Abstract
Thermodynamic principles are used to map the phase behavior of a tunable protein binding system under crowded cellular conditions. This study1 marks a significant step forward in relating molecular interactions to materials properties and cellular processes involving protein self-assembly.
Cells frequently gain spatiotemporal control over biochemical processes necessary for the proper functioning of the cell by the formation of membraneless compartments or organelles (MLOs) that are composed of proteins and nucleic acids2. The formation of MLOs inside cells can be described as a demixing process where the enthalpic interactions between components overcome the entropic loss to favor the coexistence between a dense and a dilute phase3. A detailed study of these interactions is, therefore, necessary but is still an arduous task for experiments as biomolecules involved in phase separation have several different interaction modes, which often act in tandem to provide driving forces for phase separation4,5. How these molecular interactions relate to the observed phase behavior under cellular conditions is an open question of significant interest. Heidenreich et al.1 devise a creative strategy guided by the rules of thermodynamics to provide a way of monitoring phase diagrams in vivo for a synthetic two-protein system with specific interactions between the two proteins that are known and tunable.
Recent studies have brought out qualitative aspects of how changes in protein interactions imparted by mutations in their sequence can change the observed phase behavior in silico, in vitro, and in vivo 6–8. Thermodynamic phase diagrams provide the most rigorous path to characterize the self-assembly behavior as a function of variables of interest such as protein concentrations and factors that affect intermolecular interactions such as temperature, salt, and the relative composition of different components. The intermolecular interactions can be controlled or fixed relatively quickly in a reconstituted in vitro system. But it is not easily possible to control these under cellular conditions due to changes associated with macromolecular crowding effects and a myriad of non-specific interactions between macromolecules in the milieu of a cell.
Heidenreich et al.1establish an ingenious strategy based on a synthetic two-component system, which is orthogonal to the biological system, to study how different factors like concentration, and intermolecular affinity determine multicomponent phase behavior quantitatively. They use a pair of folded proteins (lm2 and E9) whose known binding affinity for each other can be either increased or decreased using point mutations. Using dimers and tetramers of the proteins they specify the “valence” of the intermolecular interactions. By independently coexpressing these dimers and tetramers in yeast cells they are able to vary the relative compositions of these self-assembling building blocks which allows them to directly visualize the appearance of puncta formed by fluorescently labeled proteins in the form of a multcomponent phase diagram. On decreasing the interaction affinity between the two proteins using point mutations of the lm2 dimer they see a corresponding increase in the dimer and tetramer concentrations required for phase separation i.e. an upward shift of the phase diagram across the diagonal. This decrease in interaction affinity also leads to faster dynamics inside the condensate. Hence, by controlling the interaction affinity between the two molecules they are able to change the phase diagram and the material properties of the condensates. The use of statistical mechanical theory and coarse-grained simulations in providing an interpretation of the observed behavior, including the role of kinetics, highlights the synergistic role of different methods in uncovering complex phenomena (Fig. 1).
Figure 1. Studying self-assembly of well-defined protein binding systems using a unified toolset:
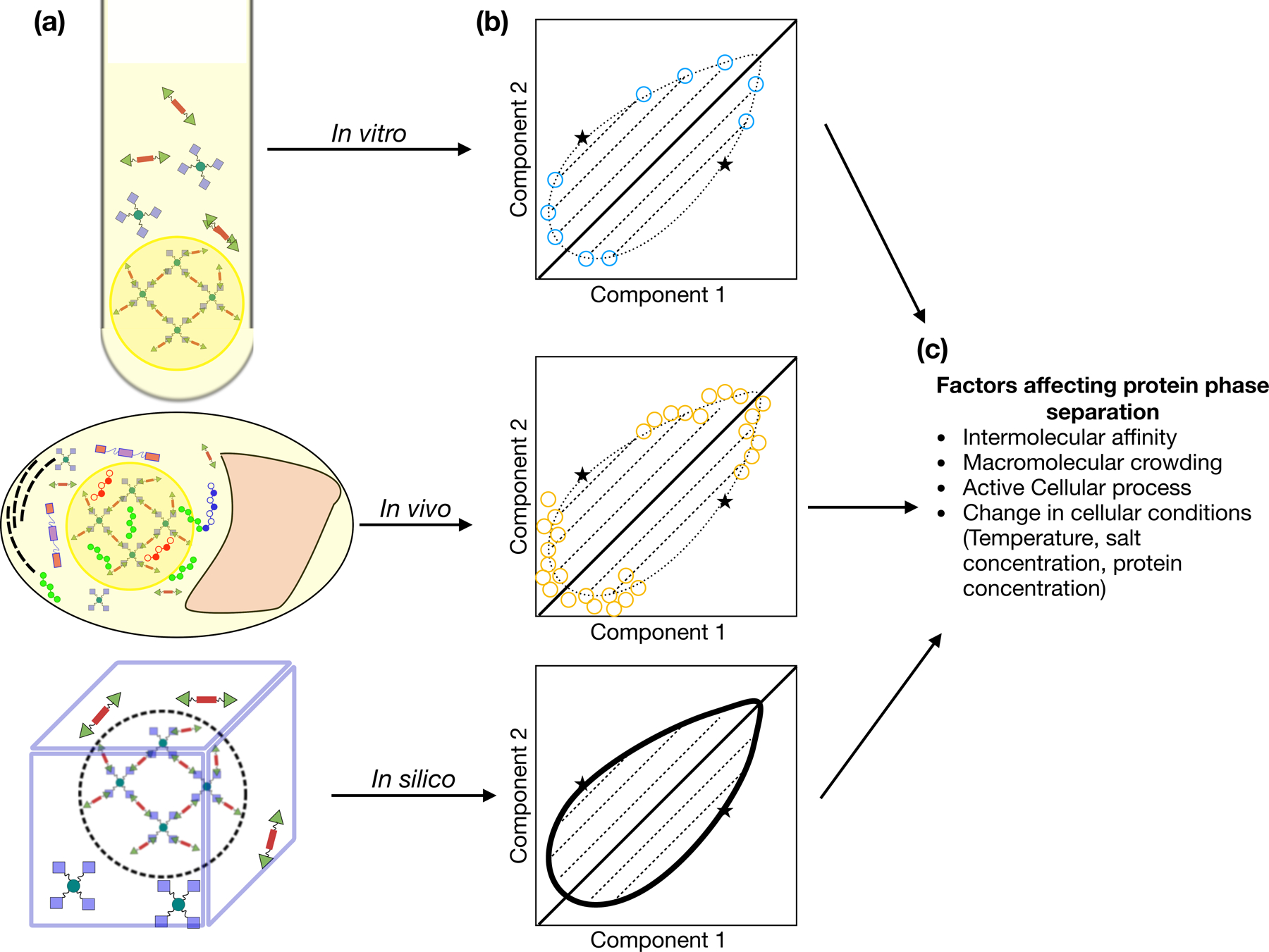
One can employ multiple methods(a) in tandem for studying self-assembly with a well-defined protein binding model as presented in Heidenreich et. al. which allows for easy transferability of information between methods and helps address their respective shortcomings to construct multicomponent phase diagrams(b) which present a unified picture of how cellular factors(c) affect protein self-assembly.
This work essentially portrays how multicomponent phase separation can be studied and understood by controlling the intermolecular interactions. But one must be careful about how inferences from such a well-designed synthetic system would apply to other biological systems where the multivalency of interactions may be much less well-defined. This particular issue maybe even more relevant inside the crowded environment of the cell where there are a multitude of biomolecules that can actively or passively affect the affinity, valence, or local concentration of phase separating components.
On the other hand, the strategy of Heidenreich et al.1 can be used to study the role of the cellular environment on the observed phase behavior by comparing the results obtained on the same synthetic two-protein system in a well-defined laboratory test tube. This can be especially helpful for deciphering the role of cellular active processes in the formation and maintenance of MLOs9. In the case of Heidenreich et al.1, they use their methodology to show how the cellular process of cotranslational assembly sufficiently directs the localization of specific RNA molecules in a synthetic condensate. As most MLOs contain RNA and protein as their major constituents10, localization of RNA and its specficity is a major topic that can be conveniently studied using the proposed methdodology.
With the advent of more sophisticated and precise experimental methods like the one proposed by Heidenreich et al.1 we can forsee a future where computational and experimental techniques can be used in tandem for estimating the contribution of different types of intermolecular interactions, hence providing greater clarity into understanding the convoluted interaction network driving phase separation in live cells.
Footnotes
COMPETING FINANCIAL INTEREST:
There is NO competing interest.
References
- 1.Heidenreich M et al. Designer protein assemblies with tunable phase diagrams in living cells 0–18 (2020). [DOI] [PubMed]
- 2.Banani SF, Lee HO, Hyman AA & Rosen MK Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brangwynne CP, Tompa P & Pappu RV Polymer physics of intracellular phase transitions. Nat. Phys 11, 899 (2015). [Google Scholar]
- 4.Murthy AC et al. Molecular interactions underlying liquid−liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol 26, 637–648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernon RM et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7, 1–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conicella AE et al. TDP-43 α-helical structure tunes liquid–liquid phase separation and function. Proc. Natl. Acad. Sci. U. S. A 117, 5883–5894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster BS et al. Identifying sequence perturbations to an intrinsically disordered protein that determine its phase-separation behavior. Proc. Natl. Acad. Sci 117, 202000223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin EW et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science (80-. ) 367, 694–699 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falahati H & Wieschaus E Independent active and thermodynamic processes govern the nucleolus assembly in vivo. Proc. Natl. Acad. Sci. U. S. A 114, 1335–1340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tauber D, Tauber G & Parker R Mechanisms and Regulation of RNA Condensation in RNP Granule Formation. Trends Biochem. Sci 1–15 (2020). doi: 10.1016/j.tibs.2020.05.002 [DOI] [PMC free article] [PubMed]


