Abstract
Background
The viral illness severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), more commonly known as Coronavirus 2019 (COVID-19), has become a global pandemic, infecting over 100 million individuals worldwide.
Objectives
The objective of this study was to compare the test characteristics of point-of-care lung ultrasound (LUS) with chest x-ray study (CXR) at radiographically detecting COVID-19 pneumonia.
Methods
This was a single-center, prospective, observational study at an urban university hospital with > 105,000 patient visits annually. Patients ≥ 18 years old, who presented to the Emergency Department with predefined signs and symptoms of COVID-19, were eligible for enrollment. Each patient received an LUS using a portable, handheld ultrasound followed by a single-view, portable anteroposterior CXR. Patients with an abnormal LUS or CXR underwent a non-contrast-enhanced computed tomography scan (NCCT). The primary outcome was the radiographic diagnosis of COVID-19 pneumonia on NCCT.
Results
One hundred ten patients underwent LUS, CXR, and NCCT; 99 LUS and 73 CXRs were interpreted as positive; 81 NCCTs were interpreted as positive, providing a prevalence of COVID-19 pneumonia of 75% (95% confidence interval [CI] 66–83.2) in our study population. LUS sensitivity was 97.6% (95% CI 91.6–99.7) vs. 69.9% (95% CI 58.8–79.5) for CXR. LUS specificity was 33.3% (95% CI 16.5–54) vs. 44.4% (95% CI 25.5–64.7) for CXR. LUS positive predictive value and negative predictive value were 81.8% (95% CI 72.8–88.9) and 81.8% (95% CI 48.2–97.7), respectively, vs. 79.5% (95% CI 68.4–88), and 32.4% (95% CI 18–49.8), respectively, for CXR.
Conclusion
LUS was more sensitive than CXR at radiographically identifying COVID-19 pneumonia.
Keywords: point-of-care ultrasound, lung ultrasound, COVID-19 pneumonia
Introduction
The novel viral illness severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), more commonly known as Coronavirus 2019 (COVID-19), has become a global pandemic infecting over 100 million people worldwide since December 2019 (1). Symptoms range from mild and nonspecific, such as cough, fever, and myalgias, to acute hypoxic respiratory failure (2, 3, 4). Similar to other viral illnesses, symptoms and physical examination findings have proven unreliable as indicators of infection (5,6). Therefore, the diagnosis typically relies on imaging or nasopharyngeal reverse-transcription polymerase chain reaction (RT-PCR) swabs.
Chest x-ray study (CXR) has limited utility for viral pneumonia with sensitivities < 70%, and it performs even worse with sensitivities < 50% for diagnosing lung pathology in critically ill respiratory patients (2,7, 8, 9). Non-contrast-enhanced computed tomography (NCCT) is considered the reference standard for the diagnosis of viral pneumonia, given its sensitivities between 97% and 100% (4,8, 9, 10, 11). Unfortunately, it is not readily available in outpatient or resource-limited settings. Furthermore, it is a time-intensive imaging modality requiring patient transportation and decontamination after each scan. Accessibility and patient instability limit its utility. Nasopharyngeal swabs are performed easily at the bedside, but studies have shown poor diagnostic sensitivities between 30% and 71%, not to mention limited availability and long wait times for results (7,12, 13, 14). Furthermore, a patient's clinical status, along with laboratory and imaging results, dictate disposition and management over a qualitative positive diagnostic test (10).
Point-of-care lung ultrasound (LUS) has become ubiquitous across most medical specialties and is utilized clinically by emergency physicians (EPs), pulmonary/critical care intensivists, and internists. Numerous protocols exist for the evaluation of unexplained dyspnea and acute respiratory distress, and much literature exists to support its clinical utility and accuracy (6,15,16). Furthermore, LUS was utilized successfully during the 2009 H1N1 outbreak, with a sensitivity of 94% for viral pneumonia (17,18).
Similar to the H1N1 outbreak, one of the most significant challenges during this pandemic is to diagnose and to provide a disposition for patients accurately and efficiently, while ensuring the safety of providers and conserving limited medical resources. Portable, handheld ultrasounds are a practical option in triage scenarios, outpatient clinics, and resource-limited settings.
To date, only anecdotal case reports, editorials, and two small retrospective, descriptive studies have looked at LUS in patients with COVID-19 pneumonia (19, 20, 21, 22). To our knowledge, no prospective studies have assessed the utility of LUS at diagnosing COVID-19 pneumonia.
The primary objective of this study is to compare the test characteristics of LUS and CXR at radiographically detecting viral/atypical pneumonia, presumed to be COVID-19 in the current pandemic, against NCCT as the reference diagnostic imaging standard.
Materials and Methods
Study Design and Setting
This was an institutional review board-approved, single-center, prospective, observational study. No funding was provided for this study. We included patients ≥ 18 years old who presented to an urban, academic, Level I emergency department (ED) over a 2-week period in April 2020. All English- and Spanish-speaking patients with one or more predefined signs and symptoms of COVID-19 were eligible to be enrolled. Predefined signs and symptoms included: cough, fever, dyspnea, myalgia, malaise, ageusia, anosmia, increased work of breathing, temperature ≥ 38°C (100.4°F), heart rate ≥ 100 beats/min, respiratory rate ≥ 16 breaths/min, and SpO2 < 94%. Patients, who provided written informed consent, were consecutively enrolled. Patients unable to consent and pregnant patients were excluded.
Study Protocol
Patients with one or more of the predefined signs and symptoms of COVID-19 were eligible for enrollment. Upon enrollment, a postgraduate year (PGY)1–3 emergency medicine resident or emergency medicine attending, unblinded to the LUS indication, performed the LUS using the portable, handheld Butterfly iQ (Guilford, CT) transducer in the lung setting. The transducer was connected to a fifth-generation Apple iPad Mini (Apple Inc., Cupertino, CA) with the Butterfly iQ application preinstalled. Prior to study commencement, our residents and faculty reviewed a 2-min video reviewing the LUS findings of viral pneumonia (17, 18, 19, 20, 21, 22). No additional training was provided to our emergency medicine residents or attendings prior to their participation. All of our emergency medicine attendings are credentialed in the core American College of Emergency Physicians (ACEP) point-of-care-ultrasound applications, including lung ultrasound (23). Each participating resident and attending had performed > 25 previous LUS per ACEP and Accreditation Council for Graduate Medical Education guidelines for emergency medicine training (23,24).
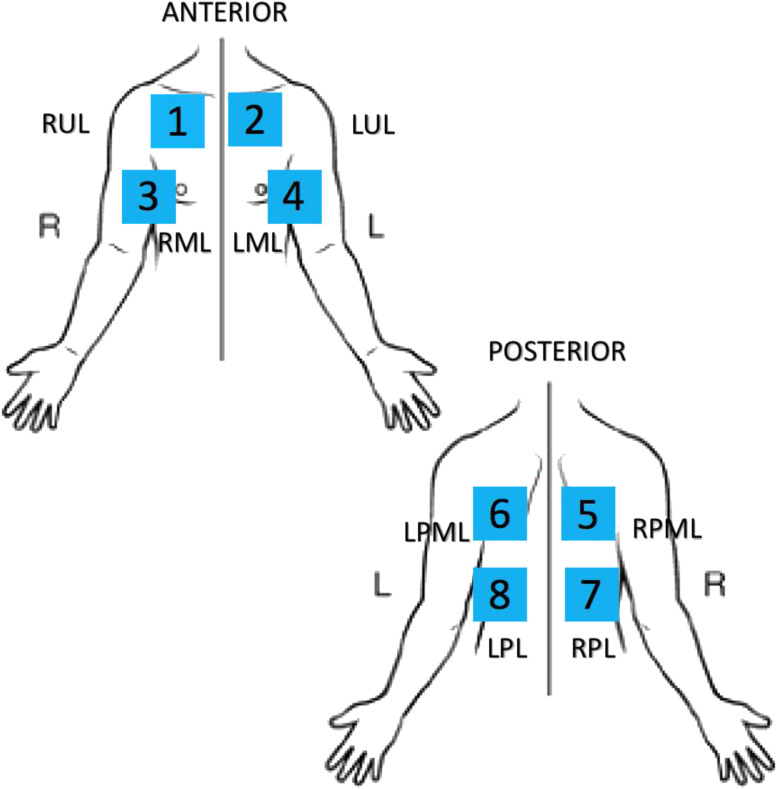
Each physician followed a predetermined standard LUS protocol (Figure 1 ) recording 6-s clips for each view and performed the LUS wearing full personal protective equipment. After each LUS, the physician disinfected the transducer and iPad following standard manufacturer sanitation protocols and ACEP guidelines (25, 26, 27). After the LUS, an unblinded radiology technician performed a single-view, portable anteroposterior CXR within 1 h of the LUS being completed. Given the risk of vector transmission, no posteroanterior/lateral CXR was performed in the radiology suite. All LUS were completed prior to CXR.
Figure 1.
COVID-19 lung ultrasound protocol. RUL = right upper lobe; LUL = left upper lobe; RML = right middle lobe; LML = left middle lobe; LPML = left posterior middle lobe; RPML = right posterior middle lobe; LPL = left posterior lower lobe; RPL = right posterior lower lobe.
Patients with abnormal LUS or CXR findings then underwent NCCT in accordance with hospital guidelines. Each NCCT was completed within 24 h (mean of 384 min, SD ± 289.3) of the LUS and CXR. The time delay was secondary to a surge in COVID-19 patients and the need to disinfect the scanner after each patient. At the discretion of the emergency medicine attending, high-risk patients with normal LUS and CXR findings had an NCCT done as well. Per departmental and hospital guidelines, one or more of the following criteria defined potentially high-risk patients: temperature ≥ 38.3°C (101°F), heart rate ≥ 110 beats/min, respiratory rate ≥ 20 breaths/min, hypoxia < 92%, absolute lymphocyte count < 1000/mm3 (1.0–4.8 K/mm3), and systolic blood pressure < 100 mm Hg. Immunocompromised patients were considered high risk as well.
Outcome Measures
Prior to study commencement, study investigators defined four LUS findings consistent with viral/atypical pneumonia: irregular pleural line, B-lines, consolidation, and pleural effusion (Figure 2 ) (17, 18, 19, 20, 21, 22). The presence of three or more B-lines was considered positive. Additionally, the presence of a single confluent B-line encompassing a third or more of the visualized distal intercostal space was considered positive (28, 29, 30). The presence of one of the aforementioned sonographic findings defined a positive zone. The presence of two or more positive zones was defined as diagnostic. The two zones could be unilateral or bilateral. Due to previous reports suggesting diffuse skip lesions of COVID-19 pneumonia, we devised an eight-view LUS protocol (Figure 1) (19, 20, 21, 22).
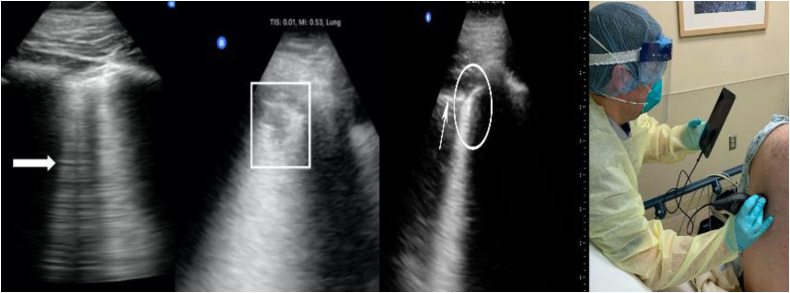
Figure 2.
Lung ultrasound findings. (Left panel) B-lines (thick arrow); consolidation with irregular pleural line (box); normal pleural line (thin arrow) and irregular pleural line (circle). (Right panel) Provider performing point-of-care lung ultrasound with personal protective equipment.
We did not mandate transducer orientation for the LUS. Physicians positioned the probe in sagittal or transverse plane based on personal preference. The emergency medicine attending of record, unblinded to the LUS indication, interpreted the LUS. After enrollment, four ultrasound-fellowship-trained emergency medicine attendings, blinded to the initial LUS, CXR, NCCT, and swab results, interpreted the LUS studies to assess the accuracy of the initial LUS interpretations, as is standard quality assurance practice at our institution. Each study had two faculty members review it. A third ultrasound faculty member was available if the initial two disagreed regarding an interpretation. However, this was not necessary during our study. Study investigators calculated a kappa coefficient to measure the interrater reliability between the non-ultrasound-fellowship-trained providers and the fellowship-trained faculty.
Predefined CXR findings included opacity, infiltrate, interstitial edema or markings, and atelectasis. The presence of one or more of these abnormalities was considered positive for viral/atypical pneumonia. Unilobar and unilateral findings were considered abnormal. Abnormalities did not have to be multilobar or bilateral to mandate an NCCT. A board-certified radiologist, blinded to LUS findings but not to the CXR indication, provided an official CXR interpretation. We used any mention of infiltrate, pneumonia, or atelectasis as being positive.
A board-certified radiologist, blinded to the LUS findings but not to the CXR findings or to the NCCT indication, interpreted the NCCT. Due to the current COVID-19 pandemic, our radiology and pulmonary/critical care departments developed hospital guidelines for NCCT interpretation in the setting of viral pneumonia. The presence of ground glass opacities was defined as Category (Cat) 1 and was consistent with viral/atypical pneumonia. Similar to CXR, abnormalities did not have to be multilobar or bilateral to be defined at Cat 1. Cat 2 was indeterminate, and Cat 3 was consistent with “other diagnosis.” Per hospital guidelines, all patients with a Cat 1 or 2 NCCT were admitted, isolated, and treated as positive for COVID-19 pneumonia. Therefore, all Cat 1 and 2 scans were considered positive for the purpose of this study. Cat 3 was negative for viral/atypical pneumonia.
A nasopharyngeal RT-PCR swab was performed on each admitted patient with suspected COVID-19. Discharged patients were not swabbed. The hospital microbiology laboratory reported results as positive or negative for COVID-19 using the Luminex (Austin, TX) NxTAG CoV nasopharyngeal swab.
Using the electronic medical record, Epic (Verona, WI), study investigators performed chart abstraction on all discharged patients 7 days after initial ED presentation to identify any patients subsequently diagnosed or admitted for COVID-19 pneumonia. Epic allows providers to query participating local health systems to share medical records.
The primary outcome was the radiographic diagnosis of viral/atypical pneumonia, presumed to be COVID-19 during the current pandemic, on NCCT. The main objective was to compare test characteristics of LUS and CXR for identifying viral/atypical pneumonia radiographically against NCCT as the diagnostic imaging standard. Secondary endpoints were the test characteristics of LUS following expert review and of CXR without including atelectasis as positive finding. Additional secondary endpoints included: the most common abnormal LUS findings, the number of positive LUS zones in relation to NCCT category, and the frequency of positive LUS zones overall. Finally, we compared test characteristics of LUS and CXR for identifying viral/atypical pneumonia radiographically against nasopharyngeal RT-PCR swab results.
Data Analysis
Using a power analysis of 80%, our sample size calculation of 98 patients was based on previous data demonstrating a 20% difference in sensitivities between LUS and CXR at diagnosing pneumonia (31). Presently, there are only limited retrospective data with respect to LUS findings in COVID-19 pneumonia. Data are presented as proportions with 95% confidence intervals (CIs). Fisher's exact and chi-squared tests were utilized.
Results
Refer to the patient flow chart in Figure 3 : we approached and enrolled 143 consecutive patients with one or more of the predefined signs and symptoms of COVID-19 pneumonia. Thirty-three patients were not included in the final analysis because an NCCT was not done. Twenty-seven of these patients were discharged home at the discretion of the attending physician. Four patients were admitted with the following alternate diagnoses: asthma exacerbation, chronic obstructive pulmonary disease exacerbation, non-ST-elevation myocardial infarction, and septic shock. Two patients left against medical advice (AMA) despite concern for COVID-19 pneumonia. Overall, 13 (39%) of the 33 patients had a positive LUS (positive for B-lines only), and only three (9%) CXRs were abnormal in this group.
Figure 3.
Patient flow chart. ED = emergency department; HR = heart rate; RR = respiratory rate; SBP = systolic blood pressure; RT-PCR = reverse-transcription polymerase chain reaction; COPD = chronic obstructive pulmonary disease.
During the predefined 7-day follow-up period, only 1 of the 27 discharged patients returned 5 days after the initial ED evaluation and was admitted for COVID-19. During the first ED visit, the patient had a normal CXR and LUS. No swab was done during the original presentation. One of the two patients who left AMA had a positive outpatient nasopharyngeal RT-PCR swab but no advanced imaging. This patient had a positive LUS and a normal CXR during the initial ED visit. All 3 patients had uncomplicated recoveries.
Ultimately, 110 patients underwent LUS, CXR, and NCCT. Table 1 summarizes patient characteristics. Ninety-nine LUS and 73 CXRs were interpreted as positive; 83 NCCT were interpreted as Cat 1 or 2, yielding a prevalence of COVID-19 pneumonia of 75% (95% CI 66.3–83.2). Table 2, Table 3 detail the test characteristics of LUS and CXR compared with NCCT and nasopharyngeal RT-PCR swab, respectively.
Table 1.
Patient Characteristics
| NCCT + Pneumonia (n = 83) | NCCT− Pneumonia (n = 27) | |
|---|---|---|
| Age, median (IQR), years | 56 (48–64) | 64 (50–74) |
| Sex, n (%) | ||
| Female | 47 (56.6) | 12 (44.4) |
| Male | 36 (43.4) | 15 (55.6) |
| Heart rate, median (IQR), beats/min | 98 (87–111) | 90 (73–107) |
| Blood pressure, median (IQR), mm Hg | ||
| Systolic | 134 (119–152) | 139 (127–156) |
| Diastolic | 82 (73–91) | 82 (79–89) |
| Respiratory rate, median (IQR), breaths/min | 20 (18–22) | 20 (18–22) |
| Pulse oximetry, median (IQR) | 95 (93–97) | 97 (95–98) |
| Temperature (°C), median (IQR) | 37.5 (36.8–38.5) | 36.6 (36.3–37.3) |
| Comorbidities, n (%) | ||
| Congestive heart failure | 13 (15.6) | 5 (18.5) |
| Diabetes mellitus | 26 (31.3) | 7 (25.9) |
| Coronary artery disease | 19 (22.9) | 5 (18.5) |
| Asthma | 12 (14.5) | 5 (18.5) |
| Chronic obstructive lung disease | 12(14.5) | 8 (29.6) |
| Interstitial lung disease | 2 (2.4) | 0 |
| End-stage renal disease | 5 (6.0) | 0 |
| Human immunodeficiency virus | 4 (4.8) | 2 (7.4) |
| Cancer | 4 (4.8) | 2 (7.4) |
NCCT = non-contrast-enhanced computed tomography; IQR = interquartile range.
Table 2.
Test Characteristics of CXR and LUS vs. NCCT
| CXR with Atelectasis | CXR without Atelectasis | LUS | LUS Expert Review | Pleural Line Irregularity | |
|---|---|---|---|---|---|
| Sensitivity | 69.9 (58.8–79.5) | 60.2 (48.9–70.8) | 97.6 (91.6–99.7) | 100 (95.7–100) | 32.5 (22.6–43.7) |
| Specificity | 44.4 (25.5–64.7) | 66.7 (46.0–83.5) | 33.3 (16.5–54.0) | 33.3 (16.5–54.0) | 66.7 (41.0–86.7) |
| Positive likelihood ratio | 1.26 (0.87–1.81) | 1.81 (1.03–3.17) | 1.46 (1.12–1.92) | 1.50 (1.15–1.96) | 0.98 (0.47–2.01) |
| Negative likelihood ratio | 0.68 (0.40–1.16) | 0.60 (0.41–0.87) | 0.07 (0.02–0.31) | 0 | 1.01 (0.71–1.45) |
| Odds ratio | 1.86 (0.77–4.48) | 3.03 (1.23–7.43) | 20.3 (4.46–n/a) | n/a | 0.96 (0.34–2.75) |
| Positive predictive value | 79.5 (68.4–88.0) | 84.7 (73.0–92.8) | 81.8 (72.8–88.9) | 82.2 (73.3–89.1) | 81.8 (64.5–93) |
| Negative predictive value | 32.4 (18–49.8) | 35.3 (22.4–49.9) | 81.8 (48.2–97.7) | 100 (66.4–100) | 17.6 (9.47–28.8) |
Data are presented as percentages with 95% confidence intervals.
CXR = single view, portable anteroposterior chest x-ray study; LUS = eight-zone point-of-care lung ultrasound; NCCT = non-contrast-enhanced computed tomography.
Table 3.
Test Characteristics of CXR and LUS vs. Nasopharyngeal RT-PCR
| CXR with Atelectasis | CXR without Atelectasis | LUS | LUS Expert Review | Pleural Line Irregularity | |
|---|---|---|---|---|---|
| Sensitivity | 67.2 (4.31–78.4) | 56.3 (43.3–68.6) | 96.9 (89.2–99.6) | 100 (94.4–100) | 26.6 (16.3–39.1) |
| Specificity | 29.7 (15.9–47.0) | 43.2 (27.1–60.5) | 13.5 (4.54–28.8) | 13.5 (4.54%–28.8) | 70.3 (53.0–84.1) |
| Positive likelihood ratio | 0.96 (0.73–1.25) | 0.99 (0.70–1.41) | 1.12 (0.98–1.28) | 1.16 (1.02–1.31) | 0.89 (0.47–1.70) |
| Negative likelihood ratio | 1.10 (0.60–2.02) | 1.01 (0.64–1.61) | 0.23 (0.05–1.13) | 0.00 | 1.05 (0.81–1.35) |
| Odds ratio | 0.87 (0.36–2.08) | 0.98 (0.43–2.22) | 4.84 (0.89–26.4) | 21.8 (1.17–407.1) | 0.85 (0.35–2.10) |
| Positive predictive value | 62.0 (55.4–68.1) | 62.8 (54.2–70.6) | 65.6 (62.5–68.6) | 66.3 (63.4–69.1) | 60.3 (44.5–74.3) |
| Negative predictive value | 34.7 (22.5–49.4) | 36.7 (26.78–48.0) | 71.8 (34.1–92.6) | 100 | 36.0 (30.3–42.1) |
Data are presented as percentages with 95% confidence intervals.
CXR = single view, portable anteroposterior chest x-ray study; LUS = eight-zone point-of-care lung ultrasound; RT-PCR = nasopharyngeal reverse-transcription polymerase chain reaction swab.
There were 27 Cat 3 NCCTs. Of those, 11 (40.7%) had no abnormalities noted on NCCT. Other findings on Cat 3 NCCT included the following: emphysematous changes (n = 7), atelectasis (n = 6), pleural effusion (n = 1), pulmonary edema (n = 1), and sarcoidosis (n = 1). Nine of the 11 NCCTs without abnormalities had a normal LUS, and eight had a normal CXR. Both LUS and CXR were abnormal in sarcoidosis and pleural effusion. LUS noted the pulmonary edema, which CXR missed.
With respect to our study population, the two false-negative LUS were read as positive on expert review (98.8% agreement, κ = 0.8901). Notably, 1 patient with a false-positive LUS and a positive COVID-19 swab test was admitted to the hospital. Expert review of the LUS agreed with the initial positive result. On hospital day #1, the patient's repeat CXR was read as markedly abnormal consistent with COVID-19 pneumonia. The inpatient provider deemed a repeat NCCT unnecessary.
Atelectasis is typically an indeterminate CXR finding, therefore, we also calculated test characteristics defining atelectasis as a negative finding for COVID-19 pneumonia. With this definition, 59 CXRs were interpreted as positive. The sensitivity of CXR using this definition was 60.2% (95% CI 48.9–70.8%). Refer to Table 2 for detailed test characteristics.
Every LUS that was interpreted as positive had B-lines. The next most common finding was an irregular pleural line (27.3%). Its presence alone improved LUS specificity to 66.7% (95% CI 41–86.7) but diminished its sensitivity to 32.5% (95% CI 22.6–43.7). The number of positive LUS zones was higher in NCCT Cats 1 and 2 (3.69 [95% CI 3.35–4.03]) compared with NCCT Cat 3 (1.92 [95% CI 1.25–2.59]). In fact, when comparing Cat 1 with Cat 2, the number of positive LUS zones was higher in Cat 1 (3.9 [95% CI 3.51–4.27]) than in Cat 2 (2.87 [95% CI 2.21–3.53]).
Two patient subgroups are worth highlighting. Congestive heart failure (CHF) and interstitial lung disease (ILD) have similar radiographic findings as COVID-19 pneumonia on CXR and LUS. In our study population, 2 patients (2%) had ILD and 18 (16%) had CHF. CXR, LUS, and NCCT (Cat 1) were each positive for both ILD patients, although neither had a positive nasopharyngeal swab. In the CHF subgroup, 8 patients had a Cat 1 NCCT, 5 patients had a Cat 2 NCCT, and 5 patients had a Cat 3 NCCT, yielding a prevalence of 72% (95% CI 46.5–90.3). LUS had a sensitivity of 92.3% (95% CI 64.0–99.8) and a specificity of 20% (95% CI 0.51–71.6) for CHF patients with radiographic evidence of COVID-19 pneumonia on NCCT (Cats 1 and 2). LUS had four false positives and one false negative, which was read as positive on expert review. CXR including atelectasis had a sensitivity of 46% (95% CI 19.2–74.9) and a specificity of 20% (95% CI 0.51–71.6). Notably, 7 of the Cat 1 CHF patients had a positive nasopharyngeal swab. Two of the Cat 2 CHF patients had a positive swab, and none of the Cat 3 CHF patients had a positive swab.
Of the 110 patients who met final inclusion criteria, 101 had nasopharyngeal RT-PCR swabs done. Of the Cat 3 NCCT patients, 9 did not have a swab performed given that the NCCT results were not consistent with viral/atypical pneumonia. The remaining 18 patients with Cat 3 NCCT results had swabs taken prior to the NCCT results. All Cat 1 and 2 patients had nasopharyngeal swabs performed. Overall, there were 64 positive nasopharyngeal RT-PCT swabs, yielding a prevalence of 63% (95% CI 53.2–72.7). Notably, 86% of Cat 1 NCCT scans had a positive COVID-19 swab, as well as 58% of Cat 2 NCCT scans. None of the Cat 3 NCCT scans had a positive COVID-19 swab. Table 3 reviews the test characteristics of LUS and CXR compared with nasopharyngeal swab.
Discussion
This study demonstrates that LUS has better sensitivity for the radiographic diagnosis of viral/atypical pneumonia than portable CXR, which is consistent with prior literature assessing its use in bacterial pneumonia (32, 33, 34). LUS is an accurate diagnostic imaging modality for pneumonia in diverse clinical settings among varied adult and pediatric patient populations (32, 33, 34). In our study, LUS had sensitivity comparable with NCCT. In fact, the 2 LUS false-negative patients were read as positive on expert review. Although CXR is widely available, it was only 70% sensitive using a generous definition of abnormalities.
LUS has become widely implemented in numerous clinical settings. More recently, inexpensive portable, handheld ultrasound units have been utilized in areas with a COVID-19 patient surge. Our hospital was experiencing such a surge, allowing us to collect these data quickly. To our knowledge, this is the first study comparing initial LUS and CXR to NCCT radiographic findings of COVID-19 pneumonia.
We chose to use NCCT as our reference imaging standard, as LUS and CXR are both looking for signs of pneumonia, not for presence of the virus. Furthermore, our hospital's treatment and disposition guidelines were based on imaging abnormalities in conjunction with the patient's clinical status. Our hospital included both Cat 1 and Cat 2 reads on NCCT to define viral/atypical pneumonia in an attempt to capture all cases. We chose to include both Cat 1 and 2 as well. As mentioned in our results, 86% of Cat 1 NCCT scans had a positive COVID-19 swab as well as 61% of Cat 2 NCCT scans. None of the Cat 3 NCCT scans had a positive COVID-19 swab.
Due to previous reports suggesting diffuse skip lesions of COVID-19 pneumonia, we devised an eight-view LUS protocol (19, 20, 21, 22). Our results suggest that this is a sufficient approach. Our 2 false-negative patients did have B-lines on expert review, although they were on the edge of the field of view, and we surmise that is why they were missed initially. We did not record the scan time, but the ease of portable, handheld ultrasounds allowed the EP to move efficiently through subsequent patients. We estimate the entire LUS took ≤ 5 min for each patient.
The specificities of both LUS and CXR were low. However, our study was designed to augment sensitivity of both LUS and CXR. We defined any CXR finding of infiltrate or atelectasis as possibly showing the radiographic changes of viral/atypical pneumonia. Similarly, we defined the presence of three or more B-lines or the presence of a single confluent B-line encompassing a third or more of the visualized distal intercostal space as positive for viral/atypical pneumonia even though these findings are often found in other pulmonary conditions, such as CHF and ILD (28, 29, 30,35, 36, 37, 38). B-lines are vertical, hyperechoic artifacts arising from the visceral pleura or alveoli extending to the bottom of the screen and are well-described artifacts of LUS (15). B-lines were the most common abnormality seen in the study group, with every positive LUS including B-lines.
Patients with ILD and CHF exacerbations present a unique challenge given that these individuals have B-lines present on LUS at baseline (15,16,35, 36, 37). Fortunately, ILD is an uncommon condition. In our study population, only 2 patients had ILD. LUS (positive for B-lines), CXR, and NCCT (Cat 1) were positive for each patient. Subpleural consolidations and effusions on LUS suggest infection. However, none of these findings were present on either patients’ LUS. With ILD patients, it is important to assess for other signs and symptoms of infection.
CHF is far more common—16% of our study patients had this comorbidity. In this subgroup, LUS identified 92% of the patients with viral/atypical pneumonia on NCCT with four false positives. Previously, we discussed the one LUS false-positive patient with a positive swab who on hospital day #1 had a CXR consistent with viral/atypical pneumonia. On expert review, the one false negative was read as positive for B-lines. CXR, including atelectasis, had a sensitivity of 46% in CHF patients with viral/atypical pneumonia. Typically, LUS in CHF demonstrates a smooth pleural line and lack of subpleural consolidations (15,16). The presence of an irregular pleural line or consolidation implies an infectious etiology. Using the finding of irregular pleural line increased our specificity to 66% but lowered our sensitivity.
Unfortunately, we were unable to include 33 patients in the final data. Twenty-seven patients were considered low risk and discharged without an NCCT at the discretion of the attending EP, 4 were admitted for alternate diagnoses, and 2 left AMA. Although we can speculate that some of these patients had coronavirus, we were unable to perform outpatient testing due to limited availability of tests. This limited our study to more clinically sick patients. It is unknown if this would change our sensitivity, but it is likely that if these patients were included, the specificity of both LUS and CXR would increase.
COVID-19 is a highly contagious infection transmitted via contact and airborne droplets (39). We chose to utilize a portable, handheld ultrasound device to perform LUS to minimize staff exposure, patient movement, and the use of personal protective equipment. Moreover, the handheld device is easily disinfected, operates on battery power, and requires less supporting infrastructure. Even a portable CXR requires one to two staff members to perform an extensive disinfection. These characteristics make portable, handheld ultrasounds ideal in diverse medical environments that are resource limited: whether in newly constructed tents in large urban areas or in remote villages far from modern facilities.
Typical barriers to broad implementation of point-of-care ultrasound (POCUS) are experience and time restraints. Our protocol took < 5 min to complete. Furthermore, a diverse group of 21 PGY1–3 emergency medicine residents and 22 attending EPs performed the LUS without additional training. If larger studies corroborate these results, LUS may be a viable choice for diagnosing COVID-19 pneumonia, especially in situations where CXR and NCCT are difficult to obtain. In outpatient settings, temporary surge facilities, or resource-limited areas, a negative LUS could obviate the need for further imaging. Although a positive LUS lacks specificity, combining this imaging technology with a rapid COVID-19 laboratory test would help determine which patients might require further imaging and treatment. Such LUS protocols are already being used, and our data lend support to these efforts (40).
It is important to note that we do not advocate that all patients with suspected COVID-19 pneumonia have an NCCT done. This was a temporary hospital policy during the initial surge in the spring of 2020. The aim was to identify patients with radiographic evidence of viral/atypical pneumonia, presumed to be COVID-19, to isolate them to particular sections of our institution. Given the similar sensitivities of LUS and NCCT, POCUS may provide a means to diagnose or at least rule out COVID-19 pneumonia given the 100% negative predictive value in our study, when utilized by fellowship-trained providers. Furthermore, the association of more positive LUS zones with NCCT Cats 1 and 2 and less positive zones with Cat 3 may allow providers to distinguish the sicker individuals from those less ill and to assess disease progression or improvement without the need for NCCT.
Limitations
This study suffers from the limitations of a single-center study. Furthermore, the EPs participating were not blinded to the LUS indication, which may have caused providers to overcall certain LUS findings and interpret them as positive. Moreover, we used a broad definition of positive findings for both LUS and CXR, which increases the sensitivity of both at the expense of specificity.
Another significant limitation was that only anteroposterior CXR was performed. This is a temporary institutional policy during the current pandemic to limit patient movement and staff exposure. Posteroanterior/lateral films are standard of care, as anteroposterior films are less accurate. However, our radiologists were unblinded to the CXR indication, which potentially caused overreading CXR findings as positive as well. Similarly, the unblinded radiologists may have overread NCCT findings, interpreting them as abnormal.
NCCT is an imperfect diagnostic standard, as is the nasopharyngeal RT-PCR swab. Nonetheless, NCCT is considered the reference standard for the diagnosis of viral pneumonia, given its sensitivities between 97% and 100% (4,8, 9, 10, 11). Therefore, we chose NCCT to assess the test characteristics of LUS and CXR at radiographically diagnosing of viral/atypical pneumonia, presumed to be COVID-19 in the current pandemic. Furthermore, our pulmonary/critical care and radiology departments mandated that all patients with suspected COVID-19 pneumonia have an NCCT done prior to admission. Furthermore, our institution's treatment guidelines were based on the NCCT findings in conjunction with patient clinical status. The finding of ground glass opacities indicated the presence of viral or atypical pneumonia. Notably, ground glass opacities are not specific to COVID-19 pneumonia. They can be found in any type of viral or atypical pneumonia. Nonetheless, it is likely that each abnormal NCCT indicated the presence of COVID-19 pneumonia given its high prevalence during the current pandemic.
The prevalence of COVID-19 pneumonia in our patient population was 75%, indicating a significant surge that may impact the positive and negative predictive values of LUS. This limits the generalizability of our results. Furthermore, we did not account for lower-risk patients who did not undergo an NCCT. LUS test characteristics may be different in lower-risk individuals and in populations with lower disease prevalence.
Lastly, our ED is not representative of the broader medical community. We have an active ultrasound division with numerous faculty and fellows. All attending EPs are credentialed in POCUS per ACEP guidelines (23,24). In our department, residents are the treating clinicians and have more ultrasound experience compared with most practicing physicians. Our results may not be generalizable to the medical community with less POCUS experience. Furthermore, our patient population is not representative of the overall population, especially given that the study was done during a significant surge time in the current COVID-19 pandemic.
Conclusion
In summary, point-of-care LUS was more sensitive than chest x-ray study at radiographically diagnosing viral/atypical pneumonia, presumed to be COVID-19, in a high-prevalence population. Both have similarly low specificities. Portable, handheld ultrasound devices could become an effective first-line imaging modality for COVID-19 pneumonia in diverse clinical settings.
Article Summary
1. Why is this topic important?
Coronavirus 2019 (COVID-19) has been a global pandemic. It is paramount to have an accurate means to diagnosis patients to expedite treatment and quarantine.
2. What does this study attempt to show?
This study attempts to show the utility of point-of-care lung ultrasound for the diagnosis of COVID-19 pneumonia.
3. What are the key findings?
Point-of-care lung ultrasound is more sensitive than chest x-ray study at diagnosing COVID-19 pneumonia.
4. How is patient care impacted?
Fewer missed diagnoses using an ultrasound first approach when assessing patients with suspected COVID-19 pneumonia.
References
- 1.World Health Organization Coronavirus disease (COVID-19) situation report-207. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Call S.A., Vollenweider M.A., Hornung C.A., et al. Does this patient have influenza? JAMA. 2005;293:987–997. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]
- 6.Eden A. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Surv Anesthesiol. 2004;48:225–226. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Wong H.Y., Lam H.Y., Fong A.H., et al. Frequency and distribution of chest radiographic findings in covid-19 positive patients. Radiology. 2020:e201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng M.Y., Lee E.Y., Yang J., et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2020;2:e200034. doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler M.H., Touw H.R., van de Ven P.M., et al. Diagnostic accuracy of chest radiograph, and when concomitantly studied lung ultrasound, in critically ill patients with respiratory symptoms: a systematic review and meta-analysis. Crit Care Med. 2018;46:e707–e714. doi: 10.1097/CCM.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 10.Caruso D., Zerunian M., Polici M., et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020:e201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y., Zhang H., Xie J., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:e115–e117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:e32–e40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Yang M., Shen C., et al. Laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv. 2020 doi: 10.1016/j.xinn.2020.100061. https://www.medrxiv.org/content/10.1101/2020.02.11.20021493v2.full.pdf+html Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Yao L., Li J., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92:903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenstein D.A., Meziere G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell F.M., Ehrman R.R., Cosby K., et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: a lung and cardiac ultrasound (Lu CUS) protocol. Acad Emerg Med. 2015;22:182–191. doi: 10.1111/acem.12570. [DOI] [PubMed] [Google Scholar]
- 17.Testa A., Soldati G., Copetti R., et al. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16:R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsung J.W., Kessler D.O., Shah V.P. Prospective application of clinician-performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: distinguishing viral from bacterial pneumonia. Crit Ultrasound J. 2012;4:1–10. doi: 10.1186/2036-7902-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poggiali E., Dacrema A., Bastoni D., et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295:e6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonsenso D., Piano A., Raffaelli F., et al. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y., Wang S., Liu Y., et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19) https://ssrn.com/abstract=3544750 Available at:
- 22.Peng Q.Y., Wang X.T., Zhang L.N. Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Int Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Emergency Physicians Policy statement. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. https://www.acep.org/patient-care/policy-statements/ultrasound-guidelines-emergency-point-of--care-and-clinical-ultrasound-guidelines-in-medicine/ Available at:
- 24.Accreditation Council of Graduate Medical Education Emergency medicine defined key index procedure minimums. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/EM_Key_Index_Procedure_Minimums_103117.pdf?ver=2017-11-10-130003-693 Available at:
- 25.American College of Emergency Physicians Guideline on COVID-19: ultrasound machine and transducer cleaning. https://www.acep.org/globalassets/new-pdfs/policy-statements/guideline-for-ultrasound-transducer-cleaning-and-disinfection.pdf Available at: [DOI] [PMC free article] [PubMed]
- 26.Butterfly iQ cleaning and disinfection guidelines. https://www.butterflynetwork.com/covid19/cleaning-and-disinfection Available at:
- 27.Apple How to clean your Apple products. https://support.apple.com/en-us/HT204172 Available at:
- 28.Anderson K.L., Fields J.M., Panebianco N.L., et al. Inter-rater reliability of quantifying pleural B-lines using multiple counting methods. J Ultra Med. 2013;32:115–120. doi: 10.7863/jum.2013.32.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Testa A., Soldati G., Copetti R., et al. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16:1–8. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpicelli G., Elbarbary M., Blaivas M., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 31.Ye X., Xiao H., Chen B., et al. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One. 2015;10(6):e0130066. doi: 10.1371/journal.pone.0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez M.A., Shams N., Ellington L.E., et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereda M.A., Chavez M.A., Hooper-Miele C.C., et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reissig A., Copetti R., Mathis G., et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest. 2012;142:965–972. doi: 10.1378/chest.12-0364. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Gargani L., Barskova T., et al. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther. 2017;19:206. doi: 10.1186/s13075-017-1409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie H.Q., Zhang W.W., Chen X.M., et al. A simplified lung ultrasound for the diagnosis of interstitial lung disease in connective tissue disease: a meta-analysis. Arthritis Res Ther. 2019;21:93. doi: 10.1186/s13075-019-1888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liteplo A.S., Marill K.A., Villen T., et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–210. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 38.Pivetta E., Goffi A., Lupia E., et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED. A SIMEU multicenter study. Chest. 2015;148:202–210. doi: 10.1378/chest.14-2608. [DOI] [PubMed] [Google Scholar]
- 39.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 40.Soldati G., Smargiassi A., Inchingolo R., et al. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients; a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39:1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]