Abstract
Purpose
Calcineurin inhibitor (CNI) use has improved lung transplantation outcomes. However, significant perioperative complications in patients receiving CNI can deteriorate the early course of lung transplantation. To date, there is no consensus regarding the optimal agent for the induction regimen after lung transplantation. We aimed to determine the efficacy of basiliximab induction with delayed CNI initiation in the prevention of acute complications without compromising immunosuppression in high-risk patients.
Materials and Methods
Between January 2013 and December 2019, 236 patients at a single lung transplant center were retrospectively reviewed. Forty-one patients (17.4%) received basiliximab induction, and 195 patients (82.6%) received a routine triple-drug regimen without induction. The primary endpoint was postoperative acute kidney injury with several other postoperative outcomes as secondary end-points.
Results
Preoperatively, the induction group had a higher proportion of patients who were admitted before transplantation (95.1% vs. 47.7%, p<0.001) and received intensive unit care (90.2% vs. 33.8%, p<0.001) and extracorporeal membrane oxygenation (ECMO) (87.8% vs. 20.0%, p<0.001) compared to the non-induction group. No significant differences were observed in the incidence of acute rejection between groups (p=0.657), although lower incidence of postoperative complications, including acute kidney injuries or culture-proven infections, were observed in the induction group. However, the differences were not statistically significant. A subgroup analysis of high-risk and preoperative ECMO support groups showed similar results.
Conclusion
Basiliximab induction with delayed CNI initiation for high-risk patients might decrease the incidence of perioperative complications, including acute renal failure, without increasing the risk of acute rejection.
Keywords: Calcineurin inhibitor, lung transplantation, basiliximab, immunosuppression
INTRODUCTION
The short-term outcomes of solid organ transplantation have markedly improved in the past few decades, largely due to improvements in immunosuppression and medical care. Calcineurin inhibitor (CNI)-based “triple-drug” maintenance regimens are routinely employed in patients receiving lung transplantation.1 The goal of immunosuppression in transplantation is long-term patient survival with healthy allografts, while minimizing complications that occur due to immunosuppressive medications. However, lifelong medication increases the risk of renal dysfunction, severe infections, malignancies, and other critical comorbidities, thereby resulting in diminished quality of life and reduced rates of long-term survival.2
CNI nephrotoxicity is an important side effect of current immunosuppression strategies. Significant rates of postoperative renal dysfunction have been described in patients undergoing lung transplantation and those who had received CNI in the perioperative period.3 An option to prevent the occurrence of postoperative renal function would be delaying CNI initiation after the early postoperative period, which is mainly characterized by hemodynamic instability; the need for blood transfusions inotropic agents, and vasopressors; and the occurrence of systemic inflammation, all of which contribute to fluctuating CNI pharmacokinetics and an increased risk of renal injury.4 During this early postoperative phase, immunosuppression is due to the rapid depression of the adaptive immunity for the prevention of acute cellular rejection in all modalities. Basiliximab is a chimeric monoclonal antibody that selectively binds to the α–subunit (CD25) of interleukin-2 receptors on T-cells that are activated as a result of an immune response to the foreign antigens of newly transplanted organs.5 Furthermore, basiliximab has shown a favorable side effect profile after solid organ transplantation.5 Therefore, the early administration of basiliximab as an induction agent would allow a delayed CNI initiation, minimizing its nephrotoxicity during the early perioperative phase.
The goal of induction therapy is to prevent acute rejection without compromising the renal function during the early postoperative period, particularly in recipients who are at a high-risk for acute renal failure. To date, there is no consensus on the optimal agent for the induction regimen after lung transplantation. Our study aimed to determine the efficacy of basiliximab induction therapy followed by delayed CNI initiation in preventing acute complications without compromising sufficient immunosuppression in the perioperative period for patients with double-lung transplantation who are at risk for postoperative renal dysfunction.
MATERIALS AND METHODS
Patient characteristics
All adult recipients of lung transplantation between January 2013 and December 2019 at the lung transplant center of Severance Hospital, Yonsei University College of Medicine were retrospectively reviewed. Initially, patients who underwent double-lung transplantation without additional procedures, such as co-transplantation of other organs, or heart surgery were included. We also excluded patients supported by cardiopulmonary bypass or central extracorporeal membrane oxygenation (ECMO) during transplantation. This study was approved by the Institutional Review Board (IRB) of Severance Hospital of Yonsei University (IRB: 4-2020-0629). The IRB waived the requirement for obtaining informed consent from the patients.
Patients with at least one of the following risk factors were categorized as high-risk patients for postoperative renal dysfunction: 1) history of renal disease such as chronic renal failure, 2) poor baseline renal function [serum creatinine (sCr) >1.5 mg/dL or estimated glomerular filtration rate calculated with MDRD formula <60 mL/min], and 3) preoperative mechanical ventilation or ECMO care. Since August 2017, patients categorized as high-risk patients have routinely received a basiliximab induction regimen, while only a few selected patients had received such induction treatment before this date. Therefore, we categorized the patients not only into the induction and non-induction groups, but also into the high-risk induction, high-risk non-induction, and low-risk groups for analysis. We also performed a subgroup analysis of the patients with preoperative ECMO support.
All patients followed our standard post-transplant protocol, including intensive unit care, administration of antibiotics, examination of blood parameters, evaluation of post-transplant status, and follow-up visits.
Immunosuppressant protocols
Our standard immunosuppressive regimen was based on a triple-drug combination of tacrolimus, mycophenolate mofetil (MMF), and corticosteroids. For the non-induction group, 1-mg tacrolimus was administered as a loading dose before surgery, and started after transplantation with target blood levels of 10–15 mg/dL. All patients received 500-mg intravenous (IV) methylprednisolone before reperfusion, followed by IV administration of 0.5 mg/kg for 3 days. Then, we gradually tapered the dose to 0.25 mg/kg. One 2000-mg MMF dose was administered preoperatively and maintained daily unless it resulted in leukopenia or liver dysfunction, in which case the dose was lowered or discontinued.
The basiliximab induction regimen consisted of IV administration of a 20-mg dose at 2 hours before lung transplantation and a second 20-mg IV dose given on postoperative day (POD) 4 without tacrolimus administration. Since basiliximab has a half-life of 7 days, our institutional policy was to resume tacrolimus on POD 7 at a dose of 1–2 mg, and gradually titrate it until the target serum levels were achieved.6
Perioperative infectious prophylaxis was based on broadspectrum antibiotics or adapted to pre-transplant resistance testing. All patients received a lifelong pneumocystic prophylaxis with trimethoprim-sulfamethoxazole. Cytomegalovirus prophylaxis with valganciclovir was maintained for at least 3 months. IV or oral antifungal therapy was administered.
Assessment of postoperative outcomes
Postoperative acute kidney injury was defined as any of the following conditions: an elevation of sCr ≥0.3 mg/dL within 48 hours or ≥1.5-fold elevation from the baseline value within 7 days; a urine volume <0.5 mg/kg/hour for 6–12 hours; or the need for renal replacement due to metabolic acidosis and electrolyte imbalance.7 Baseline sCr was measured immediately before transplantation. Postoperative sCr levels were measured immediately and 12 hours post-transplantation; on PODs 1, 2, 3, and 7; and on the day of the patient's first hospital discharge. We also collected the delta sCr (ΔsCr) values to assess renal function alterations, which were calculated as follows: postoperative sCr level-baseline sCr. An increase in serum sCr indicated a worsening renal function.
Factors such as intraoperative hemodynamic instability, intraoperative transfusion, postoperative bleeding, and infections (microbes confirmed on culture and necessitating antibiotic treatment) that are potentially related to kidney injury were also collected.
Operative data, including ischemic and operative times, were collected. Postoperative outcomes, including ECMO weaning in the operating room, length of mechanical ventilation, primary graft dysfunction at 72 hours after surgery, respiratory and other complications, were recorded. Acute rejection was defined according to the International Society of Heart and Lung Transplantation criteria.8
Statistics
Statistical analyses were performed using SPSS software version 25 (IBM Corp., Armonk, NY, USA). Continuous variables are reported as the mean±standard deviation values for parametric values and the median [interquartile range] for non-parametric values, and categorical variables are reported as frequencies (%). The Student's t test and Mann-Whitney U test were used to compare continuous variables, whereas the chi-squared or Fischer's exact test was used to compare categorical variables, when required. Differences in continuous variables among groups were examined using a one-way analysis of variance (ANOVA), followed by Bonferroni correction for multiple comparisons. Mixed ANOVA was used for comparing serial changes in sCr levels among groups, with induction treatment as the in-between factor and time as the within factor (POD when the sCr levels were measured). Statistical significance threshold was set at a p value of <0.05.
RESULTS
Demographic and clinical characteristics
Among 262 patients who underwent lung transplantation at the Department of Thoracic Surgery, Yonsei University College of Medicine from January 2013 to December 2019, 236 patients were included in the present study (Fig. 1).
Fig. 1. Flow chart describing the selection of study participants.
Preoperative clinical features of patients are described in Table 1. Of the 236 patients, 17.4% (n=41) received a basiliximab induction regimen and 82.6% (n=195) received a routine triple-drug regimen without induction agents. The median follow-up duration was 685.0 (168.5;1368.5) days and 278.0 (182.0;369.0) days for the non-induction and induction groups, respectively (p<0.001). The induction group was older than the non-induction group (mean age, 57.5±9.3 years vs. 52.7±12.5 years, p=0.006) and predominantly male (78.0% vs. 59.5%, p=0.040). In both groups, the most common pre-transplantation diagnosis was interstitial lung disease (78.0% and 72.8%). Preoperative comorbidities and kidney function of both groups were comparable. The induction group had a higher proportion of patients who had been admitted before transplantation (95.1% vs. 47.7%, p<0.001) and had a longer hospitalization before transplantation [32.0 (23.0;72.0) days vs. 0.0 (0.0;20.0) days, p<0.001]. Besides, the induction group frequently received more care in the intensive care unit (ICU) (90.2% vs. 33.8%, p<0.001) and spent a significantly longer duration in the ICU [16.0 (11.0;28.0) days vs. 0.0 (0.0;8.0) days, p<0.001). Moreover, the induction group had a higher proportion of patients receiving pre-transplantation ECMO support (87.8% vs. 20.0%, p<0.001), and had longer durations of pre-transplantation ECMO support [12.0 (6.0;22.0) days vs. 0.0 (0.0;0.0) days, p<0.001).
Table 1. Clinical and Demographic Characteristics of the Patients.
| Non-induction group (n=195) | Induction group (n=41) | p value | |
|---|---|---|---|
| Male sex | 116 (59.5) | 32 (78.0) | 0.040 |
| Age (yr) | 52.7±12.5 | 57.5±9.3 | 0.006 |
| BMI | 20.8±3.7 | 22.7±4.8 | 0.017 |
| Diagnosis | 0.065 | ||
| COPD and emphysema | 6 (3.1) | 4 (9.8) | |
| Interstitial lung disease | 142 (72.8) | 32 (78.0) | |
| Bronchiectasis | 13 (6.7) | 3 (7.3) | |
| Others | 34 (17.4) | 2 (4.9) | |
| Comorbidities | |||
| Diabetes mellitus | 26 (13.3) | 11 (26.8) | 0.054 |
| Hypertension | 15 (7.7) | 7 (17.1) | 0.075 |
| Chronic renal disease | 19 (9.7) | 4 (9.8) | 1.000 |
| Malignancies | 29 (14.9) | 1 (2.4) | 0.056 |
| Baseline creatinine levels (mg/dL) | 0.65±0.22 | 0.53±0.20 | 0.003 |
| Time on waiting list (days) | 82.0 [24.0;171.0] | 121.0 [29.0;244.0] | 0.102 |
| Pretransplantational admission | 93 (47.7) | 39 (95.1) | <0.001 |
| Pretransplantational admission duration (days) | 0.0 [0.0;2.0] | 32.0 [23.0;72.0] | <0.001 |
| Pretransplantational ICU care | 66 (33.8) | 37 (90.2) | <0.001 |
| Pretransplantational ICU care duration (days) | 0.0 [0.0;8.0] | 16 [11.0;28.0] | <0.001 |
| Pretransplantational ventilator care | 60 (30.8) | 37 (90.2) | <0.001 |
| Pretransplantational ventilator care duration (days) | 0.0 [0.0;5.5] | 14.0 [2.0;21.0] | <0.001 |
| Pretransplantational ECMO support | 39 (20.0) | 36 (87.8) | <0.001 |
| Pretransplantational ECMO support duration (days) | 0.0 [0.0;0.0] | 12.0 [6.0;22.0] | <0.001 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Data are presented as mean±standard deviation or n (%).
Operative and postoperative outcomes
Table 2 shows the operative details and postoperative outcomes of all groups. There were no significant differences in the total operative time, intraoperative ECMO weaning rate, postoperative ICU and hospital stay durations, and operative mortality between the induction and non-induction groups. Greater amounts of estimated blood loss and intraoperative transfusion were observed in the induction group [2800.0 (2000.0; 5000.0) vs. 2000.0 (1200.0;3450.0), p=0.003, and 2910.0 (2100.0; 5350.0) vs. 2330.0 (1440.0;3600.0), p=0.010, respectively). The target tacrolimus level was reached earlier in the non-induction group [POD 4.0 (3.0;9.0) vs. 14.0 (10.0;17.0), p<0.001, respectively], and the tacrolimus level was higher on the day the target level was achieved in the non-induction group [12.0 (10.8; 13.9) vs. 10.8 (10.5;11.6), p<0.001, respectively]. Regarding postoperative complications, there were no statistically significant differences in the incidence of acute rejection or acute kidney injury between the two groups. Culture-proven infection was less frequent in the induction than in the non-induction group (7.3% vs. 22.1%), although this difference was not statistically significant.
Table 2. Operative and Postoperative Outcomes.
| Non-induction group (n=195) | Induction group (n=41) | p value | |
|---|---|---|---|
| Total operative time (hours) | 5.0 [4.5;6.0] | 6.0 [5.5;7.0] | 0.001 |
| Intraoperative ECMO weaning | 114 (58.5) | 25 (61.0) | 0.902 |
| Intraoperative transfusion amount (mL) | 2330.0 [1440.0;3600.0] | 2910.0 [2100.0;5350.0] | 0.010 |
| Estimated blood loss (mL) | 2000.0 [1200.0;3450.0] | 2800.0 [2000.0;5000.0] | 0.003 |
| Postoperative day when the target tacrolimus level was achieved (days) | 4.0 [3.0;9.0] | 14.0 [10.0;17.0] | <0.001 |
| Tacrolimus level on the day the target level was achieved (mg/dL) | 12.0 [10.8;13.9] | 10.8 [10.5;11.6] | <0.001 |
| Postoperative complications | |||
| Acute rejection | 7 (3.6) | 2 (4.9) | 0.657 |
| Postoperative bleeding requiring re-operation | 27 (13.8) | 8 (19.5) | 0.493 |
| Respiratory failure requiring tracheostomy | 49 (25.1) | 15 (36.6) | 0.318 |
| Bronchial dehiscence | 16 (8.2) | 1 (2.4) | 0.334 |
| Pulmonary artery stenosis | 11 (5.6) | 1 (2.4) | 0.647 |
| Acute kidney injury | 62 (31.8) | 9 (22.0) | 0.288 |
| Postoperative renal replacement therapy | 40 (20.5) | 7 (17.1) | 0.775 |
| Infection (culture proven) | 43 (22.1) | 3 (7.3) | 0.051 |
| Neurological complications | 13 (6.7) | 2 (4.9) | 0.941 |
| Gastrointestinal complications | 43 (22.1) | 4 (9.8) | 0.115 |
| Wound dehiscence | 8 (4.1) | 2 (4.9) | 1.000 |
| Postoperative ICU stay (first admission) (days) | 8.0 [5.0;15.0] | 7.0 [5.0;12.0] | 0.489 |
| Duration of postoperative hospital stay (days) | 39.0 [25.0;76.5] | 52.0 [30.0;96.0] | 0.121 |
| Duration of total hospital stay (days) | 54.0 [32.0;98.5] | 109.0 [61.0;160.0] | 0.001 |
| Surgery-related mortality (within 30 days) | 10 (5.1) | 1 (2.4) | 0.738 |
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Data are presented as n (%).
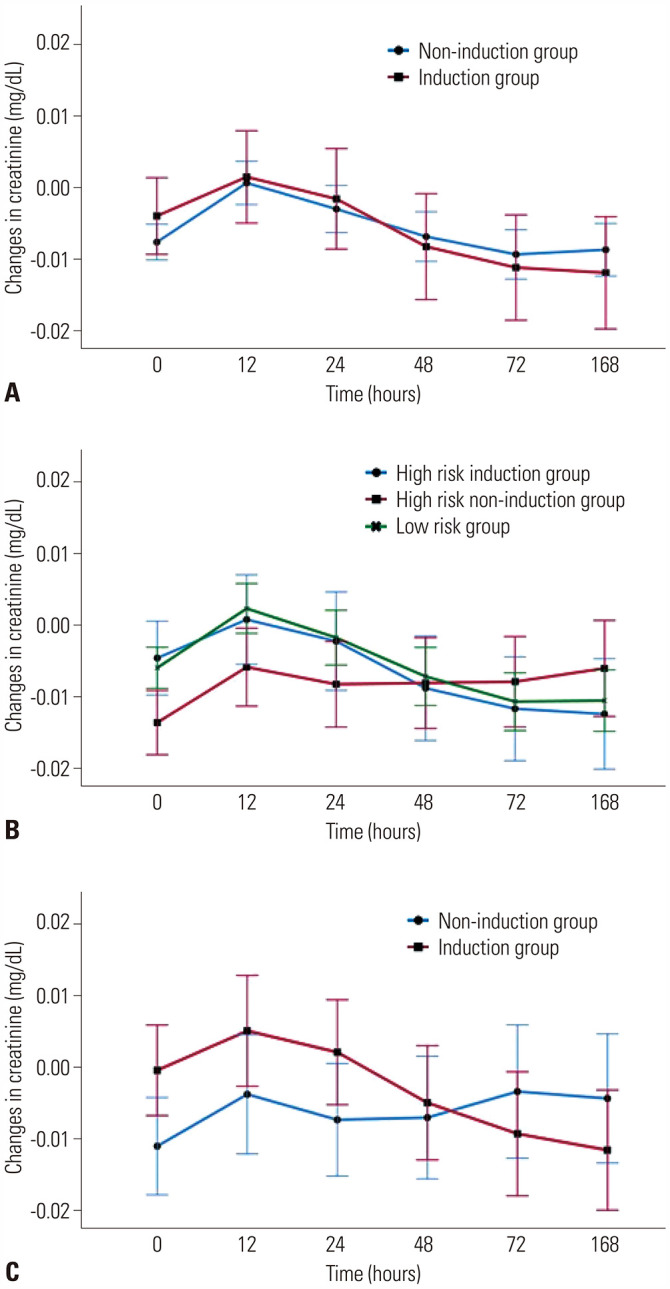
The serial changes in sCr levels of both groups are presented in Fig. 2A. Changes in sCr levels were comparable between the induction and non-induction groups (p=0.440).
Fig. 2. Changes in serum creatinine levels. (A) Comparison between the induction and non-induction groups, (B) comparison among groups categorized according to results of a risk analysis, and (C) comparison between the induction and non-induction groups receiving ECMO support. ECMO, extracorporeal membrane oxygenation.
Comparison of high-risk groups
We also divided the entire cohort into three groups according to a preoperative risk analysis for acute kidney injury. Of all patients included in the study, 55.9% (n=132) were categorized to the low-risk group, 17.4% (n=41) to the high-risk induction group undergoing a basiliximab induction regimen, and 26.7% (n=63) to the high-risk non-induction group undergoing a traditional triple-drug immunosuppression regimen. We performed an analysis in the high-risk induction and high-risk non-induction groups, and presented the results in Table 3. The data from the low-risk group are shown as control values. The high-risk induction group had been listed for transplantation for a longer duration [121.0 (29.0;244.0) days vs. 26.0 (11.5; 97.0) days, p=0.001] and was admitted for longer periods [32.0 (23.0;72.0) days vs. 23.0 (13.0;37.0) days, p=0.003] before transplantation compared to the high-risk non-induction group. The incidence and duration of pre-transplantation ECMO support were higher in the high-risk induction group [87.8% vs. 61.9%, p=0.008; 12.0 (6.0;22.0) days vs. 6.0 (0.0;14.5) days, p=0.003], whereas the incidence of admitting ICU was higher in the high-risk non-induction group (93.7% vs. 90.2%, p=0.709). The amount of estimated blood loss was greater in the high-risk induction group [2800.0 (2000.0;5000.0) mL vs. 2100.0 (1400.0; 3500.0) mL, p=0.066]. Regarding the postoperative complications, the incidence of acute rejection was not statistically significant between the two groups (4.9% vs. 7.9%, p=0.701). The high-risk non-induction group showed a higher incidence of acute kidney injury (42.9% vs. 22.0%, p=0.048) and culture-proven infection (30.2% vs. 7.3%, p=0.011). The incidence of neurologic and gastrointestinal complications was also higher in the high-risk non-induction group (neurologic, 11.1% vs. 4.9%, p=0.477; gastrointestinal, 22.2% vs. 9.8%, p=0.169), although the difference was not statistically significant. Serial changes in sCr levels in all groups are presented in Fig. 2B. At 2 days after surgery, ΔsCr in the high-risk induction and low-risk groups presented negative values, whereas that of the high-risk non-induction group was increased; however, the difference between groups was not statistically significant (p=0.723).
Table 3. Analysis according to Preoperative Risk.
| High-risk induction (n=41) | High-risk non-induction (n=63) | p value | Low-risk (n=132) | |
|---|---|---|---|---|
| Baseline creatinine levels (mg/dL) | 0.53±0.20 | 0.58±0.26 | 0.293 | 0.67±0.19 |
| Time on waiting list (days) | 121.0 [29.0;244.0] | 26.0 [11.5;97.0] | 0.001 | 96.0 [46.0;189.5] |
| Pretransplantational admission | 39 (95.1) | 63 (100.0) | 0.153 | 30 (22.7) |
| Pretransplantational admission duration (days) | 32.0 [23.0;72.0] | 23.0 [13.0;37.0] | 0.003 | 0.0 [0.0;0.0] |
| Pretransplantational ICU care | 37 (90.2) | 59 (93.7) | 0.709 | 7 (5.3) |
| Pretransplantational ICU care duration (days) | 16.0 [11.0;28.0] | 14.0 [8.0;21.0] | 0.109 | 0.0 [0.0;0.0] |
| Pretransplantational ECMO support | 36 (87.8) | 39 (61.9) | 0.008 | 0 (0.0) |
| Pretransplantational ECMO support duration (days) | 12.0 [6.0;22.0] | 6.0 [0.0;14.5] | 0.003 | 0.0 [0.0;0.0] |
| Total operative time (hours) | 6.0[5.5;7.0] | 5.5 [5.0;6.0] | 0.020 | 5.0 [4.25;6.0] |
| Intraoperative ECMO weaning | 25 (61.0) | 32 (50.8) | 0.413 | 82 (62.1) |
| Intraoperative transfusion amount (mL) | 2910.0 [2100.0;5350.0] | 3100.0 [1935.0;4485.0] | 0.808 | 2030.0 [1220.0;3255.0] |
| Estimated blood loss (mL) | 2800.0 [2000.0;5000.0] | 2100.0 [1400.0;3500.0] | 0.066 | 1800.0 [1100.0;3350.0] |
| Postoperative day when the target tacrolimus level was achieved (days) | 14.0 [10.0;17.0] | 4.0 [3.0;9.0] | 0.001 | 4.0 [3.0;9.0] |
| Tacrolimus level on the day the target level was achieved (mg/dL) | 10.8 [10.5;11.6] | 12.1 [10.8;13.8] | 0.001 | 11.9 [10.8;14.0] |
| Postoperative complications | ||||
| Acute rejection | 2 (4.9) | 5 (7.9) | 0.701 | 2 (1.5) |
| AKI | 9 (22.0) | 27 (42.9) | 0.048 | 35 (26.5) |
| Infection (culture proven) | 3 (7.3) | 19 (30.2) | 0.011 | 24 (18.2) |
| Neurologic complications | 2 (4.9) | 7 (11.1) | 0.477 | 6 (4.5) |
| Gastrointestinal complications | 4 (9.8) | 14 (22.2) | 0.169 | 29 (22.0) |
| Duration of postoperative ICU stay (first admission; days) | 7.0 [5.0;12.0] | 10.0 [5.0;19.0] | 0.197 | 7.0 [5.0;13.0] |
| Duration of postoperative hospital stay (days) | 52.0 [30.0;96.0] | 53.0 [33.5;91.5] | 0.960 | 35.0 [25.0;60.5] |
| Duration of total hospital stay (days) | 109.0 [61.0;160.0] | 79.0 [55.0;121.0] | 0.126 | 40.5 [27.0;78.0] |
| Surgery-related mortality (within 30 days) | 1 (2.4) | 6 (9.5) | 0.240 | 4 (3.0) |
AKI, acute kidney injury; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
Data are presented as n (%).
Comparison of induction and non-induction groups receiving preoperative ECMO support
Patients who received preoperative ECMO support were separately analyzed, and the results are shown in Table 4. Among the 75 patients who received preoperative ECMO support, 36 (48%) received basiliximab induction treatment. Similar results were observed in the subgroup analysis. The incidence of acute rejection was not statistically different between the induction and non-induction groups (5.6% vs. 7.7%, p=1.000), but the non-induction group tended to have a higher incidence of acute kidney injury during the early postoperative phase (19.4% vs. 41.0%, p=0.076). Although not statistically different, the non-induction group also tended to have a higher incidence of culture-proven infection and neurologic and gastrointestinal complications.
Table 4. Subgroup Analysis of Patients with Preoperative ECMO Support.
| Non-induction group (n=39) | Induction group (n=36) | p value | |
|---|---|---|---|
| Baseline creatinine levels (mg/dL) | 0.59±0.30 | 0.52±0.18 | 0.003 |
| Time on waiting list (days) | 26.0 [11.0;89.0] | 105.0 [28.5;237.5] | 0.003 |
| Pretransplantational admission duration (days) | 30.0 [16.5;39.5] | 32.0 [23.0;71.5] | 0.064 |
| Pretransplantational ICU care duration (days) | 16.0 [9.5;21.0] | 16.5 [11.5;27.0] | 0.316 |
| Pretransplantational ECMO support duration (days) | 2.0 [9.0;18.5] | 13.0 [9.5;22.5] | 0.286 |
| Total operative time (hours) | 5.5 [5.0;6.8] | 6.0 [5.5;7.0] | 0.254 |
| Intraoperative ECMO weaning | 16 (41.0) | 21 (58.3) | 0.205 |
| Intraoperative transfusion amount (mL) | 3400.0 [1875.0;4770.0] | 2880.0 [2072.5;5775.0] | 0.958 |
| Estimated blood loss (mL) | 2400.0 [1825.0;3900.0] | 2700.0 [1900.0;5050.0] | 0.311 |
| Postoperative day when the target tacrolimus level was achieved (days) | 5.0 [3.0;10.0] | 14.0 [10.0;17.0] | <0.001 |
| Tacrolimus level on the day the target level was achieved (mg/dL) | 11.5 [10.8;13.6] | 10.8 [10.2;11.8] | 0.024 |
| Postoperative complications | |||
| Acute rejection | 3 (7.7) | 2 (5.6) | 1.000 |
| AKI | 16 (41.0) | 7 (19.4) | 0.076 |
| Infection (culture proven) | 11 (28.2) | 3 (8.3) | 0.056 |
| Neurologic complications | 3 (7.7) | 2 (5.6) | 1.000 |
| Gastrointestinal complications | 9 (23.1) | 4 (11.1) | 0.288 |
| Duration of postoperative ICU stay (first admission) (days) | 12.0 [6.0;20.5] | 6.5 [5.0;12.0] | 0.031 |
| Duration of postoperative hospital stay (days) | 44.0 [31.5;83.5] | 51.5 [30.0;93.5] | 0.687 |
| Duration of total hospital stay (days) | 79.0 [57.0;118.5] | 107.5 [61.0;156.5] | 0.215 |
| Surgery-related mortality (within 30 days) | 4 (10.3) | 1 (2.8) | 0.360 |
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Data are presented as n (%).
Changes in the average ΔsCr levels according to the presence or absence of an induction regimen are shown in Fig. 2C. Changes in the sCr levels were not significantly different between the induction and non-induction groups. Initially, the sCr levels of the induction group seemed to be elevated, but they stabilized over time.
DISCUSSION
The key findings of our study suggest that the basiliximab induction regimen allows for a delayed initiation of tacrolimus, and thereby lessens the risk of acute renal injury, even in patients who are at an increased risk of postoperative renal impairment. In addition, there was no evidence of increased acute rejection with this regimen. This favorable early experience could be applied as a routine protocol for lung transplant recipients at risk for postoperative renal dysfunction.
The rationale for immunosuppression induction with delayed of CNI agent initiation in lung transplantation is based on the outcomes of previous studies on other solid organ transplantations.5,9,10 However, researchers have not yet reached a consensus regarding the application of immunosuppression induction for lung transplantation patients. Few studies have investigated this issue; however, these studies had small sample sizes and were retrospective in nature. Several reports have demonstrated the efficacy of induction agents, such as basiliximab, with relatively low cumulative burdens in comparison to conventional maintenance, which is the reason why basilisimab induction is still not a routine regimen for lung transplantation.1,11 To the best of our knowledge, no study has been conducted to date regarding immunosuppression induction with delayed CNI initiation in the prevention of acute kidney injury during the perioperative phase of lung transplantation.
Tacrolimus is the first-line CNI, and it is typically initiated on the first POD and maintained throughout the lifetime of the patient. A second CNI became available for use in 1997.2 While acute and chronic rejection may be improved with tacrolimus, adverse effects, such as nephrotoxicity, affect survival.12,13 Tacrolimus leads to renal impairment as it causes vasoconstriction of the afferent and efferent glomerular arterioles, and evidence has demonstrated that elevated whole-blood tacrolimus peak concentrations are associated with acute kidney injury during the early postoperative period.3,13 In this regard, discrepancies exist regarding the initiation of tacrolimus when there is evidence of postoperative renal complications.14 As the acuity and severity of end-stage respiratory failure are worsened in patients on waiting lists, more patients are likely to possess the risk factors for compromised renal function. Therefore, in these patients, early postoperative management of lung transplantation becomes more delicate. The basiliximab induction strategy described here could provide an alternative immunosuppression regimen to avoid acute renal impairment observed with a CNI regimen in patients at increased risk of renal failure.
This study has some limitations. It is a retrospective analysis using single-center data, and the study cohort is relatively small. We resumed tacrolimus on POD 7 in the induction group since the half-life of basiliximab is 7 days, and in references to other solid organ transplantations; however, there are no exact criteria for when to resume tacrolimus administration in lung transplant patients with basiliximab induction.12,15,16 A prospective, randomized study is needed to provide deeper insights into the role and standard of basiliximab induction treatment. Furthermore, the effect of the era in which treatment was provided cannot be excluded, since basiliximab induction was introduced recently. It is possible that the results might partially reflect the recent improvements in surgical strategies and postoperative care. Another limitation of this study is that perioperative renal injury was assessed by changes in sCr levels and reductions in urine output, rather than by Cr clearance, proteinuria, or histopathologic confirmation from renal biopsies. This could have resulted in missed tubulointerstitial injury, and may have affected the evaluation of renal injury.
In conclusion, basiliximab induction with delayed tacrolimus initiation in patients who are at risk for renal impairment may reduce the incidence of acute renal failure during the early postoperative period, without increasing the risk of acute rejection. Further studies using prospective, randomized clinical trials and long-term follow-up data are warranted.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Ha Eun Kim and Jin Gu Lee.
- Data curation: Ha Eun Kim.
- Formal analysis: Ha Eun Kim and Jin Gu Lee.
- Funding acquisition: Ha Eun Kim and Jin Gu Lee.
- Investigation: Ha Eun Kim and Jin Gu Lee.
- Methodology: Ha Eun Kim.
- Project administration: Hyo Chae Paik and Jin Gu Lee.
- Resources: Hyo Chae Paik, Su Jin Jeong, Moo Suk Park, Song Yee Kim, and Jin Gu Lee.
- Software: Ha Eun Kim.
- Supervision: Jin Gu Lee.
- Validation: Ha Eun Kim.
- Visualization: Ha Eun Kim.
- Writing—original draft: Ha Eun Kim.
- Writing—review & editing: all authors .
- Approval of final manuscript: all authors.
References
- 1.Benazzo A, Schwarz S, Muckenhuber M, Schweiger T, Muraközy G, Moser B, et al. Alemtuzumab induction combined with reduced maintenance immunosuppression is associated with improved outcomes after lung transplantation: a single centre experience. PLoS One. 2019;14:e0210443. doi: 10.1371/journal.pone.0210443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039–1053. doi: 10.3978/j.issn.2072-1439.2014.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikma MA, Hunault CC, van de Graaf EA, Verhaar MC, Kesecioglu J, de Lange DW, et al. High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury. Eur J Clin Pharmacol. 2017;73:573–580. doi: 10.1007/s00228-017-2204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikma MA, van Maarseveen EM, van de Graaf EA, Kirkels JH, Verhaar MC, Donker DW, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015;15:2301–2313. doi: 10.1111/ajt.13309. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez CB, Marino IR. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther. 2007;7:137–148. doi: 10.1517/14712598.7.1.137. [DOI] [PubMed] [Google Scholar]
- 6.McKenna GJ, Klintmalm GBG. Induction and maintenance of Immunosuppression. In: Busuttil RW, Klintmalm GBG, editors. Transplantation of the liver. 3rd ed. Philadelphia: Saunders; 2015. [Google Scholar]
- 7.Abelha FJ, Botelho M, Fernandes V, Barros H. Determinants of postoperative acute kidney injury. Crit Care. 2009;13:R79. doi: 10.1186/cc7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg PB, Vriesendorp AE, Drazner MH, Dries DL, Kaiser PA, Hynan LS, et al. Induction therapy with basiliximab allows delayed initiation of cyclosporine and preserves renal function after cardiac transplantation. J Heart Lung Transplant. 2005;24:1327–1331. doi: 10.1016/j.healun.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90:1511–1515. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 11.Coiffard B, Piloni D, Boucekine M, Morosini M, Meloni F, Kessler R, et al. Effect of induction therapy on peripheral blood lymphocytes after lung transplantation: a multicenter international study. Transpl Immunol. 2018;48:47–54. doi: 10.1016/j.trim.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1–15. doi: 10.1111/j.1399-0012.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 13.Ivulich S, Dooley M, Kirkpatrick C, Snell G. Clinical challenges of tacrolimus for maintenance immunosuppression post-lung transplantation. Transplant Proc. 2017;49:2153–2160. doi: 10.1016/j.transproceed.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; Focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Lange NW, Salerno DM, Sammons CM, Jesudian AB, Verna EC, Brown RS., Jr Delayed calcineurin inhibitor introduction and renal outcomes in liver transplant recipients receiving basiliximab induction. Clin Transplant. 2018;32:e13415. doi: 10.1111/ctr.13415. [DOI] [PubMed] [Google Scholar]
- 16.Rafael-Valdivia L, Mendoza MA, Martinez-Saldivar B, Sanchez-Fueyo A, Brunet M, Garcia-Valdecasas JC, et al. How long should initiation of calcineurin inhibitors be delayed to protect renal function in liver transplantation? Transplant Proc. 2011;43:697–698. doi: 10.1016/j.transproceed.2011.01.091. [DOI] [PubMed] [Google Scholar]