Abstract
Background
The clinical impact of severe coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in immunocompromised patients has not been systematically evaluated.
Methods
We reviewed current literature reporting on COVID-19 in cancer (CA), hematopoietic cell (HCT), and solid organ transplant (SOT) patients and compared their clinical data and outcomes to the general population. For adult CA, HCT and SOT patients, an extensive search strategy retrieved all articles published until July 20, 2020 by combining the terms coronavirus, coronavirus infection, COVID-19, and SARS-CoV-2 in PubMed, Cochrane, and Web of Science, and following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. For the pediatric CA cohort, a global COVID-19 registry was used. For the general population cohort, a large meta-analysis was used to compare pooled prevalence estimates, and two large meta-analyses were utilized to serve as pooled comparators for hospitalized COVID-19 patients.
Findings
Compared to the general population, adult CA and SOT patients with COVID-19 had higher comorbidities, greater levels of inflammatory markers at diagnosis, and higher rates of intensive care and hospital mortality. Pediatric CA patients and HCT patients with COVID-19 tended to have clinical presentations and outcomes similar to the general population.
Interpretation
To our knowledge, this is the first systematic review evaluating COVID-19 phenotype and outcomes in immunocompromised patients and comparing them to the general population, which shows that hospital outcomes appear to be worse in adult CA and SOT patients, potentially due to their higher co-morbidity burden.
Funding
None
Key points
Question: Do immunocompromised patients with coronavirus disease 2019 (COVID-19) have different clinical presentations and outcomes than the general population?
Findings: In this systematic review of 4942 adult and pediatric cancer (CA), hematopoietic cell (HCT) and solid organ transplant (SOT) patients with COVID-19, adult CA and SOT patients had higher co-morbidities and worse hospital outcomes versus the general population with COVID-19. Pediatric CA and HCT patients with COVID-19 tended to have either milder or similar presentation and hospital outcomes than the general population with COVID-19.
Meaning: Clinical outcomes in immunocompromised patients with COVID-19 may vary based upon co-morbidity burden.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has afflicted millions of people around the world, resulting in nearly one million deaths.1 COVID-19 is primarily characterized by fever and lower respiratory symptoms but can range in severity from asymptomatic to critical illness with acute respiratory distress syndrome (ARDS), septic shock, multiorgan failure and death. While the strongest predictor of hospitalization and death is age >65 years, patients with comorbidities at any age are at higher risk of severe disease.2, 3, 4, 5 As our understanding of COVID-19 evolves, it is becoming apparent that there are two phases of the disease – an initial immunosuppressive state allowing viral replication followed by a hyperinflammatory response leading to worsening respiratory failure and shock.6 , 7 Viral escape from immune surveillance is demonstrated by both quantitative8, 9, 10, 11 and qualitative12 , 13 suppression of NK cells and depletion of CD8+ T cells14, 15, 16 in patients with severe disease. Therefore, stunting the immune response early in the infection could potentially enable uncontrolled viral replication and clinical progression to severe disease. Given this pathophysiology, immunocompromised patients may be at higher risk for severe COVID-19.
Cancer (CA), hematopoietic cell transplant (HCT), and solid organ transplant (SOT) patients compromise a significant proportion of immunocompromised patients, a heterogeneous population with primary (inheritable) and secondary (acquired) immune abnormalities.17 In 2018, an estimated 1735,350 people were newly diagnosed with CA in the United States.18 In the same year, 36,529 SOTs19 and 14,006 autologous and 9028 allogeneic HCTs20 were performed in the United States. Immune-based pharmaceutical and cellular therapies have transformed outcomes in these patients by reducing malignant disease relapse,21 preventing or attenuating deleterious alloreactivity (i.e., graft rejection or graft-versus-host disease) and inflammation,22 or augmenting antimicrobial immunity.23 Some of these same immunomodulatory therapies have also been used to treat severe COVID-19.24 , 25
Despite an emerging literature on COVID-19 in immunocompromised patients, a comprehensive review defining the clinical impact of COVID-19 in CA, HCT and SOT patients and comparing outcomes with the general population has not been performed. Therefore, we performed a systematic review of COVID-19 defining clinical features, laboratory testing, therapeutic interventions and outcomes in immunocompromised patients and then compared these results to the general population with COVID-19.
Methods
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines26 to conduct a literature search from each individual database inception until the completion of this review (July 20, 2020). In efforts to maintain a broad and comprehensive search, all studies published in a peer-reviewed, academic journal, regardless of study design were eligible for inclusion in this review. The entry terms used were coronavirus, COVID-19, and SARS-CoV-2 plus the following: cancer, heart transplant, immunocompromised, kidney transplant, lung transplant, liver transplant, solid organ transplant, stem cell transplant, from PubMed, Cochrane, and Web of Science. References from any relevant study or review, whether meeting inclusion criteria or ineligible and studies published during time of analysis were manually searched to optimize inclusion of any additional research that may have been missed in the online search. Four independent authors (J.A.B., B.P.T., R.S., and M.G.L.) screened articles. When research produced more than one published study utilizing the same data, only articles that presented disparate enough data were included. Otherwise, only the more comprehensive and recent study was included.
Publications meeting the initial search criteria were evaluated for inclusion in the current study. To be included, the study must have reported clinical data on adult (A-CA) or pediatric (P-CA) cancer patients and SOT or HCT recipients with confirmed COVID-19. Our inclusion and exclusion criteria identified 64 SOT studies,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 11 HCT studies,91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101 25 A-CA studies,102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126 and one global P-CA database,127 the COVID-19 and Childhood Cancer Registry (CCCR) (Fig. 1 )(Supplemental Table 1). The CCCR is a global COVID-19 observatory resource center through the Global COVID-19 Observatory and Resource Center for Childhood Cancer, a collaborative effort between St. Jude Children's Research Hospital (SJCRH) and the International Society of Paediatric Oncology (SIOP). The database was initially accessed in June 2020 and data was extracted through July 20, 2020, which included patient demographics and COVID-19 risk factors, illness severity, clinical/laboratory data, outcomes, and treatment. The CCCR was used for P-CA data given its volume of reported patients and the incomplete data reported in case reports and small case series, which compromise the majority of the published COVID-19 experience in the P-CA population, resulting in the inability to ensure that data from these publications were not duplicated in the registry.
Fig. 1.
PRISMA Diagram.
In order to compare characteristics of COVID-19 with the immunocompromised cohorts, a large meta-analysis of COVID-19 patients (n = 61,742)128 was selected as the general population cohort, given the publication presented patient data in a manner comparable to our study with similar pooled prevalence analyses as described below. Within this cohort, 2•7% (95% CI 0•4–7•4) of patients had CA and where thus immunocompromised, which is within the incidence rate for CA within the general population (8•3%, standard error 0•17).129 Therefore, the CA patient data was not separated from the general population data. For a comparator from hospitalized COVID-19 patients from the general population, we combined data from 2 large meta-analyses130 , 131 (total n = 11,721) with patient data similar to our meta-analysis.
Data extraction
Following initial screen, three independent reviewers (J.A.B., R.S., M.G.L.) extracted available data. Discrepancies were resolved by discussion and consensus with a fourth independent reviewer (J.J.A). Each study was evaluated for patient demographic data including patient age at time of COVID-19 diagnosis, comorbidities, time from transplant to COVID-19 diagnosis (SOT and HCT), organ transplanted (SOT), transplant indication (HCT), transplant-related immunosuppression in the (SOT), and type of malignancy (A-CA and P-CA). Similarly, each study was evaluated for prevalence of COVID-19 symptoms (fever, fatigue, myalgia, cough, dyspnea, abdominal pain, vomiting, diarrhea, anosmia/dysgeusia, and neurologic symptoms such as headache). However, data from a given study was only utilized if the study explicitly referenced the presence or absence of a given symptom.
Laboratory data including hematologic indices and inflammatory markers [procalcitonin (PCT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimer, ferritin, lactose dehydrogenase (LDH), and interleukin 6 (IL-6)] during a patient's course of COVID-19 were also extracted. Leukopenia and lymphopenia were defined as white blood cell count (WBC) less than 4500/μL and absolute lymphocyte count (ALC) less than 1000/μL, respectively.
Each study was evaluated for highest COVID-19 severity experienced by reported patients during their illness. Where studies did not explicitly state the severity of their cohort, attempts were made to classify patients based upon their reported clinical data according to the Infectious Disease Society of America criteria as either mild/moderate or severe (pneumonia, ARDS or intensive care management). If not possible, studies were excluded from analyses of COVID-19 severity. Data on hospitalization rates, need for intensive care unit (ICU), and mortality were also recorded. Where not explicitly stated as being in the ICU, patients were included as receiving “intensive care” if they needed invasive or non-invasive ventilatory support or inotropic therapy. Type and frequency of use for all attempted COVID-19 therapeutics were extracted, including modification of baseline immunosuppression (SOT) or of antineoplastic therapies (A-CA and P-CA). The HCT literature did not report alteration in immunosuppression.
Data analysis
Pooled proportional estimates and their 95% confidence intervals (CI) for symptoms, comorbidities, and outcomes were calculated using a random effect model with Freeman-Tukey double arcsine transformation.132 , 133 Heterogeneity of included studies were assessed by calculating I2 statistics. Nearly every pooled analysis showed some degree of heterogeneity among the included studies with the degree of heterogeneity varying widely (Supplemental Table 2). Continuous variables, such as lab values, were presented as medians and interquartile ranges (IQR) either as listed explicitly in the studies or calculated from individual patient values. These variables were summarized descriptively. Demographic data and disease characteristics were summarized descriptively. Statistical analyses were performed using the base R package (R Foundation for Statistical Computing, Vienna, Austria) with the “meta” package and GraphPad Prism 8 (GraphPad Software, San Diego, California).
Role of the funding source
There was no funding source for this study.
Results
Patient demographics
The SOT cohort included kidney (n = 675, 59•7%), liver (n = 295, 26•1%), heart (n = 77, 6•8%), multiple/other (n = 34, 3•0%) and lung (n = 50, 4•4%). Median patient age ranged from 0•5 to 73•6 years with 50% studies having median age >57 years. Median time from SOT to COVID-19 diagnosis ranged from 0 to 26 years with 50% of studies reporting a median time from transplant >four years.
Within the HCT cohort, 74•2% (n = 23) were allogeneic and 22•6% (n=seven) were autologous transplants with one chimeric antigen receptor T cell recipient reported. Median patient age ranged from 10•5 to 64 years with 50% of studies reporting median age ≥59 years. Median time from HCT to COVID-19 diagnosis ranged from 77 days to three years with 50% of studies reporting median time to infection ≥255 days.
The cancer (CA) cohort included adult (A) and pediatrics (P) patients. A-CA patients primarily had solid tumors (n = 2360, 74•8%) and hematologic malignancies (n = 653, 20•7%). Median patient age ranged from 32 to 76 years with 50% of studies reporting a median age ≥63 years. P-CA patients had acute lymphoblastic leukemia (n = 316, 50•7%), other hematologic malignancy (n = 101, 16•2%), extracranial solid tumor (n = 159, 25•6%), and central nervous system malignancy (n = 47, 7•5%). Nearly 60% of pediatric patients were less than ten years with the remainder between the ages of ten and 18. Patient characteristics are displayed in Supplemental Figure 1.
Patient co-morbidities
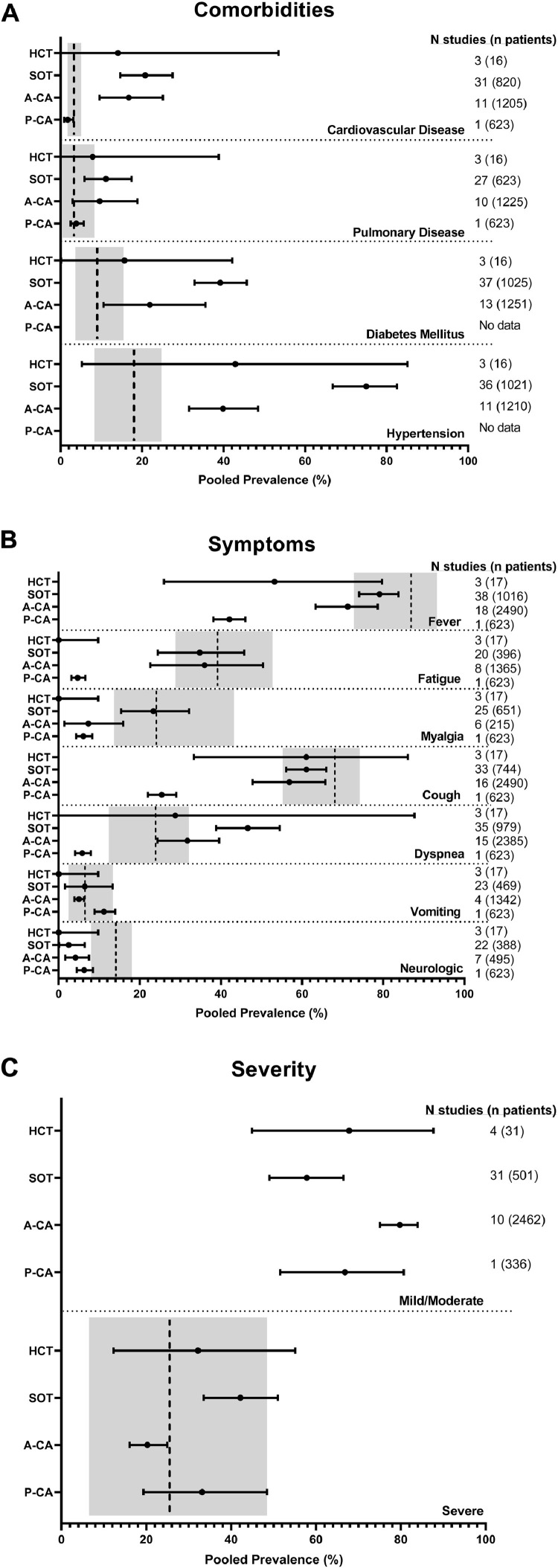
Consistent with the general population,128 hypertension was the most common comorbidity in adult immunocompromised patients with COVID-19 (Fig. 2 A). In general, immunocompromised adults had more baseline comorbidities than general population COVID-19 patients as represented by the gray rectangles. Specifically, the SOT and A-CA had more comorbid cardiovascular disease [20•76% (95% CI 14•63–27•49) and 16•71% (95% CI 9•54–25•12), respectively] and hypertension [74•97% (95% CI 66•75–82•49) and 39•86% (95% CI 31•56–48•43), respectively] than the general population. In addition, SOT patients had a higher incidence of diabetes 39•19% (95% CI, 32•86–45•69). P-CA patients had lesser amounts of comorbidities among the immunocompromised. However, the pediatric population was as comorbid in the areas of cardiovascular and pulmonary disease as adults with COVID-19 from the general population.
Fig. 2.
Characteristics of immunocompromised patients with COVID-19 compared to the general population with COVID-19. Pooled prevalence estimates from multiple studies in HCT, SOT, A-CA, and P-CA patients for (Panel A) patient comorbidities, (Panel B) COVID-19 symptoms, and (Panel C) COVID-19 severity are depicted as dots with bars indicating the 95% confidence intervals. The vertical hatched line indicates the comparable pooled prevalence for the similar measure in the general population with 95% confidence intervals indicated by the gray box as reported by Pormohammad et al.128 For Fig. 2C, data from the Pormohammad et al. meta-analysis only permitted pooled prevalence comparison for severe disease, not mild/moderate.
COVID-19 symptoms
In general, the most frequent symptoms reported in immunocompromised patients were fever and cough, consistent with what is reported in the general population with COVID-19 (Fig. 2B). SOT patients experienced markedly more dyspnea [46•63% (95% CI 38•83–54•5)] than the general population. P-CA patients had lower pooled prevalence of fever, fatigue, myalgia, cough, dyspnea and neurologic symptoms versus both other immunocompromised cohorts and the general population. Anosmia and dysgeusia were inconsistently and rarely reported. With the exception of the P-CA cohort in whom 29•46% patients are reported in the CCCR to have asymptomatic infection,127 the rate of asymptomatic disease in the other immunocompromised cohorts could not be estimated given the wide variation in reporting.
Disease severity
Data sufficient to distinguish patients in the severe COVID-19 category from the combined category of mild/moderate disease was present in 4 HCT studies (31 patients), 31 SOT studies (501 patients), ten A-CA studies (2462 patients), and 336 of the patients entered into the CCCR (Fig. 2C). The estimated pooled prevalence of severe disease was 20•24% (95% CI 16•07–24•94) among P-CA patients, 32•19% (95% CI 12•31–55•07) among HCT patients, 33•16% (95% CI 19•35–48•43) among A-CA patients, and 42•16 (95% CI 33•52–51•00) among SOT patients.
Laboratory evaluations
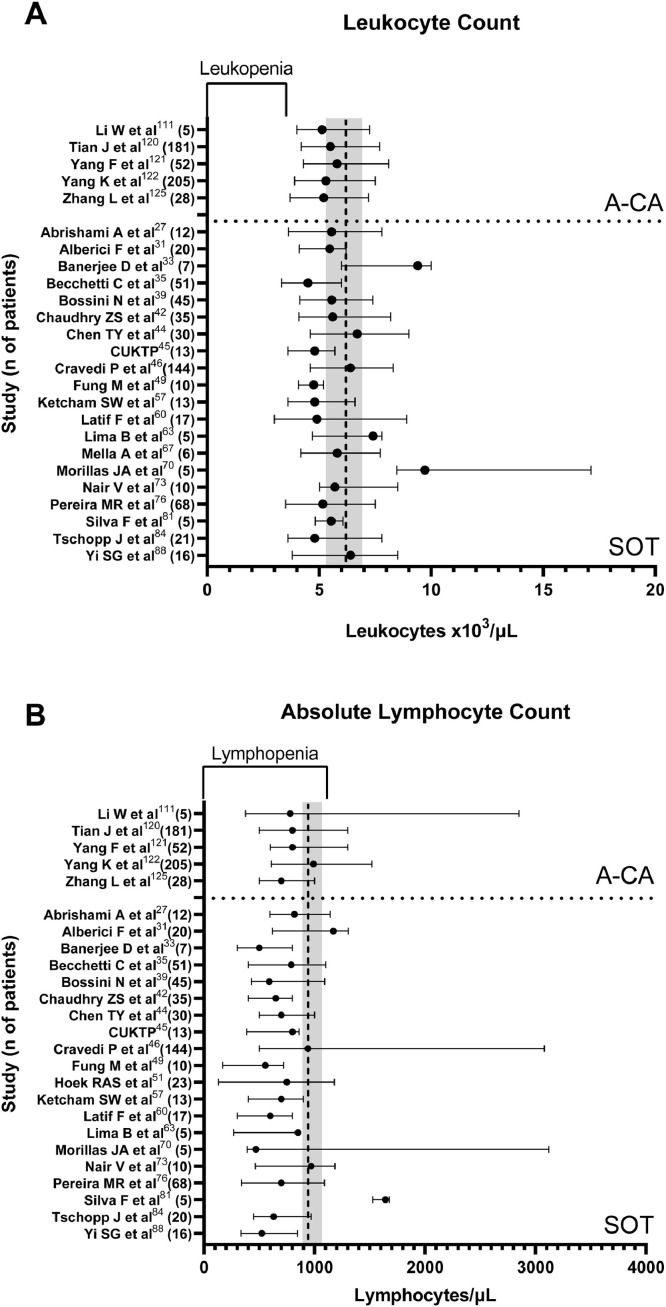
Despite receiving immunosuppressive therapies, A-CA (n=five) and SOT (n = 20) studies reporting WBC indices at time of COVID-19 diagnosis had median leukocyte counts within the normal range and comparable to those reported in the general population with COVID-19 (Fig. 3 A). However, all A-CA studies and all but one31 SOT study reported low ALC, again comparable to the general population (Fig. 3B). Similarly, data for P-CA patients showed 51•5% patients (n = 565 with leukocyte indices reported) having an ALC<1000 cells/μL. While not reported in a manner permitting calculation of interquartile ranges, six HCT studies92 , 94 , 99, 100, 101 reported ALC data with only one101 showing a median ALC above the lymphopenia range.
Fig. 3.
Leukocyte indices of SOT recipients and A-CA patients with COVID-19. The reported median (filled circle) and interquartile range (line) for (Panel A) leukocyte count (leukocytes x103/μL) and (Panel B) absolute lymphocyte counts (lymphocytes/μL) as reported in studies where this data was reported as such or could be calculated from individual patient data. These time of collection of these laboratory values differed amongst studies. Where multiple values were reported, the value with greatest severity was selected for comparison. The vertical hatched line indicates the comparable median in the general population with interquartile range indicated by the gray box as reported by Pormohammad et al.128 The inverted bracket on the upper x axis indicates the leukopenia and lymphopenia ranges for A and B, respectively. CUKTP: Columbia University Kidney Transplant Program.
Most studies reported elevated inflammatory markers among immunocompromised cohorts. All A-CA (n=three) and SOT (n = 19) studies reporting CRP data reported medians above the normal CRP range (Supplemental Figure 2A). Of these, nine SOT31 , 42 , 44 , 45 , 49 , 67 , 73 , 76 , 134 studies and one A-CA study135 reported median CRP values above the 95% confidence interval for the general population.128 Of 18 SOT studies and one A-CA study reporting ferritin levels, only one SOT study63 reported a median ferritin level in the normal range with the remainder of studies having elevated values (Supplemental Figure 2B). All but one31 of the SOT studies reporting LDH data (n = 16) had median values above the normal range (Supplemental Figure 2C). In contrast, only one120 of the three A-CA studies reporting on LDH had a median elevated level and this one just above the cutoff for normal. Only one HCT study reported on inflammatory markers.101 Among this eight patient cohort the median CRP, ferritin, and LDH were all elevated.
Given the thrombotic complications from COVID-19, d-dimer levels were analyzed (Supplemental Figure 2D). Among SOT studies, one A-CA study, and one HCT study reporting d-dimer levels, only one of these studies39 reported a median within the normal range. In all the other studies, the d-dimer level was elevated. Of note, d-dimer was not among the data reported in CCCR data on P-CA patients. Lastly, given its association with COVID-19-associated cytokine release syndrome (CRS) and multisystem inflammatory system in children (MIS-C), IL-6 levels were analyzed (Supplemental Figure 2E). All eight SOT studies and all four A-CA studies including data on IL-6 showed median IL-6 levels above the normal range with several studies reporting levels above those reported in the general population with COVID-19. No HCT studies reviewed reported on IL-6 levels, which were also not reported in the CCCR for P-CA patients.
Therapeutic management
Multiple therapies were given to immunocompromised patients with COVID-19 (Table 1 ). The most common therapy was hydroxychloroquine. Azithromycin and corticosteroids were the next most commonly utilized therapies. Multiple antiviral agents were trialed among the immunocompromised including remdesivir, lopinavir-ritonavir, darunavir-cobicistat, oseltamivir, umifenovir, ribavirin, favipiravir, and ganciclovir. IL-6 blockade was reported most frequently in the SOT population; and convalescent plasma was rarely trialed.
Table 1.
COVID-19-directed therapies used in immunocompromised patients.
| SOT (n = 1018) | HCT (n = 30) | A-CA (2243) | P-CA (n = 623) | |
|---|---|---|---|---|
| Immunosuppression | ||||
| Hydroxychloroquine | 636 | 11 | 306 | 28 |
| Azithromycin | 300* | 3 | 293 | 53 |
| Corticosteroids | 425 | 1 | 118 | NA |
| Ruxolitinib | 0 | 2 | 0 | NA |
| Unspecified immunomodulation | NA | NA | 85 | NA |
| Antiviral | ||||
| Remdesivir | 20 | 2 | 0 | 1 |
| Lopinavir-ritonavir | 134 | 4 | 77 | 10 |
| Darunavir-cobicistat | 24 | 0 | 0 | NA |
| Oseltamivir | 10 | 0 | 45 | NA |
| Umifenovir | 9 | 0 | 171 | NA |
| Ribavirin | 3 | 0 | 51 | NA |
| Favipiravir | 1 | 0 | 0 | NA |
| Leronlimab | 6 | 0 | 0 | NA |
| Ganciclovir | 0 | 0 | 9 | NA |
| Unspecified | 0 | 0 | 184 | NA |
| Monoclonal antibody | ||||
| Tocilizumab | 120 | 0 | 12 | NA |
| Anakinra | 11 | 2 | 0 | NA |
| Cytokine | ||||
| Interferon-α | 11 | 0 | 77 | NA |
| Interferon-β | 3 | 0 | 0 | NA |
| Plasma | ||||
| IVIG | 44 | 3 | 73 | 16 |
| Convalescent Plasma | 4 | 2 | 0 | 2 |
n = 864 (one study listed “antibiotics”, but did not specify azithromycin).
One common approach to COVID-19 management was modifying baseline immunosuppression or delaying further immunosuppressive therapies (Supplemental Figure 3). Pooled prevalence estimates for modification/discontinuation by immunosuppressive agent were as follows: 64•7% (95% CI 11•5–100) for mTOR inhibitors, 49•2% (95% CI 32•6–66•0) for calcineurin inhibitors, and 84•5% (95% CI 75•8–91•9) for antimetabolites. Delays in antineoplastic therapies occurred in 56•3% (95% CI 11•4–96•2) of A-CA and 28•4% (95% CI 24•9–32•1) of P-CA patients.
Outcomes in hospitalized patients
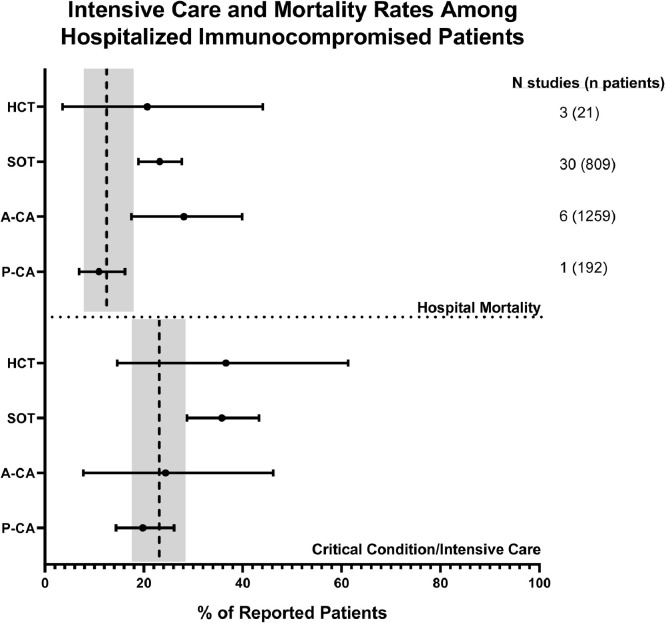
To evaluate severity in a single context that was most uniform across all studies evaluated, we evaluated outcomes data for only hospitalized patients. We calculated the pooled prevalence of critical condition/need for intensive care as well as hospital mortality among hospitalized immunocompromised patients with COVID-19 and compared similarly calculated data from two large studies of hospitalized general population patients with COVID-19,130,131 (Fig. 4 ). The SOT COVID-19 population distinguished itself from its general population counterpart by having a significantly higher need for intensive care support [35•8% (95% CI 28•7–43•3) and 23•1% (95% CI 18•0–28•6), respectively] and greater mortality [23•2% (95% CI 18•9–27•7) and 12•5% (95% CI 8•3–17•4)], respectively. No other population varied significantly from the general population, including the P-CA cohort. However, within immunocompromised patients, the P-CA cohort did vary significantly from the A-CA cohort in terms of mortality [10•9% (95% CI 6•9–16•2) and 28•1% (95% CI 17•5–28•1), respectively].
Fig. 4.
Intensive care and mortality rates among hospitalized immunocompromised patients. Pooled prevalence estimates of need for intensive care and mortality were calculated from patient data exclusively on hospitalized immunocompromised patients from those studies where such data was explicitly reported. The pooled prevalence is depicted as a filled circle with the bar representing 95% confidence intervals. Data from two large studies130,131 (total n = 11,721) of hospitalized COVID-19 patients from the general population were utilized to calculate comparable pooled prevalence estimates (vertical hatched line) and 95% confidence intervals (respective gray bars).
Discussion
To our knowledge, the current study is the largest meta-analysis analyzing clinical and laboratory data as well as outcomes among different immunocompromised patient populations with COVID-19 and comparing results to a robust general population cohort with COVID-19. As recent meta-analyses have focused exclusively on cancer136 or SOT patients.137 Herein, results show differences among the immunocompromised and general population cohorts after COVID-19. First, SOT patients with COVID-19 had more comorbidities (cardiovascular disease, diabetes, and hypertension), dyspnea and required intensive care more often, and had higher mortality despite having similar disease severity versus the general population with COVID-19. Second, A-CA patients with COVID-19 also had more comorbidities (cardiovascular disease, hypertension) and higher mortality than the general population with COVID-19. Furthermore, at the time of COVID-19 diagnosis, both A-CA and SOT patients had similar levels of WBCs and lymphopenia, despite higher levels of ferritin and d-dimers than the general population with COVID-19. Notably, SOT patients with COVID-19 also had higher IL-6 levels. Third, P-CA patients with COVID-19 had similar comorbidities, but less symptomatology compared to A-CA, SOT and the general population with COVID-19. Despite having similar severity in COVID-19 as A-CA and SOT patients, P-CA patients had lower mortality versus their adult immunocompromised counterparts,136 but similar mortality risk and need for ICU as the general population with COVID-19. Fourth, HCT patients with COVID-19 seem similar to the general population with COVID-19 with respect to disease severity and outcomes for hospitalized patients. However, the literature on COVID-19 in HCT patients was limited.
Fung and Babik performed the original literature review on immunosuppressed patients with COVID-19, which suggested that SOT and CA patients may be at increased risk for severe disease and adverse outcomes.138 The current meta-analysis supports these preliminary findings and further enables subgroup analysis of cancer patients by age. Specifically, P-CA have less fever, fatigue, myalgia, cough and dyspnea than their adult counterparts. Additionally, P-CA patients have less hospital-associated mortality than A-CA. Both findings are consistent with recent limited P-CA139 and more extensive pediatric COVID-19140 literature, suggesting more mild or asymptomatic infection in pediatric patients.
In a recent prospective, multi-center cohort study involving 482 SOT patients, Kates et al. observed a 28-day mortality rate of 20•5% among inpatients (n = 376), which associated with patient age and co-morbidities rather than intensity of immunosuppressive therapy.141 Like the current meta-analysis, modifications in immunosuppression were the most frequent intervention (337, 70% patients) and hydroxychloroquine the most frequent COVID-19 directed therapy. In contrast to the Kates et al. study, the current meta-analysis showed increased pooled prevalence for both intensive care and mortality in hospitalized SOT patients (n = 809), exceeding the respective prevalence in the general population with COVID-19. Potentially, the SOT populations may be different between studies given differences in pooled prevalence of co-morbidities and disease severity, likely reflecting the over two-fold difference in analyzed hospitalized SOT patients between studies. Furthermore, SOT patients with COVID-19 in the current meta-analysis had profound lymphopenia and higher background inflammation (CRP, ferritin) at the time of COVID-19 diagnosis versus the general population. In particular, IL-6 levels were noted to be higher in SOT patients with COVID-19. As a risk factor for COVID-19-related mortality in hospitalized patients,142 elevated IL-6 is a biomarker for the underlying immune dysregulation associated with severe COVID-19143 as exemplified by impairment in NK cell function and restoration with tocilizumab.144
Only 30 HCT patients with COVID-19 were included in the current analysis, given limited literature published. A retrospective, single-institution experience on COVID-19 in 77 adult cell therapy recipients [35 allogeneic HCT, 37 autologous HCT, and five chimeric antigen receptor (CAR) T cell] was recently published and reported favorable clinical outcomes in patients without active malignant disease.145 Median time between COVID-19 diagnosis and cell therapy was 782 days (IQR 354–1611 days). Most patients had fever and lower respiratory tract symptoms (cough, dyspnea) with chest imaging showing infiltrate. Only 44% patients required hospitalization, and 50% did not require oxygen. Of the 47% patients receiving COVID-19 treatment, most patients received hydroxychloroquine (32%). Of 15 patients having IL-6 levels assessed, eight received tocilizumab or siltuximab for pre-treatment median IL-6 176•7 pg/mL (49•5–1578•4 pg/mL). Overall survival at day 30 post-COVID-19 diagnosis was 78% (95% CI 68–91%).
Outcomes in cancer and transplant patients are improving, given the use of biologic therapies targeting immune response against malignant disease146 and improvements in the field of transplantation, including enhanced supportive care and use of cellular therapies.147 , 148 Remarkably, these same immunomodulatory and cellular therapies149 are being applied to patients with severe COVID-19, including pediatric patients.150 Yet alterations in immunosuppression as reported in the current meta-analysis may negatively impact primary disease outcomes in cancer and transplant patients with COVID-19.
By synthesizing the current literature reporting on clinical outcomes in CA, HCT and SOT patients with COVID-19, the current study provides a framework for future research, revealing gaps in knowledge that reflect the lack of detailed, homogenous reporting and the need for longer post-infection follow-up to assess direct (viral pathology) and indirect (alterations in immunosuppression) effects of SARS-CoV-2 on primary disease outcomes. Common gaps in knowledge across immunocompromised patient populations with COVID-19 include detailed viral epidemiology, including sensitivity and specificity of diagnostic assays, duration of viral shedding, and efficacy of therapeutics like remdesivir. Specific knowledge deficits relevant to both solid organ and hematopoietic cell transplant recipients with COVID-19 include defining need for modification in immunosuppressive therapy, viral effects on graft function, and potential viral induction of deleterious alloreactivity targeting the graft, including graft rejection/failure and even the host like graft-versus-host disease.
This study has several limitations, including significant study heterogeneity largely due to inherent differences among the immunocompromised patient populations but also differences in data collection among studies. Secondly, due to sample size limitations, subset analyses like effect of therapeutic agent or graft type on outcome could not be performed. Thirdly, the follow-up period varied significantly across studies, affecting outcomes like mortality. Lastly, the study could not perform univariable and multivariable analyses for constructing risk models for severe COVID-19 disease.
Notwithstanding these limitations, the work is the first systematic review summarizing COVID-19 in distinct immunocompromised patient populations and comparing data with the general population. In so doing, immunocompromised patients were found to have more comorbidities than the general population, have worse outcomes among patients requiring hospitalization for COVID-19 compared to the general population, and experience alterations in the management of their primary disease that may place them at risk for worse outcomes. Future study directions must focus on reporting identified gaps presented herein and ensuring uniform data capture.
Author contributions
J.A.B. and B.P.T. performed data literature review, data extraction, and primarily wrote the manuscript. M.G.L. and R.S. performed literature review and data extraction. J.R.S. performed statistical analysis, and J.J.A. conceptualized the scope of the manuscript, co-wrote the manuscript, and provided intellectual contributions. All authors critically reviewed and approved the final submitted manuscript.
Declaration of Competing Interest
The authors report no conflicts of interest relevant to the published work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.01.022.
Appendix. Supplementary materials
References
- 1.Organization W.H. WHO Coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/(accessed 9/11/2020.
- 2.Zhou Y., Yang Q., Chi J. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabret N., Britton G.J., Gruber C. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B Z.X., Qiu Y., Feng F., Feng J., Jia Y., Zhu H., Hu K., Liu J., Liu Z., Wang S., Gong Y., Zhou C., Zhu T., Cheng Y., Liu Z., Deng H., Tao F., Ren Y., Cheng B., Gao L., Wu X., Yu L., Huang Z., Mao Z., Song Q., Zhu B., Wang J. Clinical characteristics of 82 death cases with COVID-19. medRxiv2020.
- 9.Chen X L.J., Mo P., Zhang Y., Jiang Q., Ma Z., Cao Q., Hu W., Zou S., Chen L., Yao L., Luo M., Chen T., Deng L., Liang K., Song S., Yang R., Zheng R., Gao S., Gui X., Ke H., Hou W., Lundkvist Å., Xiong Y. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. medRxiv2020.
- 10.Liu J O.L., Guo P., Wu H., Fu P., Chen Y., Yang D., Han X., Cao Y., Alwalid O., Tao J., Peng S., Shi H., Yang F., Zheng C. Epidemiological, clinical characteristics and outcomes of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv2020.
- 11.Kuri-Cervantes L., Pampena M.B., Meng W. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng M., Gao Y., Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilk A.J., Rustagi A., Zhao N.Q. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du R.H., Liang L.R., Yang C.Q. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urra J.M., Cabrera C.M., Porras L., Rodenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020:1–11. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinn I.K., Orange J.S. Immunodeficiency disorders. Pediatr Rev. 2019;40(5):229–242. doi: 10.1542/pir.2017-0308. [DOI] [PubMed] [Google Scholar]
- 18.Society A.C. American Cancer Society; Atlanta: 2018. Cancer Facts and Figures 2018. [Google Scholar]
- 19.(SRTR) OPaTNOaSRoTR. OPTN/SRTR 2018 annual data report. in: department of health and human services HRaSA, editor.; 2020.
- 20.D'Souza A., Fretham C., Lee S.J. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transpl. 2020;26(8):e177–ee82. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroger N., Stubig T., Atanackovic D. Immune-modulating drugs and hypomethylating agents to prevent or treat relapse after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20(2):168–172. doi: 10.1016/j.bbmt.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 22.McDonald-Hyman C., Turka L.A., Blazar B.R. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7(280):280rv2. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houghtelin A., Bollard C.M. Virus-specific T cells for the immunocompromised patient. Front Immunol. 2017;8:1272. doi: 10.3389/fimmu.2017.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.America IDSo. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 9/12/2020. [DOI] [PMC free article] [PubMed]
- 25.Health TNIo. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/ (accessed 9/12/2020.
- 26.Tricco A.C., Lillie E., Zarin W. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 27.Abrishami A., Samavat S., Behnam B., Arab-Ahmadi M., Nafar M., Sanei Taheri M. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78(2):281–286. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahluwalia M., Givertz M.M., Mehra M.R. A proposed strategy for management of immunosuppression in heart transplant patients with COVID-19. Clin Transplant. 2020:e14032. doi: 10.1111/ctr.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S.H., Smith R., Camilleri B. Belatacept, kidney transplantation and COVID-19: successful management of the first reported case within the United Kingdom. Clin Transplant. 2020:e14026. doi: 10.1111/ctr.14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arpali E., Akyollu B., Yelken B., Tekin S., Turkmen A., Kocak B. Case report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020;22(4):e13296. doi: 10.1111/tid.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartiromo M., Borchi B., Botta A. Threatening drug-drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID-19) Transpl Infect Dis. 2020;22(4):e13286. doi: 10.1111/tid.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becchetti C., Zambelli M.F., Pasulo L. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belli L.S., Duvoux C., Karam V. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5(8):724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch F., Borner N., Kemmner S. Attenuated early inflammatory response in solid organ recipients with COVID-19. Clin Transpl. 2020:e14027. doi: 10.1111/ctr.14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bossini N., Alberici F., Delbarba E. Kidney transplant patients with SARS-CoV-2 infection: the Brescia Renal COVID task force experience. Am J Transpl. 2020 doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bush R., Johns F., Acharya R., Upadhyay K. Mild COVID-19 in a pediatric renal transplant recipient. Am J Transpl. 2020 doi: 10.1111/ajt.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussalino E., De Maria A., Russo R., Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient with SARS-CoV-2 pneumonia: a case report. Am J Transpl. 2020;20(7):1922–1924. doi: 10.1111/ajt.15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhry Z.S., Williams J.D., Vahia A. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a case-control study. Am J Transpl. 2020 doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen D., Yang B., Zhang Y. Withdrawing mycophenolate mofetil in treating a young kidney transplant recipient with COVID-19: a case report. Medicine (Baltimore) 2020;99(24):e20481. doi: 10.1097/MD.0000000000020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T.Y., Farghaly S., Cham S. COVID-19 pneumonia in kidney transplant recipients: focus on immunosuppression management. Transpl Infect Dis. 2020:e13378. doi: 10.1111/tid.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Columbia University Kidney Transplant P Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cravedi P., Suraj S.M., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transpl. 2020 doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crespo M., Perez-Saez M.J., Redondo-Pachon D. COVID-19 in elderly kidney transplant recipients. Am J Transpl. 2020 doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Ruiz M., Andres A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transpl. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fung M., Chiu C.Y., DeVoe C. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transpl. 2020 doi: 10.1111/ajt.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillen E., Pineiro G.J., Revuelta I. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transpl. 2020;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoek R.A.S., Manintveld O.C., Betjes M.G.H. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020 doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J.F., Zheng K.I., George J. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transpl. 2020;20(7):1907–1910. doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Husain S.A., Dube G., Morris H. Early outcomes of outpatient management of kidney transplant recipients with Coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(8):1174–1178. doi: 10.2215/CJN.05170420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang J., Miao Y., Zhao Y. Convalescent plasma therapy: helpful treatment of COVID-19 in a kidney transplant recipient presenting with serve clinical manifestation and complex complications. Clin Transpl. 2020:e14025. doi: 10.1111/ctr.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kates O.S., Fisher C.E., Stankiewicz-Karita H.C. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transpl. 2020;20(7):1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller B.C., Le A., Sobhanie M. Early COVID-19 infection after lung transplantation. Am J Transpl. 2020 doi: 10.1111/ajt.16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ketcham S.W., Adie S.K., Malliett A. Coronavirus Disease-2019 in Heart Transplant Recipients in Southeastern Michigan: a Case Series. J Card Fail. 2020;26(6):457–461. doi: 10.1016/j.cardfail.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolonko A., Dudzicz S., Wiecek A., Krol R. COVID-19 infection in solid organ transplant recipients: a single-center experience with patients immediately after transplantation. Transpl Infect Dis. 2020:e13381. doi: 10.1111/tid.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lagana S.M., De Michele S., Lee M.J. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 60.Latif F., Farr M.A., Clerkin K.J. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee B.T., Perumalswami P.V., Im G.Y., Florman S., Schiano T.D., Group C.S. COVID-19 in liver transplant recipients: an initial experience from the US epicenter. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H., Mantell B.S., Richmond M.E. Varying presentations of COVID-19 in young heart transplant recipients: a case series. Pediatr Transpl. 2020:e13780. doi: 10.1111/petr.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lima B., Gibson G.T., Vullaganti S. COVID-19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis. 2020:e13382. doi: 10.1111/tid.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B., Wang Y., Zhao Y., Shi H., Zeng F., Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transpl. 2020;20(7):1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marx D., Moulin B., Fafi-Kremer S. First case of COVID-19 in a kidney transplant recipient treated with belatacept. Am J Transpl. 2020;20(7):1944–1946. doi: 10.1111/ajt.15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattioli M., Fustini E., Gennarini S. Heart transplant recipient patient with COVID-19 treated with tocilizumab. Transpl Infect Dis. 2020:e13380. doi: 10.1111/tid.13380. [DOI] [PubMed] [Google Scholar]
- 67.Mella A., Mingozzi S., Gallo E. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020:e13348. doi: 10.1111/tid.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montagud-Marrahi E., Cofan F., Torregrosa J.V. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single center cohort of kidney recipients. Am J Transpl. 2020 doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morand A., Roquelaure B., Colson P. Child with liver transplant recovers from COVID-19 infection. A case report. Arch Pediatr. 2020;27(5):275–276. doi: 10.1016/j.arcped.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morillas J.A., Marco Canosa F., Srinivas P. Tocilizumab therapy in 5 solid and composite tissue transplant recipients with early ARDS due to SARS-CoV-2. Am J Transpl. 2020 doi: 10.1111/ajt.16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller H., Kniepeiss D., Stauber R. Recovery from COVID-19 following hepatitis C, human immunodeficiency virus infection, and liver transplantation. Am J Transpl. 2020 doi: 10.1111/ajt.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Myers C.N., Scott J.H., Criner G.J. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020:e13364. doi: 10.1111/tid.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nair V., Jandovitz N., Hirsch J.S. COVID-19 in kidney transplant recipients. Am J Transpl. 2020;20(7):1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ning L., Liu L., Li W. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transpl. 2020;20(7):1864–1868. doi: 10.1111/ajt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patrono D., Lupo F., Canta F. Outcome of COVID-19 in liver transplant recipients: a preliminary report from Northwestern Italy. Transpl Infect Dis. 2020:e13353. doi: 10.1111/tid.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transpl. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Saez M.J., Blasco M., Redondo-Pachon D. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transpl. 2020 doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin J., Wang H., Qin X. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020 doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 79.Russell M.R., Halnon N.J., Alejos J.C., Salem M.M., Reardon L.C. COVID-19 in a pediatric heart transplant recipient: emergence of donor-specific antibodies. J Heart Lung Transpl. 2020;39(7):732–733. doi: 10.1016/j.healun.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seminari E., Colaneri M., Sambo M. SARS Cov-2 infection in a renal-transplanted patient: a case report. Am J Transpl. 2020;20(7):1882–1884. doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silva F., Cipriano A., Cruz H. SARS-CoV-2 infection in kidney transplant recipients: early report of five cases. Transpl Infect Dis. 2020:e13394. doi: 10.1111/tid.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thammathiwat T., Tungsanga S., Tiankanon K. A case of successful treatment of severe COVID-19 pneumonia with favipiravir and tocilizumab in post-kidney transplant recipient. Transpl Infect Dis. 2020:e13388. doi: 10.1111/tid.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Travi G., Rossotti R., Merli M. Clinical outcome in solid organ transplant recipients with COVID-19: a single-center experience. Am J Transpla. 2020;20(9):2628–2629. doi: 10.1111/ajt.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tschopp J., L'Huillier A.G., Mombelli M. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss transplant cohort study. Am J Transpl. 2020 doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verleden G.M., Godinas L., Lorent N. COVID-19 in lung transplant patients: a case series. Am J Transpl. 2020 doi: 10.1111/ajt.16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J., Li X., Cao G., Wu X., Wang Z., Yan T. COVID-19 in a kidney transplant patient. Eur Urol. 2020;77(6):769–770. doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi S.G., Rogers A.W., Saharia A. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020 doi: 10.1097/TP.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transpl. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu L., Gong N., Liu B. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foss F.M., Rubinowitz A., Landry M.L. Attenuated novel SARS coronavirus 2 infection in an allogeneic hematopoietic stem cell transplant patient on ruxolitinib. Clin Lymphoma Myeloma Leuk. 2020 doi: 10.1016/j.clml.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J., Lin H., Wu Y. COVID-19 in posttransplant patients-report of 2 cases. Am J Transpl. 2020;20(7):1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Innes A.J., Cook L.B., Marks S. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br J Haematol. 2020 doi: 10.1111/bjh.16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanellopoulos A., Ahmed M.Z., Kishore B. COVID-19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020;190(2):e67–e70. doi: 10.1111/bjh.16856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karatas A., Inkaya A.C., Demiroglu H. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lupo-Stanghellini M.T., Messina C., Marktel S. Following-up allogeneic transplantation recipients during the COVID-19 pandemic. Lancet Haematol. 2020;7(8):e564–e5e5. doi: 10.1016/S2352-3026(20)30176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malard F., Genthon A., Brissot E. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transpl. 2020 doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nazon C., Velay A., Radosavljevic M., Fafi-Kremer S., Paillard C. Coronavirus disease 2019 3 months after hematopoietic stem cell transplant: a pediatric case report. Pediatr Blood Cancer. 2020:e28545. doi: 10.1002/pbc.28545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niu A., McDougal A., Ning B. COVID-19 in allogeneic stem cell transplant: high false-negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transpl. 2020 doi: 10.1038/s41409-020-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saraceni F., Scortechini I., Mancini G. Severe COVID-19 in a patient with chronic graft-versus-host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib. Transpl Infect Dis. 2020:e13401. doi: 10.1111/tid.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vicent M.G., Martinez A.P., Trabazo Del Castillo M. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish group of transplant (GETMON/GETH) Pediatr Blood Cancer. 2020:e28514. doi: 10.1002/pbc.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.HO Al-Shamsi, Coomes E.A., Alrawi S. Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aries J.A., Davies J.K., Auer R.L. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020;190(2):e64–ee7. doi: 10.1111/bjh.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Assaad S., Avrillon V., Fournier M.L. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaidos A., Katsarou A., Mustafa C., Milojkovic D., Karadimitris A. Interleukin 6-blockade treatment for severe COVID-19 in two patients with multiple myeloma. Br J Haematol. 2020;190(1):e9–e11. doi: 10.1111/bjh.16787. [DOI] [PubMed] [Google Scholar]
- 106.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhakal B., D'Souza A., Chhabra S., Hari P. Multiple myeloma and COVID-19. Leukemia. 2020;34(7):1961–1963. doi: 10.1038/s41375-020-0879-9. [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez-Lugo J.D., Bachier-Rodriguez L., Goldfinger M. A case series of monoclonal gammopathy of undetermined significance and COVID-19. Br J Haematol. 2020;190(3):e130–e1e3. doi: 10.1111/bjh.16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee L.Y., Cazier J.B., Angelis V. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W., Wang D., Guo J. COVID-19 in persons with chronic myeloid leukaemia. Leukemia. 2020;34(7):1799–1804. doi: 10.1038/s41375-020-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin-Moro F., Marquet J., Piris M. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190(1):e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehta V., Goel S., Kabarriti R. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyashita H., Mikami T., Chopra N. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Omarini C., Maur M., Luppi G. Cancer treatment during the coronavirus disease 2019 pandemic: do not postpone, do it! Eur J Cancer. 2020;133:29–32. doi: 10.1016/j.ejca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ouyang W., Yu J., Zhang J., Xie C. Alert to potential contagiousness: a case of lung cancer with asymptomatic severe acute respiratory syndrome Coronavirus 2 infection. J Thorac Oncol. 2020;15(6):e82–ee3. doi: 10.1016/j.jtho.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robilotti E.V., Babady N.E., Mead P.A., et al. Determinants of severity in cancer patients with COVID-19 illness. medRxiv2020.
- 119.Thibaud S., Tremblay D., Bhalla S., Zimmerman B., Sigel K., Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020;190(2):e73–ee6. doi: 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tian J., Yuan X., Xiao J. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 122.Yang K., Sheng Y., Huang C. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang H., Xie C., Huang Y. Treatment and outcome of a patient with lung cancer infected with severe acute respiratory syndrome Coronavirus-2. J Thorac Oncol. 2020;15(5):e63–ee4. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X., Song K., Tong F. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.St. Jude's children's research hospital and the international society of paediatric oncology (SIOP). COVID-19 and childhood cancer registry. https://global.stjude.org/en-us/global-covid-19-observatory-and-resource-center-for-childhood-cancer/registry.html (accessed 7/20/2020 2020).
- 128.Pormohammad A., Ghorbani S., Baradaran B. Clinical characteristics, laboratory findings, radiographic signs and outcomes of 61,742 patients with confirmed COVID-19 infection: a systematic review and meta-analysis. Microb Pathog. 2020 doi: 10.1016/j.micpath.2020.104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Villarroel M.A., Blackwell D.L., Jen A. Tables of summary health statistics for U.S. Adults. 2018 Natl Health Interv Survey. 2019 https://www.cdc.gov/nchs/nhis/SHS/tables.htm accessed September 10, 2020. [Google Scholar]
- 130.Fried M.W., Crawford J.M., Mospan A.R. Patient characteristics and outcomes of 11,721 patients with COVID19 hospitalized across the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller J.J. The inverse of the Freeman-Tukey Doubl Arcsine Transformation. Am Stat. 1978;32(4):138. [Google Scholar]
- 133.Balduzzi S., Rucker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perez-Saez M.J., Blasco M., Redondo-Pachon D. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant. 2020 doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shi Q Z.K., Yu J., Jiang F., Feng J., Zhao K., Zhang X., Chen X., Hu P., Hong Y., Li M., Liu F., Chen C., Wang W. Clinical characteristics of 101 COVID-19 nonsurvivors in Wuhan, China: a retrospective study. medRxiv2020.
- 136.Vijenthira A., Gong I.Y., Fox T.A. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020 doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raja M.A., Mendoza M.A., Villavicencio A. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transpl Rev. 2020 doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fung M., Babik J.M. COVID-19 in Immunocompromised Hosts: what We Know So Far. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bisogno G., Provenzi M., Zama D. Clinical characteristics and outcome of SARS-CoV-2 infection in Italian pediatric oncology patients: a study from the infectious diseases working group of the AIEOP. J Pediatr Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Castagnoli R., Votto M., Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 141.Kates O.S., Haydel B.M., Florman S.S. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020 [Google Scholar]
- 142.Laguna-Goya R., Utrero-Rico A., Talayero P. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146(3):518–534. doi: 10.1016/j.jaci.2020.07.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mazzoni A., Salvati L., Maggi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shah G.L., DeWolf S., Lee Y.J. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020 doi: 10.1172/JCI141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aggarwal R.S. What's fueling the biotech engine-2012 to 2013. Nat Biotechnol. 2014;32(1):32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- 147.Chabannon C., Kuball J., Bondanza A. Hematopoietic stem cell transplantation in its 60s: a platform for cellular therapies. Sci Transl Med. 2018;10(436) doi: 10.1126/scitranslmed.aap9630. [DOI] [PubMed] [Google Scholar]
- 148.Black C.K., Termanini K.M., Aguirre O., Hawksworth J.S., Sosin M. Solid organ transplantation in the 21(st) century. Ann Transl Med. 2018;6(20):409. doi: 10.21037/atm.2018.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cancio M., Ciccocioppo R., Rocco P.R.M. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22(9):474–481. doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dulek D.E., Fuhlbrigge R.C., Tribble A.C. Multidisciplinary guidance regarding the use of immunomodulatory therapies for acute COVID-19 in pediatric patients. J Pediatr Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.