Key Points
Question
Are nonspecialist providers (such as lay counselors, nurses, midwives, and teachers with no formal training in counseling interventions) effective at preventing and treating perinatal depression and anxiety, and what are the relevant implementation processes for nonspecialist-delivered interventions?
Findings
This systematic review of 46 trials (18 321 participants) and meta-analysis of 44 trials (18 101 participants) found that, compared with control groups, nonspecialist-delivered interventions were associated with lower depressive and anxiety symptoms for both preventive and treatment interventions, but there was high heterogeneity among the included trials. The majority of interventions were implemented in Australia, UK, and US, conducted by nurses and midwives, and delivered in person, in person combined with the telephone, or via telephone only, with only 2 interventions delivered online.
Meaning
This study found evidence in high-income countries to support that nonspecialist providers may be effective in preventing and treating perinatal depressive and anxiety symptoms, which suggests that integrating nonspecialist providers to deliver evidence-based counseling interventions has the potential to address the significant burden of perinatal depression and anxiety worldwide.
Abstract
Importance
Task sharing—or training of nonspecialist providers with no formal training in counseling—is an effective strategy to improve access to evidence-based counseling interventions and has the potential to address the burden of perinatal depression and anxiety.
Objectives
To identify the relevant implementation processes (who, what, where, and how) and to assess the effectiveness of counseling interventions delivered by nonspecialist providers for perinatal depression and anxiety in high-income countries.
Data Sources
CINAHL, Ovid MEDLINE, Ovid MEDLINE In-Process, PsycINFO, Web of Science, Cochrane Central Register of Controlled Trials, and Embase through December 31, 2019. Relevant systematic reviews were also considered.
Study Selection
Randomized clinical trials of counseling interventions that assessed depression or anxiety after intervention, delivered by a nonspecialist provider for adults, and that targeted perinatal populations in a high-income country were included. Self-help interventions that did not include a provider component were excluded.
Data Extraction and Synthesis
Four researchers independently reviewed abstracts and full-text articles, and 2 independently rated the quality of included studies. Random-effects meta-analysis was used to estimate the benefits of the interventions. The Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline was followed.
Main Outcomes and Measures
For implementation processes, the frequencies represented by a total or percentage were estimated, where the denominator is the total number of eligible trials, unless otherwise indicated. For effectiveness, primary and secondary outcome data of depression, anxiety, or both symptoms were used, with separate analyses for prevention and treatment, stratified by depression or anxiety. Subgroup analyses compared outcome types (anxiety vs depression) and study objectives (treatment vs prevention).
Results
In total, 46 trials (18 321 participants) were included in the systematic review; 44 trials (18 101 participants) were included in the meta-analysis. Interventions were implemented across 11 countries, with the majority in Australia, UK, and US. Two-thirds (65%) of counseling interventions were provided by nurses and midwives, lasted a mean of 11.2 weeks (95% CI, 6.4-16.0 weeks), and most were delivered face to face (31 [67.4%]). Only 2 interventions were delivered online. A dearth of information related to important implementation processes, such as supervision, fidelity, and participant sociodemographic characteristics, was observed in many articles. Compared with controls, counseling interventions were associated with lower depressive symptoms (standardized mean difference [SMD], 0.24 [95% CI, 0.14-0.34]; 43 trials; I2 = 81%) and anxiety scores (SMD, 0.30 [95% CI, 0.11-0.50]; 11 trials; I2 = 80%). Treatment interventions were reported to be effective for both depressive symptoms (SMD, 0.38 [95% CI, 0.17-0.59]; 15 trials; I2 = 69%) and anxiety symptoms (SMD, 0.34 [95% CI, 0.09-0.58]; 6 trials; I2 = 71%). However, heterogeneity was high among the trials included in this analysis.
Conclusions and Relevance
This study found evidence in high-income countries indicating that nonspecialist providers may be effective in delivering counseling interventions. Additional studies are needed to assess digital interventions and ensure the reporting of implementation processes to inform the optimal delivery and scale-up of these services.
This systematic review and meta-analysis assesses whether individuals in high-income countries with no formal training in mental health, such as lay counselors, nurses, midwives, and teachers, are effective in delivering preventive and treatment interventions to manage perinatal depression and anxiety symptoms.
Introduction
An estimated 10% to 15% of women experience depression during pregnancy or in the year following childbirth.1,2 In addition, approximately 15% to 20% of women experience anxiety symptoms perinatally.3 Many of those symptoms begin during the antenatal period,4 with annual costs amounting to more than $45.9 billion.5
Counseling interventions, notably cognitive, behavioral, and interpersonal therapies, are widely effective in preventing and treating major depression and anxiety disorders in perinatal women.6,7 Although the US Preventive Services Task Force has endorsed counseling interventions for women at risk of perinatal mood disorders,8 fewer than 20% of women with perinatal depression have access to these interventions.9 The poor dissemination and uptake of effective counseling interventions is due, in part, to the limited number of skilled mental health professionals.
Task sharing is the “rational redistribution of tasks”10 and has been used worldwide to improve access to health care. Nonspecialist providers (NSPs)—individuals with no formal training in mental health, such as lay counselors, nurses, midwives, and teachers—have been trained to prevent and treat perinatal depressive and anxiety symptoms worldwide.11,12 In low- and middle-income countries, task sharing has wide currency,13 with NSPs considered an important human resource because they are widely available, are cost-effective, and have regular, frequent contact with mothers.14,15
In high-income countries (HICs), the concept of NSPs for mental health care delivery has its own unique history, dating back to the paraprofessional movement in the United States and in the United Kingdom. More recently, NSPs have been successfully trained to address perinatal mental health in HIC contexts.16,17 Thus, NSPs may have the potential to address the startling treatment gap for depression18 and anxiety.19
In low- and middle-income countries, previous syntheses of NSP-delivered psychological interventions for perinatal populations11,20 have been conducted. Examining these processes and their effectiveness may be helpful to improve the implementation and scale-up of counseling interventions to potentially address the significant burden of perinatal depression and anxiety across HICs.
Our primary objective was to conduct a systematic review and meta-analysis to answer the following 2 questions: (1) What are the relevant implementation processes associated with the who (type of NSP), how (training and supervision), what (type of treatment), and where (type of setting) of NSP-delivered counseling interventions for perinatal depressive or anxiety symptoms in HICs? (2) Are NSP-delivered counseling interventions associated with effective prevention or treatment of depressive or anxiety symptoms among perinatal women residing in HICs?
Methods
Protocol Registration
This study used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline21 (eTable 1 in the Supplement) and followed the procedures of a recent review by members of our team of NSP-delivered interventions.11 This study is registered with PROSPERO.22
Search Strategy
A member of our team (B.A.K.) conducted the electronic search for articles, with no time or language restrictions. Literature sources included CINAHL, Ovid MEDLINE, Ovid MEDLINE In-Process, PsycINFO, Web of Science, Cochrane Central Register of Controlled Trials, and Embase through December 31, 2019. Bibliographies of 108 systematic reviews of psychological interventions for perinatal populations in HICs were also considered.23,24,25 Information was collected from primary trial articles and secondary articles (trial protocols, treatment development articles, or secondary analyses).
Eligibility Criteria
The inclusion criteria were as follows:
An HIC setting, defined by the World Bank Group in 2015 at the time of the trial,26 and exceptions made for Hong Kong and Taiwan;
The counseling intervention involved a 2-way interaction between an NSP therapist and a client that focused on changing one’s patterns and improving skills27;
A diagnosis or assessment using a validated tool in which symptoms of depression or anxiety were the primary or secondary outcome (after intervention);
Included pregnant or postpartum (up to 1 year after delivery) adult women; and
Evaluated through a randomized clinical trial (RCT).
The 2 exclusion criteria were self-help treatments without an NSP delivery component and published materials from books, conference papers, and theses.
Trial Selection and Data Extraction
Members of our team (J.W.J., Z.M., C.R., and N.Z.) systematically screened titles and abstracts to identify potentially eligible studies, of which full texts were then retrieved for further examination. A standardized data extraction form was used by those 4 team members to extract information regarding implementation processes (Box). Articles deemed ineligible or disagreements regarding eligibility were verified by another team member and, if needed, by the study leaders (D.R.S. and A.L.). The κ scores were calculated to estimate interrater reliability between researchers, resulting in a good score of κ = 0.75.
Box. Checklist of Extracted Key Implementation Processes.
Where?
Country
Geographical scope
-
Intervention setting
Rationale of intervention setting
Barriers and facilitators
Who?
-
Delivery agent
-
Who delivered the treatment?
Delivery agent rationale
-
-
Specialist
What was the role of the specialist?
-
Participants
Target population
Age
Marital status
No. of children
Sociodemographic variables (educational, race/ethnicity, and income levels)
Were other family members involved in the intervention?
What?
Treatment theory
Treatment rationale
How?
-
Treatment characteristics
Treatment delivery method
-
Overall duration of treatment
No. of sessions (intended and completed)
Duration of each session
Was there sustained delivery past end of trial?
-
Training
-
How were delivery agents trained?
Training content
Length of training
Competency evaluations
Treatment quality/fidelity assessment
-
-
Supervision
-
Who was the supervisor?
How was supervision conducted?
How frequently?
-
For the meta-analysis, we extracted mean (SD) values of the primary end points for both the intervention and the control groups and their respective sample sizes. When mean (SD) values were not available, we extracted the binary outcome data, with which we were able to estimate the effect size using an online calculator.28 For studies reporting median values and ranges, we estimated the effect size via a second online calculator.29 Effective sample sizes were used for all cluster trials, using reported or estimated intraclass correlation coefficients. When multiple control groups were available, data from active control groups were prioritized for extraction.
Assessment of Risk of Bias
The Cochrane Collaboration tool for assessing risk of bias30 was used by a minimum of 2 independent coauthors (Z.M., C.R., or N.Z.) to review the included studies (κ = 0.79). This included random sequence generation, allocation concealment, selective reporting, masking of research personnel and participants, masking of outcome assessors, attrition bias, and other biases. Studies meeting 3 or more high-risk criteria or missing details were considered low quality according to previously established criteria31 (eFigure 1 in the Supplement).
Statistical Analysis
We estimated the frequencies of all implementation processes, represented by a total or percentage, where the denominator was the total number of eligible trials, unless otherwise indicated (eg, when data were not specified or were missing for a particular variable). When possible, the mean was calculated along with the 95% CI. When ranges were provided for a particular variable (eg, 6-10 sessions), the mean was used (eg, 8 sessions). Outliers were identified, and analyses were repeated without these outliers.
For the meta-analyses, we used all available primary and secondary outcome data for perinatal depression, anxiety, or both for each trial. Analyses were performed using Review Manager, version 5.3,32 with the results presented as forest plots of standardized mean differences (SMDs), their 95% CIs, and relative weights calculated as the inverse of the variance and accounted for both original within-study variance and between-study variance τ.33 The SMDs were estimated using Hedges g,34 with between-group postintervention mean values.35 We used a random-effects analysis36 because of the expected heterogeneity. A test for subgroup differences was conducted comparing prevention and treatment trials, evidence-based (eg, interpersonal psychotherapy [IPT] and cognitive-behavioral therapy [CBT]) vs non–evidence-based interventions (supportive counseling); sample age demographic characteristics (adult only vs mixed adolescents and adults); and outcome measure (clinical diagnostic tool vs self-report). We conducted a post hoc sensitivity analysis using leave-1-out analyses to test the effect of excluding single trials that had the largest and smallest sample size and the largest and smallest effect sizes. This was conducted separately for treatment and prevention trials and for outcomes of interest (depression and anxiety).
Results
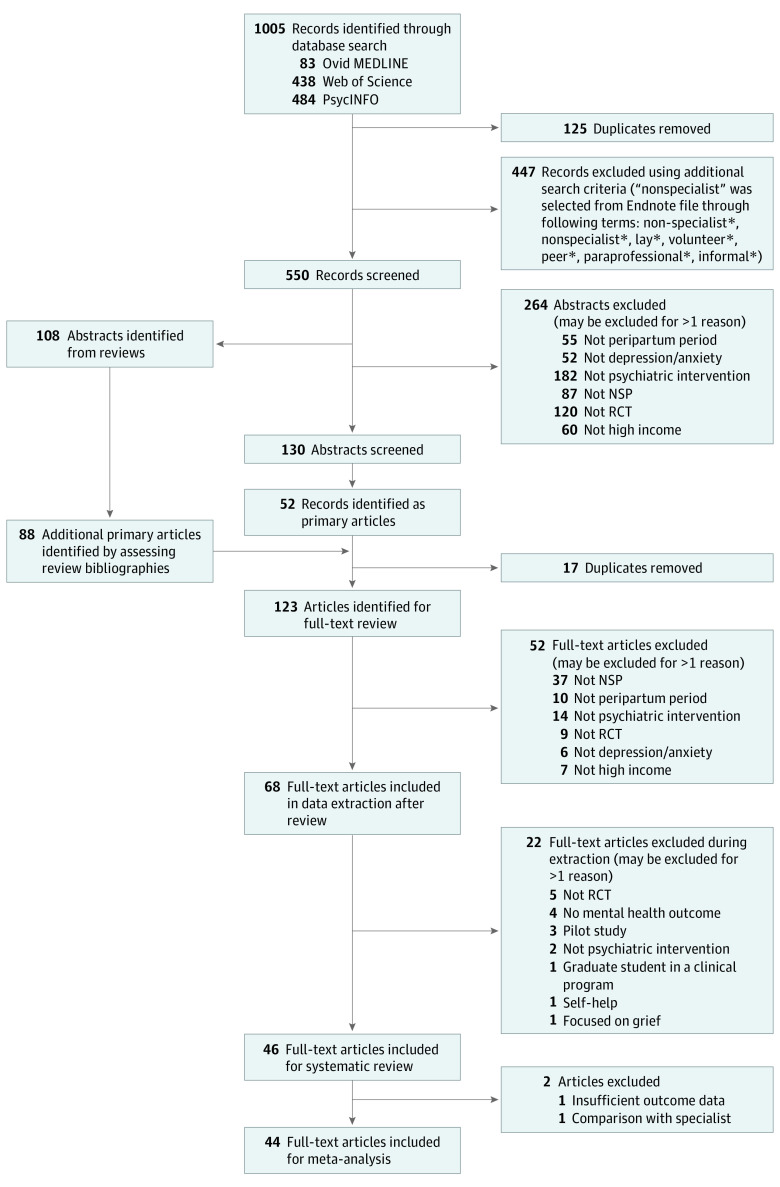
Figure 1 shows the flowchart of eligible articles. In total, 46 trials (18 321 participants) were included in the systematic review, and 44 trials (18 101 participants) were included in the meta-analysis. Two trials were excluded from the meta-analysis because the active control group included specialists37 and because of insufficiently reported outcomes.38
Figure 1. Flowchart for Identifying Eligible Articles.
NSP indicates nonspecialist provider; RCT, randomized clinical trial.
Trial Characteristics
Of the eligible trials (eTable 2 in the Supplement), 42 were individual RCTs, and 4 were cluster RCTs. The trials were conducted in 11 countries, including Australia (12 trials [26.1%]), the United Kingdom (10 trials [21.7%]), the United States (10 trials [21.7%]), Canada (3 trials [6.5%]), Scotland (3 trials [6.5%]), Sweden (2 trials [4.3%]), and Singapore (2 trials [4.3%]) and 1 trial (2.2%) each in Hong Kong, Finland, Norway, and Taiwan. Participants were recruited primarily from primary care settings (44 trials [95.7%]) followed by online methods (2 trials [4.3%]). The median trial sample size was 186 participants (range, 37-2064 participants). Participants were primarily from an urban population (31 trials [67.4%]) followed by semiurban (7 trials [15.2%]) and rural (2 trials [4.3%]) populations. Most participants were selected based on a self-report measure of depression (35 trials [76.1%]) rather than a diagnostic interview (11 trials [23.9%]). Most studies focused on the prevention of maternal mental health symptoms (28 trials [60.9%]), treatment (17 trials [37.0%]), or both (1 trial [2.2%]).
Delivery Agents, Specialists, and Participants
Delivery Agents
The most common type of NSP were midwives (16 trials) followed by nurses (14 trials), peers or community members (10 trials), health visitors (4 trials) or junior research staff (4 trials), occupational therapists (3 trials), family physicians (2 trials), and community health workers (1 trial), with at least 5 trials using a combination of NSP cadre previously mentioned. The NSPs were selected owing to their involvement with perinatal populations in an existing health care service (11 trials [23.9%]); however, most studies (35 [76.1%]) did not provide a rationale for NSP selection.
Specialists
The primary roles of mental health specialists included acting as a supervisor (16 trials [34.8%]), a trainer in the selected treatment (14 trials [30.4%]), or a research evaluator (12 trials [26.1%]) or providing referrals (4 trials [8.7%]).
Participants
The target population typically included general primary care attendees (31 trials [67.4%]), most of whom were recruited from obstetrical units (n = 21) if specified, primary care attendees who were at high risk (15 trials [32.6%]), or general perinatal populations outside of the hospital (2 trials [4.3%]). Primary care settings included obstetrical, family medicine, or mental health clinics or a ward within a hospital (31 trials [67.4%]), a collection of clinics within a certain area (8 trials [17.4%]), other community health programs (3 trials [6.5%]), general practitioners (2 trials [4.3%]), or an unspecified health-related program (1 trial [2.2%]). Those considered at high risk were identified based on a self-reported risk scale (7 of 15 trials [46.7%]) or by being part of a low-income group (5 trials [33.3%]) or an ethnic minority (3 trials [20%]), having a traumatic birth experience (2 trials [13.3%]), or having a history of mental illness (1 trial [6.7%]).
All studies included adult women between 18 and 45 years of age; however, 9 trials also included adolescent participants,39,40,41,42,43,44,45,46,47 some as young as 14 years. Marital status was reported in 34 trials, in which most participants were married (19 trials [55.9%]) but a sizable number were divorced or separated (14 trials [41.2%]). The mean (SD) number of children in reported studies (24 trials) was 1.88 (0.86) children per participant. Most studies did not report important socioeconomic variables, such as educational level (24 trials [52.2%]) or race/ethnicity (19 trials [41.3%]). Among studies that did, most participants had completed some form of secondary education (16 of 25 trials [64.0%]); the majority of the sample was categorized as White (12 of 20 trials [60.0%]) followed by Latinx (4 trials [20.0%]), Black (2 trials [10.0%]), and Asian (2 trials [10.0%]). Of 46 trials, 10 (21.7%) reported involving either the participant’s spouse or partner (5 [50.0%]) or her child (5 [50.0%]).
Intervention Content
Most interventions were described as supportive counseling (18 of 46 trials [39.1%]) or as an evidence-based psychological treatment (17 trials [37.0%]), such as CBT (n = 12), IPT (n = 3), or behavioral activation (n = 2) or as some combination of psychoeducation related to maternal mental health and parenting and self-efficacy (10 trials [21.7%]) or stress debriefing (2 trials [4.3%]). The rationale for selecting a particular treatment modality was identified in 26 trials (56.5%) and included being a contextually relevant treatment (7 of 26 trials [26.9%]), maintaining maternal or child health during or after pregnancy (6 trials [23.1%]), providing support (5 trials [19.2%]), using an evidence-based treatment (4 trials [15.4%]), being cost-effective (2 trials [7.7%]), reaching at-risk groups (n = 2), and deploying existing resources (n = 2).
Intervention Setting
The intervention setting was reported in 38 trials. More than half the trials were conducted within nonmental health primary care settings (20 of 38 trials [52.6%]), including child health or obstetric clinics, general practice, and other locations within the hospital, at home or by telephone (16 trials [42.1%]), online (1 trial [2.6%]), or within (1 trial [2.6%]) the community. In the minority of trials that mentioned why the particular setting was selected (n = 6), patient centeredness and flexibility were listed (4 [66.7%]), followed by feasibility (2 [33.3%]). For those trials that reported barriers in current care (n = 5), they reported an intervention delivered via telephone when it was intended for in-person treatment (1 [20%]), language barriers (1 [20%]), attitudinal barriers such as stigma (1 [20%]), or preference for in-person sessions (2 [40%]). Facilitators (n = 2) included the provision of food for the study participant and peer connection.
Intervention Delivery and Monitoring
Treatment Characteristics
Most treatments were delivered face to face (31 trials [67.4%]), through a combination of face to face and telephone (10 trials [21.7%]), or via telephone only (3 trials [6.5%]). Only 1 study (2.2%) delivered treatment via the internet,48 and 1 other study (2.2%) delivered treatment via a combination of the internet (email and communication applications) and the telephone.49 Almost all studies (42 trials [91.3%]) reported whether a group or individual format was used, with most delivering treatment individually (30 of 42 trials [71.4%]) or in group formats (12 trials [28.6%]). Treatments lasted a mean of 11.2 weeks (95% CI, 6.4-16.0 weeks), with a mean of 5.9 (95% CI, 4.9-7.0) intended sessions compared with a mean of 4.8 (95% CI, 3.8-5.8) actual sessions completed; however, this information was only reported by half of the eligible trials (n = 23). In 6 of 23 studies (26.1%) reporting treatment dosage, the number of sessions was variable, determined by the NSP and not specified.
Training and Supervision
Training methods were only reported for half of the trials (23 [50%]). Most trials reportedly used didactic practices (12 of 23 [52.2%]) or a mix of didactics and practice approaches (9 [39.1%]). The duration of training was reported in 15 of 46 trials (32.6%). Training typically lasted between 0.5 and 5 days (10 trials [66.7%]), but 3 trials (20%) indicated that training lasted between 1 week and 1 month,38,43,50 1 trial (6.7%)51 reported 3 months’ training, and 1 trial (6.7%)52 conducted training that lasted a year, in which the NSPs received training during a year-long counseling course. Only 8 trials reported an assessment of treatment quality through fidelity ratings, and only 2 trials mentioned a requirement of a competency evaluation.
Supervision methods were reported by only 6 of 46 trials (13.0%). Most of those methods involved observing sessions (3 trials [50%]), listening to audio-recorded sessions (1 trial [16.7%]), or both (1 trial [16.7%]) or providing consultation on an ad hoc basis (1 trial [16.7%]). Only 4 studies reported using a supervision format, including group supervision (1 of 4 [25%]), individual supervision (2 [50%]), or a combination of both (1 [25%]). Supervision frequency was reported in 8 trials and was conducted weekly (5 trials [62.5%]) or biweekly (1 trial [12.5%]) or the frequency varied (2 trials [25.0%]). Supervision was typically provided by a mental health expert, such as a psychiatrist or psychologist. No trials used peer supervision models.
Control groups were described by 44 of 46 (95.6%) trials. Most trials reported using treatment as usual or routine care without specifying what either involved (25 of 44 [56.8%]), treatment as usual with routine home or clinic visits (10 [22.7%]), treatment as usual with provision of community resources and referrals (4 [9.1%]), and treatment as usual with provision of postpartum education (2 [4.5%]). Of 44 trials, 3 (6.8%) reported delivering support to the control group, including peer support (1 [2.3%]),43 a single debriefing session (1 [2.3%]),53 and interpersonal therapy by specialists (1 [2.3%]).37
Effectiveness of NSP-Delivered Interventions
We included 44 trials in the meta-analysis. The most common outcome assessment tool used for the primary outcome of depression was the Edinburgh Postnatal Depression Scale,54 which was used in 30 of 44 trials (68.2%); 7 trials (15.9%) included a diagnostic interview. In 11 trials, anxiety was assessed using a self-report measure, such as the State-Trait Anxiety Inventory55 (4 trials [36.4%]), the Generalized Anxiety Disorder 7-item scale56 (2 trials [18.2%]), or the Depression Anxiety Stress Scales57 (3 trials [27.3%]). Compared with controls, counseling interventions were associated with lower depressive symptoms (SMD, 0.24 [95% CI, 0.14-0.34]; 43 trials; I2 = 81%) and anxiety scores (SMD, 0.30 [95% CI, 0.11-0.50]; 11 trials; I2 = 80%). However, heterogeneity was high among the trials included in this analysis.
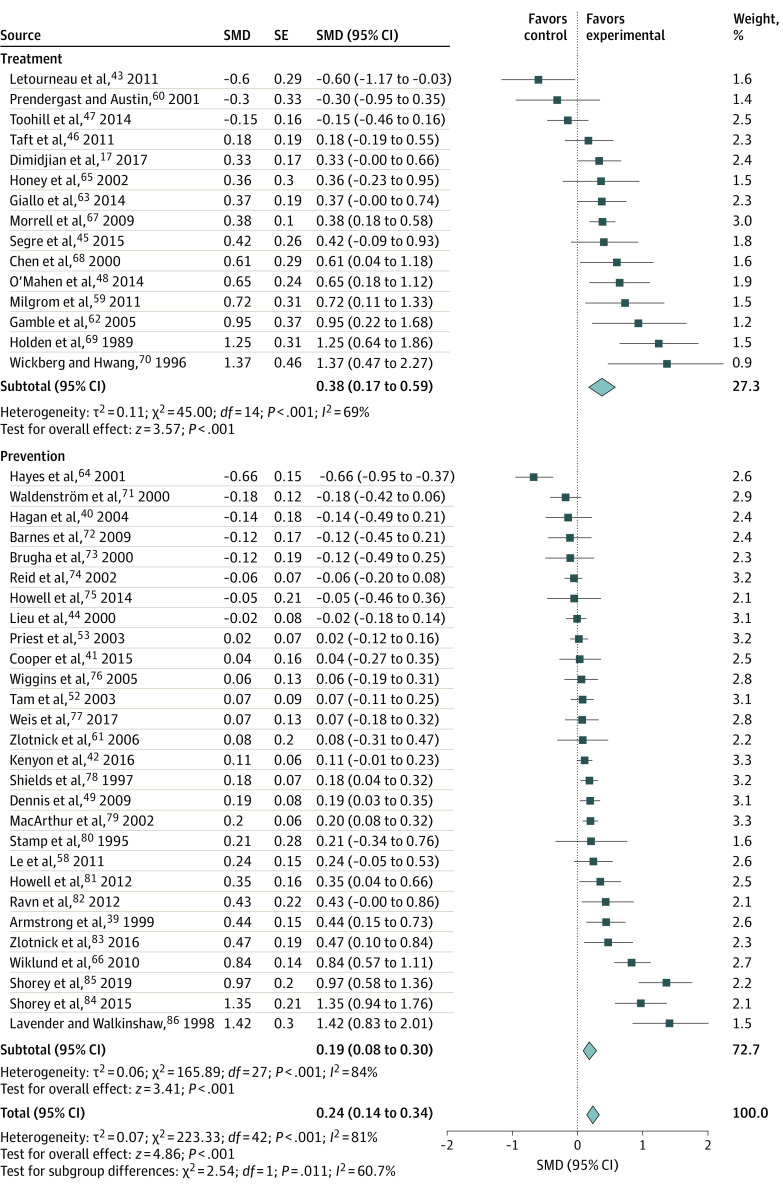
Figure 2 presents the forest plot of the effectiveness analyses for 15 trials17,32,39,40,42,43,44,45,46,47,48,49,52,53,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86 that focused on treatment of depression as the primary or secondary outcome. In those trials, the SMD was 0.38 (95% CI, 0.17-0.59; I2 = 69%). Figure 2 also presents the forest plot of the effectiveness analysis for 28 trials focused on prevention that reported depression as a primary or secondary outcome. For those trials, the SMD was 0.19 (95% CI, 0.08-0.30), favoring the intervention, with the inconsistency measure (I2 = 81%) suggesting substantial heterogeneity among the trials.
Figure 2. Effectiveness of Counseling Interventions on Depression, Stratified by Treatment and Prevention.
Random-effects models with inverse variance weighting were used. Each square shows the effect size for a single study with the horizontal error bars representing the width of the 95% CI. Each diamond shows the summary effect size, with the diamond width indicating the overall 95% CIs. SMD indicates standardized mean difference.
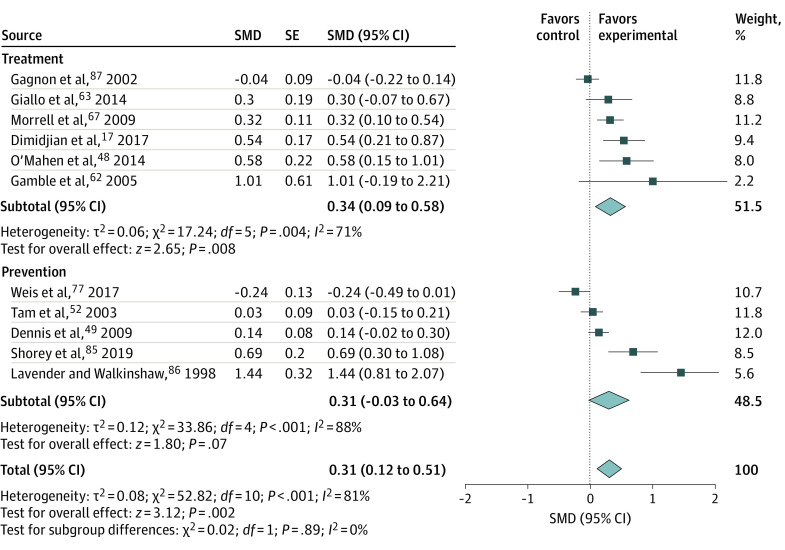
Figure 317,48,49,52,62,63,67,77,85,86,87 presents the effectiveness analyses for 6 trials focusing on treatment of anxiety as the primary or secondary outcome. For those trials, the SMD was 0.34 (95% CI, 0.09-0.58; I2 = 71%). Figure 3 also presents the effectiveness analyses for 5 trials focusing on prevention that reported anxiety as a primary or secondary outcome. For those trials, the SMD was 0.31 (95% CI, −0.03 to 0.64; I2 = 88%). The effective sample sizes and mean (SD) values for all studies are given in eTable 3 in the Supplement. Leave-1-out analyses are presented in the eAppendix in the Supplement.
Figure 3. Effectiveness of Counseling Interventions on Anxiety, Stratified by Treatment and Prevention.
Random-effects models with inverse variance weighting were used. Each square shows the effect size for a single study with the horizontal error bars representing the width of the 95% CI. Each diamond shows the summary effect size, with the diamond width indicating the overall 95% CIs. SMD indicates standardized mean difference.
The Table provides the SMD, 95% CIs, and high heterogeneity estimates (I2) for all trials and for subgroup analyses, by condition, treatment vs prevention, evidence-based treatment, sample age, and outcome measure. There were no statistically significant differences when comparing diagnostic vs self-reported outcomes (eFigure 2 in the Supplement); however, there were stronger effect sizes for evidence-based treatments (eg, CBT, IPT, and BA) compared with non–evidence-based treatments (eg, supportive counseling). Trials including mixed age samples (both adolescents and adults) showed no treatment benefit for depression, whereas samples of only adults showed significant benefit of treatment for depression (eFigure 3 in the Supplement).
Table. Effect Sizes and Heterogeneity Estimates for All 44 Trials and Grouped by Outcome, Intervention Type, Evidence-Based Treatment, Age, and Measurement Type for the Primary Mental Health Outcome With Subgroup Comparisons.
| Outcome and comparison | Standardized mean difference (95% CI) | I2, % | Subgroup comparisons | ||
|---|---|---|---|---|---|
| χ2 | P value | I2, % | |||
| Comparisons of depression vs anxiety by prevention or treatment | |||||
| Depression only | |||||
| Prevention or treatment (n = 44) | 0.24 (0.14 to 0.34) | 81 | 0.30 | .59 | 0 |
| Anxiety only | |||||
| Prevention or treatment (n = 11) | 0.30 (0.11 to 0.50) | 80 | |||
| Depression or anxiety | |||||
| Treatment only (n = 16) | 0.34 (0.14 to 0.55) | 74 | 1.75 | .19 | 42.9 |
| Prevention only (n = 28) | 0.19 (0.08 to 0.30) | 84 | |||
| Depression only | |||||
| Treatment only (n = 15)a | 0.38 (0.17 to 0.59) | 69 | 0.16 | .69 | 0 |
| Anxiety only | |||||
| Treatment only (n = 6)b | 0.32 (0.07 to 0.56) | 68 | |||
| Depression only | |||||
| Prevention only (n = 28)a | 0.19 (0.08 to 0.30) | 84 | 0.41 | .52 | 0 |
| Anxiety only | |||||
| Prevention only (n = 5)b | 0.31 (−0.03 to 0.64) | 88 | |||
| Comparisons of evidence-based vs non–evidence-based treatments | |||||
| Depression or anxiety/prevention or treatment | |||||
| Evidence-based treatment (n = 15) | 0.30 (0.08 to 0.52) | 82 | 0.63 | .43 | 0 |
| Non–evidence-based treatment (n = 29) | 0.20 (0.10 to 0.30) | 80 | |||
| Depression treatment only | |||||
| Evidence-based treatment (n = 8) | 0.43 (0.30 to 0.56) | 0 | 0.32 | .57 | 0 |
| Non–evidence-based treatment (n = 7) | 0.29 (−0.18 to 0.75) | 82 | |||
| Depression prevention only | |||||
| Evidence-based treatment (n = 7) | 0.20 (−0.23 to 0.63) | 92 | 0.06 | .81 | 0 |
| Non–evidence-based treatment (n = 21) | 0.27 (−0.04 to 0.57) | 99 | |||
| Comparisons of mixed age samples (adolescent and adult) vs adult only samples | |||||
| Depression or anxiety/prevention or treatment | |||||
| Mixed ages (n = 9) | 0.05 (−0.09 to 0.20) | 57 | 6.43 | .01 | 84.4 |
| Adults (n = 35) | 0.29 (0.18 to 0.41) | 83 | |||
| Comparison of diagnostic interviews vs self-report outcome measures | |||||
| Depression or anxiety/prevention or treatment | |||||
| Diagnostic interview (n = 7) | 0.20 (−0.8 to 0.47) | 62 | 0.05 | .83 | 0 |
| Self-report (n = 43) | 0.23 (0.13 to 0.32) | 81 | |||
Subgroup comparison for depression treatment vs prevention: χ2 = 2.54, P = .11, I2 = 60.7%.
Subgroup comparison for anxiety treatment vs prevention: χ2 = 0.2, P = .89, I2 = 0%.
A systematic assessment of risk bias was conducted (eTable 2 and eFigure 1 in the Supplement). We found low risk of bias on randomization and outcome blinding. Although the most commonly used random allocation method was opaque envelopes containing computer-generated random numbers, details related to allocation concealment and masking of participants and personnel were frequently lacking.
Discussion
The present study examined the implementation processes and effectiveness of counseling interventions delivered by NSPs for perinatal depression and for anxiety in HICs. Our results highlight findings relevant to the delivery and scalability of effective NSP-delivered interventions.
First, the present study found an impressive and growing evidence base of 46 RCTs examining NSP-delivered interventions for perinatal populations, highlighting the importance of task sharing in HIC contexts. Since having conducted our search, there has been at least 1 RCT showing the effectiveness of NSP-delivered counseling interventions in perinatal populations.88 We highlight that NSPs can be trained to fulfill an important gap in the provision of effective psychological interventions for both depression and anxiety treatments. Consistent with the wider literature,89 effect sizes were stronger for treatments compared with preventive interventions.
Second, most studies included herein trained nurses and midwives to deliver counseling interventions. These findings are consistent with a recent qualitative study that independently found that nurses and midwives were considered to be the most preferred nonspecialist provider to deliver counseling interventions for perinatal interventions.90 This reflects the contextual reality of HICs, with nurses and midwives being frontline workers who can provide adequate and effective mental health care to perinatal populations.
Third, we found stronger effect sizes for evidence-based treatments (eg, CBT, IPT, and behavioral activation) compared with non–evidence-based treatments (eg, supportive counseling). This is similar to other analyses that have suggested CBT and IPT are superior to other interventions in both treatment6 and prevention91 of perinatal depression and anxiety. Considering our findings that NSPs can effectively deliver manualized evidence-based psychotherapies, such as CBT and IPT,17,37,38,40,45,48,58,59,60,61,62,63,64,65,66 these approaches should be advocated for NSPs, just as they are for specialists. Although “supportive” interventions may be simpler for training, further research is needed to expand the evidence base for these approaches.
Fourth, similar to effective NSP-delivered interventions for perinatal populations in low- and middle-income countries,11 we found that most studies relied on conventional face-to-face methods for intervention delivery, training, and supervision. No trial used peer supervision methods despite their potential to address the bottleneck imposed by relying on expert supervisors.92 Digital platforms for intervention delivery, the provision of training and supervision, and the demonstration of the reliability and validity of peer supervision all offer potential solutions to facilitate scaling up of quality-ensured interventions. This is particularly relevant during the ongoing coronavirus pandemic in which one of the most important lessons is how much of our daily roles and activities can be moved to digital platforms. To address the burden of perinatal depression and anxiety, it will be essential to offer and assess evidence-based counseling interventions through digital platforms and for RCTs to compare their relative effectiveness with traditional in-person models of delivery and supervision.
Limitations
In addition to the high rates of heterogeneity observed among the included trials, a major limitation of this study is the dearth of relevant indicators related to important implementation processes reported by authors. For example, only half of eligible trials reported treatment dosages and less than 15% of eligible trials reported key processes related to supervision. We recommend that authors of trials systematically report key implementation details (Box), as has been proposed for other public health, behavior change interventions.93 Our review was limited to samples composed of adults, which included some studies with mixed adult and adolescent populations. We found no observed benefit for studies that included both adolescents and adults compared with adult-only studies. This highlights the need for future reviews of NSP-delivered interventions for adolescents, a highly vulnerable group for perinatal depression and anxiety.
Conclusions
In sum, this study synthesizes a compelling evidence base that suggests that NSPs effectively deliver preventive and treatment interventions to manage perinatal depression and anxiety symptoms in HICs. The potential for such approaches is now widely accepted for mental health care globally94 and increasingly being advocated for in high-resource contexts.13 This delivery strategy may address one of the most significant gaps in mental health care (ie, access to evidence-based counseling interventions) to influence perinatal populations worldwide.
eTable 1. PRISMA Checklist
eFigure 1. Risk Assessment
eTable 2. Trial Characteristics (N = 46)
eTable 3. Data Used to Calculate the Standardized Mean Difference and Standard Error for Each Outcome From Each Study, N = 43
eFigure 2. Forest Plot Examining a Subgroup Analysis of Outcome Tool (Diagnostic vs Self-Assessment)
eFigure 3. Forest Plot Examining a Subgroup Analysis of Age (Mixed Age vs Adult)
eAppendix. Leave-One-Out Analyses
eReferences
References
- 1.Falah-Hassani K, Shiri R, Dennis C-L. Prevalence and risk factors for comorbid postpartum depressive symptomatology and anxiety. J Affect Disord. 2016;198:142-147. doi: 10.1016/j.jad.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 2.O'hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37-54. doi: 10.3109/09540269609037816 [DOI] [Google Scholar]
- 3.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315-323. doi: 10.1192/bjp.bp.116.187179 [DOI] [PubMed] [Google Scholar]
- 4.Wisner KL, Sit DK, McShea MC, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490-498. doi: 10.1001/jamapsychiatry.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer A, Knapp M, Parsonage M. Lifetime costs of perinatal anxiety and depression. J Affect Disord. 2016;192:83-90. doi: 10.1016/j.jad.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 6.Sockol LE, Epperson CN, Barber JP. A meta-analysis of treatments for perinatal depression. Clin Psychol Rev. 2011;31(5):839-849. doi: 10.1016/j.cpr.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. 2013;9:379-407. doi: 10.1146/annurev-clinpsy-050212-185612 [DOI] [PubMed] [Google Scholar]
- 8.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(6):580-587. doi: 10.1001/jama.2019.0007 [DOI] [PubMed] [Google Scholar]
- 9.Byatt N, Xiao RS, Dinh KH, Waring ME. Mental health care use in relation to depressive symptoms among pregnant women in the USA. Arch Womens Ment Health. 2016;19(1):187-191. doi: 10.1007/s00737-015-0524-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization, PEPFAR & UNAIDS. Task Shifting: Rational Redistribution of Tasks Among Health Workforce Teams: Global Recommendations and Guidelines. World Health Organization; 2007. [Google Scholar]
- 11.Singla DR, Kohrt BA, Murray LK, Anand A, Chorpita BF, Patel V. Psychological treatments for the world: lessons from low- and middle-income countries. Annu Rev Clin Psychol. 2017;13(1):149-181. doi: 10.1146/annurev-clinpsy-032816-045217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeft TJ, Fortney JC, Patel V, Unützer J. Task-sharing approaches to improve mental health care in rural and other low-resource settings: a systematic review. J Rural Health. 2018;34(1):48-62. doi: 10.1111/jrh.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett ML, Lau AS, Miranda J. Lay health worker involvement in evidence-based treatment delivery: a conceptual model to address disparities in care. Annu Rev Clin Psychol. 2018;14:185-208. doi: 10.1146/annurev-clinpsy-050817-084825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singla D, Lazarus A, Atif N, et al. “Someone like us”: delivering maternal mental health through peers in two South Asian contexts. J Affect Disord. 2014;168:452-458. doi: 10.1016/j.jad.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Mahen HA, Grieve H, Jones J, McGinley J, Woodford J, Wilkinson EL. Women's experiences of factors affecting treatment engagement and adherence in internet delivered behavioural activation for postnatal depression. Internet Interventions. 2015;2(1):84-90. doi: 10.1016/j.invent.2014.11.003 [DOI] [Google Scholar]
- 16.Glavin K, Smith L, Sørum R, Ellefsen B. Supportive counselling by public health nurses for women with postpartum depression. J Adv Nurs. 2010;66(6):1317-1327. doi: 10.1111/j.1365-2648.2010.05263.x [DOI] [PubMed] [Google Scholar]
- 17.Dimidjian S, Goodman SH, Sherwood NE, et al. A pragmatic randomized clinical trial of behavioral activation for depressed pregnant women. J Consult Clin Psychol. 2017;85(1):26-36. doi: 10.1037/ccp0000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornicroft G, Chatterji S, Evans-Lacko S, et al. Undertreatment of people with major depressive disorder in 21 countries. Br J Psychiatry. 2017;210(2):119-124. doi: 10.1192/bjp.bp.116.188078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso J, Liu Z, Evans-Lacko S, et al. ; WHO World Mental Health Survey Collaborators . Treatment gap for anxiety disorders is global: results of the World Mental Health Surveys in 21 countries. Depress Anxiety. 2018;35(3):195-208. doi: 10.1002/da.22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman A, Fisher J, Bower P, et al. Interventions for common perinatal mental disorders in women in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(8):593-601I. doi: 10.2471/BLT.12.109819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The effectiveness and underlying implementation processes of non-specialist-delivered interventions for perinatal mental health: a critical review and meta-analysis. PROSPERO identifier: CRD42018090124. Updated May 23, 2018. Accessed December 14, 2020. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=90124
- 23.Dennis CL, Hodnett E. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev. 2007;(4):CD006116. doi: 10.1002/14651858.CD006116.pub2 [DOI] [PubMed] [Google Scholar]
- 24.Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. Cochrane Database Syst Rev. 2007;(3):CD006309. doi: 10.1002/14651858.CD006309 [DOI] [PubMed] [Google Scholar]
- 25.O’Connor E, Senger CA, Henninger ML, Coppola E, Gaynes BN. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(6):588-601. doi: 10.1001/jama.2018.20865 [DOI] [PubMed] [Google Scholar]
- 26.The World Bank. World Bank country and lending groups. Published 2016. Accessed August 1, 2016. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 27.Cuijpers P, Karyotaki E, Reijnders M, Purgato M, Barbui C. Psychotherapies for depression in low- and middle-income countries: a meta-analysis. World Psychiatry. 2018;17(1):90-101. doi: 10.1002/wps.20493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson DB. George Mason University. Practical meta-analysis effect size calculator. Accessed December 14, 2020. https://campbellcollaboration.org/escalc/html/EffectSizeCalculator-SMD10.php
- 29.VassarStats . Website for statistical computation. Accessed December 14, 2020. http://vassarstats.net/
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: a meta-analysis. Clin Psychol Rev. 2014;34(2):130-140. doi: 10.1016/j.cpr.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 32.Cochrane Training. Cochrane Review Manager RevMan. Accessed August 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman
- 33.Meta-analysis.com . Meta-analysis: fixed effect vs. random effects. Published 2007. Accessed August 2020. https://www.meta-analysis.com/downloads/M-a_f_e_v_r_e_sv.pdf
- 34.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Statistics. 1981;6(2):107-128. doi: 10.2307/1164588 [DOI] [Google Scholar]
- 35.Cuijpers P, Weitz E, Cristea IA, Twisk J. Pre-post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci. 2017;26(4):364-368. doi: 10.1017/S2045796016000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37.Field T, Diego M, Delgado J, Medina L. Peer support and interpersonal psychotherapy groups experienced decreased prenatal depression, anxiety and cortisol. Early Hum Dev. 2013;89(9):621-624. doi: 10.1016/j.earlhumdev.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saisto T, Salmela-Aro K, Nurmi JE, Könönen T, Halmesmäki E. A randomized controlled trial of intervention in fear of childbirth. Obstet Gynecol. 2001;98(5, pt 1):820-826. doi: 10.1016/s0029-7844(01)01552-6 [DOI] [PubMed] [Google Scholar]
- 39.Armstrong KL, Fraser JA, Dadds MR, Morris J. A randomized, controlled trial of nurse home visiting to vulnerable families with newborns. J Paediatr Child Health. 1999;35(3):237-244. doi: 10.1046/j.1440-1754.1999.00348.x [DOI] [PubMed] [Google Scholar]
- 40.Hagan R, Evans SF, Pope S. Preventing postnatal depression in mothers of very preterm infants: a randomised controlled trial. BJOG. 2004;111(7):641-647. doi: 10.1111/j.1471-0528.2004.00165.x [DOI] [PubMed] [Google Scholar]
- 41.Cooper PJ, De Pascalis L, Woolgar M, Romaniuk H, Murray L. Attempting to prevent postnatal depression by targeting the mother-infant relationship: a randomised controlled trial. Prim Health Care Res Dev. 2015;16(4):383-397. doi: 10.1017/S1463423614000401 [DOI] [PubMed] [Google Scholar]
- 42.Kenyon S, Jolly K, Hemming K, et al. Lay support for pregnant women with social risk: a randomised controlled trial. BMJ Open. 2016;6(3):e009203. doi: 10.1136/bmjopen-2015-009203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letourneau N, Stewart M, Dennis CL, Hegadoren K, Duffett-Leger L, Watson B. Effect of home-based peer support on maternal-infant interactions among women with postpartum depression: a randomized, controlled trial. Int J Ment Health Nurs. 2011;20(5):345-357. doi: 10.1111/j.1447-0349.2010.00736.x [DOI] [PubMed] [Google Scholar]
- 44.Lieu TA, Braveman PA, Escobar GJ, Fischer AF, Jensvold NG, Capra AM. A randomized comparison of home and clinic follow-up visits after early postpartum hospital discharge. Pediatrics. 2000;105(5):1058-1065. doi: 10.1542/peds.105.5.1058 [DOI] [PubMed] [Google Scholar]
- 45.Segre LS, Brock RL, O’Hara MW. Depression treatment for impoverished mothers by point-of-care providers: a randomized controlled trial. J Consult Clin Psychol. 2015;83(2):314-324. doi: 10.1037/a0038495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taft AJ, Small R, Hegarty KL, Watson LF, Gold L, Lumley JA. Mothers’ AdvocateS In the Community (MOSAIC)—non-professional mentor support to reduce intimate partner violence and depression in mothers: a cluster randomised trial in primary care. BMC Public Health. 2011;11(1):178. doi: 10.1186/1471-2458-11-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toohill J, Fenwick J, Gamble J, et al. A randomized controlled trial of a psycho-education intervention by midwives in reducing childbirth fear in pregnant women. Birth. 2014;41(4):384-394. doi: 10.1111/birt.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Mahen HA, Richards DA, Woodford J, et al. Netmums: a phase II randomized controlled trial of a guided internet behavioural activation treatment for postpartum depression. Psychol Med. 2014;44(8):1675-1689. doi: 10.1017/S0033291713002092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis CL, Hodnett E, Kenton L, et al. Effect of peer support on prevention of postnatal depression among high risk women: multisite randomised controlled trial. BMJ. 2009;338:a3064. doi: 10.1136/bmj.a3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong KL, Fraser JA, Dadds MR, Morris J. Promoting secure attachment, maternal mood and child health in a vulnerable population: a randomized controlled trial. J Paediatr Child Health. 2000;36(6):555-562. doi: 10.1046/j.1440-1754.2000.00591.x [DOI] [PubMed] [Google Scholar]
- 51.Cooper AA, Conklin LR. Dropout from individual psychotherapy for major depression: a meta-analysis of randomized clinical trials. Clin Psychol Rev. 2015;40:57-65. doi: 10.1016/j.cpr.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 52.Tam WH, Lee DT, Chiu HF, Ma KC, Lee A, Chung TK. A randomised controlled trial of educational counselling on the management of women who have suffered suboptimal outcomes in pregnancy. BJOG. 2003;110(9):853-859. doi: 10.1111/j.1471-0528.2003.02412.x [DOI] [PubMed] [Google Scholar]
- 53.Priest SR, Henderson J, Evans SF, Hagan R. Stress debriefing after childbirth: a randomised controlled trial. Med J Aust. 2003;178(11):542-545. doi: 10.5694/j.1326-5377.2003.tb05355.x [DOI] [PubMed] [Google Scholar]
- 54.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 55.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Scale. Consulting Psychologists; 1983. [Google Scholar]
- 56.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 57.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343. doi: 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- 58.Le H-N, Perry DF, Stuart EA. Randomized controlled trial of a preventive intervention for perinatal depression in high-risk Latinas. J Consult Clin Psychol. 2011;79(2):135-141. doi: 10.1037/a0022492 [DOI] [PubMed] [Google Scholar]
- 59.Milgrom J, Holt CJ, Gemmill AW, et al. Treating postnatal depressive symptoms in primary care: a randomised controlled trial of GP management, with and without adjunctive counselling. BMC Psychiatry. 2011;11(1):95. doi: 10.1186/1471-244X-11-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prendergast J, Austin MP. Early childhood nurse-delivered cognitive behavioural counselling for post-natal depression. Australasian Psychiatry. 2001;9(3):255-259. doi: 10.1046/j.1440-1665.2001.00330.x [DOI] [Google Scholar]
- 61.Zlotnick C, Miller IW, Pearlstein T, Howard M, Sweeney P. A preventive intervention for pregnant women on public assistance at risk for postpartum depression. Am J Psychiatry. 2006;163(8):1443-1445. doi: 10.1176/ajp.2006.163.8.1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gamble J, Creedy D, Moyle W, Webster J, McAllister M, Dickson P. Effectiveness of a counseling intervention after a traumatic childbirth: a randomized controlled trial. Birth. 2005;32(1):11-19. doi: 10.1111/j.0730-7659.2005.00340.x [DOI] [PubMed] [Google Scholar]
- 63.Giallo R, Cooklin A, Dunning M, Seymour M. The efficacy of an intervention for the management of postpartum fatigue. J Obstet Gynecol Neonatal Nurs. 2014;43(5):598-613. doi: 10.1111/1552-6909.12489 [DOI] [PubMed] [Google Scholar]
- 64.Hayes BA, Muller R, Bradley BS. Perinatal depression: a randomized controlled trial of an antenatal education intervention for primiparas. Birth. 2001;28(1):28-35. doi: 10.1046/j.1523-536x.2001.00028.x [DOI] [PubMed] [Google Scholar]
- 65.Honey KL, Bennett P, Morgan M. A brief psycho-educational group intervention for postnatal depression. Br J Clin Psychol. 2002;41(pt 4):405-409. doi: 10.1348/014466502760387515 [DOI] [PubMed] [Google Scholar]
- 66.Wiklund I, Mohlkert P, Edman G. Evaluation of a brief cognitive intervention in patients with signs of postnatal depression: a randomized controlled trial. Acta Obstet Gynecol Scand. 2010;89(8):1100-1104. doi: 10.3109/00016349.2010.500369 [DOI] [PubMed] [Google Scholar]
- 67.Morrell CJ, Slade P, Warner R, et al. Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: pragmatic cluster randomised trial in primary care. BMJ. 2009;338:a3045. doi: 10.1136/bmj.a3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CH, Tseng YF, Chou FH, Wang SY. Effects of support group intervention in postnatally distressed women: a controlled study in Taiwan. J Psychosom Res. 2000;49(6):395-399. doi: 10.1016/S0022-3999(00)00180-X [DOI] [PubMed] [Google Scholar]
- 69.Holden JM, Sagovsky R, Cox JL. Counselling in a general practice setting: controlled study of health visitor intervention in treatment of postnatal depression. BMJ. 1989;298(6668):223-226. doi: 10.1136/bmj.298.6668.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord. 1996;39(3):209-216. doi: 10.1016/0165-0327(96)00034-1 [DOI] [PubMed] [Google Scholar]
- 71.Waldenström U, Brown S, McLachlan H, Forster D, Brennecke S. Does team midwife care increase satisfaction with antenatal, intrapartum, and postpartum care? a randomized controlled trial. Birth. 2000;27(3):156-167. doi: 10.1046/j.1523-536x.2000.00156.x [DOI] [PubMed] [Google Scholar]
- 72.Barnes J, Senior R, MacPherson K. The utility of volunteer home-visiting support to prevent maternal depression in the first year of life. Child Care Health Dev. 2009;35(6):807-816. doi: 10.1111/j.1365-2214.2009.01007.x [DOI] [PubMed] [Google Scholar]
- 73.Brugha TS, Wheatley S, Taub NA, et al. Pragmatic randomized trial of antenatal intervention to prevent post-natal depression by reducing psychosocial risk factors. Psychol Med. 2000;30(6):1273-1281. doi: 10.1017/S0033291799002937 [DOI] [PubMed] [Google Scholar]
- 74.Reid M, Glazener C, Murray GD, Taylor GS. A two-centred pragmatic randomised controlled trial of two interventions of postnatal support. BJOG. 2002;109(10):1164-1170. doi: 10.1111/j.1471-0528.2002.01306.x [DOI] [PubMed] [Google Scholar]
- 75.Howell EA, Bodnar-Deren S, Balbierz A, et al. An intervention to reduce postpartum depressive symptoms: a randomized controlled trial. Arch Womens Ment Health. 2014;17(1):57-63. doi: 10.1007/s00737-013-0381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiggins M, Oakley A, Roberts I, et al. Postnatal support for mothers living in disadvantaged inner city areas: a randomised controlled trial. J Epidemiol Community Health. 2005;59(4):288-295. doi: 10.1136/jech.2004.021808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weis KL, Lederman RP, Walker KC, Chan W. Mentors offering maternal support reduces prenatal, pregnancy-specific anxiety in a sample of military women. J Obstet Gynecol Neonatal Nurs. 2017;46(5):669-685. doi: 10.1016/j.jogn.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 78.Shields N, Reid M, Cheyne H, et al. Impact of midwife-managed care in the postnatal period: an exploration of psychosocial outcomes. J Reprod Infant Psychol. 1997;15(2):91-108. doi: 10.1080/02646839708404537 [DOI] [Google Scholar]
- 79.MacArthur C, Winter HR, Bick DE, et al. Effects of redesigned community postnatal care on womens’ health 4 months after birth: a cluster randomised controlled trial. Lancet. 2002;359(9304):378-385. doi: 10.1016/S0140-6736(02)07596-7 [DOI] [PubMed] [Google Scholar]
- 80.Stamp GE, Williams AS, Crowther CA. Evaluation of antenatal and postnatal support to overcome postnatal depression: a randomized, controlled trial. Birth. 1995;22(3):138-143. doi: 10.1111/j.1523-536X.1995.tb00689.x [DOI] [PubMed] [Google Scholar]
- 81.Howell EA, Balbierz A, Wang J, Parides M, Zlotnick C, Leventhal H. Reducing postpartum depressive symptoms among black and Latina mothers: a randomized controlled trial. Obstet Gynecol. 2012;119(5):942-949. doi: 10.1097/AOG.0b013e318250ba48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ravn IH, Smith L, Smeby NA, et al. Effects of early mother-infant intervention on outcomes in mothers and moderately and late preterm infants at age 1 year: a randomized controlled trial. Infant Behav Dev. 2012;35(1):36-47. doi: 10.1016/j.infbeh.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 83.Zlotnick C, Tzilos G, Miller I, Seifer R, Stout R. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affect Disord. 2016;189:263-268. doi: 10.1016/j.jad.2015.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shorey S, Chan SW, Chong YS, He HG. A randomized controlled trial of the effectiveness of a postnatal psychoeducation programme on self-efficacy, social support and postnatal depression among primiparas. J Adv Nurs. 2015;71(6):1260-1273. doi: 10.1111/jan.12590 [DOI] [PubMed] [Google Scholar]
- 85.Shorey S, Chee CYI, Ng ED, Lau Y, Dennis CL, Chan YH. Evaluation of a technology-based peer-support intervention program for preventing postnatal depression (part 1): randomized controlled trial. J Med Internet Res. 2019;21(8):e12410. doi: 10.2196/12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavender T, Walkinshaw SA. Can midwives reduce postpartum psychological morbidity? a randomized trial. Birth. 1998;25(4):215-219. doi: 10.1046/j.1523-536X.1998.00215.x [DOI] [PubMed] [Google Scholar]
- 87.Gagnon AJ, Dougherty G, Jimenez V, Leduc N. Randomized trial of postpartum care after hospital discharge. Pediatrics. 2002;109(6):1074-1080. doi: 10.1542/peds.109.6.1074 [DOI] [PubMed] [Google Scholar]
- 88.Dennis CL, Grigoriadis S, Zupancic J, Kiss A, Ravitz P. Telephone-based nurse-delivered interpersonal psychotherapy for postpartum depression: nationwide randomised controlled trial. Br J Psychiatry. 2020;216(4):189-196. doi: 10.1192/bjp.2019.275 [DOI] [PubMed] [Google Scholar]
- 89.Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015;177:7-21. doi: 10.1016/j.jad.2015.01.052 [DOI] [PubMed] [Google Scholar]
- 90.Singla DR, Lemberg-Pelly S, Lawson A, Zahedi N, Thomas-Jacques T, Dennis CL. Implementing psychological interventions through nonspecialist providers and telemedicine in high-income countries: qualitative study from a multistakeholder perspective. JMIR Ment Health. 2020;7(8):e19271. doi: 10.2196/19271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(4):388-406. doi: 10.1001/jama.2015.18948 [DOI] [PubMed] [Google Scholar]
- 92.Singla DR, Ratjen C, Krishna RN, Fuhr DC, Patel V. Peer supervision for assuring the quality of non-specialist provider delivered psychological intervention: lessons from a trial for perinatal depression in Goa, India. Behav Res Ther. 2020;130:103533. doi: 10.1016/j.brat.2019.103533 [DOI] [PubMed] [Google Scholar]
- 93.Pinnock H, Epiphaniou E, Sheikh A, et al. Developing standards for reporting implementation studies of complex interventions (StaRI): a systematic review and e-Delphi. Implement Sci. 2015;10(1):42. doi: 10.1186/s13012-015-0235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization. mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-Specialized Health Settings. World Health Organization; 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PRISMA Checklist
eFigure 1. Risk Assessment
eTable 2. Trial Characteristics (N = 46)
eTable 3. Data Used to Calculate the Standardized Mean Difference and Standard Error for Each Outcome From Each Study, N = 43
eFigure 2. Forest Plot Examining a Subgroup Analysis of Outcome Tool (Diagnostic vs Self-Assessment)
eFigure 3. Forest Plot Examining a Subgroup Analysis of Age (Mixed Age vs Adult)
eAppendix. Leave-One-Out Analyses
eReferences