Key Points
Question
How do facility characteristics affect deimplementation of 4 low-value breast cancer operations in the Choosing Wisely campaign?
Findings
In this cohort study in response to national recommendations to avoid 4 low-value procedures, use of 2 procedures decreased significantly while 2 other procedures increased in use. Academic research programs and high-volume facilities demonstrated the greatest reduction in use, with significant interfacility variation for each low-value procedure.
Meaning
Facility-level characteristics were associated with use of low-value breast cancer operations.
Abstract
Importance
Through the Choosing Wisely campaign, surgical specialties identified 4 low-value breast cancer operations. Preliminary data suggest varying rates of deimplementation and have identified patient-level and clinician-level determinants of continued overuse. However, little information exists about facility-level variation or determinants of differential deimplementation.
Objective
To identify variation and determinants of persistent use of low-value breast cancer surgical care.
Design, Setting, and Participants
Retrospective cohort study in which reliability-adjusted facility rates of each procedure were calculated using random-intercept hierarchical logistic regression before and after evidence demonstrated that each procedure was unnecessary. The National Cancer Database is a prospective cancer registry of patients encompassing approximately 70% of all new cancer diagnoses from more than 1500 facilities in the United States. Data were analyzed from November 2019 to August 2020. The registry included women 18 years and older diagnosed as having breast cancer between 2004 and 2016 and meeting inclusion criteria for each Choosing Wisely recommendation.
Main Outcomes and Measures
Rate of each low-value breast cancer procedure based on facility type and breast cancer volume categories before and after the release of data supporting each procedure’s omission.
Results
The total cohort included 920 256 women with a median age of 63 years. Overall, 86% self-identified as White, 10% as Black, 3% as Asian, and 4.5% as Hispanic. Most women in this cohort were insured (51% private and 47% public), were living in a metropolitan or urban area (88% and 11%, respectively), and originated from the top half of income-earning households (65.5%). While there was significant deimplementation of axillary lymph node dissection and lumpectomy reoperation in response to guidelines supporting omission of these procedures, rates of contralateral prophylactic mastectomy and sentinel lymph node biopsy in older women increased during the study period. Academic research programs and high-volume facilities overall demonstrated the greatest reduction in use of these low-value procedures. There was significant interfacility variation for each low-value procedure. Facility-level axillary lymph node dissection rates ranged from 7% to 47%, lumpectomy reoperation rates ranged from 3% to 62%, contralateral prophylactic mastectomy rates ranged from 9% to 67%, and sentinel lymph node biopsy rates ranged from 25% to 97%. Pearson correlation coefficient for each combination of 2 of the 4 procedures was less than 0.11, suggesting that hospitals were not consistent in their deimplementation performance across all 4 procedures. Many were high outliers in one procedure but low outliers in another.
Conclusions and Relevance
Interfacility variation demonstrates a performance gap and an opportunity for formal deimplementation efforts targeting each procedure. Several facility-level characteristics were associated with differential deimplementation and performance.
This study identifies variation and determinants of persistent use of low-value breast cancer surgical care.
Introduction
The provision of services without a clinically meaningful benefit is a national epidemic, costing the United States more than $100 billion dollars annually.1,2 Deimplementation is the science of eliminating low-value practices through evidenced-based processes.3,4,5 One prominent initiative to promote the deimplementation of low-value services is the Choosing Wisely campaign, which is a campaign by the American Board of Internal Medicine Foundation to identify unnecessary medical and surgical services.6 Seventeen surgical societies have participated in Choosing Wisely and identified 24 surgical procedures for deimplementation.7 However, despite general enthusiasm for reducing low-value surgery, clear gaps in evidence-based practice remain.
Examining the natural trend in de-escalation of surgical treatment for early-stage breast cancer offers a unique opportunity to identify determinants of deimplementation specific to low-value surgical procedures. Early-stage breast cancer is highly prevalent, carries an excellent prognosis, and multiple clinical trials support de-escalation of various treatments.8,9,10 Through Choosing Wisely, the American College of Surgeons, the Society for Surgical Oncology (SSO), and the American Society for Breast Surgeons have identified 4 low-value breast cancer treatments for elimination: (1) axillary lymph node dissection (ALND) for limited nodal disease in patients receiving lumpectomy and radiotherapy, (2) lumpectomy re-excision for close but negative margins for invasive cancer, (3) contralateral prophylactic mastectomy (CPM) in average-risk women with unilateral cancer, and (4) sentinel lymph node biopsy (SLNB) in clinically node-negative women 70 years and older with hormone receptor–positive (HR+) cancer.
Despite similar high-quality evidence supporting these recommendations, deimplementation has been inconsistent. Preliminary studies have shown a decrease in rates of ALND and lumpectomy re-excision at both institutional and national levels.2,11,12 In contrast, other studies suggest more than 80% of women 70 years and older with HR+ breast cancer receive SLNB2,13,14,15 and that CPM rates for patients with unilateral cancer are increasing.2,16 While previous studies have examined some tumor-level, patient-level, and clinician-level determinants of persistent use,17 to our knowledge, variation of deimplementation across facilities and procedures has not been described. Furthermore, the contribution of facility-level factors to variable use of unnecessary procedures has not been determined. Therefore, our aims are to (1) compare deimplementation rates and facility-level variation across procedures and (2) assess for facility-level determinants of deimplementation across procedures.
Methods
Data Source and Study Population
The National Cancer Database (NCDB) is based on hospital registry data collected from more than 1500 Commission on Cancer (CoC)–accredited facilities18 and captures approximately 70% of newly diagnosed cancer cases. All data are deidentified and Health Insurance Portability and Accountability Act–compliant. Because of the use of deidentified data, this study was deemed exempt by the University of Michigan institutional review board and patient consent was not obtained.
Using the NCDB, we identified women 18 years and older diagnosed as having breast cancer from 2004 to 2016. Four cohorts were created to evaluate ALND, lumpectomy margin reoperation, CPM, and SLNB rates. Women who received treatment outside of a CoC reporting facility or who received neoadjuvant systemic therapy were excluded. Detailed information regarding inclusion and exclusion criteria for each of the 4 low-value surgical targets is available in eAppendix 1 in the Supplement. Briefly, the ALND study cohort was based on the inclusion criteria of the American College of Surgeons Oncology Group Z0011 trial (n = 47 174).9 The lumpectomy margin reoperation cohort was based on the 2014 SSO/American Society for Radiation Oncology (ASTRO) consensus statement of negative margin as “no tumor on ink” (n = 487 443)19; reoperation was used as a proxy for re-excision similar to other NCDB studies.12,20 The CPM cohort included women with unilateral in situ or invasive stage 0 to II breast cancer who underwent mastectomy (n = 372 561). The SLNB cohort included women 70 years and older with clinically node negative, stage I to II, HR+ invasive breast cancer (n = 212 733).21
Analysis of Procedure Variation at the Facility Level
We performed reliability adjustment using empirical Bayes methods to calculate hospital-level rates of each procedure from 2004 to 2016. To obtain an accurate estimate of facility performance, only hospitals with at least 10 patients per year were included. We created an interrupted time series hierarchical logistic regression model with an interrupted intercept, slope, and quadratic term, as well as a random intercept for facility, to account for clustering within facilities. We compared facility performance before and after publication of the data supporting omission of each procedure (2011 for ALND based on American College of Surgeons Oncology Group Z0011,9 2014 for lumpectomy reoperation based on the SSO/ASTRO consensus statement,19 2007 for CPM based on the SSO consensus statement that CPM for average risk patients with unilateral breast cancer is unnecessary,22 and 2013 for SLNB in women 70 years and older with HR+ breast cancer based on the 10-year CALGB 9343 results demonstrating that SLNB did not improve survival).23
We performed further analyses based on hospital volume and facility type. Hospital volume was based on average annual breast cancer case count and categorized as low (10-99 breast cancer cases), medium (100-199 cases), or high (≥200 cases). These cutoffs were chosen based on previously published ranges and to ensure an adequate number of facility-level and patient-level data across groups for multilevel analysis.24,25 A histogram detailing the proportion of hospitals and patients analyzed by hospital volume category for each procedure is provided in eAppendix 2 in the Supplement.
To determine hospital-level factors associated with successful deimplementation, we limited the sample to patients diagnosed from 2014 to 2016 when the maximum level of deimplementation could be expected. For each of the 4 procedures, reliability-adjusted hospital quintiles based on procedure rates were compared by cross-tabulation between surgical procedures using the Cochran-Armitage, Pearson χ2, and Fisher exact tests as appropriate. We created a Pearson correlation matrix of reliability-adjusted rates to analyze whether trends existed between any 2 pairs of procedures.
A P value less than .05 was considered significant, and all P values were 2-sided. The P values represent differences in annual procedure rates or change in procedure rate over time using a hierarchal logistic model. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Trends in deimplementation of low-value breast cancer operations over time are shown in Figure 1. Consistent with prior data, we found ALND and lumpectomy reoperation rates decreased rapidly in response to evidence demonstrating the safety of their omission. The ALND rates decreased from 63% (95% CI, 61.6-64.0) in 2004 to 14% (95% CI, 13.5-14.5) in 2016 (relative reduction of 78%). The greatest rate of change occurred from 2010 to 2011 (from 62% to 31%), corresponding to dissemination of Z0011 trial results (P < .001). Reoperation rates after lumpectomy decreased from 19% (95% CI, 18.8-19.7) in 2004 to 15% (95% CI, 14.4-15.1) in 2016 (relative reduction of 24%). The greatest rate of change was from 2013 (18%) to 2014 (16%), corresponding to the year of release of the SSO/ASTRO consensus statement designating a negative margin as “no tumor on ink” (P < .001). For every 100 000 women, a 2% reduction corresponds to sparing 2000 women from the procedure.
Figure 1. Trends in Deimplementation of Low-Value Breast Cancer Operations Over Time for Patients Meeting Criteria for Omission of Procedure.
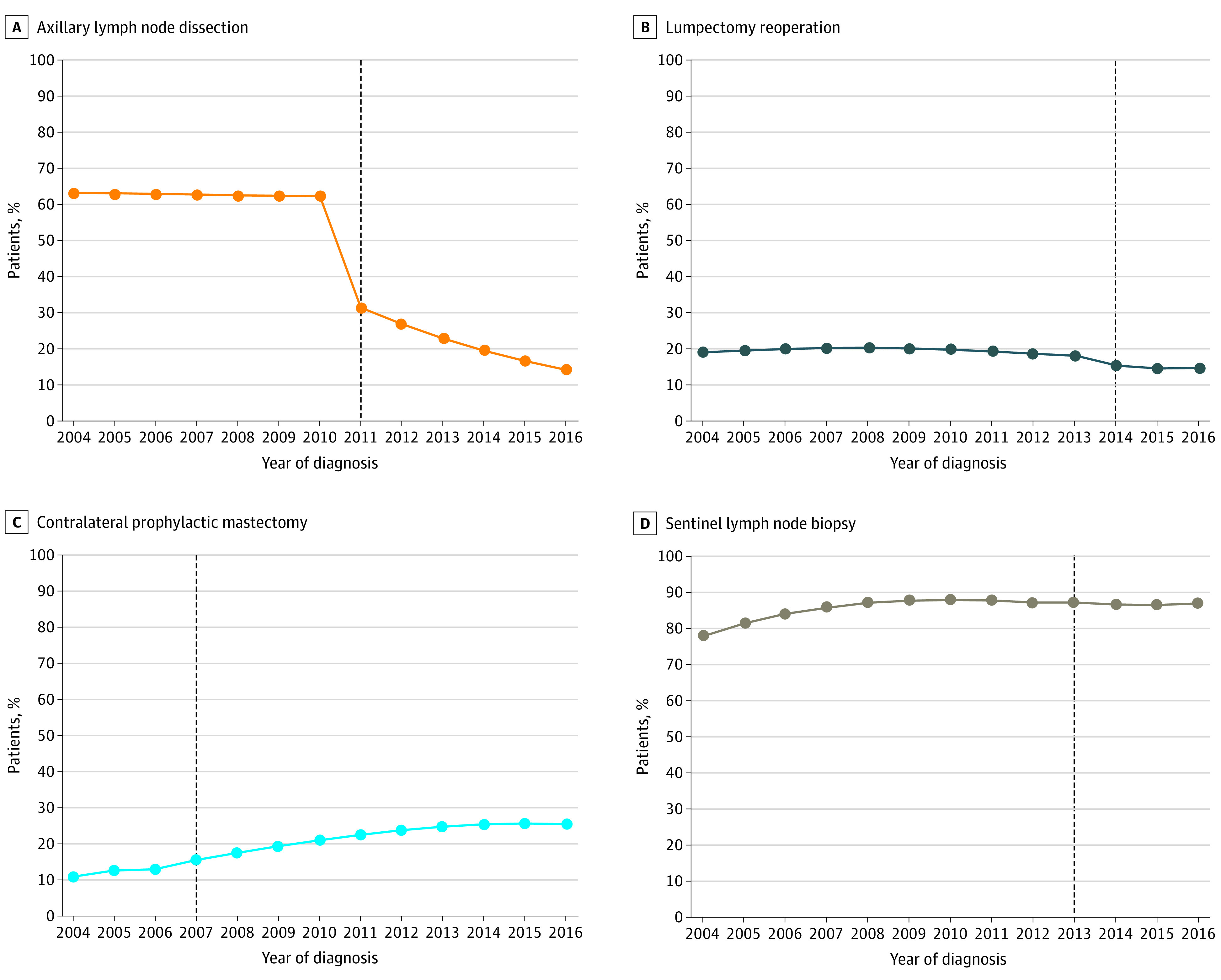
A, Axillary lymph node dissection for lumpectomy patients with 1 to 2 positive nodes receiving radiotherapy. B, Lumpectomy reoperation for patients receiving radiotherapy. C, Contralateral prophylactic mastectomy for patients with unilateral breast cancer. D, Sentinel lymph node biopsy for patients 70 years and older with clinically node-negative hormone receptor positive cancer. Axillary lymph node dissection and lumpectomy reoperation rates for patients meeting eligibility criteria decreased significantly after the release of guidelines supporting procedure omission. In contrast, rates of contralateral prophylactic mastectomy and sentinel lymph node biopsy for older women have increased from 2004 to 2016.
By comparison, rates of CPM for patients with unilateral breast cancer and SLNB for older women have steadily increased since 2004. In 2016, 26% of women (95% CI, 24.7-25.9) with unilateral breast cancer undergoing mastectomy received CPM despite SSO guidelines in 2007 to avoid CPM for average-risk women,22 representing a nearly 2.5-fold increase since 2004 when the rate was 11% (95% CI, 10.3-11.2). The increase in CPM rates was statistically significant both before, in the year of, and after publication of the guidelines, with the greatest rate of change from 2007 to 2016 (from 16% to 26%; P < .001). Similarly, rates of SLNB in women 70 years and older with clinically node-negative HR+ breast cancer increased from 78% (95% CI, 76.9-79.3) in 2004 to 87% (95% CI, 86.8-88.0) in 2012. The SLNB rates remained relatively stable from 2013 (88%) to 2016 (87%), despite evidence from the CALGB 9343 trial in 2013 showing no survival benefit.
Hospital-Level Variation in Rates of Low-Value Breast Cancer Operations
The hospital-level variation in rates of low-value breast cancer operations can be found in Figure 2. From 2014 to 2016, 389 hospitals performed at least 10 of each procedure per year with significant interfacility variation for each low-value procedure. Hospital-level ALND rates ranged from 7% to 47% (mean [SD], 17.8 [5.8]), lumpectomy reoperation rates ranged from 3% to 62% (mean [SD], 16.8 [7.0]), CPM rates ranged from 9% to 67% (mean [SD], 31.8 [11.3]), and SLNB rates ranged from 25% to 97% (mean [SD], 85.2 [9.0]). The ranked order of adjusted rates across hospitals is displayed in caterpillar plots. The maximum Pearson correlation coefficient between any 2 of 4 procedures was less than 0.11, suggesting little correlation in hospital performance across procedures. For ALND and lumpectomy reoperation, hospitals in the top vs bottom quintile did not differ based on breast cancer volume or facility type. However, significantly more integrated network cancer programs were in the highest quintile for CPM rates compared with community cancer programs (23% vs 2%; P < .001). Additionally, more comprehensive community cancer programs were in the highest quintile for SLNB rates for women 70 years and older with HR+ breast cancer compared with academic research programs (48% vs 21%; P = .05). Further detail is provided in eAppendix 3 in the Supplement.
Figure 2. Caterpillar Plots of Reliability-Adjusted Rate for Each Hospital and Breast Cancer Surgery for Patients Meeting Criteria for Omission of Procedure From 2014 to 2016.
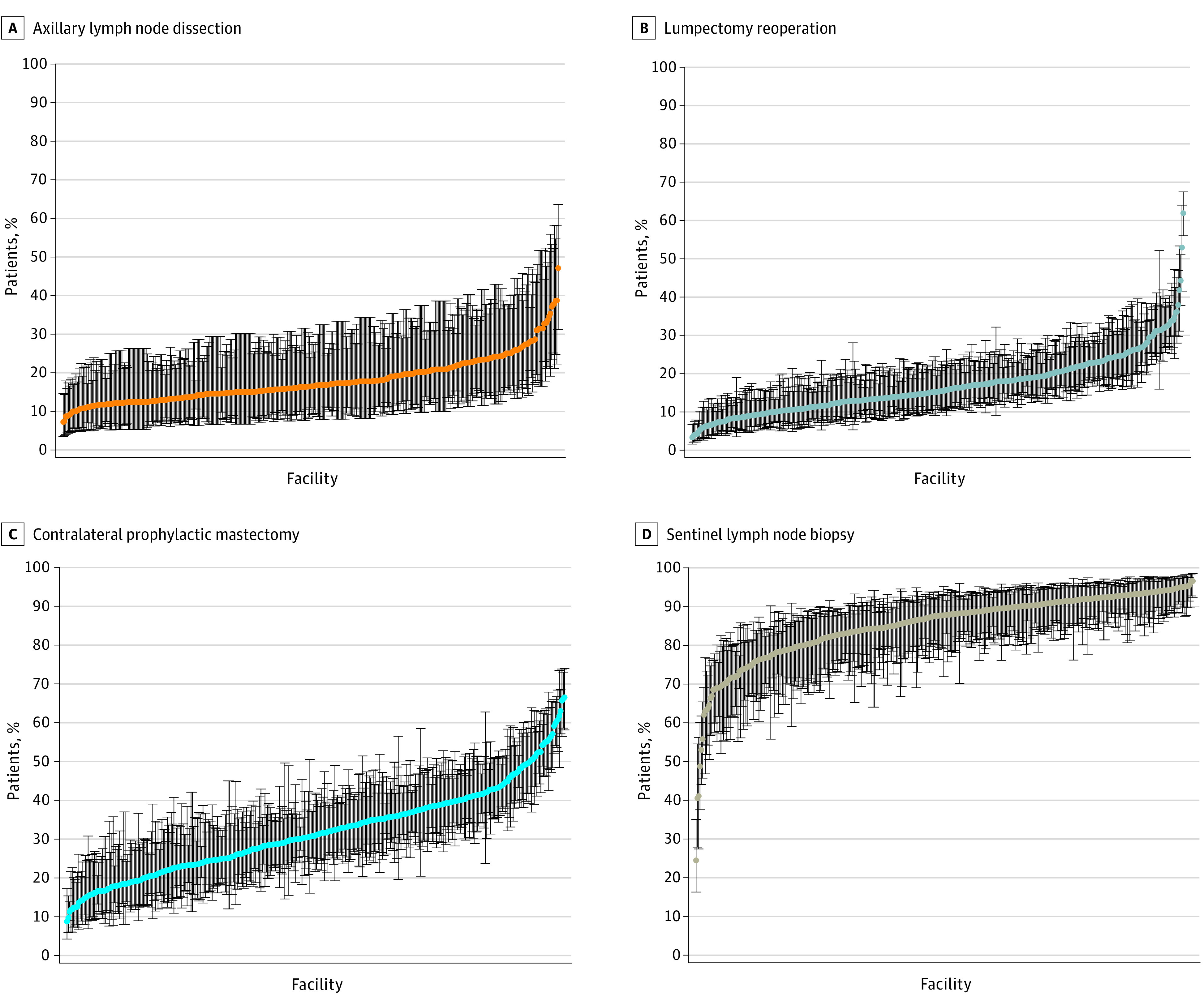
Axillary lymph node dissection (A), lumpectomy reoperation (B), contralateral prophylactic mastectomy (C), and sentinel lymph node biopsy (D) for patients 70 years and older with hormone receptor–positive cancer. Hospitals are ranked from lowest to highest rate of procedure use, with lowest procedure rates on the left. There is large variation in facility-level rates of each procedure. Bars represent 95% confidence intervals.
Trends in Performance of Low-Value Breast Cancer Operations Based on Facility Type
Trends in the performance of low-value breast cancer operations based on facility type can be found in Figure 3. For rates of ALND, deimplementation ranged from a relative reduction of 57% (42% in 2004 to 18% in 2016) for community cancer programs to a relative reduction of 79% (68% in 2004 to 15% in 2016) for academic research programs (P = .055 for academic vs nonacademic). Academic research programs were the only facility type to have a significant decrease in ALND rates prior to Z0011 (from 68% to 65%; P < .001). From 2010 to 2011 and from 2011 to 2016, all facility types except for community cancer programs had a significant decrease in ALND rates. For lumpectomy reoperation, deimplementation ranged from a relative reduction of 20% (19% in 2004 to 15% in 2016) for community cancer programs to a relative reduction of 30% (22% in 2004 to 15% in 2016) for academic research programs (P = .002 for academic vs nonacademic). The trend in deimplementation occurred prior to the release of the SSO/ASTRO guidelines in 2014 with a significant decrease in lumpectomy reoperation for every facility type except for community cancer programs. During the year of guideline release from 2013 to 2014, there was a significant decrease in rates for every facility type. Rates decreased from 19% to 16% in community cancer programs, from 19% to 17% in comprehensive community cancer programs, from 19% to 17% in academic research programs, and from 19% to 17% in integrated network cancer programs (P < .001). For CPM, academic research programs had the lowest rates at the end of the study period (26% in 2016) while integrated network cancer programs had the highest (32% in 2016; P < .001 for academic vs nonacademic). Similarly, academic research programs had significantly lower rates of SLNB in older women with HR+ breast cancer at the end of the study period (84% in 2016) compared with all other facility types (87%-88% in 2016; P < .001 for academic vs nonacademic). Further detail is provided in eAppendix 4 in the Supplement.
Figure 3. Trends in Performance of Low-Value Breast Cancer Operations for Patients Meeting Criteria for Omission of Procedure Based on Facility Type.
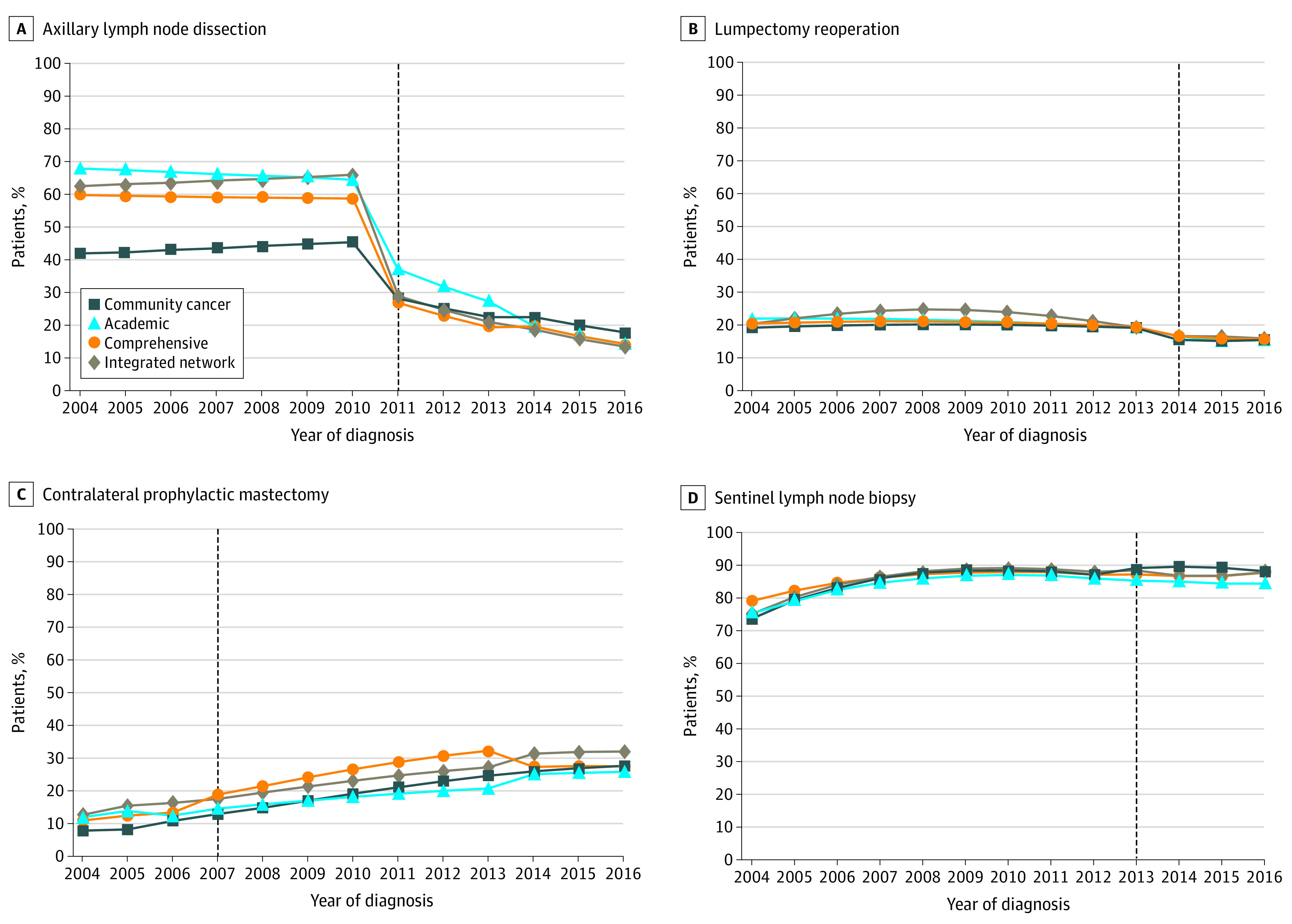
Axillary lymph node dissection (A), lumpectomy reoperation (B), contralateral prophylactic mastectomy (C), and sentinel lymph node biopsy (D) for patients 70 years and older with hormone receptor–positive cancer. Academic research programs have the greatest deimplementation of axillary lymph node dissection and lumpectomy reoperation in response to national guidelines supporting omission of these procedures. Similarly, despite an increase in contralateral prophylactic mastectomy and sentinel lymph node biopsy in older women over time, academic research facilities have the lowest rates of these 2 low-value procedures at the conclusion of the study period.
Trends in Performance of Low-Value Breast Cancer Operations Based on Facility Annual Breast Cancer Volume
Trends in the performance of low-value breast cancer operations based on facility annual breast cancer volume can be found in Figure 4. High-volume hospitals had the greatest decrease in ALND rates (from 65% in 2004 to 14% in 2016; relative reduction, 79%). Medium-volume hospitals had the smallest decrease in ALND rates and the highest ALND rates at the end of the study period (from 62% in 2004 to 17% in 2016; relative reduction 72%; P < .001 for high- vs medium-volume facilities). Whereas ALND rates only decreased at high-volume facilities before 2011, all facilities had significant decreases in ALND rates after Z0011. For lumpectomy reoperation, high-volume hospitals had the greatest reduction but also had the highest reoperation rates at the end of the study period (from 22% in 2004 to 16% in 2016; relative reduction, 28%; P = .002 for high- vs medium-volume facilities). All hospitals by volume category decreased reoperation rates during the year of guideline release. Rates decreased from 18% to 15% in low-volume facilities, from 18% to 16% in medium-volume facilities, and from 21% to 18% in high-volume facilities (P < .001). Although CPM rates increased for all facilities regardless of hospital volume, low-volume hospitals had the lowest rates of CPM during the study period (from 7% in 2004 to 24% in 2016), whereas high-volume facilities had the highest rates of CPM (from 14% in 2004 to 29% in 2016; P < .001 for high- vs low-volume facilities). Conversely, high-volume hospitals had the lowest rates of SLNB during the study period (from 77% in 2004 to 86% in 2016), while low-volume hospitals had the highest rates (from 79% in 2004 to 88% in 2016; P = .04 for high- vs low-volume facility rates in 2016). Further detail is provided in eAppendix 5 in the Supplement.
Figure 4. Trends in Performance of Low-Value Breast Cancer Operations for Patients Meeting Criteria for Omission of Procedure Based on Facility Annual Breast Cancer Volume.
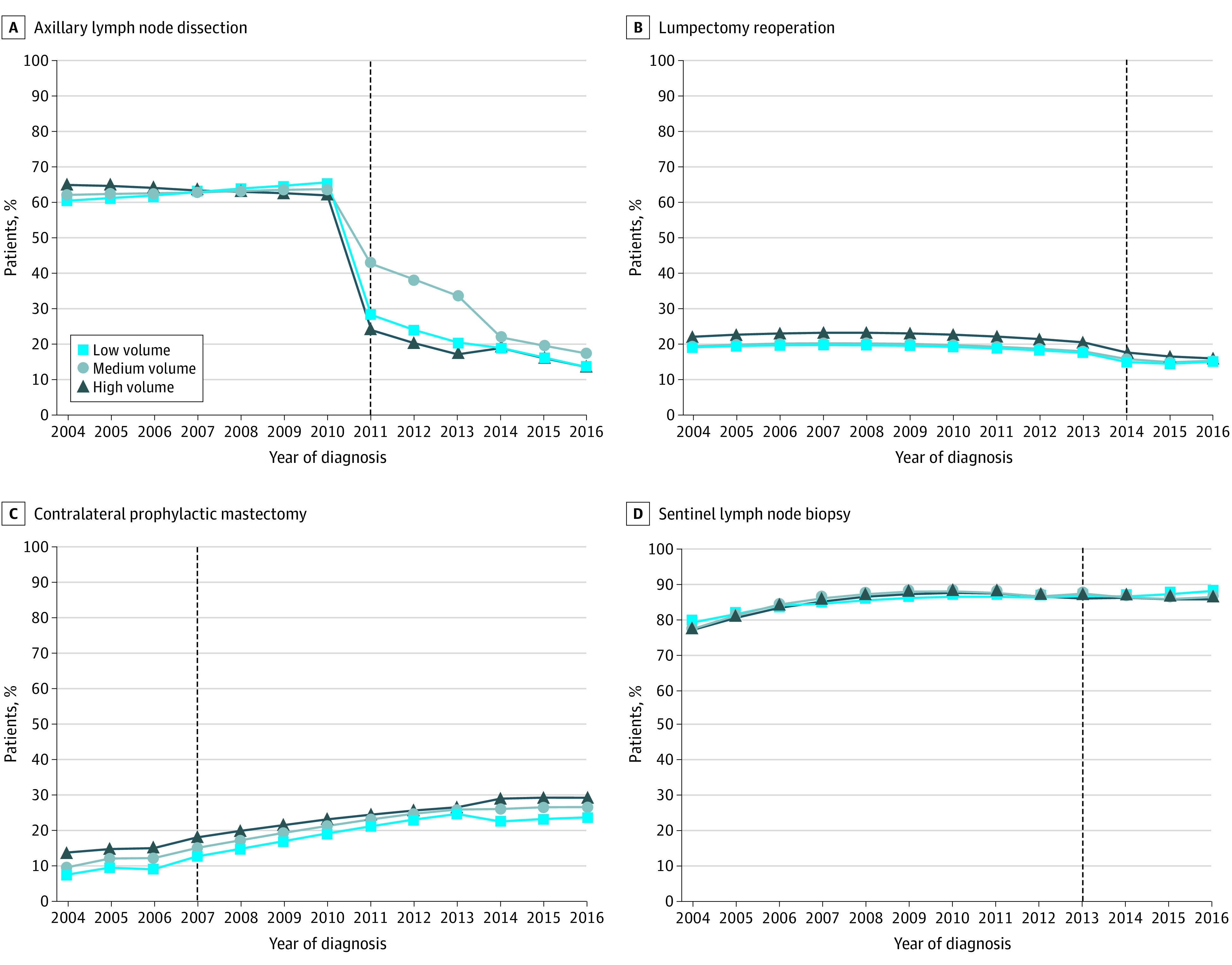
Axillary lymph node dissection (A), lumpectomy reoperation (B), contralateral prophylactic mastectomy (C), and sentinel lymph node biopsy (D) for patients 70 years and older with hormone receptor–positive cancer. High-volume hospitals have the lowest rates of sentinel lymph node biopsy in older women at the end of the study period and the greatest deimplementation of axillary lymph node dissection and lumpectomy reoperation in response to national guidelines supporting omission of these procedures. However, high-volume facilities also have the highest rates of contralateral prophylactic mastectomy.
Discussion
To our knowledge, this is the first study to explore facility-level variation and determinants of differential deimplementation of low-value surgery in a single disease. We identify 3 findings to inform future efforts to reduce overtreatment. First, natural deimplementation of the 4 procedures was variable despite similar levels of evidence supporting treatment de-escalation. Second, we demonstrated significant interfacility variation in deimplementation; being a positive outlier of deimplementation for some procedures did not translate to being a positive outlier for others. Finally, several facility-level characteristics were associated with deimplementation performance, suggesting strategies to reduce low-value care can be tailored to institutional factors.
This study confirms prior data demonstrating that deimplementation of ALND and lumpectomy reoperation occurred rapidly after published evidence supported omission of these procedures. This finding is notable considering it takes 17 years, at least historically, for clinical practice to change in light of research findings.26 Conversely, SLNB rates in older women with early-stage HR+ breast cancer and CPM rates in average-risk women with unilateral breast cancer have steadily increased since 2004. Thus, understanding the factors contributing to the early deimplementation of ALND and lumpectomy reoperation may help identify strategies to reduce other low-value surgical procedures performed at persistently high rates.
In prior qualitative work, we and others identified patient and clinician-related factors facilitating deimplementation.15,17 For ALND, both surgeons and patients viewed lymphedema risk as significant, which likely contributes to eagerness to omit ALND.17,27 Notably, this finding is mirrored by the rapid deimplementation of completion lymph node dissection in patients with melanoma after the Multi-center Selective Lymphadenectomy Trial-II (MSLT-II) demonstrated no overall survival benefit.28,29 Additionally, clinicians have cited the strong evidence base and wide dissemination of the Z0011 trial for ALND and SSO/ASTRO recommendation of a negative margin as “no tumor on ink” as reasons why these practices have decreased.17,30,31 This is supported by our study, which found considerable variation in ALND and lumpectomy reoperation rates by facility type before the respective guidelines but minimal variation afterwards, suggesting these recommendations provided clarity on appropriate indications for ALND and lumpectomy re-excision where there had previously been uncertainty. In contrast, qualitative studies found surgeons are not convinced about the quality of the evidence supporting SLNB omission, are not familiar with national recommendations to avoid SLNB, and feel the procedure adds minimal time and risk to a patient’s operation.17 This suggests quantifying and communicating overtreatment harms to clinicians and patients (eg, the care cascades associated with unnecessary SLNB or financial toxicity and increased risk of complications associated with CPM or unindicated lumpectomy re-excision) may be effective strategies for deimplementation.
While factors contributing to the dramatic increase in CPM rates over the last decade have been investigated, nearly all efforts to eliminate this low-value practice have focused on demand-side, or patient-level, factors.32,33 Contralateral prophylactic mastectomy has traditionally been viewed as a patient preference-sensitive procedure,33 and high-procedure rates are attributed to patient-level factors including younger age, insurance status, desire for peace of mind, fear of recurrent disease, and misperceptions about its influence on survival rates.32,34,35,36 However, our study shows a direct association with hospital volume and facility type, suggesting significant supply-side contributions to this trend. The finding that high-volume hospitals have the highest CPM rates likely reflects wider availability of breast reconstruction, which is known to be strongly correlated.37,38 However, despite having access to breast reconstruction and likely high-volume breast cancer surgery practices, academic research facilities had the lowest rates of CPM at the conclusion of the study period.
Importantly, our study demonstrates facilities are inconsistent in deimplementation performance, suggesting that reducing overtreatment is not an inherent trait associated with a particular facility. Given these findings, strategies for deimplementation should target each procedure individually, with attention to the varied stakeholders involved. One potential approach is to develop a deimplementation toolkit adapted to an individual hospital’s performance across multiple metrics because not all facilities will require the same interventions. Although hospitals are not consistently positive or negative outliers in deimplementation performance, there are some key differences in deimplementation based on facility type and volume. Facility characteristics play a key role in eliminating overtreatment through organizational culture, leadership, and resources.3 Recognizing and targeting specific facility-level factors is an attractive strategy for reducing low-value services because implementing change in health care frequently occurs at the hospital-level.39 Academic research programs had the lowest rates of CPM and SLNB in older women at the end of the study period, and by some measures were the most successful in deimplementing ALND. Additionally, high-volume facilities had the lowest rate of each low-value procedure except for CPM.
Some of the trends noted in our study may reflect the diversity of clinicians caring for patients with breast cancer. Unlike some cancers that are largely centralized to academic medical centers, most patients with breast cancer are treated in nonacademic settings, where there is variation in clinician training and procedural volume.40,41 Whereas specialty-trained or high-volume surgeons may be more comfortable omitting therapies, clinicians with less oncologic experience may be more aggressive owing to concerns about errors from omission. Studies supporting this hypothesis have associated higher-volume breast surgeons with implementation of national quality metrics including high rates of breast-conserving surgery, oncoplastic surgery, and improved patient satisfaction.42,43,44,45 Additionally, high-volume breast surgeons may concentrate in academic facilities, whose resources, culture, and payment structure encourage multidisciplinary and evidence-based care.46,47
Limitations
While major strengths of the NCDB include breadth of the patient population and facility-level data, it is limited by its retrospective nature and available variables. As a result, eligible patient cohorts were based on surrogate measures available through NCDB (eg, the use of reoperation rather than re-excision without definitive knowledge of the lumpectomy margin status). However, methods in this study have been used in prior literature.20,48,49,50,51,52,53 There may be important facility-level characteristics contributing to use of low-value services not represented in this data set (eg, reimbursement structures). Because the NCDB is composed of CoC facilities, this database may be skewed toward more complex diagnoses. However, the NCDB is a comprehensive database that encompasses approximately 70% of patients diagnosed with breast cancer, and we would expect that CoC hospitals are excellent targets for assessing gaps in evidence-based practice. Increased genetic testing (which is not an available variable through NCDB) may result in higher CPM rates, but the prevalence of pathogenic germline mutations is low and is unlikely to account for these increases alone.54,55 Finally, we note that an ideal rate of deimplementation has not been established and may vary by procedure. Even in the case of ALND, where surgeons and patients recognize the significant complication risks, 15% to 20% of patients continue to undergo ALND. This is likely owing to factors that cannot be quantified by a large data set such as the NCDB.
Conclusions
Despite similar evidence and national recommendations supporting the omission of 4 low-value breast cancer procedures, only 2 have been successfully deimplemented. Several facility-level characteristics were associated with deimplementation performance, with academic research facilities and facilities with a high volume of patients with breast cancer demonstrating the greatest reduction in use of these low-value procedures. However, hospitals were not uniform in their deimplementation performance across all 4 procedures, suggesting that success at reducing overtreatment is not an inherent trait associated with a particular hospital. Significant interfacility variation demonstrates a performance gap for many centers and room for formal deimplementation efforts targeting each procedure.
eAppendix 1. Detailed description of study cohorts
eAppendix 2. Methods: Volume Categories
eAppendix 3. Comparison of facility type and breast cancer volume for hospitals ranking in the bottom versus top quintile for procedure performance
eAppendix 4. Trends in performance of low-value breast cancer operations based on facility type
eAppendix 5. Trends in performance of low-value breast cancer operations based on facility volume
References
- 1.Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019;322(15):1501-1509. doi: 10.1001/jama.2019.13978 [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Baskin AS, Dossett LA. Deimplementation of the Choosing Wisely recommendations for low-value breast cancer surgery: a systematic review. JAMA Surg. 2020;(Jun). doi: 10.1001/jamasurg.2020.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2. doi: 10.1186/s13012-019-0960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimshaw JM, Patey AM, Kirkham KR, et al. De-implementing wisely: developing the evidence base to reduce low-value care. BMJ Qual Saf. 2020;29(5):409-417. doi: 10.1136/bmjqs-2019-010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Sabel MS, Dossett LA. A framework for de-implementation in surgery. Ann Surg. 2020. doi: 10.1097/SLA.0000000000004325 [DOI] [PubMed] [Google Scholar]
- 6.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. doi: 10.1001/jama.2012.476 [DOI] [PubMed] [Google Scholar]
- 7.Antunez AG, Telem DA, Dossett LA. Assessment of surgical specialty societies’ Choosing Wisely recommendations. JAMA Surg. 2019;154(10):971-973. doi: 10.1001/jamasurg.2019.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-1241. doi: 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569-575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes KS, Schnaper LA, Berry D, et al. ; Cancer and Leukemia Group B; Radiation Therapy Oncology Group; Eastern Cooperative Oncology Group . Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971-977. doi: 10.1056/NEJMoa040587 [DOI] [PubMed] [Google Scholar]
- 11.Havel L, Naik H, Ramirez L, Morrow M, Landercasper J. Impact of the SSO-ASTRO margin guideline on rates of re-excision after lumpectomy for breast cancer: a meta-analysis. Ann Surg Oncol. 2019;26(5):1238-1244. doi: 10.1245/s10434-019-07247-5 [DOI] [PubMed] [Google Scholar]
- 12.Landercasper J, Bennie B, Ahmad HF, Linebarger JH. Opportunities to reduce reoperations and to improve inter-facility profiling after initial breast-conserving surgery for cancer: a report from the NCDB. Eur J Surg Oncol. 2019;45(11):2026-2036. doi: 10.1016/j.ejso.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 13.Downs-Canner SM, Gaber CE, Louie RJ, et al. Nodal positivity decreases with age in women with early-stage, hormone receptor-positive breast cancer. Cancer. 2020;126(6):1193-1201. doi: 10.1002/cncr.32668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chagpar AB, Horowitz N, Sanft T, et al. Does lymph node status influence adjuvant therapy decision-making in women 70 years of age or older with clinically node negative hormone receptor positive breast cancer? Am J Surg. 2017;214(6):1082-1088. doi: 10.1016/j.amjsurg.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Baskin A, Miller J, et al. Trends in breast cancer treatment de-implementation in older patients with hormone receptor-positive breast cancer: a mixed methods study. Ann Surg Oncol. 2020;(Jul). doi: 10.1245/s10434-020-08823-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9-16. doi: 10.1001/jamasurg.2014.2895 [DOI] [PubMed] [Google Scholar]
- 17.Smith ME, Vitous CA, Hughes TM, Shubeck SP, Jagsi R, Dossett LA. Barriers and facilitators to de-implementation of the Choosing Wisely guidelines for low-value breast cancer surgery. Ann Surg Oncol. 2020;27(8):2653-2663. doi: 10.1245/s10434-020-08285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Surgeons . National Cancer Database. Accessed August 28, 2020. https://www.facs.org/quality-programs/cancer/ncdb
- 19.Moran MS, Schnitt SJ, Giuliano AE, et al. ; Society of Surgical Oncology; American Society for Radiation Oncology . Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507-1515. doi: 10.1200/JCO.2013.53.3935 [DOI] [PubMed] [Google Scholar]
- 20.Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014;149(12):1296-1305. doi: 10.1001/jamasurg.2014.926 [DOI] [PubMed] [Google Scholar]
- 21.Boughey JC, Haffty BG, Habermann EB, Hoskin TL, Goetz MP. Has the time come to stop surgical staging of the axilla for all women age 70 years or older with hormone receptor-positive breast cancer? Ann Surg Oncol. 2017;24(3):614-617. doi: 10.1245/s10434-016-5740-z [DOI] [PubMed] [Google Scholar]
- 22.Giuliano AE, Boolbol S, Degnim A, Kuerer H, Leitch AM, Morrow M. Society of Surgical Oncology: position statement on prophylactic mastectomy, approved by the Society of Surgical Oncology Executive Council, March 2007. Ann Surg Oncol. 2007;14(9):2425-2427. doi: 10.1245/s10434-007-9447-z [DOI] [PubMed] [Google Scholar]
- 23.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382-2387. doi: 10.1200/JCO.2012.45.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenup RA, Obeng-Gyasi S, Thomas S, et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267(2):375-381. doi: 10.1097/SLA.0000000000002095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen TW, Pezzin LE, Li J, Sparapani R, Laud PW, Nattinger AB. Effect of hospital volume on processes of breast cancer care: a National Cancer Data Base study. Cancer. 2017;123(6):957-966. doi: 10.1002/cncr.30413 [DOI] [PubMed] [Google Scholar]
- 26.Lomas J. Words without action? the production, dissemination, and impact of consensus recommendations. Annu Rev Public Health. 1991;12:41-65. doi: 10.1146/annurev.pu.12.050191.000353 [DOI] [PubMed] [Google Scholar]
- 27.Galper SR, Lee SJ, Tao ML, et al. Patient preferences for axillary dissection in the management of early-stage breast cancer. J Natl Cancer Inst. 2000;92(20):1681-1687. doi: 10.1093/jnci/92.20.1681 [DOI] [PubMed] [Google Scholar]
- 28.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211-2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bredbeck BC, Mubarak E, Zubieta DG, et al. Management of the positive sentinel lymph node in the post-MSLT-II era. J Surg Oncol. 2020;(Sep). doi: 10.1002/jso.26200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gainer SM, Hunt KK, Beitsch P, Caudle AS, Mittendorf EA, Lucci A. Changing behavior in clinical practice in response to the ACOSOG Z0011 trial: a survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012;19(10):3152-3158. doi: 10.1245/s10434-012-2523-z [DOI] [PubMed] [Google Scholar]
- 31.DeSnyder SM, Hunt KK, Smith BD, Moran MS, Klimberg S, Lucci A. Assessment of practice patterns following publication of the SSO-ASTRO consensus guideline on margins for breast-conserving therapy in stage i and ii invasive breast cancer. Ann Surg Oncol. 2015;22(10):3250-3256. doi: 10.1245/s10434-015-4666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ager B, Butow P, Jansen J, Phillips KA, Porter D, Group CDA; CPM DA Advisory Group . Contralateral prophylactic mastectomy (CPM): a systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast. 2016;28:107-120. doi: 10.1016/j.breast.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 33.Baker SK, Mayer DK, Esposito N. The contralateral prophylactic mastectomy decision-making process. Plast Surg Nurs. 2013;33(1):11-21. doi: 10.1097/PSN.0b013e3182842424 [DOI] [PubMed] [Google Scholar]
- 34.Greener JR, Bass SB, Lepore SJ. Contralateral prophylactic mastectomy: a qualitative approach to exploring the decision making process. J Psychosoc Oncol. 2018;36(2):145-158. doi: 10.1080/07347332.2017.1395940 [DOI] [PubMed] [Google Scholar]
- 35.Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Ann Surg Oncol. 2012;19(8):2600-2606. doi: 10.1245/s10434-012-2299-1 [DOI] [PubMed] [Google Scholar]
- 36.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16(10):2697-2704. doi: 10.1245/s10434-009-0641-z [DOI] [PubMed] [Google Scholar]
- 37.Agarwal S, Kidwell KM, Kraft CT, et al. Defining the relationship between patient decisions to undergo breast reconstruction and contralateral prophylactic mastectomy. Plast Reconstr Surg. 2015;135(3):661-670. doi: 10.1097/PRS.0000000000001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112(3):489-494. doi: 10.1002/cncr.23214 [DOI] [PubMed] [Google Scholar]
- 39.Allanson ER, Tunçalp Ö, Vogel JP, et al. Implementation of effective practices in health facilities: a systematic review of cluster randomised trials. BMJ Glob Health. 2017;2(2):e000266. doi: 10.1136/bmjgh-2016-000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pass HA, Klimberg SV, Copeland EM III. Are “breast-focused” surgeons more competent? Ann Surg Oncol. 2008;15(4):953-955. doi: 10.1245/s10434-008-9835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheetz KH, Nuliyalu U, Nathan H, Sonnenday CJ. Association of surgeon case numbers of pancreaticoduodenectomies vs related procedures with patient outcomes to inform volume-based credentialing. JAMA Netw Open. 2020;3(4):e203850. doi: 10.1001/jamanetworkopen.2020.3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilligan MA, Neuner J, Zhang X, Sparapani R, Laud PW, Nattinger AB. Relationship between number of breast cancer operations performed and 5-year survival after treatment for early-stage breast cancer. Am J Public Health. 2007;97(3):539-544. doi: 10.2105/AJPH.2005.075663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roohan PJ, Bickell NA, Baptiste MS, Therriault GD, Ferrara EP, Siu AL. Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88(3):454-457. doi: 10.2105/AJPH.88.3.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694-3698. doi: 10.1200/JCO.2007.10.9272 [DOI] [PubMed] [Google Scholar]
- 45.Hiotis K, Ye W, Sposto R, Skinner KA. Predictors of breast conservation therapy: size is not all that matters. Cancer. 2005;103(5):892-899. doi: 10.1002/cncr.20853 [DOI] [PubMed] [Google Scholar]
- 46.Chaudhry R, Goel V, Sawka C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ. 2001;164(2):183-188. [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson LC, Tian L, Voti L, et al. The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health. 2006;96(1):160-166. doi: 10.2105/AJPH.2004.053579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard DH, Soulos PR, Chagpar AB, Mougalian S, Killelea B, Gross CP. Contrary to conventional wisdom, physicians abandoned a breast cancer treatment after a trial concluded it was ineffective. Health Aff (Millwood). 2016;35(7):1309-1315. [DOI] [PubMed] [Google Scholar]
- 49.Mann JM, Wu X, Christos P, Nagar H. The state of surgical axillary management and adjuvant radiotherapy for early-stage invasive breast cancer in the modern era. Clin Breast Cancer. 2018;18(4):e477-e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kantor O, Pesce C, Kopkash K, et al. Impact of the society of surgical oncology-american society for radiation oncology margin guidelines on breast-conserving surgery and mastectomy trends. J Am Coll Surg. 2019;229(1):104-114. doi: 10.1016/j.jamcollsurg.2019.02.051 [DOI] [PubMed] [Google Scholar]
- 51.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in contralateral prophylactic mastectomy rates according to racial groups in young women with breast cancer, 1998 to 2011: a report from the National Cancer Data Base. J Am Coll Surg. 2015;221(1):187-196. doi: 10.1016/j.jamcollsurg.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 52.Albornoz CR, Matros E, Lee CN, et al. Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: the role of breast reconstruction. Plast Reconstr Surg. 2015;135(6):1518-1526. doi: 10.1097/PRS.0000000000001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominici LS, Sineshaw HM, Jemal A, Lin CC, King TA, Freedman RA. Patterns of axillary evaluation in older patients with breast cancer and associations with adjuvant therapy receipt. Breast Cancer Res Treat. 2018;167(2):555-566. [DOI] [PubMed] [Google Scholar]
- 54.Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3(9):1190-1196. doi: 10.1001/jamaoncol.2017.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543-561. doi: 10.2147/CLEP.S206949 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Detailed description of study cohorts
eAppendix 2. Methods: Volume Categories
eAppendix 3. Comparison of facility type and breast cancer volume for hospitals ranking in the bottom versus top quintile for procedure performance
eAppendix 4. Trends in performance of low-value breast cancer operations based on facility type
eAppendix 5. Trends in performance of low-value breast cancer operations based on facility volume


