Marine species, such as the whale shark, are being subjected to unprecedented levels of human influence in their natural habitats. They may be vulnerable to obtaining injuries while spending time in surface waters. This study explores injury characteristics and timeframes of injury recovery with a view to inform management.
Keywords: Healing rate, whale shark, wildlife injuries, wound healing, anthropogenic activities, conservation management
Abstract
Wound healing is important for marine taxa such as elasmobranchs, which can incur a range of natural and anthropogenic wounds throughout their life history. There is evidence that this group shows a high capacity for external wound healing. However, anthropogenic wounds may become more frequent due to increasing commercial and recreational marine activities. Whale sharks are particularly at risk of attaining injuries given their use of surface waters and wildlife tourism interest. There is limited understanding as to how whale sharks recover from injuries, and often insights are confined to singular opportunistic observations. The present study makes use of a unique and valuable photographic data source from two whale shark aggregation sites in the Indian Ocean. Successional injury-healing progression cases were reviewed to investigate the characteristics of injuries and quantify a coarse healing timeframe. Wounds were measured over time using an image standardization method. This work shows that by Day 25 major injury surface area decreased by an average of 56% and the most rapid healing case showed a surface area reduction of 50% in 4 days. All wounds reached a point of 90% surface area closure by Day 35. There were differences in healing rate based on wound type, with lacerations and abrasions taking 50 and 22 days to reach 90% healing, respectively. This study provides baseline information for wound healing in whale sharks and the methods proposed could act as a foundation for future research. Use of a detailed classification system, as presented here, may also assist in ocean scale injury comparisons between research groups and aid reliable descriptive data. Such findings can contribute to discussions regarding appropriate management in aggregation areas with an aim to reduce the likelihood of injuries, such as those resulting from vessel collisions, in these regions or during movements between coastal waters.
Introduction
Exogenous wound healing is an essential physiological adaptation for vertebrate species (Han et al., 2005; Belacortu and Paricio, 2011; Jazwinska and Sallin, 2016). For marine vertebrates, the ability to heal from external wounds is fundamental for survival and allows animals to regain motor function and minimize any prolonged impacts of injury while also managing water-borne microflora (McCallum et al., 2003). Elasmobranchs (sharks and rays) are known to exhibit injury resilience and high healing capacity (Bird, 1978; Reif, 1978; Chin et al., 2015; McGregor et al., 2019), which has been partly explained by their unique adaptive immune responses (Marra et al., 2017). However, with current expansion of ocean-based human activities, elasmobranchs now inhabit an environment where human interactions, which can result in physical wounds, are increasing (Lester et al., 2020)—a circumstance that may test their natural resilience.
The incurrence of external wounds is a natural manifestation throughout the life history of elasmobranchs (Kajiuraet al., 2000; Chin et al., 2015). Routine natural injuries tend to be minor abrasions resulting from behaviours such as courtship, copulation or aggression (Carrier et al., 1994; Kajiura et al., 2000; Fitzpatrick et al., 2006; Domeier and Nasby-Lucas, 2007) and can occur as early as parturition, where both the female and the neonate may conclude this process with scars (Chin et al., 2015). Injuries of anthropogenic origin can result from discarded marine debris (Stelfoxet al., 2016; Parton et al., 2019), targeted and non-targeted fishing practices (Campana et al., 2009; Danylchuk et al., 2014; François et al., 2019), research (Heupel et al., 1998; Chin et al., 2015) and vessel strike (Speed et al., 2008; McGregor et al., 2019; Lester et al., 2020). Anthropogenic injuries are likely to be more complex and destructive than those obtained naturally and have the potential to induce long-term physiological adjustments, behavioural alterations and, in the worst case, death. It is important, therefore, to establish a solid baseline understanding of the characteristics and healing processes of physical wounds, from as many origins as sample size allows, so as to better identify and inform conservation opportunities where injury instance can be managed more effectively.
Systematic assessment of wound healing in free-swimming animals is a challenge as it relies on repeated observations of recovering individuals. Furthermore, in elasmobranch research there is a distinct lack of specimens for investigation as many species are negatively buoyant which may cause carcasses to sink following lethal traumatic events (Speed et al., 2008; Campana et al., 2016). Consequently, there are few studies that have reported on wound characteristics and change over time in detail. Fishing-related injuries (Bansemer and Bennett, 2010; Danylchuk et al., 2014; François et al., 2019), the impact of tagging procedures and neonatal scar healing (Chin et al., 2015) have been explored, and more recently injuries that resulted from vessel collisions were monitored over time to determine healing rates (McGregor et al., 2019). However, these accounts are limited to fairly small sample sizes and as a result several knowledge gaps remain in this group. Most notably, baseline knowledge related to how the type of tissue damage (e.g. superficial break in dermal surface or tear penetrating deeper sub-dermal layers) may influence healing rates is lacking.
Baseline information on wound characteristics and healing can have broad management applications, from developing appropriate animal handling procedures (Chin et al., 2015) to accurately quantifying human-related threats (McGregor et al., 2019). For marine mammals and reptiles, which have been the focus of a number of targeted injury impact studies (Laist et al., 2001; Hazel and Gyuris 2006; Hodgson and Marsh 2007; Rommel et al., 2007; Zasloff, 2011; Redfern et al., 2013; Dwyer et al., 2014; Martin et al., 2016), information of this kind has provided a useful base to inform sustainable management of wildlife populations. In particular, information on the amount of damage individuals can sustain without suffering mortality and improved understanding of the wound characteristics, healing capacity and long-term survivorship of elasmobranchs could greatly enhance targeted conservation. This information will be especially relevant for species that spend large portions of time in surface waters for activities such as feeding and basking [e.g. whale shark (Rhincodon typus; e.g. Tyminski et al., 2015); basking shark, (Cetorhinus maximus; e.g. Sims et al., 2005); reef manta ray (Mobula alfredi; e.g. Braun et al., 2014)], where animals might be vulnerable to attaining a range of natural and anthropogenic wounds.
Presented here is a systematic assessment of the healing capabilities and recovery processes of whale sharks, which aims to provide a greater understanding of the natural resilience and recovery from wounds experienced by this species. The whale shark is the largest extant species of fish (Martin, 2007) and one of the world’s most wide-ranging marine-vertebrates (Castro et al., 2007; Sequeira et al., 2014; Guzman et al., 2018) that utilizes a diverse range of habitats including shallow coastal waters and deep open ocean (Tyminski et al., 2015). This species is commonly encountered at the surface and is a good candidate to address questions related to injury resilience and healing since their broad range overlaps with a number of areas where potentially injury inducing activities also occur (Cagua et al., 2014). A variety of external injuries have been observed on whale sharks (Rowat et al., 2006; Speed et al., 2008; Ramírez-Macías et al., 2012; Araujo et al., 2014; Lester et al., 2020), but few studies have explicitly explored how individuals heal from these afflictions (Fitzpatrick et al., 2006; Womersley et al., 2016). Long-term monitoring programmes established at key coastal aggregation sites give access to extensive historical photographic and descriptive records of individual sightings and accompanying injuries. Coastal regions with high individual shark fidelity are particularly valuable as the fine scale temporal changes of wounds can be monitored in close succession. Here, a quantitative approach of documenting wound healing utilizing photographic records of whale shark injuries over time was adopted, with a focus on those which were sighted multiple instances over a short period. The main objectives of the study were to (1) develop a standardized method of measuring wound healing in free swimming whale sharks ensuring clear classification of injuries, (2) broadly explore wound characteristics and ascertain healing rates of injuries and (3) begin to investigate what factors may influence the rate of healing.
Methods
Data collection
Whale shark sighting records from regions surrounding the Gulf of Tadjourah, Djibouti (11°35’ N; 42°48′ E) and South Ari Atoll, Maldives (03°28’ N; 72°51′ E) were examined for injury-healing progression cases. Dedicated yearly photo-identification surveys of varying duration have been conducted by Marine Conservation Society Seychelles (MCSS) in Djibouti and the Maldives Whale Shark Research Programme (MWSRP) in the Maldives since 2003 and 2006 respectively. Detailed information about the study areas and data collection are described in Rowat et al. (2006) and Riley et al. (2010). A thorough search of descriptive encounter data was performed to locate instances where individuals with notable scarring were sighted on multiple occasions. Each highlighted injury case consisted of information on individual shark identification (ID) obtained by comparing the unique spot markings on each flank, from the 5th gill slit to the trailing edge of the pectoral fin, using I3S software (Van Tienhoven et al., 2007). Cases also included animal size (sampled with varying accuracy depending on technique), sex (determined by the presence or absence of claspers) and descriptions of notable features such as obvious scarring. Injury and scar encounter descriptions largely followed the standardized terminology described by Speed et al. (2008) with wound severity, type, location on the body and source described where possible. Cases were reviewed in further detail to highlight those where clear images of the wound were available from each encounter. Upon further inspection of injury images occasional classification and terminology inconsistencies were found and further standardization was required.
An updated classification system, which accounts for a larger range of injury types and a more detailed severity grouping, was developed to assist in more accurate determination of wound characteristics and improve on descriptive data in this study (Table 1, Fig. 1, and online supplementary material Table SI). In each case injury source and type were assigned by the presence or absence of key visual characteristics (Table 1). For example, clean cut incisions presenting as a parallel, repeating pattern of lacerations were indicative of a strike from a vessel propeller (Rommel et al., 2007), while strikes from a vessel hull were characterized by large wounds including blunt traumas or large abrasions unlikely to result from any other natural or human cause (Laist et al., 2001) (see online supplementary Table SI for examples of injuries that might result from a range of natural and anthropogenic origins). In the case of minor injuries care was taken to determine the source, although on many occasions wounds were too small to display enough notable features to accurately determine cause. Therefore, in this study minor injuries were included for comparisons of healing rate between severities but assigned as source undetermined, and source was not taken into account for the subsequent rate analysis.
Table 1.
Table to assist in the type and severity classification of whale shark (R. typus) injuries from external visual assessment in-water and using encounter photographs. Detailed type descriptions, severity conditions and photographic examples (⇒) are provided of ‘abrasion’, ‘amputation’, ‘bite’, ‘blunt trauma’, ‘abnormality, ‘entanglement’, ‘laceration’ and ‘nick’ wounds.

|
Figure 1.

Examples of injuries (⇒) of varying type, location and severity, attributed to both natural and anthropogenic sources, portraying severity within four type groups. Images (1A)–(4A) portray ‘major’ wounds which were determined based on quantifiable characteristics (Table 1) and images (1B)–(4B) portray ‘minor’ wounds.
Injury severity was the final stage of assessment and based on quantifiable characteristics designed to be either accurately measured (as in this study) or estimated in water (potential future application to increase viability of in-water encounter data). Severity of each case was determined for the first injury in the photographic series. Here, severity classification was not associated with potential impact on whale shark survival, as further knowledge on the relationship between injury extent and mortality is required before any assumptions of survivorship are made. Instead, severity was related to potential impact on resource allocation and the changes in physiology and/or behaviour needed to manage the injury. Major wounds were broadly defined as ‘potentially requiring significant resource allocation or physiological/behavioural adjustments’. Minor wounds were defined as insignificant with no apparent influence on resource allocation, changes in physiology and/or behaviour.
Key determinants for severity included a combination of descriptive indicators (wound type, location, skin colour around the wound in relation to surrounding area), quantitative indicators (relative size of the wound in relation to body features) and subjective indicators (potential influence on normal swimming and foraging ability) (Table 1 and Fig. 1). For example, in order for a laceration wound to be considered major it was present on the main body of the animal (lacerations present on appendages were considered minor, as in Fig. 1B, due to the thinner dermal layers present in these regions) and the total length of skin incisions was greater than or equal to the vertical height of the first dorsal fin (i.e. the combined length of multiple lacerations or one singular wound). If the injury did not meet these criteria but an area of discoloured skin was visible around the wound site relative to surrounding tissues or there was evidence of tissue necrosis (online supplementary material Fig. SI), the wound was considered major; this may be a sign of infection or healing complications with, as yet, unknown consequences. For abrasions to be considered major the surface area of the wound was greater than or equal to 50% of the surface area of the first dorsal fin and the perimeter was greater than or equal to that of the first dorsal fin, or the perimeter was greater than or equal to 200% of the first dorsal fin perimeter (Table 1). In Fig. 1 images (1A) and (1B) are examples of `laceration' injuries: (1A) deep laceration to the caudal peduncle region indicative of a propeller strike and (1B) superficial lacerations to the first dorsal fin of undetermined source. (2A) and (2B) are examples of ‘abnormalities’: (2A) the majority of the first dorsal fin is rolled over to the right side of undetermined source and (2B) irregular white markings on the trailing edge of the right pectoral fin of undetermined source. (3A) and (3B) portray examples of ‘amputations’: (3A) the majority of the first dorsal fin has been removed which is indicative of an anthropogenic wound (online supplementary material Table SI) that may have resulted from a vessel strike or potential opportunistic finning attempt and (3B) tip of left pectoral is missing of undetermined source. (4A) and (4B) portray examples of ‘abrasions’: (4A) abrasion covering large area of the flank indicative of a boat hull strike and (4B) superficial abrasion covering small section of the first dorsal fin of undetermined source.
These classification methods were applicable to this dataset and considered most representative of the physical characteristics of whale shark wounds likely to be assessed in water and visible in encounter photographs. Since citizen science is such a valuable input to this type of research, quantitative parameters were carefully developed to ensure they could be reasonably estimated in-water by trained personnel.
Injury measurements
Each healing case was selected for assessment and included in the analysis if there was sufficient photographic evidence to track injury progression from an open wound to a fully healed scar, with a minimum of three images on different dates (original wound sighting, intermediate date, fully healed scar). There were three exceptions to this rule which included a case of potential appendage regeneration, which was only sighted on two instances and two minor injuries which were sighted multiple times within a 12-day period, but were not sighted with a fully healed scar. For a given image to be included in an injury progression series and used for analysis, it needed to be as close as possible to the same relative angle as other images in the series. An open wound was defined as a wound where any tissue other than the epidermis, or outermost skin layer, was visible. Thus, if any white hypodermal tissues or deeper red musculature tissues were evident in images then the injury was classed as open. A fully healed scar was defined as an injury where the epidermis had reformed, concealing previously exposed sub-dermal or muscular tissue layers. Open wounds may include regions of healing and without direct observation of the event which resulted in an injury the exact start date of wound healing cannot be accurately determined. In the absence of this data healing progression was reviewed from the first instance that an open wound was sighted rather than when the injury was obtained. As a consequence, the healing rates presented in this study represent an estimate. Injury photographs were imported into the open-source image processing software FIJI by ImageJ version 2.0.0-rc-59/1.51 k (Schindelin et al., 2012). Prior to measuring, all images from each injury case were manually inspected to locate one unchanging epidermal spot marking to act as a relative scale. The rectangle tool was used to measure the surface area and perimeter of the chosen anchor point in pixels (Fig. 2).
Figure 2.

Example of methodology implemented to quantify wound change over time. The rectangle and freehand tools in FIJI by ImageJ were used to measure wound surface area and perimeter in pixels relative to the selected scale marking.
The outline of the wound was traced using the freehand selection tool and measurements of the surface area and perimeter were taken in pixels (Fig. 2). When the wound was broken into sections, separate measurements of each section were taken and summed together to give totals. This method was chosen to provide a healing rate based on trauma that resulted from a single event and to account for wounds that began as one whole and split into smaller sections during the healing process. The total wound surface area was then divided by the surface area of the anchor point in each image to give a ratio; this allowed for standardized comparisons between image specific pixel density in each case. The same method was used for the perimeter measurements. These two-dimensional measurements did not account for total wound volume which was not possible with the dataset available. This was overcome by comparing all measurements to the first wound sighting to define relative change over time irrespective of initial injury actual size or volume. The first instance that an injury was photographed was assigned day zero and each subsequent image assigned a number of days since initial sighting. Injury measurements were compiled with associated injury locations, types, severities and individual shark ID. Healing rate was calculated on each sighting event to determine the relative rate of change between sightings at a given stage of the recovery process:
 |
where yi is the measured rate on the ith day since initial sighting, a0 is initial wound surface area on first sighting, ai is wound surface area after i days since initial sighting, am is the surface area of the measured relative scale i days since initial sighting (ami) and on initial sighting (am0) and ti is i number of days since initial sighting.
Modelled healing rates
A minimum adequate mixed effect model was run in R package ‘lme4’ (Bates et al., 2014; Team RC, 2019) to explore the effect of injury type, location and severity on the logit transformed proportion of wound change since initial sighting. Shark ID and injury case were fitted as random effects. For this analysis, surface area was chosen as the primary dependent variable and records where the injury was 100% healed were removed to standardize between injuries that were opportunistically sighted close to the time of full recovery and those that were not. This resulted in 22 usable cases with 70 observations in total. Model selection was performed by stepwise removal of non-significant terms and interactions to give a minimum adequate model (Engqvist, 2005). An exponential model was fitted using a nonlinear least squares regression from the R package ‘nls2’ (Grothendieck, 2013):
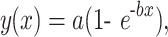 |
where y is the proportion of healing, 𝑎 is the value of the horizontal asymptote and b is the exponential rate of growth. A quadratic plateau model using a nonlinear least squares regression (Grothendieck, 2013) was also fitted to calculate on which day the rate of wound change between sightings plateaued:
 |
where y is the rate of change, a is the rate of change at Day 0, b is the exponential rate of decay and xcl represents the critical value of days (x) where rate of change (y) plateaus. For the exponential and quadratic plateau models any injury sighting that was over 150 days was omitted to gain a clearer temporal resolution; this allowed for partially healed cases to remain included but records that were observed as virtually healed after the cut off time frame were removed. Negative values were also removed from the rate plateau models. Package ‘rcompanion’ (Mangiafico, 2019) was used to assess model fit using a pseudo-r2 (Nagelkerke) to calculate improvements of the fitted sum of squared errors from the null.
Results
Injury observations
Database searches resulted in 6 suitable cases from Djibouti and 21 cases from the Maldives (online supplementary material Table SIIa, b). Although databases consisted of an extensive range of wounds of varied types from numerous sources (both natural and anthropogenic in origin), many were only encountered on one occasion or did not have suitable accompanying images for healing assessment. Of the 27 cases from 18 male individuals, the average number of images per progression series was 5.15 (± 0.49 S.E., n = 27). The average number of days for injuries to fully heal, not including appendage fusion or regeneration cases, was 169.57 (± 36.13 S.E., n = 21). Major injuries, all of which were attributed to vessel collisions (online supplementary material Table SIIa, b; injuries of alternate source, such as entanglement and bite wounds, were encountered but did not have enough appropriate images for a detailed assessment of healing), took an average of 210.5 (± 50.61 S.E., n = 14) days to heal and minor injuries took an average of 87.71 (± 16.69 S.E., n = 7) days (not including the two cases which did not reach a defined healing point). By Day 25 major injury surface area had decreased by 56.45% (± 6.06 S.E., n = 12), and minor injury surface area had decreased by 79.95% (± 7.60 S.E., n = 7). The most rapid healing case recorded was that of a minor first dorsal abrasion that reduced in surface area by 50.47% in 4 days after initial sighting.
Lacerations dissecting appendages showed signs of tissue fusion re-joining disconnected tissues (online supplementary material Fig. SII), where fibrous matter appeared to support the void left by severed appendages (Fig. 3). Lacerations to the main body showed the epidermis folding inward around the wound perimeter while the exposed sub-dermal tissue began to heal (Fig. 4). Generally, amputations showed little capacity to regrow lost tissue, however given that in many cases individuals with these afflictions were repeatedly sighted, it would seem that partial and complete fin amputations appear to have a minimal impact on short-term survivorship. In one instance a juvenile male from the Djibouti aggregation was sighted in 2006 missing the tip of the first dorsal fin. When this individual was re-sighted approximately 5 years later (2011) it appeared to have re-grown the previously amputated first dorsal fin tip (Fig. 5). The fin showed an overall surface area increase of 6.39% and a perimeter increase of 1.09% over a 3237 day (~106 month) period (Fig. 5). This is the first reported case of an elasmobranch re-growing a part of an appendage, and was not noted, to this degree, in any other case. Without a more in-depth tissue analysis it cannot be said as to what extent tissue regeneration is involved in the processes of healing in this species; however, it was observed that the tissues reforming around wounded areas, whether they be scar tissue or regenerated tissue, appeared melanized (darker) in relation to natural body pigmentation in most cases and showed some lightening over time. Black pigmentation marks often appeared in the scar region and in a few instances the white spot markings were shown to reform on a previously wounded area (Fig. 6). When seemingly recent injuries were observed with blood still visible, the outer tissue layer around the wound site appeared pallid, which may be a sign of infection (Fig. 3); however, without an improved knowledge of established signs of septicity in free-swimming elasmobranchs, such as ulceration and inflammation in captive individuals (Garner, 2013), this observation remains inconclusive.
Figure 3.

Examples of open injuries to the pectoral (A) and first dorsal (B) fins with a notable pallid epidermis surrounding the wound site and fibrous tissue present at the open wound site.
Figure 4.

Example of the epidermis folding inward around a major laceration perimeter 16 days (B) after the wound was initially sighted (A) in December 2016.
Figure 5.

Example of appendage regeneration. In 2006 (A,C) the individual is sighted with the tip of the first dorsal fin missing. Approximately 5 years later in 2011 (B,D) tissue appears to have regenerated to fill the previously severed area and reform the natural curved shape of the first dorsal fin.
Figure 6.

Example of white spot marking pigmentation returning to a previously wounded area over the course of ~ 5 months. Markings visible in (E) were not present on first sighting (A) where the pigmented outer skin layer had been removed.
A case study of a juvenile male whale shark (WS198, ~ 4 m in total length upon first sighting) from the Maldives dataset, which was repeatedly observed throughout the healing process, is presented here (Fig. 7). The individual was encountered on 1 June 2015 with no apparent injuries. When re-sighted 23 days later (24 June) the individual had incurred a series of major lacerations to the right flank. Owing to the large, clean cut incisions which presented as a parallel pattern of lacerations, the injury was attributed to a propeller strike (online supplementary Table SI)(Rommel et al., 2007; Dwyer et al., 2014), possibly by an outboard motor, which are commonly used by tourist vessels in the surrounding area (Cagua et al., 2014; J. Hancock, personal communication). The lacerations were estimated to be at least a couple of days old (maximum 23 days based on pre injury encounters) as there was no blood visible and the outer epidermis had begun to fold inward around the wound perimeter. The collective wound measurements equated to 88% of the first dorsal fin surface area and 430% of the perimeter. This was the maximum wound extent relative to the first dorsal fin measured and was therefore the largest wound a whale shark was recorded surviving in this dataset. After 16 days (10 July) the wound showed signs of extensive tissue repair; most of the lacerations had fused together and little exposed sub-dermal tissue remained, however the individual appeared progressively more emaciated over the course of early observations. After a maximum of 6 months (159 days) the injury had fully closed and was classified as healed. From this date onward it showed little change, but based on visual estimates, the individual looked more robust and in improved condition. WS198 was repeatedly encountered in the years following and went on to obtain three major vessel-related injuries (one on 27 March 2016 and two on 10 May 2017) involving multiple lacerations, suggesting three distinct collision events in a three-year period. By relative Day 66 of the recovery process of the three subsequent injuries, wound surface area had reduced by 97.48%, 100% and 95.44% respectively (Fig. 8).
Figure 7.

Example of an individual (WS198) which was sighted with a series of open vessel-induced lacerations to the right flank on 24 June 2015 (A). Less than 1 month later (B) the wounds showed signs of extensive healing with considerably less sub-dermal tissue visible and after approximately 6 months (C) the injury was classified as fully healed with the pigmented epidermal layers covering all tissues- In the years following (D and E) the scar showed little further change other than mild pigmentation alterations.
Figure 8.

Temporal evolution (days since initial wound sighting) of wound healing showing total surface area reduction (%) of four distinct vessel-related injuries inflicted on WS198 over a three-year period.
Cases of tissue fusion were quantified (online supplementary material Fig. SII). This included three instances of a severed first dorsal fin where appendage fusion occurred as wound margins were drawn together. In these instances, although open wounds closed, there was no defined or standardized point of healing and therefore a linear measurement of tissue reattachment was adopted to quantify the percentage reattachment of total severed length over time. Tissue reattachment occurred an average of 82.8% (± 4.7 S.E., n = 3) along the original severed length, with a small nick remaining in all cases. By Day 125 an average of 57.31% (± 10 S.E., n = 3) of tissue had reattached.
Modelled healing rates
Wound severity (χ2 = 0.01, df = 1, P = 0.91) and location on the body (χ2 = 3.2, df = 2, P = 0.202) had no significant influence on the proportion of surface area reduction over time. The type of injury was significant (χ2 = 5.01, df = 1, P = 0.025*), with abrasions exhibiting a higher proportion of surface area reduction in significantly fewer days when compared to lacerations. The percentage change in wound surface area since initial sighting was well fitted by an exponential model (Fig. 9 and Table 2). From the model, 90% wound surface area closure had occurred by Day 34.84 and wound perimeter closure reached 90% by Day 52.71 (Fig. 9 and Table 2). The rate of wound surface area change between sighting occasions plateaued at 33.1 days (± 6.27 S.E., t = 5.28, P < 0.001***, r2 = 0.52) and the rate of wound perimeter change plateaued at 39.93 days (± 10.04 S.E., t = 3.98, P < 0.001***, r2 = 0.44) (Table 2). When the type of injury was explored individually, surface area reached 90% closure by Day 50.17 for lacerations and Day 22.13 for abrasions (Fig. 10). Perimeter measurements reached 90% closure by Day 69.22 for lacerations and 34.82 for abrasions (Fig. 10). Rate of surface area change between sightings plateaued at 12.85 (± 2.1 S.E., t = 6.12, P < 0.001***, r2 = 0.7) days for lacerations and 40.33 (± 15.43 S.E., t = 2.61, P < 0.05*, r2 = 0.4) days for abrasions (Fig. 11). Rate of perimeter change between sightings plateaued at 38.66 (± 13.28 S.E., t = 2.91, P < 0.05*, r2 = 0.43) days for lacerations and 42.25 (± 15.83 S.E., t = 2.67, P < 0.05*, r2 = 0.44) days for abrasions (Fig. 11 and Table 2).
Figure 9.
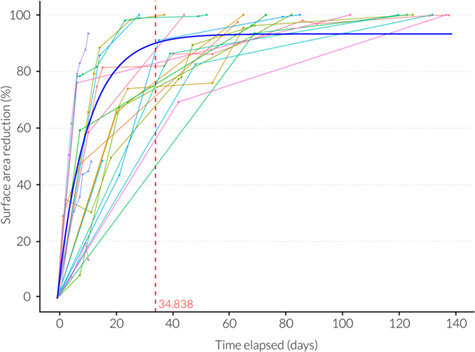
Temporal evolution (days since initial wound sighting) of wound healing showing total surface area reduction (%). Best fit exponential model is shown (blue line) where the x intercept equals 34.84 days when 90% healing is reached (red dotted line).
Table 2.
Outputs from the exponential and quadratic plateau models. Statistical significance is indicated by asterisks (‘.’ for P < 0.1, ‘*’ for P < 0.5, ‘**’ for P < 0.01, ‘***’ for P < 0.001).
| Dataset | Measurement | a | b | Xcl | Xcl SE | Xcl T | Xy = 90 | r2 |
|---|---|---|---|---|---|---|---|---|
| All | Area | 93.251*** | 0.096*** | na | na | na | 34.838 | 0.837 |
| All | Perimeter | 94.428*** | 0.058*** | na | na | na | 52.713 | 0.816 |
| All | Area rate | 9.306*** | −0.513*** | 33.100*** | 6.265 | 5.283 | na | 0.518 |
| All | Perimeter rate | 9.769*** | −0.445** | 39.931*** | 10.037 | 3.978 | na | 0.436 |
| Abrasion | Area | 94.804*** | 0.135*** | na | na | na | 22.128 | 0.841 |
| Abrasion | Perimeter | 100.000*** | 0.066*** | na | na | na | 34.819 | 0.813 |
| Abrasion | Area rate | 8.146*** | −0.374. | 40.334* | 15.434 | 2.613 | na | 0.404 |
| Abrasion | Perimeter rate | 9.267*** | −0.405. | 42.253* | 15.825 | 2.670 | na | 0.442 |
| Laceration | Area | 98.124*** | 0.050*** | na | na | na | 50.167 | 0.925 |
| Laceration | Perimeter | 96.936*** | 0.038*** | na | na | na | 69.216 | 0.875 |
| Laceration | Area rate | 15.087*** | −2.117*** | 12.851*** | 2.099 | 6.124 | na | 0.695 |
| Laceration | Perimeter rate | 10.421*** | −0.488. | 38.656** | 13.282 | 2.910 | na | 0.427 |
Figure 10.
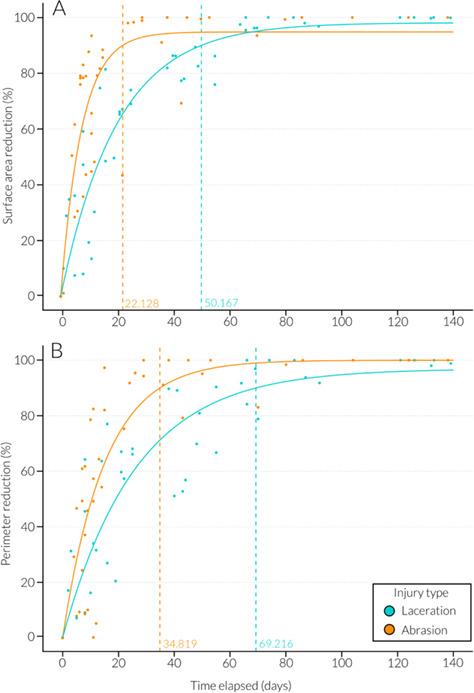
Temporal evolution (days since initial wound sighting) of wound healing showing (A) total surface area reduction (%) for lacerations (teal) and abrasions (orange) where x intercepts are 50.17 (laceration) and 22.13 (abrasion) days when 90% healing is reached (dotted line) and (B) total perimeter reduction for lacerations (teal) and abrasions (orange) where x intercepts are 69.22 (laceration) and 34.82 (abrasion) days when 90% healing is reached (dotted line).
Figure 11.
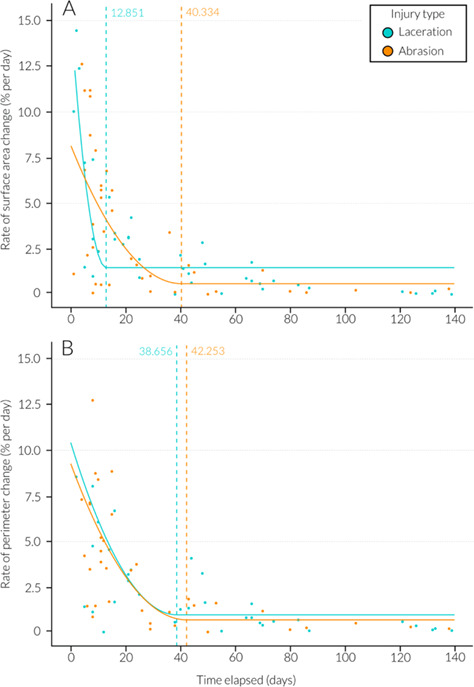
Temporal evolution (days since initial wound sighting) of wound healing showing rate of (A) surface area change between sightings (%) on a given day for lacerations (teal) and abrasions (orange) where critical x intercepts are 12.85 (laceration) (P < 0.001***) and 40.33 (abrasion) (P < 0.05*) days (dotted line) and (B) perimeter change between sightings on a given day for lacerations (teal) and abrasions (orange) where critical x intercepts are 38.66 (laceration) (P < 0.01**) and 42.25 (abrasion) (P < 0.05*) days (dotted line).
Discussion
Here a detailed report on injury healing in whale sharks is presented. The successional image measurement methods and classification system proved a viable way of comparing injury change over time. Photographic data suggest that this species has a high capacity to tolerate and recover from injuries resulting in extensive tissue damage (maximum recorded 88% and 430% of the first dorsal surface area and perimeter, respectively) with many individuals surviving wounds that were unmistakably the result of a vessel collision (n = 16; online supplementary Table SIIa, b). Wounds showed initial rapid healing reaching a point of 90% surface area and perimeter closure by Days 35 and 53, respectively, and slowing over time following a positive exponential curve. This suggests the majority of healing takes place in the early stages of the entire healing process which took an average of 170 days. Wounds classified as major showed a decrease in surface area of 56% in 25 days. Minor injuries were shown to reduce in surface area by as much as 80% in the first 25 days, with the fastest rate recorded showing a surface area reduction of 50% in 4 days. Lacerations, which are the most likely wound types to result from a vessel propeller collision, took significantly longer to heal than abrasions. From the models, lacerations took approximately 1 month longer than abrasions to reach 90% healed, and surface area closure between sightings reached a slower rate plateau approximately 1 month earlier than abrasions (13 days compared to 40 days), extending their recovery process. As well as providing valuable insight into wound healing processes in wild marine fauna, specifically elasmobranchs, these findings also have significant implications for conservation and the application of appropriate management to limit human-wildlife conflicts.
The rapid wound healing recorded in whale sharks supports a growing body of evidence denoting that the exogenous trauma recovery mechanisms of elasmobranchs are highly developed and able to promote healing from a number of injury types. Observed rates are similar to those recorded in captive nurse sharks (Ginglymostoma cirratum) and leopard sharks (Triakis semifasciata) where dermal denticle reformation over surgically removed areas took four months (Reif, 1978). Findings also agree with systematic observations of wild black tip reef sharks (Carcharhinus melanopterus) where vitellin scar surface area was shown to decrease by 94% in 24 days in neonatal individuals; a bite wound on an adult closed within 3 days and was completely healed within 40 days; and a major, deep (25 cm across and 3–5 cm deep) wound from a suspected vessel collision closed fully within 27 days (Chin et al., 2015). Similarly, a male sicklefin lemon shark (Negaprion acutidens) was photographed with a 20 cm vertical laceration on the second dorsal fin which had healed significantly within two months and was difficult to distinguish a year later (Buray et al., 2009). Pelagic stingrays (Pteroplatytrygon violacea) have been shown to expel circle hooks and exhibit wound recovery over a 28 day period (François et al., 2019) and shark bites on reef manta rays (M. alfredi) were reported as completely healed within 126 to 225 days (Marshall and Bennett, 2010). Much slower healing rates have also been documented in reef manta rays where a series of vessel strike wounds had closed by 37% after 33 days and 93% by Day 295 (McGregor et al., 2019). Perhaps in this case healing rates are better reflected in the tissue fusion instances presented here, where 57% reattachment occurred over total severed length by Day 125 and a small nick remained in all cases. Considerably longer healing timeframes have been recorded in wild juvenile white sharks (Carcharodon carcharias) where a major laceration (25 cm across) caused by a boat propeller appeared to take nine months to completely heal (Towner et al., 2012) and timeframes of over six months have been recorded in grey nurse sharks (Carcharias taurus) following hook injuries (Bansemer and Bennett, 2010).
Inherently, the rate of elasmobranch healing likely depends on individual physiology and adaptive immunology (Lueret al., 2004; Marra et al., 2017). Morphological adaptations such as the thicker epidermal layers exhibited in female blue sharks (Prionace glauca) (Nakano and Stevens, 2008) and small-spotted catsharks (Scyliorhinus canicula) (Crooks et al., 2013) may assist in intrinsic healing capacity in the females of these species. Additionally, neonatal individuals may have a higher rate of healing than adults associated with the importance of allocating resources and energy effectively during early life stages to optimize growth and maximize survival (Chin et al., 2015). Since all sharks in this study were male, sex-related differences in healing remain unexplored in this species. Rates of recovery may also be influenced by the external environment. For example, early studies on the wound healing rate of teleost fish revealed that tissue generated more quickly across wound sites in warmer conditions (Anderson and Roberts, 1975). Reduced healing capacity in cooler waters observed in grey nurse sharks was explained as a consequence of reduced metabolic rates (Bansemer and Bennett, 2010). This may also explain why white sharks exhibited healing rates of several months when recovering from minor abrasions at Guadalupe Island where temperatures range from ∼18–20 °C (Domeier and Nasby-Lucas, 2007) and a reef manta ray occupying a region between ~ 21–24 °C exhibited slower healing rates (McGregor et al., 2019). Meanwhile, black tip reef sharks and sicklefin lemon sharks in tropical waters exhibit similar healing rates to the whale shark (Buray et al., 2009; Chin et al., 2015). Although whale sharks have been shown to oscillate throughout a broad range of temperatures and perform deep dives into the bathypelagic zone (Tyminski et al., 2015), they spend a large proportion of time in shallow surface waters and have a preference for warmer temperatures (Sequeira et al., 2013). These behaviours lend support to the role of temperature in the rapid healing capacity of this species, and may also go on to explain why scarred individuals are frequently encountered in warmer surface waters (Authors, personal observation). While interspecific variation or environmental factors may influence the rate and characteristics of injury recovery, little is yet known about the relative importance of these variables for wound healing in elasmobranchs.
The instance of tissue regeneration observed in this study is a novel example of an elasmobranch regenerating a part of an amputated appendage and the first recorded form of tissue regeneration in whale sharks. Although a more in-depth analysis of tissue structure is required for a complete understanding of this case, it can be noted that this unique observation was visually distinct from conventional wound healing as tissue was regrown in response to a partial amputation as opposed to the closing up of an injury site with a scar of unknown tissue composition. Similar capabilities have been observed in whitespotted bamboo sharks (Chiloscyllium plagiosum) which can regenerate at least two thirds of their liver (Lu et al., 2013) and many species of shark routinely regenerate and replace their teeth throughout their lives (Rasch et al., 2016). Complete limb regeneration is a notable physiological adaptation in some higher vertebrate species, and serves as a mechanism to regain functional use of an appendage following amputation (Han et al., 2005). In amphibians, regeneration is a local response of the cells at the amputation site and results in a perfect replacement limb regardless of the level of amputation (Han et al., 2005). This is unlikely to be the case in whale sharks given that from the numerous instances of partial amputation recorded, this regeneration case was unique. Therefore, no firm conclusions regarding the species’ capacity to regrow appendages can be drawn here. Factors such as the mobility of the species and dynamism of the appendage, the amount of tissue removed and the fitness of the individual likely play a part in whether appendage regeneration will take place. Nevertheless, amputation injuries should be monitored where possible to better understand this phenomenon.
The repeated observations of injured individuals prompt further questions regarding the sublethal impacts of trauma which remain largely unexplored. Here, a maximum wound extent (WS198) in relation to first dorsal fin size is provided, which can serve as an initial estimate for the amount of damage that an individual can survive. However, changes to swimming and foraging ability, internal tissue structure alterations, or infection/inflammation resulting from injuries may significantly reduce individual fitness and post-injury survival. Previously injured individuals have been shown to exhibit less evasive behaviours and be involved in longer tourism encounters than non-injured individuals (Quiros, 2007; Haskell et al., 2014), which suggests that injured whale sharks may have a reduced level of agility as a direct result of their previous injuries or are selecting warmer surface waters to speed up the healing process. Perhaps the energetic costs of seeking new foraging areas while harbouring a debilitating wound forces individuals to remain close to the foraging areas where they obtained the injury, and by default in close proximity to the initial injury source. The extent to which previous injuries influence behaviour and habitat selection or the likelihood of attaining a further injury and the healing rate of subsequent injuries requires further exploration. In addition to the immediate and prolonged behavioural/physical effects of injuries, recurring human activities around sharks has the potential to influence levels of stress. Stress-related behaviours such as changing direction or diving have been shown to be more common directly following vessel and swimmer approaches, which may carry energetic costs and inhibit recovery (Montero-Quintana et al., 2018). These areas would benefit from veterinarian expertise to provide independent evaluation of the long-term potential risk of injuries and recurring human activities to shark stress, health and potential survivorship. This type of work could better inform severity classifications, sub-lethal impacts and mortality estimates which would greatly enhance population level conclusions in this field.
The current study provides the first coarse injury healing timeframe for whale sharks and can be used to infer the maximum number of days since an injury was obtained based on the healing status of a wound. For elasmobranch species in general, ecological applications of this information include evaluating wound characteristics to potentially deduce date of birth for neonatal sharks based on the healing status of their vitelline scar (Aubrey and Snelson, 2007; Rowat et al., 2007; Chin et al., 2015) and determining the timing of copulation (Chin et al., 2013; Mourier et al., 2013; Chin et al., 2015) or the location of key nursery areas (McCallister et al., 2013). Regrettably, these applications are difficult to apply to whale shark ecology given that encountering individuals at crucial life stages, such as adults engaged in mating activities or neonates, is extremely rare (Rowatet al., 2007; Ramírez-Macías et al., 2017). Additionally, significant knowledge gaps in whale shark reproductive ecology impede conclusions related to these areas. Though present lack of knowledge may limit firm ecological applications, information on injury healing timeframes may have far reaching conservation outcomes for this species, most notably in relation to managing stakeholder usage in coastal aggregation sites. Given that frequency of vessel-related injuries recorded on whale sharks is increasing in coastal regions alongside mounting recreational and wildlife tourism activities (Lester et al., 2020), and a high number of individuals have been observed with external injuries (Rowat et al., 2006; Ramírez-Macías et al., 2012; Araujo et al., 2014), it follows that further management is needed to protect individuals occupying or passing through coastal areas. Regional researchers should be encouraged to collect photographic records of external injuries, including those which have already healed, with the aim of evaluating wound characteristics and documenting the entire healing timeframe. By examining the features of injuries present on individuals the source and potential date of injury infliction can likely be ascertained. This information, along with regional domain knowledge related to stakeholder use, can have important implications for better understanding local threats to whale sharks. Furthermore, in conjunction with targeted education and awareness programmes, the improved accuracy of initial injury timing estimations can be used to support marine park management strategies (Schoeman et al., 2020) and strengthen attitudes towards regulation development and sustainable compliance, as well as monitoring the success of conservation initiatives.
In summary, this study indicates that whale sharks are resilient to a range of external wounds, including those that result from vessel collisions, by showing that whale sharks exhibit rapid wound healing and long-term survival from major injuries. This species has also demonstrated the capacity to regenerate tissues in order to regrow part of an amputated appendage; a previously unobserved phenomenon. These are important findings for whale shark and wider elasmobranch conservation, especially considering the whale shark is now listed as "Endangered" by the IUCN Red List (Pierce and Norman, 2016) and up to a quarter of all shark and ray species worldwide are threatened with extinction (Dulvy et al., 2014). Nevertheless, the long-term effects of trauma to the overall condition of whale sharks remain undetermined and although individuals may appear resilient to external injuries, certain traumas may have less recognizable effects such as internal damage, reduced swimming capacity resulting in higher average energetic output or behavioural changes that may significantly reduce individual fitness. Similarly, it is likely that additional energy would be assigned towards healing processes, shifting allocation away from growth (Perry et al., 2018), feeding or other essential aspects of the species’ aerobic metabolic scope. The post injury emancipation observed in WS198 yields anecdotal support for this statement. As a consequence, it is essential that strategies are developed to minimize the likelihood of whale sharks attaining an injury as well as reducing further stress on newly injured individuals. Future research should aim to overcome the data limitations of the current study by conducting dedicated cicatrization studies or exploring areas related to the sub-lethal effects of injuries on overall condition or behaviour. Additionally, a large-scale assessment of whale shark space use and overlap with human activity could provide vital insight into high risk regions where this species may be vulnerable to attaining injuries and where mitigation efforts could be focused. Researchers involved in long term whale shark monitoring programmes should also be encouraged to document injuries and healing rates whenever possible. The improved understanding of whale shark resilience and response to injuries along with the impact on long-term health and survival will be an invaluable asset in refining mitigation and safeguarding the future of this endangered species.
Author contributions
F.W. and D.R. conceived the study and F.W. designed the study. F.W., D.R., J.H. and C.P. contributed to field-based data collection. F.W. reviewed and analysed the collected data and drafted the paper. All authors contributed to subsequent drafts.
Competing interests
The authors declare no competing interests.
Supplementary Material
Acknowledgements
We thank all who were involved in the fieldwork and data collection including the numerous photographers, citizen scientists and the local whale shark tourism industry for image contributions. Particular thanks are given to NGOs DECAN and Megaptera, the management and crew of MV Deli and Savinien Leblond for their assistance in the Djibouti surveys. We also extend specific thanks to members of the MWSRP team including Richard Rees, Basish Mohmamed, Ibrahim Shameel and Irithasham Zareer for their support in data collection and facilitation of data sharing.
References
- Anderson CD, Roberts RJ (1975) A comparison of the effects of temperature on wound healing in a tropical and a temperate teleost. J Fish Biol 7: 173–182. [Google Scholar]
- Araujo G, Lucey A, Labaja J, So CL, Snow S, Ponzo A (2014) Population structure and residency patterns of whale sharks, Rhincodon typus, at a provisioning site in Cebu, Philippines. PeerJ 2: e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey CW, Snelson FF Jr (2007) Early life history of the spinner shark in a Florida nursery In CT McCandless, NE Kohler, HL Pratt Jr, eds, Shark Nursery Grounds of the Gulf of Mexico and the East Coast Waters of the United States. American Fisheries Society, Symposium 50, Bethesda, MD, pp. 175–179.
- Bansemer CS, Bennett MB (2010) Retained fishing gear and associated injuries in the east Australian grey nurse sharks (Carcharias taurus): implications for population recovery. Mar Freshwater Res 61: 97–103. [Google Scholar]
- Bates D, Mächler B, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- Belacortu Y, Paricio N (2011) Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev Dynam 240: 2379–2404. [DOI] [PubMed] [Google Scholar]
- Bird PM (1978) Tissue regeneration in three carcharhinid sharks encircled by embedded straps. Copeia 1978: 345. [Google Scholar]
- Braun CD, Skomal GB, Thorrold SR, Berumen ML (2014) Diving behavior of the reef manta ray links coral reefs with adjacent deep pelagic habitats. PLoS One 9: e88170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buray N, Mourier J, Planes S, Clua E (2009) Underwater photo-identification of sicklefin lemon sharks, Negaprion acutidens, at Moorea (French Polynesia). Cybium 33: 21–27. [Google Scholar]
- Cagua EF, Collins N, Hancock J, Rees R (2014) Whale shark economics: a valuation of wildlife tourism in south Ari Atoll, Maldives. PeerJ 2: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana SE, Joyce W, Fowler M, Showell M (2016) Discards, hooking, and post-release mortality of porbeagle (Lamna nasus), shortfin mako (Isurus oxyrinchus), and blue shark (Prionace glauca) in the Canadian pelagic longline fishery. ICES J Mar Sci 73: 520–528. [Google Scholar]
- Campana SE, Joyce W, Francis MP, Manning MJ (2009) Comparability of blue shark mortality estimates for the Atlantic and pacific longline fisheries. Mar Ecol Prog Ser 396: 161–164. [Google Scholar]
- Carrier JC, Pratt HL, Martin LK (1994) Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum. Copeia 1994: 646–656. [Google Scholar]
- Castro AL, Stewart BS, Wilson SG, Hueter RE, Meekan MG, Motta PJ, Bowen BW, Karl SA (2007) Population genetic structure of earth's largest fish, the whale shark (Rhincodon typus). Mol Ecol 16: 5183–5192. [DOI] [PubMed] [Google Scholar]
- Chin A, Heupel MR, Simpfendorfer CA, Tobin AJ (2013) Ontogenetic movements of juvenile blacktip reef sharks: evidence of dispersal and connectivity between coastal habitats and coral reefs. Aquat Conserv 23: 468–474. [Google Scholar]
- Chin A, Mourier J, Rummer JL (2015) Blacktip reef sharks (Carcharhinus melanopterus) show high capacity for wound healing and recovery following injury. Conserv Physiol 3. doi: 10.1093/conphys/cov062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks N, Babey L, Haddon WJ, Love AC, Waring CP (2013) Sexual dimorphisms in the dermal denticles of the lesser-spotted catshark, scyliorhinus canicula (linnaeus, 1758). PLoS One 8: e76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danylchuk AJ, Suski C, Mandelman JW, Murchie KJ, Haak CR, Brooks AM, Cooke SJ (2014) Hooking injury, physiological status and short-term mortality of juvenile lemon sharks (Negaprion bevirostris) following catch-and-release recreational angling. Conserv Physiol 2.doi: 10.1093/conphys/cot036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeier ML, Nasby-Lucas N (2007) Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar Biol 150: 977–984. [Google Scholar]
- Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LN, Fordham SV, Francis MP et al. (2014) Extinction risk and conservation of the world's sharks and rays. eLife 3: e00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer SL, Kozmian-Ledward L, Stockin KA (2014) Short-term survival of severe propeller strike injuries and observations on wound progression in a bottlenose dolphin. New Zeal J Mar Fresh 48:294–302. [Google Scholar]
- Engqvist L (2005) The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav 70: 967–971. [Google Scholar]
- Fitzpatrick B, Meekan M, Richards A (2006) Shark attacks on a whale shark (Rhincodon typus) at Ningaloo reef, Western Australia. Bull Mar Sci 78: 397–402. [Google Scholar]
- François P, Sidonie C, Caroline C, Jean-Marc G (2019) The effect of hook type and trailing gear on hook shedding and fate of pelagic stingray (Pteroplatytrygon violacea): new insights to develop effective mitigation approaches. Mar Policy 107: 103594. [Google Scholar]
- Garner MM (2013) A retrospective study of disease in elasmobranchs. Vet Pathol 50: 377–389. [DOI] [PubMed] [Google Scholar]
- Grothendieck G (2013) Non-linear regression with brute force. R package version 0.2.
- Guzman HM, Gomez CG, Hearn A, Eckert SA (2018) Longest recorded trans-pacific migration of a whale shark (Rhincodon typus). Mar Biodivers Rec 11: 8. [Google Scholar]
- Han M, Yang X, Taylor G, Burdsal CA, Anderson RA, Muneoka K (2005) Limb regeneration in higher vertebrates: developing a roadmap. Anat Rec B New Anat 287: 14–24. [DOI] [PubMed] [Google Scholar]
- Haskell PJ, McGowan A, Westling A, Méndez-Jiménez A, Rohner A, Collins K, Rosero-Caicedo M, Salmond J, Monadjem A, Marshall AD, et al. (2014) Monitoring the effects of tourism on whale shark Rhincodon typus behaviour in Mozambique. Oryx 49: 492–499. [Google Scholar]
- Hazel J, Gyuris E (2006) Vessel-related mortality of sea turtles in Queensland, Australia. Wildl Res 33: 149–154. [Google Scholar]
- Heupel MR, Simpfendorfer CA, Bennett MB (1998) Analysis of tissue responses to fin tagging in Australian carcharhinids. J Fish Biol 52: 610–620. [Google Scholar]
- Hodgson AJ, Marsh H (2007) Response of dugongs to boat traffic: the risk of disturbance and displacement. J Exp Mar Biol Ecol 340: 50–61. [Google Scholar]
- Jazwinska A, Sallin P (2016) Regeneration versus scarring in vertebrate appendages and heart. J Pathol 238: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiura SM, Sebastian AP, Tricas TC (2000) Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environ Biol Fishes 58: 23–31. [Google Scholar]
- Laist DW, Knowlton AR, Mead JG, Collet AS, Podesta M (2001) Collisions between ships and whales. Mar Mam Sci 17: 35–75. [Google Scholar]
- Lester E, Meekan MG, Barnes P, Raudino H, Rob D, Waples K, Speed CW (2020) Multi-year patterns in scarring, survival and residency of whale sharks in Ningaloo Marine Park, Western Australia. Mar Ecol Prog Ser 634: 115–125. [Google Scholar]
- Lu C, Zhang J, Nie Z, Chen J, Zhang W, Ren X, Yu W, Liu L, Jiang C, Zhang Y et al. (2013) Study of micrornas related to the liver regeneration of the whitespotted bamboo shark, Chiloscyllium plagiosum. Biomed Res Int 2013: 795676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luer CA, Walsh CJ, Bodine AB (2004) The immune system of sharks, skates and rays In JC Carrier, JA Musick, HR Heithaus, eds, Biology of Sharks and Their Relatives. CRC Press, Boca Raton, FL, pp. 369–395. [Google Scholar]
- Mangiafico S (2019) Rcompanion: Functions to support extension education program evaluation. R package version 2.0.10.
- Marra NJ, Richards VP, Early A, Bogdanowicz SM, Pavinski Bitar PD, Stanhope MJ, Shivji MS (2017) Comparative transcriptomics of elasmobranchs and teleosts highlight important processes in adaptive immunity and regional endothermy. BMC Genomics 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AD, Bennett MB (2010) The frequency and effect of shark-inflicted bite injuries to the reef manta ray Manta alfredi. Aff J Mar Sci 32: 573–580. [Google Scholar]
- Martin J, Sabatier Q, Gowan TA, Giraud C, Gurarie E, Calleson CS, Ortega-Ortiz JH, Deutsch CJ, Rycyk A, Koslovsky SM et al. (2016) A quantitative framework for investigating risk of deadly collisions between marine wildlife and boats. Methods Ecol Evol 7: 42–50. [Google Scholar]
- Martin RA (2007) A review of behavioural ecology of whale sharks (Rhincodon typus). Fish Res 84: 10–16. [Google Scholar]
- McCallister M, Ford R, Gelsleichter J (2013) Abundance and distribution of sharks in Northeast Florida waters and identification of potential nursery habitat. Mar Coast Fish 5: 200–210. [Google Scholar]
- McCallum H, Harvell D, Dobson A (2003) Rates of spread of marine pathogens. Ecol Lett 6: 1062–1067. [Google Scholar]
- McGregor F, Richardson AJ, Armstrong AJ, Armstrong AO, Dudgeon CL (2019) Rapid wound healing in a reef manta ray masks the extent of vessel strike. PLoS One 14: e0225681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Quintana AN, Vázquez-Haikin JA, Merkling T, Blanchard P, Osorio-Beristain M (2018) Ecotourism impacts on the behaviour of whale sharks: an experimental approach. Oryx 54: 270–275. [Google Scholar]
- Mourier J, Buray N, Schultz JK, Clua E, Planes S (2013) Genetic network and breeding patterns of a sicklefin lemon shark (Negaprion acutidens) population in the Society Islands, French Polynesia. PLoS One 8: e73899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Stevens JD (2008) The biology and ecology of the blue shark, Prionace glauca In Sharks of the Open Ocean: Biology, Fisheries and Conservation, pp. 140–151. Oxford: Blackwell Scientific Publications.
- Parton KJ, Galloway TS, Godley BJ (2019) Global review of shark and ray entanglement in anthropogenic marine debris. Endanger Species Res 39: 173–190. [Google Scholar]
- Perry CT, Figueiredo J, Vaudo JJ, Hancock J, Rees R, Shivji M (2018) Comparing length-measurement methods and estimating growth parameters of free-swimming whale sharks (Rhincodon typus) near the south Ari Atoll, Maldives. Mar Fresh Res 69: 1487–1495. [Google Scholar]
- Pierce SJ, Norman B (2016) Rhincodon typus The IUCN Red List of Threatened Species 2016. e.T19488A2365291.
- Quiros AL (2007) Tourist compliance to a code of conduct and the resulting effects on whale shark (Rhincodon typus) behavior in Donsol, Philippines. Fish Res 84: 102–108. [Google Scholar]
- Ramírez-Macías D, Meekan M, De La Parra-Venegas R, Remolina-Suarez F, Trigo-Mendoza M, Vazquez-Juarez R (2012) Patterns in composition, abundance and scarring of whale sharks Rhincodon typus near Holbox Island, Mexico. J Fish Biol 80: 1401–1416. [DOI] [PubMed] [Google Scholar]
- Ramírez-Macías D, Queiroz N, Pierce SJ, Humphries NE, Sims DW, Brunnschweiler JM (2017) Oceanic adults, coastal juveniles: tracking the habitat use of whale sharks off the pacific coast of Mexico. PeerJ 5: e3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch LJ, Martin KJ, Cooper RL, Metscher BD, Underwood CJ, Fraser GJ (2016) An ancient dental gene set governs development and continuous regeneration of teeth in sharks. Dev Biol 415: 347–370. [DOI] [PubMed] [Google Scholar]
- Redfern JV, Mckenna MF, Moore TJ, Calambokidis J, Deangelis ML, Becker EA, Barlow J, Forney KA, Fiedler PC, Chivers SJ (2013) Assessing the risk of ships striking large whales in marine spatial planning. Conserv Biol 27: 292–302. [DOI] [PubMed] [Google Scholar]
- Reif WE (1978) Wound healing in sharks. Zoomorphologie 90: 101–111. [Google Scholar]
- Riley MJ, Hale MS, Harman A, Rees RG (2010) Analysis of whale shark Rhincodon typus aggregations near South Ari Atoll, Maldives archipelago. Aquat Biol 8: 145–150. [Google Scholar]
- Rommel SA, Costidis AM, Pitchford TD, Lightsey JD, Snyder RH, Haubold EM (2007) Forensic methods for characterizing watercraft from watercraft-induced wounds on the Florida manatee (Trichechus manatus latirostris). Mar Mam Sci 23: 110–132. [Google Scholar]
- Rowat D, Gore MA, Baloch BB, Islam Z, Ahmad E, Ali QM, Culloch RM, Hameed S, Hasnain SA, Hussain B et al. (2007) New records of neonatal and juvenile whale sharks (Rhincodon typus) from the Indian Ocean. Environ Biol Fishes 82: 215–219. [Google Scholar]
- Rowat D, Meekan MG, Engelhardt U, Pardigon B, Vely M (2006) Aggregations of juvenile whale sharks (Rhincodon typus) in the Gulf of Tadjoura, Djibouti. Environ Biol Fishes 80:465–472. [Google Scholar]
- Schindelin J, Arganda-carreras I, Frise E, Kaying V, Longair M, Pietzsch T, Preibsch S, Rueden C, Saalfeld S, Schmid B et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman RP, Patterson-Abrolat C, Plön S (2020) A global review of vessel collisions with marine animals. Front Mar Sci 7. [Google Scholar]
- Sequeira AM, Mellin C, Meekan MG, Sims DW, Bradshaw CJ (2013) Inferred global connectivity of whale shark Rhincodon typus populations. J Fish Biol 82: 367–389. [DOI] [PubMed] [Google Scholar]
- Sequeira AM, Mellin C, Fordham DA, Meekan MG, Bradshaw CJ (2014) Predicting current and future global distributions of whale sharks. Glob Chang Biol 20: 778–789. [DOI] [PubMed] [Google Scholar]
- Sims DW, Southall EJ, Tarling GA, Metcalfe JD (2005) Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. J Anim Ecol 74: 755–761. [Google Scholar]
- Speed CW, Meekan MG, Rowat D, Pierce SJ, Marshall AD, Bradshaw CJA (2008) Scarring patterns and relative mortality rates of indian ocean whale sharks. J Fish Biol 72: 1488–1503. [Google Scholar]
- Stelfox M, Hudgins J, Sweet M (2016) A review of ghost gear entanglement amongst marine mammals, reptiles and elasmobranchs. Mar Pollut Bull 111: 6–17. [DOI] [PubMed] [Google Scholar]
- Team RC (2019) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Towner A, Smale MJ, Jewell O (2012) Boat strike wound healing in Carcharodon carcharias In ML Domeier, ed, Global Perspectives on the Biology and Life History of the White Shark. CRC Press, Boca Raton, FL, pp. 77–84. [Google Scholar]
- Tyminski JP, de la Parra-Venegas R, González Cano J, Hueter RE (2015) Vertical movements and patterns in diving behavior of whale sharks as revealed by pop-up satellite tags in the eastern Gulf of Mexico. PLoS One 10: e0142156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tienhoven AM, Den Hartog JE, Reijns RA, Peddemors VM (2007) A computer-aided program for pattern-matching of natural marks on the spotted raggedtooth shark Carcharias taurus. J Appl Ecol 44: 273–280. [Google Scholar]
- Womersley FC, Leblond ST, Rowat DRL (2016) Scarring instance and healing capabilities of whale sharks and possible implications. The 4th International Whale Shark Conference. Hamad bin Khalifa University Press; 2016: 67. [Google Scholar]
- Zasloff M (2011) Observations on the remarkable (and mysterious) wound-healing process of the bottlenose dolphin. J Invest Dermatol 131: 2503–2505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


