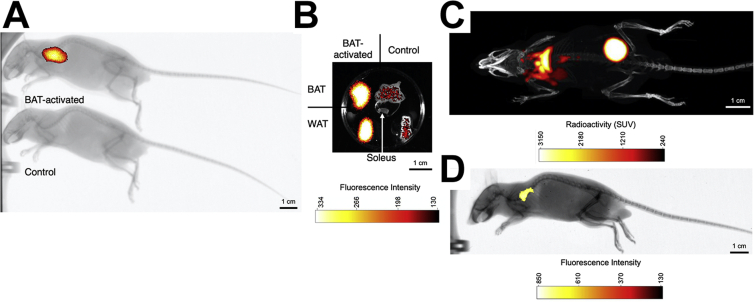
Brown adipose tissue (BAT) is a mitochondrial dense tissue capable of regulating body temperature and energy balance (1). BAT is a potential therapeutic target for metabolic diseases including obesity and type 2 diabetes (1). Determining in vivo BAT metabolic activity is a powerful tool in translational research. Positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) is the standard technique for imaging BAT glucose uptake as a proxy for thermogenic activity (2). However, PET is limited by the requirement for radioisotope tracers, associated costs, and a lack of functionality to detect concurrent metabolic processes within the same animal. Multimodal imaging can overcome these limitations. We combined FDG PET with fluorescence optical imaging, a promising technique, not yet widely used in BAT studies (3). We induced BAT activity in C57BL6 mice with CL316,243, a highly specific beta 3-adrenoreceptor agonist, with 1 mg/kg subcutaneous injection for 3 days. We intravenously injected a commercially available fluorescent probe, RediJect 2-DG (100 μl), 3 h before imaging with an Xtreme II optical imaging system (Bruker, Ettlingen) in CL316,243-treated BAT-activated animals or saline-injected controls (panel A). Anatomical regions of interest were used in analysis of fluorescence optical imaging. Animals treated with beta 3-adrenoreceptor agonist had higher uptake of RediJect 2-DG in BAT, which we confirmed with ex vivo optical imaging of harvested tissues including BAT, subcutaneous white adipose tissue (WAT), and soleus muscle (panel B). Next, we compared RediJect 2-DG to FDG to determine if RediJect 2-DG was a suitable alternative to FDG and to establish the impact of co-injection. We co-injected RediJect 2-DG and FDG into a mouse with induced BAT activity. In succession, we imaged the same mouse with PET/computed tomography to detect the FDG (panel C) and then used optical imaging to detect the RediJect 2-DG (panel D). RediJect 2-DG optical imaging identifies increased activity in the BAT anatomical region as was observed with PET and validated ex vivo using optical imaging and gamma-counter biodistribution analysis. This study is an important step to progress onto wider multitracer work. Simultaneous co-injection of a radioisotope and fluorescent probe could expand current BAT in vivo imaging modalities and facilitate the future detection of multiple concurrent metabolic processes in a single animal.
EQUIPMENT: Albira Si PET/SPECT/CT (Bruker), Xtreme II optical imaging system (Bruker)
REAGENTS: XenoLight RediJect 2-DeoxyGlucosone (DG) (PerkinElmer), CL316,243 (Sigma)
Acknowledgments
Author ORCIDs
Jurgen E. Schneider https://orcid.org/0000-0003-0999-5684
Lee D. Roberts https://orcid.org/0000-0002-1455-5248
Funding and additional support
This work was supported by grants from the Medical Research Council (MR/R014086/1) and the British Heart Foundation.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Townsend K., Tseng Y.-H. Brown adipose tissue. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borga M., Virtanen K.A., Romu T., Leinhard O.D., Persson A., Nuutila P., Enerbäck S. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography) Meth. Enzymol. 2014;537:141–159. doi: 10.1016/B978-0-12-411619-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 3.Rice D.R., White A.G., Leevy W.M., Smith B.D. Fluorescence imaging of interscapular brown adipose tissue in living mice. J. Mater. Chem. B. 2015;3:1979–1989. doi: 10.1039/C4TB01914H. [DOI] [PMC free article] [PubMed] [Google Scholar]