Abstract
Background: COVID-19 commonly presents with upper respiratory symptoms; however, studies have shown that SARS-CoV-2 infection affects multiple organ systems. Here, we review the pathophysiology and imaging characteristics of SARS-CoV-2 infection in organ systems throughout the body and explore commonalities.
Objective: Familiarity with the underlying pathophysiology and imaging characteristics is essential for the radiologist to recognize these findings in patients with COVID-19 infection. Though pulmonary findings are the most prevalent presentation, COVID-19 may have multiple manifestations and recognition of the extrapulmonary manifestations is especially important because of the potential serious and long-term effects of COVID-19 on multiple organ systems.
KEY WORDS: COVID-19, SARS-CoV-2; thrombosis; ACE2; cytokine storm; imaging; inflammation
Abbreviations: COVID-19, Coronavirus-19; ACE2, Angiotensin-converting enzyme 2; IL, Interleukin; TNF, Tumor necrosis factor; CT, Computed tomography; MRI, Magnetic resonance imaging; MRA, Magnetic resonance angiography; US, Ultrasound; PE, Pulmonary embolism; AKI, Acute kidney injury; RAAS, Renin-angiotensin-aldosterone system; MCA, Middle cerebral artery; PCA, Posterior cerebral artery; PICA, Posterior inferior cerebral artery; DIC, Diffuse intravascular coagulation; MIS-C, Multisystem inflammatory syndrome in children; MIS-A, Multisystem inflammatory syndrome in adults; CRP, C-reactive protein; RT-PCR, Reverse transcriptase-polymerase chain reaction
BACKGROUND
The novel SARS-CoV-2 virus is responsible for the Coronavirus-19 (COVID-19) illness, which has affected millions worldwide and continues to spread. The virus was first identified in connection with an outbreak of pneumonia in Wuhan City, Hubei province, China in December 2019 (1,2). The most common initial presentation includes fever and dry cough, and other early symptoms include dyspnea, fever, malaise, muscle pain, loss of taste and/or smell, and myalgia (3, 4, 5,6). More severe illness includes respiratory distress, thrombosis, and multiorgan failure (7).
SARS-CoV-2 is a large, enveloped, single-stranded RNA virus which enters the body via nasal and bronchial epithelial cells and pneumocytes. A sentinel paper in Cell by Hoffman, et al. demonstrated by sequence analysis that SARS-CoV-2 uses the ACE2 receptor in a manner similar to the SARS-CoV virus. Their analysis showed conservation of the receptor binding motif which is known to make contact with the ACE2 receptor. To further support ACE2 mediated entry, they expressed ACE2 on BHK-21 cells, not otherwise susceptible to SARS-CoV-2 infection and found that this facilitated viral entry into cells (8). Viral replication results in breakdown of the epithelial-endothelial barrier, leading to an inflammatory response with resultant pulmonary damage. In severe COVID-19 infection, there is fulminant activation of the coagulation cascade. This is the proposed etiology of multiorgan dysfunction (9, 10, 11).
While the virus’ effects on the pulmonary system have been extensively studied, effects on other organ systems are also being actively investigated (12) (Table 1 ). A unifying theme appears to be widespread increases in acute phase reactants and activation of the coagulation cascade (13,14). As severe illness and multiorgan failure portend mortality, a more comprehensive understanding of SARS-CoV-2 pathophysiology is needed. By elucidating the unifying pathophysiology, targeted therapies may be able to combat infection throughout multiple organs simultaneously.
Table 1.
Overview of COVID-19 Clinical Manifestations by Organ System
| Organ System | Manifestations |
|---|---|
| Cardiothoracic | Cough Dyspnea Sore throat Rhinorrhea Chest pain |
| Gastrointestinal | Nausea Vomiting Diarrhea Abdominal pain |
| Genitourinary | Elevated creatinine Proteinuria Hematuria Hyperkalemia |
| Neurologic/Neurovascular | Headache Altered mentation Anosmia Lethargy Stroke Confusion Hemiparesis Paresthesia Aphasia |
| Vascular | Edema Pulseleness Palor Skin change Necrosis |
Radiology plays a key role in evaluating the effects of COVID-19 in each organ system (Table 2 ). Extensive work has been done to characterize these radiographic findings. To our knowledge, this is the first comprehensive description of multi-system radiographic findings of COVID-19. Here, we present a system-based review of the clinical presentation, underlying pathophysiology, and radiographic manifestations of COVID-19 highlighting unifying themes so as to progress our understanding of SARS-CoV-2 infection.
Table 2.
Key Radiographic Findings by Organ System
| Organ System | Radiographic Findings |
|---|---|
| Cardiothoracic | Lower lobe and peripheral predominant opacities Ground glass opacities or consolidations in advanced disease Septal thickening Air bronchograms Crazy paving Pleural effusions Pulmonary embolism Heart strain Myocarditis Acute coronary syndrome |
| Gastrointestinal | Bowel wall thickening Inflammation of adjacent mesenteric fat Small volume ascites Small bowel obstruction Mesenteric vessel occlusion and bowel ischemia |
| Genitourinary | Enlarged, echogenic kidneys Renal infarcts – wedge-shaped parenchymal defects |
| Neurologic/Neurovascular | Vasculitis pattern – punctate enhancement with extensive ischemic lesions Restricted diffusion throughout centrum semiovale, corpus callosum, basal ganglia, cerebellum Ischemic stroke Venous sinus thrombosis Hemorrhagic stroke PRES Microhemorrhages |
| Vascular | Venous or arterial thrombus |
CARDIOTHORACIC SYSTEM
SARS-CoV-2 is primarily transmitted by respiratory droplets (8) and has a variable incubation period ranging from 0-14 days with a mean of 4-5 days. This presents the clinical challenge of many presymptomatic carriers who can spread the disease (15). COVID-19 often presents predominantly as a pneumonia (16). While the most common cardiothoracic symptoms include cough and dyspnea, less common symptoms include sore throat, rhinorrhea, and chest pain (15). Approximately 80% of patients have mild respiratory illness not requiring hospitalization, 20% require hospitalization, and 5% of these require intensive care (15). Severe pulmonary disease manifests as acute hypoxic respiratory failure (16). In addition to pulmonary parenchymal disease, pulmonary barotrauma has emerged as a frequent side effect of serious COVID-19 infection. A recent study in Radiology demonstrated a 15% rate of barotrauma in COVID-19 positive patients in invasive mechanical ventilation, compared to a 0.5% rate of barotrauma in patients without COVID-19 (17). While this study included a large cohort and provided convincing evidence of increased barotrauma in COVID-19 patients, detailed information regarding ventilator settings and comorbid conditions was not provided, leaving many unanswered questions regarding the real risk imposed by the virus itself in patients with COVID-19.
As the pandemic progresses, there is increasing recognition that the virus causes long-term damage to the lung parenchyma. An article in Lancet Respiratory Medicine reviewed early evidence suggesting fibrotic change in the lungs of patients post COVID-19 infection and suggested the use of antifibrotic medication such as pirfenidone (18). Barisione, et al. performed a study on transbronchial biopsy autopsy specimens in patients who succumbed to COVID-19. Their data demonstrated phases of alveolar damage secondary to SARS-CoV-2, with the final phase culminating in interstitial fibroblast proliferation with sparse collagen fiber deposition (19). These data are in concert with recent radiology data demonstrating the development of pulmonary fibrosis on chest CT. Fang, et al. presented a case series of 12 patients in the recovery period of severe COVID-19 infection in which there was gradually development of increasing fibrosis (20). While a large sample size is needed to confirm these results, these data suggest serious long term pulmonary sequelae from COVID-19 infection.
Though cardiac manifestations of COVID-19 are less common than thoracic findings, they represent serious disease and require prompt recognition by clinicians and radiologists. COVID-19 myocardial injury was observed early in the study of the pandemic (4). Myocarditis has emerged as a rare, albeit serious sequalae of the disease (21,22). Acute coronary syndrome stemming from the prothrombotic state has also been reported (23). A recent case report of a COVID-19 positive patient presenting with acute myocardial infarction diagnosed on coronary angiography and cardiac MR underscored the key role that radiology plays in decision making for patients with elevated myocardial markers (24). Cardiac indices are image-based parameters that have shown predictive value for increased morbidity and mortality in a variety of conditions. A recent study by Eslami, et al. evaluated the utility of cardiac indices in patients infected with COVID-19. They found that an increased cardiothoracic ratio was associated with an increased hazard ratio of death and that extensive lung involvement was associated with an elevated cardiothoracic ratio. These data indicate that cardiothoracic indices may be a powerful predictor of mortality in patients with COVID-19 (25).
SARS-CoV-2 enters type I and II alveolar epithelial cells, bronchial epithelial cells, and vascular endothelial cells through ACE-2 receptor cell mediated endocytosis, rendering the bronchial tree an ideal entry point for infection (26). Once inside, the virus initiates a cascade of pro-inflammatory cytokines including interleukin (IL)-1B, IL-6, and tumor necrosis factor leading to cytokine storm and sepsis (16). This aggressive inflammatory response results in direct lung damage. Further, infected cells produce IL-8 which serves as a chemoattractant for cells of both the innate and adaptive immune system. This amplifies the immune response resulting in additional cellular damage (27). Ultimately, unless medical treatment can interrupt this cascade of events, the patient will go on to develop acute respiratory distress syndrome from the pulmonary edema and inflammation. Radiographically this manifests as pulmonary parenchymal opacities representing acute respiratory distress syndrome.
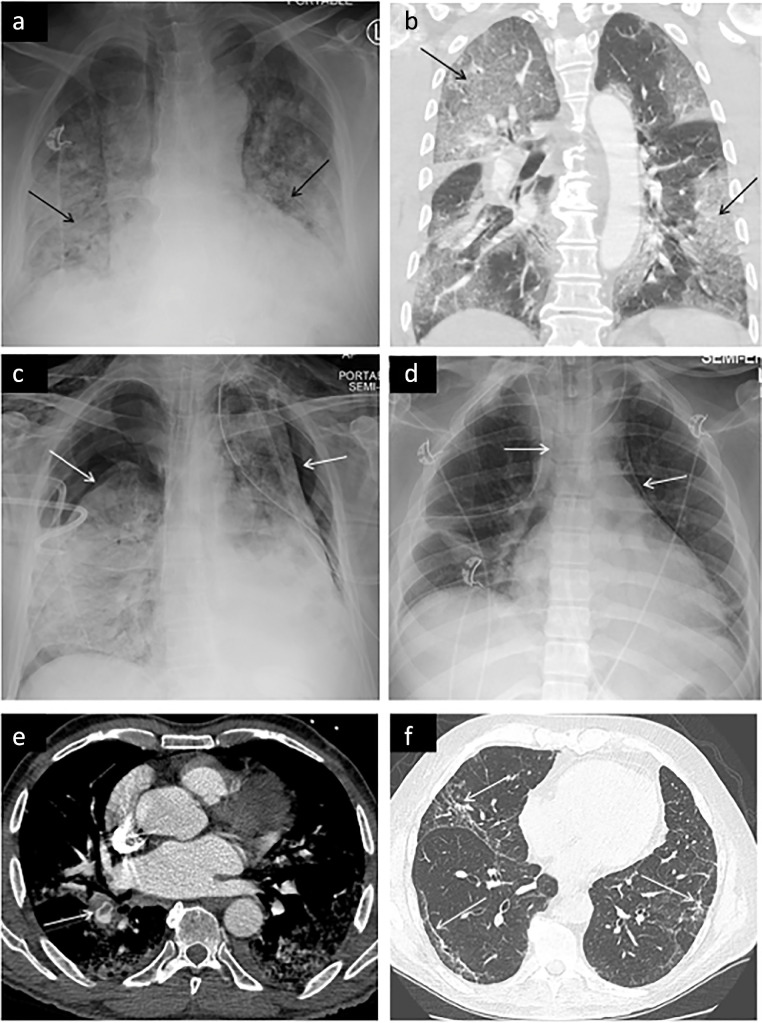
Chest imaging of COVID-19 has been extensively characterized and studied (28, 29–30). Chest radiographic features include lower lobe and peripheral predominant opacities (Fig 1 a) (31). Chest radiography is less sensitive than CT with a reported sensitivity of approximately 69% (32). 40-66.7% of cases with negative radiograph demonstrated abnormal findings on chest CT (33). While multiple professional societies have recommended against radiography for routine monitoring of patients with COVID-19, critically ill patients often undergo daily exams as part of their intensive care (34). Pleural effusions are relatively uncommon with one metanalysis reporting an incidence of 5.9% (35). CT demonstrates a similar distribution of ground glass opacities which may become confluent in advanced disease (Fig 1b). Less common features include septal thickening, air bronchograms, and crazy paving (28,33,36,37). Pulmonary barotrauma in the form of pneumothorax (Fig 1c) and pneumomediastinum (Fig 1d) can be seen on both chest radiography and CT. These findings are essential for the radiologist to identify because patients may require urgent intervention.
Figure 1.
Demonstrates thoracic manifestations of COVID-19. (a) Characteristic chest radiograph findings of bilateral peripheral and lower lobe predominant airspace opacities (arrows) which appear as ground glass opacities or consolidation on CT (b). Pneumothorax (c) frequently occurs in critically ill COVID-19 patients, as seen in this case of bilateral pneumothoraces. (d) Pneumomediastinum is also frequently observed, as shown here. (e) Pulmonary embolism is a common vascular complication of COVID-19 infection, as shown here in the right lower lobar segmental artery of a patient infected with COVID-19. (f) Demonstrates pulmonary fibrosis, a long term sequalae of COVID-19 infection, manifesting as peripheral reticulations and traction bronchiolectasis.
A major thrombotic complication of COVID-19 infection seen on chest CT is pulmonary embolism (38,39). In one retrospective study, 22% of patients who underwent CT angiography were diagnosed with PE (Fig 1e). Of those with PE, 55% had segmental PE, 31% had lobar PE, 13% had central PE and 5.5% had subsegmental PE. Radiographic evidence of right heart strain was seen in 11% of patients (38). In the long term, COVID-19 infection results in scarring of the pulmonary parenchyma manifesting as fibrotic change on chest CT (Fig 1f). Patients presenting with this long term sequala will likely become more prevalent as time progresses and more people recover from the acute phase of the disease (20).
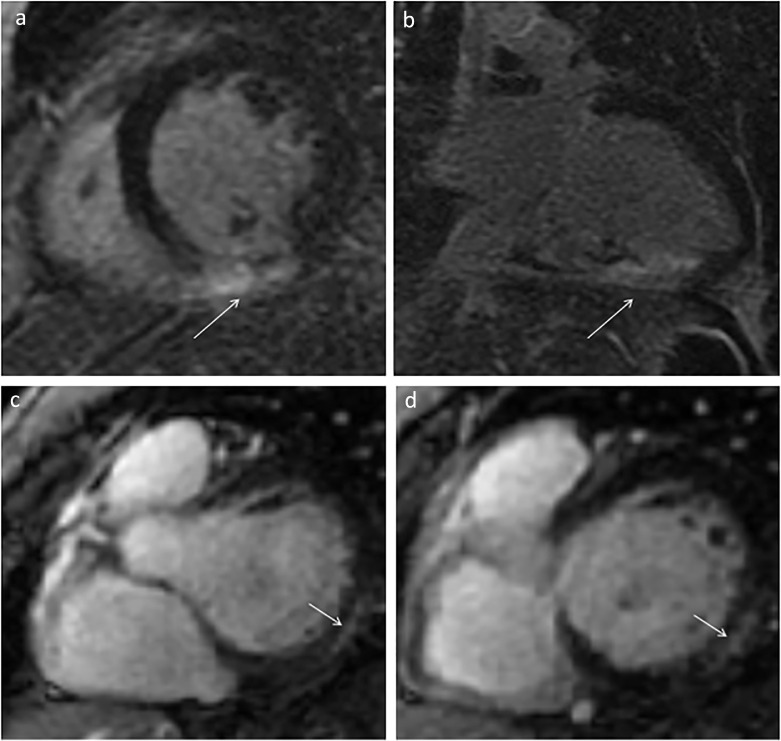
Cardiac manifestations of COVID-19 are also important to identify on imaging. Cardiac MRI is the best modality for identifying the subtle findings of myocardial injury secondary to COVID-19 and its thrombotic complications. As described in Capaccione, et al., in the setting of elevated markers of myocardial injury, cardiac imaging can serve as a critical branch point for diagnosing myocarditis versus myocardial infarction. Given that treatment diverges significantly at this branch point, cardiothoracic imaging can be critical in appropriately diagnosing and treating the patient (24). Figure 2 a, b demonstrates representative axial and coronal images cardiac MR images of a patient with myocarditis. Figure 2c, d are serial short axis images of a cardiac MR in a patient with acute myocardial infarction in the setting of COVID-19 infection.
Figure 2.
Demonstrates cardiac manifestations of COVID-19. (a) Short axis and (b) three chamber post-gadolinium views of a patient with RCA thrombus resulting in infarction secondary to untreated COVID-19 infection. Serial chart axis images (c, d) demonstrating delayed gadolinium enhancement of the myocardium in a patient with COVID-19 myocarditis.
Cardiothoracic imaging plays a key role in the diagnosis and management of patients with COVID-19 infection.
GASTROINTESTINAL SYSTEM
Research has shown that approximately 18% of COVID-19 patients experience gastrointestinal symptoms (40). These may be clinically evident before respiratory disease (41). Mild symptoms are nonspecific and include nausea, vomiting, diarrhea, and abdominal pain (42,43). Importantly, acute mesenteric ischemia can manifest acutely or subacutely, and can be seen in patients with or without cardiovascular risk factors or a history of arteriosclerotic disease (44). Mesenteric ischemia thought to arise from the prothrombotic state of patients with COVID-19 has been reported as a severe complication (45). Numerous studies have shown hepatic injury manifesting as elevated liver function tests is common in SARS-CoV-2 infected patients. The mechanism of injury is currently unclear, but is hypothesized to be related to direct hepatocyte injury or indirectly via immune-related cytokine storm or hypoxia related injury (46). Multiple case reports have described pancreatitis in patients with COVID-19 (47,48). Though a causal relationship between COVID-19 and pancreatitis has not been established, the systemic inflammatory response is likely contributory.
Similar to infection of the respiratory system, SARS-CoV-2 enters enteric epithelial cells through ACE2 receptor binding (49). Diarrhea and other gastrointestinal symptoms observed in many COVID-19 patients are likely the clinical manifestations of cellular injury which alters intestinal cell permeability and causes enterocyte dysfunction.
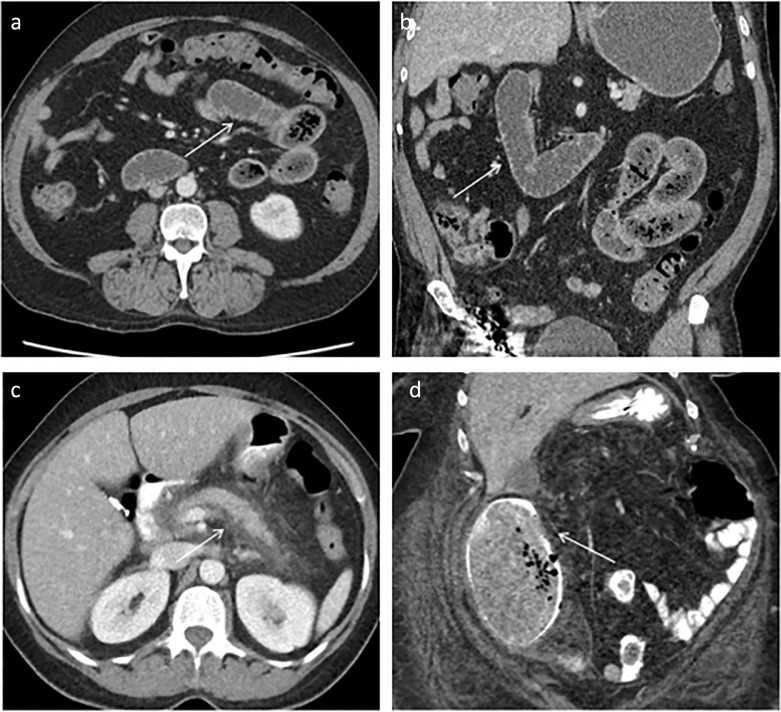
The imaging features of the gastrointestinal system in COVID-19 patients are nonspecific for COVID-19 but characteristic of disease patterns such as enteritis or mesenteric ischemia. Patients with mild infectious colitis/enteritis may have bowel wall thickening, inflammation of adjacent mesenteric fat or a small amount of ascites. Some patients present with small bowel obstruction (50) (Fig 3 a, b). As above, pancreatitis is increasingly being recognized in patients infected with COVID-19, and manifests as peripancreatic inflammation and edema (Fig 3c). The hypercoagulable state may lead to mesenteric vessel occlusion and bowel ischemia (Fig 3d). Studies have shown that pulmonary COVID-19 is often identified incidentally on abdominopelvic imaging for other pathology. A study by Dane, et al. identified 23 patents undergoing abdominal imaging who were incidentally noted to have pulmonary findings. The indication of the study for the majority of these cases was “abdominal pain”. RT-PCR testing for SARS-CoV-2 was positive in 17 of the cases, underscoring the importance of recognizing COVID-19 even in unexpected cases and on extrathoracic imaging (51).
Figure 3.
Demonstrates gastrointestinal manifestations of COVID-19. (a), (b) Multiple dilated, fluid filled loops of small bowel (white arrows) compatible with small bowel obstruction in the axial and coronal planes, respectively. (c) Edematous pancreas with extensive surrounding fat stranding and edema compatible with acute pancreatitis (white arrows). (d) Ascending colonic dilation and paracolonic changes with pneumatosis representing acute bowel ischemia.
GENITOURINARY SYSTEM
Similar to the GI system, the renal system can be infected with COVID-19. Hirsch, et al., demonstrated that acute kidney injury (AKI) developed in 37% of hospitalized patients with COVID-19, and of these, 14% required dialysis (52). Rates of AKI were particularly high among those admitted to intensive care units, ranging from 78 to 83%, with many patients requiring renal replacement therapy (53). Proteinuria was reported in 87% (54), hematuria in 41% (52), and hyperkalemia in 12.5% (55).
As in other organ systems, SARS-CoV-2 enters host cells via ACE2 (56). ACE2 is also a key enzyme in the renin-angiotensin-aldosterone system. ACE2 is expressed on mesangial cells, podocytes, and the brush border of the proximal tubular cells of the kidney (57). In a study of 26 autopsy specimens, Hua, et al. analyzed renal abnormalities in patients infected with COVID-19 using ultrastructural analysis and immunostaining. They identified proximal tubule injury, loss of brush border, and in some cases, necrosis. A limitation of the study was the small sample size given the challenges of performing autopsies on patients infected with SARS-CoV-2. Despite this, these findings provide valuable insight into how the virus causes clinical and radiographic findings of COVID-19 in the kidney (57). Infection of the cells described above results in acute tubular injury, erythrocyte aggregation leading to obstruction in glomerular and peritubular capillary loops, and microvascular dysfunction from endothelial cell injury (55,58,59). Acute kidney injury may also result from local disruption in renin-angiotensin-aldosterone system homeostasis. ACE2 plays a critical role in this system given that it cleaves angiotensin II into angiotensin 1-7 which in turn has vasodilatory effects (60). Finally, the cytokine storm induced by the virus with its resultant multisystem dysfunction contributes to AKI.
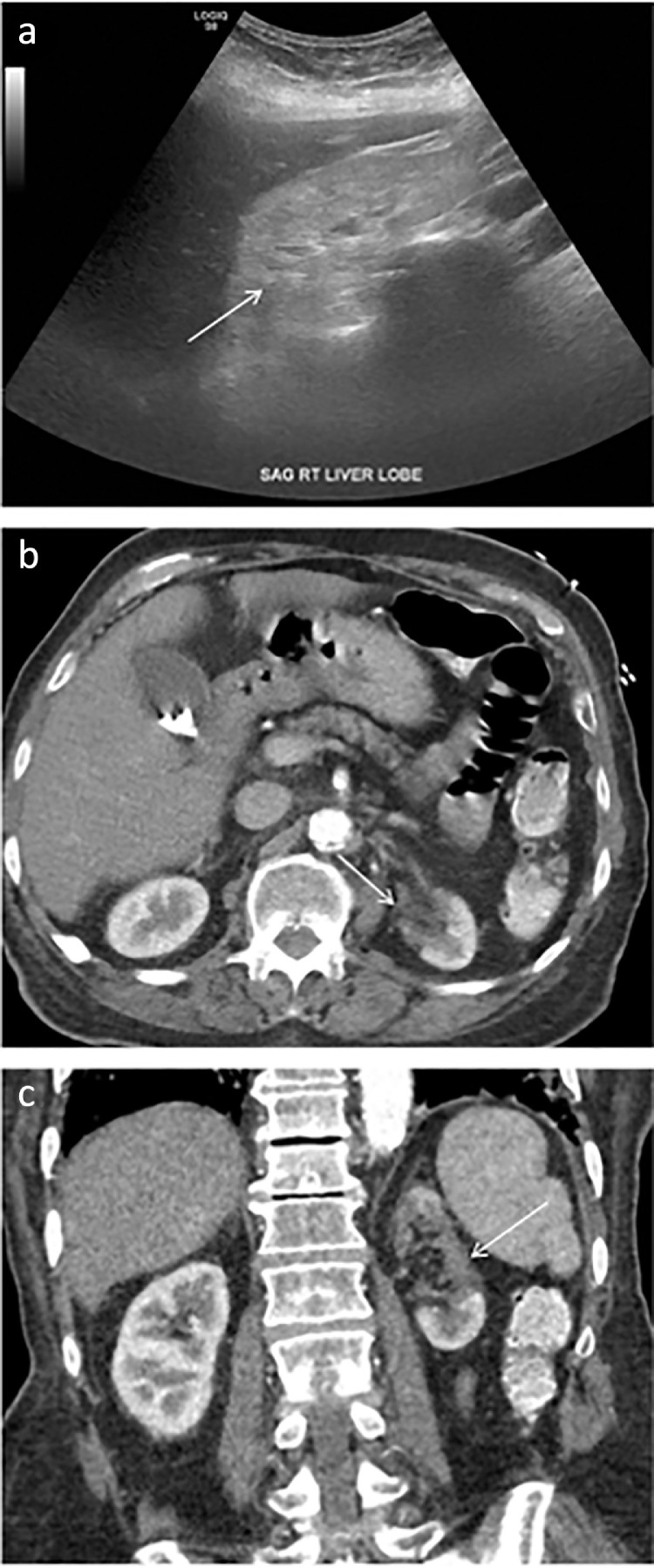
Renal sonography in AKI associated with COVID-19 infection may be normal or demonstrate enlarged, echogenic kidneys (Fig 4 a) (61). Renal infarcts due to severe hypercoagulopathy associated with the virus (62,63) are best seen on postcontrast CT imaging as focal, wedge-shaped regions of decreased enhancement involving both the cortex and medulla (Fig 4b, c). While less common than pulmonary or GI manifestations of COVID-19 infection, renal complications can be severe and have long lasting clinical sequela rendering it critical for radiologists to promptly recognize these findings in COVID-19 infected patients.
Figure 4.
Demonstrates renal manifestations of COVID-19. (a) Representative ultrasound image demonstrating diffusely increased echogenicity of the right kidney (arrow) compared to the liver compatible with renal parenchymal disease. (b), (c) Contrast-enhanced CT images of the abdomen in the axial and coronal planes, respectively, demonstrate a large focal region of decreased enhancement involving the upper and middle left kidney (arrow), the result of thrombosis.
NEUROLOGIC SYSTEM
Similar to the respiratory system, neurologic manifestations of COVID-19 can be due to parenchymal disease or thrombotic complications of COVID-19. Neurologic symptoms associated with COVID-19 infection are varied and complex ranging from mild headache, anosmia, and taste impairment to stroke, meningitis, encephalitis, and seizures (64). A systematic review of the literature by Pan, et al. evaluated 61 studies with a total of 711 patients infected with COVID-19 with cross sectional imaging (CT or MRI) of the brain. The study analyzed the patients according to severity of respiratory symptoms. Predominant neurologic findings in patients with mild respiratory symptoms included cerebral infarction, cranial nerve abnormalities including olfactory bulb involvement, and white matter abnormalities. Individuals with severe respiratory disease had increased rates of cerebral infarction and white matter abnormalities, as well as hemorrhagic events. These findings suggest that neurologic sequela of COVID-19 exist on a continuum corresponding to severity of disease. In patients with severe disease there should be heightened clinical suspicion for hemorrhagic events (65).
As in other cells of the body, the SARS-CoV-2 virus enters via the ACE2 receptor, which is expressed in the neurologic system on glial cells, neurons, capillary endothelium and the olfactory bulbs (66). Neurologic clinical manifestations may be the result of intracranial viral invasion from hematogenous spread, direct extension through the olfactory bulb (67), or may be secondary to immune-mediated damage from cytokine storm (68). This massive release of cytokines can alter the blood-brain barrier leading to inflammation of the brain parenchyma. Thromboembolic events, endothelial cell and neurovascular injury via ACE2 mediated cell entry, and hemorrhage also contribute to the serious neurologic effects of COVID-19 infection (69).
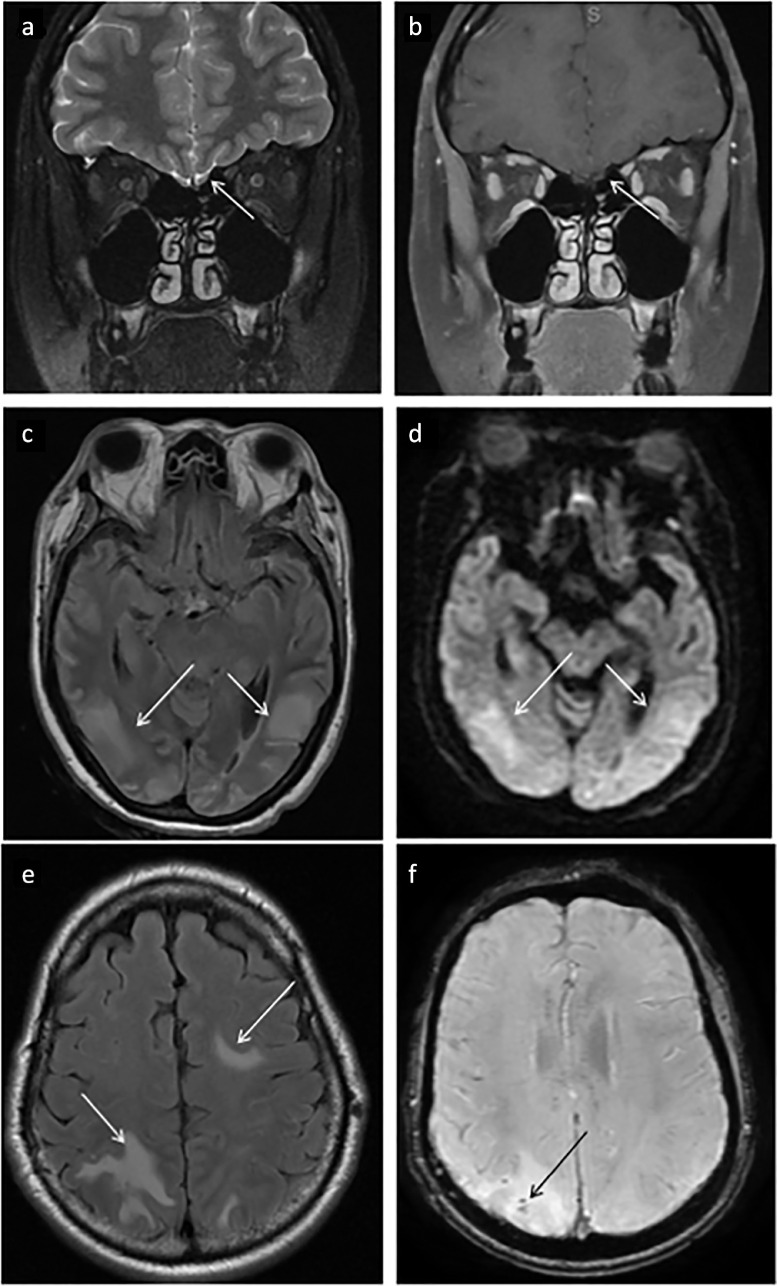
Neuroimaging may be essential for diagnosis of neuropathology in patients with COVID-19. The instability of COVID-19 patients, poor renal function limiting the use of contrast agents, and contamination of the scanner and/or imaging suite present significant challenges for obtaining studies (70). Neurologic imaging manifestations are protean and may be related to direct SARS-CoV-2 CNS infection or the sequela of COVID-19 infection and associated therapy, including hypoxemic injury, cytokine release syndrome, mechanical ventilation, or extracorporeal membrane oxygenation (71,72). A common presenting clinical symptom is anosmia (73), with imaging showing increased T2/FLAIR signal of the olfactory bulb on MRI (Fig 5 a, b). PRES (posterior reversible encephalopathy syndrome) with and without hemorrhage (Fig 5c,d) has also been identified as a neurologic manifestation of COVID-19 infection (74,75). COVID-19 related leukoencephalopathy (Fig 5e, f) encompasses various patterns of white matter signal abnormality (71) with some cases demonstrating associated parenchymal microhemorrhages (76). Additional neuropathology that has been associated with COVID-19 infection includes hypoxic encephalopathy, cranial nerve pathologies, cytotoxic lesions of the corpus callosum, demyelinating lesions, leptomeningeal enhancement, and cortical signal abnormality (71). More studies are needed to clarify whether neurologic imaging findings are direct COVID-19 encephalopathic changes or the sequela of hypoxic/ischemic encephalopathy or post-viral demyelination.
Figure 5.
Demonstrates non-vascular neurologic manifestations of COVID-19. Minimal (a) T2 / (b) FLAIR hyperintensity in the left greater than right olfactory bulbs which correlated clinically with loss of smell in a COVID-19 positive patient. PRES manifesting as asymmetric T2/FLAIR hyperintensities (c) of the occipital lobes which exhibited restricted diffusion on DWI sequences (d). Leukoencephalopathy manifesting as confluent subcortical T2/FLAIR hyperintensity within the bilateral parietal, bilateral occipital, and left frontal lobes (e) is also commonly seen. In many cases as with this patient, microhemorrhages were identified on SWI sequences (f).
Thromboembolic complications of COVID-19 infection are similarly an important cause of severe morbidity and mortality in COVID-19 infection (77–79). As in other organ systems, SARS-CoV-2 infection results in vascular thrombosis and endothelial inflammation. This manifests clinically as large vessel occlusions leading to ischemic stroke, venous sinus thrombosis, hemorrhagic strokes, and, less commonly, central nervous system vasculitides (78, 79–80). Clinical presentation incudes a myriad of nonspecific neurological symptoms including headaches, altered mentation, anosmia, lethargy, and confusion. Additionally, patients may present with localizing symptoms such as hemiparesis, paraesthesias, and aphasia (81). Less commonly, non-traumatic sub-arachnoid hemorrhage and parenchymal hemorrhage has been reported (72,82). A vasculitis-like imaging pattern has also been identified, manifesting as punctate enhancement with extensive ischemic lesions with restricted diffusion throughout the centrum semiovale, corpus callosum, basal ganglia, and cerebellum. These may or may not have a detectable intra- or extra-cranial vessel abnormality on MRA (83).
COVID-19 thrombotic complications in the brain have similar mechanisms to thrombosis in other vessels. Slow flow of blood within microcirculation promotes viral entry into capillary endothelial cells via ACE2. Infection triggers inflammation secondary to cytokine storm damaging brain parenchyma (66,78,84). In addition to local inflammation, this cytokine storm promotes a prothrombotic state leading to vascular thromboses. Infected endothelial cells upregulate cell signaling molecules which activate thrombotic pathways and cause microangiopathy. Thrombocytopenia, elevated D-dimer, and elevated C-reactive protein (CRP) in patients with severe COVID-19 and stroke support this hypothesis. The pathophysiology behind vasculitis is proposed to be a process similar to that of varicella with viral replication in cerebral arterial walls triggering local inflammation and apoptosis (81,84).
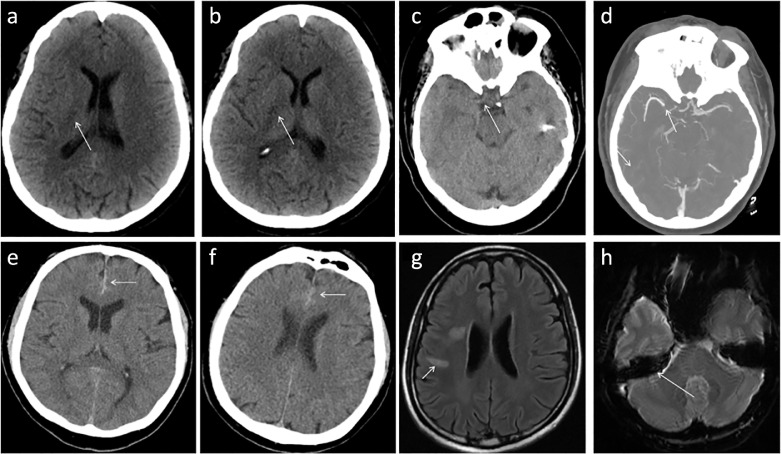
Strokes were among the most common neurologic imaging findings across multiple studies. Yoon, et. al. demonstrated that the most frequently involved vascular territory was the middle cerebral artery (MCA) followed by posterior circulation (posterior cerebral artery (PCA)), posterior inferior cerebral artery (PICA)), multiterritorial infarcts, and watershed infarcts (85). Radiographically, stroke is often first imaged with emergent non-contrast CT which may exhibit foci of hypoattenuation (Fig 6 a,b), loss of the insular ribbon sign, blurred margins of the lentiform nucleus, or the dense MCA sign (Fig 6c). Acute vascular occlusion is better seen on CTA, often obtained immediately after CT suggestive of stroke (Fig 6d). On MRI, findings of acute stroke in the setting of COVID-19 infection are identical to stroke in other contexts, manifesting as regions of restricted diffusion. Other vascular neuroradiology manifestations of COVID-19 infection include subdural hematoma (Fig 6e, f) and venous sinus thrombosis (Fig 6g, h). Neuroradiology plays a key role in diagnosis of these and less common neuropathology. Radiologists should have a high index of suspicion for abnormalities when evaluating neuroimaging in patients with COVID-19.
Figure 6.
Demonstrates neurovascular manifestations of COVID-19. (a), (b) Serial images in an emergent non-contrast head CT demonstrating multiple foci of decreased attenuation in the right posterior limb of the internal capsule compatible with acute infarct (arrows). (c) Representative head CT image of a COVID-19 patient with symptoms suspicious for stroke, found to have a dense right MCA (arrow); subsequent CTA (d) confirmed this finding (arrow). (e) Represents initial head CT of a COVID-19 patient with altered mental status found to have an acute subdural hemorrhage (arrow); follow-up imaging of the same patient (f) demonstrated stability (arrow). (g) Venous sinus thrombosis has also been identified in patients with COVID-19 manifesting as T2/FLAIR hyperintensity secondary to vascular congestion resulting in edema; on SWI (h) blooming artifact confirms thrombus in the right sigmoid sinus in this patient, likely the sequala of hypercoagulability in the setting of COVID-19.
VASCULAR SYSTEM
Given that solid organ damage secondary to thrombosis and hypoxic injury are a hallmark of COVID-19 infection, it is not surprising that numerous reports have detailed both arterial and venous thrombosis of the extremities in patients with COVID-19. A large study of 3,334 patients including 829 ICU and 2,505 non-ICU patients assessing all types of thrombosis found an overall arterial thrombosis rate of 11.1%, with systemic thromboembolism in 1.0% (86) and deep venous thrombosis in 3.9% (86). These numbers likely underestimate the true incidence of thrombosis. Surveillance imaging of COVID-19 positive patients with bilateral leg ultrasound found the rate of deep venous thrombosis as high as 65% - 69% (87,88).
The underlying pathophysiology of arterial and venous thrombosis is the same as in solid organ systems; ACE2 receptors are a key entry point for viral infection. Electron microscopy studies have demonstrated endothelial cell invasion by SARS-CoV-2 (89,90). As in solid organs systems, this results in endothelial cell injury, impaired fibrinolytic function, and release of prothrombotic von-Willebrand factor as well as proinflammatory IL-6 (91). Abnormal blood flow contributes to thrombosis via stasis which may induce further endothelial injury (92). Severe COVID-19 results in platelet dysfunction, complement activation, and cytokine storm resulting in systemic inflammation and severe illness (93, 94–95).
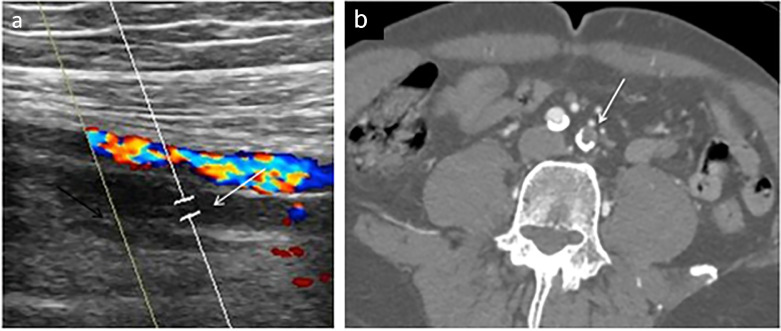
Ultrasound is the imaging modality of choice for suspected venous thrombus. Positive cases demonstrate non-compressible veins with increased internal echogenicity. Color and spectral Doppler will be absent in the setting of occlusive thrombus (Fig 7 a). Arterial thrombus may also be visualized sonographically with absent color and spectral Doppler flow in the occluded artery. On post contrast CT or MRI, vascular thrombus manifests as a focal contrast filling defect on CT and MRI, and can have associated vessel wall thickening or perivascular edema (Fig 7b). On noncontrast MRI, absence of the expected flow related signal void in arterial structures is suggestive of arterial thrombus.
Figure 7.
Demonstrates vascular manifestations of COVID-19 infection. (a) Occlusive deep vein thrombosis manifested by absent color Doppler flow in the proximal femoral vein. (b) Acute arterial thrombus, manifested by a filling defect (arrow) in the left common iliac artery on CT angiogram. Thrombus in this patient extended throughout the left lower extremity arterial system. (Color version of figure is available online.)
COVID-19 IN THE PEDIATRIC POPULATION
Acute infection in pediatric patients is similar yet often more mild than adult COVID-19 symptoms. Decreased prevalence of pulmonary symptoms is hypothesized to be the result of lower ACE2 gene expression in pediatric lungs (96). However, the post infectious period is distinct. Multisystem Inflammatory Syndrome in Children (MIS-C) emerges approximately one month after acute COVID-19 infection and presents clinically as a Kawasaki-like disease causing multi-organ system damage. Cough and shortness of breath are infrequently observed in MIS-C, in contradistinction to acute COVID-19 infection. MIS-C patients most commonly present with fever, abdominal pain, diarrhea, vomiting, conjunctivitis, rash, headache and/or sore throat (97,98). Most of these patients have positive SARS-CoV-2 antibodies rather than positive RT-PCR, indicating that this syndrome occurs after acute infection (99).
The molecular pathophysiology of MIS-C is thought to be postinfectious cytokine storm (100). These patients have elevated inflammatory markers including CRP, D-dimer, procalcitonin, ferritin, and elevated erythrocyte sedimentation rate (ESR) (100). Kawasaki disease is characterized by elevated levels of tumor necrosis factor, IL-6, and IL-1β, similar to adult patients with COVID-19 (101,102). Recently there have been several case reports of a Kawasaki-like multisystem inflammatory syndrome in adults (MIS-A) (103,104). Subsequently, a comprehensive case series collected 27 adults and analyzed commonalities in order to understand the syndrome in adults better. Elevated CRP and D-dimer were characteristic laboratory findings, and a fever was to most common symptom. They concluded that although MIS-A is rare, any adult with COVID-19 is at risk for this syndrome and it should be considered clinically (105). As an increasing number of COVID-19 related multisystem inflammatory syndrome cases come to light in both adults and children, a better understanding of the molecular pathophysiology may lead to improved treatment strategies.
On imaging, acute COVID-19 infection in children is similar to that of adults, with subpleural lower lobe peripheral predominant ground glass opacities on chest imaging (106). A study by Bayramoglu, et al. demonstrated that nearly half of children with COVID-19 had ground glass opacities either with or without accompanying consolidation. Further, they identified the feeding vessel sign, halo sign, pleural thickening, interlobular interstitial thickening, and lymphadenopathy as other frequent chest imaging signs in children with COVID-19 (107). A meta-analysis evaluating imaging findings of COVID-19 in 850 children demonstrated a 61.5% rate of ground glass opacities or consolidation. They also identified additional pediatric chest imaging signs, including the halo sign, interstitial opacities, bronchial wall thickening, and crazy-paving sign (108). Although MIS-C is largely a clinical diagnosis, some characteristic imaging findings have been reported (97,98,109). Though the imaging findings are overall nonspecific, MIS-C should be considered in the differential diagnosis in a pediatric patient with prior COVID-19 exposure.
COVID-19: SINGLE PATHOPHYSIOLOGY, MULTIPLE MANIFESTATIONS
While initial reports of SARS-CoV-2 infection pointed to a highly transmissible pneumonia, extensive clinical experience and research have demonstrated that COVID-19 is a complex multisystem disorder. On a molecular level, infection via the ACE2 receptor initiates a cascade of cell signaling events resulting in generation of inflammatory cytokines, prothrombotic molecules, and acute phase reactants. These serve to both amplify the immune system's response as well as damage the surrounding tissue. Clinical and radiographic findings are the macroscopic manifestations of these microscopic findings: edema secondary to increased tissue permeability and immune cell response, micro and macrothrombi with distal ischemia, and tissue inflammation. Understanding symptoms in terms of these tissue effects can direct research into treatments aimed at addressing underlying pathophysiology, as well as inform diagnostic imaging of patients with COVID-19. Further, by pursuing therapies aimed at breaking the molecular cycle of inflammation and thrombosis, therapeutic agents can provide systemic benefit.
Though COVID-19 has proved a devastating disease extending to all corners of the globe, tremendous progress in understanding and treatment of the disease has resulted in improved clinical outcomes and decreased mortality (110). By understanding the underlying pathophysiology and imaging manifestations which extend across multiple systems of the body, we can better prepare ourselves to identify key radiographic findings and best serve our patients as we battle this pandemic together.
FUNDING
No funding was received for the completion of this project.
DECLARATION OF COMPETING INTEREST
Mary M. Salvatore- Speaker and Consultant: Genentech, Boehringer Ingelheim. Grant funding: Genentech, Boehringer Ingelheim. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, IA E, Madani TA. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020;41(3) doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersinga WJ, Rhodes A, Cheng AC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 9.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thachil J, Tang N, Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaim S, Chong JH, Sankaranarayanan V. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:19. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher-Sandersjoo A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azkur AK, Akdis M, Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thevarajan I, Buising KL, Cowie BC. Clinical presentation and management of COVID-19. Med J Aust. 2020;213(3):134–139. doi: 10.5694/mja2.50698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KD, Harris C, Cain JK. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front Med (Lausanne) 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuinness G, Zhan C, Rosenberg N. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–EE62. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory Medicine. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barisione E, Grillo F, Ball L. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Archiv. 2020 doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y, Zhou J, Ding X. Pulmonary fibrosis in critical ill patients recovered from COVID-19 pneumonia: preliminary experience. Am J Emerg Med. 2020;38(10):2134–2138. doi: 10.1016/j.ajem.2020.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juusela A, Nazir M, Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020;2(2) doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: what do we know so far? CJC Open. 2020;2(4):278–285. doi: 10.1016/j.cjco.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangalore S, Sharma A, Slotwiner A. ST-Segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capaccione KM, Leb JS, D'Souza B. Acute myocardial infarction secondary to COVID-19 infection: a case report and review of the literature. Clin Imaging. 2020;72:178–182. doi: 10.1016/j.clinimag.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eslami V, Abrishami A, Zarei E. The association of CT-measured cardiac indices with lung involvement and clinical outcome in patients with COVID-19. Academic radiology. 2021;28(1):8–17. doi: 10.1016/j.acra.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borczuk AC, Salvatore SP, Seshan SV. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng M-Y, Lee EY, Yang J. Imaging Profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothoracic Imaging. 2020;2(1) doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernheim A, Mei X, Huang M. Chest CT findings in Coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan F, Ye T, Sun P. Time course of lung changes at chest CT during recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vancheri SG, Savietto G, Ballati F. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;30(11):6161–6169. doi: 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobi A, Chung M, Bernheim A. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Homsi M, Chung M, Bernheim A. Review of chest CT manifestations of COVID-19 infection. Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin GD, Ryerson CJ, Haramati LB. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao C, Liu X, Zhang H. coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou S, Zhu T, Wang Y. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. 2020;30(10):5446–5454. doi: 10.1007/s00330-020-06879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hani C, Trieu NH, Saab I. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyiadji N, Cormier P, Patel PY. Acute pulmonary embolism and COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capaccione KM, Li G, Salvatore MM. Pulmonary embolism rate in patients infected with SARS-CoV-2. Blood Res. 2020;55(4):275–278. doi: 10.5045/br.2020.2020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung KS, Hung IFN, Chan PPY. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y, Rong L, Nian W. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao R, Qiu Y, He J-S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan BE. COVID-19-associated thromboembolic events causing acute mesenteric ischaemia. Academic radiology. 2020;27(12):1788–1789. doi: 10.1016/j.acra.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parry AH, Wani AH, Yaseen M. Acute mesenteric ischemia in severe Coronavirus-19 (COVID-19): possible mechanisms and diagnostic pathway. Acad Radiol. 2020;27(8):1190. doi: 10.1016/j.acra.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel KP, Patel PA, Vunnam RR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aloysius MM, Thatti A, Gupta A. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20(5):1026–1027. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadiparthi C, Bassi M, Yegneswaran B. Hyperglycemia, hypertriglyceridemia, and acute pancreatitis in COVID-19 infection: clinical implications. Pancreas. 2020;49(7):e62–ee3. doi: 10.1097/MPA.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sokolowska M, Lukasik ZM, Agache I. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives-a report of the European Academy of Allergy and Clinical Immunology (EAACI) Allergy. 2020;75(10):2445–2476. doi: 10.1111/all.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhayana R, Som A, Li MD. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297(1):E207–EE15. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dane B, Brusca-Augello G, Kim D. Unexpected findings of Coronavirus Disease (COVID-19) at the lung bases on abdominopelvic CT. American Journal of Roentgenology. 2020;215(3):603–606. doi: 10.2214/AJR.20.23240. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch JS, Ng JH, Ross DW. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Argenziano MG, Bruce SL, Slater CL. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cummings MJ, Baldwin MR, Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Penninger JM, Li Y. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su H, Yang M, Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabarre P, Dumas G, Dupont T. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amraei R, Rahimi N. COVID-19. Renin-Angiotensin System and Endothelial Dysfunction. Cells. 2020;9(7):1652. doi: 10.3390/cells9071652. Published 2020 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuba K, Imai Y, Ohto-Nakanishi T. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lushina N, Kuo JS, Shaikh HA. Pulmonary, cerebral, and renal thromboembolic disease in a patient with COVID-19. Radiology. 2020;296(3):E181–E1E3. doi: 10.1148/radiol.2020201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basara Akin I, Altay C, Eren Kutsoylu O. Possible radiologic renal signs of COVID-19. Abdom Radiol (NY) 2020:1–4. doi: 10.1007/s00261-020-02671-8. [published online ahead of print, 2020 Jul 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan S, Chen WC, Baal JD. Neuroradiological features of mild and severe SARS-CoV-2 infection. Academic Radiology. 2020;27(11):1507–1514. doi: 10.1016/j.acra.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baig AM, Khaleeq A, Ali U. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 67.Chougar L, Shor N, Weiss N. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin YY, Lee KY, Ro LS. Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biomed J. 2019;42(3):178–186. doi: 10.1016/j.bj.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azizi SA, Azizi S-A. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J Neurovirol. 2020;26(5):631–641. doi: 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khalili N, Haseli S, Bahrami-Motlagh H. Neurologic involvement in COVID-19: radiologists' perspective. Academic radiology. 2020;27(7):1051–1053. doi: 10.1016/j.acra.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gulko E, Oleksk ML, Gomes W. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin E, Lantos JE, Strauss SB. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York city. AJNR Am J Neuroradiol. 2020;44(11):2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strauss SB, Lantos JE, Heier LA. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. AJNR Am J Neuroradiol. 2020;41(10):1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Princiotta Cariddi L, Tabaee Damavandi P, Carimati F. Reversible Encephalopathy Syndrome (PRES) in a COVID-19 patient. J Neurol. 2020;267(11):3157–3160. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franceschi AM, Ahmed O, Giliberto L. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(7):1173–1176. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radmanesh A, Derman A, Lui YW. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223–E2E7. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kihira S, Schefflein J, Pawha P. Neurovascular complications that can be seen in COVID-19 patients. Clin Imaging. 2020;69:280–284. doi: 10.1016/j.clinimag.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franceschi AM, Arora R, Wilson R. Neurovascular complications in COVID-19 infection: case series. AJNR Am J Neuroradiol. 2020;41(9):1632–1640. doi: 10.3174/ajnr.A6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Najjar S, Najjar A, Chong DJ. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020;17(1):231. doi: 10.1186/s12974-020-01896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poillon G, Obadia M, Perrin M. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.05.003. S0150-9861(20)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellul MA, Benjamin L, Singh B. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Saiegh F, Ghosh R, Leibold A. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91(8):846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 83.Hanafi R, Roger PA, Perin B. COVID-19 Neurologic complication with CNS Vasculitis-Like pattern. AJNR Am J Neuroradiol. 2020;41(8):1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reddy ST, Garg T, Shah C. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. 2020;12(2):199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoon BC, Buch K, Lang M. Clinical and neuroimaging correlation in patients with COVID-19. AJNR Am J Neuroradiol. 2020;41(10):1791–1796. doi: 10.3174/ajnr.A6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilaloglu S, Aphinyanaphongs Y, Jones S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nahum J, Morichau-Beauchant T, Daviaud F. Venous thrombosis among critically ill patients with Coronavirus Disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Llitjos JF, Leclerc M, Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perico L, Benigni A, Casiraghi F. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2020;17(1):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Escher R, Breakey N, Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maier CL, Truong AD, Auld SC. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holter JC, Pischke SE, de Boer E. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proceedings of the National Academy of Sciences. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wool GD, Miller JL. The impact of COVID-19 disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y, Shen C, Li J. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hameed S, Elbaaly H, Reid CEL. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blumfield E, Levin TL, Kurian J. Imaging findings in Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19. AJR Am J Roentgenol. 2021;216(2):507–517. doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 99.Belhadjer Z, Meot M, Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;144(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 100.Shulman ST. Pediatric Coronavirus Disease-2019-associated multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9(3):285–286. doi: 10.1093/jpids/piaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujimaru T, Ito S, Masuda H. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine. 2014;70(2):156–160. doi: 10.1016/j.cyto.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Nakra NA, Blumberg DA, Herrera-Guerra A. Multi-System Inflammatory Syndrome in Children (MIS-C) following SARS-CoV-2 Infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaigany S, Gnirke M, Guttmann A. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396(10246):e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sokolovsky S, Soni P, Hoffman T. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. The American Journal of Emergency Medicine. 2021;39 doi: 10.1016/j.ajem.2020.06.053. 253.e1–253.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morris SB, Schwartz NG, Patel P. Case Series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foust AM, Phillips GS, Chu WC. International Expert Consensus Statement on Chest Imaging in Pediatric COVID-19 patient management: imaging findings, imaging study reporting and imaging study recommendations. Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bayramoglu Z, Canıpek E, Comert RG. Imaging features of pediatric COVID-19 on Chest Radiography and chest CT: a retrospective. Single-Center Study. Acad Radiol. 2021;28(1):18–27. doi: 10.1016/j.acra.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katal S, Johnston SK, Johnston JH. Imaging findings of SARS-CoV-2 infection in pediatrics: a systematic review of coronavirus disease 2019 (COVID-19) in 850 patients. Acad Radiol. 2020;27(11):1608–1621. doi: 10.1016/j.acra.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winant AJ, Blumfield E. Thoracic imaging findings of Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19: what radiologists need to know now. Radiol Cardiothorac Imaging. 2020;2(4) doi: 10.1148/ryct.2020200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horwitz LI, Jones SA, Cerfolio RJ. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16(2):90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]