Abstract
It is becoming increasingly clear that virtually all types of human cancers harbor a small population of stem-like cancer cells (i.e., cancer stem cells, CSCs). These CSCs preexist in primary tumors, can self-renew and are more tolerant of standard treatments, such as antimitotic and molecularly targeted agents, most of which preferentially eliminate differentiated and proliferating cancer cells. CSCs are therefore postulated as the root of therapy resistance, relapse and metastasis. Aside from surgery, radiation, and chemotherapy, immunotherapy is now established as the fourth pillar in the therapeutic armamentarium for patients with cancer, especially late-stage and advanced cancers. A better understanding of CSC immunological properties should lead to development of novel immunologic approaches targeting CSCs, which, in turn, may help prevent tumor recurrence and eliminate residual diseases. Here, with a focus on CSCs in solid tumors, we review CSC regulation programs and recent transcriptomics-based immunological profiling data specific to CSCs. By highlighting CSC antigens that could potentially be immunogenic, we further discuss how CSCs can be targeted immunologically.
Keywords: Cancer stem cells, Immunogenicity, Immunotherapy, Prostate cancer stem cells, Stem cell genomics
1. Introduction
Human cancer is a heterogeneous disease with most tumors containing phenotypically and functionally distinct subsets of cells [1]. Mounting evidence has established the presence of a relatively small population of cancer cells with stem-like properties in most, if not all, untreated human malignancies. These cells biologically resemble normal stem cells (SCs) found in the same tissue, such as the capacity of self-renewal and differentiation, and are thus frequently termed ‘cancer stem/stem-like cells’ (CSCs) [1, 2]. Functionally, CSCs are believed to be the subpopulation among bulk tumor cells with the ability to initiate and long-term repopulate tumors with recapitulation of the lineage/cellular heterogeneity seen in parental tumors [1–3]. Since the first identification of CSCs in human acute myeloid leukemia (AML) [4, 5], CSCs have been isolated from many different malignancies, including cancers of the breast, prostate, colon, brain, pancreas, lung, liver, bladder, ovary and others (reviewed in [2, 3, 6–8]).
Despite advances in cancer detection and treatment, most advanced cancers still remain lethal. Compared to primary tumors that generally can be effectively treated, and, in some cases, cured (e.g., by a combination of surgical resection and standard therapies), cases of advanced cancer (where surgical intervention is infeasible), recurrent and metastatic disease are in desperate need of immediate care. Most standard of care therapies (e.g., chemotherapy, radiotherapy, and molecularly targeted therapy) target relatively differentiated and proliferating cancer cells, leaving behind CSCs that are mostly dormant and have been documented to resist many clinical treatments, subsequently resulting in tumor relapse and metastasis (Fig. 1) [8, 9]. Furthermore, in multiple cancer types, the frequency of CSCs increases as tumors progress. This expansion occurs, for example, in PSA-/lo CSCs in prostate cancer (PCa) [10] and in PKH26+ slow-cycling CSCs in breast cancer (BCa) [11], highlighting the importance of CSCs as a therapeutic target, especially for advanced cancers. Increasing evidence has unraveled diverse mechanisms by which CSCs utilize to survive under hostile conditions leading to therapy resistance and tumor relapse. Cell quiescence, overexpression of multifunctional efflux transporters, enhanced DNA-repair capacity, aberrant activation of developmental pathways, high levels of anti-oxidant proteins, overexpression of anti-apoptotic proteins, underexpression of the molecular targets of targeted therapies (e.g., androgen receptor (AR) in PCa and estrogen receptor (ER) in BCa), and interactions with the tumor microenvironment (TME) are all examples of intensively studied survival mechanisms in CSCs (reviewed in [2, 3, 9, 12–16]). A deeper understanding of these mechanisms, coupled with uncovering novel mechanisms underlying CSC therapy resistance, will improve the efficacy of current anticancer treatments and aid the development of novel CSC-specific therapeutic strategies including immunotherapies (Fig. 1).
Figure 1. Targeting CSCs to effectively treat cancer.
The inability of conventional therapies to target CSCs represents a significant factor contributing to current cancer treatment failure. A combination of conventional therapy and CSC-specific therapy is expected to achieve a better clinical outcome by inhibiting tumor regrowth mediated by drug-resistant CSCs.
Immunotherapy has recently regained global attention and has emerged as the ‘new hope’ for cancer treatment [17]. This is due, in large part, to the appreciation of immune evasion as a hallmark of cancer [18] and the success in developing immune checkpoint inhibitors for the treatment of aggressive cancer, such as melanoma. Solid tumors escape immunosurveillance and evade eradication via avoiding detection by the immune system or limiting the extent of immunological killing. As advocated in the ‘cancer immunoediting hypothesis’ [19], tumor cells, as well as CSCs, have developed a myriad of strategies to circumvent the immune attack, including genetic and nongenetic alterations that lead to reduced immune recognition, activation of oncogenic pathways that lead to enhanced tolerance to cytotoxic effects of immunity, loss of tumor antigen expression, and promotion of a protective immunosuppressive microenvironment. Multiple lines of evidence have emerged to demonstrate the immunoresistance of CSCs in different cancer types [20, 21]. In this review, with a focus on CSCs in solid tumors, we summarize the current understanding of CSCs with respect to their mechanistic regulatory network and immunological properties. By highlighting the immune gene expression profile of CSCs and CSC-associated markers and tumor-associated antigens (TAAs) that could potentially be used for immunotherapy, we discuss CSC immunogenicity and the potential immunological approaches to target them.
2. CSC regulation programs
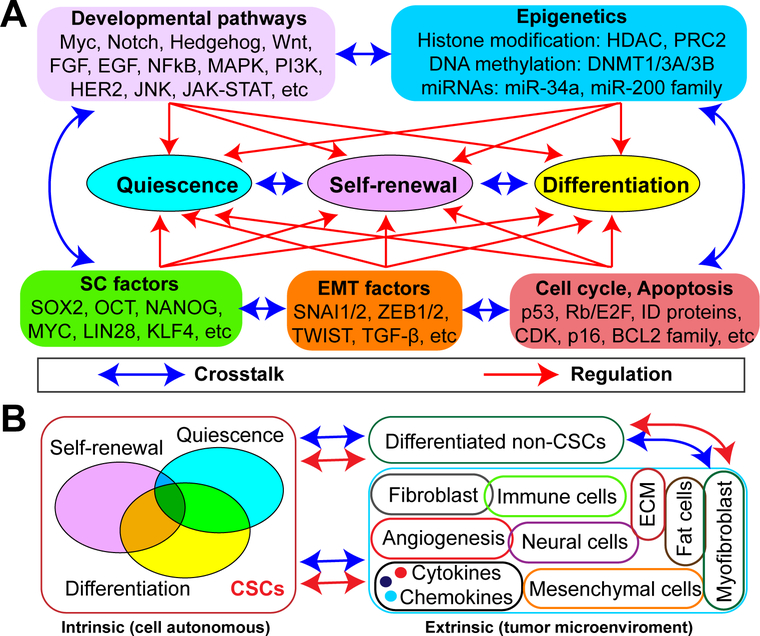
Tumorigenesis resembles abnormal organogenesis. Like normal tissue SCs, CSCs display three cell-intrinsic principal properties: self-renewal (deregulated active state for maintenance), quiescence (non-active state for survival) and differentiation (state of losing stemness). Therefore, any programs (genetic and/or non-genetic) that can regulate one or more of these three properties could theoretically have an impact on CSC biology. Genetics is a ‘primer’ that modulates CSC phenotypes via various mechanisms. Aside from metabolism, which represents the functional outcome of CSC regulation, developmental pathways, SC factors, cell cycle regulation and apoptosis, epithelial-mesenchymal transition (EMT) and epigenetics represent the five most frequently reported mechanisms governing CSC biology (Fig. 2A). Of note, owing to intricate interactions and overlap between and among the mechanistic programs that regulate CSCs, definition of the five mechanisms (Fig. 2A) is somewhat arbitrary, as they could all eventually converge on transcriptional regulation driven by specific and cohorts of transcription factors.
Figure 2. Regulation of CSCs.
(A) Intrinsic CSC regulatory mechanisms. Depicted are the five frequently reported mechanisms (Developmental pathways, Epigenetics, SC factors, EMT factors, and Cell cycle/Apoptosis) that control the main properties of CSCs (Self-renewal, Differentiation, and Quiescence). Although genetics is not specifically highlighted, it serves as a ‘primer’ and, as such, genetic alterations will impact CSC properties via affecting one or more of these mechanisms. Quiescence, self-renewal and differentiation are the key features of CSCs and are dynamically regulated by diverse factors. Each one of these mechanisms may modulate all three CSC traits and the mechanisms and CSC properties are constantly and reciprocally cross-talking with and to each other to form an intricate regulatory network.
(B) Extrinsic CSC regulatory mechanisms. A clinically overt tumor contains CSCs, differentiated/bulk non-CSCs and a tumor microenvironment (TME). Depicted is the cellular basis of the crosstalk between these three components, highlighting the reciprocal interactions and regulation between them.
Many signaling pathways operative in normal SCs during development and homeostasis are frequently found deregulated in CSCs [3, 22–24] including Myc, Notch, Hedgehog (Hh), Wnt, FGF/FGFR, EGF/EGFR, NF-κB, MAPK, PTEN/PI3K, HER2, JAK/STAT and others. Almost all of them have documented “oncogenic” roles in tumorigenesis. To a certain degree, cancer cells transcriptionally resemble embryonic stem cells (ESCs) [25]. It is therefore not surprising that several well-known ESC factors (e.g., Sox2, Nanog, Oct4, Myc, Lin28, Klf4) are often overexpressed and essential for CSC maintenance. This notion is supported by evidence showing that ectopic expression of ESC factors alone or in combination is sufficient to reprogram non-tumorigenic cells or bulk cancer cells into functional CSCs. Using PCa as a model, we have shown that Nanog is upregulated in PSA-/lo prostate CSCs [10] and its knockdown markedly inhibits tumor development [26], while its overexpression promotes CSC characteristics and resistance to androgen deprivation therapy (ADT), the current mainstay treatment for men with locally advanced PCa [27, 28]. Additionally, the EMT program has been linked to the acquisition of aggressive traits as well as stem-like properties in cancer cells, as it confers mesenchymal properties in epithelial cells [29]. A set of pleiotropic EMT transcription factors (e.g., Snail1/2, Zeb1/2, Twist) together with EMT inducers (e.g., TGF-β) have been proven to contribute to and manipulate the functional properties of CSCs (reviewed in [30]). Furthermore, altered cell cycle regulation can play a role in CSC quiescence, proliferation and apoptosis [31, 32] as many cell cycle regulators are frequently lost (e.g., p53, Rb, p16/CDKN2A, CDKN1B) or amplified (e.g., CCND1, CDK4, CCNE1) in human cancer genomes and function as tumor suppressors or oncogenes, respectively [33]. A number of typical cell cycle-related genes are reported to be dysregulated in CSCs and an altered cell cycle program helps them resist therapy-induced apoptosis [31]. Finally, epigenetics has been implicated in many, if not all, aspects of cancer biology and its role in SC (especially ESC) biology has been extensively studied [34]. Cellular differentiation is, by definition, epigenetic. Molecular determinants involved in various types of epigenetic modification, including DNA methylation, histone modification (e.g., acetylation and methylation), chromatin remodeling, and RNA interference mediated by small (e.g., siRNA, miRNA and piRNA) and long non-coding RNA (lncRNA), have been intensely investigated. For example, DNA methyltransferases 1 (DNMT1) is essential for mammary and CSC maintenance and tumorigenesis [35]. EZH2, a key subunit of polycomb repressive complex 2 (PRC2), promotes a stem-like phenotype and is overexpressed in lethal castration-resistant prostate cancer (CRPC) [36]. Inhibition of EZH2 reverses the stem-like aggressiveness of PCa and re-sensitizes cancer cells to ADT [37]. miRNAs are also well known epigenetic regulators of gene expression. For instance, miR-200 family members have the ability to modulate EMT and thus tumor invasion and metastasis [38]. Recent work from our group has shown that miR-34a and miR-141 (a miR-200 family member) can directly target prostate CSCs to inhibit tumor progression and metastasis [39, 40]. Last but not the least, it is important to note that all regulatory mechanisms (Fig. 2A) are constantly interacting with each other within an intrinsic transcriptional CSC regulatory network. In many cases, this network stems from altered transcriptional programs affected by oncogenes and/or tumor suppressors. Interestingly, these oncogenes and tumor suppressors can impact the antitumor immune response (see below).
Aside from intrinsic factors, extrinsic factors also control CSC behavior. Like normal SCs, CSCs reside in and rely on a specialized niche called TME, which represents another layer of complexity in CSC regulation (Fig. 2B). The CSC niche in solid tumors is composed of non-CSCs and a variety of non-cancer cells including inflammatory cells, immune cells, vascular endothelial cells, fibroblasts, smooth muscle cells, mesenchymal cells, adipocytes, nerve fibers and neural cells, together with extracellular matrix (ECM) (Fig. 2B) [14]. These various components collaboratively interact with each other via networks of cytokines, chemokines and growth factors to create a hypoxic, inflammatory, and immunosuppressive environment that facilitates tumor growth and progression (reviewed in [14]). Thus, the TME, like cancer cells, is dynamically evolving as the tumor develops and progresses [41]. The importance of TME in regulating CSC biology is highlighted by the use of clinical drugs that target angiogenesis, hypoxia-inducible factors (HIF) and the NF-κB inflammatory signaling pathway. New immunotherapies that target the TME are discussed briefly below.
3. Immunological properties of CSCs
3.1. CSC markers and TAAs: antigens for potential immunotherapy
Rational immune targeting of CSCs depends on identification of (i) unique CSC markers to facilitate isolation and (ii) antigens that are uniquely or preferentially expressed by CSCs compared to their differentiated non-CSC progeny and/or normal cells. Though the lineage relationship between CSCs and tissue SCs remains obscure in most tumor systems, most CSC markers have been identified based on our understanding of the underlying SC biology of relevant tissues from which the tumor originates. In addition to functional assays (e.g., Aldefluor, mammosphere, prostatosphere, neurosphere and organoid), side population (SP) and cell surface marker coupled with fluorescence-activated cell sorting (FACS) have been the main methods employed to identify and isolate putative CSC populations. Since the initial identification of the marker profile CD34+CD38- for AML CSCs [4], markers for potential CSCs have been reported in many other human malignancies (reviewed in [2, 3, 6–8]) (Table 1). These CSC markers represent promising targets for CSC immunotherapy, such as antibody therapy targeting CD44, CD133 and HER2 (see below). However, potential safety issues should be considered when utilizing these markers as CSCs often share phenotypic marker profiles with normal tissue SCs. Additionally, in some solid cancers, a limited number of CSC markers are currently available and in general the biological function of these markers are less characterized.
Table 1.
CSC markers reported in different human cancers
| Cancer type | CSC markers |
|---|---|
| Leukemia | CD34+CD38-, CD96+, ALDH+, CD47+, CD44+,CD123+, TIM-3+, CD32+ and CD25+, CLL-1+ |
| Breast | CD44+CD24-, PKH26+, CD49fli, ALDH+, SP, CD133+, CD90hi, CD44+CD49fhiCD133/2hi |
| Prostate | SP, ABCG2+, CD44+, ALDH+, CD44+α2β1hi, CXCR4+ and CD133+, CD44+α2β1hiCD133+, NANOG+, PSA-/lo, CK187CK19- (HLAI-), TRA-1–60+CD151+CD166+, ALDHhiCD44+α2β1+ |
| Bladder | CD44+CK5+CK20-, EMA-CD44v6+, SP, 67LR+CD66c-CK17+, ALDH+, SOX2+ |
| Ovarian | ALDH+ and ALDH+CD133+, ALDH+ and CD44+, SP, CD117/c-kit+, CD44+ and MyD88+, CD24+, CD133+CXCR4+, |
| Colon | CD133+, CD44+, EpCAMhiCD44+ and/or CD166+, ALDH+, SP, LGR5+, ABCB5+ |
| Liver | CD133+, ALDH+, EpCAM+ and CD90+, CD45-CD90+, CD133+CD44+,CD13+, ICAM-1+, SALL4+ |
| Lung | SP, CD133+, ALDH+, CD117/c-kit+, OCT4/NANOG+, CD44+, TPBG/5T4+, CD166+, CD166+CD44+ and CD166+EpCAM+ |
| Pancreatic | CD44v6+, α6β4+, TSPAN8+ and CXCR4+, CD44+CD24+ESA+, c-Met+, ALDH+, CD133+CXCR4+ |
| Glioblastoma | CD133+, SP and ABCG2+, SSEA-1+, SOX2+, BMI1+ and MUSASHI1+, NESTIN+, OLIG2+, CD49f+, A2B5+CD133-, L1CAM+, EGFR+, CD44+ and CD44hiID1hi, MYC+, ALDH+ |
CSCs and their more differentiated progeny in many tumor types display distinct transcriptomic profiles and therefore express different antigens. CSCs are known to express a variety of TAAs that potentially may be recognized by the host immune system [42]. Different subgroups of TAAs have been described in CSCs [21, 43, 44] including: (i) overexpressed antigens that are minimally expressed by normal tissues but constitutively overrepresented by tumors as part of their malignant phenotype (e.g., hTERT, EGFR, survivin); (ii) cancer/testis (CT) antigens that are normally restricted to adult testicular germ cells and placenta but aberrantly activated in tumors (e.g., NY-ESO1, MAGE-A3, -A4); (iii) mutated antigens (neoantigens) derived from somatic mutations in cancer genomes leading to entirely new epitopes recognizable by immune cells (e.g., MUM-1 and CDK4 in melanoma); and (iv) differentiation antigens specific to a certain type of tissue and expressed by both cancer and nonmalignant cells. Many lineage-specific markers are considered differentiation antigens (e.g., PSA in PCa and MART-1 in melanoma). To a certain extent, CSC markers (Table 1) can be considered as merely overexpressed antigens, although not all of these markers may be expressed minimally in normal cells. Though lineage differentiation antigens are generally expressed at low levels in CSCs, CT antigens and neoantigens are considered the best candidates for CSC-targeting immunotherapy [43]. In a gene-expression analysis comparing SP and non-SP cells purified from colon, lung and breast cancer cells, Yamada et al. found 18 CT antigens that were preferentially expressed in CSCs, many of which had been reported as targets of cytotoxic T lymphocytes (CTLs) [45]. For example, the CT antigen HSP40 family member DNAJB8 is preferentially expressed in renal and colon CSCs and has an important functional role in maintaining CSCs [46, 47]. A DNAJB8-specific CTL response could be induced by a DNA vaccination in renal cancer models [47] and by DNAJB8-derived antigenic peptide in colon cancer models [46]. For DNA mutation-created neoantigens, they can originate from either ‘driver’ or ‘passenger’ mutations. Although ‘drivers’ are much more attractive as targets, cancer cells can be theoretically targeted by any mutation regardless of its oncologic significance. High mutational index has been suggested to be the underlying mechanism behind the success of immune checkpoint inhibitor therapy in certain cancer types. It is as yet unclear whether the mutation index of CSCs may be distinct from that of non-CSCs, as no report has unraveled the CSC-specific mutational landscape so far. Despite the paucity in reported CSC-specific neoantigens, there is data suggesting that neoantigens are present in colorectal cancer, in both CSCs and non-CSCs, in a manner that is targetable by neoepitope-specific CD8+ T cells [48].
3.2. Immunological profile and immunogenicity of CSCs
A deep understanding of the immunological properties of CSCs is the key step towards successful CSC-specific immunotherapies. Unfortunately, knowledge in this regard is limited. Further characterization of the CSC immunological profile, including antigen processing and presenting molecules (e.g., major histocompatibility complex (MHC) encoded by human leukocyte antigen (HLA) genes), cytokines, TAAs, and co-stimulatory and co-inhibitory molecules, in various cancer types, will be essential in developing efficacious immunotherapies [42]. Previously, comprehensive immune profiling in CSCs and autologous non-CSCs isolated from glioblastoma multiforme (GBM) assessed the expression of MHC-I and -II, antigen-processing machinery (APM) molecules and ligands of NKG2D (MICA/B and ULBPs) that engage NKG2D receptors on NKs or T lymphocytes [49]. Results revealed defective expression of these molecules and low sensitivity of CSCs to interferon stimulation (which is generally effective in enhancing APM gene expression) [49]. Consequently, GBM CSCs failed to elicit a T cell mediated response, and actually suppressed T cell proliferation and induced their differentiation toward a TH2 phenotype in co-culture experiments with autologous circulating lymphocytes [49]. This example highlights the low immunogenicity of CSCs and shows how the immunological profile dictates immunosuppressive activity. Similarly, tumorigenic ABCB5+ melanoma CSCs preferentially inhibited IL-2–dependent T-cell activation in a CD86-dependent manner and induced CD4+CD25+FoxP3+ regulatory T cells (Tregs) [50]. Molecularly, melanoma CSCs displayed lower levels of MHC-I (but not MHC-II) and melanoma-associated antigens (e.g., MART-1, ML-IAP, NY-ESO-1, MAGE-A), and higher levels of co-stimulatory molecules CD86 and PD-1, leading to their immune-evasive capacity [50]. While the trend of defective expression in HLA molecules and APM components in CSCs in various types of cancers is generally recognized, controversial and conflicting results exist. For instance, colon CSCs isolated as sphere-forming cells expressed lower levels of MHC class I relative to non-CSCs [51]; whereas CSCs isolated as the SP cells from colon cell lines expressed HLA class I at the same level as the main-population cells [52]. Wei et al. reported that GBM CSCs isolated as sphere-forming cells expressed high levels of MHC I and low levels of CD86 and CD40, but not MHC II or CD80, indicating a potential lack of the capacity for antigen presentation necessary to stimulate T-cell activation or proliferation in these CSCs [53]. With regard to APM gene expression, CSCs isolated as spheres from 12 human solid tumor cell lines showed equal or higher mRNA levels of APM molecules (e.g., LMP2, LMP7 and MECL-1, and TAP1 and TAP2 transporters), but downregulation or loss of HLA-I molecules in spheres observed in 8 of 10 cell lines [54]. Interestingly, sphere-cultured cells derived from human primary GBM and colon cancer samples exhibited low or no expression of APM molecules [49, 55]. Collectively, it seems that, despite varying immunological profiles in different contexts (e.g., different CSC isolation methods and culture conditions; CSCs in different tumor types), one way or another, the antigen presentation function is compromised in CSCs as both MHC and APM are required for an efficient antigen presentation.
We investigated the immunological profiles of normal prostate SCs and prostate CSCs (PCSCs). First, we employed the TCGA RNA-seq PRAD data to survey the expression levels of a large panel of key cancer immunity-related genes (Fig. 3A) in primary PCa compared with normal/benign prostate tissues [56]. Interestingly, we observed differential expression in only a few genes (Fig. 3B), which is consistent with a low immunogenicity typically found in PCa and low responsiveness of patient prostate tumors to current mono-immunotherapies. We recently interrogated the whole-genome transcriptomes of AR- basal/stem vs. differentiated AR+ luminal cells and found that human basal cells molecularly resemble ESCs [57]. Interestingly, the prostate basal/stem cells overexpress immune-inhibitory factors (e.g., PDL2 and TGF- β) while underexpress many HLA molecules (Fig. 3C). ESCs express little MHC-I and no MHC-II genes [58]. Importantly, we previously defined a PCSC population based on the lack of expression of the prostate differentiation gene PSA (e.g., PSA-/lo) [10]. Here we observed the downregulation of HLA molecules and upregulation of IL-4 in freshly purified PSA-/lo PCa cells compared with the corresponding PSA+ cells (Fig. 3D). A study in colon cancer revealed an immune-suppressive role for CSC-associated IL-4 in T cell function [59]. Clinically, neuroendocrine PCa (NEPC) represents the most stem-like and lethal subtype of PCa. By reanalyzing a published RNA-seq dataset [60] encompassing CRPC that were histologically characterized as adenocarcinomas (CRPC-Adeno) and NEPC-like CRPC (i.e., CRPC-NE), we observed that CRPC-NE, compared to CRPC-Adeno, under-expressed PD-1, co-stimulatory molecules (e.g., CD86 and CD28) and many HLA genes (Fig. 3E), suggesting an overall low immunogenicity for NEPC cells. These results imply that the current anti-PD1 drugs may have limited therapeutic efficacy against NEPC cells. On the other hand, NEPC overexpressed CD133 and MAGEA3 compared to CRPC-Adeno (Fig. 3E), implicating these molecules as potential novel targets for immunotherapy. Interestingly, a recent study shows that the inefficient response of CRPC to chemotherapy is mediated by PCSCs that intrinsically resist Docetaxel, and lack differentiation markers (e.g., PSA, CK18, CK19) and HLA class I (HLA-I) antigens, but overexpress the Notch and Hedgehog signaling components [61]. These HLA-I- PCa cells are highly tumorigenic and their abundance correlates with tumor aggressiveness and poor patient prognosis. Reanalysis of this dataset [61] reveals that most selected immune-related genes, especially the HLA genes, are downregulated in Docetaxel-resistant DU145 (Fig. 3F) and 22Rv1 (Fig. 3G) cells. Thus, our analysis (Fig. 3) demonstrates an overall low immunogenicity in PCSCs. Similarly, a report in head and neck squamous cell carcinoma (HNSCC) examining the heterogeneous responses to chemotherapy indicates that chemotherapeutic agents cause downregulation of HLA class I genes and a significant enrichment of CSCs in relapses [62]. Collectively, these observations suggest that CSCs are generally characterized by low immunogenicity and are endowed with a unique immunological profile that can be further modulated by conventional anticancer treatments leading to new challenges as well as opportunities.
Fig. 3. Immunological profiles of prostate SC and PCSCs.
(A) Representative key cancer immune-related genes that are functionally classified as Cytokines, Antigen Processing & Presentation (MHC, Major Histocompatibility Complex; APM, Antigen Processing Machinery), and Immunotherapy targets (Co-stim.; Co-stimulatory).
(B) Heatmap of differentially expressed immune-related genes in primary PCa and benign/normal prostate tissues in TCGA [56].
(C-G) Heatmaps of immune-related DEGs (differentially expressed genes) in (C) normal human prostate basal/stem cells over differentiated luminal cells [57], (D) PSA-/lo PCSC population over differentiated PSA+ cells [10], (E) CRPC-NE (i.e., NEPC) over CRPC-Adeno (i.e., CRPC with adenocarcinoma phenotype) [60], (F) Docetaxel-resistant over parental DU145 cells [61], and (G) Docetaxel-resistant vs. parental 22Rv1 cells [61]. Both Docetaxel-resistant DU145 and 22Rv1 cells display CSC characteristics [61].
Scale bars in (B-G) indicate fold change (FC). Note that B, C and E were RNA-seq data whereas D, F and G were based on microarray data.
3.3. Immunomodulation and immunoresistance of CSCs
In normal adult tissues, immune evasion has been recently identified as an intrinsic property of quiescent SCs resulting from systemic downregulation of the APM, including MHC class I and TAP proteins [63]. Similarly, multiple lines of evidence have emerged to suggest the immunoresistance of CSCs in different cancer types [20, 21]. Many cell-autonomous and non-autonomous factors have been described to confer CSC immunomodulating properties. Autonomously, CSCs exhibit a deregulated immunological gene-expression profile (e.g., Fig. 3), which may predispose them to an “immune-privileged” state. Furthermore, various oncogenic pathways, in addition to governing stemness, may regulate immunological behavior in CSCs. For example, c-Myc, a pluripotency transcription factor commonly overexpressed in the majority of human cancers, transcriptionally upregulates the expression of the innate immune inhibitor CD47 and adaptive immune checkpoint molecule PD-L1 [64]. Loss of tumor suppressor PTEN leads to reduced expression of neoantigens that demonstrate strong immunoreactivity and thus associates with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma [65]. STAT3 signaling, as a stemness pathway constitutively activated in GBM CSCs, can functionally render CSCs immunosuppressive as inhibition of STAT3 can restore T-cell function [53]. Non-autonomously, CSCs isolated from a number of solid tumors have been shown to release a variety of cytokines and soluble immunosuppressive factors [21] such as VEGF, GDF-15, TGF-β, IL-4, IL-6, IL-10, IL-13, prostaglandin E2 (PGE2), galectin 3 (LGALS3), PD-1, TNF, and others. Several mechanisms underlying the role of these cytokines in CSCs have been reported. For example, upregulated IL-4 signaling can directly impair cytotoxic T cell function and/or promote generation of Tregs and of myeloid-derived suppressor cells (MDSCs) through the release of TGF-β [66]. Also, many of the above-mentioned immunosuppressive factors can help recruit suppressive immune cells such as Tregs and M2 type macrophages to the tumor. CSCs can also reciprocally affect the TME components and subsequently remodel the TME to establish an immuno-suppressive environment [17, 19, 21]. For instance, CSCs can promote local angiogenesis by releasing VEGF, induce inflammation through IL-6, and regulate mesenchymal cells to enhance immune tolerance by recruiting Tregs [21].
Together, these data suggest that CSCs may not only cell-autonomously evade immune attacks but also actively suppress immune responses. This highlights that reversing the immune dysfunction induced by CSCs may impact the efficacy of CSC-targeting immunotherapies. Indeed, targeting of oncogenic Myc signaling by epigenetic means (e.g., JQ1) in a Myc-driven B cell lymphoma mouse model significantly diminished expression of PD-L1; thus, a combination of anti-PD-1 antibody and JQ1 caused synergistic immune responses [67]. Similarly, dual-epigenetic therapy (with DNMTi and HDACi) depleted Myc signaling in non-small-cell lung cancer (NSCLC) and increased the production of T cell chemoattractant CCL5, leading to a substantial anti-tumor response associated with a reversion of an immune evasion phenotype [68]. Additionally, targeting CSC-associated IL-4 by monoclonal antibodies restored the immunogenicity of CSCs, leading to efficient T cell proliferation and induction of anti-CSC Th1 type response in colorectal cancer [55]. Clinically, several studies combining VEGF or VEGFR inhibitors with checkpoint immunotherapies have reported enhancements in tumor immune responses with increased clinical benefit [69].
Among the CSC regulation programs (Fig. 2A), EMT is of particular interest as it enriches CSCs, is a key step toward metastasis, and has been proposed as a major mechanism of resistance (i.e., tumor plasticity) including immune escape (reviewed in [70]). Studies in BCa have suggested that acquisition of EMT in human cancer cells is associated with the CD44high/CD24low phenotype and an inhibition of CTL-mediated tumor cell lysis [71]. Similarly, Snail-induced EMT program accelerates cancer metastasis through not only enhanced invasion but also induction of immunosuppression in melanoma cells due to induced regulatory T cells and impaired dendritic cell (DC) maturation in vitro and in vivo [72]. A correlation study in NSCLC has revealed that an EMT-gene signature is associated with increased expression of diverse immune inhibitory ligands and receptors (e.g., PD-L1/2, PD-1, TIM-3, LAG-3, B7-H3, BTLA, CTLA-4). Importantly, tumors with EMT features displayed higher levels of Th1-inflammation markers (e.g., IFNγ and CXCL-10) and an enrichment of CD4+/FoxP3+ immune-suppressive Tregs than epithelial-like malignancies [73]. Consistently, Ricciardi et al. found that enhanced EMT features after exposure to inflammatory cytokines (i.e., TGF-β, IFN-γ, TNF-α) elicited multiple immune-regulatory effects in cancer cells resulting in NK and T-cell apoptosis, inhibition of lymphocyte proliferation and stimulation of regulatory T and B cells [74]. Collectively, these data suggest that EMT profoundly alters the susceptibility of cancer cells to immune surveillance and that targeting cancer cell plasticity may represent a novel strategy to better control the emergence of resistant variants. In support, pre-clinical animal studies have demonstrated that pharmacologic targeting of PD-L1 with antibody prevents growth and metastasis of ZEB1-overexpressing lung tumors [75]. Mechanistically, ZEB1 transcriptionally upregulates PD-L1 in lung cancer [75]. This may have important clinical implications, as the subset of patients wherein malignant progression is driven by EMT might respond to treatment with PD-L1 antagonists. Therefore, it is tempting to speculate that inhibition of EMT, in general, may boost the efficacy of current immunotherapies. Encouragingly, disruption of TGF-β signaling using the A83–01 inhibitor in MCF7-EMT cells resulted in a significant reversal of the EMT phenotype and a decrease in their resistance to CTLs [76]. Furthermore, EMT induces loss of E-cadherin, which is a preferred ligand of CD103; and CD103 is preferentially expressed by tumor-infiltrating lymphocytes (TILs) [70]. It is thus expected that restored expression of E-cadherin would enhance CTL functions. These discussions suggest that, given the broad range of biological consequences caused by EMT, mechanism-based combination therapies involving EMT-inhibiting agents and CSC immunotherapy may represent an efficient strategy to target CSC plasticity.
4. Immunological targeting of CSCs
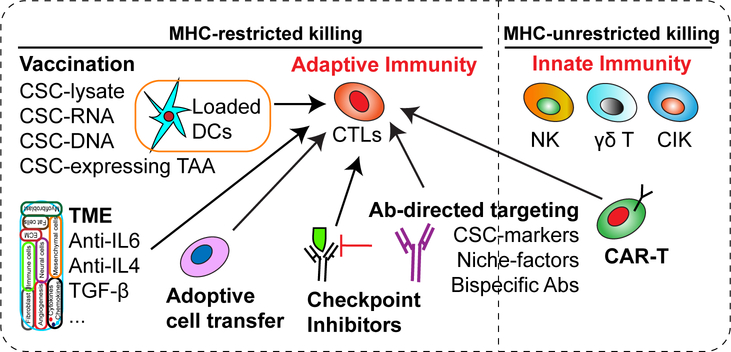
Cancer and CSC-specific immunotherapies include those that elicit tumor-targeting cytolytic lymphocytes, alter the immunosuppressive TME, and disrupt immune-repressive regulatory networks [77]. A variety of immunological approaches are being explored to target cancer cells in general and CSCs in particular (Fig. 4). Notably, as of yet, the majority of CSC-targeting immunotherapy strategies are still in early preclinical phases (Table 2). Nonetheless, based on the abundant evidence embedded in the literature, we summarize below the potential CSC immunotherapies that have shown in vitro and/or in vivo efficacies at least in experimental settings.
Figure 4. Novel immunological approaches targeting CSCs.
Immunological approaches may target CSCs via unleashing the power of either MHC-restricted and/or -unrestricted tumor cell killing by the host immune system. Except for innate immunotherapies, the majority of other immunotherapies aim to increase the number and function of CTLs.
Abbreviations: CSCs, cancer stem cells; DCs, dendritic cells; TAA, tumor-associated antigens; TME, tumor microenvironment; CTLs, cytotoxic T lymphocytes; NK, natural killer cells; CIK, cytokine-induced killer cells; CAR-T, chimeric antigen receptor-expressing T cells; Ab, antibody
Table 2.
Examples of CSC-targeting strategies
| Type | Cancer | Effects | Ref |
|---|---|---|---|
| NK cells | Breast/Glioblastomas/Pancreatic | Allogeneic activated human NK cells preferentially target CSCs isolated from a wide variety of human cancer cell lines in vitro and dissociated primary cancer specimens ex vivo | [82] |
| Glioblastomas | Neural stem cells isolated from tumor specimens resist to freshly isolated NK cells, but were highly susceptible to lysis mediated by both allogeneic and autologous IL-2 (or IL-15)-activated NK cells | [83] | |
| CSC-lysate DC vaccine | Melanoma/ squamous cell carcinoma* | Significantly prevented lung metastasis of melanoma cells, inhibited SCC growth compared to immunization with bulk tumor cells | [97] |
| Melanoma/ squamous cell carcinoma | CSC-DC vaccine was administered in the adjuvant setting after localized radiation therapy of established tumors. Vaccination significantly inhibited tumor growth, reduced ALDHhigh CSC frequency in primary tumors, and metastases via induction of humoral immune responses against CSCs | [99] | |
| Breast | DCs migrated to the spleen, activated CD8+ and CD45+ T cells, induced CTL antitumor responses | [98] | |
| Prostate | Induced a tumor-specific immune response more robust than differentiated tumor cells, delayed tumor growth in mice challenged with prostate CSCs, caused tumor regression in TRAMP mice | [86] | |
| Melanoma/ squamous cell carcinoma | In the adjuvant setting, more effectively limited local tumor recurrence and spontaneous pulmonary metastasis, prolonged host survival, which was further enhanced by simultaneous PD-L1 blockade | [100] | |
| Glioblastomas | Elicited antigen-specific T-cell responses against CSCs and prolonged survival in animals | [95] | |
| CSC-mRNA DC vaccine | Glioblastomas* | An immune response induced by vaccination was identified in all seven patients treated with a mRNA-DC vaccine (NCT00846456) | [153] |
| DNA vaccine | Renal cell carcinoma | Compared with immunization with the tumor-associated antigen survivin, which was expressed in both CSCs and non-CSCs, immunization with DNAJB8 expression plasmids yielded stronger antitumor effects | [47] |
| CSC-primed T cells | Lung | ALDHhigh-CD8+T cells conferred more significant antitumor effects, resulting in the inhibition of tumor growth and prolonged survival | [106] |
| Head and neck | ALDH1A1-specific CD8+ T cells recognize and eliminate ALDHhi CSCs in vitro, inhibit xenograft growth and metastases in vivo, and prolong survival | [105] | |
| CSC-CAR T | Glioblastomas | Anti-CD133 CAR T cells killed patient-derived GBM CSCs in vitro and in an orthotopic tumor model | [109] |
| Prostate | EpCAM-specific CAR T cells had significant anti-tumor efficacy in vitro and in animal models | [113] | |
| mAb | Breast | Anti-CD44 antibodies inhibit growth of murine breast tumors and induce apoptosis | [119] |
| Melanoma | Anti-CD44 antibodies inhibit human melanoma metastasis and prolong the survival of tumor-bearing animals | [120] | |
| Pancreatic /liver | CIK cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133high CSC and inhibit the growth of pancreatic and hepatic cancer cells in vitro and in vivo | [122] |
Related clinical trial studies have been conducted. Note that based on the findings of [97], multiple phase 1 CSC-vaccine studies have been conducted (e.g., NCT02084823 for lung cancer, NCT02074046 for pancreatic cancer, NCT02089919 for hepatocellular Cancer, NCT02176746 for colorectal Cancer), but no results have been posted.
Abbreviations: CSC, cancer stem cells; NK, natural killer cells; DC, dendritic cells; CAR T, chimeric antigen receptor T cells, mAb, monoclonal antibodies; TRAMP, transgenic adenocarcinoma of the mouse prostate; CIK, cytokine-induced killer.
4.1. Innate immune response to CSCs
As the first-line effectors to defend cancer cells, innate immune cells, mainly natural killer (NK) cells, γδ T cells and others [78], constitute the primary cell types of cytotoxic lymphocytes responsible for recognizing and killing cancer cells in an MHC-unrestricted manner. The low or no expression of MHC class I molecules on CSCs (see Section 3.2), in theory, should render them preferentially susceptible to innate immune response, especially the NK cell-mediated killing. However, the role of NK cells in anti-CSC immune surveillance remains uncertain and somewhat controversial, partly due to the fact that NK cells represent only a minor fraction of the human lymphocyte population and their activation relies on signaling of natural cytotoxicity receptors (NCRs). There have been conflicting reports on the expression of NK ligands on different CSCs. For example, some CSCs frequently express low levels of NK cell activating ligands [79], suggesting that CSCs may preferentially escape NK cell-mediated innate immune-surveillance. The majority of CD133+ brain tumor stem cells do not express detectable MHC-I or NK cell activating ligands, which may render them resistant to innate immune surveillance [80]. Stimulation of the expression of these molecules by IFN-γ caused CD133+ CSCs sensitive to NK cell-mediated lysis in vitro [80]. MICA and MICB (MHC class I-related chain A and B), two ligands for the stimulatory NK cell receptor NKG2D, are downregulated in human breast CSCs due to aberrant expression of oncogenic miR-20a [81]. On the other hand, glioma CSCs have also been reported to express various ligands of NK cell activation receptors that can mediate NK cell cytotoxicity [82, 83]. Moreover, colorectal CSCs appear to express even higher levels of ligands for the NCRs (than non-CSCs) resulting in higher sensitivities to NK cell killing [51]. Oral squamous carcinoma CSCs are significantly more susceptible to NK cell mediated cytotoxicity whereas their differentiated counterpart is significantly more resistant [84]. However, activated NK cells displayed similar efficacy in killing malignant melanoma CSCs and non-CSCs based on different combinations of activating NK receptors between the two [85]. In the case of PCSCs, one study in mouse model suggests that they might be targets for NK cell-mediated cytotoxicity, as the ‘PCSC lines’ established from the TRAMP (transgenic adenocarcinoma of the mouse prostate) tumors express PCa-associated antigens, MHC-I and -II molecules, as well as ligands for NK cell receptors [86]. Together, these discussions suggest that CSC susceptibility to NK cell cytotoxicity might be tumor type dependent. However, since conflicting results have been reported for CSCs from the same tumor type (e.g., glioma CSCs [80, 83]), the discordance between different studies might also be related to how CSCs are defined and to the well-known heterogeneity of CSC subpopulations [2].
CSCs in different tumor systems also seem to manifest different sensitivities to the killing by unconventional non-MHC-restricted γδ T cells of the Vγ9/Vδ2 phenotype, which represent another group of innate immune effector cells. Human Vγ9/Vδ2 T cells have been observed to kill CSCs derived from colon cancer [87], ovarian cancer [88], and neuroblastoma [89]. In contrast, breast CSCs express relatively low levels of MHC-I and CD54 and are more resistant to killing by Vγ9/Vδ2 T cells [90]. The resistance of breast CSCs to γδ T cells could be overcome upon pretreatment with γδ T-cell agonist zoledronate, resulting in increased cytotoxicity of γδ T cells [88]. Interestingly, zoledronate-activated γδ T-cells can synergistically enhance the killing activity of CD8+ T cells through the IFN-γ-driven upregulation of MHC-I and ICAM-1 [88]. In the clinic, adoptive transfer of ex vivo expanded γδ T cells and in vivo therapeutic manipulation of γδ T cells by lymphocyte activators (e.g., phosphoantigens and aminobisphosphonates) with low-dose IL-2, have been reported to potentiate anti-tumor activities of γδ T cells in patients with CRPC [91]. Combination therapy involving Vγ9/Vδ2 T cells with zoledronate is feasible in patients with advanced solid tumors including melanoma, ovarian, colon, breast, and cervical cancers [92]. Further studies are needed to confirm direct targeting of CSCs by γδ T cells in these clinical trials.
4.2. CSC-based vaccines
Active immunotherapy strategies are based on the endogenous activation of T-cell responses to cancer via delivering TAAs to patients, which can be achieved with vaccines (e.g., whole cell, peptide, DC, genetic). Currently, DC vaccination is one of the most studied and most effective strategies to prevent diseases (Fig. 4). In practice, DCs, the professional antigen-presenting cells (APCs), are induced ex vivo from peripheral blood monocytes or marrow cells, pulsed with tumor antigens, maturated, and finally administered to the patient. Experimental evidence has shown that the use of DCs to initiate tumor-specific T-cell responses represents a promising cancer vaccination approach [79, 93, 94]. In particular, CSCs have been used as antigen sources to elicit DC-mediated tumor specific immune responses [95, 96]. Conceptually, the use of CSC lysates as source of antigen could allow simultaneous targeting of multiple antigens, and thus would be less susceptible to antigen loss as a means of tumor escape [79]. In fact, enriched CSCs are immunogenic and more effective as an antigen source than unselected tumor cells or non-CSCs in inducing antitumor immunity against CSC epitopes. For instance, in various syngeneic immunocompetent mouse tumor models, a CSC-DC vaccination significantly prevents lung metastasis of melanoma cells and inhibits growth of squamous carcinoma compared to immunization with bulk tumor cells [97]. The administration of DCs pulsed with irradiated PCSC lines from the above-mentioned TRAMP tumors induced a tumor-specific immune response that was more robust than that induced by DCs pulsed with differentiated prostate tumor cells, and importantly, the CSC-DC vaccine delayed tumor growth in mice challenged with CSCs and caused tumor regression in TRAMP mice [86]. Also, a breast CSC-DC vaccine was shown to activate CD8+ and CD45+ T cells, and induce CTL-antitumor response [98]. Interestingly, it has been demonstrated that CSC-DC vaccines exhibit maximum utility when deployed in an adjuvant setting after surgical removal of the bulk tumor mass in order to target microscopic residual CSCs, or as combinatorial therapy with radiation and/or chemotherapy in treating macroscopic tumors. For example, CSC-DC was more efficacious than DCs pulsed with non-CSCs in treating microscopic tumors in established murine melanoma D5 and squamous cancer SCC7 tumor models [99]. Additionally, use of ALDHhigh CSC-DC vaccines in the adjuvant setting more effectively limited local tumor recurrence and spontaneous pulmonary metastasis, as compared with traditional DC vaccines, with increased host survival further accentuated by simultaneous PD-L1 blockade [100].
In contrast to cell-based vaccines, DNA vaccination is relatively a new strategy for protecting against cancer by injection with genetically engineered DNA (mostly given as a plasmid) so cells directly produce an antigen, leading to a protective immunological response [101]. Many clinical trials have demonstrated the tolerability of DNA immunization and their ability to elicit antigen-specific T cells. In human CRPC, both PAP (prostate acid phosphatase) and PSA have been targeted using DNA-based vaccination, with a DNA vaccine encoding PAP currently being evaluated in a randomized phase II trial [102, 103]. In the setting of targeting CSCs, Nishizawa et al. showed that, compared to immunization with the TAA survivin (commonly expressed by both CSCs and non-CSCs in renal cell carcinoma), immunization with CSC-specific DNAJB8 expression plasmids yielded stronger antitumor effects [47]. Recently, an experimental DNA vaccine against the stem factor Sox2 was developed and an antitumor effect was recorded after immunization against mouse oncogenic Sox2-expressing lung TC-1/B7 cancer cells [104]. Albeit studies on CSC-specific DNA vaccination are limited, we speculate that it could serve as a novel immunotherapy, given that the technique is relatively easy and can be used theoretically to target any antigens.
4.3. T cell-based immunotherapy
A successful adoptive T cell therapy requires the generation of effector T cells followed by adoptive transfer of CD8+ T cells back into patients. Ways of generating CSC-specific T cells have been described, including CSC-primed T cells and chimeric antigen receptors (CAR)-engineered T cells. For CSC-primed T cells, studies have demonstrated that CSCs derived from multiple human cancer cell lines, including breast, head and neck, and pancreatic, could be used to induce a CD8+ T cell response [105]. For example, the ALDH-specific CD8+ T cells can be induced/expanded by in vitro stimulation of human CD8+ T cells isolated from peripheral blood of normal HLA-A2+ donors with ALDH1A1 peptide-pulsed autologous DCs. Strikingly, these ALDH-specific CD8+ T cells can recognize and eliminate ALDHhi CSCs (decreased by 60%–89%) in vitro, inhibit xenograft growth and metastasis in vivo, and prolong animal survival after adoptive transfer in preclinical models [105]. Similarly, Luo et al. isolated lung CSCs as ALDHhi population and utilized their lysate-pulsed DCs to stimulate CD8+ T cells by co-cultivation [106]. Subsequently, these ALDHhigh-CD8+ T cells exhibited significant antitumor effects, resulting in inhibition of tumor growth and extended survival [106]. Additionally, CSCs purified as SP from bone malignant fibrous histiocytoma (MFH) expressed HLA-I molecules and autologous CTL can be obtained by in vitro co-culture with SP cells [107]. Interestingly, a CTL clone preferentially recognized SP cells rather than non-SP cells, indicating that this CTL clone recognizes MFH CSC-specific antigens [107]. However, it is worth noting that antigens recognized by CSC-primed T cells remain largely unknown.
Alternatively, CAR T cells represent a unique and promising cancer immunotherapy. The tremendous success and advance in the treatment of hematological malignancies using adoptive transfer of CAR T cells has generated substantial interests in applying this therapy for solid cancers. Using ex vivo gene transfer, T cells from patients can be genetically engineered to express a novel T cell receptor or CAR to specifically recognize a TAA and thereby selectively kill tumor cells. Therefore, CAR T cells permit recognition of a cell-surface protein in an MHC-unrestricted manner and can be engineered to target virtually any TAAs [108]. In preclinical models of solid tumors, CAR T cells have been designed to target CSC-associated antigens, such as CD133 in glioblastoma [109], CSPG4 in many cancer types [110], EGFRvIII [111] and IL13Rα2 [112] in gliomas, and EpCAM in PCa [113]. Some of these reports have demonstrated the antitumor effects of CAR T cells by targeting CSCs, although overall studies in this area are rather limited. Importantly, a high rate of severe toxicities has been observed for CAR T therapies targeting TAAs including CAIX in renal cell carcinoma [114] and ERBB2 in metastatic colon cancer [115], presumably due to shared expression of targeted antigens by both cancer and normal cells. In addition to the technical challenge of isolating and expanding T cells restricted to specific TAAs, future effort towards identification of CSC-specific antigens is likely to be key in pursuing this approach to target CSCs. Neoantigens that can be recognized efficiently by T cells without self-tolerance mechanisms make them promising candidates for developing CAR T therapy. Although a current understanding of CSC genomics is lacking, these studies suggest that CSC-specific T cells can be generated and expanded in vitro for subsequent adoptive transfer into tumor-bearing hosts to target CSCs and eliminate tumors in vivo. In principle, in vitro CSC-primed T cells may represent a novel and feasible immunotherapy to specifically target CSC [79].
4.4. Monoclonal antibody-based immunotherapy
Monoclonal antibodies (mAb)-based treatments are considered to be one of the most successful strategies in cancer therapy during the past two decades. Antibody–drug conjugates are powerful new treatment options for many tumor types [116], and immunomodulatory antibodies, exemplified by anti-PD-1, anti-PD-L1 and anti-CTLA-4 antibodies that target immune checkpoints, have also recently achieved remarkable clinical success (discussed separately below). CSCs express various markers (Table 1) at levels that could be substantially different from the bulk tumor cell population, providing attractive targets for antibody-based therapies. Indeed, attempts to target CSCs with specific antibodies have indicated an improvement in therapeutic responses. For example, an anti-CD44 mAb suppresses tumor progression and causes apoptosis of leukemic stem cells [117, 118]. Anti-CD44 antibodies have been shown to inhibit growth of murine breast tumors and induce apoptosis [119], and decrease human melanoma metastasis and increase animal survival in SCID mice [120]. In vitro proliferation and in vivo tumor growth of CD133+ hepatocellular and gastric CSCs can be inhibited by drug-conjugated anti-CD133 antibody [121]. An asymmetric bispecific antibody consisting of CD133 and CD3 antibodies exhibited a strong anti-tumor efficacy in several cancers [122, 123]. Studies have shown that a CSC-specific antibody-incorporated liposomal nanoparticle delivery system loaded with drugs or a suicide gene can significantly improve anti-tumor ability in solid tumors [124, 125]. By targeting HER2, an important regulator of breast CSC self-renewal, trastuzumab (a HER2-targeting antibody) dramatically reduces BCa recurrence [126]. In addition to trastuzumab, other HER2-targeting agents, including the monoclonal antibody pertuzumab and the immunotoxin conjugate ado-trastuzumab emtansine (TDM-1), have further improved the efficacy of HER2-targeting in the clinic [127, 128]. Notably, HER2 expression can also define a subset of CSCs in GBM [129], suggesting the use of HER2 targeting agents in other tumor types. As the ‘CSC markers’ may not be truly specific or exclusive to CSCs [116], single agent mAb therapy (i.e., targeting just one marker) may cause side effects in normal cells. Combination therapy using antibody cocktails targeting multiple CSC markers could potentially lower doses of individual antibodies to achieve better killing of CSCs while reducing toxicities in normal tissues caused by high concentrations of single anti-CSC mAbs.
5. Immune checkpoint blockade: Old players on a new playground
Immunological checkpoints control cells of the innate and adaptive immune system. Stimulatory checkpoint molecules promote activation of naive T cells, as well as effector, memory and regulatory T cell responses, whereas inhibitory checkpoint molecules expressed by innate and adaptive immune cells inhibit their activities and limit adaptive immunity [130]. Based on current knowledge, the success of immune checkpoint therapies is determined by cancer specific TMEs. Tumors such as melanoma, bladder cancer, and NSCLC are considered “hot” due to their inflamed TME, significant T-cell infiltration, increased PD-L1 expression, and high neoantigen load [131]. Other cancers such as PCa are considered “cold” with minimal T-cell infiltrates and very limited response to single-agent checkpoint inhibition [132]. Immune checkpoint blockade therapies have demonstrated great successes in inhibiting tumor growth and even completely eliminating tumors in certain cancers, although overall only a small percentage patients experience durable responses. Whether checkpoint blockade can specifically target and abrogate the ability of CSCs to regenerate tumors, and whether the responsiveness seen in some patients treated with immune blockade is attributable to its effect on CSC populations are currently unknown.
Intriguingly, the preferential PD-L1 expression in CSCs, which is speculated as an immune evasion mechanism, may provide a rationale for targeting the PD-1 axis. In primary human head and neck squamous cell carcinomas (HNSCC), PD-L1 expression is elevated on CD44+ CSCs [133]. High expression of PD-L1 has also been reported on CD133+ colorectal [134], gastric [135], breast and colon CSCs [136]. Experimentally, a streptavidin-granulocyte-macrophage-colony stimulating factor (SA-GM-CSF) surface-modified bladder CSC vaccine effectively induced specific immune responses towards CSCs but also upregulated the PD-L1 expression in tumor cells, resulting in subsequent immune resistance [137]. Interestingly, adding PD-1 blockade to the CSC vaccine significantly enhanced the functions of tumor-specific T lymphocytes and improved the cure rate in mice [178]. While these results are exciting, there is little pre-clinical data or clinical evidence to indicate that the clinical efficacy of immune checkpoint inhibitors is acting via an anti-CSC mechanism. Assessment of CSCs in currently ongoing and future clinical trials involving checkpoint blockade is thus necessary to determine whether these therapies specifically target CSCs. Furthermore, in addition to its established role in the immune response, PD‐L1 expression may have intrinsic effects on cancer cells themselves. A recent study demonstrated that PD‐L1 maintains breast CSCs by sustaining PI3K/AKT pathway activation to promote Oct4 and Nanog expression [138]. This may have important clinical implications as immune checkpoint therapies may impact CSC biology and thus direct future novel drug development. As such, combination of immune checkpoint blockade with CSC targeting therapies, including small-molecule inhibitors and immunotherapies such as antibodies and vaccines, may enhance the clinical utility of each approach.
6. Immunological targeting of CSCs through TME
CSCs reside in a niche within the tumor, which dictates their biological behavior. The critical role of TME in modulating CSC activities and promoting tumor growth and progression has been intensively studied and reviewed [3, 8, 14]. Different immune cell subsets are recruited into the TME via interactions between chemokines and chemokine receptors, and these populations have distinct effects on tumor biology [139]. Many tumors are infiltrated by immune-suppressive Tregs and/or MDSCs. Interestingly, MDSCs may endow cancer cells with CSC properties and are linked with cancer stemness [140, 141]. A breast CSC niche is supported by juxtacrine signaling from tumor-associated monocytes and macrophages [142], which may directly regulate CSCs through inflammatory cytokines IL-1, IL-6, and IL-8 in the CSC niche and drive CSC self-renewal [143]. Moreover, these cytokines activate STAT3 and NF-κB pathways in both tumor and stromal cells, which in turn stimulate further cytokine production, generating positive feedback loops that contribute to CSC self-renewal [144]. Collectively, TME is generally immunosuppressive and targeting the unique aberrant TME of CSCs may represent an alternative strategy for immunotherapies, given the fact that the anti-tumor immune activation in solid tumors is commonly inefficient.
Recent attempts to target components of the CSC niche, aiming to alter the types of cells that might be present at the tumor site and/or disrupt the tumor vasculature, have shown some promises. Antibodies that target the angiogenesis factors, mainly VEGF and their receptors VEGFRs, have been shown to inhibit tumor angiogenesis and tumor growth [145]. Furthermore, selectively targeting chemokine–chemokine receptor signaling can potentially complement and increase the efficacy of current immunotherapies. This may be important in specifically targeting CSCs. A previous study demonstrated the selective targeting and elimination of breast CSCs in vitro and in xenografts by a CXCR1-blocking antibody and a CXCR1 inhibitor [146]. Immunologically, blockade of the IL-8 receptor CXCR1 using antibody or repertaxin (a CXCR1/2 small-molecule inhibitor), selectively depleted the CSC population in human BCa cell lines in vitro, followed by the induction of massive apoptosis in the bulk tumor population via FASL/FAS signaling [146]. A recent study revealed that cytokines produced by BCa cells after chemotherapy withdrawal activate both Wnt/β-catenin and NF-κB pathways, which in turn further promote BCa cells to produce and secrete cytokines, forming an autocrine inflammatory forward-feedback loop to facilitate the enrichment of drug-resistant breast CSCs [147]. This CSC enrichment could be effectively blocked by inhibition of Wnt/β-catenin and NF-κB signaling, as well as by an IL-8-neutralizing antibody or reparixin [159]. In addition, IL-6 has been implicated as a direct regulator of CSCs in multiple cancer types and anti-IL-6 antibody abolishes JAK1-STAT3-OCT4 signaling axis, thus inhibiting CSCs [148]. These studies suggest that components in CSC niche represent additional and attractive immunotherapeutic targets.
7. Challenges & Perspective
Due to their unique biological stem-like features, such as intrinsic conventional therapy resistance and frequent immune-privilege (low immunogenicity), CSCs are evinced to be intimately involved in tumor maintenance, progression, metastasis and relapse. Therefore, targeting CSCs is essential to treat residual disease and prevent recurrence. Immunotherapy is promising, as the results of multiple clinical trials have demonstrated benefits for patients with certain types of cancer. Nevertheless, the objective response rate varies significantly and a durable response is often limited to a small number of patients. Notably, the outcome of current mono-immunotherapies in solid tumors is generally disappointing. One underlying reason for this inefficiency might be attributable to the presence of CSCs that are not effectively targeted by the current immunotherapeutic regimens. CSC-specific immunotherapy, still in its infancy, has demonstrated efficacy in a few preclinical and clinical settings (Table 2), but challenges exist.
Biologically, an in-depth genomic, biological, and immunological characterization of CSCs, together with their interactions with immune cells in the TME, is essential to devise more efficacious strategies and novel therapeutics. Though we have gained much knowledge on human CSC properties, most of our current understanding is derived from xenograft studies in immune compromised mice lacking a fully functional immune system. The future use of humanized mice, immunodeficient strains with engrafted human immune systems, may help resolve this problem [149]. Another big challenge facing efficient immunotherapy is the heterogeneity in CSC populations and plasticity of cancer cells. CSCs are known to be heterogeneous [2] reflected by the fact the multiple subpopulations expressing different phenotypic markers have been reported for CSCs in one cancer type (Table 1). For instance, our work has demonstrated that prostate CSCs are mainly PSA−/lo but this PSA−/lo pool is rather heterogeneous harboring tumorigenic subsets that can be prospectively purified out using different markers [150]. The relationship between various CSC subsets within the same cancer type remains largely unknown, and whether similar immunological properties are shared by these CSC subpopulations is yet to be addressed. This may lead to a situation that a CSC-specific immunological treatment only eliminates a subset of CSCs, but not all. Moreover, CSCs and non-CSCs can manifest diverse plasticity (not discussed in this review but reviewed in [2]). This tumor cell plasticity presents a huge hurdle in the development of durable targeted cancer therapies, as therapeutic eradication of existing CSC populations might be followed by their regeneration from non-CSCs within the tumor under treatment pressures [2]. It is exciting to see that several agents that target TME components have demonstrated clinical values in treating cancers, but our current understanding of the complex TME is limited. Importantly, recent studies have suggested that conventional anticancer treatments are prone to enrich CSCs and reshape the TME that may alter the immunotherapy-responsiveness of CSCs [3]. For example, chemotherapy increased the frequency of CSCs in tumors and downregulated the expression of HLA class I molecules in PCa and HNSCC [61, 62], which may potentially lead to immunoresistance. In aggregate, a CSC represents a ‘moving target’, as it will constantly evolve, along with the tumor evolution, during progression, and, particularly, upon treatment.
Immune targeting of CSCs holds significant promise in helping achieve our ultimate goal of curing patients with cancer. Although therapies that efficiently and selectively eliminate CSCs are still far away from clinical application, various immunotherapeutic strategies to target CSCs (Fig. 4) are in development and many of them have displayed efficacy in inhibiting tumor growth and metastasis in preclinical and clinical settings. Addressing issues associated with the relatively low immunogenicity and negative immunomodulating effects of CSCs is a top priority in our future efforts. In addition, the majority of the reported CSC markers and TAAs are not CSC-exclusive, and therefore identification of CSC-specific antigens is critical for the success of antigen-dependent immunotherapies. This will also aid in avoiding potential safety concerns and achieve treatment specificity. The immunosuppressive functions of CSCs may present a new opportunity for intervention, as immunomodulating agents that can revert/inhibit the CSC-associated escape from immunosurveillance may allow new design of immunotherapy strategies/protocols to target CSCs.
As with all other monotherapies, mono-immunotherapy is unlikely to cure cancer, and strategies that combine conventional therapies to debulk tumors and CSC-specific immunotherapies would be desirable to defeat cancer (Fig. 1). By combining two or more therapeutic agents that target different subsets and/or properties of tumor cells, combination therapy is expected to reduce drug resistance and cancer cell plasticity and achieve better efficacy compared to the mono-therapies, ultimately leading to precision medicine. In theory, the immunogenicity of CSCs can be enhanced by inhibiting negative immunoregulatory pathways and by upregulating HLA I and APM components via combinatorial therapies with IFNs, chemotherapy, radiotherapy, and/or epigenetic treatments [21]. For example, CAR T cells specific to CSCs combined with other therapies will be effective to enhance their anti-tumor efficacy [151]. Recently, epigenetic drugs have been observed to modulate the expression of immune-related genes either on tumor cells and/or tumor-associated immune cells in a manner that restores immune recognition and immunogenicity. For instance, in NSCLC cells, HDAC6 inhibitor ricolinostat promoted T-cell activation and improved APC functions by increasing the expression of MHC molecules, whereas JQ1, a BET bromodomain inhibitor, attenuated CD4+FOXP3+ Treg cell suppressive functions and facilitated immune-mediated tumor growth arrest [152]. Therefore, epigenetic therapy combined with immunotherapy may represent a new direction. In near future, rigorous evaluation of these strategies alone or in combination with other treatments is needed to provide insights into the optimization and development of novel anti-cancer immunotherapy protocols.
Acknowledgements
Work in the authors’ lab was supported by grants from the US National Institutes of Health (NIH) (R01-CA155693), Department of Defense (W81XWH-14-1-0575 and W81XWH-16-1-0575), and the Chinese Ministry of Science and Technology (MOST) Grant 2016YFA0101203 (D.G.T). This work was also supported by Roswell Park Comprehensive Cancer Center and National Cancer Institute (NCI) grant P30CA016056.
Footnotes
Conflict of interest
None.
References:
- [1].Shackleton M, Quintana E, Fearon ER, Morrison SJ, Heterogeneity in cancer: cancer stem cells versus clonal evolution, Cell 138(5) (2009) 822–9. [DOI] [PubMed] [Google Scholar]
- [2].Tang DG, Understanding cancer stem cell heterogeneity and plasticity, Cell Res 22(3) (2012) 457–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Visvader JE, Lindeman GJ, Cancer stem cells: current status and evolving complexities, Cell Stem Cell 10(6) (2012) 717–28. [DOI] [PubMed] [Google Scholar]
- [4].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE, A cell initiating human acute myeloid leukaemia after transplantation into SCID mice, Nature 367(6464) (1994) 645–8. [DOI] [PubMed] [Google Scholar]
- [5].Bonnet D, Dick JE, Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell, Nat Med 3(7) (1997) 730–7. [DOI] [PubMed] [Google Scholar]
- [6].Chen X, Rycaj K, Liu X, Tang DG, New insights into prostate cancer stem cells, Cell Cycle 12(4) (2013) 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kreso A, Dick JE, Evolution of the cancer stem cell model, Cell Stem Cell 14(3) (2014) 275–91. [DOI] [PubMed] [Google Scholar]
- [8].Vlashi E, Pajonk F, Cancer stem cells, cancer cell plasticity and radiation therapy, Semin Cancer Biol 31 (2015) 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rycaj K, Tang DG, Cancer stem cells and radioresistance, Int J Radiat Biol 90(8) (2014) 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, Calhoun-Davis T, Li H, Palapattu GS, Pang S, Lin K, Huang J, Ivanov I, Li W, Suraneni MV, Tang DG, The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration, Cell Stem Cell 10(5) (2012) 556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP, Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content, Cell 140(1) (2010) 62–73. [DOI] [PubMed] [Google Scholar]
- [12].Maugeri-Sacca M, Vigneri P, De Maria R, Cancer stem cells and chemosensitivity, Clin Cancer Res 17(15) (2011) 4942–7. [DOI] [PubMed] [Google Scholar]
- [13].Moore N, Lyle S, Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance, J Oncol 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Plaks V, Kong N, Werb Z, The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?, Cell Stem Cell 16(3) (2015) 225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ramos EK, Hoffmann AD, Gerson SL, Liu H, New Opportunities and Challenges to Defeat Cancer Stem Cells, Trends Cancer 3(11) (2017) 780–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun Y, Tumor microenvironment and cancer therapy resistance, Cancer Lett 380(1) (2016) 205–15. [DOI] [PubMed] [Google Scholar]
- [17].Fakhrejahani F, Tomita Y, Maj-Hes A, Trepel JB, De Santis M, Apolo AB, Immunotherapies for bladder cancer: a new hope, Curr Opin Urol 25(6) (2015) 586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [19].Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science 331(6024) (2011) 1565–70. [DOI] [PubMed] [Google Scholar]
- [20].Reim F, Dombrowski Y, Ritter C, Buttmann M, Hausler S, Ossadnik M, Krockenberger M, Beier D, Beier CP, Dietl J, Becker JC, Honig A, Wischhusen J, Immunoselection of breast and ovarian cancer cells with trastuzumab and natural killer cells: selective escape of CD44high/CD24low/HER2low breast cancer stem cells, Cancer Res 69(20) (2009) 8058–66. [DOI] [PubMed] [Google Scholar]
- [21].Maccalli C, Parmiani G, Ferrone S, Immunomodulating and Immunoresistance Properties of Cancer-Initiating Cells: Implications for the Clinical Success of Immunotherapy, Immunol Invest 46(3) (2017) 221–238. [DOI] [PubMed] [Google Scholar]
- [22].Regenbrecht CR, Lehrach H, Adjaye J, Stemming cancer: functional genomics of cancer stem cells in solid tumors, Stem Cell Rev 4(4) (2008) 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okamoto OK, Cancer stem cell genomics: the quest for early markers of malignant progression, Expert Rev Mol Diagn 9(6) (2009) 545–54. [DOI] [PubMed] [Google Scholar]
- [24].Karamboulas C, Ailles L, Developmental signaling pathways in cancer stem cells of solid tumors, Biochim Biophys Acta 1830(2) (2013) 2481–95. [DOI] [PubMed] [Google Scholar]
- [25].Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH, A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs, Cell 143(2) (2010) 313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG, Functional evidence that the self-renewal gene NANOG regulates human tumor development, Stem Cells 27(5) (2009) 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG, NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation, Oncogene 30(36) (2011) 3833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jeter CR, Liu B, Lu Y, Chao HP, Zhang D, Liu X, Chen X, Li Q, Rycaj K, Calhoun-Davis T, Yan L, Hu Q, Wang J, Shen J, Liu S, Tang DG, NANOG reprograms prostate cancer cells to castration resistance via dynamically repressing and engaging the AR/FOXA1 signaling axis, Cell Discov 2 (2016) 16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA, The epithelial-mesenchymal transition generates cells with properties of stem cells, Cell 133(4) (2008) 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scheel C, Weinberg RA, Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links, Semin Cancer Biol 22(5–6) (2012) 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chappell J, Dalton S, Altered cell cycle regulation helps stem-like carcinoma cells resist apoptosis, BMC Biol 8 (2010) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG, Cyclins and cell cycle control in cancer and disease, Genes Cancer 3(11–12) (2012) 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Helsten T, Kato S, Schwaederle M, Tomson BN, Buys TP, Elkin SK, Carter JL, Kurzrock R, Cell-Cycle Gene Alterations in 4,864 Tumors Analyzed by Next-Generation Sequencing: Implications for Targeted Therapeutics, Mol Cancer Ther 15(7) (2016) 1682–90. [DOI] [PubMed] [Google Scholar]
- [34].Chen T, Dent SY, Chromatin modifiers and remodellers: regulators of cellular differentiation, Nat Rev Genet 15(2) (2014) 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S, Veeranan-Karmegam R, Arjunan P, Gnana-Prakasam JP, Sadanand F, Pei L, Chang CS, Choi JH, Shi H, Manicassamy S, Prasad PD, Sharma S, Ganapathy V, Jothi R, Thangaraju M, DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis, Nat Commun 6 (2015) 6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM, The polycomb group protein EZH2 is involved in progression of prostate cancer, Nature 419(6907) (2002) 624–9. [DOI] [PubMed] [Google Scholar]
- [37].Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbe DP, Gomez EC, Wang J, Long HW, Xu B, Brown M, Loda M, Sawyers CL, Ellis L, Goodrich DW, Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance, Science 355(6320) (2017) 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ, The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat Cell Biol 10(5) (2008) 593–601. [DOI] [PubMed] [Google Scholar]
- [39].Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG, The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44, Nat Med 17(2) (2011) 211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu C, Liu R, Zhang D, Deng Q, Liu B, Chao HP, Rycaj K, Takata Y, Lin K, Lu Y, Zhong Y, Krolewski J, Shen J, Tang DG, MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes, Nat Commun 8 (2017) 14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rycaj K, Li H, Zhou J, Chen X, Tang DG, Cellular determinants and microenvironmental regulation of prostate cancer metastasis, Semin Cancer Biol 44 (2017) 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maccalli C, Volonte A, Cimminiello C, Parmiani G, Immunology of cancer stem cells in solid tumours. A review, Eur J Cancer 50(3) (2014) 649–55. [DOI] [PubMed] [Google Scholar]
- [43].Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N, Immune responses to human cancer stem-like cells/cancer-initiating cells, Cancer Sci 107(1) (2016) 12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ilyas S, Yang JC, Landscape of Tumor Antigens in T Cell Immunotherapy, J Immunol 195(11) (2015) 5117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yamada R, Takahashi A, Torigoe T, Morita R, Tamura Y, Tsukahara T, Kanaseki T, Kubo T, Watarai K, Kondo T, Hirohashi Y, Sato N, Preferential expression of cancer/testis genes in cancer stem-like cells: proposal of a novel sub-category, cancer/testis/stem gene, Tissue Antigens 81(6) (2013) 428–34. [DOI] [PubMed] [Google Scholar]
- [46].Morita R, Nishizawa S, Torigoe T, Takahashi A, Tamura Y, Tsukahara T, Kanaseki T, Sokolovskaya A, Kochin V, Kondo T, Hashino S, Asaka M, Hara I, Hirohashi Y, Sato N, Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells, Cancer Sci 105(4) (2014) 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, Kanaseki T, Kamiguchi K, Asanuma H, Morita R, Sokolovskaya A, Matsuzaki J, Yamada R, Fujii R, Kampinga HH, Kondo T, Hasegawa T, Hara I, Sato N, HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells, Cancer Res 72(11) (2012) 2844–54. [DOI] [PubMed] [Google Scholar]
- [48].Mennonna D, Maccalli C, Romano MC, Garavaglia C, Capocefalo F, Bordoni R, Severgnini M, De Bellis G, Sidney J, Sette A, Gori A, Longhi R, Braga M, Ghirardelli L, Baldari L, Orsenigo E, Albarello L, Zino E, Fleischhauer K, Mazzola G, Ferrero N, Amoroso A, Casorati G, Parmiani G, Dellabona P, T cell neoepitope discovery in colorectal cancer by high throughput profiling of somatic mutations in expressed genes, Gut 66(3) (2017) 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM, Galli R, Parmiani G, Maccalli C, Immunobiological characterization of cancer stem cells isolated from glioblastoma patients, Clin Cancer Res 16(3) (2010) 800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH, Modulation of T-cell activation by malignant melanoma initiating cells, Cancer Res 70(2) (2010) 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, Mandelboim O, Stassi G, Di Fabrizio E, Parmiani G, Moretta A, Dieli F, Karre K, Carbone E, Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules, J Immunol 190(5) (2013) 2381–90. [DOI] [PubMed] [Google Scholar]
- [52].Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, Nakatsugawa M, Nishizawa S, Tamura Y, Tsuruma T, Terui T, Kondo T, Ishitani K, Hasegawa T, Hirata K, Sato N, Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells, Am J Pathol 178(4) (2011) 1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF, Heimberger AB, Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway, Mol Cancer Ther 9(1) (2010) 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Busse A, Letsch A, Fusi A, Nonnenmacher A, Stather D, Ochsenreither S, Regenbrecht CR, Keilholz U, Characterization of small spheres derived from various solid tumor cell lines: are they suitable targets for T cells?, Clin Exp Metastasis 30(6) (2013) 781–91. [DOI] [PubMed] [Google Scholar]
- [55].Volonte A, Di Tomaso T, Spinelli M, Todaro M, Sanvito F, Albarello L, Bissolati M, Ghirardelli L, Orsenigo E, Ferrone S, Doglioni C, Stassi G, Dellabona P, Staudacher C, Parmiani G, Maccalli C, Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4, J Immunol 192(1) (2014) 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].N. Cancer Genome Atlas Research, The Molecular Taxonomy of Primary Prostate Cancer, Cell 163(4) (2015) 1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang D, Park D, Zhong Y, Lu Y, Rycaj K, Gong S, Chen X, Liu X, Chao HP, Whitney P, Calhoun-Davis T, Takata Y, Shen J, Iyer VR, Tang DG, Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer, Nat Commun 7 (2016) 10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N, Characterization of the expression of MHC proteins in human embryonic stem cells, Proc Natl Acad Sci U S A 99(15) (2002) 9864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Francipane MG, Alea MP, Lombardo Y, Todaro M, Medema JP, Stassi G, Crucial role of interleukin-4 in the survival of colon cancer stem cells, Cancer Res 68(11) (2008) 4022–5. [DOI] [PubMed] [Google Scholar]
- [60].Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F, Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer, Nat Med 22(3) (2016) 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C, Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells, Cancer Cell 22(3) (2012) 373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Grau JJ, Mesia R, de la Iglesia-Vicente M, Williams ES, Taberna M, Caballero M, Larque AB, de la Oliva J, Cordon-Cardo C, Domingo-Domenech J, Enrichment of Cells with Cancer Stem Cell-Like Markers in Relapses of Chemoresistant Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma, Oncology 90(5) (2016) 267–72. [DOI] [PubMed] [Google Scholar]
- [63].Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, Brown BD, Quiescent Tissue Stem Cells Evade Immune Surveillance, Immunity 48(2) (2018) 271–285 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gutgemann I, Eilers M, Felsher DW, MYC regulates the antitumor immune response through CD47 and PD-L1, Science 352(6282) (2016) 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter SL, Hammerman P, Freeman GJ, Wu CJ, Ott PA, Wong KK, Van Allen EM, Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma, Immunity 46(2) (2017) 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yoshimura A, Muto G, TGF-beta function in immune suppression, Curr Top Microbiol Immunol 350 (2011) 127–47. [DOI] [PubMed] [Google Scholar]
- [67].Hogg SJ, Vervoort SJ, Deswal S, Ott CJ, Li J, Cluse LA, Beavis PA, Darcy PK, Martin BP, Spencer A, Traunbauer AK, Sadovnik I, Bauer K, Valent P, Bradner JE, Zuber J, Shortt J, Johnstone RW, BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1, Cell Rep 18(9) (2017) 2162–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RC, Wenzel A, Hicks J, Ballew M, Stone M, Tran PT, Zahnow CA, Hellmann MD, Anagnostou V, Strissel PL, Strick R, Velculescu VE, Baylin SB, Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer, Cell 171(6) (2017) 1284–1300 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hughes PE, Caenepeel S, Wu LC, Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer, Trends Immunol 37(7) (2016) 462–476. [DOI] [PubMed] [Google Scholar]
- [70].Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S, New insights into the role of EMT in tumor immune escape, Mol Oncol 11(7) (2017) 824–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Hung NT, Thiery JP, Mami-Chouaib F, Chouaib S, Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis, Cancer Res 73(8) (2013) 2418–27. [DOI] [PubMed] [Google Scholar]
- [72].Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y, Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells, Cancer Cell 15(3) (2009) 195–206. [DOI] [PubMed] [Google Scholar]
- [73].Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, Rodriguez JC, Hwu P, Wistuba II, Heymach JV, Gibbons DL, Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma, Clin Cancer Res 22(14) (2016) 3630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, Bifari F, Krampera M, Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells, Br J Cancer 112(6) (2015) 1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX, Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression, Nat Commun 5 (2014) 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M, Chouaib S, Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression, Oncogene 34(17) (2015) 2261–71. [DOI] [PubMed] [Google Scholar]
- [77].Rekoske BT, McNeel DG, Immunotherapy for prostate cancer: False promises or true hope?, Cancer 122(23) (2016) 3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shiao SL, Chu GC, Chung LW, Regulation of prostate cancer progression by the tumor microenvironment, Cancer Lett 380(1) (2016) 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS, Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches, Stem Cells 33(7) (2015) 2085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall WA, Low WC, Ohlfest JR, Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy, J Neurooncol 83(2) (2007) 121–31. [DOI] [PubMed] [Google Scholar]
- [81].Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, Yang J, Xu SL, Ye XZ, Xu C, Yang L, Qian C, Wang JM, Cui YH, Zhang X, Bian XW, Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells, Cancer Res 74(20) (2014) 5746–57. [DOI] [PubMed] [Google Scholar]
- [82].Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S, Monjazeb AM, Fragoso RC, Sayers TJ, Murphy WJ, NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype, J Immunol 195(8) (2015) 4010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F, Moretta L, Moretta A, Corte G, Bottino C, NK cells recognize and kill human glioblastoma cells with stem cell-like properties, J Immunol 182(6) (2009) 3530–9. [DOI] [PubMed] [Google Scholar]
- [84].Tseng HC, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, Alva JA, Walter G, Head C, Ishikawa TO, Herschman HR, Cacalano N, Pyle AD, Park NH, Jewett A, Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells, PLoS One 5(7) (2010) e11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pietra G, Manzini C, Vitale M, Balsamo M, Ognio E, Boitano M, Queirolo P, Moretta L, Mingari MC, Natural killer cells kill human melanoma cells with characteristics of cancer stem cells, Int Immunol 21(7) (2009) 793–801. [DOI] [PubMed] [Google Scholar]
- [86].Jachetti E, Mazzoleni S, Grioni M, Ricupito A, Brambillasca C, Generoso L, Calcinotto A, Freschi M, Mondino A, Galli R, Bellone M, Prostate cancer stem cells are targets of both innate and adaptive immunity and elicit tumor-specific immune responses, Oncoimmunology 2(5) (2013) e24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G, Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes, J Immunol 182(11) (2009) 7287–96. [DOI] [PubMed] [Google Scholar]
- [88].Lai D, Wang F, Chen Y, Wang C, Liu S, Lu B, Ge X, Guo L, Human ovarian cancer stem-like cells can be efficiently killed by gammadelta T lymphocytes, Cancer Immunol Immunother 61(7) (2012) 979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nishio N, Fujita M, Tanaka Y, Maki H, Zhang R, Hirosawa T, Demachi-Okamura A, Uemura Y, Taguchi O, Takahashi Y, Kojima S, Kuzushima K, Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human gammadelta T cells, J Immunother 35(8) (2012) 598–606. [DOI] [PubMed] [Google Scholar]
- [90].Chen HC, Joalland N, Bridgeman JS, Alchami FS, Jarry U, Khan MWA, Piggott L, Shanneik Y, Li J, Herold MJ, Herrmann T, Price DA, Gallimore AM, Clarkson RW, Scotet E, Moser B, Eberl M, Synergistic targeting of breast cancer stem-like cells by human gammadelta T cells and CD8(+) T cells, Immunol Cell Biol 95(7) (2017) 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC, Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer, Cancer Res 67(15) (2007) 7450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M, Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours, Br J Cancer 105(6) (2011) 778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Huang C, Ramakrishnan R, Trkulja M, Ren X, Gabrilovich DI, Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines, Cancer Immunol Immunother 61(4) (2012) 573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Karan D, Formulation of the bivalent prostate cancer vaccine with surgifoam elicits antigen-specific effector T cells in PSA-transgenic mice, Vaccine 35(43) (2017) 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS, Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens, Stem Cells 27(8) (2009) 1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhou L, Lu L, Wicha MS, Chang AE, Xia JC, Ren X, Li Q, Promise of cancer stem cell vaccine, Hum Vaccin Immunother 11(12) (2015) 2796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, Prince ME, Xu Y, Zhang XL, Huang S, Chang AE, Li Q, Cancer stem cell vaccination confers significant antitumor immunity, Cancer Res 72(7) (2012) 1853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Phuc PV NN, Thuy DT, Effects of breast cancer stem cell extract primed dendritic cell transplantation on breast cancer tumor murine models. Ann Rev Res Biol (2011). [Google Scholar]
- [99].Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, Egenti M, Owen J, Moyer JS, Prince ME, Huang S, Wicha MS, Xia JC, Li Q, Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells, Oncoimmunology 4(3) (2015) e990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, Hurt E, Owen J, Moyer JS, Prince ME, Dai F, Bao Y, Wang Y, Whitfield J, Xia JC, Huang S, Wicha MS, Li Q, Therapeutic Efficacy of Cancer Stem Cell Vaccines in the Adjuvant Setting, Cancer Res 76(16) (2016) 4661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G, Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer, J Clin Oncol 27(25) (2009) 4047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, Masucci G, Wersall P, Nilsson S, Pisa P, A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer, Br J Cancer 91(4) (2004) 688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM, Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer, Clin Cancer Res 20(14) (2014) 3692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Polakova I, Duskova M, Smahel M, Antitumor DNA vaccination against the Sox2 transcription factor, Int J Oncol 45(1) (2014) 139–46. [DOI] [PubMed] [Google Scholar]
- [105].Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, Brand RE, Ferrone CR, Whiteside TL, Ferrone S, DeLeo AB, Wang X, Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells, Clin Cancer Res 17(19) (2011) 6174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Luo H, Zeng C, Fang C, Seeruttun SR, Lv L, Wang W, A new strategy using ALDHhigh-CD8+T cells to inhibit tumorigenesis, PLoS One 9(8) (2014) e103193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kano M, Tsukahara T, Emori M, Murase M, Torigoe T, Kawaguchi S, Wada T, Yamashita T, Sato N, Autologous CTL response against cancer stem-like cells/cancer-initiating cells of bone malignant fibrous histiocytoma, Cancer Sci 102(8) (2011) 1443–7. [DOI] [PubMed] [Google Scholar]
- [108].Jena B, Dotti G, Cooper LJ, Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor, Blood 116(7) (2010) 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G, Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57, Oncotarget 6(1) (2015) 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Beard RE, Zheng Z, Lagisetty KH, Burns WR, Tran E, Hewitt SM, Abate-Daga D, Rosati SF, Fine HA, Ferrone S, Rosenberg SA, Morgan RA, Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells, J Immunother Cancer 2 (2014) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, Zhang W, Fine HA, Rosenberg SA, Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma, Hum Gene Ther 23(10) (2012) 1043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, Barish ME, Forman SJ, Jensen MC, Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells, Clin Cancer Res 18(8) (2012) 2199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ, Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM, BMC Immunol 16 (2015) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, Gratama JW, Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity, Mol Ther 21(4) (2013) 904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA, Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2, Mol Ther 18(4) (2010) 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Santamaria S, Delgado M, Kremer L, Garcia-Sanz JA, Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible?, Front Immunol 8 (2017) 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC, HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells, Leuk Res 28(10) (2004) 1089–96. [DOI] [PubMed] [Google Scholar]
- [118].Liu J, Jiang G, CD44 and hematologic malignancies, Cell Mol Immunol 3(5) (2006) 359–65. [PubMed] [Google Scholar]
- [119].Ghatak S, Misra S, Toole BP, Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway, J Biol Chem 277(41) (2002) 38013–20. [DOI] [PubMed] [Google Scholar]
- [120].Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS, Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody, Cancer Res 54(6) (1994) 1561–5. [PubMed] [Google Scholar]
- [121].Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL, Moore PA, Ruben SM, Carter PJ, CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers, Br J Cancer 99(1) (2008) 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, Wicha MS, Chang AE, Li Q, Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo, Clin Immunol 149(1) (2013) 156–68. [DOI] [PubMed] [Google Scholar]
- [123].Zhao L, Yang Y, Zhou P, Ma H, Zhao X, He X, Wang T, Zhang J, Liu Y, Zhang T, Targeting CD133high Colorectal Cancer Cells In Vitro and In Vivo With an Asymmetric Bispecific Antibody, J Immunother 38(6) (2015) 217–28. [DOI] [PubMed] [Google Scholar]
- [124].Wang AZ, Langer R, Farokhzad OC, Nanoparticle delivery of cancer drugs, Annu Rev Med 63 (2012) 185–98. [DOI] [PubMed] [Google Scholar]
- [125].Jain RK, Stylianopoulos T, Delivering nanomedicine to solid tumors, Nat Rev Clin Oncol 7(11) (2010) 653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, Breast G Cancer International Research, Adjuvant trastuzumab in HER2-positive breast cancer, N Engl J Med 365(14) (2011) 1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, Group ES, Trastuzumab emtansine for HER2-positive advanced breast cancer, N Engl J Med 367(19) (2012) 1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J, Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study, Lancet Oncol 14(6) (2013) 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S, HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors, Clin Cancer Res 16(2) (2010) 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sharpe AH, Introduction to checkpoint inhibitors and cancer immunotherapy, Immunol Rev 276(1) (2017) 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Gajewski TF, The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment, Semin Oncol 42(4) (2015) 663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bilusic M, Madan RA, Gulley JL, Immunotherapy of Prostate Cancer: Facts and Hopes, Clin Cancer Res 23(22) (2017) 6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, Sunwoo JB, CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1, Clin Cancer Res 22(14) (2016) 3571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zhi Y, Mou Z, Chen J, He Y, Dong H, Fu X, Wu Y, B7H1 Expression and Epithelial-To-Mesenchymal Transition Phenotypes on Colorectal Cancer Stem-Like Cells, PLoS One 10(8) (2015) e0135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yang Y, Wu KE, Zhao E, Li W, Shi L, Xie G, Jiang B, Wang Y, Li R, Zhang P, Shuai X, Wang G, Tao K, B7-H1 enhances proliferation ability of gastric cancer stem-like cells as a receptor, Oncol Lett 9(4) (2015) 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wu Y, Chen M, Wu P, Chen C, Xu ZP, Gu W, Increased PD-L1 expression in breast and colon cancer stem cells, Clin Exp Pharmacol Physiol 44(5) (2017) 602–604. [DOI] [PubMed] [Google Scholar]
- [137].Shi X, Zhang X, Li J, Mo L, Zhao H, Zhu Y, Hu Z, Gao J, Tan W, PD-1 blockade enhances the antitumor efficacy of GM-CSF surface-modified bladder cancer stem cells vaccine, Int J Cancer (2017). [DOI] [PubMed] [Google Scholar]
- [138].Almozyan S, Colak D, Mansour F, Alaiya A, Al-Harazi O, Qattan A, Al-Mohanna F, Al-Alwan M, Ghebeh H, PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation, Int J Cancer 141(7) (2017) 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Nagarsheth N, Wicha MS, Zou W, Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy, Nat Rev Immunol 17(9) (2017) 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, Kotarski J, Tarkowski R, Wicha M, Cho K, Giordano T, Liu R, Zou W, Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2, Immunity 39(3) (2013) 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanislawek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I, Myeloid-Derived Suppressor Cells Endow Stem-like Qualities to Breast Cancer Cells through IL6/STAT3 and NO/NOTCH Cross-talk Signaling, Cancer Res 76(11) (2016) 3156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, Weinberg RA, A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages, Nat Cell Biol 16(11) (2014) 1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Korkaya H, Liu S, Wicha MS, Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots, Clin Cancer Res 17(19) (2011) 6125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Korkaya H, Liu S, Wicha MS, Breast cancer stem cells, cytokine networks, and the tumor microenvironment, J Clin Invest 121(10) (2011) 3804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH, Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients, Clin Cancer Res 15(6) (2009) 2148–57. [DOI] [PubMed] [Google Scholar]
- [146].Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS, CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts, J Clin Invest 120(2) (2010) 485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Jia D, Li L, Andrew S, Allan D, Li X, Lee J, Ji G, Yao Z, Gadde S, Figeys D, Wang L, An autocrine inflammatory forward-feedback loop after chemotherapy withdrawal facilitates the repopulation of drug-resistant breast cancer cells, Cell Death Dis 8(7) (2017) e2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, Lee YJ, Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells, Cell Signal 25(4) (2013) 961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Morton JJ, Bird G, Refaeli Y, Jimeno A, Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap, Cancer Res 76(21) (2016) 6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liu X, Chen X, Rycaj K, Chao HP, Deng Q, Jeter C, Liu C, Honorio S, Li H, Davis T, Suraneni M, Laffin B, Qin J, Li Q, Yang T, Whitney P, Shen J, Huang J, Tang DG, Systematic dissection of phenotypic, functional, and tumorigenic heterogeneity of human prostate cancer cells, Oncotarget 6(27) (2015) 23959–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Guo Y, Feng K, Wang Y, Han W, Targeting cancer stem cells by using chimeric antigen receptor-modified T cells: a potential and curable approach for cancer treatment, Protein Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Adeegbe DO, Liu Y, Lizotte PH, Kamihara Y, Aref AR, Almonte C, Dries R, Li Y, Liu S, Wang X, Warner-Hatten T, Castrillon J, Yuan GC, Poudel-Neupane N, Zhang H, Guerriero JL, Han S, Awad MM, Barbie DA, Ritz J, Jones SS, Hammerman PS, Bradner J, Quayle SN, Wong KK, Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-Small Cell Lung Cancer, Cancer Discov 7(8) (2017) 852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, Saeboe-Larssen S, Sandberg C, Brinchmann JE, Helseth E, Rasmussen AM, Lote K, Aamdal S, Gaudernack G, Kvalheim G, Langmoen IA, Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma, Cancer Immunol Immunother 62(9) (2013) 1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]