Abstract
Purpose of review
A complex network of hormones and other effectors characterize the hypermetabolic response in critical illness; these mediators work together to induce numerous pathophysiologic alterations. Increased incidence of infection, multiorgan failure, long-term debilitation, delays in rehabilitation, and death result from an inability to meet the prohibitively elevated protein and energy requirements, which occur during illness and can persist for several years. Pharmacologic interventions have been successfully utilized to attenuate particular aspects of the hypermetabolic response; these modalities are a component of managing critically ill patients – including those patients with severe burns. Here, we review recent advances in pharmacologically attenuating the hypermetabolic and catabolic responses.
Recent findings
Propranolol, a nonspecific β-adrenergic receptor antagonist, is one of the most widely used anticatabolic therapies. Oxandrolone, testosterone, and intensive insulin therapy represent anabolic pharmacological strategies. Promising therapies, such as metformin, glucagon-like peptide 1, peroxisome proliferator-activated receptor agonists, are currently being investigated.
Summary
Profound metabolic derangements occur in critically ill patients; this hypermetabolic response is a major contributor to adverse outcomes. Despite the pharmacological therapies currently available to counteract this devastating cascade, future studies are warranted to explore new multimodality agents that will counteract these effects while maintaining glycemic control and preventing unfavorable complications.
Keywords: anabolic, anticatabolic, burns, critical illness, hypermetabolism, insulin, metformin, oxandrolone, propranolol
INTRODUCTION
Critical illness or injury results in an extensive and persistent hypermetabolic response which drives catabolism far longer than the duration of the initial insult [1]. The hallmarks of this response are supraphysiologic metabolic rates, including profoundly accelerated proteolysis, lipolysis, glycolysis, liver dysfunction, insulin resistance, and loss of total and lean body mass; fatal physiologic exhaustion occurs if this hypermetabolic response is left untreated [2–4]. Mediators of these complex responses are cytokines, acute-phase and constitutive proteins, as well as hormones. All these mediators are altered upon onset of any acute critical illness and remain abnormal for a much more prolonged period of time than previously thought [3,5].
When circulating levels of gluconeogenic hormones, glucagon, cortisol, and catecholamines are elevated in response to critical injury, inefficient liver glucose production is stimulated alongside substantially increased lipolysis, leading to futile substrate cycling [3,4]. Recent studies in critically ill and severely burned patients demonstrate significant derangements in energy-producing and mitochondrial pathways, inclusive of increased gluconeogenesis, glycogenolysis, lipolysis, and elevated glucogenic precursor circulation. Impaired insulin sensitivity and hyperglycemia result, which leads to postreceptor insulin resistance [6,7]. Lactate, the anaerobic glucose oxidation end product, is recycled to the liver to stimulate production of more glucose via gluconeogenic pathways [8]. Significant elevations in serum insulin and serum glucose remain [3], and characteristics of insulin resistance persist for at least 3 years postburn [6].
Lipid metabolism is another significantly altered metabolic pathway as a result of the hypermetabolic response. Lipolysis, characterized by the reduction of triacylglycerol into glycerol and free fatty acids (FFAs), contributes to postburn morbidity and mortality, and also organ infiltration and altered glucose metabolism [9]. FFAs specifically hamper insulin-stimulated glucose uptake [10,11] and, through inhibition of glucose transport, induce insulin resistance [12]. Increased abundance of FFAs, in the context of type 2 diabetes, is predictive not only of the incidence but also of the disease severity[13]. Modulation of plasma FFA concentrations can be the result of hypoalbuminemia or elevations in intracellular FFA turnover, despite increased lipolysis. This is part of the futile cycle involving the generation of FFA from muscle and adipose triglycerides. In general, the anabolic effect of insulin is countered by catabolic hormones causing significant lipolysis, proteolysis, and hyperglycemia [6,7]. In an attempt to fulfill unmet metabolic and energy requirements, the body inefficiently utilizes lipids and proteins after critical illness [6,7]. Additionally, as the body fails to recognize fat as a source of energy, skeletal muscle is utilized as the major obligatory fuel, resulting in substantial muscle protein catabolism [3]. Owing to the extensive depletion of net protein, consequential muscle wasting and loss of body mass occur, ostensibly contributing to reduced strength and an inability to completely rehabilitate [14]. In addition to changes in tissue loss, protein degradation also interferes with cross-leg and whole-body nitrogen balance [8,14]. Hence, protein catabolism is directly associated with elevated metabolic rate [15]. The loss of body mass affects other key processes needed for recovery: a reduction of total body mass by 10% induces immune dysfunction, wound healing is compromised with decreases of 20%, severe infections result from loss of 30%, and a 40% loss becomes fatal [16].
Anabolic and anticatabolic treatment options
Elevations in circulating glucagon, cortisol, and catecholamines perpetuate the extensive alterations in metabolic rate, physiology, and growth observed following critical illness or severe injury. Over the past several decades, the utility of pharmacologic agents to increase anabolism has been studied, including recombinant human growth hormone (rhGH), insulin, the combination of insulin-like growth factor 1 (IGF-1) with IGF-binding protein-3 (IGFBP-3), testosterone, and oxandrolone. A catecholamine surge is a hallmark of critical illness, the effects of which can be circumvented with the administration of propranolol, a nonselective β-adrenergic receptor antagonist. The administration of anabolic or anticatabolic therapies to the critically ill has led to significant decreases in protein catabolism when given in addition to the current standards of care.
Recombinant human growth hormone and insulin-like growth factor 1
Significant improvements in cardiac function, bone mineral content, lean body mass, height velocities, and weight gain result from rhGH administration 0.2 mg/kg via daily intramuscular injection to severely burned children [17,18]. The hepatic acute-phase response is favorably influenced by rhGH as indicated by the increase in serum IGF-1 concentrations in the serum [4,19]. Furthermore, muscle protein kinetics improved, muscle growth is maintained [17,20], time to donor site healing reduces by 1.5 days [21], and cardiac output and resting energy expenditure is decreased [22]. IGF mediates these beneficial effects of rhGH. Relative to healthy controls, serum IGFBP-3 and IGF-1 increased 100% in patients receiving rhGH [21]. However, these findings did not translate well into critically ill adult patients. The results from a prospective, multicenter, double-blind, randomized, placebo-controlled trial of 0.10±0.02 mg/kg body weight rhGH administered to 285 critically ill nonburned patients demonstrated that these relatively high rhGH doses were associated with greater morbidity and mortality [21–23] and hyperglycemia and insulin resistance [21,22]. Further examination of the short-term compared with the long-term administration of rhGH in severely burned children revealed that there was no increased risk of mortality in this patient population [22].
Taking into consideration that the effects of growth hormone are mediated by IGF-1, the infusion of equimolar doses of recombinant human IGF-1 and IGFBP-3 effectively improved protein metabolism without inducing hypoglycemia, such as seen with rhGH administration in catabolic pediatric study participants and adults [21,24,25]. The combination of recombinant human IGF-1 (rhIGF-1) and IGFBP-3 diminishes muscle catabolism and concurrently improves gut mucosal integrity in severely burned children [21,25]. The attenuation of the type 1 and type 2 hepatic acute-phase responses improves ratios of circulating constitutive proteins that modify the hypercatabolic usage of body proteins [25,26]. The administration of IGF-1 alone as an independent therapy should be cautioned against as studies by Langouche and van den Berghe [27] showed that in nonburned, critical care patients, IGF-1 alone lacks efficacy.
Insulin
Insulin remains one of the most extensively studied therapeutic agents, leading to the discovery of novel uses for this hormone. Apart from decreasing blood glucose via mediation of peripheral glucose uptake into adipose tissue and skeletal muscle, or suppressing hepatic gluconeogenesis, insulin also increases DNA replication and protein synthesis via modulating amino acid uptake, increasing fatty acid synthesis, and decreasing proteolysis [28]. Owing to the latter property, insulin is particularly attractive as an antihyperglycemia therapy in severely burned patients in light of the findings that muscle protein synthesis increases, donor site healing accelerates, and lean body mass loss and the acute-phase response each decrease when insulin is administered during acute hospitalization [29–31]. The European multicenter trial Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis found that when administered to patients with severe infections and sepsis, insulin administration did not affect mortality; however, severe hypoglycemia was four-fold higher with intensive insulin as opposed to conventional therapy [32]. Another large multicenter study reported a dramatic increase in serious hypoglycemic episodes with the use of a continuous hyperinsulinemic–euglycemic clamp throughout ICU stay [33]. The gap in knowledge that remains is to determine the ideal target glucose range, although clinical trials to determine ideal glucose levels for ICU and burned patients are underway. Suggestions for reduction of glucose levels to 140 mg/dl or less [34] or to less than 150 mg/dl [35] have been made. For severely burned patients, reductions to 140 mg/dl resulted in decreased morbidity and mortality, whereas attenuation of the hypermetabolic response was seen with blood glucose levels 130 mg/dl or less [36].
Although maintaining a continuous hyperinsulinemic–euglycemic clamp in burn patients is particularly difficult because of the necessity for continuous enteral feeding to maintain euglycemia, the occasional halt in enteral feeding because of daily dressing changes or weekly operations may disrupt gastrointestinal motility and increase the incidence of hypoglycemia [8].
Testosterone, ketoconazole, and oxandrolone
Despite the profound improvements anabolic agents have on improving lean body mass, physical activity is imperative to developing strength [37]. Burn patients have significant reductions in testosterone such that severely burned men have similar concentrations of serum testosterone to women [38]. Exogenous testosterone administration for 2 weeks resulted in a two-fold reduction in muscle protein breakdown and improved net muscle balance and protein synthesis efficiency [38]. Similarly, the antifungal imidazole ketoconazole reduces steroid synthesis by blocking P450-dependent enzyme systems [39,40]. However, in burned children, although cortisol levels were reduced to a normal range for up to 60 days after injury, ketoconazole administration did not impact inflammation, hormone secretion (IGF-1, rhGH, and IGFBP-3), hypermetabolism, organ function, or net protein balance/synthesis [41].
Administration of the testosterone analog oxandrolone leads to increased muscle protein catabolism via improved protein synthesis efficiency [42], better donor site wound healing times, and reduced weight loss [43]. Length of hospital stay resulted from the administration of 10 mg of oxandrolone every 12 h in a prospective randomized study [44]. In a large single-center, prospective, double-blinded, randomized trial, 0.1 mg/kg of oxandrolone administered every 12 h, in an age-independent manner, reduced length of hospital stay, maintained lean body mass, and improved hepatic protein synthesis [45,46]. Compliance with long-term administration of oxandrolone is good because of the oral, and not injected, route of administration. The effects of burn-associated hypermetabolism on body tissues are significantly reduced, leading to significant increases in total body mass, lean body mass, and bone mineral content throughout the 1-year administration period [47]; resting energy expenditure and the rate pressure product were reduced [48], whereas lung function improved [49■] with the 1-year administration of oxandrolone. In a 5-year follow-up study, the improvements in bone mineral content with oxandrolone were found to persist for up to 5 years postburn – despite cessation of oxandrolone treatment 1 year after injury [48]. Children treated with oxandrolone gained greater height percentiles as well. The combination of exercise with oxandrolone was even more effective, leading to greatest efficacy in children between the ages of 7 and 18 years, with significant increases in lean body mass and muscle strength. Duration of administration of oxandrolone directly impacts the outcomes; patients receiving oxandrolone for up to 2 years postburn had significantly greater increases in bone mineral content, bone mineral density, and height velocities compared with control patients. Interestingly, when compared with patients treated with oxandrolone for up to 1 year, those patients in the cohort receiving oxandrolone for 2 years had significantly improved bone mineral density and content [50■■]. Thus, despite the elusive benefits of steroid hormones such as testosterone or ketoconazole, oxandrolone is superior in terms of improving outcomes in severely injured patients.
Propranolol
Propranolol has shown great promise in terms of reversing the effects of injury. Propranolol administration to severely burned patients reduced resting energy expenditure, marked tachycardia, and thermogenesis [4,51,52]. With a reduction in heart rate of 20%, cardiac work decreased significantly [51,52]. Propranolol prevents peripheral lipolysis by blocking the activation of the β-2-adrenergic receptor by catecholamines, which are elevated to supraphysiologic levels postburn [4,51,53,54]. At the same time, a significant decrease in fatty infiltration of the liver occurs with propranolol administration [55]. Decreased skeletal muscle wasting and improvements in lean body mass were found with propranolol administration [51]. Despite the numerous benefits, the mechanisms underlying propranolol administration have yet to be fully elucidated. The efficacy of propranolol may be a result of increased protein synthesis taking place in an environment that promotes reduction in peripheral lipolysis and persistent protein breakdown [56]. Glucose levels, typically elevated in severely burned patients, can be normalized with propranolol administered 4 mg/kg body weight/q24, significantly reducing the amount of insulin needed [52]. Taken together, propranolol is a promising treatment to counteract postburn insulin resistance. The advantages attributed to postburn propranolol administration persist beyond the acute hospitalization period. In patients receiving propranolol for 1 year following burn injury, significant reductions in the percentage of predicted resting energy expenditure and in the percentage of the predicted heart rate were found [57]. Additionally, central mass and central fat accumulation were significantly reduced. Long-term administration of propranolol also improved the accretion of lean body mass and prevented bone loss over the course of 1 year.
Novel agents
In severely injured patients, metformin, a member of the biguanide family, has recently emerged as an alternative therapy for the management of hyperglycemia [58]. Metformin counteracts the major metabolic processes that drive injury-induced hyperglycemia via preventing gluconeogenesis and impaired peripheral insulin sensitivity [59]. In addition, metformin is not typically associated with inducing hypoglycemic events associated with the administration of exogenous insulin [60■■]. A small, randomized study showed the reduction of glucose concentrations and of endogenous glucose production alongside faster glucose clearance with metformin treatment [58]. Metformin increased the muscle protein fractional synthetic rate and improved net muscle protein balance as well [59]. In a recent phase II randomized control trial, metformin administered to adult burn patients demonstrated safety and efficacy and appears to be an alternative to insulin [60■■]. As a replacement for insulin to control blood glucose, metformin is a promising alternative that has proven efficacy for counteracting hyperglycemia and muscle protein wasting in critically injured patients. Despite therapeutic potential and numerous benefits, metformin is associated with lactic acidosis [61]. Metformin-associated lactic acidosis can be avoided by not administering to patients with a potential risk for impaired lactate elimination (such as occurs with renal or hepatic failure) or tissue hypoxia. Caution should be used when administering metformin to subacute burn patients.
Other agents are being trialed as antihyperglycemia agents in severely burned patients, including glucagon-like peptide 1 (GLP-1) and the peroxisome proliferator-activated receptor gamma agonists such as the thiazolidinedione pioglitazone. Cree et al. [62] demonstrated in a prospective, double-blind, placebo-controlled randomized trial that the peroxisome proliferator-activated receptor alpha agonist fenofibrate significantly improved mitochondrial glucose oxidation, increased insulin sensitivity, and reduced plasma glucose. Improved insulin receptor signaling was found in muscle with fenofibrate treatment via greater tyrosine phosphorylation of the insulin receptor 1 after hyperinsulinemic– euglycemic clamp compared with placebo patients [62]. Similarly, exogenous administration of GLP-1 decreases blood glucose by counteracting glucagon, stimulating insulin, and suppressing gastric emptying, which are not associated with hypoglycemia. In a recent study, Deane and colleagues investigated seven mechanically ventilated critically ill patients with no known history of diabetes, and showed that acute exogenous GLP-1 infusion significantly attenuated the glycemic response to enteral nutrition. These results suggest that GLP-1 and/or its analogues may have potential in the management of hyperglycemia in critically ill patients [63,64].
CONCLUSION
Critical illness induces profound metabolic derangements, which are associated with persistent changes that underlie adverse outcome in this patient population. Oxandrolone and propranolol have been shown to have great promise in improving long-term outcomes and reducing catabolism individually. Additionally, other pharmacological treatments have proven successful in attenuating the hypermetabolic response, including IGF and growth hormone (Table 1, Fig. 1). Although maintaining blood glucose levels at 130 mg/dl with intensive insulin therapy reduces mortality and morbidity in critically ill patients, associated hypoglycemic events have led to the investigation of alternative strategies such as metformin and fenofibrate. Nevertheless, additional investigations are warranted in critically ill patients to determine ideal glucose ranges, the safety and efficacy of new therapies, and whether the coadministration of these therapies could yield greater improvements.
Table 1.
Summary of the main effects of various pharmacologic interventions to alter the hypermetabolic response to burn injury
| Drug | Inflammatory response |
Stress hormones |
Body composition |
Net protein balance | Insulin resistance/glucose metabolism | Cardiac work |
|---|---|---|---|---|---|---|
| rhGH | Improved | No difference | Improved | No difference | Hyperglycemia | No difference |
| IGF-1 | Improved | No difference | Improved | Improved | Improved | No difference |
| Oxandrolone | Improved | No difference | Improved | Improved | No difference | No difference |
| Insulin | Improved | No difference | Improved | Improved | Improved | No difference |
| Fenofibrate | No difference | No difference | No difference | No difference | Improved | No difference |
| GLP-1 | Unknown | Unknown | Unknown | Unknown | Improved (indirect) | Unknown |
| Metformin | Improved | Unknown | Unknown | Improved | Improved | Unknown |
| Propranolol | Improved | Improved | Improved | Improved | Improved | Improved |
| Ketoconazole | Unknown | Improved | Unknown | Unknown | Unknown | Unknown |
| rhGH + propranolol | Improved | Improved | Improved | Improved | Improved | Improved |
| Oxandrolone + propranolol | Improved (preliminary) | Improved (preliminary) | Improved (preliminary) | Improved (preliminary) | Improved (preliminary) | Improved (preliminary) |
FIGURE 1.
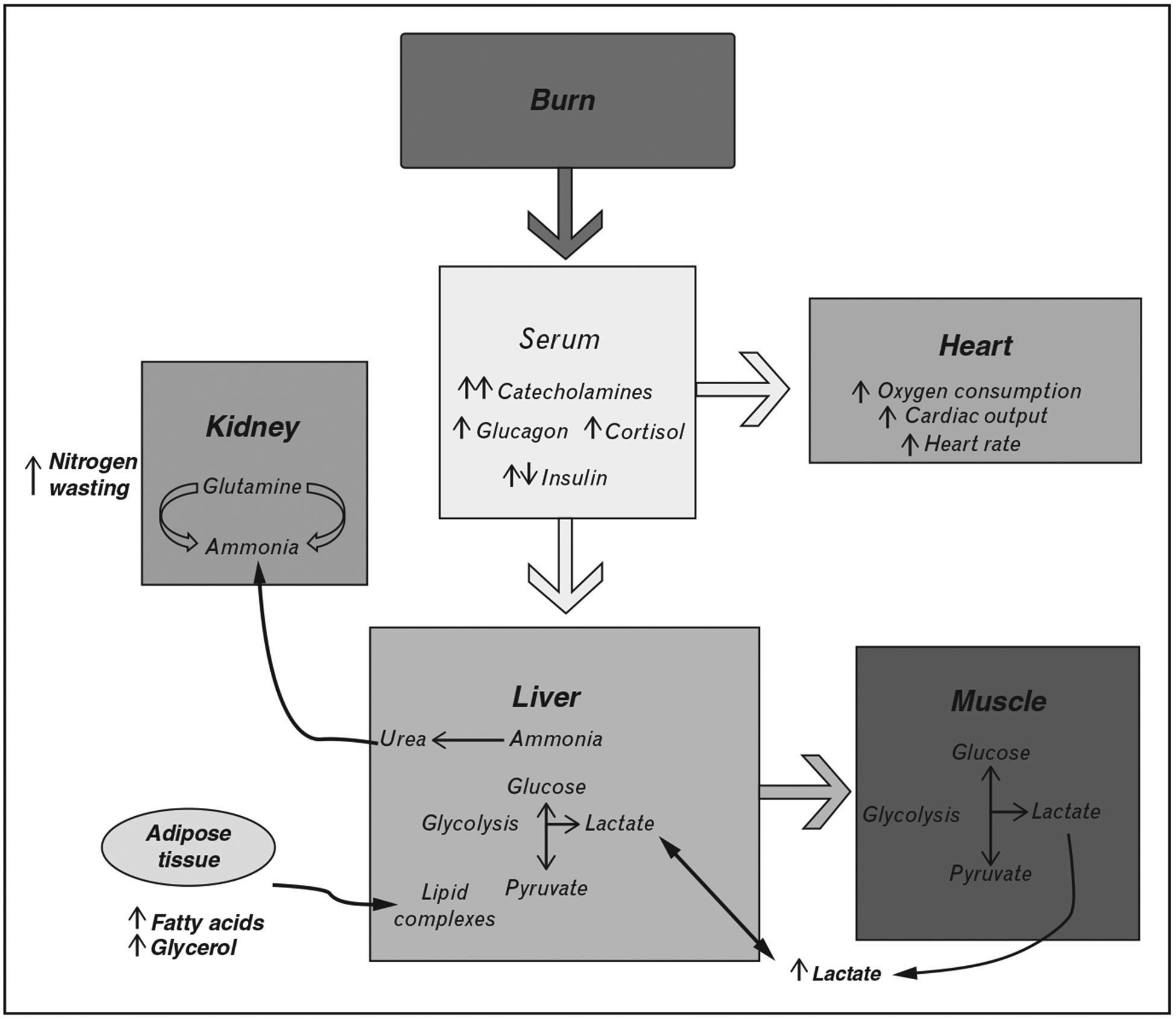
Pathophysiological alterations in multiple organs after burn trauma.
KEY POINTS.
A complex network of hormones and other effectors characterize the hypermetabolic response in critical illness, resulting in myriad pathophysiological alterations.
Presently, propranolol and oxandrolone represent the most efficacious anticatabolic therapies.
Novel pharmacological approaches have emerged to counteract hypermetabolism and catabolism in burn and critically ill patients, including metformin, GLP-1, and fenofibrate.
Financial support and sponsorship
The work is supported by grants from the National Institutes of Health (R01 GM087285-01), CIHR Funds (123336), and CFI Leader’s Opportunity Fund (Project #25407) M.G.J. Dr Finnerty is supported by a pilot grant from the UTMB Department of Surgery, grants from the National Institutes for Health (R01-GM056687, P50-GM060338, and R01-GM112936), the National Institute for Disability, Independent Living, and Rehabilitation Research (90DP0043-01-00), the Anderson Foundation, the Gillson Longebaugh Foundation, and from the Shriners Hospitals for Children (84080, 79141, 71008). The project was conducted with the support of UTMB’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences (NIH).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Mongardon N, Singer M. The evolutionary role of nutrition and metabolic support in critical illness. Crit Care Clin 2010; 26:443–450. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress 2007; 10:109–120. [DOI] [PubMed] [Google Scholar]
- 3.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg 2008; 248:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg 2009; 36:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011; 6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauglitz GG, Herndon DN, Kulp GA, et al. Abnormal insulin sensitivity persists up to three years in pediatric patients postburn. J Clin Endocrinol Metab 2009; 94:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeschke MG, Barrow RE, Herndon DN. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg 2004; 139:641 – 647. [DOI] [PubMed] [Google Scholar]
- 8.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004; 363:1895–1902. [DOI] [PubMed] [Google Scholar]
- 9.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963; 1:785–789. [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Ruiz J, et al. Insulin receptor down-regulation and impaired antilipolytic action of insulin in diabetic patients after pancreas/kidney transplantation. J Clin Endocrinol Metab 1994; 78:657–663. [DOI] [PubMed] [Google Scholar]
- 11.Shah P, Vella A, Basu A, et al. Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes 2002; 51:301–310. [DOI] [PubMed] [Google Scholar]
- 12.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999; 103:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankow JS, Duncan BB, Schmidt MI, et al. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care 2004; 27:77–82. [DOI] [PubMed] [Google Scholar]
- 14.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery 2000; 128:312–319. [DOI] [PubMed] [Google Scholar]
- 15.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg 2000; 232:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology 1992; 130:43–52. [DOI] [PubMed] [Google Scholar]
- 17.Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Critical care medicine 2001; 29:1318–1324. [DOI] [PubMed] [Google Scholar]
- 18.Mlcak RP, Suman OE, Murphy K, Herndon DN. Effects of growth hormone on anthropometric measurements and cardiac function in children with thermal injury. Burns 2005; 31:60–66. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Herndon DN, Wolf SE. Growth hormone down-regulation of interleukin-1beta and interleukin-6 induced acute phase protein gene expression is associated with increased gene expression of suppressor of cytokine signal-3. Shock 2003; 19:314–320. [DOI] [PubMed] [Google Scholar]
- 20.Aili Low JF, Barrow RE, Mittendorfer B, et al. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns 2001; 27:447–452. [DOI] [PubMed] [Google Scholar]
- 21.Williams FN, Jeschke MG, Chinkes DL, et al. Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg 2009; 208:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branski LK, Herndon DN, Barrow RE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg 2009; 250:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. New Engl J Med 1999; 341:785–792. [DOI] [PubMed] [Google Scholar]
- 24.Heszele MF, Price SR. Insulin-like growth factor I: the yin and yang of muscle atrophy. Endocrinology 2004; 145:4803–4805. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Crit Care Med 2000; 28:1578–1584. [DOI] [PubMed] [Google Scholar]
- 26.Spies M, Wolf SE, Barrow RE, et al. Modulation of types I and II acute phase reactants with insulin-like growth factor-1/binding protein-3 complex in severely burned children. Crit Care Med 2002; 30:83–88. [DOI] [PubMed] [Google Scholar]
- 27.Langouche L, van den Berghe G. Glucose metabolism and insulin therapy. Crit Care Clin 2006; 22:119–129. [DOI] [PubMed] [Google Scholar]
- 28.Dimitriadis G, Mitrou P, Lambadiari V, et al. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 2011; 93 (Suppl 1):S52–S59. [DOI] [PubMed] [Google Scholar]
- 29.Jeschke MG, Barrow RE, Mlcak RP, Herndon DN. Endogenous anabolic hormones and hypermetabolism: effect of trauma and gender differences. Ann Surg 2005; 241:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg 2004; 239:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged eu-glycemic hyperinsulinemia on lean body mass after severe burn. Surgery 2002; 132:341–347. [DOI] [PubMed] [Google Scholar]
- 32.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139. [DOI] [PubMed] [Google Scholar]
- 33.Langouche L, Vander Perre S, Wouters PJ, et al. Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J Clin Endocrinol Metab 2007; 92:3890–3897. [DOI] [PubMed] [Google Scholar]
- 34.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA 2003; 290:2041–2047. [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36:296–327. [DOI] [PubMed] [Google Scholar]
- 36.Jeschke MG, Kraft R, Emdad F, et al. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg 2010; 252:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suman OE, Thomas SJ, Wilkins JP, et al. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol (1985) 2003; 94:2273–2281. [DOI] [PubMed] [Google Scholar]
- 38.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med 2001; 29:1936–1942. [DOI] [PubMed] [Google Scholar]
- 39.Loose DS, Kan PB, Hirst MA, et al. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest 1983; 71:1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pont A, Williams PL, Loose DS, et al. Ketoconazole blocks adrenal steroid synthesis. Ann Intern Med 1982; 97:370–372. [DOI] [PubMed] [Google Scholar]
- 41.Jeschke MG, Williams FN, Finnerty CC, et al. The effect of ketoconazole on post-burn inflammation, hypermetabolism and clinical outcomes. PLosS One 2012; 7:e35465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg 2001; 233:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care 2000; 15:12–17. [DOI] [PubMed] [Google Scholar]
- 44.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res 2006; 27:131–141. [DOI] [PubMed] [Google Scholar]
- 45.Jeschke MG, Finnerty CC, Suman OE, et al. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007; 246:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demling RH, DeSanti L. The rate of restoration of body weight after burn injury, using the anabolic agent oxandrolone, is not age dependent. Burns 2001; 27:46–51. [DOI] [PubMed] [Google Scholar]
- 47.Murphy KD, Thomas S, Mlcak RP, et al. Effects of long-term oxandrolone administration in severely burned children. Surgery 2004; 136:219–224. [DOI] [PubMed] [Google Scholar]
- 48.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg 2012; 214:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.■.Sousse LE, Herndon DN, Mlcak RP, et al. Long-term administration of & oxandrolone improves lung function in pediatric burned patients. J Burn CareRes 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; In addition to the well documented benefits of oxandrolone improving hypermetabolism, bone mineral content, and lean body mass in pediatric burn patients, this study demonstrates that long-term administration also improves pulmonary function.
- 50.■■.Reeves PT, Herndon DN, Tanksley JD, et al. Five-year outcomes after long-term oxandrolone administration in severely burned children: a randomized clinical trial. Shock 2016; 45:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on the findings of this clinical trial, long-term administration of oxandrolone in pediatric burn patients resulted in improvements in bone mineral content, density, height velocity, and fewer patients meeting the criteria for osteoporosis 5 years postburn.
- 51.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 2001; 345:1223–1229. [DOI] [PubMed] [Google Scholar]
- 52.Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery 2010; 149:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hart DW, Wolf SE, Chinkes DL, et al. Beta-blockade and growth hormone after burn. Ann Surg 2002; 236:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeschke MG, Finnerty CC, Kulp GA, et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med 2008; 9:209–216. [DOI] [PubMed] [Google Scholar]
- 55.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma 2001; 51:736–739. [DOI] [PubMed] [Google Scholar]
- 56.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp 2007; 280:238–248. [DOI] [PubMed] [Google Scholar]
- 57.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg 2012; 256:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gore DC, Wolf SE, Herndon DN, Wolfe RR. Metformin blunts stress-induced hyperglycemia after thermal injury. J Trauma 2003; 54:555–561. [DOI] [PubMed] [Google Scholar]
- 59.Gore DC, Wolf SE, Sanford A, et al. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg 2005; 241:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.■■.Jeschke MG, Abdullahi A, Burnett M, et al. Glucose control in severely burned patients using metformin- an interim safety and efficacy analysis of a Phase II randomized controlled trial. Ann Surg 2016. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]; This interim analysis is the first clinical trial investigating the benefits of metformin in adult burn patients. It supports a safety and efficacy that is comparable to insulin with fewer hypoglycemic episodes and ease of administration.
- 61.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334:574–579. [DOI] [PubMed] [Google Scholar]
- 62.Cree MG, Zwetsloot JJ, Herndon DN, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg 2007; 245:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deane AM, Chapman MJ, Fraser RJ, et al. The effect of exogenous glucagon- like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomised double-blind placebo-controlled cross over study. Crit Care 2009; 13:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minnich A, Tian N, Byan L, Bilder G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab 2001; 280:E270–E279. [DOI] [PubMed] [Google Scholar]


