cortex to be a critical component of antidepressant response to conventional antidepressants (Adachi et al., 2008; Rantamaki et al., 2007; Schmidt and Duman, 2010). The rapid antidepressant-like effects of (R,S)-ketamine have been shown in one preclinical study to depend on the rapid synthesis of BDNF (Autry et al., 2011). In this study, (R,S)-ketamine was shown to rapidly increase TrkB phosphorylation, an indicator of TrkB activation, in the hippocampus, suggesting BDNF-TrkB signalling in this brain region is also involved in the antidepressant response to ketamine. This is in agreement with previous work showing the acute antidepressant effects of (R,S)-ketamine administration are associated with increased BDNF protein levels in the hippocampus (Garcia et al., 2008).
In the study by Autry et al. (2011), the ketamine-mediated suppression of resting NMDA receptor activity was also shown to deactivate eukaryotic elongation factor 2 (eEF2) kinase, resulting in reduced eEF2 phosphorylation and augmentation of BDNF synthesis. Subsequent findings confirmed the importance of this signalling pathway in the antidepressant response to ketamine as eEF2 kinase knockout mice, administered an acute low dose of (R,S)-ketamine did not show an antidepressant response or an increase in BDNF protein expression in the hippocampus (Nosyreva et al., 2013). In addition to the hippocampus, BDNF in the mPFC may also be an important site of action as preclinical work has found that an infusion of a BDNF neutralising antibody into the mPFC abolishes ketamine’s antidepressant-like effects (Lepack et al., 2014). Additional preclinical work demonstrated that (R,S)-ketamine-induced antidepressant effects are associated with upregulation of BDNF and mTORC in the hippocampus and prefrontal cortex, mediated by AMPA receptors (Zhou et al., 2014).
Considering the individual enantiomers, in a chronic social defeat stress and learned helplessness models of depression, a TRkB antagonist was able to block the antidepressant effects of both (S)-ketamine (Yang et al., 2018a) and (R)-ketamine (Yang et al., 2015). Interestingly, (R)-ketamine induced greater effects on reduced dendritic spine density, BDNF-TrkB signalling and synaptogenesis in the prefrontal cortex and hippocampus compared with (S)-ketamine and (R)-ketamine showed a greater potency and longer-lasting antidepressant effect than (S)-ketamine in this model (Yang et al., 2015).
Mammalian target of rapamycin complex and extracellular signal-regulated kinase
The mammalian target of rapamycin complex 1 (mTORC1) and extracellular signal-regulated kinase (ERK) are key signalling molecules in pathways that regulate protein synthesis with roles in synaptic development and plasticity (Ignacio et al., 2016; Mendoza et al., 2011). The function of mTORC1 and ERK in the antidepressant actions of ketamine and its enantiomers are not completely clear. Work in rodents initially demonstrated that (R,S)-ketamine rapidly activated the mTORC pathway, leading to increased synaptic signalling proteins and synaptic spine density (Li et al., 2010). Furthermore, intracerebroventricular administration of an mTORC1 inhibitor, rapamycin, has been shown to block ketamine-induced synaptogenesis and antidepressant-like effects (Li et al., 2010, 2011). Other work has shown that (R,S)-ketamine administration did not alter levels of phosphorylated mTOR in the hippocampi of control or BDNF-knockout mice, neither were the antidepressant effects of (R,S)-ketamine blocked by intraperitoneally administered rapamycin (Autry et al., 2011). However, this study did report reduced phosphorylation of ribosomal protein s6 kinase in brain tissues, a pharmacodynamic readout of mTORC1 inhibition, following rapamycin administration. One explanation for the failure of rapamycin to block the antidepressant effects of (R,S)-ketamine in the study by Autry et al. (2011) is that the peripheral route of administration may not have achieved sufficient central nervous system exposure compared to studies where intracortical rapamycin administration resulted in adequate mTORC1 inhibition to block (R,S)-ketamine’s antidepressant effects (Li et al., 2010, 2011).
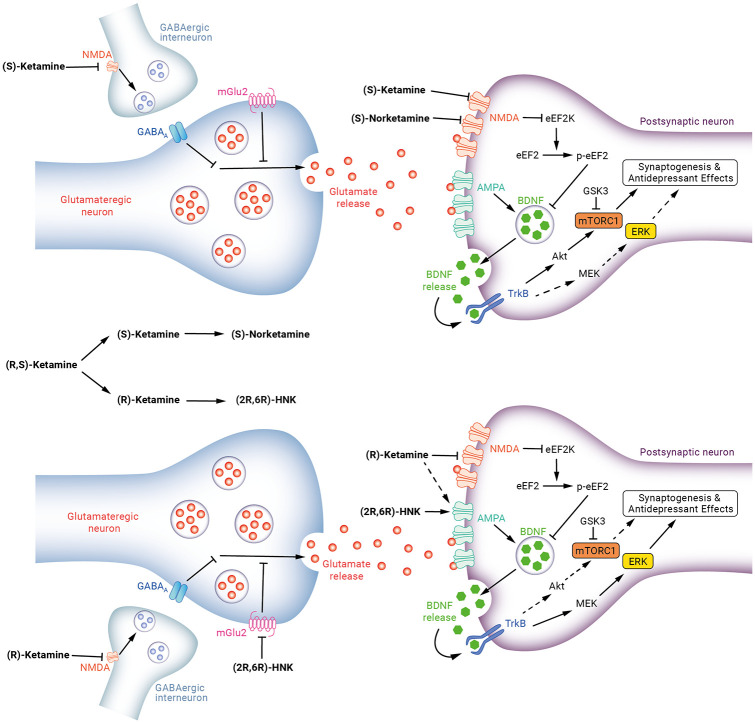
Further preclinical work demonstrated that the antidepressant effects of (S)-ketamine but not (R)-ketamine were blocked by mTORC1 inhibition and that (S)-ketamine, but not (R)-ketamine, significantly attenuated decreased phosphorylation of mTOR in the prefrontal cortex of mice in a chronic social defeat stress model (Yang et al., 2018b). This same study showed that pre-treatment with an ERK inhibitor blocked the antidepressant effects of (R)-ketamine but not (S)-ketamine and, furthermore, (R)-ketamine but not (S)-ketamine significantly attenuated the reduced phosphorylation of ERK in the prefrontal cortex and hippocampi of susceptible mice using the same model (Yang et al., 2018b). It is interesting to note that the antidepressant effects of (S)-norketamine, (S)-ketamine’s predominant metabolite, have also been shown to be blocked by the mTORC1 inhibitor rapamycin (Yang et al., 2018a). Taken together this suggests that mTORC1 has a role in antidepressant effects of (S)-ketamine but less so for (R)-ketamine and that ERK activation could instead mediate the antidepressant effects of (R)-ketamine (Figure 3).
Figure 3.
Proposed signalling pathways underlying the antidepressant actions of ketamine enantiomers and metabolites. Top: (S)-ketamine causes glutamate release via disinhibition of γ-aminobutyric acid (GABA) interneurons. Resulting glutamate surge stimulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors leading to release of brain-derived neurotrophic factor (BDNF) with resulting activation of tropomyosin kinase B (TrkB)-Akt-mammalian target of rapamycin complex 1 (mTORC1) signalling. This leads to increased synthesis of proteins required for synaptogenesis. (S)-ketamine and (S)-norketamine suppress resting N-Methyl-D-Aspartate (NMDA) receptor activity, deactivating eukaryotic elongation factor 2 (eEF2) kinase, resulting in reduced eEF2 phosphorylation, augmentation of BDNF synthesis and TrkB-mTORC1 activation. Bottom: (R)-ketamine causes glutamate release via disinhibition of GABA interneurons with activation of AMPA receptors and BDNF release but there may be an alternative pathway by which (R)-ketamine stimulates AMPA receptor transmission that still needs to be elucidated. (R)-ketamine may cause preferential activation of TrkB-MEK-ERK signalling pathway leading to synaptogenesis. (2R,6R)-HNK directly activates AMPA receptors and inhibition of group II metabotropic glutamate (mGlu2) receptors may also be involved in this metabolite’s antidepressant actions.
In a recent randomised double-blind cross-over study, Abdallah et al. (2020) explored whether pre-treatment with rapamycin could attenuate the rapid antidepressant effects of (R,S)-ketamine in individuals with MDD. Surprisingly, rapamycin did not alter the acute antidepressant effects of (R,S)-ketamine but instead prolonged the antidepressant effects (Abdallah et al., 2020). Two weeks after ketamine administration there were significantly higher response and remission rates following rapamycin + ketamine compared to placebo + ketamine. The authors hypothesised that the failure to block ketamine’s effects by rapamycin may have been due to the dosage used and peripheral route of administration, mirroring the findings from preclinical work that also utilised peripheral rapamycin administration (Autry et al., 2011). A key difference between this human work and the preclinical work, however, is follow-up time. None of the ketamine + rapamycin animal studies discussed could have discovered findings as shown in the study by Abdallah et al. (2020) as antidepressant effects were not followed up for long enough (Autry et al., 2011; Li et al., 2010, 2011). Abdallah et al. (2020) also hypothesised that rapamycin may extend the antidepressant effects of ketamine via an mTORC1-dependent anti-inflammatory mechanism (Thomson et al., 2009), protecting newly made synapses from inflammatory processes that cause synaptic elimination and undermine the antidepressant effects of ketamine, or by enhancing autophagy (a crucial mTORC1 regulated process involved in normal cellular plasticity, which involves degrading and recycling toxic or dysfunctional cellular components) (Abdallah et al., 2020). Alternatively, it is possible to speculate rapamycin may have mTORC1-independent effects that contribute to the antidepressant effects of ketamine. For example, rapamycin may attenuate an unacknowledged homeostatic mechanism that normally contributes to relapse. A final speculative consideration is whether rapamycin may promote longer-lasting antidepressant effects of (R)-ketamine via increased ERK signalling as low concentrations of rapamycin have been shown to increase Akt and ERK activation in vitro through an mTORC1-dependent mechanism (Chen et al., 2010). Although interesting, these preliminary findings should be interpreted with caution and replication in future studies is needed, before back translation to animal studies (Abdallah and Krystal, 2020), alongside work to determine any differential effects of rapamycin on each of ketamine’s enantiomers.
Glycogen synthase kinase 3
Activation of mTORC1 signalling has been linked to phosphorylation (deactivation) of glycogen synthase kinase-3 (GSK-3) and inhibition of GSK-3 has been shown to be necessary for the rapid antidepressant-like effects of (R,S)-ketamine in mice (Beurel et al., 2011). Furthermore, administration of (R,S)-ketamine in combination with lithium, a non-selective GSK-3 inhibitor, resulted in rapid activation of the mTORC1 signalling pathway, increased inhibitory phosphorylation of GSK-3, increased synaptic spine density and potentiated antidepressant-like responses in rodents (Liu et al., 2013). Ketamine-induced inhibition of GSK-3 has also been linked to AMPA receptor upregulation and stabilisation at the cell surface. In a preclinical study it was demonstrated that (R,S)-ketamine-induced inhibition of GSK-3 resulted in reduced phosphorylation of post-synaptic density-95 (which regulates AMPA receptor trafficking), diminishing the internalisation of AMPA GluA1 subunits (Beurel et al., 2016). This could ultimately allow for augmented signalling through AMPA receptors following ketamine treatment.
In clinical work, testing the GSK-3 inhibition hypothesis, lithium continuation therapy showed no benefit over placebo at 2 weeks following the cessation of four (R,S)-ketamine infusions in individuals with TRD (Costi et al., 2019). In patients with treatment-resistant bipolar depression, maintained on either therapeutic-dose lithium or valproate before receiving (R,S)-ketamine versus placebo, a significant improvement in depressive symptoms was seen in both mood stabiliser groups, and although ketamine’s antidepressant effect size relative to placebo was larger for lithium (d=2.27) than valproate (d=0.79), there was no significant difference observed between these two agents (Xu et al., 2015). Furthermore, neither serum lithium nor valproate levels correlated with ketamine’s antidepressant efficacy.
Although some evidence highlights GSK-3 as an important regulatory target for ketamine’s antidepressant effects, clinical studies have not yet confirmed preclinical findings. Further evaluation of the role of GSK-3 in the antidepressant effects of ketamine’s individual enantiomers and metabolites is still required.
Translocation of Gs alpha subunit from lipid rafts
An increase in intracellular cyclic adenosine monophosphate (cAMP) that acts to upregulate neurotrophic factors and increase synaptogenesis has been associated with conventional antidepressant action (Dwivedi and Pandey, 2008; Gass and Riva, 2007). Gαs is a subunit of the G protein GS that stimulates the generation of cAMP by activating adenylyl cyclase. Localisation of Gαs within lipid raft microdomains in the plasma membrane acts to regulate cellular signalling, and indeed production of cAMP is diminished when Gαs is localised to lipid raft microdomains (Allen et al., 2009). A number of preclinical studies have demonstrated increases in cAMP through translocation of Gαs from lipid raft domains into non-raft regions, augmenting interaction between Gαs and adenylyl cyclase, following administration of various classes of antidepressants (Czysz et al., 2015; Toki et al., 1999; Zhang and Rasenick, 2010).
Wray et al. (2019) have since reported that (R,S)-ketamine administration to C6 glioma cells led to immediate translocation of Gαs from lipid raft domains to non-raft domains and an increase in cAMP, followed by an increase in BDNF expression after 24 hours. The (R,S)-ketamine induced increase in cAMP was found to persist after knocking out the NMDA receptor indicating an NMDA receptor-independent mechanism. Further, administration of the ketamine metabolite (2R,6R)-HNK also resulted in redistribution of Gαs from lipid rafts and an increase cAMP production. These findings suggest the translocation of Gαs from lipid rafts is a reliable hallmark of antidepressant action; however, further research is needed to examine to what degree this mechanism contributes to the antidepressant effect of the individual enantiomers of ketamine.
Monoaminergic systems
Several studies suggest that 5-hydroxytryptamine (5-HT) signalling plays a role in the antidepressant effects of ketamine. Preclinical work has demonstrated that the antidepressant-like action of (R,S)-ketamine is blocked by pre-treatment with a 5-HT-depleting agent (Fukumoto et al., 2014, 2016; Gigliucci et al., 2013). (R,S)-ketamine has been found to inhibit serotonin transporter (SERT) function in vitro (Zhao and Sun, 2008) and a positron emission tomography (PET) study in conscious monkeys further reported that subanaesthetic (R,S)-ketamine selectively enhanced serotonergic transmission by inhibition of SERT activity (Yamamoto et al., 2013). Alongside SERT inhibition, increased mPFC 5-HT release via AMPA receptor stimulation in the dorsal raphe nucleus may be involved in the antidepressant effects of (R,S)-ketamine (Chaki and Fukumoto, 2019; Pham et al., 2017). Moreover, it has been demonstrated that an mPFC infusion of a 5-HT1a receptor antagonist blocks the antidepressant-like effects of (R,S)-ketamine in mice and attenuates ketamine-induced increases in phosphorylation of Akt (Fukumoto et al., 2018). (R,S)-ketamine antidepressant effects were mimicked by intra-mPFC, but not systemic, administration of a 5-HT1a receptor agonist and both the antidepressant effects of ketamine and the 5-HT1a receptor agonist were blocked by the mTORC1 inhibitor rapamycin (Fukumoto et al., 2018). Finally, in a recent study, infusion of a selective 5-HT1A receptor agonist into the mPFC produced ketamine-like rapid synaptic and antidepressant-like behavioural responses in a rodent model that were blocked by co-infusion of an AMPA receptor antagonist (Fukumoto et al., 2020). Taken together, it appears another route via which (R,S)-ketamine may cause its antidepressant effects is through 5-HT1A receptor activation in the mPFC, by AMPA receptor-dependent 5-HT release, with downstream convergence on signalling mechanisms. These include the Akt/mTORC1 pathway but may also include ERK signalling, which is also activated by direct 5HT1A receptor stimulation (Buritova et al., 2009; Newman-Tancredi et al., 2009).
Considering the individual enantiomers, an in vivo microdialysis study has shown that both (R)- and (S)-ketamine acutely increase 5-HT release in the prefrontal cortex, with (R)-ketamine causing a greater increase than (S)-ketamine (Ago et al., 2019). Although the (S)-ketamine-induced 5-HT release was attenuated by an AMPA receptor antagonist, (R)-ketamine-induced 5-HT release was not affected by AMPA receptor blockade. Although preclinical work has demonstrated that 5-HT depletion abolishes the antidepressant-like actions of (S)-ketamine in a genetic model of depression (du Jardin et al., 2016), other work has shown that 5-HT depletion does not alter the antidepressant effects of (R)-ketamine in a chronic social defeat stress model (Zhang et al., 2018). This suggests that 5-HT may not play as major a role in antidepressant effects of (R)-ketamine. The reason for these differences is not entirely clear and further work is needed to explore the role of 5-HT in the effects of ketamine and its enantiomers.
The dopamine system has also been implicated in depression and the antidepressant effects of ketamine; however, the mechanism underlying the action of ketamine or its enantiomers on this system has not been fully established. Acute subanaesthetic (R,S)-ketamine administration is associated with significantly increased dopamine levels in the cortex, striatum and nucleus accumbens in rodents (Kokkinou et al., 2018) and there is also in vivo PET imaging evidence that (R,S)-ketamine and (S)-ketamine administration leads to increased striatal dopamine release in humans as indexed by D2/D3 receptor tracer binding (Breier et al., 1998; Smith et al., 1998; Vollenweider et al., 2000). A further PET study found that IV (S)-ketamine administration, but not (R)-ketamine, led to a significant reduction of binding availability of dopamine D2/D3 receptor in the monkey striatum and suggests that unlike (R)-ketamine, (S)-ketamine can cause dopamine release in the striatum that may contribute to the psychotomimetic/dissociative side effects in humans (Hashimoto et al., 2017). Other groups found that (R,S)-ketamine-induced reductions of D2/D3 binding in humans only occurred in combination with amphetamine, suggesting ketamine may enhance the sensitivity of the dopamine system but not lead to direct dopamine release (Aalto et al., 2002, 2005; Kegeles et al., 2002).
In a study examining the effects of (R,S)-ketamine and metabolites on evoked striatal dopamine release and dopamine receptors, (R,S)-ketamine did not alter the magnitude or kinetics of electrical stimulation-evoked dopamine release in the nucleus accumbens of anesthetised mice and neither ketamine’s enantiomers nor its metabolites had affinity for dopamine receptors or the dopamine transporter (Can et al., 2016). This suggests the side effects and antidepressant actions of ketamine (or its metabolites) may not be associated with direct effects on mesolimbic dopaminergic neurotransmission. An alternative hypothesis is that ketamine produces indirect effects through NMDA receptor antagonism on GABAergic interneurons, resulting in disinhibition of glutamatergic projections on to dopamine neurons in the midbrain, an increase in glutamate release, subsequent activation of dopaminergic neurons and increased dopamine levels in targets such as the striatum and cortex (Stone et al., 2007).
Additional research findings indicate that dopamine D1 receptor activity in the mPFC are necessary for the rapid antidepressant actions of (R,S)-ketamine using optogenetic stimulation in a mouse model (Hare et al., 2019). Another potential convergence from a signal transduction perspective may involve NMDA and D1 receptor-dependent induction of mTORC/ERK and inactivation of eEF2 kinase resulting in increased protein synthesis (David et al., 2020). Further work found that pre-treatment with a dopamine D1 receptor antagonist did not block the antidepressant effects of (R)-ketamine in a chronic social defeat stress model (Chang et al., 2020). Furthermore, although (S)-ketamine has been shown to cause a robust increase in dopamine release compared with (R)-ketamine, the antidepressant-like effects were more potent and longer acting following (R)-ketamine administration in a mouse model (Ago et al., 2019). Taken together, these findings suggest that activation of the dopamine system may be required for the antidepressant actions of (S)- but not (R)-ketamine.
Inhibition of lateral habenula bursting
Increasing lines of preclinical and clinical evidence highlight a major role for the lateral habenula, an anti-reward centre, in the pathophysiology of depression. It is suggested that abnormal increases in neuronal activity in this region signal downregulation of brainstem dopaminergic and serotonergic firing, resulting in depressive symptomatology including anhedonia, helplessness and excessive focus on negative experiences (Gold and Kadriu, 2019).
Recent work from Yang et al. (2018c) found that blockade of NMDA receptor-dependent bursting activity in the lateral habenula mediated the antidepressant actions of (R,S)-ketamine in rodent models of depression. It was demonstrated that lateral habenula bursting required both NMDA receptors and low-voltage-sensitive T-type calcium channels. Furthermore, administration of T-type calcium channel inhibitors (ethosuximide and mibefradil) caused rapid antidepressant-like effects in both the forced swim test and sucrose preference test (Yang et al., 2018c). In contrast, preclinical work utilising a chronic social defeat stress model failed to demonstrate antidepressant effects of ethosuximide whereas (R)-ketamine showed rapid and long-lasting antidepressant actions in this model (Tian et al., 2018). Furthermore, a recent double-blind RCT in medication-free patients with depression found no significant reductions in depression and anxiety scores following treatment with ethosuximide, suggesting T-type calcium channel inhibitors are unlikely to exert ketamine-like robust antidepressant actions (Zhang et al., 2020a).
It should be noted that inhibition of lateral habenula bursting as a mechanism of antidepressant action has only been assessed acutely at 1 hour post (R,S)-ketamine infusion (Yang et al., 2018c). Whether this mechanism is active later during (R,S)-ketamine’s antidepressant effects (>24 hours) or indeed if there are differential effects of (S)- and (R)-ketamine on lateral habenula bursting remains unknown.
Opioid receptor system
Ketamine interacts with mu, kappa and, to a lesser extent, delta-opioid receptors (Ki = 42.1, 28.1, and 272 mΜ, respectively) (Hirota et al., 1999; Zanos et al., 2018). The affinity of (S)-ketamine for the mu and kappa opioid receptors is two to fourfold that of (R)-ketamine (Hirota et al., 1999; Hustveit et al., 1995). Recent work demonstrated that pre-treatment with naltrexone, an opioid receptor antagonist, significantly blocked the antidepressant and anti-suicidal effects of (R,S)-ketamine in TRD, suggesting that opioid system activation was necessary for the rapid-acting antidepressant and antisuicidal effects of (R,S)-ketamine (Williams et al., 2018, 2019). There are a number of important limitations to these studies including the small sample size (only 12 participants completing both naltrexone and placebo pre-treatment conditions and only seven of the 12 meeting response criteria during the ketamine plus placebo condition), lack of a placebo control arm for the (R,S)-ketamine infusion (i.e., naltrexone + IV saline and placebo + IV saline) and finally that participants may have experienced a noxious, nocebo type of response to the naltrexone + ketamine treatment, which influenced subsequent depression ratings (Mathew and Rivas-Grajales, 2019). Other work has demonstrated that naltrexone pre-treatment did not affect the antidepressant effects of (R,S)-ketamine in depressed individuals with alcohol use disorder (Yoon et al., 2019) and an earlier study in healthy individuals found that the behavioural effects of an antidepressant dose of ketamine were potentiated by pre-treatment with naltrexone (Krystal et al., 2006). Finally, in patients with TRD, concurrent use of buprenorphine and methadone (high affinity mu opioid receptor agonists) was not associated with reductions in antidepressant efficacy of (R,S)-ketamine (Marton et al., 2019).
In rodent models of depression (chronic social defeat stress and inflammation induced), it was shown that naltrexone pre-treatment did not block the antidepressant effects of (R,S)-ketamine (Zhang and Hashimoto, 2019). However, in a subsequent preclinical study, it was shown that opioid antagonists abolish the ability of (R,S)-ketamine to reduce depression-like behavioural and lateral habenula cellular hyperactivity (Klein et al., 2020). The authors suggested the opioid system is ‘necessary but not sufficient’ for the antidepressant actions of (R,S)-ketamine in rodents as activation by morphine, a mu-opioid agonist, at a dose high enough to induce a hedonic response, did not mimic the rapid antidepressant-like effects of ketamine or reduce lateral habenula neuronal activity (Klein et al., 2020). The authors argued that in their studies of lateral habenula cellular activity, (R,S)-ketamine did not appear to act as a mu-opioid agonist but that some mu-opioid receptor activity was necessary for NMDA receptor antagonism. In brain regions, including the habenula, NMDA receptors and opioid receptors display colocalisation (Rodriguez-Munoz et al., 2012) and NMDA receptor activation can be modulated by actions of opioid receptors (Kow et al., 2002; Martin et al., 1997). Taken together, this suggests a potential interaction that may be explained by direct ‘crosstalk’ between the glutamatergic and the opioid receptor systems (Chartoff and Connery, 2014), or by convergence at downstream signalling pathways.
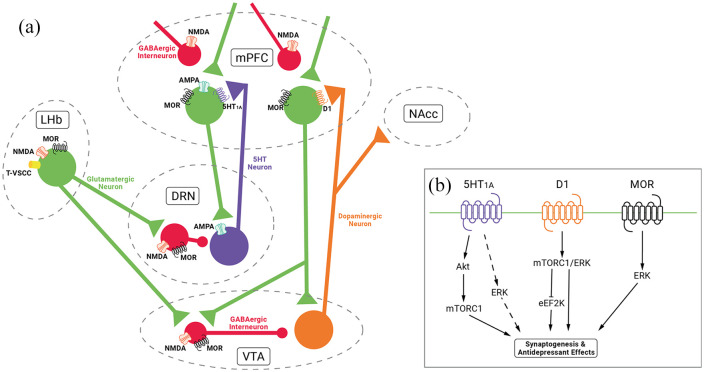
There are several potential convergences between opioid signalling and other mechanisms implicated in the antidepressant action of ketamine. For example, administration of endogenous opioids has been shown to upregulate BDNF expression in the frontal cortex, hippocampus and amygdala (Zhang et al., 2006). Moreover, these effects were reversed by naltrexone administration. Acute mu-opioid receptor activation has been shown to result in rapid activation of ERK signalling (Bian et al., 2015; Zheng et al., 2008) and acute treatment with ketamine enhances the levels of opioid-induced ERK phosphorylation in cells that endogenously express mu-opioid receptors (Gupta et al., 2011). Further, administration of an opioid receptor antagonist, naloxone, has been shown to inhibit mu-opioid-induced ERK activation in a dose-dependent manner in C6 glioma cell lines (Gutstein et al., 1997). Finally, there are a number of functional interactions between opioid receptors and monoaminergic systems relevant to mood control (Lutz and Kieffer, 2013). Specifically, activation of mu-opioid receptors expressed in the dorsal raphe nucleus and ventral tegmental area, via GABAergic interneurons, disinhibit 5-HT (Fadda et al., 2005; Tao and Auerbach, 2002) and dopamine neurons (Le Merrer et al., 2009) with projections including the prefrontal cortex and nucleus accumbens (Lutz and Kieffer, 2013) (Figure 4).
Figure 4.
Hypothesised monoamine and opioid mechanisms and potential convergences with signalling pathways implicated in the antidepressant actions of ketamine. (a) (R,S)-ketamine inhibits lateral habenula (LHb) bursting via actions on N-Methyl-D-Aspartate (NMDA)/low voltage sensitive t-type channels (T-VSCC)/mu-opioid receptors (MOR). This results in disinhibition of monoamine release via γ-aminobutyric acid (GABA)-ergic interneurons in the dorsal raphe nucleus (DRN) and ventral tegmental area (VTA) to projections including the medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc). Action of (R,S)-ketamine on NMDA/MOR on GABAergic interneurons in the DRN and VTA may be a further mechanism of disinhibition of 5-HT and dopamine release. 5-HT release in mPFC may also occur via α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor stimulation in DRN for (R,S)-ketamine and (S)-ketamine but might not be as relevant for (R)-ketamine. (b) Stimulation of postsynaptic 5-HT1A receptors via 5-HT in mPFC results in activation of Akt/mammalian target of rapamycin complex 1 (mTORC1) and potentially ERK signalling. Stimulation of postsynaptic D1 receptor via dopamine may result in activation of mTORC1/ERK and inactivation of eukaryotic elongation factor 2 (eEF2) kinase. Postsynaptic MOR activation may also potentiate the ERK signalling pathway.
The role of the opioid system in the antidepressant effect of ketamine remains controversial and a topic of debate (Amiaz, 2019; Heifets et al., 2019; Krystal et al., 2019b; Sanacora, 2019). Further work with rigorous trial design and parallel mechanistic studies are required to understand the function of the ketamine-opioid receptor interaction and subsequent signalling cascades in the antidepressant effect of each of ketamine and its individual enantiomers.
Conclusion
The discovery of the rapid antidepressant effects of (R,S)-ketamine, including in treatment-resistant patients, has appropriately been hailed ‘the most important discovery in half a century’ in depression research (Duman and Aghajanian, 2012). Through the drug development and clinical trials process, the (S)-ketamine nasal spray, SpravatoTM, has been approved in both the United States and Europe, although some concerns remain regarding efficacy and side effects. The first pilot study of (R)-ketamine in TRD has demonstrated encouraging results and, considering preclinical findings, it appears (R)-ketamine may have a more favourable safety profile than (S)-ketamine. Accumulating preclinical evidence also suggests (R)-ketamine to have more potent and longer-lasting antidepressant effects than both (R,S)-ketamine and (S)-ketamine. As studies of (R)-ketamine progress through Phase I and Phase II, results from direct comparison studies of the safety and efficacy of (R)-ketamine and (S)-ketamine in TRD will be crucial. Other key outstanding questions are outlined in Figure 5.
Figure 5.
Outstanding questions.
Although NMDA receptor inhibition and subsequent AMPA receptor activation have a role in the antidepressant effects of ketamine, further mechanistic work is building a more nuanced understanding of the distinct molecular and cellular mechanisms of ketamine, its enantiomers and metabolites, including BDNF-TrkB, mTORC1 and ERK signalling. Although there may be a role for monoaminergic and opioid receptor systems in the antidepressant effects or detrimental side effects of ketamine, further work examining the effects of each of the component enantiomers on these systems is required. All the while, new pieces of the ketamine puzzle are being discovered and other potential future directions of enquiry include examining the role of the transforming growth factor β1 system (Zhang et al., 2020b) and the brain-gut-microbiome axis (Huang et al., 2019; Yang et al., 2017b) in the antidepressant effects of ketamine and its enantiomers.
As we further our understanding of the similarities and differences in the signalling pathways associated with (S)-ketamine, (R)-ketamine and their metabolites, we should bear in mind potential complementary or synergistic antidepressant effects that might arise via distinct mechanisms. A deeper understanding of the precise molecular and cellular mechanisms underlying the antidepressant effects and negative side effects of (R,S)-ketamine, (S)-ketamine and (R)-ketamine will be invaluable as we seek to develop future rapid-acting antidepressants with favourable safety profiles, alongside treatment strategies to maintain adequate response.
Acknowledgments
The authors thank Ashleigh Earl for the illustrations in Figures 1, 3 and 4.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LAJ declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. In the last 3 years, JMS has been principle investigator or sub-investigator on studies sponsored by Takeda, Janssen and Lundbeck Plc. He has attended an Investigators’ meeting run by Allergan Plc. AHY is employed by King’s College London; Honorary Consultant SLaM (NHS United Kingdom). Paid lectures and advisory boards for the following companies with drugs used in affective and related disorders: Astrazenaca, Eli Lilly, Lundbeck, Sunovion, Servier, Livanova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma. He is also a consultant to Johnson & Johnson and to Livanova. He has received honoraria for attending advisory boards and presenting talks at meetings organised by LivaNova. He was principal investigator in the Restore-Life VNS registry study funded by LivaNova; ESKETINTRD3004: An open-label, long-term, safety and efficacy study of intranasal esketamine in treatment-resistant depression; the effects of psilocybin on cognitive function in healthy participants; and on the safety and efficacy of psilocybin in participants with treatment-resistant depression. Grant funding (past and present): National Institute of Mental Health (NIMH) (United States); Canadian Institutes of Health Research (CIHR) (Canada); National Alliance for Research on Schizophrenia and Depression (NARSAD) (United States); Stanley Medical Research Institute (United States); Medical Research Council (MRC) (United Kingdom); Wellcome Trust (United Kingdom); Royal College of Physicians (Scotland); British Medical Association (BMA) (United Kingdom); University of British Columbia and Vancouver General Hospital (UBC-VGH) Foundation (Canada); Western Economic Development Consortia (WEDC) (Canada); Coast Capital Savings (CCS) Depression Research Fund (Canada); Michael Smith Foundation for Health Research (MSFHR) (Canada); National Institute for Health Research (NIHR) (United Kingdom); and Janssen (United Kingdom). The authors have no shareholdings in pharmaceutical companies.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report represents independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Dr Luke A Jelen is a Medical Research Council (MRC) Clinical Research Training Fellow (MR/T028084/1).
ORCID iDs: Luke A Jelen  https://orcid.org/0000-0001-6398-5239
https://orcid.org/0000-0001-6398-5239
James M Stone  https://orcid.org/0000-0003-3051-0135
https://orcid.org/0000-0003-3051-0135
References
- Aalto S, Hirvonen J, Kajander J, et al. (2002) Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology (Berl) 164: 401–406. [DOI] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, et al. (2005) Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 182: 375–383. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Krystal JH. (2020) Ketamine and rapid acting antidepressants: Are we ready to cure, rather than treat depression? Behav Brain Res 390: 112628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Gueorguieva R, et al. (2020) Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Barrot M, Autry AE, et al. (2008) Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry 63: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Tanabe W, Higuchi M, et al. (2019) (R)-ketamine induces a greater increase in prefrontal 5-HT release than (S)-ketamine and ketamine metabolites via an AMPA receptor-independent mechanism. Int J Neuropsychopharmacol 22: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harbi KS. (2012) Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6: 369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Dave RH, et al. (2009) Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol Pharmacol 76: 1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R. (2019) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism: Is it a ketamine-specific effect? Am J Psychiatry 176: 250–251. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017) Ketamine for depression, 4: In what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry 78: e852–e857. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. (2011) Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16: 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Amadei C, et al. (2016) Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord 18: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian JM, Wu N, Su RB, et al. (2015) Phosphatidylethanolamine-binding protein is not involved in micro-opioid receptor-mediated regulation of extracellular signal-regulated kinase. Mol Med Rep 11: 3368–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, et al. (1998) Effects of NMDA antagonism on striatal dopamine release in healthy subjects: Application of a novel PET approach. Synapse 29: 142–147. [DOI] [PubMed] [Google Scholar]
- Buritova J, Berrichon G, Cathala C, et al. (2009) Region-specific changes in 5-HT1A agonist-induced extracellular signal-regulated kinases 1/2 phosphorylation in rat brain: A quantitative ELISA study. Neuropharmacology 56: 350–361. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, et al. (2016) Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther 359: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, et al. (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175: 620–630. [DOI] [PubMed] [Google Scholar]
- Chaki S. (2017) Beyond ketamine: New approaches to the development of safer antidepressants. Curr Neuropharmacol 15: 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Fukumoto K. (2019) Role of serotonergic system in the antidepressant actions of mGlu2/3 receptor antagonists: Similarity to ketamine. Int J Mol Sci 20: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, et al. (2019) Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav 181: 53–59. [DOI] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, et al. (2020) Lack of dopamine D1 receptors in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Eur Arch Psychiatry Clin Neurosci 270: 271–275. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Connery HS. (2014) It’s MORe exciting than mu: Crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XG, Liu F, Song XF, et al. (2010) Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinog 49: 603–610. [DOI] [PubMed] [Google Scholar]
- Costi S, Soleimani L, Glasgow A, et al. (2019) Lithium continuation therapy following ketamine in patients with treatment resistant unipolar depression: A randomized controlled trial. Neuropsychopharmacology 44: 1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Laws KR. (2015) The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum Psychopharmacol 30: 152–163. [DOI] [PubMed] [Google Scholar]
- Czysz AH, Schappi JM, Rasenick MM. (2015) Lateral diffusion of Gαs in the plasma membrane is decreased after chronic but not acute antidepressant treatment: Role of lipid raft and non-raft membrane microdomains. Neuropsychopharmacology 40: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, et al. (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 75: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Janik A, et al. (2019) Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 76: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Barrera I, Gould N, et al. (2020) D1 dopamine receptor activation induces neuronal eEF2 pathway-dependent protein synthesis. Front Mol Neurosci 13: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, et al. (2012) Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Muller HK, et al. (2016) Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl) 233: 2813–2825. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2012) Synaptic dysfunction in depression: Potential therapeutic targets. Science 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. (2009) Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr Dis Treat 5: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. (2008) Adenylyl cyclase-cyclicAMP signaling in mood disorders: Role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr Dis Treat 4: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, et al. (1997) Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 333: 99–104. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, et al. (2005) Dopamine and serotonin release in dorsal striatum and nucleus accumbens is differentially modulated by morphine in DBA/2J and C57BL/6J mice. Synapse 56: 29–38. [DOI] [PubMed] [Google Scholar]
- Farmer CA, Gilbert JR, Moaddel R, et al. (2020) Ketamine metabolites, clinical response, and gamma power in a randomized, placebo-controlled, crossover trial for treatment-resistant major depression. Neuropsychopharmacology 45: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, et al. (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: Results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, et al. (2015) Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology 40: 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S. (2014) Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231: 2291–2298. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S. (2016) The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaca MV, Liu RJ, et al. (2019) Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci USA 116: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaca MV, Liu RJ, et al. (2020) Medial PFC AMPA receptor and BDNF signaling are required for the rapid and sustained antidepressant-like effects of 5-HT1A receptor stimulation. Neuropsychopharmacology 45: 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, et al. (2018) Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, et al. (2017) Antidepressant potential of (R)-ketamine in rodent models: Comparison with (S)-ketamine. J Pharmacol Exp Ther 361: 9–16. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, et al. (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32: 140–144. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. (2007) CREB, neurogenesis and depression. Bioessays 29: 957–961. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Pothula S, Liu RJ, et al. (2020) GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest 130: 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, et al. (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228: 157–166. [DOI] [PubMed] [Google Scholar]
- Gold PW, Kadriu B. (2019) A major role for the lateral habenula in depressive illness: Physiologic and molecular mechanisms. Front Psychiatry 10: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, et al. (2019) Ketamine metabolite pilot study in a suicidal depression trial. J Psychiatr Res 117: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I. (2011) Potentiation of mu-opioid receptor-mediated signaling by ketamine. J Neurochem 119: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein HB, Rubie EA, Mansour A, et al. (1997) Opioid effects on mitogen-activated protein kinase signaling cascades. Anesthesiology 87: 1118–1126. [DOI] [PubMed] [Google Scholar]
- Hantouche E, Angst J, Azorin JM. (2010) Explained factors of suicide attempts in major depression. J Affect Disord 127: 305–308. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, et al. (2019) Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 10: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. (2004) Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev 45: 104–114. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, et al. (2017) Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: A PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Williams NR, Bentzley BS, et al. (2019) Rigorous trial design is essential to understand the role of opioid receptors in ketamine’s antidepressant effect. JAMA Psychiatry 76: 657–658. [DOI] [PubMed] [Google Scholar]
- Hirota K, Okawa H, Appadu BL, et al. (1999) Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 90: 174–182. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Hua D, Zhan G, et al. (2019) Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav 176: 93–100. [DOI] [PubMed] [Google Scholar]
- Hustveit O, Maurset A, Oye I. (1995) Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol 77: 355–359. [DOI] [PubMed] [Google Scholar]
- Ignacio ZM, Reus GZ, Arent CO, et al. (2016) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 82: 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, et al. (2019) Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J Affect Disord 243: 516–524. [DOI] [PubMed] [Google Scholar]
- Jelen LA, King S, Stone JM. (2018) Alternatives to ketamine in depression: State-of-the-art and future perspectives. Ther Adv Psychopharmacol 8: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Kochan LD, et al. (2002) NMDA antagonist effects on striatal dopamine release: Positron emission tomography studies in humans. Synapse 43: 19–29. [DOI] [PubMed] [Google Scholar]
- Ketamine-Clinics-Directory (2020) Ketamine Clinics Directory. Available at: https://ketamineclinicsdirectory.com (accessed 10 June 2020).
- Kishimoto T, Chawla JM, Hagi K, et al. (2016) Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: A meta-analysis of efficacy, safety and time trajectories. Psychol Med 46: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Chandra J, Sheriff S, et al. (2020) Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci USA 117: 2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. (1998) Ketamine: Teaching an old drug new tricks. Anesth Analg 87: 1186–1193. [DOI] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, Howes OD. (2018) The effects of ketamine on dopaminergic function: Meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Commons KG, Ogawa S, et al. (2002) Potentiation of the excitatory action of NMDA in ventrolateral periaqueductal gray by the mu-opioid receptor agonist, DAMGO. Brain Res 935: 87–102. [DOI] [PubMed] [Google Scholar]
- Kraus C, Wasserman D, Henter ID, et al. (2019) The influence of ketamine on drug discovery in depression. Drug Discov Today 24: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryst J, Kawalec P, Pilc A. (2020) Efficacy and safety of intranasal esketamine for the treatment of major depressive disorder. Expert Opin Pharmacother 21: 9–20. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Yoon G, Petrakis IL. (2019. b) Rigorous trial design is essential to understand the role of opioid receptors in ketamine’s antidepressant effect-reply. JAMA Psychiatry 76: 658–659. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Sanacora G, et al. (2019. a) Ketamine: A paradigm shift for depression research and treatment. Neuron 101: 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Madonick S, Perry E, et al. (2006) Potentiation of low dose ketamine effects by naltrexone: Potential implications for the pharmacotherapy of alcoholism. Neuropsychopharmacology 31: 1793–1800. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, et al. (2009) Reward processing by the opioid system in the brain. Physiol Rev 89: 1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal GC, Bandeira ID, Correia-Melo FS, et al. (2020) Intravenous arketamine for treatment-resistant depression: Open-label pilot study. Eur Arch Psychiatry Clin Neurosci. Epub ahead of print 20 February 2020. DOI: 10.1007/s00406-020-01110-5. [DOI] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, et al. (2017) Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 81: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, et al. (2014) BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, et al. (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, et al. (2013) GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 38: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. (2013) Opioid receptors: Distinct roles in mood disorders. Trends Neurosci 36: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, et al. (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63: 349–352. [DOI] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. (1997) mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J Neurosci 17: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton T, Barnes DE, Wallace A, et al. (2019) Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry 85: e75–e76. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Rivas-Grajales AM. (2019) Does the opioid system block or enhance the antidepressant effects of ketamine? Chronic Stress (Thousand Oaks) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathisen LC, Skjelbred P, Skoglund LA, et al. (1995) Effect of ketamine, an NMDA receptor inhibitor, in acute and chronic orofacial pain. Pain 61: 215–220. [DOI] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. (2011) The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci 36: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, et al. (2010) A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 82: 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assie MB, et al. (2009) Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol 156: 338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, et al. (2013) Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 33: 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, et al. (2020) Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am J Geriatr Psychiatry 28: 121–141. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: 7–23. [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, et al. (2018) Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84: e3–e6. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, et al. (2017) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112: 198–209. [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, et al. (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am J Psychiatry 176: 428–438. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, et al. (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, et al. (2007) Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 32: 2152–2162. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, et al. (2013) Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27: 444–450. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, et al. (2012) The mu-opioid receptor and the NMDA receptor associate in PAG neurons: Implications in pain control. Neuropsychopharmacology 37: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G. (2019) Caution against overinterpreting opiate receptor stimulation as mediating antidepressant effects of ketamine. Am J Psychiatry 176: 249. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. (2010) Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35: 2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K. (2018) Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: Comparison with (R)-ketamine. Int J Neuropsychopharmacol 21: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma PR, Johns B, Kuskowski M, et al. (2014) Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155: 123–129. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, et al. (2016. a) Intravenous esketamine in adult treatment-resistant depression: A double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80: 424–431. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, et al. (2016. b) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173: 816–826. [DOI] [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, et al. (1998) Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology 18: 18–25. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. (2007) Glutamate and dopamine dysregulation in schizophrenia—A synthesis and selective review. J Psychopharmacol 21: 440–452. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. (2002) GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther 303: 704–710. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR, Raimondi G. (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Dong C, Zhang K, et al. (2018) Lack of antidepressant effects of low-voltage-sensitive T-type calcium channel blocker ethosuximide in a chronic social defeat stress model: Comparison with (R)-ketamine. Int J Neuropsychopharmacol 21: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Donati RJ, Rasenick MM. (1999) Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of Gsα from plasma membrane. J Neurochem 73: 1114–1120. [DOI] [PubMed] [Google Scholar]
- Towers SK, Gloveli T, Traub RD, et al. (2004) Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol 559: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EH. (2019) Esketamine for treatment-resistant depression: Seven concerns about efficacy and FDA approval. Lancet Psychiatry 6: 977–979. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, et al. (1997) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7: 25–38. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, et al. (2000) Effects of (S)-ketamine on striatal dopamine: A [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34: 35–43. [DOI] [PubMed] [Google Scholar]
- Wajs E, Aluisio L, Holder R, et al. (2020) Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: Assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 81. [DOI] [PubMed] [Google Scholar]
- White PF, Ham J, Way WL, et al. (1980) Pharmacology of ketamine isomers in surgical patients. Anesthesiology 52: 231–239. [DOI] [PubMed] [Google Scholar]
- White PF, Schuttler J, Shafer A, et al. (1985) Comparative pharmacology of the ketamine isomers: Studies in volunteers. Br J Anaesth 57: 197–203. [DOI] [PubMed] [Google Scholar]
- Widman AJ, McMahon LL. (2018) Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci USA 115: E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, et al. (2018) The effect of a single dose of intravenous ketamine on suicidal ideation: A systematic review and individual participant data meta-analysis. Am J Psychiatry 175: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Bentzley BS, et al. (2019) Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry 24: 1779–1786. [DOI] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, et al. (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, et al. (2019) NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry 24: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AJ, Niciu MJ, Lundin NB, et al. (2015) Lithium and valproate levels do not correlate with ketamine’s antidepressant efficacy in treatment-resistant bipolar depression. Neural Plast 2015: 858251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi JI, Toki H, Qu Y, et al. (2018) (2R,6R)-hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology 43: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, et al. (2013) Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: A PET study in conscious monkeys. Neuropsychopharmacology 38: 2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kobayashi S, Nakao K, et al. (2018. a) AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry 84: 591–600. [DOI] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, et al. (2017. a) (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82: e43–e44. [DOI] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, et al. (2017. b) Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 7: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, et al. (2018. b) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83: 18–28. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, et al. (2015) R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, et al. (2018. c) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554: 317–322. [DOI] [PubMed] [Google Scholar]
- Yoon G, Petrakis IL, Krystal JH. (2019) Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 76: 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, et al. (2019) (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA 116: 6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2018) Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 70: 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Nelson ME, Highland JN, et al. (2017) A negative allosteric modulator for α5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. (2012. b) Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry 71: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, et al. (2012. a) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, et al. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, et al. (2006) Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects. Eur J Neurosci 23: 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K. (2014) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116: 137–141. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K. (2019) Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 85: e25–e27. [DOI] [PubMed] [Google Scholar]
- Zhang K, Dong C, Fujita Y, et al. (2018) 5-hydroxytryptamine-independent antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Jia G, Xia L, et al. (2020. a) Efficacy of anticonvulsant ethosuximide for major depressive disorder: A randomized, placebo-control clinical trial. Eur Arch Psychiatry Clin Neurosci. Epub ahead of print 1 February 2020. DOI: 10.1007/s00406-020-01103-4. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yang C, Chang L, et al. (2020. b) Essential role of microglial transforming growth factor-β1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-β1. Transl Psychiatry 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rasenick MM. (2010) Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: A 5-hydroxytryptamine transporter-independent action of this antidepressant compound. J Pharmacol Exp Ther 332: 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun L. (2008) Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci 15: 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol 73: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhou YL, Liu WJ, et al. (2018) Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res 106: 61–68. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, et al. (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29: 419–423. [DOI] [PubMed] [Google Scholar]