Abstract
Purpose
We investigated whether antineutrophil cytoplasmic antibody (ANCA) positivity is associated with vascular manifestations at diagnosis of Behçet's disease (BD) and poor outcomes during follow-up.
Materials and Methods
We retrospectively reviewed the medical records of 1060 patients with BD. Among them, 808 patients could be diagnosed with BD based on the revised version of the International Criteria for Behçet's Disease (ICBD) in 2014 (2014 ICBD criteria) and 588 patients could be diagnosed with BD based on the International Study Group (ISG) criteria proposed in 1990 (1990 ISG criteria). We examined the sites and patterns of vascular involvement in the BD patients at diagnosis and evaluated adverse outcomes during follow up, such as all-cause mortality, acute coronary syndrome, and deep vein thrombosis.
Results
Among the 808 patients with BD based on the 2014 ICBD criteria, the rate of ANCA positivity at diagnosis was 2.2%. ANCA-positive BD patients exhibited a higher frequency of overall vascular manifestations (22.2% vs. 6.1%) and higher frequencies of vascular involvement in the upper extremities and visceral arteries than ANCA-negative BD patients (5.6% vs. 0.1% and 5.6% vs. 0.1%). Among the 588 BD patients based on the 1990 ISG criteria, similarly, ANCA-positive BD patients exhibited a higher frequency of vascular manifestations than ANCA-negative BD patients. ANCA positivity, however, did not seem to be associated with poor outcomes in BD patients during follow up.
Conclusion
ANCA positivity in BD patients was found to be associated with cross-sectional vascular involvement in the upper extremities and visceral arteries at diagnosis but was not predictive of poor outcomes during follow-up.
Keywords: Antineutrophil cytoplasmic antibody, Behçet, vasculitis
INTRODUCTION
Behçets disease (BD) is classified as variable vessel vasculitis that affects small, medium, and large-sized arteries or veins based on the 2012 Chapel Hill Consensus Conference Nomenclature of Vasculitis (the 2012 CHCC definitions).1 BD is a chronic and multi-systemic syndrome that manifests with hallmark features of recurrent oral and genital ulcers and skin lesions. It can also affect ocular, cardiovascular, neurological, gastrointestinal (GI), and musculoskeletal systems.2 In addition to heterogeneous and multiorgan manifestations in BD, there is no universally accepted diagnostic test for BD; thus, diagnosis of BD has heavily relied on the identification of several clinical features and the exclusion of other medical conditions.3 For these reasons, various clinical classification criteria for BD have been suggested and validated, among which two are the most widely recognized: The International Study Group (ISG) Criteria in 1990 (the 1990 ISG criteria) and the revised version of the International Criteria for Behçet's Disease (ICBD) in 2014 (the 2014 ICBD criteria).4,5 There are two obvious differences between the two criteria: unlike the 1990 ISG criteria, the 2014 ICBD criteria uses a scoring system and includes vascular and neurological manifestations as its point-rating items.6
Since many other inflammatory diseases may share some clinical features of BD, such as systemic lupus erythematosus, inflammatory bowel disease, sarcoidosis, and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), they should be distinguished from BD.3 Among these medical conditions, AAV, which affects small-sized vessels, has overlapping clinical features with BD; however, it can be classified and distinguished from BD by the 2012 CHCC definitions and the classification algorithm proposed by the European Medicine Agency in 2007 (the 2007 EMA algorithm).1,7 Detection of ANCA against myeloperoxidase (MPO) and proteinase 3 (PR3) also plays a supportive role in distinguishing the two diseases.8 Nevertheless, ANCA positivity cannot be an absolute marker to discriminate BD from AAV, because a few cases of ANCA positivity in BD have been previously reported: one of the previous case-series studies also reported that ANCA-positive patients with BD did not exhibit any arterial or venous thrombosis and arterial aneurysms.9 However, there has been no extensive study to explore the clinical significance of ANCA, particularly in the context of vascular manifestations, on a large number of patients with BD. In this study, we investigated whether ANCA positivity might be associated with cross-sectional vascular manifestations at diagnosis of BD and whether ANCA positivity could be assessed as a potential predictor of poor outcomes, in particular any vascular complications during follow up, in patients with BD.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical records of 1060 patients with BD who were selected based on the following inclusion and exclusion criteria: 1) patients who were initially diagnosed with BD the Division of Rheumatology, the Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, from March 1990 to August 2019; 2) patients with BD as the primary diagnosis, which was confirmed by the 10th revised International Classification Diseases codes recorded in the clinical data repository (CDR) of Severance Hospital; 3) patients who had the results for ANCA tests; 4) patients who had medical records complete enough to collect and review clinical and laboratory data at diagnosis and to apply the two BD diagnosis criteria; 5) patients who had ANCA, but were not classified with AAV based on both the 2007 EMA algorithm and the 2012 CHCC definitions;1,7 6) patients who, at diagnosis, had no serious medical conditions mimicking BD, such as autoimmune diseases, inflammatory bowel diseases, and systemic vasculitides other than BD;3 and 7) patients who had been followed up for more than 6 months until the time of inclusion in this study. For reclassification, the 1990 ISG criteria and the 2014 ICBD criteria were applied to all of them. Finally, 808 of 1060 BD patients could be reclassified with BD based on the 2014 ICBD criteria.5 In addition, 588 of the 1060 BD patients could be reclassified with BD based on the 1990 ISG criteria.4 All patients who fulfilled the 1990 ISG criteria met the 2014 ICBD criteria (Supplementary Fig. 1, only online). No ANCA-positive BD patients were reclassified as AAV (Supplementary Table 1, only online). All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board (IRB) of Severance Hospital (4-2019-1331). The need for written informed consent from the patients was waived by the approving IRB, as this was a retrospective study.
ANCA tests
ANCA tests were conducted either or both by indirect immunofluorescence assays (IIA) for perinuclear (P)-ANCA and cytoplasmic (C)-ANCA and antigen-specific assays for MPO-ANCA and PR3-ANCA, and the results were also included in the medical records. First, we used the IIA as the primary screening method for ANCA.10 The antigen-specific assays for MPO-ANCA and PR3-ANCA were performed by using novel anchor coated highly sensitive Phadia ELiA (Thermo Fisher Scientific/Phadia, Freiburg, Germany) that contains human native antigens, and its levels were measured with a Phadia250 analyzer. For those patients who had no results from antigen-specific assays, but had ANCA positivity by the IIA, we considered them to have MPO-ANCA (or P-ANCA) or PR3-ANCA (or C-ANCA).
Clinical and laboratory data
We collected demographic data regarding age at diagnosis and sex. We obtained the results from ANCA tests that were performed within 6 weeks before and after diagnosis. We reviewed any clinical manifestations related to the criteria for BD and comorbidities, such as diabetes mellitus, hypertension, dyslipidemia, aortic valve replacement, percutaneous coronary intervention, and other vascular intervention at the time of diagnosis, and then we counted the number of patients having each clinical manifestation. During follow up, all-cause mortality, cerebral vascular accident, acute coronary syndrome, deep vein thrombosis (DVT), GI involvement, and ocular involvement were evaluated as adverse outcomes. The frequency of the use of six medications [glucocorticoid, colchicine, azathioprine, methotrexate, cyclosporine, and anti-tumor necrosis factor alphas inhibitor (TNF-α blockade)] were also reviewed. The follow-up duration was defined as the period between the date of diagnosis of BD and the date of the last visit for surviving patients. For the deceased patients, the follow-up duration was defined as the period between the diagnosis of BD and the time of death. For patients who had any adverse outcomes, it was defined as the period starting from the diagnosis of BD until an adverse outcome appeared.
Sites and patterns of vascular manifestations at diagnosis
We investigated BD patients for any sites and/or patterns of vascular involvement that includes arterial or venous thrombosis and arterial aneurysms. We categorized 8 sites as follows: thrombosis at the lower extremities; vena cava thrombosis; cerebral venous sinus thrombosis; pulmonary artery involvement; intracardiac thrombosis; aorta involvement; and extra-pulmonary artery involvement, including the lower extremities, upper extremities, and visceral arteries.11
Statistical analyses
All statistical analyses were conducted using SPSS software (version 23 for Windows; IBM Corp., Armonk, NY, USA). Continuous variables are expressed as medians (interquartile range), and categorical variables are expressed as numbers and percentages. Significant differences in categorical variables between the two groups were analyzed using the chi-square and Fisher's exact tests. Significant differences in continuous variables between the two groups were compared using the Mann-Whitney test. Comparisons of cumulative overall survival rate and adverse outcome-free survival rates between two groups were analyzed by the Kaplan-Meier survival analysis. p values less than 0.05 were considered statistically significant.
RESULTS
Characteristics of the 808 BD patients based on the 2014 ICBD criteria
The baseline and follow-up characteristics of the 808 BD patients who met the 2014 ICBD criteria are described in Table 1. At the time of diagnosis of BD, the median age at diagnosis was 39.0 years and 27.5% of them were male. The rate of ANCA positivity was 2.2%: MPO-ANCA (or P-ANCA) was detected in 12 patients, and PR3-ANCA (or C-ANCA) was noted in 6 patients. The most common BD-related clinical manifestations at diagnosis were oral ulcers (99.5%), followed by genital ulcers (79.6%). Vascular manifestations were observed in 52 patients (6.4%). The most common comorbidity at diagnosis was hypertension (14.4%), followed by dyslipidemia (14.0%) and diabetes mellitus (9.0%). Among 808 BD patients, 588 patients also fulfilled the 1990 ISG criteria (72.8%). During the follow-up period, the most common adverse outcome was GI involvement (12.4%), followed by ocular involvement (6.6%) and cerebrovascular disease (4.5%). Thirty-two patients (4.0%) and 29 patients (3.6%) exhibited acute coronary syndrome and DVT. Three patients died during follow-up. Glucocorticoid was administered to 636 BD patients (78.7%), while TNF-alpha blockade was provided to 17 patients (2.1%).
Table 1. Characteristics of 808 BD Patients Based on the 2014 ICBD Criteria.
| Variables | Values |
|---|---|
| At diagnosis | |
| Demographic data | |
| Age at diagnosis (yr) | 39.0 (17.0) |
| Male sex | 222 (27.5) |
| ANCA positivity | |
| MPO-ANCA (or P-ANCA) | 12 (1.5) |
| PR3-ANCA (or C-ANCA) | 6 (0.7) |
| MPO-ANCA (or P-ANCA) and PR3-ANCA (or C-ANCA) | 0 (0) |
| ANCA negative | 790 (97.8) |
| BD-related clinical manifestation | |
| Oral ulcer | 804 (99.5) |
| Genital ulcer | 643 (79.6) |
| Ocular manifestation | 344 (42.6) |
| Skin manifestation | 565 (69.9) |
| Neurologic manifestation | 67 (8.3) |
| Vascular manifestation | 52 (6.4) |
| Pathergy test positivity | 12 (1.5) |
| Comorbidities | |
| Diabetes mellitus | 73 (9.0) |
| Hypertension | 116 (14.4) |
| Dyslipidemia | 113 (14.0) |
| Aortic valve replacement | 11 (1.4) |
| Percutaneous coronary intervention | 11 (1.4) |
| Other vascular Intervention | 22 (2.7) |
| Reclassification | |
| 1990 ISG criteria fulfilled | 588 (72.8) |
| 2014 ICBD criteria fulfilled | 808 (100) |
| During follow-up | |
| Poor outcomes | |
| All-cause mortality | 3 (0.6) |
| CVA | 36 (4.5) |
| ACS | 32 (4.0) |
| DVT | 29 (3.6) |
| GI involvement | 100 (12.4) |
| Ocular involvement | 53 (6.6) |
| Medications administered during follow-up | |
| Glucocorticoid | 636 (78.7) |
| Colchicine | 603 (74.6) |
| Azathioprine | 188 (23.3) |
| Methotrexate | 46 (5.7) |
| Cyclosporine | 35 (4.3) |
| TNF-α blockade | 17 (2.1) |
BD, Behçet's disease; ICBD, International Criteria for Behçet's Disease; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; P, perinuclear; PR3, proteinase 3; C, cytoplasmic; ISG, International Study Group; CVA, cerebrovascular accident; ACS, acute coronary syndrome; DVT, deep vein thrombosis; GI, gastrointestinal; TNF, tumor necrosis factor.
Values are expressed as a median (interquartile range) and number (percentage).
Comparison of variables at diagnosis and follow-up between ANCA-positive and ANCA-negative BD patients based on the 2014 ICBD criteria
Among BD-related clinical manifestations at the diagnosis of BD, ANCA-positive BD patients exhibited vascular manifestations more frequently than ANCA-negative BD patients (22.2% vs. 6.1%, p=0.024). Also, among comorbidities at diagnosis, aortic valve replacement was performed more often in ANCA-positive BD patients than ANCA-negative BD patients (15.4% vs. 1.1%, p=0.023). In regard to the poor outcomes during follow up, DVT appeared more frequently in ANCA-positive BD patients than ANCA-negative BD (11.1% vs. 3.4%), although this was statistically insignificant. A larger portion of ANCA-positive BD patients had ever received azathioprine after diagnosis than ANCA-negative BD patients, and this also had no statistical significance (Table 2).
Table 2. Comparison of Variables at Diagnosis and during Follow-Up between ANCA-Positive and ANCA-Negative BD Patients Based on the 2014 ICBD Criteria.
| Variables | ANCA-positive BD patients (n=18) | ANCA-negative BD patients (n=790) | p value |
|---|---|---|---|
| At diagnosis | |||
| Demographic data | |||
| Age (yr) | 34.5 (24.0) | 40.0 (17.0) | 0.346 |
| Male sex | 7 (38.9) | 215 (27.2) | 0.273 |
| BD-related clinical manifestation | |||
| Oral ulcer | 18 (100) | 786 (99.5) | 1.000 |
| Genital ulcer | 12 (66.7) | 631 (80.0) | 0.169 |
| Ocular manifestation | 10 (55.6) | 334 (42.3) | 0.260 |
| Skin manifestation | 11 (61.1) | 554 (70.1) | 0.410 |
| Neurologic manifestation | 0 (0) | 67 (8.5) | 0.389 |
| Vascular manifestation | 4 (22.2) | 48 (6.1) | 0.024 |
| Pathergy test positivity | 1 (5.6) | 11 (1.4) | 0.238 |
| Comorbidities at diagnosis | |||
| Diabetes mellitus | 2 (15.4) | 71 (9.0) | 0.673 |
| Hypertension | 4 (30.8) | 110 (13.9) | 0.304 |
| Dyslipidemia | 4 (30.8) | 108 (13.7) | 0.297 |
| Aortic valve replacement | 2 (15.4) | 9 (1.1) | 0.023 |
| Percutaneous coronary intervention | 0 (0) | 11 (1.4) | 1.000 |
| Other vascular Intervention | 2 (15.4) | 20 (2.5) | 0.082 |
| During follow-up | |||
| Follow-up duration (months) | 132.0 (99.6) | 113.4 (79.5) | 0.624 |
| Poor outcomes | |||
| All-cause mortality | 0 (0) | 3 (0.4) | 1.000 |
| Follow-up duration for death (months) | 132.0 (99.6) | 113.4 (79.5) | 0.624 |
| CVA | 0 (0) | 36 (4.6) | 1.000 |
| Follow-up duration for CVA (months) | 132.0 (99.6) | 112.1 (80.4) | 0.494 |
| ACS | 1 (5.6)) | 31 (3.9) | 1.000 |
| Follow-up duration for ACS (months) | 132.0 (104.5) | 112.2 (79.3) | 0.574 |
| DVT | 2 (11.1) | 27 (3.4) | 0.075 |
| Follow-up duration for DVT (months) | 118.5 (105.7) | 112.2 (80.0) | 0.934 |
| GI involvement | 2 (11.1) | 98 (12.4) | 0.680 |
| Follow-up duration for GI disease (months) | 118.5 (105.7) | 107.5 (81.2) | 0.552 |
| Ocular involvement | 2 (11.1) | 51 (6.4) | 0.574 |
| Follow-up duration for ocular disease (months) | 132.0 (99.6) | 111.2 (79.9) | 0.485 |
| Medications administered | |||
| Glucocorticoid | 14 (77.8) | 622 (78.7) | 1.000 |
| Colchicine | 12 (66.7) | 591 (74.8) | 0.432 |
| Azathioprine | 8 (44.4) | 180 (22.8) | 0.032 |
| Methotrexate | 0 (0) | 46 (5.8) | 0.618 |
| Cyclosporine | 0 (0) | 35 (4.4) | 1.000 |
| TNF-α blockade | 0 (0) | 17 (2.2) | 1.000 |
BD, Behçet's disease; ANCA, antineutrophil cytoplasmic antibody; ICBD, International Criteria for Behçet's Disease; CVA, cerebrovascular accident; ACS, acute coronary syndrome; DVT, deep vein thrombosis; GI, gastrointestinal; TNF, tumor necrosis factor.
Values are expressed as a median (interquartile range) and number (percentage).
Comparison of the sites and patterns of vascular manifestations at diagnosis between ANCA-positive and ANCA-negative BD patients based on the 2014 ICBD criteria
At diagnosis of BD, 52 patients out of a total of 808 BD patients exhibited 57 events of vascular manifestations: 4 patients among 18 ANCA-positive BD patients had five vascular events, while 48 patients out of a total of 790 ANCA-negative patients showed 52 vascular events. ANCA-positive BD patients exhibited higher frequencies of vascular involvement in the upper extremities and visceral arteries than ANCA-negative BD patients (5.6% vs. 0.1%, p=0.044 and 5.6% vs. 0.1%, p=0.044) (Table 3).
Table 3. Comparison of the Sites and Pattern of Vascular Manifestation at Diagnosis between ANCA-Positive and ANCA-Negative BD Patients Based on the 2014 ICBD Criteria.
| Vascular manifestation | Total (n=808) | ANCA-positive BD patients (n=18) | ANCA-negative BD patients (n =790) | p value |
|---|---|---|---|---|
| Lower extremity vein thrombosis | ||||
| Deep vein | 13 (1.6) | 0 (0) | 13 (1.6) | 1.000 |
| Superficial vein | 12 (1.5) | 1 (5.6) | 11 (1.4) | 0.238 |
| Vena cava thrombosis | ||||
| Inferior vena cava | 0 (0) | 0 (0) | 0 (0) | N/A |
| Superior vena cava | 0 (0) | 0 (0) | 0 (0) | N/A |
| Cerebral venous sinus thrombosis | 2 (0.2) | 0 (0) | 2 (0.3) | 1.000 |
| Pulmonary artery involvement | 2 (0.2) | 0 (0) | 2 (0.3) | 1.000 |
| Intracardiac thrombosis | 4 (0.5) | 1 (5.6) | 3 (0.4) | 0.086 |
| Aorta involvement | ||||
| Aortic aneurysm | 7 (0.9) | 0 (0) | 7 (0.9) | 1.000 |
| Carotid artery aneurysm | 2 (0.2) | 0 (0) | 2 (0.3) | 1.000 |
| Extra-pulmonary artery involvement | ||||
| Lower extremity artery | 11 (1.4) | 1 (5.6) | 10 (1.3) | 0.221 |
| Upper extremity artery | 2 (0.2) | 1 (5.6) | 1 (0.1) | 0.044 |
| Visceral artery | 2 (0.2) | 1 (5.6) | 1 (0.1) | 0.044 |
| Comments | N=52, E=57 | N=4, E=5 | N=48, E=52 | |
| Number of patients with vascular manifestation (N) and vascular events (E) | ||||
BD, Behçet's disease; ANCA, antineutrophil cytoplasmic antibody; ICBD, International Criteria for Behçet's Disease; E, event.
Values are expressed as number (percentage).
Comparison of cumulative adverse outcome-free survival rates between ANCA-positive and ANCA-negative BD patients based on the 2014 ICBD criteria
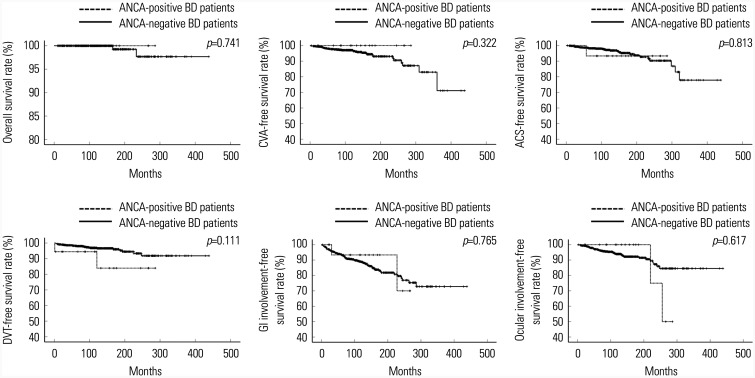
Among the adverse outcomes, ANCA-positive BD patients tended to exhibit a lower cumulative DVT-free survival rate than ANCA-negative BD patients, although the difference was not statistically significant (p=0.111). ANCA positivity did not seem to be associated with adverse outcomes in BD patients during follow up (Fig. 1).
Fig. 1. Comparison of the cumulative adverse outcome-free survival rates between ANCA-positive and ANCA-negative BD patients based on the 2014 ICBD criteria. ANCA positivity did not seem to be associated with adverse outcomes in BD patients during follow up. ANCA, antineutrophil cytoplasmic antibody; BD, Behçet's disease; ICBD, the International Criteria for Behçet's Disease; CVA, cerebrovascular accident; ACS, acute coronary syndrome; DVT, deep vein thrombosis; GI, gastrointestinal.
Characteristics of the 588 BD patients based on the 1990 ISG criteria and comparisons of variables at diagnosis and during follow up between ANCA-positive and ANCA-negative BD patients
Using the above strategies, we investigated the baseline and follow-up characteristics of the 588 BD patients who met the 1990 ISG criteria (Supplementary Table 2, only online). ANCA was detected in 13 patients out of the 588 BD patients (2.2%): MPO-ANCA (or P-ANCA) was detected in 8 patients and PR3-ANCA (or C-ANCA) was recorded in 5 patients. Vascular manifestations were observed in 26 patients (4.4%). Among BD-related clinical manifestations at diagnosis, ANCA-positive BD patients exhibited a higher frequency of vascular manifestations than ANCA-negative BD patients (23.1% vs. 4.0%, p=0.016). Also, among comorbidities at diagnosis, aortic valve replacement and vascular intervention, except coronary intervention, were performed more often in ANCA-positive BD patients than in ANCA-negative BD patients (15.4% vs. 0.5%, p=0.004 and 15.4% vs. 1.6%, p=0.022). In respect to adverse outcomes during follow up, DVT showed a tendency to develop more often in ANCA-positive BD patients than in ANCA-negative BD patients (15.4% vs. 3.3%), although it was not statistically significant. There were no differences between the two groups of BD patients in regards to administered medications (Table 4).
Table 4. Comparison of Variables at Diagnosis and during Follow-Up between ANCA-Positive and ANCA-Negative BD Patients Based on the 1990 ISG Criteria.
| Variables | ANCA-positive BD patients (n=13) | ANCA-negative BD patients (n=575) | p value |
|---|---|---|---|
| At diagnosis | |||
| Demographic data | |||
| Age (yr) | 34.0 (24.0) | 39.0 (15.0) | 0.449 |
| Male sex | 7 (53.8) | 165 (28.7) | 0.049 |
| Clinical manifestation | |||
| Oral ulcer | 13 (100) | 575 (100) | N/A |
| Genital ulcer | 8 (61.5) | 490 (85.2) | 0.019 |
| Ocular manifestation | 9 (69.2) | 290 (50.4) | 0.262 |
| Skin manifestation | 11 (84.6) | 519 (90.3) | 0.373 |
| Neurologic manifestation | 0 (0) | 38 (6.6) | 1.000 |
| Vascular manifestation | 3 (23.1) | 23 (4.0) | 0.016 |
| Pathergy test positivity | 1 (7.7) | 10 (1.7) | 0.220 |
| Comorbidities at diagnosis | |||
| Diabetes mellitus | 2 (15.4) | 51 (8.9) | 0.330 |
| Hypertension | 4 (30.8) | 81 (14.9) | 0.104 |
| Dyslipidemia | 4 (30.8) | 79 (13.7) | 0.097 |
| Aortic valve replacement | 2 (15.4) | 3 (0.5) | 0.004 |
| Percutaneous coronary intervention | 0 (0) | 10 (1.7) | 1.000 |
| Other vascular Intervention | 2 (15.4) | 9 (1.6) | 0.022 |
| During follow-up | |||
| Follow-up duration (months) | 156.1 (108.9) | 116.0 (80.0) | 0.103 |
| Poor outcomes | |||
| All-cause mortality | 0 (0) | 2 (0.35) | 1.000 |
| Follow-up duration for death (months) | 156.1 (108.9) | 116.0 (80.0) | 0.103 |
| CVA | 0 (0) | 27 (4.7) | 1.000 |
| Follow-up duration for CVA (months) | 156.1 (108.9) | 114.6 (80.0) | 0.080 |
| ACS | 0 (0) | 25 (4.3) | 1.000 |
| Follow-up duration for ACS (months) | 156.1 (108.9) | 114.7 (80.1) | 0.083 |
| DVT | 2 (15.4) | 19 (3.3) | 0.075 |
| Follow-up duration for DVT (months) | 132.0 (151.9) | 113.8 (80.3) | 0.391 |
| GI involvement | 2 (15.4) | 74 (12.9) | 0.680 |
| Follow-up duration for GI disease (months) | 143.6 (129.4) | 110.9 (81.2) | 0.219 |
| Ocular involvement | 1 (7.7) | 36 (6.3) | 0.574 |
| Follow-up duration for ocular disease (months) | 156.1 (103.5) | 113.7 (80.3) | 0.077 |
| Medications administered | |||
| Glucocorticoid | 12 (92.3) | 459 (79.8) | 0.707 |
| Colchicine | 8 (61.5) | 446 (77.6) | 0.173 |
| Azathioprine | 5 (38.5) | 139 (24.2) | 0.236 |
| Methotrexate | 0 (0) | 33 (5.7) | 1.000 |
| Cyclosporine | 0 (0) | 21 (3.7) | 1.000 |
| TNF-α blockade | 0 (0) | 9 (1.6) | 1.000 |
BD, Behçet's disease; ANCA, antineutrophil cytoplasmic antibody; ISG, International Study Group; CVA, cerebrovascular accident; ACS, acute coronary syndrome; DVT, deep vein thrombosis; GI, gastrointestinal; TNF, tumor necrosis factor.
Values are expressed as a median (interquartile range) and number (percentage).
Comparison of sites and pattern of vascular manifestations at diagnosis between ANCA-positive and ANCA-negative BD patients based on the 1990 ISG criteria
At diagnosis, 26 patients out of the 588 BD patients experienced 30 events of vascular manifestations: 3 patients among the 13 ANCA-positive BD patients had four vascular incidents, whereas 26 patients out of the 575 ANCA-negative patients had 26 vascular-featured events. Compared to the results from 808 BD patients who met the 2014 ICBD criteria, analogous results were obtained from the 588 BD patients who met the 1990 ISG criteria: ANCA-positive BD patients exhibited higher frequencies of vascular involvement in the upper extremities and visceral arteries than ANCA-negative BD patients (7.7% vs. 0.2%, p=0.044 and 7.7% vs. 0%, p=0.022) (Supplementary Table 3, only online).
Comparison of the cumulative adverse outcome-free survival rates between ANCA-positive and ANCA-negative BD patients based on the 1990 ISG criteria
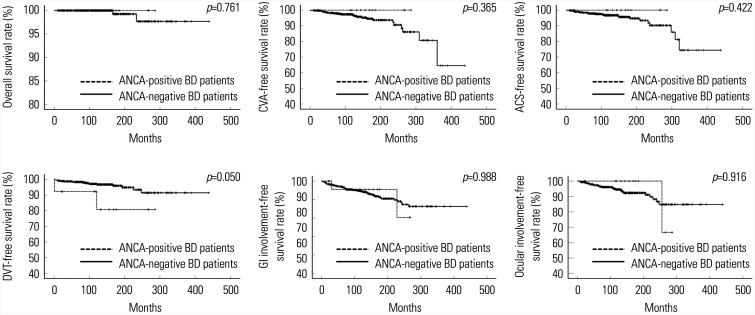
Among the adverse outcomes we assessed, ANCA-positive BD patients tended to exhibit a lower cumulative DVT-free survival rate than ANCA-negative BD patients, but the difference was lacked statistical significance (p=0.050). ANCA positivity did not seem to be associated with adverse outcomes in BD patients during follow up, regardless of diagnosis criteria for BD (Fig. 2).
Fig. 2. Comparison of the cumulative adverse outcome-free survival rates between ANCA-positive and ANCA-negative BD patients based on the 1990 ISG criteria. ANCA positivity did not seem to be associated with adverse outcomes in BD patients during follow-up. ANCA, antineutrophil cytoplasmic antibody; BD, Behçet's disease; ISG, the International Study Group; CVA, cerebrovascular accident; ACS, acute coronary syndrome; DVT, deep vein thrombosis; GI, gastrointestinal.
DISCUSSION
In this study, we investigated whether ANCA positivity might be associated with cross-sectional vascular manifestations at diagnosis and whether ANCA positivity could be assessed to predict the likelihood of adverse outcomes occurring, including vascular complications, during follow up in patients with BD. First, we identified the association of ANCA positivity with vascular manifestations at diagnosis, as ANCA-positive BD patients exhibited a significantly higher frequency of vascular manifestations than ANCA-negative BD patients, regardless of the criteria for BD. In particular, we found that ANCA-positive BD patients had more vascular manifestations in the upper extremities and visceral arteries with higher frequencies of extra-pulmonary arterial involvement than ANCA-negative BD patients.
According to a previous study, the most common sites and patterns of vascular involvement at the first event in BD patients were DVT, followed by central venous thrombosis, pulmonary arterial involvement, and extra-pulmonary arterial involvement. However, as the frequency of vascular involvement increased, the proportion of DVT gradually decreased, whereas extra-pulmonary arterial involvement increased.11 Since vascular manifestations at diagnosis of BD were reviewed in this study, a frequency of extra-pulmonary arterial involvement was theoretically expected to be lower than frequencies of DVT and pulmonary arterial involvement. In the present study, the most common vascular manifestation was DVT, as was expected, but vena cava involvement was not prominent. Interestingly, both DVT and pulmonary artery involvement were observed only in ANCA-negative BD patients. Nevertheless, vascular involvement in the upper extremities and visceral arteries, which belong to extra-pulmonary arterial involvement, were found more often in ANCA-positive BD patients than in ANCA-negative BD patients. Therefore, we speculate that ANCA might participate in or accelerate aneurysm and/or thrombosis in the lower extremities and visceral arteries in BD patients.
As for large-sized vessel involvement, BD can encroach large vessels, such as in aortic valve and/or ascending aorta involvement and vena cava thrombosis, which may lead to fatal outcomes.12,13 Although large vessel vasculitis is likely to be a consequence of BD involvement, AAV can also provoke large vessel vasculitis: so far, several case series have reported that large vessel vasculitis might be associated with AAV and have indicated that large vessel vasculitis can occur in a spectrum of various clinical features.14,15,16 Thus, vascular manifestations that characterize large vessel vasculitis in ANCA-positive BD patients are likely to contributable to both BD and AAV. In our study, we did not notice any ANCA-positive BD patients who exhibited vena cava thrombosis or aorta involvement. Therefore, this point further supports our assumption that ANCA might be associated with vascular involvement in the upper extremities and visceral arteries in BD patients.
Secondly, a previous study reported that the incidence of arterial and venous thrombosis was greater in AAV patients than in the corresponding age- and gender-matched non-AAV population, and that trend was obvious within a 1-year follow-up. Particularly, at baseline, MPO-ANCA was detected more often in AAV patients with arterial thromboembolism than those without (58% vs. 35%, p=0.011); however, the detection of MPO-ANCA did not differ in AAV patients, regardless of having venous thromboembolism.17 Based on these results, although the disease type was different, at least at the entry of this study, we expected that ANCA-positive BD patients would exhibit a higher cumulative rate of thrombotic events than ANCA-negative BD patients during follow up. However, we found that ANCA positivity had no predictive potential on the occurrence of any arterial or venous thrombotic events in BD patients who met either the 2014 ICBD criteria or the 1990 ISG criteria.
Although the most common vascular involvement at diagnosis was DVT among all BD patients we investigated, DVT was not present in any ANCA-positive BD patients at diagnosis. During follow up, however, DVT occurred in 2 of 18 ANCA-positive BD patients who met the 2014 ICBD criteria and 2 of 13 ANCA-positive BD patients who met the 1990 ISG criteria. ANCA positivity tended to reduce the cumulative DVT-free survival rate, compared to ANCA negativity, in 588 BD patients who fulfilled the 1990 ISG criteria for BD. Nevertheless, there were no significant differences in the cumulative thrombotic event-free survival rates between ANCA-positive and ANCA-negative BD patients. Based these results, we developed two hypotheses: first, ANCA positivity does not contribute to the occurrence of thrombotic complications in BD patients. Second, ANCA positivity may contribute to, but does not surpass, the thrombotic potential of BD itself.
Since vascular involvement in BD, which is a focus of our current study, is one of the items in the 2014 ICBD criteria, but not in the 1990 ISG criteria, we applied both criteria to previously diagnosed BD patients in this study.4,5 Accordingly, we expected different sites and patterns of vascular involvement in BD patients classified based on the two different criteria; however, vascular involvement was similar between BD patients based on the 2014 ICBD criteria and those based on the 1990 ISG criteria. Therefore, we could generalize our results in BD patients regardless of either criteria system.
In this study, we counted the number of patients who had no results of antigen-specific assay, but had ANCA positivity by IIF, as ANCA-positive for two reasons: one is that the specificity of antigen-specific assay is higher than that of IIF, whereas its sensitivity is lower than that of IIF. Therefore, concerns over false-positives for ANCA of IIF and false-negatives for ANCA of antigen-specific assays are still raised in real clinical settings. Another reason is that ANCA by IIF is currently used in the classification of AAV, and we use the IIF as the primary screening method for ANCA. Patients who were negative for ANCA on antigen-specific assay but positive by IIF are considered to have MPO-ANCA or PR3-ANCA when AAV is strongly suspected by the clinical and laboratory features.18
Our study has several limitations. 1) Our study was designed as a retrospective study, and thus, we cannot assuage concerns about missing data and could not repeat ANCA tests during follow up. 2) The number of ANCA-positive BD patients was not large enough to yield more statistically significant results. 3) Only patients who had BD as the primary diagnosis and fulfilled either the 1990 ISG or the 2014 ICBD criteria for BD were included in this study; we did not include clinically suspected BD patients. 4) This study did not include inflammation of smaller vessels, such as uveitis, other than for eight categories.11 Since ANCA may affect small vessels more often than larger vessels, these vascular manifestations should not be ignored. 5) With regard to missing data of BD patients without the results of ANCA tests, because clinical data were abstracted and collected by a CDR system with several terms, including BD and ANCA results, the number and clinical characteristics of BD patients without ANCA results could not be obtained. 6) Since patients who had no results of antigen-specific assay but had ANCA positivity by IIF were considered to have MPO-ANCA (or P-ANCA) or PR3-ANCA (or C-ANCA), there might be false-positive for ANCA. However, our study has a strength, as we demonstrated the clinical significance of ANCA positivity in a large number of BD patients for the first time. Also, we believe that as a pilot study, our findings will encourage researchers to further evaluate the clinical implications of ANCA positivity in BD patients. In addition, future prospective studies with serial results of repeated ANCA tests will clarify the exact mechanism underlying the association between ANCA positivity and vasculitis involvement in BD.
In conclusion, the rate of ANCA positivity in BD patients was 2.2%, and ANCA positivity at diagnosis was associated with cross-sectional vascular involvement in the upper extremities and visceral arteries. It was, however, not predictive of adverse outcomes during follow up.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang-Won Lee, Minyoung Kevin Kim, and Hyeok Chan Kwon.
- Data curation: Minyoung Kevin Kim and Hyeok Chan Kwon.
- Formal analysis: Minyoung Kevin Kim and Sang-Won Lee.
- Funding acquisition: Sang-Won Lee.
- Investigation: Sang-Won Lee, Minyoung Kevin Kim, and Hyeok Chan Kwon.
- Methodology: Minyoung Kevin Kim and Hyeok Chan Kwon.
- Project administration: Sang-Won Lee.
- Resources: Sang-Won Lee, Minyoung Kevin Kim, and Hyeok Chan Kwon.
- Software: Minyoung Kevin Kim and Hyeok Chan Kwon.
- Supervision: Sang-Won Lee.
- Validation: all authors.
- Visualization: Sang-Won Lee, Minyoung Kevin Kim, and Hyeok Chan Kwon.
- Writing—original draft: Minyoung Kevin Kim and Hyeok Chan Kwon.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Algorithm for selecting the study population. A list of 1060 patients with BD as the primary diagnosis was collected by the clinical data repository of Severance Hospital. When the revised version of the ICBD in 2014 (the 2014 ICBD criteria) was applied, 252 patients were excluded from this study. When The ISG criteria in 1990 (the 1990 ISG criteria) were applied, 472 patients were excluded from this study. Finally, 808 of 1060 BD patients could be reclassified as BD based on the 2014 ICBD criteria. In addition, 588 of 1060 BD patients could be reclassified as BD based on the 1990 ISG criteria. BD, Behçet's disease, ICBD, International Criteria for Behçet's Disease; ISG, International Study Group; ANCA, antineutrophil cytoplasmic antibody.
Application of the 2007 EMA Algorithm for AAV and cPAN Based on the 2012 CHCC Definitions to 18 BD Patients with ANCA
Characteristics of 588 BD Patients Based on the 1990 ISG Criteria
Comparison of the Sites and Pattern of Vascular Manifestation at Diagnosis between ANCA-Positive and ANCA-Negative BD Patients Based on the 1990 ISG Criteria
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behçet's disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17:567–575. doi: 10.1016/j.autrev.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose NL, Haskard DO. Differential diagnosis and management of Behçet syndrome. Nat Rev Rheumatol. 2013;9:79–89. doi: 10.1038/nrrheum.2012.156. [DOI] [PubMed] [Google Scholar]
- 4.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 5.International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD) The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338–347. doi: 10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 6.Davatchi F, Sadeghi Abdollahi B, Chams-Davatchi C, Shahram F, Shams H, Nadji A, et al. The saga of diagnostic/classification criteria in Behcet's disease. Int J Rheum Dis. 2015;18:594–605. doi: 10.1111/1756-185X.12520. [DOI] [PubMed] [Google Scholar]
- 7.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millet A, Pederzoli-Ribeil M, Guillevin L, Witko-Sarsat V, Mouthon L. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis. 2013;72:1273–1279. doi: 10.1136/annrheumdis-2013-203255. [DOI] [PubMed] [Google Scholar]
- 9.Duzgun N, Sahin M, Ayaslioglu E. Anti-neutrophil cytoplasmic antibody in Behçet's disease. Int J Biomed Sci. 2006;2:49–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin L, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017;13:683–692. doi: 10.1038/nrrheum.2017.140. [DOI] [PubMed] [Google Scholar]
- 11.Tascilar K, Melikoglu M, Ugurlu S, Sut N, Caglar E, Yazici H. Vascular involvement in Behçet's syndrome: a retrospective analysis of associations and the time course. Rheumatology (Oxford) 2014;53:2018–2022. doi: 10.1093/rheumatology/keu233. [DOI] [PubMed] [Google Scholar]
- 12.Cocco G, Jerie P. Cardiac pathology and modern therapeutic approach in Behçet disease. Cardiol J. 2014;21:105–114. doi: 10.5603/CJ.a2013.0056. [DOI] [PubMed] [Google Scholar]
- 13.Springer J, Villa-Forte A. Thrombosis in vasculitis. Curr Opin Rheumatol. 2013;25:19–25. doi: 10.1097/BOR.0b013e32835ad3ca. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, Yamazaki H, Kohsaka H. A case of ANCA-associated large vessel vasculitis with multiple saccular aneurysms. J Rheumatol. 2016;43:179. doi: 10.3899/jrheum.150435. [DOI] [PubMed] [Google Scholar]
- 15.Satomura A, Fujita T, Maruyama T, Hamada H, Nozawa Y, Takayama E, et al. Aortic aneurysm as a complication of myeloperoxidase-antineutrophil cytoplasmic antibody-associated vasculitis. Open Med (Wars) 2017;12:468–473. doi: 10.1515/med-2017-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirinos JA, Tamariz LJ, Lopes G, Del Carpio F, Zhang X, Milikowski C, et al. Large vessel involvement in ANCA-associated vasculitides: report of a case and review of the literature. Clin Rheumatol. 2004;23:152–159. doi: 10.1007/s10067-003-0816-0. [DOI] [PubMed] [Google Scholar]
- 17.Kang A, Antonelou M, Wong NL, Tanna A, Arulkumaran N, Tam FWK, et al. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2019;46:285–293. doi: 10.3899/jrheum.170896. [DOI] [PubMed] [Google Scholar]
- 18.McAdoo SP, Medjeral-Thomas N, Gopaluni S, Tanna A, Mansfield N, Galliford J, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. 2019;34:63–73. doi: 10.1093/ndt/gfx378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Algorithm for selecting the study population. A list of 1060 patients with BD as the primary diagnosis was collected by the clinical data repository of Severance Hospital. When the revised version of the ICBD in 2014 (the 2014 ICBD criteria) was applied, 252 patients were excluded from this study. When The ISG criteria in 1990 (the 1990 ISG criteria) were applied, 472 patients were excluded from this study. Finally, 808 of 1060 BD patients could be reclassified as BD based on the 2014 ICBD criteria. In addition, 588 of 1060 BD patients could be reclassified as BD based on the 1990 ISG criteria. BD, Behçet's disease, ICBD, International Criteria for Behçet's Disease; ISG, International Study Group; ANCA, antineutrophil cytoplasmic antibody.
Application of the 2007 EMA Algorithm for AAV and cPAN Based on the 2012 CHCC Definitions to 18 BD Patients with ANCA
Characteristics of 588 BD Patients Based on the 1990 ISG Criteria
Comparison of the Sites and Pattern of Vascular Manifestation at Diagnosis between ANCA-Positive and ANCA-Negative BD Patients Based on the 1990 ISG Criteria