Abstract
The coronavirus disease 2019 (COVID-19) pandemic has become a global health crisis. Considering the recent food and drug administration (FDA) approval of remdesivir as the first officially approved agent for COVID-19 treatment, we performed this systematic review and meta-analysis to evaluate the efficacy and safety of remdesivir administration in COVID-19 patients. A systematic literature search was done through MEDLINE, Google Scholar, Web of Science, Scopus, Science Direct, Cochrane Library, medRxiv, and bioRxiv from their inception to December 22nd, 2020. Five randomized controlled trials (RCTs) and five non-randomized studies of intervention (NRSI) were entered into the meta-analysis. The results showed that remdesivir administration was associated with a significant improvement in the 28-day recovery (RR = 1.09, 95%CI, 1.04–1.15), low flow oxygen support through days one to 14 (RR = 2.88, 95%CI, 1.80–4.60), and invasive mechanical ventilation or extracorporeal membrane oxygenation requirement through days 14–28 of the follow-up time (RR = 5.34, 95%CI, 2.37–12.05). The risk of experiencing serious adverse drug reactions (ADRs) was significantly lower (RR = 0.75, 95%CI, 0.63–0.90) in the remdesivir group than the comparison/control group. The pooled median difference of the time to clinical improvement was 2.99 (95%CI = 2.71–3.28), which did not remain significant during the sensitivity analysis. The clinical output comparison of the 5-day and 10-day remdesivir courses revealed that the 5-day regimen might provide similar benefits while causing fewer serious ADRs than 10-day. The current meta-analysis provided an updated evaluation of scientific evidence on the use of remdesivir in COVID-19 patients. Performing adequate well-designed RCTs are needed to show more accurate results.
Keywords: Remdesivir, COVID-19, SARS-CoV-2, Meta-analysis, Systematic review, Coronavirus
1. Introduction
The pandemic of coronavirus disease 2019 (COVID-19), caused by the newly emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global health crisis (WHO, 2020b). As of December 28th, 2020, more than 81.2 million cases of COVID-19 have been confirmed worldwide, with about 1.77 million deaths (WHO, 2020a). Numerous medicines are being investigated for the management of COVID-19; among them, remdesivir has been at the center of attention and appointed the first approval of the US Food and Drug Administration (FDA) to manage COVID-19 (The U.S. Food and Drug Administration, 2020b).
Remdesivir is a ribonucleic acid (RNA)-dependent RNA polymerase inhibitor with in-vitro inhibitory activity against the coronaviruses, which was initially developed to treat Ebola. A study on infected monkeys with SARS-CoV-2 revealed that the early administration of remdesivir is associated with a significant reduction in viral load and pulmonary damage (Amirian and Levy, 2020; Sheahan et al., 2017; Williamson et al., 2020).
Based on the results of the national institute for allergy and infectious diseases (NIAID) and SIMPLE studies, the FDA approved the use of remdesivir in severe hospitalized COVID-19 patients under an emergency use authorization (EUA) on May 1st, 2020. Afterward, on August 28th, 2020, the letter was reissued with revisions to expand the authorized remdesivir administration to the non-severe COVID-19 patients. Finally, on October 22nd, 2020, remdesivir became the first drug with the FDA approval for the treatment of COVID-19 (The U.S. Food and Drug Administration, 2020a, The U.S. Food and Drug Administration, 2020b). The final approval was supported by the data analysis of the NIAID, SIMPLE, and Spinner et al. trials (Beigel et al., 2020b; Goldman et al., 2020; Spinner et al., 2020).
After the FDA approval of remdesivir in the management of COVID-19, the world health organization (WHO) SOLIDARITY therapeutics trial with approximately 12,000 patients in 500 hospital sites in over 30 countries showed that remdesivir had no statistically significant effect on the mortality rate among individuals with COVID-19 (Pan et al., 2020). Moreover, the Wang et al. trial with no overall significant promising results of remdesivir administration in the COVID-19 patients was not considered in the FDA approval process of remdesivir. Furthermore, there are some reports about the incidence of remdesivir related adverse drug reactions (ADRs) in many hospitalized patients with COVID-19. These reports have raised concerns about the safety and efficacy of remdesivir in the treatment of COVID-19 (Wang et al., 2020).
Given the conflicting results from the clinical trials investigating the administration of remdesivir in hospitalized patients with COVID-19 and considering the global emergency of the disease, we conducted the present systematic review and meta-analysis to assess the safety and efficacy of remdesivir administration in these patients.
To the best of our knowledge, the present comprehensive study is the first systematic review and meta-analysis that has considered the preliminary results of the WHO SOLIDARITY therapeutics trial and the final results of the NIAID trial.
2. Materials and methods
2.1. Study design
This research followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement for study design (Liberati et al., 2009; Moher et al., 2009). The PRISMA checklist is shown in Table S1 (available as Supplementary data).
2.2. Search strategy
Two researchers (A.R. and S.K.) conducted the literature search independently, and any doubts and disagreements were solved by negotiation with the corresponding author (T.E.). A systematic search of the literature was done through MEDLINE (PubMed), Web of Science (Clarivate Analytics), Scopus, Science Direct, Cochrane Library (Wiley), medRxiv, and bioRxiv from their inception to December 22nd, 2020, and their citations were screened using Google Scholar to find additional related studies. In addition, the reference lists of the included studies and related published reviews were hand searched and considered for relevance. Moreover, we used the weekly updates alarm of PubMed on our final search step (#3) to stay informed about new studies.
Additional research was done through Google Scholar, clinicaltrials.gov, Gilead Sciences, the world health organization (WHO), the FDA, and Hoag hospital websites. There were no location and language restrictions. We adapted the PICO process (Population, Intervention, Comparison, and Outcomes) to define inclusion and exclusion criteria for study selection. The PICO model and the PubMed database were used to arrange the concept map and identify the study keywords and subject headings. Our search terms were (“COVID-19” OR “severe acute respiratory syndrome coronavirus 2” OR “Wuhan coronavirus” OR “2019-nCoV” OR “SARS-CoV-2” OR “2019 novel coronavirus” OR “COVID19” OR “COVID-19 pandemic” OR “coronavirus disease 2019”) AND (“Remdesivir” OR “l-alanine, N-((S)-hydroxyphenoxyphosphinyl)-, 2-ethylbutyl ester, 6-ester with 2-C-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitrile” OR “2-ethylbutyl (2S)-2-(((2R, 3S, 4R, 5R)-5-(4-aminopyrrolo(2,1-f) (1,2,4)triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl) methoxy)(phenoxy) phosphoryl) amino) propanoate” OR “GS-5734″ OR “Veklury” OR “RNA replicase” OR “RNA-Directed RNA Polymerase” OR “RNA Polymerase, RNA-Directed” OR “RNA Directed RNA Polymerase” OR “RNA-Dependent RNA Polymerase” OR “RNA Dependent RNA Polymerase” OR “RNA Polymerase, RNA-Dependent”). According to each database, the alternate forms of subject headings were excluded. The detailed search strategy for each database is shown in Table S2.
2.3. Inclusion criteria
The criteria of the studies for being included in the meta-analysis were as follows: The setting of observational study and clinical trial, population/test sample of COVID-19 patients with positive laboratory tests, intervention as remdesivir administration, outcomes/objectives, including clinical improvement and its duration, virus elimination according to lab tests and viral load profiles, improvement in radiological results, evaluation of intolerable side effects, medication safety and tolerability to remdesivir, presenting worsened cases of infection, detecting recurrence frequency after completion of treatment, and examination and reporting the mortality rate. The included studies might include comparison/control sample(s) of COVID-19 patients under treatment with any medication other than remdesivir, but covering this criterion was optional.
2.4. Exclusion criteria
Duplicate publications, reviews, animal researches, case reports, in-vitro, and in-silico studies were excluded from the meta-analysis.
2.5. Risk of bias evaluation
The included studies in the current systematic review were classified into two categories: randomized controlled trial (RCT) and non-randomized study of intervention (NRSI) (Reeves et al., 2020).
The risk of bias assessment of the studies was carried out by two researchers (A.R. and S.K) independently, and any disagreements in this step were resolved by the supervisor (P.S). The utilized scales for evaluating the risk of bias according to the type of study were as follows: A revised tool for risk of bias in randomized trials (RoB 2.0) tool for the RCTs and risk of bias in non-randomized studies of interventions (ROBINS-I) tool for the NRSIs (Higgins et al., 2020; Jadad et al., 1996; Sterne et al., 2016, Sterne et al., 2019, Sterne et al., 2020). The risk of bias plots were generated using the visualization tool for risk of bias assessments in a systematic review (robvis) (McGuinness and Higgins, 2020).
2.6. Data extraction
Data extraction from selected publications was done independently by two authors (A.R. and S.K.) using a designed checklist adapted from the Cochrane Collaboration data collection form for review of RCTs and non-RCTs for the clinical trials (Li et al., 2020). The adapted checklist contained five main sections and several subsections.
2.6.1. General information
Study title or identification, the surname of the first author, the year of the publication, reference citation, publication type, and type of the study.
2.6.2. Methods
The aim of the study, study design, and duration of participation.
2.6.3. Participants
Population description, setting, inclusion criteria, exclusion criteria, the total number of participants, baseline imbalances, withdrawals and exclusions, age, sex, the severity of illness, co-morbidities, and other relevant socio-demographics.
2.6.4. Intervention group
Total number of participants, description, duration of the treatment period, administration timing, route of administration, medical providers, co-interventions, economic information, resource requirements, the integrity of delivery, and compliance.
2.6.5. Outcomes
Outcome name, time points measured, the validity of the outcome, assumed risk estimate, power.
2.7. The ongoing clinical trials of remdesivir administration in COVID-19 patients
We have searched clinicaltrials.gov from database inception to November 10th, 2020, to find the ongoing clinical trials of remdesivir in patients with COVID-19. The following data were collected for each clinical trial: study ID, setting, status, country, sample size, disease severity, comparator agent(s), and the treatment schedule and the administration route for both intervention and comparison/placebo groups.
2.8. Statistical data analysis
The gathered data were presented using the percentage (%), proportion, interquartile range (IQR), median, range, hazard ratio, and rate ratio. Confidence intervals (CI) and P-values were used for significance testing with confidence and significance levels of 95% and 0.5, respectively. All the clinical outcomes were reported in the adjusted forms unless only the unadjusted values were available in the original report. The meta-analysis was operated by the pooled event rate comparison of the remdesivir group with the no-remdesivir group for all of the included studies, estimating the pooled median and IQR values for recovery and clinical improvement time, and calculating risk ratios (RR) for studies involving no-remdesivir (comparison/control) groups. Additionally, improvement in three respiratory support levels (low-flow oxygen, high-flow oxygen or non-invasive mechanical ventilation, and invasive mechanical ventilation or extracorporeal membrane oxygenation) was evaluated using over time clinical data of both studied groups to calculate the corresponding RRs. The final data were used to generate forest plots and corresponding 95% CI and P-values.
Data analysis was performed using Microsoft Excel (2019), Comprehensive Meta-Analysis (CMA) software version 2, R statistical software version 4.0.3 metamedian package, and MedCalc statistical software version 19.5.3.
The I-squared (I2) test was employed to assess the statistical heterogeneity between studies, and the associated Tau-squared (Tau2), Q-value, degree of freedom (df), and P-value were represented in the corresponding forest plot. According to the Cochrane handbook for systematic reviews of interventions, our interpretations of the I2 test results were as follows: 0%–40%: not significant, 30%–60%: moderate, 50%–90%: substantial, and 75%–100%: significant heterogeneity (Deeks et al., 2020). In order to represent each output, the random-effects and fixed-effect modelling approaches were selected for I2 ≥ 40% and I2 < 40%, respectively. There is no difference between fixed-effect and random-effects approaches when the I2 test result is equal to zero.
The sensitivity analysis was performed by excluding one study at a time from the full meta-analysis (leave-one-out meta-analysis method) to determine whether each included study was particularly dominant or not. A study was assumed as dominant if excluding it would change the significance of the pooled RR results.
3. Results
3.1. Searching databases and study selection process
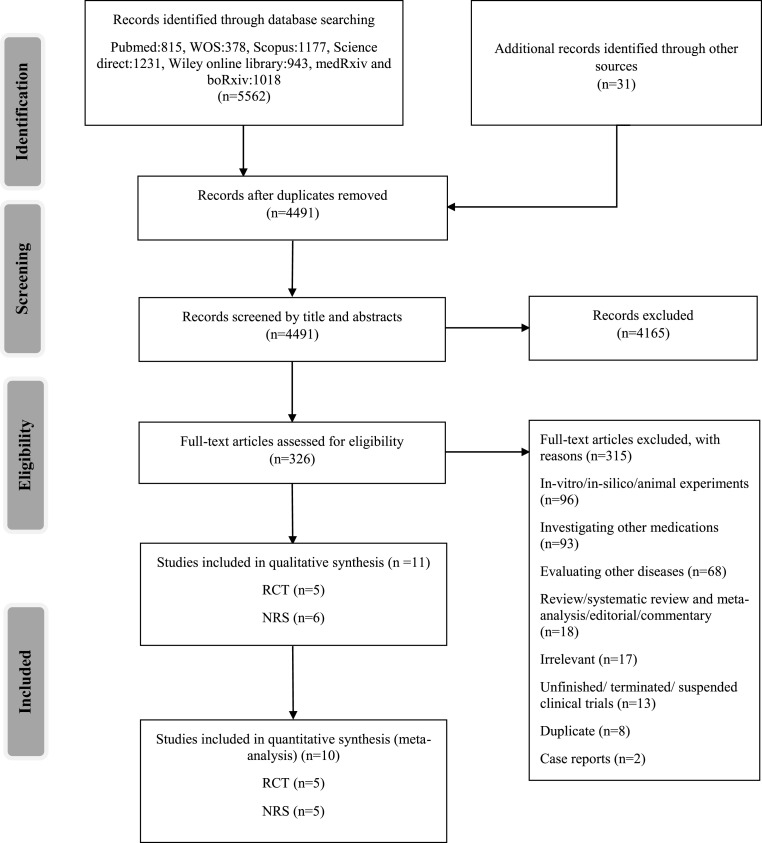
A total of 5593 studies were obtained from searching the main databases and additional resources. After duplicate removing and screening the records by their titles and abstracts, 326 studies were entered into eligibility assessment. Then, 315 records were excluded with reasons, including studies that were in-vitro/in-silico/animal experiments (n = 96), investigating other medications (n = 93), evaluating other diseases (n = 68), review/systematic review and meta-analysis/editorial/commentary (n = 18), irrelevant (n = 17), unfinished/terminated/suspended clinical trials (n = 13), duplicates (n = 8), or case reports (n = 2). Finally, ten studies, including five RCTs (Beigel et al., 2020b; Goldman et al., 2020; Pan et al., 2020; Spinner et al., 2020; Wang et al., 2020) and five NRSIs (Antinori et al., 2020; Fried et al., 2020; Grein et al., 2020; Olender et al., 2020; Pasquini et al., 2020), were entered into the meta-analysis (Fig. 1 ).
Fig. 1.
Study selection flow diagram. Preferred reporting items for systematic reviews and meta-analyses (PRISMA).
3.2. Risk of bias assessment results
The risk of bias assessment was performed using RoB 2.0 and ROBIN-I tools for the RCTs and NRSIs, respectively. The robvis tool was utilized for creating traffic light figures of the domain-level evaluations for each study. The results are shown in Fig. S1 and Fig. S2. The publication bias tests were not operated due to their low power of estimation in meta-analyses, including ten or fewer studies and the confounding issues regarding the NRSIs (Dalton et al., 2016; Page et al., 2020).
3.3. Data extraction and study characteristics
In total, there were 4217 and 2116 participants involved in the remdesivir groups of the RCTs and NRSIs, respectively. The total number of participants in the no-remdesivir groups were 3507 in the RCTs and 5076 in the NRSIs.
The treatment schedule of remdesivir in the meta-analyzed studies was a 200 mg intravenous (IV) loading dose on day one, followed by an IV maintenance dose of 100 mg/day for the subsequent four to nine days. The detailed characteristics and outputs of the included studies in our analysis are presented in Table 1, Table 2 , respectively.
Table 1.
Characteristics of the studies included in the qualitative analysis.
| Study (country) | Study design | Sample size | Age in years | Follow-up time | Additional therapy | Intervention: No. of participantsa (Percentage of severe cases)b treatment length | No-remdesivir: Treatment (Percentage of severe cases) |
|---|---|---|---|---|---|---|---|
| Wang et al. China | RCT | 233 | Range: 53-73 | 28 days | Antibiotics, corticosteroids, IFN alfa-2b, vasopressors | 155 (100) 10 days, five patients received treatment for less than five days. | Placebo provided by Gilead Sciences, US, (100) two patients received the placebo for less than five days |
| Goldman et al. (SIMPLE), Multi-country | RCT (comparison of two doses of remdesivir) | 397 | IQR: 50-71 | 14 days | Supportive therapy defined by the investigator. Details were not mentioned. | 197 (100) 10 days, 44% completed the course | None |
| 200 (100) 5 days, 86% completed the course | |||||||
| Beigel et al. (ACTT-1), Multi-country |
RCT | 1048 | Mean: 58.9 | 29 days | Defined by the written hospital policy or guidelinec | 531 (88.4)d 10 days, 39.1% completed the course | Placebo (89.1)d 43.7% completed the course |
| Spinner et al.; Multi-country | RCT | 584 | IQR: 46-66 | 28 days | Corticosteroids, lopinavir/ritonavir, hydroxychloroquine/chloroquine, tocilizumab, azithromycin | 193 (0) 10 days, 38% completed the course | Standard of care (0) |
| 191 (0) 5 days, 76% completed the course | |||||||
| Pan et al. (SOLIDARITY), Multi-country | RCT | 5451 | ˂50: 35% 50-69: 47% ≥70: 18% |
28 days | Corticosteroids, convalescent plasma therapy, Anti-IL-6 drug, IFN, antiviralse | 2743 (9.3)f 10 days, 95.8% took the medicine midway through its scheduled duration | Local standard of care (8.6)f 1.6% took the medicine midway through its scheduled duration |
| Grein et al.; Multi-country | NRSI | 53 | Range: 23-82 | 28 days | Not mentioned (may have been used) | 53 (100) 10 days, 75.5% completed the course | None |
| Antinori et al. Italy | NRSI | 35 | IQR: 49.25–75 | 28 days | Hydroxychloroquine, thirty-one patients were receiving lopinavir/ritonavir but discontinued upon the enrolment | 35 (100) 10 days, 74.3% completed the course | None |
| Olender et al.; Multi-country | NRSI | 1130 | ˂40: 10.7% 40-64: 50% ≥65: 39.3% |
14 days | Azithromycin, hydroxychloroquine group, HIV protease inhibitor, biologics, and ribavirin in both groups, experimental agents may have been used on the no-remdesivir group.g | 312 (100) 5 or 10 days (results of two groups were combined) | Standard of care (100) |
| Pasquini et al. Italy | NRSI | 51 | IQR: 59–75.5 | Median: 52 days (IQR: 46–57) | Lopinavir/ritonavir (discontinued after day one of remdesivir), tocilizumab, hydroxychloroquine | 25 (100), 10 days | Hydroxychloroquine, lopinavir/ritonavir, tocilizumab (100) |
| Fried et al. United States | NRSI | 4280 | 18-40: 9.4% 41-60: 33.9% ˃60: 56.7% |
28 days | Not mentioned | 48 (unknown)h 1–10 days, 33.3% received remdesivir for less than five days | Hydroxychloroquine (unknown)h |
| Anderson et al. United States | NRSI | 1643 | Median (IQR): 67 (56–78) | Not mentioned clearlyi | Not mentioned | 1643 (100), not defined | None |
RCT, randomized controlled trial; NRSI, non-randomized study of intervention; IQR, interquartile range; IFN, Interferon; Anti-IL-6, anti-interleukin-6; HIV, human immunodeficiency virus.
Patients with oxygen saturation levels of 94% or less were defined as severe cases.
We have used the “as-treated/safety population” sample sizes instead of the “intention-to-treat population".
According to the written hospital policies and guidelines, antibiotics, vasopressors, corticosteroids, other anti-inflammatory medications, monoclonal antibodies targeting cytokines, other biologic therapies, hydroxychloroquine, other putative SARS-CoV-2 medications, and other antiviral therapies were administered as additional therapy.
The percentage of severe cases in each group was not reported in this article, and the reported numbers were obtained from the preliminary report of the ACTT-1 study (Beigel et al., 2020a).
Lopinavir and interferon beta-1 were the trial antiviral and interferon agents, respectively. The non-trial interferons and antivirals were used as additional therapy.
Patients who were ventilated at the time of randomization were considered as severe in this study. Information about the oxygen saturation level and the type of ventilation at the time of randomization was not available.
Hydroxychloroquine group included aminoquinolines, chloroquine, hydroxychloroquine, and hydroxychloroquine sulfate. The administered biologic medications were interferons, investigational biologics, plasma, sarilumab, siltuximab, and tocilizumab.
This study did not define the patients' severity of disease at the time of admission.
The data abstraction method of this study is based on another publication; according to that publication, the presumed median follow-up time was 22.5 days (Geleris et al., 2020).
Table 2.
Outcomes of the studies included in the qualitative analysis.
| Study | Outcomes | Intervention | No-remdesivir | |
|---|---|---|---|---|
| Goldman et al. | 5-day | 10-day | – | |
| Death events on day 14, n (%) | 16 (8) | 21 (11) | ||
| Alive discharges on day 14: n (%) | 120 (60) | 103 (52) | ||
| Serious ADRsa: n (%) | 42 (21) | 68 (35) | ||
| Clinical improvementa on day 14: n (%) | 129 (64) | 107 (54) | ||
| Low flow oxygen support on day 1: n (%) | 113 (57) | 107 (54) | ||
| High flow oxygen or NIMV on day 1: n (%) | 49 (25) | 60 (30) | ||
| IMV or ECMO on day 1: n (%) | 4 (2) | 9 (5) | ||
| Low flow oxygen support on day 14: n (%) | 19 (10) | 14 (7) | ||
| High flow oxygen or NIMV on day 14: n (%) | 9 (5) | 10 (5) | ||
| IMV or ECMO on day 14: n (%) | 16 (8) | 33 (17) | ||
| Modified recoverya on day 14: n (%) | 140 (70) | 116 (59) | ||
| Median time to modified recovery: days | 9 | 10 | ||
| Hazard ratio (95% CI)b | 0.82 (0.64–1.04) | |||
| Median time to clinical improvement: days | 10 | 11 | ||
| Hazard ratio (95% CI)b | 0.79 (0.61–1.01) | |||
| Wang et al. | Clinical improvement on day 14: n (%) | 42 (27) | 18 (23) | |
| Clinical improvement on day 28: n (%) | 103 (66) | 45 (58) | ||
| Low flow oxygen support on day 1: n (%) | 129 (83) | 65 (83) | ||
| High flow oxygen or NIMV on day 1: n (%) | 28 (18) | 9 (12) | ||
| IMV or ECMO on day 1: n (%) | 0 (0) | 1 (1) | ||
| Low flow oxygen support on day 14: n (%) | 61 (39) | 28 (36) | ||
| High flow oxygen or NIMV on day 14: n (%) | 13 (8) | 8 (10) | ||
| IMV or ECMO on day 14: n (%) | 4 (3) | 7 (9) | ||
| Low flow oxygen support on day 28: n (%) | 18 (12) | 13 (17) | ||
| High flow oxygen or NIMV on day 28: n (%) | 2 (1) | 2 (3) | ||
| IMV or ECMO on day 28: n (%) | 2 (1) | 3 (4) | ||
| Alive discharges on day 14: n (%) | 39 (25) | 18 (23) | ||
| Alive discharges on day 28: n (%) | 92 (59) | 45 (58) | ||
| Serious ADRs: n (%) | 28 (18) | 20 (26) | ||
| Death events on day 14: n (%) | 15 (10) | 7 (9) | ||
| Death events on day 28: n (%) | 22 (14) | 10 (13) | ||
| Negative viral load on day 28: proportion (%) | 93/131 (71) | 49/65 (75) | ||
| Median time to clinical improvement: days (IQR) | 21 (13–28) | 23 (15–28) | ||
| Hazard ratio (95% CI)b | 1.23 (0.87–1.75) | |||
| Beigel et al. | Death events on day 14: n (%) | 35 (7) | 61 (12) | |
| Death events on day 28: n (%) | 59 (11) | 77 (15) | ||
| Serious ADRs: n (%) | 131 (25) | 163 (32) | ||
| Low flow oxygen support on day 1: n (%) | 232 (44) | 203 (39) | ||
| High flow oxygen or NIMV on day 1: n (%) | 95 (18) | 98 (19) | ||
| IMV or ECMO on day 1: n (%) | 131 (25) | 154 (30) | ||
| Low flow oxygen support on day 15c: n (%) | 53 (10) | 57 (11) | ||
| High flow oxygen or NIMV on day 15: n (%) | 23 (4) | 22 (4) | ||
| IMV or ECMO on day 15: n (%) | 83 (16) | 115 (22) | ||
| Low flow oxygen support on day 29c: n (%) | 23 (4) | 22 (4) | ||
| High flow oxygen or NIMV on day 29: n (%) | 3 (1) | 10 (2) | ||
| IMV or ECMO on day 29: n (%) | 30 (6) | 45 (9) | ||
| Recovery on day 14: n (%)d | 334 (63) | 273 (53) | ||
| Recovery on day 28: n (%) | 399 (75) | 352 (68) | ||
| Median time to recovery: days (IQR) | 10 (9–11) | 15 (13–18) | ||
| Rate ratio (95% CI)b | 1.29 (1.12–1.49) | |||
| Median time to clinical improvement: days (IQR) | 11 (10–13) | 14 (13–15) | ||
| Rate ratio (95% CI)b | 1.29 (1.12–1.48) | |||
| Spinner et al. | 5-day | 10-day | ||
| Clinical improvement on day 14: n (%) | 146 (76) | 148 (77) | 135 (68) | |
| Clinical improvement on day 28: n (%) | 171 (90) | 174 (90) | 166 (83) | |
| Low flow oxygen support on day 1: n (%) | 29 (15) | 23 (12) | 36 (18) | |
| High flow oxygen or NIMV on day 1: n (%) | 2 (1) | 1 (1) | 2 (1) | |
| IMV or ECMO on day 1: n (%) | 0 (0) | 0 (0) | 0 (0) | |
| Low flow oxygen support on day 14: n (%) | 5 (3) | 4 (2) | 8 (4) | |
| High flow oxygen or NIMV on day 14: n (%) | 4 (2) | 0 (0) | 4 (2) | |
| IMV or ECMO on day 14: n (%) | 0 (0) | 1 (1) | 5 (3) | |
| Low flow oxygen support on day 28: n (%) | 4 (2) | 0 (0) | 5 (3) | |
| High flow oxygen or NIMV on day 28: n (%) | 1 (1) | 1 (1) | 0 (0) | |
| IMV or ECMO on day 28: n (%) | 0 (0) | 1 (1) | 4 (2) | |
| Death events on day 14: n (%) | 1 (1) | 2 (1) | 4 (2) | |
| Death events on day 28: n (%) | 2 (1) | 3 (2) | 4 (2) | |
| Serious ADRs: n (%) | 9 (5) | 10 (5) | 18 (9) | |
| Alive discharges on day 14: n (%) | 146 (76) | 146 (76) | 134 (67) | |
| Alive discharges on day 28: n (%) | 170 (89) | 174 (90) | 166 (83) | |
| Recovery on day 14: n (%) | 153 (80) | 153 (79) | 145 (73) | |
| Recovery on day 28: n (%) | 175 (92) | 178 (92) | 170 (85) | |
| Median time to modified recovery: days (IQR) | 6 (4–9) | 7 (4–12) | 7 (4–14) | |
| Hazard ratio vs no-remdesivir (95% CI)b | 1.19 (0.96–1.46) | 1.10 (0.90–1.36) | – | |
| Pan et al. | Death events on day 14: n (%) | 267 (10) | 262 (10) | |
| Death events on day 28: n (%) | 301 (11) | 303 (11) | ||
| Grein et al. | Alive discharges on day 14: n (%) | 11 (21) | – | |
| Alive discharges on day 28: n (%) | 25 (47) | |||
| Clinical improvement on day 14: % | 40 | |||
| Clinical improvement on day 28e: % | 74 | |||
| Low flow oxygen support on day 1: n (%) | 10 (19) | |||
| High flow oxygen or NIMV on day 1: n (%) | 7 (13) | |||
| IMV or ECMO on day 1: n (%) | 34 (64) | |||
| Low flow oxygen support on day 14: n (%) | 1 (2) | |||
| High flow oxygen or NIMV on day 14: n (%) | 6 (11) | |||
| IMV or ECMO on day 14: n (%) | 13 (25) | |||
| Low flow oxygen support on day 28: n (%) | 0 (0) | |||
| High flow oxygen or NIMV on day 28: n (%) | 0 (0) | |||
| IMV or ECMO on day 28: n (%) | 1 (2) | |||
| Death events on day 14: n (%) | 3 (6) | |||
| Death events on day 28: n (%) | 7 (13) | |||
| Serious ADRs: n (%) | 12 (23) | |||
| Antinori et al. | Clinical improvement on day 10f: n (%) | 10 (29) | – | |
| Clinical improvement on day 28: n (%) | 22 (63) | |||
| Low flow oxygen support on day 1: n (%) | 2 (6) | |||
| High flow oxygen or NIMV on day 1: n (%) | 16 (46) | |||
| IMV or ECMO on day 1: n (%) | 16 (46) | |||
| Low flow oxygen support on day 10: n (%) | 2 (6) | |||
| High flow oxygen or NIMV on day 10: n (%) | 13 (37) | |||
| IMV or ECMO on day 10: n (%) | 10 (29) | |||
| Low flow oxygen support on day 28: n (%) | 1 (3) | |||
| High flow oxygen or NIMV on day 28: n (%) | 19 (54) | |||
| IMV or ECMO on day 28: n (%) | 3 (9) | |||
| Alive discharges on day 10: n (%) | 1 (3) | |||
| Alive discharges on day 28: n (%) | 20 (57) | |||
| Serious ADRs: n (%) | 13 (37) | |||
| Death events on day 10: n (%) | 5 (14) | |||
| Death events on day 28: n (%) | 9 (26) | |||
| Negative viral load on day 12f: proportion (%) | 21/21 (100) | |||
| Olender et al. | Recovery on day 14: n (%) | 232 (74) | 483 (59) | |
| Death events on day 14: n (%) | 24 (8) | 102 (13) | ||
| Pasquini et al. | Death eventsg: n (%) | 14 (56) | 24 (92) | |
| Fried et al. | Alive discharges on day 28: n (%) | 44 (92) | 3057 (72) | |
| Death events on day 28: n (%) | 4 (8) | 1048 (25) | ||
| Anderson et al. | Death events:h | 422 (26) | – | |
| Alive discharges: | 813 (49) | |||
ADR, adverse drug reaction; IQR, interquartile range; CI, confidence interval; NIMV, non-invasive mechanical ventilation; IMV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation.
The definitions of the evaluated clinical outputs are slightly different according to the associated studies. All of these definitions are presented in Table S3 for more information (WHO, 2020c).
Hazard and rate ratios greater than one indicate a benefit with remdesivir.
The 14-day and 28-day results were not reported in this study; therefore, the 15-day and 29-day results were used as the closest alternatives, respectively.
This data was obtained from the preliminary report of the Beigel et al. study (Beigel et al., 2020a).
This data has been revised and changed from 84% mentioned in the original article to 74% (Bonovas and Piovani, 2020).
This study did not report the 14-day results; thus, the 10-day and 12-day results were used as the closest alternatives.
The median follow-up time was 52 days (IQR: 46–57) in this study. The death event occurred in a median of 17 (IQR: 13–20) and 10 (IQR: 8–13) days after ICU admission in the remdesivir and no-remdesivir groups, respectively.
The follow-up times were not clear and uniform for all of the participants in this study.
3.4. Statistical data analysis
The follow-up durations were not the same in the meta-analyzed studies; therefore, we have used the 14-day and 28-day results for outputs with available data in these follow-up times.
The Anderson et al. study was excluded from the calculations due to the lack of information (follow-up time, dose, and duration of remdesivir therapy) in the main article (Anderson et al., 2020).
3.4.1. The incidence rate differences
3.4.1.1. RCT studies
There were significant differences between the remdesivir and no-remdesivir groups in pooled event rates of the 14-day alive discharge (P = 0.01), 14-day clinical improvement (P = 0.003), 28-day clinical improvement (P = 0.01), 14-day death (P = 0.01), 28-day death (0.02), 14-day recovery (P = 0.01), 28-day recovery (P˂0.0001), and serious ADR (P = 0.03). The detailed results of the incidence rate difference (IRD) in the RCT studies are shown in Table 3 . Additionally, the forest plots for pooling event rates of the remdesivir and no-remdesivir groups in the RCT studies are shown in Fig. S3 and Fig. S5, respectively.
Table 3.
The incidence rate difference in the RCT studies.
| Output | Event rate |
No. of studies |
No. of participants |
Difference (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Remdesivir | No-remdesivir | Remdesivir | No-remdesivir | Remdesivir | No-remdesivir | |||
| Alive discharge (14) | 52.7 | 44.1 | 3 | 2 | 936 | 278 | 8.60% (1.93–15.12%) | 0.01 |
| Alive discharge (28) | 78.2 | 72.3 | 2 | 2 | 539 | 278 | 5.90 (−0.23 to 12.31%) | 0.06 |
| Clinical improvement (14) | 55.0 | 44.7 | 3 | 2 | 936 | 278 | 10.30% (3.61–16.85%) | 0.003 |
| Clinical improvement (28) | 80.7 | 72.3 | 2 | 2 | 539 | 278 | 8.40% (2.33–14.75%) | 0.01 |
| Death (14) | 7.2 | 8.8 | 5 | 4 | 4210 | 3503 | 1.60% (0.39–2.83%) | 0.01 |
| Death (28) | 8.7 | 10.3 | 4 | 4 | 3813 | 3503 | 1.60% (0.26–2.95%) | 0.02 |
| Negative viral load | 71.0 | 75.0 | 1 | 1 | 131 | 65 | 4.0% (−9.69 to 16.18%) | 0.56 |
| Recovery (14) | 69.5 | 63.4 | 3 | 2 | 1312 | 717 | 6.10% (1.82–10.43%) | 0.01 |
| Recovery (28) | 85.3 | 77.4 | 2 | 2 | 915 | 717 | 7.90% (4.1–11.76%) | ˂0.0001 |
| Serious ADR | 16.8 | 20.5 | 4 | 3 | 1467 | 795 | 3.70% (0.37–7.17%) | 0.03 |
CI, confidence interval; (14), 14-day follow-up; (28), 28-day follow-up; ADR, adverse drug reaction.
3.5. NRSIs
There were significant differences between the remdesivir and no-remdesivir groups in pooled event rates of the 14-day death (P = 0.02), 28-day death (P˂0.0001), and 14-day recovery (P˂0.0001). The detailed results of the IRD in the NRSIs are shown in Table 4 . Additionally, the forest plots for pooling event rates of the remdesivir and no-remdesivir groups in the NRSIs are shown in Fig. S4 and Fig. S6, respectively.
Table 4.
The incidence rate difference in the NRSIs.
| Output | Event rate |
No. of studies |
No. of participants |
Difference (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Remdesivir | No-remdesivir | Remdesivir | No-remdesivir | Remdesivir | No-remdesivir | |||
| Alive discharge (28) | 68.8 | 72.0 | 3 | 1 | 136 | 4232 | 3.20% (−4.11 to 11.52%) | 0.41 |
| Death (14) | 8.4 | 13.0 | 3 | 1 | 400 | 818 | 4.60% (0.81–8.01%) | 0.02 |
| Death (28) | 22.1 | 64.5 | 4 | 2 | 161 | 4258 | 42.40% (35.23–48.29%) | ˂0.0001 |
| Recovery (14) | 74.0 | 59.0 | 1 | 1 | 312 | 818 | 15.0% (8.88–20.69%) | ˂0.0001 |
NRSI, non-randomized study of intervention; CI, confidence interval; (14), 14-day follow-up; (28), 28-day follow-up.
3.5.1. Estimating pooled median and IQR values
3.5.1.1. Time to recovery
Two no-remdesivir group enrolling studies included the time to recovery as a clinical output. Although the results of the remdesivir groups were numerically favorable compared to the no-remdesivir groups (pooled median difference = 2.56, 95%CI, −2.34 to 7.46), the recovery time difference between the remdesivir and no-remdesivir groups was not statistically significant (P = 0.31). The random-effects approach was used due to the significant heterogeneity (tau2 = 12.15, Q-value = 36.03, df = 1, P˂0.0001, I2 = 97.22%).
3.6. Time to clinical improvement
Two out of ten meta-analyzed studies enrolling no-remdesivir groups included the time to clinical improvement as a clinical output. The meta-analysis of these two studies showed that the remdesivir group had a significantly shorter time to clinical improvement than the no-remdesivir group with the pooled median difference of 2.99 (95%CI, 2.71–3.28, P˂0.0001). The fixed-effect model was used for the analysis (tau2 = 0, Q-value = 0.32, df = 1, P = 0.57, I2 = 0%).
3.6.1. The risk ratio meta-analysis
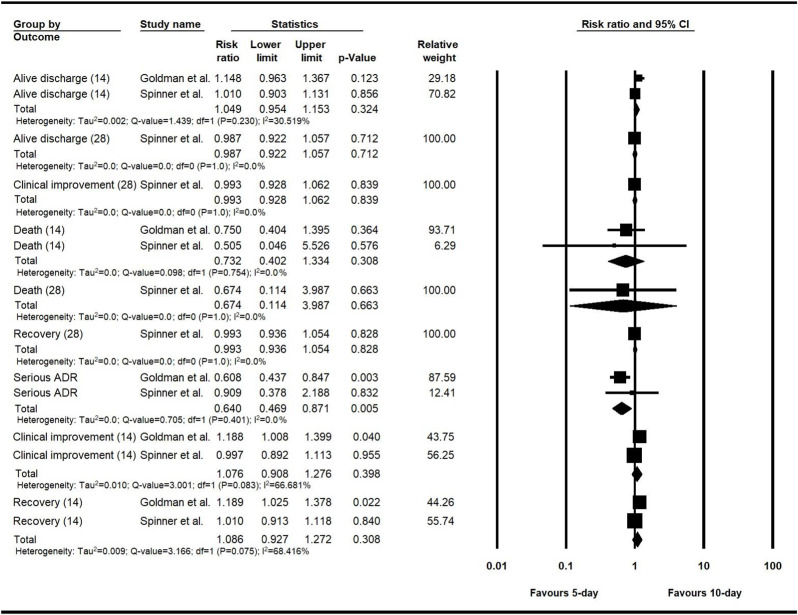
3.6.1.1. The clinical output comparison of the 5-day and 10-day remdesivir courses
The remdesivir arms of two RCT studies were divided into two groups to receive the treatment for 5-day and 10-day courses, and the clinical outputs of each group were reported separately (Goldman et al., 2020; Spinner et al., 2020). The clinical output comparison of the 5-day and 10-day groups was conducted using pooled RR. The only significant difference between the two groups was found in the serious ADRs output, which had a RR of 0.64 (n = 981, 95% CI, 0.47–0.87, P = 0.01).
The fixed-effect model was used for all the events except for the clinical improvement and recovery on the 14-day follow-up. The detailed results are shown in Fig. 2 .
Fig. 2.
The risk ratio meta-analysis for evaluating the differences between the clinical outputs of the 5-day and 10-day courses of remdesivir.
As mentioned, two RCT studies reported the clinical outputs of the 5-day and 10-day remdesivir courses separately (Goldman et al., 2020; Spinner et al., 2020). The pooled RR meta-analysis showed that the difference between these two groups was not statistically significant in the most evaluated clinical outputs. Besides, the 10-day course of remdesivir was not completed in a considerable number of patients, and the results of the 5-day and 10-day remdesivir courses were not separately reported in all studies (Table 1). Therefore, we have combined the results of the 5-day and 10-day remdesivir courses to operate the meta-analysis and generate the forest plots in the two following parts.
3.6.1.2. Generating pooled RR for overall outputs in the RCTs and NRSIs
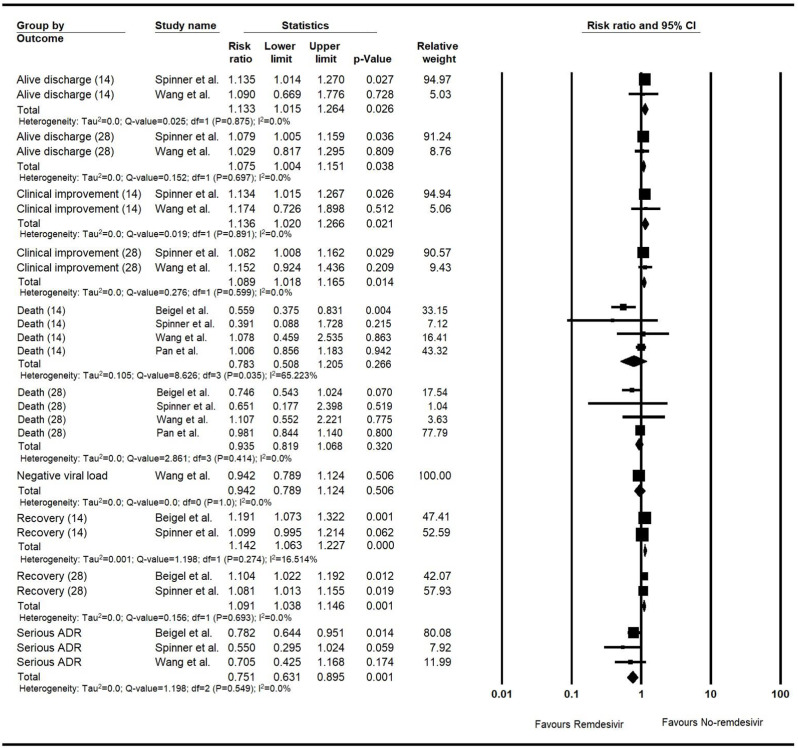
3.6.1.2.1. RCT studies
The RRs for the alive discharge output on the 14-day and 28-day follow-ups were 1.13 (n = 817, 95% CI, 1.02–1.26, P = 0.03) and 1.08 (n = 817, 95%CI, 1.0–1.15, P = 0.04), respectively. The RRs for the clinical improvement on the follow-ups of 14 and 28 days were 1.14 (n = 817, 95%CI, 1.02–1.27, P = 0.02) and 1.09 (n = 817, 95%CI, 1.02–1.17, P = 0.01), respectively. The RR values for the 14-day and 28-day recovery were 1.14 (n = 1632, 95%CI, 1.06–1.23, P˂0.001) and 1.09 (n = 1632, 95%CI, 1.04–1.15, P = 0.001), respectively.
The random-effect approach was used for all the events except for the 14-day recovery. The detailed meta-analyses are shown in the forest plot for risk ratio meta-analysis of the clinical outputs of the RCT studies (Fig. 3 ).
Fig. 3.
The forest plot for the risk ratio meta-analysis of the clinical outputs of the RCT studies.
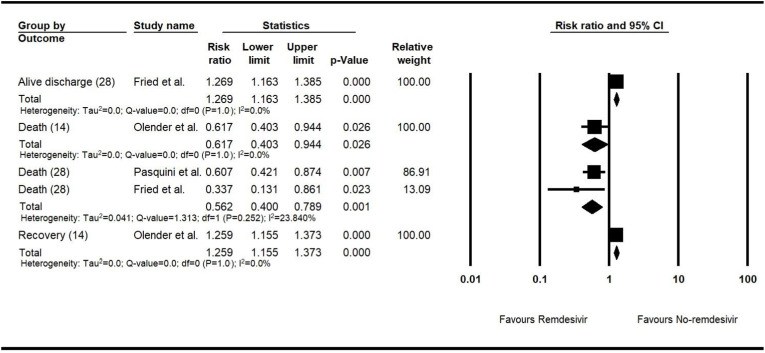
3.6.1.2.2. NRSIs
The values of RRs for the recovery and death events on the follow-up of 14 days were 1.26 (n = 1130, 95% CI, 1.16–1.37, P < 0.001) and 0.62 (n = 1130, 95% CI, 0.40–0.94, P = 0.03), respectively. The RRs of the alive discharge and death events on the 28-day follow-up had values of 1.27 (n = 4280, 95% CI, 1.16–1.39, P < 0.001) and 0.56 (n = 4331, 95% CI, 0.40–0.79, P = 0.001), respectively.
The fixed-effect approach was used for all the evaluated events. The detailed meta-analyses are shown in the forest plot for the risk ratio meta-analysis of the clinical outputs of the NRSIs (Fig. 4 ).
Fig. 4.
The forest plot for the risk ratio meta-analysis of the clinical outputs of the NRSIs.
3.6.1.3. Improvement assessment of three levels of respiratory support in patients of both remdesivir and no-remdesivir groups
3.6.1.3.1. RCT studies
The Pan et al. study did not report the respiratory support data over time in the categorical/ordinal scale and, therefore, was excluded from this part of the meta-analysis. The results of both groups were fairly comparable. The meta-analysis of the remdesivir groups showed a significant improvement over time in all evaluated categories except for the invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO) in the baseline compared with day 14 and the low-flow oxygen support in the baseline versus day 28 analysis. The random-effects approach was used for all the evaluated events.
The no-remdesivir group meta-analysis showed similar results to the remdesivir group except for the low-flow oxygen support in the baseline compared with day 14 RR, which had a statistically insignificant value. The random-effects approach was used for all the evaluated outputs except for the non-invasive mechanical ventilation (NIMV) or high flow oxygenation in the baseline versus day 28 analysis. The detailed results and corresponding forest plots are available in Fig. S7 and Fig. S8 for the remdesivir and no-remdesivir groups, respectively.
3.7. NRSIs
Two studies reported the respiratory support over time data in the categorical/ordinal scale; however, none of them enrolled a no-remdesivir group (Antinori et al., 2020; Grein et al., 2020). The meta-analysis of the remdesivir groups showed a significant improvement over time in three out of nine evaluated outputs, including the IMV or ECMO in the baseline compared with days 14 and 28 and the low-flow oxygen support in day 14 versus day 28. The random-effects approach was operated for evaluating all the outputs except for the IMV or ECMO in the baseline versus day 14 and the low-flow oxygen support in the baseline compared with days 14 and 28 analyses. The detailed results and corresponding forest plots are presented in Fig. S9.
3.8. The 5-day and 10-day remdesivir courses comparison
Remdesivir showed significant beneficial effects on all three evaluated levels of respiratory support through days one to 14 in both 5-day and 10-day regimens. The fixed-effect approach was used for all the evaluated events. (Fig. S10).
3.8.1. The sensitivity analysis results
The sensitivity analysis was performed via a leave-one-out meta-analysis to evaluate the effect of each included study. The RR meta-analysis for difference evaluation of the 5-day and 10-day remdesivir courses included two studies (Goldman et al., 2020; Spinner et al., 2020). As shown in Fig. 2, the results of this analysis would not remain robust if we excluded the Spinner et al. study from the 14-day clinical improvement and recovery and the Goldman et al. study from the serious ADR output.
The significance of the results maintained stable except for the alive discharge and clinical improvement on the follow-ups of 14 and 28 days after excluding the Spinner et al. study and the 14-day death after excluding the Pan et al. study from the meta-analyses of the RCTs (Table S4). The RR meta-analysis of the NRSIs did not include sufficient studies to run the sensitivity analysis. Only the 28-day death pooled RR result was obtained from two studies that remained stable during the sensitivity analysis (Fig. 4).
In the improvement assessment of the respiratory support levels in the NRSIs, the Grein et al. study had a significant impact on the IMV or ECMO requirement in day-one versus day-14 comparison results. Conversely, the Antinori et al. study played an influential part in defining the final values of the low flow oxygen support in the baseline versus day-14 and day-28 comparisons (Fig. S9).
In the RCTs, the Beigel et al. study showed a noticeably dominant impact on the several evaluated outputs in improvement assessment of the respiratory support levels in both remdesivir and no-remdesivir groups. The detailed results of this sensitivity analysis are shown in Tables S5 and S6 for the remdesivir and no-remdesivir groups, respectively.
The pooled results of the improvement assessment of the respiratory support levels in both 5-day and 10-day remdesivir regimens, which were reported individually in Fig. S10, would not remain stable if we excluded the Goldman et al. study from the evaluation of the IMV or ECMO requirement and NIMV or high flow oxygenation through the baseline to day 14 of the follow-up period.
Two eligible studies were included in each part of the pooled median and IQR value estimation meta-analysis, and the hazard/rate ratio values from the original reports were used for calculating the corresponding P-values. The sensitivity analysis showed that the Spinner et al. and Beigel et al. studies were particularly influential in the time to recovery and time to clinical improvement comparisons, respectively (Table S7).
3.9. The ongoing clinical trials of remdesivir administration in COVID-19 patients
We have found a total of 19 ongoing studies with available data (Table 5 ). The study sample sizes range from 30 to 4891, with a cumulative sample size of 14,888 patients. Furthermore, the clinical severity of COVID-19 ranges from mild and moderate to severe and critical. In one randomized, double-blind placebo-controlled trial, remdesivir is administered in the outpatient setting with the loading dose of 200 mg, followed by the maintenance dose of 100 mg for two following days. In most trials, the administration route is IV; however, in two trials, patients are given inhaled remdesivir. According to the disease severity and study protocols, the dose of remdesivir in these trials is 200 mg on the first day, followed by 100 mg for two to nine consecutive days.
Table 5.
Summary of the ongoing clinical trials investigating the therapeutic effects of remdesivir for COVID-19 treatment.
| ID | Status | Setting | Country | Population (N) | Intervention group(s) | Comparison/control group(s) | |
|---|---|---|---|---|---|---|---|
| NCT04257656 | Terminated | Multi-center, randomized, double-blind, placebo-controlled trial | China | Hospitalized severe COVID-19 patients (237) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 9 days | Placebo; LD, 200 mg on day 1, MD, 100 mg for 9 days | |
| NCT04560231 | Recruiting | Clinical trial | Pakistan | Moderate COVID-19 patients (30) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4–9 days | Not mentioned | |
| NCT04596839 | Recruiting | Open-label, multi-center, randomized controlled trial | Bangladesh | Severe COVID-19 patients (60) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4 days | Standard of care | |
| NCT04570982 | Recruiting | Prospective observational study | Nepal | Hospitalized COVID-19 cases (200) | Remdesivir for moderate to severe COVID-19 Convalescent plasma therapy for severe to life-threatening COVID-19 |
Not mentioned | |
| NCT04365725 | Recruiting | Multi-center, retrospective | France | Severe Covid-19 patients (200) |
Remdesivir | Not mentioned | |
| NCT04345419 | Recruiting | Randomized trial | Egypt | COVID 19 patients (120) | Remdesivir, chloroquine | Not mentioned | |
| NCT04610541 | Recruiting | Multi-center, open-label, interventional safety study | Hungary | Moderate and Severe Covid-19 cases (2000) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg on day 2 | Not mentioned | |
| NCT04252664 | Suspended | Multi-center, randomized, double-blind, placebo-controlled | China | Mild to Moderate COVID-19 cases (308) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 9 days | Placebo; LD, 200 mg on day 1, MD, 100 mg for 9 days | |
| NCT04582266 | Not yet recruiting | Observational (Pharmacokinetics and Safety study) | United States | Pregnant and non-pregnant women with COVID-19 (40) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for up to 9 days | Not mentioned | |
| NCT04410354 | Active, not recruiting | Randomized, double-blind, placebo-controlled | United States | Advanced COVID-19 cases (80) | Merimepodib 1200 mg for 10 days Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4–9 days |
Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4–9 days | |
| NCT04292899 | Completed | Open-label, randomized clinical trial | Multi-country | Severe COVID-19 cases (4891) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4 or 9 days | Standard of care | |
| NCT04480333 | Recruiting | Randomized, placebo-controlled, crossover assignment clinical trial | United States | Healthy Volunteers (45) | Remdesivir 0.10 mg/kg; inhaled nanoparticles for 5 days | Placebo; inhaled nanoparticles for 5 days | |
| NCT04501952 | Recruiting | Randomized, double-blind placebo-controlled trial | United States and Denmark | COVID-19 outpatients (1230) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 2 days | Placebo; LD, 200 mg on day 1, MD, 100 mg for 2 days | |
| NCT04539262 | Recruiting | Randomized, double-blind placebo-controlled trial | United States | Early-stage COVID-19 cases (282) | Remdesivir 31 or 62 mg; inhaled for 3–5 days | Placebo; inhaled for 3–5 days | |
| NCT04292730 | Completed | Open-label, randomized clinical trial | Multi-country | Moderate COVID-19 cases (1113) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for 4 or 9 days | Standard of care | |
| NCT04409262 | Recruiting | Randomized, double-blind, multi-center | Multi-country | Patients with Severe COVID-19 Pneumonia (500) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for up to 9 days plus tocilizumab | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for up to 9 days plus placebo | |
| NCT04431453 | Recruiting | Single-arm, open-label clinical trial | Multi-country | Children aged 0–17 years with COVID-19 (52) | Remdesivir; LD, 200 mg on day 1, MD, 100 mg for up to 9 days Remdesivir; LD, 5 mg/kg on day 1, MD, 2.5 mg/kg for up to 9 days |
Not mentioned | |
| NCT04330690 | Recruiting | Open-label, randomized clinical trial | Canada | Hospitalized COVID-19 cases (2900) | Remdesivir (LD, 200 mg on Day 1, MD, 100 mg for 9 Days), lopinavir/ritonavir, or hydroxychloroquine plus standard of care | Standard of care | |
| NCT04492501 | Completed | Factorial assignment clinical trial | Pakistan | Moderate, severe, and critical COVID-19 cases (600) | TPE in combination with remdesivir (LD, 200 mg on day 1, MD, 100 mg for 9 days), convalescent plasma therapy, tocilizumab, or mesenchymal stem cell therapy plus standard of care | Standard of care | |
LD, loading dose; MD, maintenance dose; TPE, Therapeutic plasma exchange.
4. Discussion
To the best of our knowledge, the present study is the most comprehensive systematic review and meta-analysis investigating the efficacy and safety of remdesivir in COVID-19 patients to date.
Remdesivir is a novel investigational antiviral nucleotide prodrug and currently has the FDA approval to treat hospitalized COVID-19 adult and pediatric patients with 12 years of age and older weighing at least 40 kg (The U.S. Food and Drug Administration, 2020b). However, on November 20th, 2020, due to the low certainty of evidence on beneficial effects of remdesivir on important patient outcomes, the WHO guideline development group recommended against remdesivir administration in hospitalized COVID-19 patients regardless of disease severity (Rochwerg et al., 2020b).
4.1. Potential molecular targets of remdesivir on SARS-CoV-2
There are at least eleven different strains of SARS-CoV-2 as a result of viral mutations. SARS-CoV-2 replicates inside the host cells by RNA dependent RNA polymerase (RdRp) of the virus, which is a highly conserved protein among different viral strains; thus, SARS-CoV-2 RdRp could be a potential antiviral target (Biswas and Majumder, 2020; Ferner and Aronson, 2020). Furthermore, main protease (Mpro), also known as chymotrypsin-like cysteine protease (3CLPro), which cleaves the central part of the polyproteins and releases proteins with replicative functions, plays a crucial role in coordinating the lifecycle of SARS-CoV-2 through its replication and transcription (Ziebuhr et al., 2000). Consequently, Mpro becomes another potential target for SARS-CoV-2 experimental medications.
Remdesivir has an inhibitory effect on viral RdRp and does its antiviral effects by interrupting the viral replication inside the host cell. The active metabolite of remdesivir (GS-441524) could form a good complex with SARS-CoV-2 NSP12 RdRp, terminate the RNA-chain, and stop the RNA replication. Additionally, both remdesivir and GS-441524 could bind to Mpro, which could add synergistic impacts when combined with its RdRp antagonism effects. Remdesivir binds to RdRp and Mpro through different binding mechanisms and has slightly stronger interactions with RdRp than with Mpro (Ferner and Aronson, 2020; Huynh et al., 2020; Nguyen et al., 2020; Zhang and Zhou, 2020).
4.2. Remdesivir safety and efficacy
The antiviral effects of remdesivir on SARS-CoV-2 could be detected by evaluating the patients' viral load profiles. Among the ten records included in our meta-analysis, the viral load testing was carried out only in two studies. One of these studies showed no significant differences between the remdesivir and no-remdesivir groups in the viral load reduction over the follow-up time (Wang et al., 2020), and the other had no comparison/control group (Antinori et al., 2020).
According to the RR meta-analysis of the RCT studies, the risk of experiencing serious ADRs in the remdesivir group was 25% lower than the no-remdesivir group. This finding is relatively in agreement with the previous systematic reviews and meta-analyses on this topic, except for the Sarfraz et al. study. Although, in the Sarfraz et al. review, the results were numerically favoring remdesivir but were not statistically significant (Alexander et al., 2020; Piscoya et al., 2020; Sarfraz et al., 2020; Shrestha et al., 2020; Zhu et al., 2020).
Additionally, the RCT studies showed that the 28-day recovery rate was enhanced by 9% in the remdesivir group compared to the no-remdesivir group, which was similar to the results of the only previous review evaluating this output (Shrestha et al., 2020).
The RR meta-analysis of the NRSIs showed that the risk of 28-day death was 44% lower in the remdesivir group relative to the no-remdesivir group. None of the previous reviews included the NRSIs in their meta-analysis. Although, the Olender et al. study was included in the Sarfraz et al. review as an RCT (Olender et al., 2020; Shrestha et al., 2020).
Comparison of the 5-day and 10-day remdesivir courses showed that the only significant difference between these two treatment regimens was in the serious ADRs rate, which was 36% higher in the 10-day regimen group. Although, this result did not remain robust through the sensitivity analysis. Only the Shrestha et al. study operated this comparison among the previous reviews, and their results are in agreement with ours; however, they did not perform the sensitivity analysis (Shrestha et al., 2020). Three out of six previous reviews conducted the sensitivity analysis to uncertainty quantification of their results (Alexander et al., 2020; Jiang et al., 2020; Sarfraz et al., 2020).
The improvement assessment of the respiratory support levels in the RCTs showed significant beneficial effects of remdesivir on the low flow oxygenation through the baseline to day 14 and the IMV or ECMO requirement through days 14–28 of the follow-up time. Whereas, the enhancement in the IMV or ECMO requirement through the baseline to day 28, low flow oxygenation through days 14–28, and NIMV or high flow oxygen requirement through the baseline to day 14 of the follow-up duration was significantly higher in the no-remdesivir group. Additionally, the remdesivir group showed a significant improvement on the low flow oxygen support through days 14–28 and IMV or ECMO requirement through the baseline to day 28 of the follow-up period in the NRSIs. These results remain robust through the sensitivity analysis. The utilized improvement assessment method for the respiratory support level in the current study was not comparable with the previous reviews. In the previous reviews, the results of the remdesivir and no-remdesivir groups were compared together to calculate the corresponding risk/odds ratio at each time point. We have analyzed each group's data in pre-defined follow-up periods individually to take the differences in the patients' baseline characteristics into account.
The current study is the first systematic review and meta-analysis, which includes the preliminary results of the WHO SOLIDARITY therapeutics trial and the final results of the NIAID trial (Beigel et al., 2020b; Pan et al., 2020).
The results and brief description of the previous systematic reviews and meta-analyses/network-analyses on this topic, their concurrence with the results of the current study, and the possible reasons for any conflicts are discussed in Table 6 .
Table 6.
The results and a brief description of the previous systematic reviews and meta-analysis/network-analysis.
| Review (The used model) | Meta-analyzed studies | Measured outcomesa | Results | Possible reasons for the conflicts | |
|---|---|---|---|---|---|
| Alexander et al. (The fixed-effect model was used for all the measured outcomes) | Wang et al., Beigel et al. (preliminary report) | Mortality | RR = 0.69 (95% CI, 0.49–0.99) | •Non-availability of the final report of the Beigel et al. study with the 28-day follow-up time data •Pooling the 14-day and 28-day data from the two included studies (non-uniform follow-up times) |
|
| Time to clinical improvement | Mean difference = −3·95 (95% CI, −4.05 to −3.86), P˂0.00001 | •Using the median-based approach with the proved preferable performance in the present study instead of the transformation-based approach (McGrath et al., 2019) | |||
| Serious ADRs | RR = 0.77 (95%CI, 0.63–0.94) | •Fairly concurrent | |||
| Jiang et al. (The random-effects approach was used for all the measured outcomes) | Wang et al., Beigel et al. (preliminary report), Goldman et al., Spinner et al. (preliminary report) | Clinical improvement | OR = 1.35 (95%CI, 1.09–1.67) | •Non-availability of the final reports of the Beigel et al. and Spinner et al. studies •Using non-uniform follow-up times for the pooled results |
|
| Clinical recovery | RR = 1.24 (95%CI, 1.07–1.43) | ||||
| 5-day vs. 10-day course; clinical improvement | OR = 1.33 (95%CI, 1.01–1.76) | •Non-availability of the final report of the Spinner et al. study | |||
| Piscoya et al. (The random-effects approach was used for all the measured outcomes) | Wang et al., Beigel et al. (preliminary report) | 14-day mortality | RR = 0.71 (95%CI, 0.39–1.28) | •Fairly concurrent | |
| Serious ADR | RR = 0.77 (95%CI, 0.63–0.94) | ||||
| Alive discharge | RR = 1.19 (95%CI, 1.05–1.34) | ||||
| Zhu et al. (Both random-effects and fixed-effect approaches were used for the analysis according to the P and I2 values) | Wang et al., Beigel et al. (preliminary report) | Alive discharge | RR = 1.19 (95%CI, 1.05–1.34) | •Fairly concurrent | |
| Serious ADR | RR = 0.77 (95%CI, 0.63–0.94) | ||||
| Mortality | RR = 0.64 (95%CI, 0.44–0.92) | •Non-availability of the final report of the Beigel et al. study with the 28-day follow-up time data •Non-uniform follow-up times |
|||
| Sarfraz et al. (The random-effects approach was used for all the measured outcomes) | Wang et al., Beigel et al. (preliminary report), Spinner et al. Olender et al. | 14-day mortality | RR = 0.61(95%CI, 0.45–0.82) | •Including Olender et al. study in the meta-analysis of the RCT studies •Non-availability of the final report of the Beigel et al. study with the 28-day follow-up time data •Non-uniform follow-up times |
|
| Serious ADR | RR = 0.75 (95%CI, 0.55–1.02) | ||||
| Shrestha et al. (Both random-effects and the fixed-effect approaches were used for the analysis) | Wang et al., Beigel et al. (preliminary report), Spinner et al. Goldman et al. | 14-day mortality | OR = 0.61 (95%CI, 0.41–0.91) | •Non-availability of the final report of the Beigel et al. study •Not including the Pan et al. study in this review •Different reporting of the number of the remdesivir group's 14-day mortality from the Spinner et al. study (2 in 193 patients in the Shrestha et al. review vs. 3 in 384 patients in the current review)b |
|
| 28-day alive discharge | OR = 1.35 (95%CI, 0.91–2.02) | •Different reporting of the number of the remdesivir group's alive discharges from the Spinner et al. study (174 in 193 patients in the Shrestha et al. review vs. 344 in 384 patients in the current review)b | |||
| 28-day mortality | OR = 1.02 (95%CI, 0.50–2.06) | •Fairly concurrent | |||
| 14-day clinical improvement | OR = 1.45 (95%CI, 1.00–2.08) | ||||
| 28-day clinical improvement | OR = 1.59 (95%CI, 1.06–2.39) | ||||
| 14-day recovery | OR = 1.48 (95%CI, 1.19–1.84) | ||||
| 28-day recovery | OR = 2.09 (95%CI, 1.09–4.03) | ||||
| 14-day alive discharge | OR = 1.41 (95%CI, 1.15–1.73) | ||||
| Serious ADR | OR = 0.69 (95%CI, 0.54–0.88) | ||||
| Time to clinical improvement | Mean difference = −2.51 (−4.16 to −0.85), P = 0.003 | ||||
| Time to recovery | Mean difference = −4.69 (−5.11 to −4.28), P˂0.00001 | •Using different models (fixed-effect approach in the Shrestha et al. review vs. random-effects model in the current study) | |||
| 5-day vs. 10-day course; 14-day results | |||||
| Mortality | OR = 1.41 (95%CI, 0.73–2.72) | •Fairly concurrent | |||
| Clinical improvement | OR = 0.79 (95%CI, 0.58–1.07) | ||||
| Recovery | OR = 0.75 (95%CI, 0.55–1.02) | ||||
| Serous ADR | OR = 1.77 (95%CI, 1.19–2.65) | ||||
| Alive discharge | OR = 2.11 (95%CI, 1.50–2.97) | •Different reporting of the number of alive discharges in both 10-day and 5-day remdesivir courses from the Goldman et al. study (68 and 16 in the Shrestha et al. review vs. 120 and 103 in the current review, respectively) | |||
RR, risk ratio (relative risk); CI, confidence interval; ADR, adverse drug reaction; OR, odds ratio.
We have only mentioned the mutual measured outcomes of these reviews.
We have used the combined number of 5-day and 10-day courses for the meta-analysis.
4.3. Concerns about the clinical use of remdesivir in COVID-19
There are some concerning issues about remdesivir. First, due to the pharmacokinetic and physicochemical features, it seems unlikely that remdesivir and its active metabolite could reach the therapeutic concentration in the human lung cells to inhibit SARS-CoV-2 in the current dosing and administration route. Second, based on the chemical structure of the prodrug, the active metabolite of remdesivir would be significantly accumulated in the liver, kidneys, and gastrointestinal (GI) tract. This issue precludes the administration of remdesivir in higher doses than 200 mg/day to achieve the therapeutic concentration in the lung cells due to the adverse effects related to the non-target organs and dose-related toxicities. Third, there are still no accepted contraindications to remdesivir except for the hypersensitivity to remdesivir or any component of the formulation. However, most studies (including our meta-analyzed records) recommended against the use of remdesivir in pregnancy, lactation, patients with alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels greater than five times the upper limit of normal (ULN), renally impaired patients with the estimated glomerular filtration rate (eGFR) less than 30 mL/min, or hemodialysis-requiring cases. Fourth, the effect of remdesivir in combination with other agents is not clear yet. Nevertheless, co-administration of hydroxychloroquine or chloroquine with remdesivir is not recommended due to the antagonistic effects of these agents on the intracellular metabolic activation and antiviral activity of remdesivir. Fifth, there are no certain optimal initiation time, dose, and duration for remdesivir yet. Sixth, there is too soon to approve the long-term post-marketing safety of remdesivir. Seventh, the only IV administration route of remdesivir limits its applicability to the inpatient setting. Furthermore, the blood hydrolytic enzymes cause premature serum hydrolysis of the prodrug. Finally, there are still challenges around mass production and pricing of remdesivir owing to the synthesis difficulties (Ferner and Aronson, 2020; Rochwerg et al., 2020a; The U.S. Food and Drug Administration, 2020c).
Our study did not show a significant difference between the 5-day and 10-day remdesivir courses. Additionally, the 5-day remdesivir course may provide similar benefits while causing fewer serious ADRs and lower costs than the 10-day regimen.
The FDA recently authorized experimenting with the investigational inhaled formulation of remdesivir on healthy volunteers, aiming to start study in COVID-19 patients by August 2020 (Gilead Sciences, 2020a, Gilead Sciences, 2020b). The pulmonary drug delivery solves the problems due to the IV formulation; besides, it could help reach the therapeutic concentration in the lung cells, lower the ADRs in the non-target organs, dose-related toxicities, and prodrug premature hydrolysis. However, the inhaled formulation not only can not address the challenges around the complicated synthesis of remdesivir but also could make the supply chain process even more challenging.
GS-441524 is an antiviral nucleoside, which is the main metabolite reaching the lung cells due to the premature serum hydrolysis of remdesivir. As a result of the GS-441524 bio-activation route, which relies on different enzymes and requires fewer steps than remdesivir, it would have a more homogeneous tissue distribution. Moreover, in vitro and in vivo studies evidence the notable safety profile for GS-441524; therefore, achieving the therapeutic concentration in the lung cells with high dose GS-441524 administration could be applied without being concerned about any dose-related toxicities and serious adverse effects. Furthermore, there are no statistically significant differences between the inhibitory effects of GS-441524 on the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) in human airway epithelial (HAE) cells in comparison to remdesivir (Agostini et al., 2018; Yan and Muller, 2020). In the recent pharmacokinetic study of remdesivir and GS-441524 in severe COVID-19 cases, remdesivir showed a half-life of 1 h while GS-441524 remained in detectable plasma concentration until the following remdesivir administration (Tempestilli et al., 2020). Overall, given the notable manufacturing and clinical profile of GS-441524, further research on the therapeutic and prophylactic efficacy of GS-441524 against SARS-CoV-2 is recommended.
The results of the ongoing studies, especially RCTs, could solve the current uncertainties around remdesivir. Additionally, the combination of inhaled and IV formulation of remdesivir could improve the efficacy of antiviral therapy against SARS-CoV-2; therefore, it would be beneficial to start new clinical trials using this combination.
4.4. Limitations
Although the whole adopted process in this study, including study design, search strategy, research selection, data extraction, and statistical analysis, was based on the standardized systematic review methodology (Dalton et al., 2016; Deeks et al., 2020; Higgins et al., 2020; Jadad et al., 1996; Li et al., 2020; Liberati et al., 2009; McGrath et al., 2019; Moher et al., 2009; Page et al., 2020; Reeves et al., 2020; Sterne et al., 2016, Sterne et al., 2019, Sterne et al., 2020), there were still some limitations.
A number of potentially eligible clinical trials with notable sample sizes were excluded from the review due to the unavailability of their results by the end of December 22nd, 2020 (Table 5).
The COVID-19 severity was different among the included participants that could affect the treatment output. The validity of the meta-analysis was limited by the lack of a comparison/control group in three out of ten included studies. There are no uniform guidelines for administering additional treatments and providing supportive care for COVID-19 patients in clinical trials, which may lead to inaccurate and unreliable clinical outcomes. The follow-up times were not the same in all of the meta-analyzed studies (Table 1). The extended uniform follow-up durations are preferred because they would produce more reliable final results.
5. Conclusion
The current meta-analysis provides an updated evaluation of scientific evidence on the use of remdesivir in COVID-19 patients. Findings from the RCT studies indicated a significant improvement in the 28-day recovery rate, low flow oxygen support through the baseline to day 14, and IMV or ECMO requirement through days 14–28 of the follow-up time in the remdesivir group. Additionally, the risk of experiencing serious ADRs was significantly lower in the remdesivir group than the comparison/control group.
The data from the NRSIs showed significant beneficial effects of remdesivir on the low flow oxygen support through days 14–28 and the IMV or ECMO requirement through the baseline to day 28 of the follow-up period. Moreover, the risk of 28-day death was lower in the remdesivir group relative to the no-remdesivir group.
There were no significant differences between the 5-day and 10-day remdesivir courses in any of the evaluated clinical outputs. Furthermore, the 5-day remdesivir course may provide similar benefits while causing fewer serious ADRs and lower costs than the 10-day regimen.
These results, combined with the concerning issues regarding synthesis difficulties, pharmacological characteristics, clinical, and physicochemical features of remdesivir, highlight the importance of performing adequate well-designed RCTs before it can be confidently administered in COVID-19 patients. Nevertheless, the results of ongoing clinical trials would be helpful for future systematic reviews and meta-analyses to reach more reliable results.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not required.
Transparency declarations
None to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2021.173926.
WOS, web of science; RCT, randomized controlled trial; NRSI, non-randomized study of intervention.
ADR, adverse drug reaction; (14), 14-day follow-up; (28), 28-day follow-up; CI, confidence interval; df, degree of freedom.
RCT, randomized controlled trial; ADR, adverse drug reaction; (14), 14-day follow-up; (28), 28-day follow-up; CI, confidence interval; df, degree of freedom.
NRSI, non-randomized study of intervention; (28), 28-day follow-up; (14), 14-day follow-up; CI, confidence interval; df, degree of freedom.
Supplementary data
Details of the PRISMA checklist, search strategy, definitions of the evaluated outputs, sensitivity analysis, risk of bias assessment, forest plots for pooling event rates, and the improvement assessment of the respiratory support levels are presented in Tables S1–S7 and Figs. S1–S10.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander P.E., Piticaru J., Lewis K., Aryal K., Thomas P., Szczeklik W., Fronczek J., Polok K., Alhazzani W., Mammen M. Remdesivir use in patients with coronavirus COVID-19 disease: a systematic review and meta-analysis. medRxiv. 2020 2020.2005.2023.20110932. [Google Scholar]
- Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M., Bach P., Baldwin M.R. Hospital length of stay for severe COVID-19: implications for Remdesivir's value. medRxiv. 2020 doi: 10.1007/s41669-020-00243-6. 2020.2008.2010.20171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori S., Cossu M.V., Ridolfo A.L., Rech R., Bonazzetti C., Pagani G., Gubertini G., Coen M., Magni C., Castelli A. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: clinical outcome and differences in post_treatment hospitalisation status. Pharmacol. Res. 2020;158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S. Remdesivir for the treatment of Covid-19—preliminary report. N. Engl. J. Med. 2020;383(10):993. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-d., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 — final report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas N.K., Majumder P.P. Analysis of RNA sequences of 3636 SARS-CoV-2 collected from 55 countries reveals selective sweep of one virus type. Indian J. Med. Res. 2020;151(5):450–458. doi: 10.4103/ijmr.IJMR_1125_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonovas S., Piovani D. Compassionate use of remdesivir in covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- Dalton J.E., Bolen S.D., Mascha E.J. Publication bias: the elephant in the review. Anesth. Analg. 2016;123(4):812–813. doi: 10.1213/ANE.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Higgins J.P.T., Altman D.G. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Analysing data and undertaking meta-analyses. Version 6.1. Cochrane. [Google Scholar]
- Ferner R.E., Aronson J.K. Remdesivir in covid-19. BMJ. 2020;369:m1610. doi: 10.1136/bmj.m1610. [DOI] [PubMed] [Google Scholar]
- Fried M.W., Crawford J.M., Mospan A.R., Watkins S.E., Munoz B., Zink R.C., Elliott S., Burleson K., Landis C., Reddy K.R., Brown R.S., Jr. Patient characteristics and outcomes of 11721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States. Clin. Infect. Dis. 2020:ciaa1268. doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Sciences . 2020. Gilead Sciences Statement on the Initiation of Clinical Testing of an Inhaled Solution of Remdesivir for Potential Outpatient Treatment of COVID-19.https://www.gilead.com/news-and-press/company-statements/gilead-sciences-statement-on-the-initiation-of-clinical-testing-of-an-inhaled-solution-of-remdesivir-for-potential-outpatient-treatment-of-covid19 accessed December 22th, 2020. [Google Scholar]
- Gilead Sciences . 2020. An Open Letter from Daniel O'Day, Chairman & CEO.https://stories.gilead.com//articles/an-open-letter-from-daniel-oday-june-22 accessed December 22th, 2020. [Google Scholar]
- Goldman J.D., Lye D.C., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Savović J., Page M.J., Elbers R.G., Sterne J.A.C. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Assessing risk of bias in a randomized trial. Version 6.1. Cochrane. [Google Scholar]
- Huynh T., Wang H., Luan B. In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2's main protease. J. Phys. Chem. Lett. 2020;11:4413–4420. doi: 10.1021/acs.jpclett.0c00994. [DOI] [PubMed] [Google Scholar]
- Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Chen D., Cai D., Yi Y., Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: a network meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Higgins J.P.T., Deeks J.J. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Collecting data. Version 6.1. Cochrane. [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;339:b2700. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath S., Zhao X., Qin Z.Z., Steele R., Benedetti A. One-sample aggregate data meta-analysis of medians. Stat. Med. 2019;38:969–984. doi: 10.1002/sim.8013. [DOI] [PubMed] [Google Scholar]
- McGuinness L.A., Higgins J.P.T. Risk-of-bias Visualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2020;12(1):55–61. doi: 10.1002/jrsm.1411. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.L., Thai N.Q., Truong D.T., Li M.S. Remdesivir strongly binds to both RNA-dependent RNA polymerase and main protease of SARS-CoV-2: evidence from molecular simulations. J. Phys. Chem. B. 2020;124(50):11337–11348. doi: 10.1021/acs.jpcb.0c07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olender S.A., Perez K.K., Go A.S., Balani B., Price-Haywood E.G., Shah N.S., Wang S., Walunas T.L., Swaminathan S., Slim J., Chin B., De Wit S., Ali S.M., Soriano Viladomiu A., Robinson P., Gottlieb R.L., Tsang T.Y.O., Lee I.H., Haubrich R.H., Chokkalingam A.P., Lin L., Zhong L., Bekele B.N., Mera-Giler R., Gallant J., Smith L.E., Osinusi A.O., Brainard D.M., Hu H., Phulpin C., Edgar H., Diaz-Cuervo H., Bernardino J.I. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin. Infect. Dis. 2020:ciaa1041. doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., Higgins J.P.T., Sterne J.A.C. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Assessing risk of bias due to missing results in a synthesis. Version 6.1. Cochrane. [Google Scholar]
- Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernández García C., Kieny M.P., Malekzadeh R., Murthy S., Reddy K.S., Roses Periago M., Abi Hanna P., Ader F., Al-Bader A.M., Alhasawi A., Allum E., Alotaibi A., Alvarez-Moreno C.A., Appadoo S., Asiri A., Aukrust P., Barratt-Due A., Bellani S., Branca M., Cappel-Porter H.B.C., Cerrato N., Chow T.S., Como N., Eustace J., García P.J., Godbole S., Gotuzzo E., Griskevicius L., Hamra R., Hassan M., Hassany M., Hutton D., Irmansyah I., Jancoriene L., Kirwan J., Kumar S., Lennon P., Lopardo G., Lydon P., Magrini N., Maguire T., Manevska S., Manuel O., McGinty S., Medina M.T., Mesa Rubio M.L., Miranda-Montoya M.C., Nel J., Nunes E.P., Perola M., Portolés A., Rasmin M.R., Raza A., Rees H., Reges P.P.S., Rogers C.A., Salami K., Salvadori M.I., Sinani N., Sterne J.A.C., Stevanovikj M., Tacconelli E., Tikkinen K.A.O., Trelle S., Zaid H., Røttingen J.A., Swaminathan S. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 2020;384(6):497–511. doi: 10.1056/NEJMoa2023184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini Z., Montalti R., Temperoni C., Canovari B., Mancini M., Tempesta M., Pimpini D., Zallocco N., Barchiesi F. Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU. J. Antimicrob. Chemother. 2020;75:3359–3365. doi: 10.1093/jac/dkaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscoya A., Ng-Sueng L.F., Parra del Riego A., Cerna-Viacava R., Pasupuleti V., Roman Y.M., Thota P., White C.M., Hernandez A.V. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1371/journal.pone.0243705. 2020.2005.2026.20109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves B.C., Deeks J., Higgins J.P.T., Shea B., Tugwell P., Wells G.A. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Including non-randomized studies on intervention effects. Version 6.1. Cochrane. [Google Scholar]
- Rochwerg B., Agarwal A., Zeng L., Leo Y.S., Appiah J.A., Agoritsas T., Bartoszko J., Brignardello-Petersen R., Ergan B., Ge L., Geduld H., Gershengorn H.B., Manai H., Huang M., Lamontagne F., Kanda S., Kawano-Dourado L., Kurian L., Kwizera A., Murthy S., Qadir N., Siemieniuk R., Silvestre M.A., Vandvik P.O., Ye Z., Zeraatkar D., Guyatt G. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020;370:m2924. doi: 10.1136/bmj.m2924. [DOI] [PubMed] [Google Scholar]
- Rochwerg B., Agoritsas T., Lamontagne F., Leo Y.S., Macdonald H., Agarwal A., Zeng L., Lytvyn L., Appiah J.A., Amin W., Arabi Y., Blumberg L., Burhan E., Bausch F., Calfee C.S., Cao B., Cecconi M., Chanda D., Cooke G., Du B., Dunning J., Geduld H., Gee P., Hashimi M., Hui D.S., Kabra S., Kanda S., Kawano-Dourado L., Kim Y.-J., Kissoon N., Kwizera A., Laake J.H., Machado F.R., Mahaka I., Manai H., Mino G., Nsutebu E., Pshenichnaya N., Qadir N., Sabzwari S., Sarin R., Sharland M., Shen Y., Ranganathan S.S., Souza J., Ugarte S., Venkatapuam S., Dat V.Q., Vuyiseka D., Wuewickrama A., Maguire B., Zeraatkar D., Bartoszko J., Ge L., Brignardello-Peterson R., Siemieniuk R., Guyatt G., Diaz J., Jacobs M., Vandvik P.O. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- Sarfraz A., Sarfraz Z., Sanchez-Gonzalez M., Michel J., Michel G., Frontela O., Posada J., Cardona J., Angueira E. Randomized controlled trials of remdesivir in hospitalized COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.4103/2452-2473.309139. 2020.2008.2021.20179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha D.B., Budhathoki P., Syed N.I.H., Rawal E., Raut S., Khadka S. Remdesivir: a potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2020;264:118663. doi: 10.1016/j.lfs.2020.118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M., Investigators f.t.G.-U.-.-. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. Jama. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., Carpenter J.R., Chan A.W., Churchill R., Deeks J.J., Hróbjartsson A., Kirkham J., Jüni P., Loke Y.K., Pigott T.D., Ramsay C.R., Regidor D., Rothstein H.R., Sandhu L., Santaguida P.L., Schünemann H.J., Shea B., Shrier I., Tugwell P., Turner L., Valentine J.C., Waddington H., Waters E., Wells G.A., Whiting P.F., Higgins J.P. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Sterne J.A.C., Hernán M., McAleenan A., Reeves B.C., Higgins J.P.T. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. 2020. Assessing risk of bias in a non-randomized study. Version 6.1. Cochrane. [Google Scholar]
- Tempestilli, M., Caputi, P., Avataneo, V., Notari, S., Forini, O., Scorzolini, L., Marchioni, L., Ascoli Bartoli, T., Castilletti, C., Lalle, E., Capobianchi, M.R., Nicastri, E., D'Avolio, A., Ippolito, G., Agrati, C., On behalf of the, C.I.S.G., 2020. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J. Antimicrob. Chemother. 75 (10), 2977-2980. [DOI] [PMC free article] [PubMed]
- The U.S. Food and Drug Administration COVID-19 update: FDA broadens emergency use authorization for veklury (remdesivir) to include all hospitalized patients for treatment of COVID-19. 2020. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized accessed December 22th, 2020.
- The U.S. Food and Drug Administration FDA approves first treatment for COVID-19. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 accessed December 22th, 2020.
- The U.S. Food and Drug Administration . 2020. FDA's Approval of Veklury (Remdesivir) for the Treatment of COVID-19—The Science of Safety and Effectiveness.https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness accessed December 22th, 2020. [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., Doremalen N.v., Leighton I., Yinda C.K., Pérez-Pérez L., Okumura A., Lovaglio J., Hanley P.W., Saturday G., Bosio C.M., Anzick S., Barbian K., Cihlar T., Martens C., Scott D.P., Munster V.J., Wit E.d. 2020. Clinical Benefit of Remdesivir in Rhesus Macaques Infected with SARS-CoV-2. bioRxiv. 2020.2004.2015.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ accessed December 22th, 2020. [Google Scholar]
- World Health Organization . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it accessed December 22th, 2020. [Google Scholar]
- World Health Organization . 2020. WHO R&D Blueprint: Novel Coronavirus COVID-19 Therapeutic Trial Synopsis.https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis accessed December 22th, 2020. [Google Scholar]
- Yan V., Muller F. Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment. ACS Med. Chem. Lett. 2020;11(7):1361–1366. doi: 10.1021/acsmedchemlett.0c00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhou R. Structural basis of the potential binding mechanism of remdesivir to SARS-CoV-2 RNA-dependent RNA polymerase. J. Phys. Chem. B. 2020;124:6955–6962. doi: 10.1021/acs.jpcb.0c04198. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Teng Z., Yang L., Xu S., Liu J., Teng Y., Hao Q., Zhao D., Li X., Lu S., Zeng Y. Efficacy and safety of remdesivir for COVID-19 treatment: an analysis of randomized, double-blind, placebo-controlled trials. medRxiv. 2020 2020.2006.2022.20136531. [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.