Key Points
Question
Can global longitudinal strain, an echocardiographic biomarker of cardiac function, be used in evaluation of patients with heart failure?
Findings
In this cohort study of 2186 patients, global longitudinal strain reflected the severity of chronic heart failure. In addition, global longitudinal strain was associated with cardiac and all-cause mortality independent of clinical profile, heart failure symptoms, cardiac structure, and systolic and diastolic function.
Meaning
Global longitudinal strain may be a helpful clinical tool to improve risk stratification in individuals with chronic heart failure.
Abstract
Importance
Global longitudinal strain (GLS) is an emerging echocardiographic biomarker of cardiac function in heart failure (HF). Evidence from large-scale studies comprehensively investigating GLS for its association with clinical phenotypes and mortality in asymptomatic and symptomatic chronic HF is limited.
Objective
To assess the factors associated with GLS and its prognostic value in patients with chronic HF.
Design, Setting, and Participants
The observational, prospective MyoVasc cohort study enrolled 3289 individuals with asymptomatic to symptomatic HF between January 17, 2013, and April 27, 2018. The median follow-up was 3.2 years (interquartile range, 2.0-4.0 years). Participants with stages A to D HF according to American Heart Association (AHA) criteria were examined at a dedicated study center. Echocardiography was performed with GLS measurement by independent reviewers. Data were analyzed from September 2, 2019, to January 15, 2020.
Main Outcomes and Measures
All-cause and cardiac mortality were recorded by structured follow-up and validated via death certificates.
Results
In the study sample, data on GLS were available on 2440 individuals, of whom 2186 (mean [SD] age, 65.0 [10.5] years; 1418 [64.9%] men) were classified as having AHA HF stages A to D. Mean (SD) GLS worsened across AHA stages from stage A (n = 434; −19.44 [3.15%]) to stage B (n = 629; −18.01 [3.46%]) to stages C/D (n = 1123; −15.52 [4.64%]). Age (β = −0.27; 95% CI, −0.47 to −0.067; per decade, P = .009), female sex (β = −1.2; 95% CI, −1.6 to −0.77; per decade, P < .001), obesity (β = 0.64; 95% CI, 0.25-1.0; P = .001), atrial fibrillation (β = 1.2; 95% CI, 0.69-1.6; P < .001), myocardial infarction (β = 1.5; 95% CI, 1.00-2.1; P < .001), and estimated glomerular filtration rate (β = −0.53; 95% CI, −0.73 to −0.32; per SD, P < .001) were independently associated with GLS in multivariable regression analysis. Global longitudinal strain was associated with the severity of HF as reflected by N-terminal prohormone B-type natriuretic protein (NT-proBNP) levels after additionally adjusting for cardiac structure and function (P < .001). During follow-up, GLS was associated with all-cause mortality (hazard ratio [HR] per SD, 1.55; 95% CI, 1.19-2.01; P < .001) and cardiac death (HR per SD, 2.32; 95% CI, 1.57-3.42; P < .001) independent of image quality, observer variability, clinical profile, HF medications, NYHA class, and cardiac structure and function. After further adjustment for the NT-proBNP level, GLS remained associated with cardiac death (HR per SD, 1.60; 95% CI, 1.07-2.41; P = .02) but not all-cause mortality (HR per SD, 1.26; 95% CI, 0.95-1.66; P = .11).
Conclusions and Relevance
In patients with chronic HF, GLS was associated with clinical and cardiac status, reflected neurohormonal activation, and was associated with cardiac mortality independent of clinical and cardiac status. These findings suggest that GLS may serve as a useful tool to improve risk stratification in patients with HF.
This cohort study examines the use of global longitudinal strain as a tool to assess cardiac function and prognostic factors in patients with heart failure.
Introduction
Left ventricular ejection fraction (LVEF) is one of the most frequently recorded clinical parameters in patients with heart failure (HF). Left ventricular ejection fraction is assessed to classify HF phenotypes according to current guidelines and is routinely used for medical decision-making.1 However, the prognostic value of LVEF in HF is limited.2
Global longitudinal strain (GLS) is an emerging biomarker of cardiac function that has been evaluated for risk stratification in HF. In one study of patients with acute decompensated HF, GLS provided superior prognostic value compared with LVEF and peak velocity of early (E) diastolic inflow and peak lateral early (E′) diastolic mitral annular velocity (E/E′) ratio.3 In patients with chronic HF with reduced ejection fraction (EF), GLS was reported to be independently associated with all-cause mortality and long-term outcome in addition to well-established prognostic factors.4,5 A post hoc analysis of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist trial demonstrated that impaired longitudinal strain was associated with survival in patients with HF who had preserved EF.6 Adequately powered prospective studies examining the prognostic value of GLS for all-cause mortality and cardiac death in chronic HF under consideration of the cardiovascular risk profile and systolic and diastolic function are scarce.
The factors associated with GLS in the setting of chronic HF have not been studied extensively. To date, evidence is limited to investigations in small studies7,8,9 and, to our knowledge, no comprehensive evaluation of factors associated with GLS is available for patients with HF. Against this background, the present study aimed to (1) investigate the clinical factors associated with GLS in the HF syndrome, (2) provide insights into the interdependence of GLS with established markers of cardiac systolic and diastolic function and N-terminal prohormone B-type natriuretic protein (NT-proBNP) as the standard laboratory biomarker of HF, and (3) broadly assess the association of GLS with cardiac and all-cause mortality.
Methods
Study Design and Study Population
Data from the MyoVasc Study,10 an investigator-initiated, prospective cohort study on HF located at the University Medical Center Mainz in Mainz, Germany, that was conducted from January 17, 2013, to April 27, 2018, were investigated. The study design and baseline characteristics have recently been published.11 Inpatients and outpatients at the study site who were aged 35 to 84 years and had cardiac dysfunction or established HF were contacted by the MyoVasc Study staff and informed in detail about the study. After written informed consent was obtained and if the inclusion criteria were met, the patients were invited to participate in a highly standardized, 5-hour examination in a dedicated study center (eTable 1 in the Supplement). Individuals serving as controls were recruited from a population-based sample that was drawn at random by the governmental registry office. When participants had function noted on echocardiography that was within the reference range at baseline, they were included in the control group. When participants presented with cardiac dysfunction, they were included in the HF sample as individuals at risk for developing symptomatic HF. For the present analysis, only participants categorized as having stages A to D HF according to the current HF guidelines of the American Heart Association (AHA) were included.12 The MyoVasc study, with this analysis included in the protocol, was approved by the Ethics Committee of the Rhineland-Palatinate Medical Association, and all study procedures adhered to the Declaration of Helsinki13 and the standards of good clinical and epidemiologic practice. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Assessments
All individuals underwent a physical examination by a qualified physician (S.-O.T., S.S.-T., F.M., M.W.H., S.G., S.D., and J.L.M.) following predefined standard operating procedures, including anthropometric and blood pressure measurements. Current medication, cardiovascular risk factors, comorbidities, and family history of myocardial infarction or stroke were recorded during a computer-assisted interview. The eMethods in the Supplement provide further definitions.
During echocardiography (iE33 echocardiography system with S5-1 sector array transducer; Philips Healthcare), 4 cardiac cycles were routinely obtained (mean frame rate, 50/s) and digitally transferred into an image archiving system (Xcelera; Philips Healthcare) for offline analysis. All measurements of cardiac structure and function were performed according to the current American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations.14 The LVEF was calculated using Simpson’s method in the apical 4-chamber view, left ventricular mass indexed to body height2.7 (LVMi) was calculated using the cube formula in the parasternal long-axis, and E/E′ was measured during a complete cardiac cycle.
Global longitudinal strain was measured offline (QLAB 9.0.1; Philips Healthcare) in all apical views. Aortic valve closure time was visually defined in the apical long-axis view. In each view, 2 points at the LV base and 1 point at the LV apex were manually selected for automatic tracking of the myocardium. The tracking result was visually controlled and manually readjusted if necessary. In case of persistent poor tracking quality, segments were excluded from analysis. All measurements were carried out by 9 observers (included S.-O.T., S.S.-T., and J.L.M.) and interobserver and intraobserver variability were assessed.
Information on clinical outcomes (ie, all-cause mortality and cardiac death) was assessed via annual follow-up investigations by a computer-assisted telephone interview and quarterly checks of the vital status via national registration offices. Source data (ie, death certificates with standardized coding and medical records) were obtained by a specific data team and subsequently evaluated by a clinical event committee, which included board-certified cardiologists (J.H.P. and P.S.W.).
Statistical Analysis
Continuous variables are reported as mean (SD) for parametric and median (interquartile range) for nonparametric distributions. Discrete variables are described by relative and absolute frequencies. For continuous variables, means were compared by a 2-sided, unpaired t test and medians by the Mann-Whitney test. The χ2 test was applied for dichotomous variables. Intraobserver and interobserver variability were evaluated by intraclass correlation analysis. Associations of GLS with clinical parameters were analyzed using multivariable linear regression models with adjustment for cardiovascular risk factors, comorbidities (ie, atrial fibrillation, chronic obstructive pulmonary disease, coronary artery disease, estimated glomerular filtration rate, myocardial infarction, peripheral artery disease, venous thromboembolism, and stroke), echocardiographic measures of function (ie, LVEF and E/E′ ratio) and geometry (ie, LVMi and relative wall thickness [RWT]), and use of HF medication. Cumulative incidence plots were generated.
Cox proportional hazard regression models for all-cause mortality and cardiac death (cause-specific and with competing risk analysis considering all-cause mortality) were calculated and adjusted for observer (n = 9), image quality, clinical profile, HF medications, E/E′ ratio, LVEF, LVMi, RWT, NYHA class, and log-transformed NT-proBNP level in a stepwise approach. C statistics were calculated and compared for each of the Cox proportional hazard regression models. Interaction analyses of GLS with clinical parameters were calculated in Cox proportional hazard regression models adjusted for age and sex for all-cause mortality and cardiac death. The assumption of proportional hazards was confirmed for all Cox proportional hazard regression models using the test developed by Grambsch and Therneau.15 In this analysis, a P value <.05 was considered statistically significant. Statistical analyses were performed using the R software package, version 3.6.0 (R Foundation).
Results
Sample Characteristics
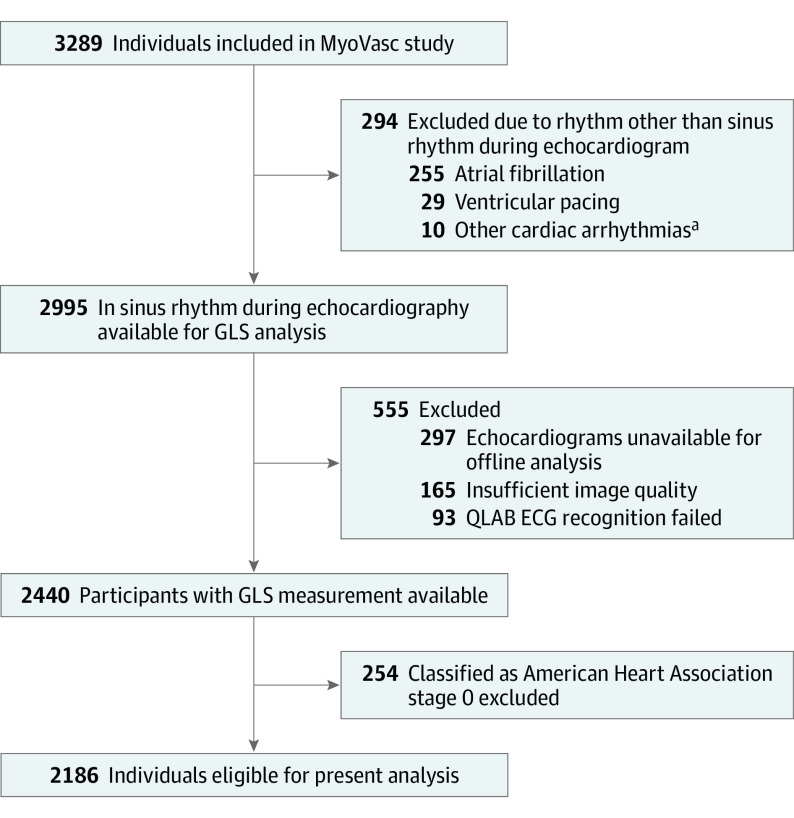
Of 2440 study participants with available data on GLS, a total of 2186 individuals with AHA HF stages A to D were included in the present analysis (Figure 1). Of these, 1418 participants were men (64.9%) and 768 were women (35.1%). Mean (SD) age was 65.0 (10.5) years. Further details on clinical characteristics are reported in Table 1. Data on GLS were not available for 514 participants with sinus rhythm classified as AHA stage A to D owing to technical or logistical reasons. These individuals were more often classified as having HF at an NYHA class greater than II, were more often obese, and showed a higher frequency of coronary artery disease (eTable 2 in the Supplement). Owing to poor tracking quality, in 466 participants (21.3%), 1 to 2 segments were excluded and, in 105 participants (4.8%), more than 2 segments were excluded of all segments from the apical views. Exclusion of participants with GLS and more than 2 missing segments in a sensitivity analysis did not relevantly affect the results. There was low mean interobserver (intraclass correlation coefficient, 0.89; 95% CI, 0.80-0.95) and intraobserver (intraclass correlation coefficient, 0.94; 95% CI, 0.87-0.97) variability for GLS. The coefficient of variation for GLS across the sample was 0.26. Of the individuals classified as having HF stage C/D (categories combined because of few individuals with stage D) (n = 1123), 420 individuals (37.4%) were categorized as having preserved EF, 250 (22.3%) with borderline preserved EF, and 184 (16.4%) with reduced EF. Of the participants with reduced EF, 90 (49.2%) had an implantable cardioverter-defibrillator and 39 (21.2%) had a pacemaker. These devices were also present in patients with borderline preserved EF (implantable cardioverter-defibrillator 39 [15.6%]; pacemaker, 28 [11.2%]) and preserved EF (implantable cardioverter-defibrillator, 8 [3.1%]; pacemaker, 11 [6.9%]). Global longitudinal strain was normally distributed in the sample (eFigure 1 in the Supplement). Mean (SD) GLS worsened across AHA HF stages A (n = 434; −19.44 [3.15%]), B (n = 629; −18.01 [3.46%]), and C/D (n = 1123; −15.52 [4.64%]). Within HF stage C/D, GLS was −17.48 (3.51%) in patients with preserved EF, −13.94 (3.46%) in patients with borderline preserved EF, and −9.53 (3.56%) in those with reduced EF.
Figure 1. Flowchart of the Sample Selection Available for Analysis.
Global longitudinal strain (GLS) was measured offline by the use of the software QLAB, version 9.0.1 (Philips Healthcare). ECG indicates electrocardiogram.
aOther cardiac arrhythmias comprised atrial tachycardia (n = 4), bigeminus (n = 3), frequent supraventricular extrasystoles (n = 2), and frequent ventricular (n = 1) extrasystoles.
Table 1. Clinical Characteristics of the 2186 Study Participantsa.
| Characteristic | Quartile of GLS, No. (%)b | |||
|---|---|---|---|---|
| First (GLS ≤−20.0) | Second (GLS >−20.0 to ≤−17.5) | Third (GLS >−17.5 to ≤−14.4) | Fourth (GLS >−14.4) | |
| Age, mean (SD), y | 64.8 (10.5) | 64.6 (10.6) | 65.5 (10.5) | 65.3 (10.4) |
| Sex | ||||
| Female | 267 (48.8) | 180 (33.0) | 183 (33.5) | 138 (25.3) |
| Male | 280 (51.2) | 366 (67.0) | 364 (66.5) | 408 (74.7) |
| NT-proBNP, median (IQR), pg/mL | 108.5 (52.9-219.2) | 117.00 (59.0-222.0) | 154.00 (70.9-309.3) | 407.5 (145.9-1099.3) |
| NYHA class ≥II | 141 (25.8) | 139 (25.5) | 171 (31.3) | 246 (45.1) |
| Cardiovascular risk factors | ||||
| Arterial hypertension | 421 (77.0) | 436 (79.9) | 450 (82.3) | 427 (78.2) |
| Type 2 diabetes | 102 (18.6) | 105 (19.2) | 134 (24.5) | 157 (28.8) |
| Dyslipidemia | 348 (63.6) | 377 (69.0) | 408 (74.6) | 436 (79.9) |
| Family history of MI or stroke | 137 (25.1) | 119 (21.8) | 121 (22.1) | 152 (27.8) |
| Obesity | 140 (25.6) | 166 (30.4) | 198 (36.2) | 210 (38.5) |
| Smoking | 65 (11.9) | 69 (12.6) | 70 (12.8) | 85 (15.6) |
| Comorbidities | ||||
| Atrial fibrillationc | 73 (13.3) | 81 (14.8) | 91 (16.6) | 128 (23.4) |
| Chronic kidney disease | 88 (16.1) | 93 (17.0) | 71 (13.0) | 109 (20.0) |
| COPD | 75 (13.7) | 65 (11.9) | 76 (13.9) | 85 (15.6) |
| Coronary artery disease | 142 (26.0) | 204 (37.4) | 225 (41.1) | 295 (54.0) |
| Myocardial infarction | 72 (13.2) | 119 (21.8) | 149 (27.2) | 202 (37.0) |
| Peripheral artery disease | 26 (4.8) | 37 (6.8) | 35 (6.4) | 55 (10.1) |
| Stroke | 35 (6.4) | 46 (8.4) | 48 (8.8) | 58 (10.6) |
| Venous thromboembolism | 46 (8.4) | 51 (9.3) | 51 (9.3) | 43 (7.9) |
| Left ventricular function | ||||
| E/E′ ratio, median (IQR) | 8.09 (6.33-10.05) | 7.82 (6.00-10.13) | 8.46 (6.61-10.93) | 10.07 (7.31-14.50) |
| LVEF, mean (SD), % | 61.1 (6.4) | 58.9 (6.5) | 55.9 (7.9) | 45.1 (11.7) |
| Left ventricular geometry | ||||
| LVEDV, median (IQR), mL | 84.0 (71.0-101.0) | 94.7 (78.0-114.7) | 99.0 (78.0-120.0) | 128.7 (97.4-170.0) |
| LVESV, median (IQR), mL | 32.5 (26.0-41.0) | 38.1 (30.0-49.0) | 42.5 (32.0-56.5) | 70.0 (46.0-103.3) |
| LVMi, median (IQR), g/m2.7 | 42.1 (35.1-49.0) | 42.2 (35.8-51.0) | 45.4 (37.4-54.1) | 53.3 (44.2-65.9) |
| RWT, mean (SD) | 0.44 (0.12) | 0.42 (0.11) | 0.44 (0.13) | 0.39 (0.12) |
| Heart failure stages according to AHA | ||||
| Stage A | 186 (34.0) | 136 (24.9) | 85 (15.5) | 27 (4.9) |
| Stage B | 179 (32.7) | 186 (34.1) | 167 (30.5) | 97 (17.8) |
| Stage C/D | 182 (33.3) | 224 (41.0) | 295 (53.9) | 422 (77.3) |
| Medicationd | ||||
| ACE inhibitor (C09A) | 151 (27.6) | 169 (31.0) | 204 (37.3) | 259 (47.4) |
| Aldosterone receptor antagonist (C03DA) | 22 (4.0) | 27 (4.9) | 44 (8.0) | 181 (33.2) |
| Angiotensin receptor blocker (C09C) | 170 (31.1) | 185 (33.9) | 172 (31.4) | 150 (27.5) |
| β-Blocker (C07) | 236 (43.1) | 274 (50.2) | 312 (57.0) | 384 (70.3) |
| Diuretic (C03) | 85 (15.5) | 107 (19.6) | 134 (24.5) | 292 (53.5) |
| Ivabradine (C01EB17) | 26 (4.8) | 25 (4.6) | 29 (5.3) | 65 (11.9) |
| Statin (C10AA) | 184 (33.6) | 233 (42.7) | 270 (49.4) | 288 (52.7) |
Abbreviations: ACE, angiotensin-converting enzyme; AHA, American Heart Association; COPD, chronic obstructive pulmonary disease; E, peak early diastolic transmitral flow velocity; E′, peak early diastolic mitral annular tissue velocity at lateral mitral annulus; GLS, global longitudinal strain; IQR, interquartile range; LVEDV, left-ventricular (LV) end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; LVMi, LV mass indexed to body height2.7; MI, myocardial infarction; NT-proBNP, N-terminal prohormone B-type natriuretic protein; NYHA, New York Heart Association; RWT, relative wall thickness.
Categorical variables are presented as relative and absolute frequency.
The first and third quartiles of GLS each included 547 patients; the second and fourth quartiles each included 546 individuals.
Individuals with atrial fibrillation were included in case sinus rhythm was present during the echocardiographic examination.
Anatomical Therapeutic Chemical classification system codes of drugs were recorded during the baseline visit in the study center; codes for the categories of medication are reported in parentheses.
GLS Outcome
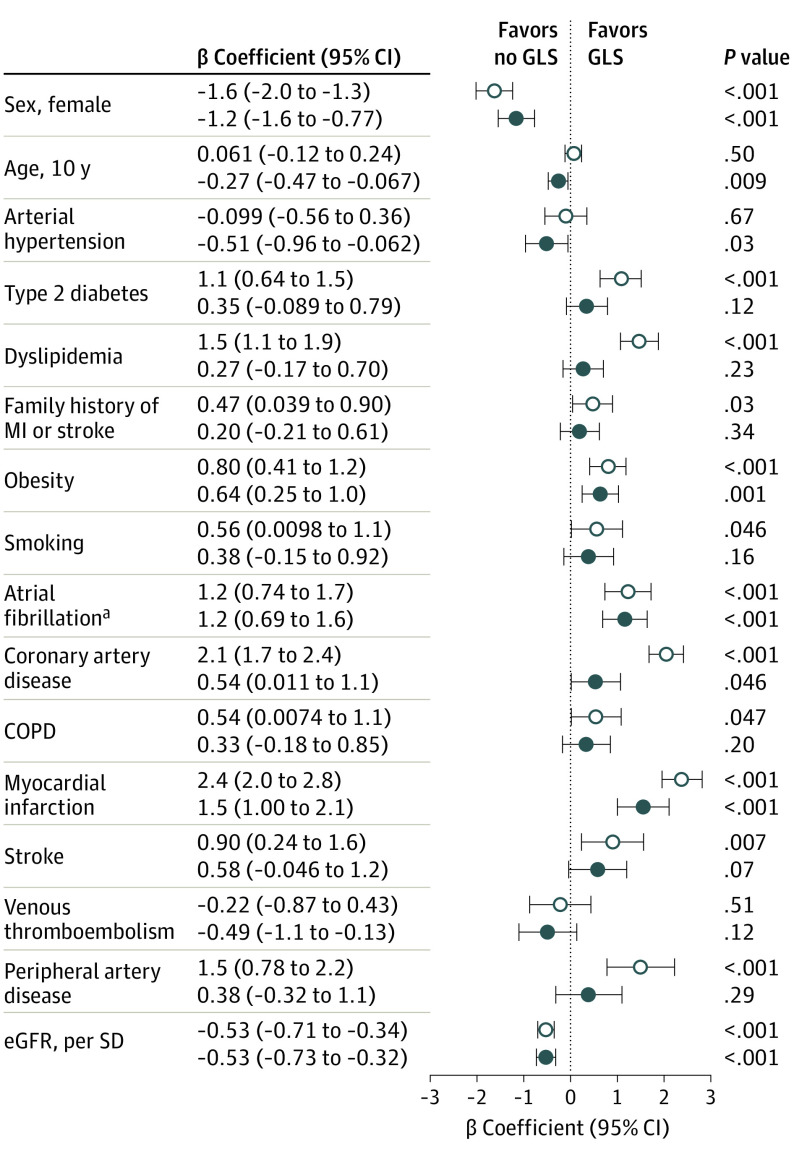
Multivariable regression models were computed to evaluate which clinical characteristics represent factors associated with GLS in individuals with HF. After adjustment for age, sex, cardiovascular risk factors, and comorbidities, age (β = −0.27; 95% CI, −0.47 to −0.067; per decade, P = .009) and female sex ((β = −1.2; 95% CI, −1.6 to −0.77; per decade, P < .001) were associated with lower GLS and obesity (β = 0.64; 95% CI, 0.25-1.0; P = .001); atrial fibrillation (β = 1.2; 95% CI, 0.69-1.6; P < .001), coronary artery disease (β = 0.54; 95% CI, 0.01-1.10; P = .046), myocardial infarction (β = 1.5; 95% CI, 1.00-2.1; P < .001), and estimated glomerular filtration rate (β = −0.53; 95% CI, −0.73 to −0.32; per SD, P < .001), were associated with higher GLS (Figure 2). When replacing the dichotomous variable type 2 diabetes with hemoglobin A1c level in the fully adjusted model, a continuously analyzed hemoglobin A1c level was associated with higher GLS (β = 0.26; 95% CI, 0.08-0.45; P = .006). Arterial hypertension was associated with lower GLS (β = −0.53; 95% CI, −0.98 to −0.093; P = .02). In a sensitivity analysis with exclusion of the upper quartile of GLS, however, arterial hypertension was no longer associated with GLS (β = 0.06; 95% CI, −0.27 to 0.40; P = .72).
Figure 2. Clinical Factors Associated With Global Longitudinal Strain (GLS).
Univariate and multivariate regression analysis with GLS as the dependent variable. The multivariate model was adjusted for all covariates shown. Filled circles represent adjustment for all covariates. Open circles represent crude values. COPD indicates chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; and MI, myocardial infarction.
aIncluded individuals with atrial fibrillation and sinus rhythm during echocardiography.
To evaluate the association between GLS and the severity of HF, a multivariate linear regression model for NT-proBNP was analyzed: after initial adjustment for age and sex, higher GLS was independently associated with higher NT-proBNP concentrations (β = 0.13; 95% CI, 0.12-0.14; P < .001). After additional adjustment for cardiovascular risk factors, comorbidities, NYHA class, and measures of cardiac function (ie, LVEF and E/E′) and cardiac structure (ie, LVMi and RWT), the effect estimate was attenuated, but a significant association between GLS and NT-proBNP level remained (β = 0.04; 95% CI, 0.03-0.05; P < .001).
In univariate analysis, all established echocardiographic measures were associated with GLS. In multivariable regression analysis with adjustment for age, sex, traditional cardiovascular risk factors, and comorbidities, as well as the cardiac measures LVEF, E/E′ ratio, RWT, and LVMi, LVEF was identified as the most significant factor associated with GLS (β = −2.31 per SD; 95% CI, −2.48 to −2.14; P < .001). In comparison with LVEF, the effect sizes per change of SD for LVMi and E/E′ ratio on GLS were substantially lower (eFigure 2 in the Supplement).
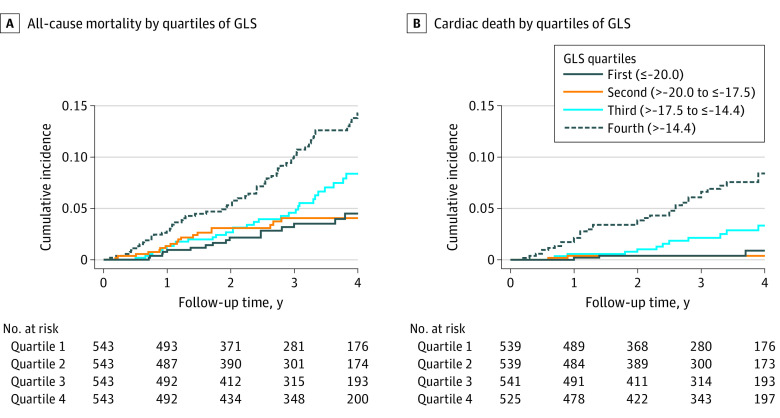
During a median follow-up time of 3.2 years (interquartile range, 2.0-4.0 years), a total of 123 study participants died, with 52 of cardiac death. The association of GLS with all-cause mortality and cardiac death is illustrated in Figure 3 and eFigure 3 in the Supplement. In Cox proportional hazards regression analysis with adjustment for age, sex, observer, and image quality, there was a 1.9-fold increased risk of all-cause mortality (HR, 1.93; 95% CI, 1.65-2.27) and a 3.0-fold increased risk for cardiac death (HR, 2.96; 95% CI, 2.30-3.82) per 1-SD higher GLS (Table 2). After further adjustment for LVEF and E/E′ ratio, the effect estimates were lower, with a 1.6-fold increased risk for mortality and 2.4-fold increased risk for cardiac death. Additional adjustment for cardiovascular risk factors, comorbidities, cardiac structure (ie, LVMi and RWT), NYHA class, and use of HF medication did not relevantly change the risk estimate of GLS for all-cause mortality (HR per SD, 1.55; 95% CI, 1.19-2.01; P < .001) and cardiac death (HR per SD, 2.32; 95% CI, 1.57-3.42; P < .001). The results were not substantially altered when replacing LVMi with end-diastolic volume or additionally adjusting for end-diastolic volume. Neither LVEF nor E/E′ ratio was associated with all-cause mortality or cardiac death independently of GLS (eTable 3 in the Supplement). In contrast, GLS remained independently associated with cardiac death even after additional adjustment for log-transformed NT-proBNP level (hazard ratio [HR] per SD for GLS, 1.60; 95% CI, 1.07-2.41; P = .02), which was not the case for all-cause mortality (HR per SD for GLS, 1.26; 95% CI, 0.95-1.66; P = .11). Details on the predictive performance (ie, C statistics) of GLS and NT-proBNP levels in the models can be found in eTable 4 in the Supplement; cause-specific HRs were not relevantly different from subdistribution HRs (eTable 5 in the Supplement). No significant interaction between GLS and the number of missing segments (ie, >2 according to current American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines) was detected (P = .34 for interaction).
Figure 3. Association of Global Longitudinal Strain (GLS) With Survival.
Cumulative incidence plots for all-cause mortality (A) and cardiac death (B), stratified by quartiles of GLS.
Table 2. Association of GLS With Mortality in Individuals With Heart Failurea.
| Variable | All-cause mortality | Cardiac death | ||
|---|---|---|---|---|
| HR (95% CI) for GLS per SD | P value | HR (95% CI) for GLS per SD | P value | |
| Adjusted for age, sex, observer, and image qualityb | 1.93 (1.65-2.27) | <.001 | 2.96 (2.30-3.82) | <.001 |
| With LVEF, E/E′ ratio | 1.63 (1.27-2.09) | <.001 | 2.36 (1.67-3.35) | <.001 |
| With cardiovascular risk factorsc | 1.60 (1.24-2.05) | <.001 | 2.37 (1.66-3.38) | <.001 |
| With comorbidities and eGFRd | 1.66 (1.29-2.13) | <.001 | 2.49 (1.75-3.56) | <.001 |
| With cardiac structure and NYHA classe | 1.55 (1.19-2.01) | .001 | 2.31 (1.60-3.34) | <.001 |
| With heart failure medicationf | 1.55 (1.19-2.01) | .001 | 2.32 (1.57-3.42) | <.001 |
Abbreviations: E, peak early diastolic transmitral flow velocity; E′, peak early diastolic mitral annular tissue velocity at lateral mitral annulus; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Multivariable Cox regression analysis with all-cause mortality was the dependent variable and GLS was the independent variable with adjustment for the denoted parameters.
Nine observers were involved in the GLS measurement.
Cardiovascular risk factors included type 2 diabetes, dyslipidemia, family history of myocardial infarction or stroke, arterial hypertension, obesity, and smoking.
Comorbidities included atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, myocardial infarction, peripheral artery disease, stroke, and venous thromboembolism.
Measures of cardiac structure comprised left ventricular mass index and relative wall thickness.
Heart failure medication comprised aldosterone receptor antagonists (C03DA), angiotensin-converting enzyme inhibitors (C09A), angiotensin receptor blockers (C09C), β-blockers (C07), and/or diuretics (C03). Anatomical Therapeutic Chemical classification system codes are reported in parentheses.
In interaction analyses using Cox proportional hazards regression models with adjustment for age and sex, atrial fibrillation was associated with an interaction with GLS and its association with all-cause mortality (P = .03 for interaction) and cardiac death (P = .04 for interaction). In further analyses, LVEF and coronary artery disease modified the association of GLS with all-cause mortality (P = .04 for LVEF interaction and P = .01 for coronary artery disease interaction), but not with cardiac death (P = .51 for LVEF interaction and P = .91 for coronary artery disease interaction). There were no interactions found for sex (P = .11), HF stages (A/B vs C/D, P = .81), or NYHA class (P = .62).
Discussion
To our knowledge, this is the largest prospective study of patients with chronic HF to evaluate GLS in the full range of the HF syndrome, from asymptomatic to symptomatic HF. The study allowed for assessing clinical factors associated with GLS and its prognostic relevance in individuals with HF after consideration of the patients’ clinical profile and measures of cardiac structure and function. The main findings of this study suggest that (1) male sex, obesity, hemoglobin A1c, atrial fibrillation, coronary artery disease, and a history of myocardial infarction are the major independent clinical factors associated with higher GLS in individuals with HF; (2) GLS is associated with the severity of HF as reflected by NT-proBNP levels independent of the clinical profile, NYHA class, and measures of cardiac structure and function; (3) GLS is associated with echocardiographic measures of structure and function, but independent of the clinical profile only for LVMi, diastolic function as reflected by E/E′ ratio, and systolic function as reflected by LVEF, with the strongest association detected with LVEF; and (4) GLS is associated with cardiac death and all-cause mortality in patients with HF independent of the clinical profile and cardiac structure and function. Of clinical importance, GLS remained associated with cardiac death after additional adjustment for the standard biomarker NT-proBNP level, suggesting a potential to improve risk stratification and management of patients with HF. However, GLS was no longer associated with all-cause mortality after adjustment for NT-proBNP levels.
Previous studies in healthy volunteers reported an age- and sex-dependency of GLS with lower values in women and younger individuals that was not observed in patients with asymptomatic hypertensive heart disease.16,17,18 However, female sex was associated with lower GLS in patients with HF who showed reduced EF.19 In the present study, sex was one of the strongest clinical characteristics associated with GLS in univariate and multivariable analyses. Although the underlying mechanism of GLS is not yet fully understood, there is evidence suggesting sex-specific differences in the cardiac pathophysiologic characteristics: the proteomic response to pressure overload in mice appears to vary with sex in favor of female mice.20 Although male humans responded to pressure overload with chamber dilation, women developed concentric hypertrophy with an increased LVEF.21 These outcomes might be mediated by estrogens and might also result in lower GLS.22 Because the association between age and GLS was mainly observed in healthy individuals, existing comorbidities may have modified the association in the present study.
The association between impaired renal function and higher GLS in hypertensive patients and individuals with chronic kidney disease was noted in the present study for patients with chronic HF.7,23 In patients with preserved EF, type 2 diabetes was associated with higher GLS in asymptomatic individuals with hypertensive heart disease and worse longitudinal strain.6,18 However, data from the community-based Framingham Heart Study showed no independent association of GLS with type 2 diabetes, but an association was noted with the homeostatic model assessment for insulin resistance.24 In the present study, type 2 diabetes was not, but the hemoglobin A1c level was, independently associated with GLS, suggesting a possible direct effect of glucose metabolism on longitudinal myocardial function. The hemoglobin A1c level also showed a nearly linear association with GLS in the Atherosclerosis Risk in the Community study.25 Hence, a dose-effect association of type 2 diabetes with GLS in patients with HF may exist and should be further explored.
In the present study, GLS was associated with LVMi and LV systolic and diastolic function. Left ventricular hypertrophy was identified as a factor associated with GLS in hypertensive patients with preserved LVEF independent of E/E′ ratio.7 Left ventricular hypertrophy was previously postulated to be directly associated with subclinical cardiac injury in the general population.26 Therefore, an increased LVMi in HF might be partly accompanied by cardiac injury, which is reflected by GLS, but not by more established measures of diastolic or systolic function.
In the current cohort with a sufficient sample size, GLS also provided information on NT-proBNP concentration in addition to the clinical profile, NYHA class, and echocardiographic measures of function and geometry. In a small retrospective study on chronic HF, an independent association of NT-proBNP level with GLS was also found,27 and similar results were reported in 137 individuals with suspected HF.28 However, owing to the small number of cases in those studies, the analyses were limited regarding the adjustment for echocardiographic measures or a small number of clinical parameters. In asymptomatic patients with hypertension without left-ventricular hypertrophy and with preserved LVEF, GLS was not associated with the NT-proBNP concentration.29
There is increasing evidence that GLS is superior to LVEF in estimating the prognosis of patients with HF.3,4,5,30In one study, GLS was superior to LVEF for estimating the probability of all-cause mortality in patients with acutely decompensated HF independent of the clinical profile and systolic function.3 Because only patients with acutely decompensated HF were included, this observation might not be readily transferable to those with chronic HF. Global longitudinal strain yielded a higher prognostic value for all-cause mortality than LVEF after adjustment for the clinical profile in a large-scale study on reduced EF in patients with HF.5 However, LVEF and GLS were not investigated in a joint model, and a measure of diastolic function was not included in the study. Another retrospective study on reduced EF in patients with HF reported a higher prognostic value of GLS for all-cause death compared with LVEF considering the clinical profile and the E/E′ ratio.4 Data for the clinical profile were obtained from a database, and prognostically important clinical variables, such as renal function, atrial fibrillation, and arterial hypertension, were not included in the analysis. In a small study including 54 patients after index hospitalization for HF with an LVEF greater than 45%, GLS denoted an increased risk for all-cause mortality after adjustment when using a cutoff level of −15.8%.30
Fewer data are available for the association of GLS with cardiac death since most studies only reported a composite end point or did not assess cardiac death.4,5,18,31,32 In patients with HF and preserved EF, longitudinal strain (measured in the apical 4-chamber view only) was associated with an increased risk for cardiovascular death after adjustment for clinical profile and E/E′ ratio.6 However, the influence of NT-proBNP level on the association of GLS with cardiac death was not investigated.
Strengths and Limitations
The primary strengths of the present study are the large sample size and the well-phenotyped cohort, which was assessed by specially trained staff in a highly standardized setting in a dedicated study center. Reproducibility of GLS was evaluated and found to be sufficient, and data on clinical outcomes were recorded in a structured manner. The study also has limitations. This research is from a single-center setting and results have not been validated externally. Whereas NT-proBNP level (an established risk prediction biomarker in chronic HF) could be measured in all patients, GLS data were not available in 17.4% of the individuals in our study, although the rate of missing GLS data in previous large clinical studies has been as high as 49%.6 Left ventricular EF was measured by a single-plane approach and diastolic function was represented only by the E/E′ ratio. Because a single-plane approach systematically underestimates LVEF,33 results could have been more pronounced if the biplane approach had been used. Variables were aggregated for parsimony of covariates in the models, which may have led to a loss of information. However, replacing the variables with disease-specific or continuous variables appeared to have no relevance to the outcomes. Specific HF end points (eg, hospitalization due to HF, transition from asymptomatic to symptomatic HF) were not available for the current analysis.
Conclusions
The findings of this study of patients with chronic HF suggest that GLS is associated with clinical and cardiac status, reflects neurohormonal activation, and is associated with cardiac mortality independent of clinical and cardiac status. Thus, GLS may serve as a useful tool to improve risk stratification in patients with HF.
eMethods. Detailed Methods
eTable 1. Inclusion and Exclusion Criteria of the Study Sample
eTable 2. Clinical Characteristics of Study Participants in Sinus Rhythm Stratified for the Availability of Data on GLS
eTable 3. Predictive Value of Left Ventricular Ejection Fraction and E/E′ Ratio in Addition to Global Longitudinal Strain for Survival
eTable 4. C Statistics of Cox Models for Predicting All-Cause and Cardiac Death
eTable 5. Cause-Specific Hazard Ratios for Global Longitudinal Strain, Left Ventricular Ejection Fraction and E/E′ Ratio For Cardiac Death
eFigure 1. Distribution of Global Longitudinal Strain in the Sample
eFigure 2. Relation Between Established Measures of Cardiac Function and Structure With Global Longitudinal Strain
eFigure 3. Cox Models With Restricted Cubic Splines for the Relation of GLS With Survival
eReference
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2.Solomon SD, Anavekar N, Skali H, et al. ; Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738-3744. doi: 10.1161/CIRCULATIONAHA.105.561423 [DOI] [PubMed] [Google Scholar]
- 3.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71(18):1947-1957. doi: 10.1016/j.jacc.2018.02.064 [DOI] [PubMed] [Google Scholar]
- 4.Sengeløv M, Jørgensen PG, Jensen JS, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8(12):1351-1359. doi: 10.1016/j.jcmg.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 5.Bertini M, Ng AC, Antoni ML, et al. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5(3):383-391. doi: 10.1161/CIRCIMAGING.111.970434 [DOI] [PubMed] [Google Scholar]
- 6.Shah AM, Claggett B, Sweitzer NK, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132(5):402-414. doi: 10.1161/CIRCULATIONAHA.115.015884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soufi Taleb Bendiab N, Meziane-Tani A, Ouabdesselam S, et al. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur J Prev Cardiol. 2017;24(14):1463-1472. doi: 10.1177/2047487317721644 [DOI] [PubMed] [Google Scholar]
- 8.Lee HH, Lee MK, Lee WH, et al. Atrial fibrillation per se was a major determinant of global left ventricular longitudinal systolic strain. Medicine (Baltimore). 2016;95(26):e4038. doi: 10.1097/MD.0000000000004038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhea IB, Morris K, Sawada S, Feigenbaum H. Prevalence, etiology, and clinical implications of reduced longitudinal systolic strain in renal transplant candidates. Echocardiography. 2016;33(11):1676-1682. doi: 10.1111/echo.13307 [DOI] [PubMed] [Google Scholar]
- 10.MyoVasc Study on the development and progression of heart failure. ClinicalTrials.gov identifier: NCT04064450. Updated August 22, 2019. Accessed August 22, 2019. https://clinicaltrials.gov/ct2/show/NCT04064450
- 11.Göbel S, Prochaska JH, Tröbs SO, et al. Rationale, design and baseline characteristics of the MyoVasc study: a prospective cohort study investigating development and progression of heart failure. Eur J Prev Cardiol. 2020;2047487320926438. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 16.Dalen H, Thorstensen A, Aase SA, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11(2):176-183. doi: 10.1093/ejechocard/jep194 [DOI] [PubMed] [Google Scholar]
- 17.Menting ME, McGhie JS, Koopman LP, et al. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography. 2016;33(11):1665-1675. doi: 10.1111/echo.13323 [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Khan F, Stoklosa T, Iannaccone A, Negishi K, Marwick TH. Prognostic implications of LV strain risk score in asymptomatic patients with hypertensive heart disease. JACC Cardiovasc Imaging. 2016;9(8):911-921. doi: 10.1016/j.jcmg.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 19.Lundorff IJ, Sengeløv M, Godsk Jørgensen P, et al. Echocardiographic predictors of mortality in women with heart failure with reduced ejection fraction. Circ Cardiovasc Imaging. 2018;11(11):e008031. doi: 10.1161/CIRCIMAGING.118.008031 [DOI] [PubMed] [Google Scholar]
- 20.Kararigas G, Fliegner D, Forler S, et al. Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J Proteome Res. 2014;13(12):5829-5836. doi: 10.1021/pr500749j [DOI] [PubMed] [Google Scholar]
- 21.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86(4):1099-1107. doi: 10.1161/01.CIR.86.4.1099 [DOI] [PubMed] [Google Scholar]
- 22.van Eickels M, Grohé C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17β-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104(12):1419-1423. doi: 10.1161/hc3601.095577 [DOI] [PubMed] [Google Scholar]
- 23.Krishnasamy R, Isbel NM, Hawley CM, et al. The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol Dial Transplant. 2014;29(6):1218-1225. doi: 10.1093/ndt/gfu004 [DOI] [PubMed] [Google Scholar]
- 24.Negishi T, Negishi K, Thavendiranathan P, et al. ; SUCCOUR Investigators . Effect of experience and training on the concordance and precision of strain measurements. JACC Cardiovasc Imaging. 2017;10(5):518-522. doi: 10.1016/j.jcmg.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 25.Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk in the Community study. Circ Heart Fail. 2015;8(3):448-454. doi: 10.1161/CIRCHEARTFAILURE.114.001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113(16):1958-1965. doi: 10.1161/CIRCULATIONAHA.105.609974 [DOI] [PubMed] [Google Scholar]
- 27.De Vecchis R, Baldi C, Di Biase G. The relation between global longitudinal strain and serum natriuretic peptide is more strict than that found between the latter and left ventricular ejection fraction: a retrospective study in chronic heart failure. J Clin Med Res. 2015;7(12):979-988. doi: 10.14740/jocmr2370w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneyama A, Koyama J, Tomita T, et al. Relationship of plasma brain-type natriuretic peptide levels to left ventricular longitudinal function in patients with congestive heart failure assessed by strain Doppler imaging. Int J Cardiol. 2008;130(1):56-63. doi: 10.1016/j.ijcard.2007.07.171 [DOI] [PubMed] [Google Scholar]
- 29.Uraizee I, Cheng S, Hung CL, et al. Relation of N-terminal pro-B-type natriuretic peptide with diastolic function in hypertensive heart disease. Am J Hypertens. 2013;26(10):1234-1241. doi: 10.1093/ajh/hpt098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W, Chai SC, Lee SGS, MacDonald MR, Leong KTG. Prognostic factors after index hospitalization for heart failure with preserved ejection fraction. Am J Cardiol. 2017;119(12):2017-2020. doi: 10.1016/j.amjcard.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 31.Nahum J, Bensaid A, Dussault C, et al. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3(3):249-256. doi: 10.1161/CIRCIMAGING.109.910893 [DOI] [PubMed] [Google Scholar]
- 32.Russo C, Jin Z, Elkind MS, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16(12):1301-1309. doi: 10.1002/ejhf.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St John Sutton M, Otterstat JE, Plappert T, et al. Quantitation of left ventricular volumes and ejection fraction in post-infarction patients from biplane and single plane two-dimensional echocardiograms: a prospective longitudinal study of 371 patients. Eur Heart J. 1998;19(5):808-816. doi: 10.1053/euhj.1997.0852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eTable 1. Inclusion and Exclusion Criteria of the Study Sample
eTable 2. Clinical Characteristics of Study Participants in Sinus Rhythm Stratified for the Availability of Data on GLS
eTable 3. Predictive Value of Left Ventricular Ejection Fraction and E/E′ Ratio in Addition to Global Longitudinal Strain for Survival
eTable 4. C Statistics of Cox Models for Predicting All-Cause and Cardiac Death
eTable 5. Cause-Specific Hazard Ratios for Global Longitudinal Strain, Left Ventricular Ejection Fraction and E/E′ Ratio For Cardiac Death
eFigure 1. Distribution of Global Longitudinal Strain in the Sample
eFigure 2. Relation Between Established Measures of Cardiac Function and Structure With Global Longitudinal Strain
eFigure 3. Cox Models With Restricted Cubic Splines for the Relation of GLS With Survival
eReference