Abstract
Outcomes of patients with burns have improved substantially over the past two decades. Findings from a 2012 study in The Lancet showed that a burn size of more than 60% total body surface area burned (an increase from 40% a decade ago) is associated with risks and mortality. Similar data have been obtained in adults and elderly people who have been severely burned. We discuss recent and future developments in burn care to improve outcomes of children.
Introduction
Major burn injury is the biggest trauma and can be classified according to cause and depth of the burns. Every year, more than half a million burn injuries happen in the USA.1 These injuries are typically not severe, although about 50 000 patients with burns still need admission and treatment at a burn centre or burn hospital. Because the effects of burns are debilitating, substantial resources have been devoted to the specialty, which has greatly improved outcomes of patients with burns.2–4 Improved outcomes can be attributed to specialised burn centres, advances in resuscitation, protocolised and specialised critical care, improved coverage of wounds and treatment of infections, better treatments for inhalation injury, and the burn-induced hypermetabolic response.4,5 Another major advance is the recent initiatives by burn care providers to hold consensus conferences and implement specific definitions of disease processes in patients who have been severely burned, which will allow appropriate multicentre trials6 and comparative studies to be done. All these changes have substantially improved morbidity and mortality after burn injury. In a recent study in The Lancet,5 we showed that the burn size associated with increased risk of mortality at a specialised centre increased from 40% total body surface area (TBSA) burned to more than 60% TBSA burned in the past decade or so.5 We would like to emphasise that the pathophysiological response to burn injury and the mortality of patients with burns are proportional to the extent of burn in a sigmoid dose-response way, and that these responses are not an all or none phenomenon beginning at 60%. The cutoff for these pathophysiological responses is around 30% TBSA burned in children (aged 0–18 years), 20% in adults (aged 18–65 years), and about 15% in elderly people (older than 65 years).
Despite advances in burn care, severe burns still damage almost every organ in the body, resulting in profound debilitating complications or even death.2–5,7 Every year, nearly 4000 cases of burns result in death from complications related to thermal injury.2,8,9 Deaths caused by burns generally happen either immediately after the injury or weeks later as a result of infection or sepsis, multisystem organ failure, or hypermetabolic catabolic responses.5,10 In the past decade, the cause of death has changed profoundly.10 10 years ago, the major cause of death in patients who had been severely burned and admitted to a burn centre was anoxic brain injury, followed by sepsis and multiple organ failure. Nowadays, the major cause of death in burned paediatric patients is sepsis followed by multiple organ failure and anoxic brain injury.10 This shift in the cause of death requires a review of the basic understanding and treatment approaches to further improve post-burn morbidity and mortality. Patient outcome and survival are directly related to the quality of the complex care that burn patients receive. Three key aspects of care exist. First, initial care at the scene, pre-hospital care, and the early hospital phase: adequate and timely response, assessment of the burns, resuscitation, and admission to a burn centre, escharotomies or fasciotomies, resuscitation, and treatment of inhalation injury. Second, after hospital phase: wound care including burn surgeries, infection control, maintenance of organ function, and attenuation of hyper metabolism. Third, long-term phase: persistent hypermetabolism, reconstruction, and rehabilitation.
Four interrelated aspects seem to be crucial for survival: burn shock and resuscitation, inhalation injury, wound closure, and burn hypermetabolism. Therefore, we discuss standard and novel treatments in these specialties. Pain control is an important aspect of burn care that affects burn outcomes and quality of life at all stages, but will not be discussed in depth. Modern pain management has substantially evolved and includes multimodal treatments such as shortacting and long acting opioids, methadone, ketamine, central-acting agents (gabapentin), non-steroidal anti-inflammatory drugs, anxiolytics, and antidepressants. Tremendous improvements have been made over the past decades, but detailed discussion of these modalities is beyond the scope of this Review.
Burn shock and resuscitation
According to guidelines from the American Burn Association, the management of a patient with burns starts after emergency medical response teams are called and the patient is assessed and transported to a burn centre. A crucial part of this phase is establishing whether a patient’s injury is survivable or futile. Futility in adults or elderly patients with burns is usually determined by the sum of age (years), burn size (%), and the presence or absence of inhalation injury (±17), with values of greater or equal to 140–150 being indicative of futility.11 However, we focus on paediatric care, and the philosophy of many paediatric burn centres is that there is no futility in children except in very rare instances—eg, a 100% TBSA full-thickness burn. Once the decision to treat is made, the initial management and therapeutic goal is preservation of limbs and prevention of organ failure, which begins with well established recognition of injury severity, first-care protocols,2,4 and surgical interventions. An essential part of this response is adequate resuscitation.12–14 Many formulas have been studied, and all have the same goal of maintenance of organ perfusion during burn shock with restoration of intravascular volume. The most commonly used formula is the Parkland Formula,13 which provides the total volume of crystalloid to be given over the first 24 h (4 mL/kg bodyweight/% TBSA burned).12,15 However, recent data suggest that the Parkland Formula provides incorrect estimates of fluid requirements in patients with large and deeper burns, inhalation injury, delays in resuscitation, alcohol or drug use, and electrical injury, resulting in inadequate and inappropriate resuscitation.12,15 The catastrophic events associated with under-resuscitation include multiple organ failure and death. Over-resuscitation induces so-called fluid creep12,13,16,17 with its inherent complications such as pulmonary oedema, pleural effusions, pericardial effusions, abdominal compartment syndrome, extremity compartment syndrome, and con version of burns to deeper wounds. Additionally, provision of more fluid than is needed in patients with burns substantially increases the risk of acute respiratory distress syndrome, pneumonia, blood stream infections, multiple organ failure, and death.18
One of the greatest challenges in resuscitation is monitoring whether the procedure is adequate and effective. The traditional endpoints of urine output (0·5–1 mL/kg bodyweight/h), mean arterial pressure (>65 mm Hg), normal base excess, and lactate concentrations are not always accurate and can be misleading.13,15,18 However, no better physiological markers exist that enable adequate resuscitation, and therefore, these parameters remain the gold standard. New attempts to improve and individualise resuscitation include use of thermal dilution catheters (PiCCO, Philips, UK)14,19 and computer-assessed closed loop resuscitation.20,21 These technologies hold promise but have not been fully established in the clinic.
Scientific literature addresses not only the amount of fluid used in resuscitation, but also the type of fluid. Crystalloids have been compared with colloids or other means of resuscitation. So far, no large prospective randomised trial has been done to establish whether crystalloids are better than colloids in resuscitation. However, most burn surgeons use crystalloids (eg, Ringer’s lactate) and add colloids (eg, albumin) as a rescue modality.13,22 Fresh frozen plasma, which is used in patients with trauma, is not given to patients with burns because experimental and clinical trials assessing the efficacy of the fluid have not been done in this patient population. Hypertonic saline showed some promise in small studies of patients with burns,23 but failed to improve outcome in patients with traumatic brain injury.24 Resuscitation has profoundly evolved over the past two decades and will continue to do so, because the procedure has a central role in survival soon after burn.
Inhalation injury
Another key component of early burn care is maintenance of adequate oxygenation and treatment of inhalation injury. A marked proportion of fire-related deaths are not attributable to burn injury, but to the toxic effects of airborne combustion byproducts.15,25–27 Many of these compounds can act together to increase mortality. Recent studies suggest that between 20% and 30% of all severe burns are associated with inhalation injury and that between 25% and 50% of patients die if they need ventilator support for more than 1 week after burn.2,4,26 Inhalation injury substantially increases mortality15,26,28 and usually needs endotracheal intubation, which increases the incidence of pneumonia. Early detection of bronchopulmonary injury is crucial to improve survival. Clinical signs of inhalation injury vary,15,26 but inhalation injury can be suspected when the patient has been exposed to smoke in an enclosed area and has physical findings of burns on the face, singed nasal vibrissae, bronchorrhoea, sooty sputum, and wheezing or rales. The best practice to diagnose inhalation injury is bronchoscopy with the inhalation injury scale of Endorf and colleagues.25 Once inhalation injury is diagnosed, treatment of the injury should start immediately. Patients with inhalation injury should not be prophylactically intubated, nor should they receive prophylactic antibiotics. Standard care protocols for inhalation injury include bronchodilators (salbutamol), nebulised heparin, nebulised acetylcysteine, and for extreme mucosal oedema, racemic adrenaline.15,26 The theoretical benefits of corticosteroid treatment include decreased mucosal oedema, reduced bronchospasm, and maintenance of surfactant function. However, in several animal and clinical studies, mortality increased with corticosteroid treatment, and bronchopneumonia was associated with more extensive abscess formation.2 Thus, the use of corticosteroids is contraindicated.
Why patients with an inhalation injury have increased mortality is not entirely clear. A recent large, multicentre trial (n=420) that had very well developed standard operating procedures at each site was undertaken to compare outcomes in patients with and without inhalation injury and to establish the effect of inhalation injury on tissue-specific changes in genomic expression (unpublished data). Clinical outcomes in this trial confirmed findings from previous trials, showing that patients with inhalation injury have increased mortality and need longer intensive-care unit stays, hospitalisations, and time on ventilation. This trial was unique in that the investigators identified the effect of inhalation injury on genomic expression in peripheral blood leucocytes. The results showed that inhalation injury was associated with only subtle alterations in 169 probe sets corresponding to 115 genes, which encode proteins known to participate in cell cycle and transcriptional control. This multicentre trial showed that inhalation injury is associated, not only with poor outcomes after burn, but also with genomic changes that are not as dramatic as one would expect. That is, inhalation injury causes only distinct changes and not larger scale changes in the genome. This finding was confirmed in a study in 2007,29 which showed that inhalation injury was not associated with major inflammatory changes, but with minimum distinct changes indicative of a slight immunosuppressive effect. However, more research needs to be focused on this injury to better understand the underlying mechanisms and to develop new treatments.
Wound closure
Closure of the burn wounds establishes length of hospital stay, risk of infection, and ultimately survival, whereas failure to get the wounds closed results in death. Treatment strategies for superficial wounds must be differentiated from treatment plans for deeper wounds. The most important factor in the improvement of patient outcome has been the implementation of early excision and grafting of burn wounds, which was first described by Janzekovic30 in the 1970s. Findings from subsequent studies clearly showed that if the source of stress and inflammation is removed early, surgical blood loss is reduced31 and survival is markedly improved.32,33 The challenge that came along with this approach was how to best cover the excised burn wounds. The gold standard is to cover these wounds with autografts, either as a sheet or meshed skin with or without coverage of allograft (cadaver skin), or synthetic materials. Several new strategies that might change how we surgically care for patients with burns are on the horizon.
Partial-thickness burns
Partial-thickness burns can be categorised as either superficial or deep burns. Superficial wounds usually heal between 7 and 14 days, whereas complete reepithelialisation of deep dermal burns can take up to 4–6 weeks, with scarring often resulting from the loss of dermis. A large variety of topical creams and agents are available for treatment, and many are silver-based for anti-infective effects. Recent studies support the use of synthetic and biosynthetic membranes—eg, Biobrane (Smith and Nephew, MA, USA), established in 1982, and Suprathel (Polymedics Innovations GmbH, Germany).34,35 These membranes decrease the number of dressing changes and the amount of pain drugs associated with these dressing changes. Several studies of Biobrane show that this membrane is efficacious for superficial burns.36–38 Suprathel is a synthetic copolymer containing more than 70% DL-lactide. Findings from prospective randomised clinical studies of partial-thickness burns and split-thickness donor sites have shown that Suprathel is associated with less pain than other commercially available membranes, although wound healing times and long-term scar qualities are similar between this synthetic membrane and other membranes.34
A novel approach to burn wound coverage is the use of biological membranes. Human amniotic membrane has a long history of use as a wound dressing. However, amnion can only be used as a temporary wound covering, not as a skin transplant. In the past 20 years, data for the use of amnion in burn wound coverage have accumulated. Some of the benefits of amnion are that it is thin, pliable, adhesive, but not prone to sticking, and easily removed. In a recent prospective study of burns in children by Branski and colleagues,39 amnion showed outstanding wound healing properties and produced excellent long-term cosmetic results. The most fascinating aspect of amniotic membrane is that it contains stem cells, which can be applied in various ways to create new treatment approaches. These approaches will be further investigated in prospective clinical trials.
Bioengineered approaches have also been tested for use in patients with partial-thickness burns. Examples include keratinocyte-fibrin sealant sprays, fibrin sealant-containing growth factors, and cell suspensions.
Full-thickness burns
Full-thickness burns are deep wounds that will either not heal or heal with a debilitating scar. These burns are treated by excision and coverage with autograft. As already mentioned, if complete autografting is not possible because the burn is large, allograft or other dermal or epidermal substitutions are needed. The scientific and commercial community agrees that harvesting autograft is the standard, although this is an ancient approach. Therefore, several new approaches have surfaced over the past two decades. The oldest and best studied dermal substitute is Integra (Integra LifeSciences Corporation, Plainsboro, NJ, USA), which was developed by a team led by surgeon John Burke from the Massachusetts General Hospital (Boston, MA, USA) and by scientist Ionnas Yannas from the Massachusetts Institute of Technology (Cambridge, MA, USA).40,41 Integra is composed of bovine collagen and glucosaminoglycans, which allow fibrovascular ingrowth. Findings from various clinical trials have shown that Integra is an effective method for burn surgeons and results in excellent cosmetic and functional outcomes.39,42 Another dermal analogue available for the treatment of full-thickness burns is Alloderm (LifeCell Corporation, Branchburg, NJ, USA). Alloderm consists of cadaveric dermis devoid of cells and epithelial element. Dermal analogue is used in a similar way to other dermal analogues, and it has produced favourable results.43
After the potential of dermal substitutes was recognised, the trend became to produce epithelial skin substitutes with or without a dermis. Cultured epithelial autografts became a surgical option in the management of patients with massive injuries involving more than 90% TBSA burned. Cultured epithelial autografts are created in vitro from autologous keratinocytes and as the name suggests, consist of keratinocytes. The promise of this technique has not been fully realised because of costs and the low quality of the neo-skin;44 however, it is regarded as a rescue modality for massive burns. A possible improvement over cultured epithelial auto grafts is ReCell (Avita Medical, Royston, UK). This spray contains autologous keratinocytes, melanocytes, fibroblasts, and Langerhans cells that are harvested from a split-thickness biopsy. ReCell is sprayed onto the wound, which is usually grafted with widely meshed autograft. Positive findings from small animal studies and clinical trials need to be confirmed in larger randomised multicentre trials.45,46 This ReCell trial is in progress and results are expected by 2014.
Another very promising bioengineered approach is the combination of autologous keratinocytes and Integra, known as cultured skin substitute. Boyce and col leagues47,48 first described this method in the 1990s. The healing and take was very good, but cultured skin substitute had several issues: no or spotty pigmentation, a long production time, and high overall costs. Since then, the investigators have added melanocytes, shortened the time of production, and with novel manipulation, introduced hair follicle and sweat glands.49,50 The addition of skin appendages might make this a highly promising method for the future care of patients with burns. Researchers are also investigating the possibility of using porcine dermis as a dermal substitute. Porcine dermal matrices are very similar to human dermal matrices. Although the porcine matrices have the dis advantages of xenografts, they represent the first choice among natural biological dermal substitutes that are not derived from human beings. Many researchers consider these matrices to be the best substitute for acellular human dermal matrices in the future.51,52 Three acellular porcine dermal matrices are on the market: Permacol (Covidien, Ireland), Strattice (Kinetic Concepts, Kidlington, UK), and Xenoderm (Healthpoint Biotherapeutics, Fort Worth, TX, USA). The efficacy of these dermal matrices needs to be proven in clinical trials.
Stem cells represent a new hope in the management of burns. These cells play an important part in wound healing, both locally and systemically, and several of the mechanisms underlying their actions in wound healing have been described. In human beings, stem cells can be found in adipose tissue, bone marrow, umbilical blood, and the blastocystic mass of embryos.53,54 Stem cells have many promising features. In view of their clonicity and pluripotency, these cells can be used to regenerate dermis and expedite re-epithelialisation. Another important characteristic of stem cells is their lack of immunogenicity, which would allow them to be transplanted with relative ease.55,56 Stem cells present in the bone marrow migrate to tissues affected by injury and help the healing and regeneration process.54 Embryonic human stem cells can be differentiated into keratinocytes in vitro and stratified into an epithelium that resembles human epidermis.57 This graft can then be applied to open wounds on patients with burns as a temporary skin substitute while autograft or other permanent coverage means become available.
Facial transplantation
Serious facial burns leave victims with substantial deformities that are difficult to treat. No evidence exists to suggest that standard treatment modalities for severe facial burns offer substantial improvements in function or scar outcome. These patients frequently become socially and personally isolated, and many suffer from psychological disorders and phobias.58,59 These patients also tend to need multiple reconstructive procedures under conditions in which minimal normal tissue (secondary to burns in other areas) is available. Facial transplantation in such patients can offer the possibility of improved quality of life. Following the lead of a surgical team in Amiens, France, in 2005,60,61 several groups in Europe, China, and the USA have successfully done composite tissue allotransplantation. This transplantation of donor facial tissue allows for the best possible functional and aesthetic outcome. Antirejection drug regimens for solid organ transplantation are well established.58,59 However, this new treatment poses unique psychological and ethical challenges that need to be addressed by a dedicated team.62 Once the large challenges posed by facial transplantation are overcome, this will become a promising treatment for patients with serious facial burns.63
Hypermetabolism
A key cause of poor outcomes after burn injury is the hypermetabolic response, which is associated with severe alterations in glucose, lipid, and aminoacid metabolism.3,7,64,65 Hypermetabolism leads to severe catabolism, which is associated with protein breakdown in muscle and in organs, leading to multiple organ dysfunction. Therefore, hypermetabolism, organ function, and consequently survival, seem to be closely linked. The burn-induced hypermetabolic response that occurs in the ebb phase (48 h after burn) and flow phase (>96–144 h after burn) is profound, extremely complex, and most likely induced by stress and inflammation.3,7,64,65 The reason for this response is not entirely clear, but persistent increases in catecholamines, glucocorticoids, glucagon, and dopa-mine secretion are thought to participate in activating cascades that trigger the hyper metabolic response and subsequent catabolism.66–73 Additionally, coagulation and complement cascades and cytokines, endotoxin, neutrophil-adherence complexes, reactive oxygen species, and nitric oxide can modulate the hypermetabolic response.74 After activation, the pathways upstream of the hypermetabolic response seem to contribute to prolonged hypermetabolism with changes in glucose, lipid, and aminoacid metabolism.7,64 These metabolic changes were previously thought to resolve shortly after wound closure was complete. However, we have recently shown that burn-induced hypermetabolism seems to last a much longer time, as seen by a 3 year increase in energy requirements, catecholamines, urine cortisol, and serum cytokines, and impairment in glucose metabolism and insulin sensitivity.7,64,75 These results underscore the importance of long-term follow-up and treatment of individuals with serious burns.
The hypermetabolic response involves many pathways. However, two in particular seem to most profoundly affect outcomes after burn injury: glucose metabolism with insulin resistance and hyperglycaemia76–79 and lipid metabolism with increased lipolysis.80–83 Early after burn injury, concentrations of glucose increase and glucose removal is impaired, leading to an overall rise in glucose and lactate.84,85 Hyperglycaemia in patients with burns is associated with increased frequency of infections, sepsis, incidence of pneumonia, catabolism, hypermetabolism, and most importantly, mortality.76–79,86,87 The notion that hyperglycaemia is detrimental to patients with burns is further supported by findings from a prospective randomised trial79 showing that glucose control increases survival and improves organ function. Lipid metabolism is also markedly altered during hyper metabolism after burn injury, an outcome that might be linked to changes in insulin resistance. Lipolysis consists of the breakdown (hydrolysis) of triacylglycerol into free fatty acids and glycerol. Lipolysis and free fatty acids contribute to morbidity and mortality after burn injury through fatty infiltration of various organs.88 Fatty liver is very common after burn injury and is associated with an increase in clinical morbidities and metabolic alterations. Findings from pathology analyses89,90 and spectroscopy studies have shown that children with burns have a three times to five times increase in hepatic triglycerides.91,92 This increase is associated with infection, sepsis, and poor outcome.80 Although this relation is clear, the mechanism by which lipids induce insulin resistance is not well understood.
Treatment of the hypermetabolic response
Various data suggest that hypermetabolism is a major contributor to poor outcome after burn and that treatment or alleviation of the hypermetabolic response is beneficial for patient outcomes. Treatment options include pharmacological and non-pharmacological strategies.3
The main goal of nutritional support is to provide an adequate energy supply and the nutrients necessary to maintain organ function and survival. Early adequate enteral nutrition alleviates catabolism and improves outcomes;93 however, overfeeding in the form of excess calories or protein, or both, is associated with hyperglycaemia, carbon dioxide retention, fatty infiltration of organs, and azotaemia (figure).95 Therefore, accurate calculation of the caloric requirements is imperative. Resting energy requirements of patients with burns are commonly estimated with equations that incorporate body mass, age, and sex. Although these equations are based on patient-specific factors, caloric requirements can still be greatly overestimated, increasing the risk of overfeeding.96,97 The adapted Toronto equation seems to be the best formula to calculate resting energy expenditure, because the calculated results very closely match the measured values.98 Generally, adequate nutrition is an essential component of burn care and should be initiated within 12 h after injury.99
Figure: Alleviation of hypermetabolic response to improve outcomes after burn injury with early excision and grafting, high ambient temperatures, exercise, and diet.
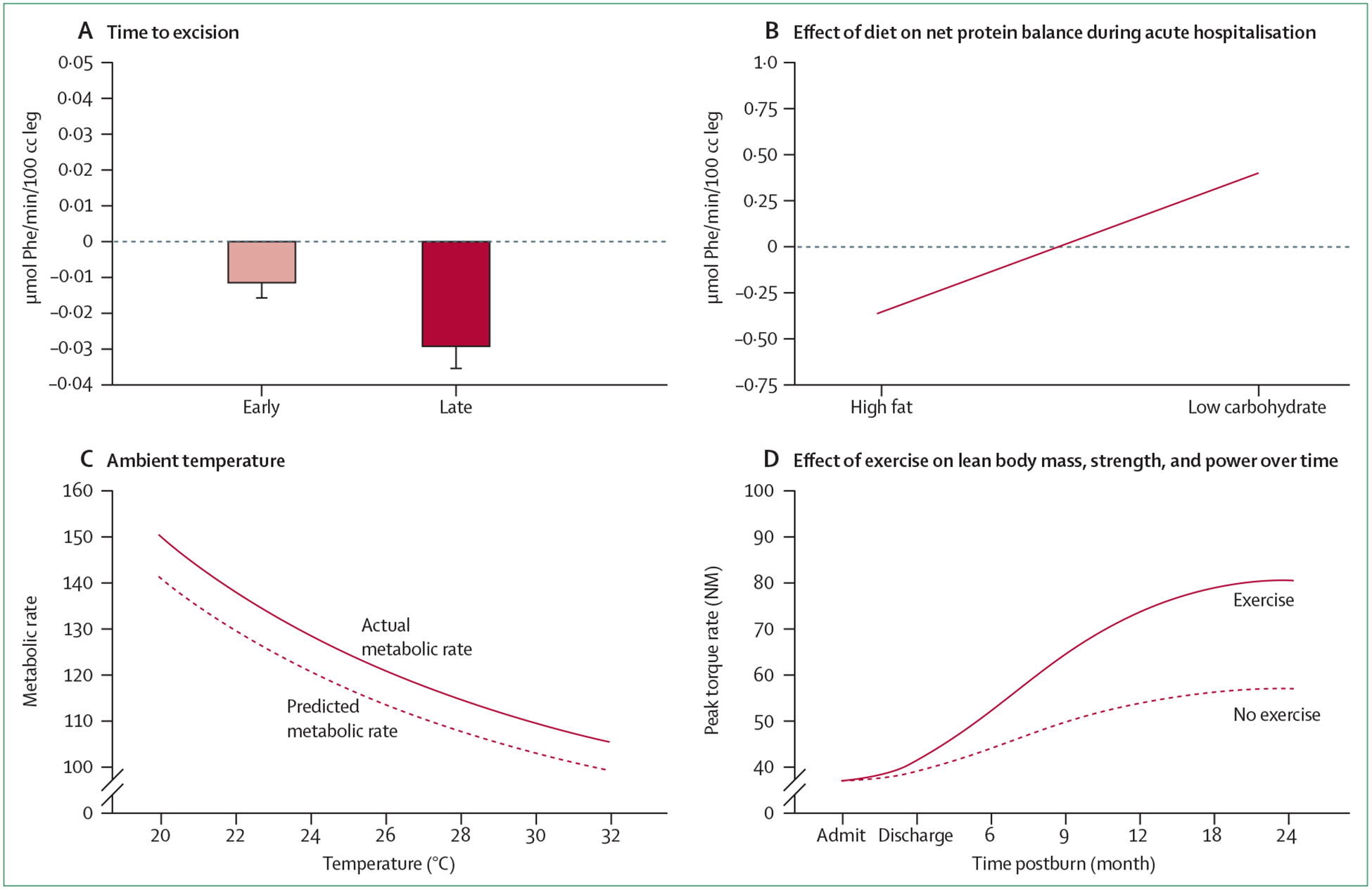
(A) Muscle protein net balance in paediatric patients with severe burns. Early excision alleviates muscle protein loss compared with late excision. Error bars show standard error of the mean. (B) A high carbohydrate diet is beneficial for muscle protein synthesis compared with a high fat diet. (C) High room temperatures can reduce the metabolic needs of patients with burns (ie, hypermetabolism). The higher the room temperature the lower the metabolic demand. (D) Long-term exercise can substantially increase strength and decrease hypermetabolism. Reproduced from Williams and colleagues,94 by permission of Elsevier.
No ideal nutrition or gold standard for patients with burns exists. We and others4 recommend nutrition that is high in glucose, high in protein and aminoacid, and low in fat with some unsaturated fatty acids. Carbohydrates and aminoacids should serve as the main energy source, sparing protein from oxidation for energy and allowing it to be effectively used by the skin and organs. Supplementation of single aminoacids, especially alanine and glutamine, is controversial. After burn injury, glutamine is quickly depleted from serum and muscle.100,101 However, this depletion happens mainly intracellularly, and effective delivery of glutamine to the cells is very difficult. Findings from small studies in patients with burns show that glutamine supplementation decreases incidence of infection, length of hospital stay, and mortality.100,101 Therefore, glutamine supplementation might be beneficial. A multicentre trial (REDOX; NCT00133978) is addressing this question, and the results are expected in the next 4–5 years. However, preliminary data102 suggest that, in critically ill patients, glutamine has no benefit in terms of outcomes. Published work of alanine is even sparser, and no data are available for whether alanine should be given. Finally, dietary components that have gained more recent attention are vitamins, micro nutrients, and trace elements.103 Plasma concentrations of vitamins and trace elements are substantially decreased for prolonged periods after the acute burn injury because of increased urinary excretion and substantial cutaneous losses. Replacement of these micronutrients reduces morbidity in patients with severe burns.104–110 Therefore, a complete daily multi vitamin and mineral supplement should be given.
Other non-pharmacological strategies
Early wound excision and closure have been the biggest advances in burn care in the past few decades (figure). Early excision and grafting has substantially reduced basal energy expenditure, mortality, and costs.2,31–33,111 The early excision of burn wounds and coverage of the excised areas with temporary cover materials or autologous skin is imperative. This process diminishes burn-induced inflammatory and stress responses, and in turn decreases hypermetabolism.
The hypermetabolic response is believed to arise, at least partly, to compensate for dissipation of heat resulting from water loss. Accordingly, the skin and core body temperature are raised by 2°C. It is not often realised that increasing ambient room temperature is a simple approach to counter acting this response to burn injury.112 In fact, a change in temperature from 25°C to 33°C reduces resting energy expenditure from 2·0 times predicted resting energy expenditure to 1·4 times predicted resting energy expenditure in patients with serious burns (figure).2
Providing patients with burns with physical therapy is a crucial yet easy intervention that can ameliorate metabolic disruptions and prevent contractures of the burn wound. Progressive resistance exercises have been shown to promote muscle protein synthesis, increase body mass, strengthen muscles, and build endurance (figure).97,113 Resistance exercises are safe for burned children who do not have exercise-related hyperpyrexia.96,112
Drugs
Drugs are used as an adjunct for the treatment of various aspects of the hypermetabolic response. In the past two decades, several agents have been tested; some are more effective and promising than others. Almost all drugs are associated with beneficial effects but also side-effects, some of them severe. The table shows drugs currently in use.
Table:
Drugs to treat the hypermetabolic response
| Dose | Benefit | Side-effects | Survival effect | |
|---|---|---|---|---|
| Recombinant human growth hormone | 0·1–0·2 mg/kg per day | Accelerates donor site healing,114 anti-inflammatory,115 increases concentration of insulin-like growth factor 1,116 decreases basal energy expenditure and cardiac output,117 anabolic and preserves growth,118 long-term beneficial in terms of development73 | Increased mortality in critically ill patients,119 hyperglycaemia and insulin resistance120 | None in children,73,121 not in use. Contraindication to give recombinant human growth hormone when an infection or sepsis is present |
| Insulin-like growth factor 1 / binding protein-3 | 1–4 mg/kg per day | Decreases muscle protein catabolism,122 ameliorates integrity of gut mucosa,123 reduces acute phase and inflammatory response124 | Hypoglycaemia, neuropathies | Unknown |
| Oxandrolone | Adults: 5–10 mg twice a day; children: 0·05–0·1 mg/kg per day | Increases muscle protein synthesis,125 reduces weight loss and promotes wound healing,126,127 decreases hospital stay,128 increases lean body mass,129 long-term beneficial in terms of development, especially for children130,131 | Virilising, clitoral hypertrophy, increase adult respiratory distress syndrome in critically ill patients | Improved survival in adults, unknown in children128 |
| Propranolol | Adults: 10–40 mg three times a day; children: 1–4 mg/kg per day | Anti-inflammatory and antistress,132 anticatabolic and increases lean body mass,133 decreases hyperglycaemia,134 long-term better growth, less hypermetabolism, less fat accumulation, prevents loss of bone135 | Hypotension, bradycardia, increased insulin sensitivity | Unknown, but propranolol is an interesting and effective agent to treat hypermetabolic stress responses caused by catecholamine; recently the Shriners Hospitals for Children, the National Institutes of Health, and American Burn Association funded multicentre trials to identify the effects of propranolol in children and adults with severe burns |
| Insulin | Varies, glucose target 110–150 mg/dL136 | Improves insulin resistance,79 anti-inflammatory,69 increased wound healing,137 improved organ function,138 anabolic,139 decreased infections and sepsis78 | Hypoglycaemia | Hyperglycaemia increases mortality,76 hypoglycaemia increases mortality in critically ill140 |
| Metformin | 500–1000 mg twice a day | Decreases gluconeogenesis and increases peripheral insulin sensitivity,141 good glucose control in burn,142 alleviates catabolism143 | Lactic acidosis, hepatic failure | Unknown |
| Glucagon-like peptide-1 | To be defined | Glucose control in burn144 | Not sufficient as monotherapy144 | Unknown |
| Fenofibrate | To be defined | Alleviates lipolysis and improves insulin resistance,91 improves mitochondrial respiration91 | Not sufficient as monotherapy | Unknown |
Outcome measures
The ultimate goal of intensive burn care is to keep the patient alive, an outcome that is dependent on coverage of burn wounds, maintenance of organ function, control of infection and sepsis, and alleviation of hyper metabolism. The ability to predict patient outcomes, identify patients at risk, or even individualise patient care is highly desirable. However, no predictors exist that would allow for any such identification. In a recent study145 of children with more than 30% TBSA burned, our group investigated whether trajectories differed between survivors and non-survivors. We noted that these groups showed profound differences in important markers of inflammation and metabolism at each timepoint. Serum concentrations of interleukin 6, interleukin 8, granulocyte colony-stimulating factor, monocyte chemoattractant protein-1, C-reactive protein, glucose, insulin, blood urea nitrogen, creatinine, and bilirubin were higher in patients who did not survive. The patients also had a heightened hypermetabolic response accompanied by a greater frequency of sepsis and organ dysfunction.145 These findings, which were the first of their kind, are important because they will enable the development of models that can predict patient outcome and treatments to improve patient outcomes. A similar study on predicting burns mortality was done at the time; however, it centred on spline modelling.146,147 Findings from this study showed that mortality could be reliably predicted by the combination of information about protein abundance with clinical covariates in a multi variate adaptive regression splines classifier. Finally, novel and exciting results are expected from the Inflammation and the Host Response to Injury Collaborative Research programme by Glue Grant. More than 500 patients with burns have been enrolled in this study, and the genomic and proteomic changes in patients with various outcomes and morbidities are being analysed. Preliminary data suggest that patients who die from burns have a distinct genomic profile compared with survivors. Similarly, patients with sepsis, pneumonia, multiple organ failure, and non-healing wounds all have a different genomic signature, suggesting that the genome plays a central part in the determination of outcome of an individual. The results of this huge trial will be published over the next 3–4 years and could lead to novel treatment avenues for patients with severe burns. A substantial effort is underway to identify genomic and proteomic predictors of good and poor outcome. Such predictors will be indispensable for the development of individualised medicine, and we believe that the future of burn care is closely linked to understanding of these patient trajectories. Nevertheless, survival after burn injury depends on implementation of fundamental aspects of burn care including wound coverage, infection control, and reduction of the hypermetabolic response.
Conclusions
Burn injury triggers a plethora of pathophysiological responses associated with detrimental outcomes. Novel treatment strategies such as early excision and grafting, early and adequate nutrition, alleviation of the hypermetabolic response, treatment of hyperglycaemia, and the catecholamine surge with use of β blockers, improved ventilation strategies, and exercise improve survival and outcomes in patients with severe burns. Large multicentre trials with protocolised care will improve morbidity and mortality after burn injury, as shown by the Inflammation and the Host Response to Injury research programme. However, burn injury still causes many deaths, and hope fully, novel treatment modalities, individualised medicine, and genomic and proteomic profiles will further increase the lethal dose in terms of TBSA burn (LD 50) for patients with burns. Because of the shift in basic theory to the expectation that patients with severe burns will survive, burn care providers are faced with new challenges, especially with regard to quality of life and long-term outcomes in these patients. Recently, a system was developed by investigators from Shriners Hospital in Boston (MA, USA) to assess and quantify various aspects of functional recovery in convalescent patients with burns.148,149 This system allows departures from the anticipated trajectory of recovery for various functional indices to be identified, which ultimately enables researchers to identify changes needed to the rehabilitation programme.148,149 This Review provides insights into existing and novel therapeutic approaches to further improve burn outcomes. We further discuss existing and novel challenges, not only for burn care providers, but also for medical professionals looking after patients with burns. In summary, it is becoming more apparent that a burn is not over once burn wounds are healed, and that profound pathophysiological responses persist for a substantially longer time than previously thought.7,64 In view of this understanding, a change in direction and philosophy for how we treat burns is needed.
Search strategy and selection criteria.
We searched PubMed for papers published in any language between Jan 1, 2008, and Dec 18, 2012, with the following search terms: “large clinical trials in burns”, “resuscitation, inhalation injury”, “wound care”, “burn wounds”, “infection”, “organ function”, “hypermetabolism”, and “predictors of mortality”.
Acknowledgments
This study was supported by Shriners Hospitals for Children (8490, 71008, 8640, 8760, 84080, and 79135), National Institutes of Health (R01-GM56687, R01-GM087285, T32-GM008256, and P50-GM60338), National Institute of Disability and Rehabilitation Research (H133A020102 and H133A70019), the Canada Foundation for innovation Leader’s Opportunity Fund (Project 25407), Canadian Institutes of Health Research Grant (123336), and Physicians’ Services Incorporated Foundation—Health Research Grant Programme. None of the funding sources had involvement in the collection, analysis, or interpretation of data. We thank Eileen Figueroa and Kasie Cole for assistance in manuscript preparation.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
For more on Glue Grant see http://www.gluegrant.org
References
- 1.WHO. The injury chartbook: a graphical overview of the global burden of injuries. Geneva: World Health Organization, 2002. [Google Scholar]
- 2.Herndon DN. Total burn care. 3rd edn Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 3.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004; 363: 1895–902. [DOI] [PubMed] [Google Scholar]
- 4.Jeschke MG, Kamolz L, Sjoeberg F, Wolf SE. Handbook of burns, vol 1 Vienna: Springer; 2012. [Google Scholar]
- 5.Kraft R, Herndon DN, Al-Mousawi AM, Williams FN, Finnerty CC, Jeschke MG. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet 2012; 379: 1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhalgh DG, Saffle JR, Holmes JH 4th, et al. , and the American Burn Association Consensus Conference on Burn Sepsis and Infection Group. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007; 28: 776–90. [DOI] [PubMed] [Google Scholar]
- 7.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg 2008; 248: 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011; 37: 1087–100. [DOI] [PubMed] [Google Scholar]
- 9.Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns 2012; 38: 630–37. [DOI] [PubMed] [Google Scholar]
- 10.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care 2009; 13: R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts G, Lloyd M, Parker M, et al. The Baux score is dead. Long live the Baux score: a 27-year retrospective cohort study of mortality at a regional burns service. J Trauma Acute Care Surg 2012; 72: 251–56. [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh DG. Burn resuscitation. J Burn Care Res 2007; 28: 555–65. [DOI] [PubMed] [Google Scholar]
- 13.Greenhalgh DG. Burn resuscitation: the results of the ISBI/ABA survey. Burns 2010; 36: 176–82. [DOI] [PubMed] [Google Scholar]
- 14.Kraft R, Herndon DN, Branski LK, Finnerty CC, Leonard KR, Jeschke MG. Optimized fluid management improves outcomes of pediatric burn patients. J Surg Res 2013; 181: 121–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med 2009; 37: 2819–26. [DOI] [PubMed] [Google Scholar]
- 16.Pruitt BA Jr. Protection from excessive resuscitation: “pushing he pendulum back”. J Trauma 2000; 49: 567–68. [DOI] [PubMed] [Google Scholar]
- 17.Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res 2007; 28: 382–95. [DOI] [PubMed] [Google Scholar]
- 18.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg 2007; 245: 622–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branski LK, Herndon DN, Byrd JF, et al. Transpulmonary thermodilution for hemodynamic measurements in severely burned children. Crit Care 2011; 15: R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinas J, Chung KK, Mann EA, et al. Computerized decision support system improves fluid resuscitation following severe burns: an original study. Crit Care Med 2011; 39: 2031–38. [DOI] [PubMed] [Google Scholar]
- 21.Salinas J, Drew G, Gallagher J, et al. Closed-loop and decision-assist resuscitation of burn patients. J Trauma 2008; 64 (suppl): S321–32. [DOI] [PubMed] [Google Scholar]
- 22.Faraklas I, Lam U, Cochran A, Stoddard G, Saffle J. Colloid normalizes resuscitation ratio in pediatric burns. J Burn Care Res 2011; 32: 91–97. [DOI] [PubMed] [Google Scholar]
- 23.Belba MK, Petrela EY, Belba GP. Comparison of hypertonic vs isotonic fluids during resuscitation of severely burned patients. Am J Emerg Med 2009; 27: 1091–96. [DOI] [PubMed] [Google Scholar]
- 24.Bulger EM, May S, Brasel KJ, et al. , and the ROC Investigators. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA 2010; 304: 1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res 2007; 28: 80–83. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri TL, Warner P, Mlcak RP, et al. Inhalation injury in children: a 10 year experience at Shriners Hospitals for Children. J Burn Care Res 2009; 30: 206–08. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan RL, Hess D. Inhaled nitric oxide in inhalation injury. J Burn Care Res 2009; 30: 162–64. [DOI] [PubMed] [Google Scholar]
- 28.Erdman AR. Is hydroxocobalamin safe and effective for smoke inhalation? Searching for guidance in the haze. Ann Emerg Med 2007; 49: 814–16. [DOI] [PubMed] [Google Scholar]
- 29.Finnerty CC, Herndon DN, Jeschke MG. Inhalation injury in severely burned children does not augment the systemic inflammatory response. Crit Care 2007; 11: R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janzekovic Z Early surgical treatment of the burned surface. Panminerva Med 1972; 14: 228–32. [PubMed] [Google Scholar]
- 31.Desai MH, Herndon DN, Broemeling L, Barrow RE, Nichols RJ Jr, Rutan RL. Early burn wound excision significantly reduces blood loss. Ann Surg 1990; 211: 753–59, discussion 759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herndon DN, Barrow RE, Rutan RL, Rutan TC, Desai MH, Abston S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg 1989; 209: 547–52, discussion 552–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma 1986; 26: 149–52. [DOI] [PubMed] [Google Scholar]
- 34.Rahmanian-Schwarz A, Beiderwieden A, Willkomm LM, Amr A, Schaller HE, Lotter O. A clinical evaluation of Biobrane(®) and Suprathel(®) in acute burns and reconstructive surgery. Burns 2011; 37: 1343–48. [DOI] [PubMed] [Google Scholar]
- 35.Wood F, Martin L, Lewis D, et al. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane® synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns 2012; 38: 830–39. [DOI] [PubMed] [Google Scholar]
- 36.Lesher AP, Curry RH, Evans J, et al. Effectiveness of Biobrane for treatment of partial-thickness burns in children. J Pediatr Surg 2011; 46: 1759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns 2007; 33: 946–57. [DOI] [PubMed] [Google Scholar]
- 38.Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg 2000; 105: 62–65. [DOI] [PubMed] [Google Scholar]
- 39.Branski LK, Herndon DN, Celis MM, Norbury WB, Masters OE, Jeschke MG. Amnion in the treatment of pediatric partial-thickness facial burns. Burns 2008; 34: 393–99. [DOI] [PubMed] [Google Scholar]
- 40.Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981; 194: 413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982; 215: 174–76. [DOI] [PubMed] [Google Scholar]
- 42.Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 2003; 24: 42–48. [DOI] [PubMed] [Google Scholar]
- 43.Wainwright DJ, Bury SB. Acellular dermal matrix in the management of the burn patient. Aesthet Surg J 2011; 31 (suppl): 13S–23S. [DOI] [PubMed] [Google Scholar]
- 44.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns 2006; 32: 395–401. [DOI] [PubMed] [Google Scholar]
- 45.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns 2007; 33: 966–72. [DOI] [PubMed] [Google Scholar]
- 46.Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns 2012; 38: 44–51. [DOI] [PubMed] [Google Scholar]
- 47.Boyce ST. Skin substitutes from cultured cells and collagen-GAG polymers. Med Biol Eng Comput 1998; 36: 791–800. [DOI] [PubMed] [Google Scholar]
- 48.Boyce ST, Kagan RJ, Meyer NA, YakuboffK P, Warden GD. The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J Burn Care Rehabil 1999; 20: 453–61. [DOI] [PubMed] [Google Scholar]
- 49.Boyce ST, Rice RK, Lynch KA, et al. Assessment of replication rates of human keratinocytes in engineered skin substitutes grafted to athymic mice. Wound Repair Regen 2012; 20: 544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sriwiriyanont P, Lynch KA, Maier EA, Hahn JM, Supp DM, Boyce ST. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol 2012; 21: 783–85. [DOI] [PubMed] [Google Scholar]
- 51.Drago H, Marín GH, Sturla F, et al. The next generation of burns treatment: intelligent films and matrix, controlled enzymatic debridement, and adult stem cells. Transplant Proc 2010; 42: 345–49. [DOI] [PubMed] [Google Scholar]
- 52.Ge L, Sun L, Chen J, et al. The viability change of pigskin in vitro. Burns 2010; 36: 533–38. [DOI] [PubMed] [Google Scholar]
- 53.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009; 35: 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010; 28: 905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev 2010; 19: 491–502. [DOI] [PubMed] [Google Scholar]
- 56.Trojahn Kølle SF, Oliveri RS, Glovinski PV, Elberg JJ, Fischer-Nielsen A, Drzewiecki KT. Importance of mesenchymal stem cells in autologous fat grafting: a systematic review of existing studies. J Plast Surg Hand Surg 2012; 46: 59–68. [DOI] [PubMed] [Google Scholar]
- 57.Guenou H, Nissan X, Larcher F, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet 2009; 374: 1745–53. [DOI] [PubMed] [Google Scholar]
- 58.Pomahac B, Nowinski D, Diaz-Siso JR, et al. Face transplantation. Curr Probl Surg 2011; 48: 293–357. [DOI] [PubMed] [Google Scholar]
- 59.Pomahac B, Pribaz J, Eriksson E, et al. Three patients with full facial transplantation. N Engl J Med 2012; 366: 715–22. [DOI] [PubMed] [Google Scholar]
- 60.Devauchelle B, Badet L, Lengelé B, et al. First human face allograft: early report. Lancet 2006; 368: 203–09. [DOI] [PubMed] [Google Scholar]
- 61.Petruzzo P, Testelin S, Kanitakis J, et al. First human face transplantation: 5 years outcomes. Transplantation 2012; 93: 236–40. [DOI] [PubMed] [Google Scholar]
- 62.Soni CV, Barker JH, Pushpakumar SB, et al. Psychosocial considerations in facial transplantation. Burns 2010; 36: 959–64. [DOI] [PubMed] [Google Scholar]
- 63.Pushpakumar SB, Barker JH, Soni CV, et al. Clinical considerations in face transplantation. Burns 2010; 36: 951–58. [DOI] [PubMed] [Google Scholar]
- 64.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011; 6: e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001; 17: 107–24. [DOI] [PubMed] [Google Scholar]
- 66.Coombes EJ, Batstone GF. Urine cortisol levels after burn injury. Burns 1982; 8: 333–37. [DOI] [PubMed] [Google Scholar]
- 67.Goodall M, Stone C, Haynes BW Jr. Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg 1957; 145: 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg 2000; 232: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg 2004; 239: 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg 2006; 244: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norbury WB, Herndon DN. Modulation of the hypermetabolic response after burn injury In: Herndon DN, ed. Total burn care, 3rd edn New York: Saunders Elsevier; 2007: 420–33. [Google Scholar]
- 72.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma 2006; 60: 968–71. [DOI] [PubMed] [Google Scholar]
- 73.Przkora R, Herndon DN, Suman OE, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg 2006; 243: 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheridan RL. A great constitutional disturbance. N Engl J Med 2001; 345: 1271–72. [DOI] [PubMed] [Google Scholar]
- 75.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab 2009; 94: 1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma 2001; 51: 540–44. [DOI] [PubMed] [Google Scholar]
- 77.Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med 2002; 30: 2438–42. [DOI] [PubMed] [Google Scholar]
- 78.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery 2008; 144: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeschke MG, Kulp GA, Kraft R, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med 2010; 182: 351–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma 2001; 51: 736–39. [DOI] [PubMed] [Google Scholar]
- 81.Barrow RE, Wolfe RR, Dasu MR, Barrow LN, Herndon DN. The use of beta-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg 2006; 243: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martini WZ, Irtun O, Chinkes DL, Rasmussen B, Traber DL, Wolfe RR. Alteration of hepatic fatty acid metabolism after burn injury in pigs. JPEN J Parenter Enteral Nutr 2001; 25: 310–16. [DOI] [PubMed] [Google Scholar]
- 83.Morio B, Irtun O, Herndon DN, Wolfe RR. Propranolol decreases splanchnic triacylglycerol storage in burn patients receiving a high-carbohydrate diet. Ann Surg 2002; 236: 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gore DC, Ferrando A, Barnett J, et al. Influence of glucose kinetics on plasma lactate concentration and energy expenditure in severely burned patients. J Trauma 2000; 49: 673–77. [DOI] [PubMed] [Google Scholar]
- 85.Wolfe RR, Miller HI, Spitzer JJ. Glucose and lactate kinetics in burn shock. Am J Physiol 1977; 232: E415–18. [DOI] [PubMed] [Google Scholar]
- 86.Jeschke MG, Klein D, Thasler WE, et al. Insulin decreases inflammatory signal transcription factor expression in primary human liver cells after LPS challenge. Mol Med 2008; 14: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pham TN, Warren AJ, Phan HH, Molitor F, Greenhalgh DG, Palmieri TL. Impact of tight glycemic control in severely burned children. J Trauma 2005; 59: 1148–54. [DOI] [PubMed] [Google Scholar]
- 88.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963; 1: 785–89. [DOI] [PubMed] [Google Scholar]
- 89.Barrow RE, Hawkins HK, Aarsland A, et al. Identification of factors contributing to hepatomegaly in severely burned children. Shock 2005; 24: 523–28. [DOI] [PubMed] [Google Scholar]
- 90.Jeschke MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med 2009; 15: 337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cree MG, Newcomer BR, Herndon DN, et al. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab (Lond) 2007; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 2004; 89: 3864–71. [DOI] [PubMed] [Google Scholar]
- 93.Mochizuki H, Trocki O, Dominioni L, Brackett KA, Joffe SN, Alexander JW. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 1984; 200: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams FN, Jeschke MG, Chinkes DL, et al. Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg 2009; 208: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saffle JR, Graves C. Nutritional support of the burned patient In: Herndon DN, ed. Total burn care, 3rd edn London: Saunders Elsevier; 2007: 398–419. [Google Scholar]
- 96.Gore DC, Rutan RL, Hildreth M, Desai MH, Herndon DN. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil 1990; 11: 400–04. [DOI] [PubMed] [Google Scholar]
- 97.Suman OE, Mlcak RP, Chinkes DL, Herndon DN. Resting energy expenditure in severely burned children: analysis of agreement between indirect calorimetry and prediction equations using the Bland-Altman method. Burns 2006; 32: 335–42. [DOI] [PubMed] [Google Scholar]
- 98.Royall D, Fairholm L, Peters WJ, Jeejeebhoy KN, Allard JP. Continuous measurement of energy expenditure in ventilated burn patients: an analysis. Crit Care Med 1994; 22: 399–406. [DOI] [PubMed] [Google Scholar]
- 99.Gore DC, Chinkes D, Sanford A, Hart DW, Wolf SE, Herndon DN. Influence of fever on the hypermetabolic response in burn-injured children. Arch Surg 2003; 138: 169–74. [DOI] [PubMed] [Google Scholar]
- 100.Wischmeyer PE. The glutamine story: where are we now? Curr Opin Crit Care 2006; 12: 142–48. [DOI] [PubMed] [Google Scholar]
- 101.Wischmeyer PE, Lynch J, Liedel J, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med 2001; 29: 2075–80. [DOI] [PubMed] [Google Scholar]
- 102.Heyland D, Muscedere J, Wischmeyer PE, et al. , and the Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013; 368: 1489–97. [DOI] [PubMed] [Google Scholar]
- 103.Gamliel Z, DeBiasse MA, Demling RH. Essential microminerals and their response to burn injury. J Burn Care Rehabil 1996; 17: 264–72. [PubMed] [Google Scholar]
- 104.Berger MM. Can oxidative damage be treated nutritionally? Clin Nutr 2005; 24: 172–83. [DOI] [PubMed] [Google Scholar]
- 105.Berger MM. Antioxidant micronutrients in major trauma and burns: evidence and practice. Nutr Clin Pract 2006; 21: 438–49. [DOI] [PubMed] [Google Scholar]
- 106.Berger MM, Baines M, Raffoul W, et al. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am J Clin Nutr 2007; 85: 1293–300. [DOI] [PubMed] [Google Scholar]
- 107.Berger MM, Binnert C, Chiolero RL, et al. Trace element supplementation after major burns increases burned skin trace element concentrations and modulates local protein metabolism but not whole-body substrate metabolism. Am J Clin Nutr 2007; 85: 1301–06. [DOI] [PubMed] [Google Scholar]
- 108.Berger MM, Eggimann P, Heyland DK, et al. Reduction of nosocomial pneumonia after major burns by trace element supplementation: aggregation of two randomised trials. Crit Care 2006; 10: R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berger MM, Shenkin A. Vitamins and trace elements: practical aspects of supplementation. Nutrition 2006; 22: 952–55. [DOI] [PubMed] [Google Scholar]
- 110.Berger MM, Shenkin A. Trace element requirements in critically ill burned patients. J Trace Elem Med Biol 2007; 21 (suppl 1): 44–48. [DOI] [PubMed] [Google Scholar]
- 111.Solomon JR. Early surgical excision and grafting of burns including tangential excision. Prog Pediatr Surg 1981; 14: 133–49. [PubMed] [Google Scholar]
- 112.Wilmore DW, Mason AD Jr, Johnson DW, Pruitt BA Jr. Effect of ambient temperature on heat production and heat loss in burn patients. J Appl Physiol 1975; 38: 593–97. [DOI] [PubMed] [Google Scholar]
- 113.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol 2001; 91: 1168–75. [DOI] [PubMed] [Google Scholar]
- 114.Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg 1995; 221: 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeschke MG, Herndon DN, Wolf SE, et al. Recombinant human growth hormone alters acute phase reactant proteins, cytokine expression, and liver morphology in burned rats. J Surg Res 1999; 83: 122–29. [DOI] [PubMed] [Google Scholar]
- 116.Jeschke MG, Chrysopoulo MT, Herndon DN, Wolf SE. Increased expression of insulin-like growth factor-I in serum and liver after recombinant human growth hormone administration in thermally injured rats. J Surg Res 1999; 85: 171–77. [DOI] [PubMed] [Google Scholar]
- 117.Branski LK, Herndon DN, Barrow RE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg 2009; 250: 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aili Low JF, Barrow RE, Mittendorfer B, Jeschke MG, Chinkes DL, Herndon DN. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children. Burns 2001; 27: 447–52. [DOI] [PubMed] [Google Scholar]
- 119.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 1999; 341: 785–92. [DOI] [PubMed] [Google Scholar]
- 120.Demling R Growth hormone therapy in critically ill patients. N Engl J Med 1999; 341: 837–39. [DOI] [PubMed] [Google Scholar]
- 121.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 1998; 228: 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 1999; 229: 713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.CioffiWG Gore DC, Rue LW 3rd, et al. Insulin-like growth factor-1 lowers protein oxidation in patients with thermal injury. Ann Surg 1994; 220: 310–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeschke MG, Barrow RE, Herndon DN. Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Ann Surg 2000; 231: 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg 2001; 233: 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Demling RH, DeSanti L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns 2003; 29: 793–97. [DOI] [PubMed] [Google Scholar]
- 127.Demling RH, Seigne P. Metabolic management of patients with severe burns. World J Surg 2000; 24: 673–80. [DOI] [PubMed] [Google Scholar]
- 128.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res 2006; 27: 131–39. [DOI] [PubMed] [Google Scholar]
- 129.Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007; 246: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg 2012; 214: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics 2007; 119: e109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jeschke MG, Norbury WB, Finnerty CC, Branski LK, Herndon DN. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J Trauma 2007; 62: 676–81. [DOI] [PubMed] [Google Scholar]
- 133.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 2001; 345: 1223–29. [DOI] [PubMed] [Google Scholar]
- 134.Brooks NC, Song J, Boehning D, et al. Propranolol improves impaired hepatic phosphatidylinositol 3-kinase/akt signaling after burn injury. Mol Med 2012; 18: 707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg 2012; 256: 402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jeschke MG, Kraft R, Emdad F, Kulp GA, Williams FN, Herndon DN. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg 2010; 252: 521–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma 1998; 44: 342–45. [DOI] [PubMed] [Google Scholar]
- 138.Klein D, Schubert T, Horch RE, Jauch KW, Jeschke MG. Insulin treatment improves hepatic morphology and function through modulation of hepatic signals after severe trauma. Ann Surg 2004; 240: 340–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Finfer S, Liu B, Chittock DR, et al. , and the NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108–18. [DOI] [PubMed] [Google Scholar]
- 141.Moon RJ, Bascombe LA, Holt RI. The addition of metformin in type 1 diabetes improves insulin sensitivity, diabetic control, body composition and patient well-being. Diabetes Obes Metab 2007; 9: 143–45. [DOI] [PubMed] [Google Scholar]
- 142.Gore DC, Wolf SE, Herndon DN, Wolfe RR. Metformin blunts stress-induced hyperglycemia after thermal injury. J Trauma 2003; 54: 555–61. [DOI] [PubMed] [Google Scholar]
- 143.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg 2005; 241: 334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mecott GA, Herndon DN, Kulp GA, et al. The use of exenatide in severely burned pediatric patients. Crit Care 2010; 14: R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg 2013; published online April 10. DOI: 10.1097/SLA.0b013e31828dfbf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Finnerty CC, Qian WJ, Kaushal A, et al. Determination of burn patient outcome by large scale quantitative discovery proteomics. Crit Care Med 2013; 41: 1421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Finnerty CC, Ju H, Spratt H, et al. Proteomics improves the prediction of burns mortality: results from regression spline modeling. Clin Transl Sci 2012; 5: 243–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ryan CM, Schneider JC, Kazis LE, et al. Benchmarks for multidimensional recovery after burn injury in young adults: The development, validation, and testing of the American Burn Association/Shriners Hospitals for Children Young Adult Burn Outcome Questionnaire. J Burn Care Res 2013; 34: e121–e42. [DOI] [PubMed] [Google Scholar]
- 149.Tompkins RG, Liang MH, Lee AF, Kazis LE, and the Multi-Center Benchmarking Study Working Group. The American Burn Association/Shriners Hospitals for Children Burn Outcomes Program: a progress report at 15 years. J Trauma Acute Care Surg 2012; 73 (suppl 2): S173–78. [DOI] [PubMed] [Google Scholar]


