Abstract
Bladder cancer (BC), a common urologic cancer, is the fifth most frequently diagnosed tumor worldwide. hsa-miR-34a displays antitumor activity in several types of cancer. However, the functional mechanisms underlying hsa-miR-34a in BC remains largely unknown. We observed that hsa-mir-34a levels were significantly and negatively associated with clinical disease stage as well as regional lymph node metastasis in human BC. In a series of in vitro investigations, overexpression of hsa-miR-34a inhibited cell migration and invasion in BC cell lines 5637 and UMUC3 as detected by Transwell assays. We further found that hsa-miR-34a inhibited cell migration and invasion by silencing matrix metalloproteinase-2 (MMP-2) expression and thus interrupting MMP-2-mediated cell motility. Our analysis of BC datasets from The Cancer Genome Atlas database revealed a negative correlation between hsa-miR-34a and MMP-2. Moreover, higher MMP-2 protein expression was observed in the BC tissues when compared with that noted in the normal tissue. MMP-2 levels were also significantly associated with clinical disease stage and poor survival rate in human BC. These findings indicate that MMP-2 plays a critical role in regulating BC progression. Therefore, hsa-miR-34a is a promising treatment to target MMP-2 for the prevention and inhibition of cell migration and invasion in BC.
Keywords: bladder cancer, microRNA-34a-5p, migration, invasion, MMP-2
Introduction
Bladder cancer (BC) is the fifth most commonly diagnosed tumor worldwide and the second leading cause of death in patients with genitourinary tract malignancies (1). Annually, approximately 430,000 new BC cases and 165,000 cancer deaths are reported globally, according to the International Agency for Research on Cancer and the World Health Organization (2). The vast majority (an estimated 80%) of BC is diagnosed as nonmuscle invasive (NMIBC), also known as superficial BC, and the remaining 20% are muscle (i.e., muscularis propria)-invasive BC (MIBC) (3). The overall survival rate of patients diagnosed with NMIBC is high, although 30–50% of NMIBC will recur after transurethral resection and 10–20% will progress to MIBC (4). MIBC is responsible for the vast majority of BC-specific deaths, and approximately 50% of patients with MIBC present a higher incidence of distant metastasis than those with NMIBC at the time of diagnosis (5). Currently, transurethral resection of a bladder tumor (TURBT) and intravesical chemotherapy are still considered the standard of care for bladder cancer. However, not all patients are suitable for chemotherapy. Viable treatment alternatives are warranted in the treatment of BC.
Tumor metastasis is a complex and continuous multistep process encompassing local adhesion, migration, and invasion that also accompany a variety of proteases, particularly matrix metalloproteinases (MMPs) (6). MMPs, a group of calcium (Ca2+)- and zinc (Zn2+)-dependent endopeptidases, are responsible for the tissue remodeling and degradation of the extracellular matrix (ECM) leading to tumor cell migration and invasion in the metastatic environment (7,8). In total, 24 genes encoding MMPs in humans have been identified and many are implicated in cancer (9). For instance, MMP-1, MMP-3, and MMP-9 expression levels are high in human chondrosarcoma cells, and MMP-9 is positively correlated with clinical outcome parameters in chondrosarcoma (10). The secretion of MMP-2 and MMP-9 is elevated in various types of human cancers, and their expression is closely related to clinical staging, lymph node metastasis, and poor prognosis (11–13).
MicroRNAs (miRNAs/miRs), consisting of approximately 22 nucleotides, are non-coding RNAs (14) that bind to target mRNAs and suppress protein translation by either blocking the translation or destabilizing mRNA levels (15). Many miRNAs are classified as either tumor-suppressive (TS) miRNAs or oncogenic miRNAs, also known as oncomirs (16). miR-34a is a TS miRNA, and its expression is epigenetically silenced in several human cancers (17,18). Expression of hsa-miR-34a stimulates cell apoptosis, cell cycle arrest, cellular senescence, epithelial-mesenchymal transition (EMT) repression, and impedes cell proliferation in cancer stem cells (19). Moreover, hsa-miR-34a is downregulated in BC tissues and has been demonstrated to inhibit migration and invasion by silencing Notch1 (20) or CD44 (21). Overexpression of hsa-miR-34a may also inhibit cell migration and invasion and thus suppress metastasis in hepatocellular carcinoma (22). In breast cancer, low hsa-miR-34a expression was found to advance cell invasion in vitro and distant metastasis in vivo by directly silencing proto-oncogene Fos-related antigen-1 (Fra-1) expression (23). These findings highlight hsa-miR-34a as a potential therapeutic target and a clinical biomarker of cancer progression and metastasis.
The present study revealed that hsa-miR-34a expression is negatively associated with clinical disease stage, regional lymph node metastasis, and overall survival rate in patients with BC. In vitro evidence revealed that hsa-miR-34a suppressed MMP-2 expression, leading to inhibition of BC cell motility. Another critical clinical characteristic of MMP-2 was its higher expression in late stage than in early stage BC. Moreover, high expression of MMP-2 was found to be significantly associated with a lower survival rate for patients with BC. This study provides insight into the mechanisms underlying hsa-miR-34a-mediated cell migration and invasion through MMP-2 silencing in BC.
Materials and methods
Cell culture
Human BC cell lines (5637 and UMUC3) and human bladder epithelial cell line (SV-HUC-1) were obtained from Bioresource Collection and Research Center (Hsinchu, Taiwan) and incubated at 37°C under 5% CO2. SV-HUC-1 cells were cultured in F-12 medium (Gibco; Thermo Fisher Scientific, Inc.). 5637 and UMUC3 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). All culture media were supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMAX-1, 100 U/ml penicillin, and 100 µg/ml streptomycin.
hsa-miR-34a stable clones in BC cells
MicroRNA expression vectors were constructed by annealing a paired oligonucleotide consisting of mature hsa-miR-34a-5p sequences and cloning it into a small-RNA expression vector (pSM-vector), as previously described (24). Culture cells with 70% confluence were transfected with pSM-34a-5p in 6-well plates for 24 h prior to the administration of G418 selection antibiotic. The transfection was performed using polymer-based transfection reagents (Ultra293, GeneDirex, Taipei, Taiwan) according to manufacturer's instructions. The cell viability of each transfected cell line was tested to investigate whether miRNA disrupted BC cell proliferation.
Transfection of hsa-miR-34a mimic and inhibitor
MISSION synthetic negative control (NC) mimic (5′-GGUUCGUACGUACACUGUUCA-3′), hsa-miR-34a-5p mimic (5′-UGGCAGUGUCUUAGCUGGUUGU-3′), NC inhibitor (5′-GGUUCGUACGUACACUGUUCA-3′) and hsa-miR-34a-5p inhibitor (5′-ACAACCAGCUAAGACACUGCC-3′) were purchased from Sigma-Aldrich/Merck KGaA. Cells were seeded in 6-well dishes with 2 ml culture medium. Viromer® BLUE (Lipocalyx GmbH, Germany), miRNA transfection reagent, was used to transfect hsa-miR-34a mimic (100 nM) or inhibitor (100 nM) in cells for 24 h. Cell samples were then evaluated for MMP-2 expression and the ability of cell migration and invasion by Western blot analysis and Transwell migration assay, respectively.
Luciferase reporter assay
Wild-type (5′…CACUGCC…3′) and mutant (5′…GAGAGGC…3′) plasmids of human MMP-2 3′-UTR (untranslated region) containing the miR-34a-5p binding site were constructed in pmiR-GLO (Promega Corp.) by MDBio, Inc. (Taipei, Taiwan). BC cells were seeded in 6-well dishes and transfected with the plasmid (1 µg/µl), using Viromer® RED (Lipocalyx GmbH), as per manufacturer's protocol. After 24 h of transfection, cell lysates were harvested and the activities of firefly and Renilla luciferase were detected using Dual-Luciferase kit (Promega Corp.). Firefly luciferase activity was normalized to Renilla luciferase activity.
Western blot analysis
Total proteins were extracted from BC cell lines. The samples containing 20 µg of total protein were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and proteins were transferred to Immobilon polyvinyldifluoride membranes. Bovine serum albumin (4%) was used to block membranes for 1 h at room temperature; the membranes were then probed with primary anti-MMP-2 (dilution 1:3,000; GeneTex; cat. no. GTX104577), anti-b-actin (dilution 1:5,000; Merck; cat. no. MAB1501) and anti-GAPDH (dilution 1:5,000; Abcam; cat. no. ab8245) antibodies at 4°C overnight. The membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (dilution 1:3,000; Cell Signaling; cat. no. 7074S) or anti-mouse antibody (dilution 1:3,000; Sigma-Aldrich/Merck KGaA; A9044) at 37°C for 1 h. Enhanced chemiluminescence was then added to the blots, which were imaged using a ChemiDoc-It Imaging System (UVP Inc.).
Quantitative real-time polymerase chain reaction
Total RNA extracted from BC cells was reverse transcribed and subjected to quantitative real-time polymerase chain reaction (qPCR) to detect MMP-2 and GAPDH as previously described (25).
Migration and invasion assays
Transwell inserts in 24-well dishes (8-µm pore size; Costar) were used to perform cell migration and invasion. For invasion assays, Transwell inserts were precoated with 30 µl Matrigel basement membrane matrix (BD Biosciences) for 30 min. The human BC cells in 200 µl serum-free medium were seeded in Transwell inserts with a 300 µl medium containing 1% FBS and placed in the lower chamber. Cell migration and invasion were imaged under ×200 magnification by Eclipse Ti2 microscope (Nikon) after 24 h.
Analysis of publicly available database
Using the TCGA dataset of bladder urothelial carcinoma, the correlations among MMP-2, hsa-mir-34a expression, race, tumor histology, molecular subtype, clinical stage, and nodal metastasis status were analyzed for each tumor sample through the UALCAN web server (http://ualcan.path.uab.edu/) (26). Overall survival rate between patients with BC with high and low hsa-miR-34a or MMP-2 expression was analyzed by OncoLnc (http://www.oncolnc.org/).
Statistical analysis
Statistical analyses were performed using SigmaPlot 10.0 (Systat Software, Inc.) and GraphPad Prism 7 (GraphPad Software. Inc.). The values are expressed as the mean ± standard deviation (SD). All differences between experimental groups and controls were assessed for significance using Student's t-test. Between-group differences were considered to be significant if the P-value was <0.05.
Results
Clinical importance of hsa-miR-34a in human BC based on the TCGA Database
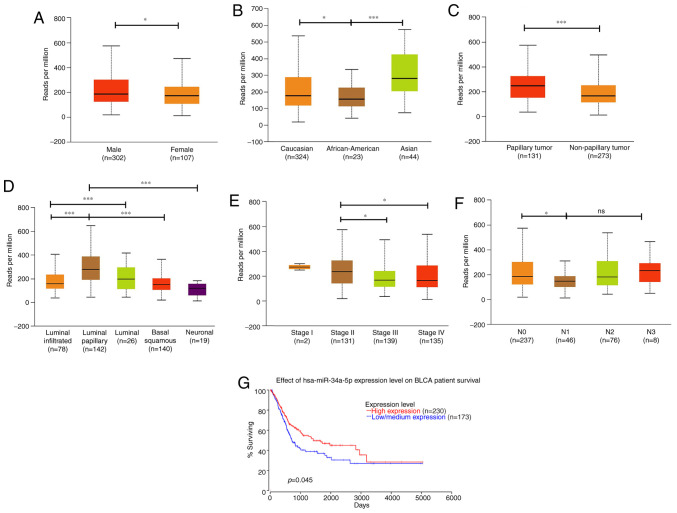
Numerous studies have classified hsa-mir-34a as a tumor suppressor because it inhibits tumorigenesis, and hsa-mir-34a is considered a potential therapeutic candidate for cancer (27). However, the clinical importance of hsa-mir-34a in human BC is largely unknown. We therefore analyzed the expression pattern of hsa-mir-34a by using different variables from The Cancer Genome Atlas (TCGA) Urothelial Bladder Carcinoma datasets through the UALCAN web server. Results revealed that hsa-miR-34a had a lower expression in female individuals diagnosed with BC (n=107) than in male individuals (n=302) (P=0.04628; Fig. 1A). Notably, hsa-miR-34a expression was more enhanced in Asians (n=44) than in Caucasians (n=324; P=0.0195) and African-Americans (n=23; P=0.00007; Fig. 1B). This result indicates that hsa-miR-34a expression varies according to genetic differences between races. We further analyzed the expression pattern of hsa-miR-34a in tumor histology and molecular subtypes of BC. Papillary urothelial carcinoma (n=131), which progresses more slowly and has a more straightforward treatment and a more favorable prognosis than other types of BC (28), exhibited higher hsa-miR-34a expression than nonpapillary samples (n=273; P=0.00061; Fig. 1C). Moreover, MIBC has five molecular subtypes, namely luminal-papillary, luminal-infiltrated, luminal, basal-squamous, and neuronal (28). We found that the luminal-papillary subtype (n=142) exhibited higher levels of hsa-miR-34a expression than did the other four molecular subtypes (Fig. 1D). By contrast, the neuronal subtype (n=19), which has the poorest clinical outcome of all the subtypes, exhibited lower levels of hsa-miR-34a expression than did the luminal-papillary subtype (P=0.000002; Fig. 1D). These data indicate that hsa-miR-34a may be positively associated with favorable clinical outcomes.
Figure 1.
UALCAN portal analysis of BC samples based on the TCGA database. (A) Comparison of hsa-miR-34a expression between male and female individuals in BC samples. (B-F) Expression of hsa-miR-34a for different races, tumor histology, molecular subtypes, clinical stages, and nodal metastasis status of BC samples. (G) Overall survival rate between patients with BC with high and low hsa-miR-34a expression. All data are expressed as the mean ± the SD of triplicate samples. *P<0.05, ***P<0.001 compared with the control group; ns, not significant. BC, bladder cancer; TCGA, The Cancer Genome Atlas.
Next, we analyzed hsa-miR-34a expression in clinical stages and nodal metastasis status of BC samples. Results indicated that late stage (stage III and IV) had lower hsa-miR-34a expression than did early stage (stage II) tumors (stage II vs. III: P=0.025879; stage II vs. IV: P=0.046764; Fig. 1E). Moreover, significant associations were observed between low hsa-miR-34a expression levels and regional lymph node metastasis (N0 vs. N1: P=0.010535; Fig. 1F). OncoLnc, a new TCGA data portal, focuses on survival correlations by using mRNA or miRNA expression data. Using OncoLnc analysis, we found that low hsa-miR-34a expression levels were significantly correlated with a low survival rate for patients with BC (P=0.045; Fig. 1G). These data indicate that hsa-miR-34a, as a tumor suppressor, has significant associations with clinical and histopathologic characteristics in patients with BC.
hsa-miR-34a impedes cell migration and invasion in BC cells
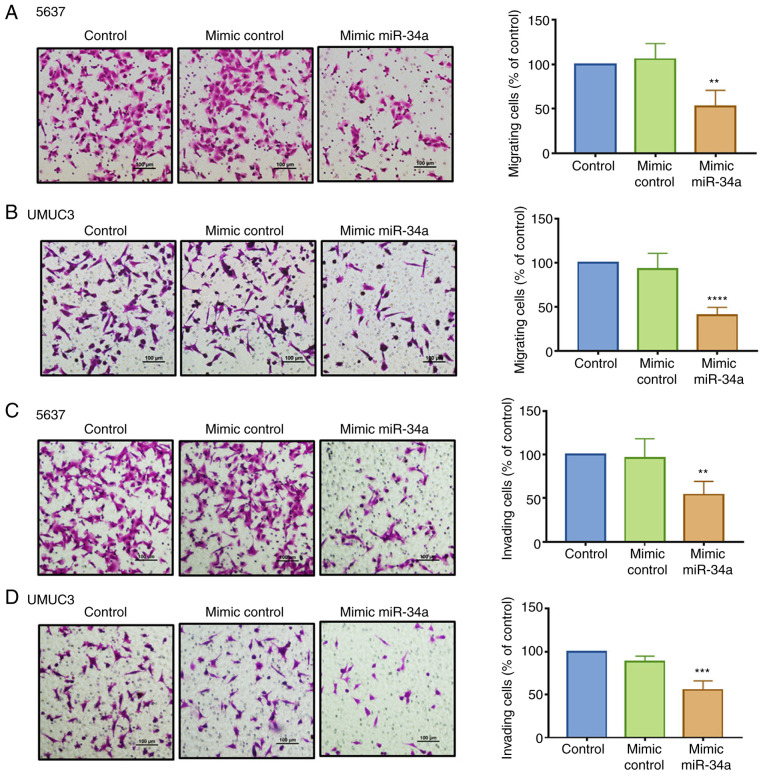
hsa-miR-34a can inhibit cancer cell proliferation and migration (29). Therefore, we investigated whether hsa-miR-34a exhibits antitumor effects in inhibiting BC cell motility. An hsa-miR-34a mimic was used to analyze its function in regulating cell migration and invasion by employing a Transwell assay. We found that compared with the control mimic, hsa-miR-34a mimic significantly suppressed cell migration (Fig. 2A and B) and invasion ability (Fig. 2C and D) in the 5637 and UMUC3 cell lines. The groups between the control and control mimic exhibited no such effects. The successful transfection of hsa-miR-34a mimic was confirmed (Fig. S1A). These data demonstrated that hsa-miR-34a reduced cell migration and invasion in BC cells.
Figure 2.
hsa-miR-34a affects the functions of cell migration and invasion in BC cells. (A-D) Cells were transfected with control or hsa-miR-34a mimic for 24 h after which cell migration and invasion were performed using Transwell assays. The number of migrated or invaded cells were quantified. All data are expressed as the mean ± SD of triplicate samples. **P<0.01, ***P<0.001, ****P<0.0001 compared with the control group. BC, bladder cancer.
hsa-miR-34a directly targets MMP-2
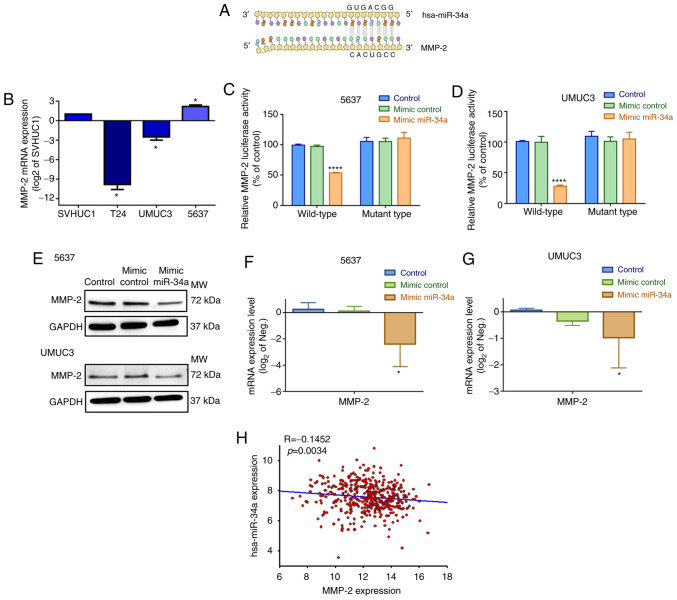
The action of miRNAs appears to involve mRNA silencing by binding to the 3′-UTRs (untranslated regions) at the miRNA recognition elements of target mRNAs (30). Subsequently, we investigated which target mRNA hsa-mir-34a binds to when impeding cell motility. On the basis of an analysis using the miRNA target prediction program (microRNA.org), we confirmed that hsa-miR-34a directly targets the 3′-UTR of MMP-2 (Fig. 3A). Therefore, we hypothesized that hsa-miR-34a affects BC cell migration and invasion through MMP-2 silencing. First, we assessed basal MMP-2 expression levels in three BC cell lines. The 5637 and UMUC3 cells exhibited significantly higher MMP-2 mRNA and protein expression than T24 cells (Figs. 3B and S1B).
Figure 3.
hsa-miR-34a suppresses MMP-2 expression through direct binding. (A) An miRNA target prediction program (microRNA.org) was used to identify miRNAs that potentially bind to the MMP-2 3′-UTR. (B) Levels of MMP-2 mRNA expression in different BC cell lines were determined by qPCR assay. (C and D) Cells were cotransfected with wild-type or mutant MMP-2 3′-UTR (1 µg/µl) and control mimic or hsa-miR-34a mimic (100 nM) for 24 h, and luciferase activities were measured. (E-G) Cells were transfected with control mimic or hsa-miR-34a mimic for 24 h; the levels of MMP-2 protein and mRNA expression were measured using western blot analysis and qPCR assay, respectively. (H) Correlation between hsa-miR-34a and MMP-2 expression in BC samples. All data are expressed as the mean ± the SD of triplicate samples. *P<0.05, ****P<0.0001 compared with the mimic control group. MMP-2, matrix metalloproteinase-2; BC, bladder cancer.
To confirm that hsa-miR-34a directly binds to the 3′-UTR of MMP-2 and inhibits MMP-2 mRNA translation, we constructed wild-type and mutant MMP-2-3′-UTR luciferase plasmids containing hsa-miR-34a binding sites. After transfection of 5637 and UMUC3 cells with wild-type luciferase plasmids, we found that the hsa-miR-34a mimic inhibited luciferase activity, but the mutant type had no such affect (Fig. 3C and D). Moreover, the hsa-miR-34a mimic diminished the levels of endogenous MMP-2 protein and mRNA expression in 5637 and UMUC3 cells (Fig. 3E-G). hsa-miR-34a expression was also negatively correlated with MMP-2 mRNA in human BC samples (Fig. 3H). According to our data, high hsa-miR-34a expression levels are associated with improved clinical outcomes and significant suppression of MMP-2 expression by combining with the 3′-UTR region of MMP-2 mRNA.
Hsa-miR-34a regulates cell motility by suppressing MMP-2 expression
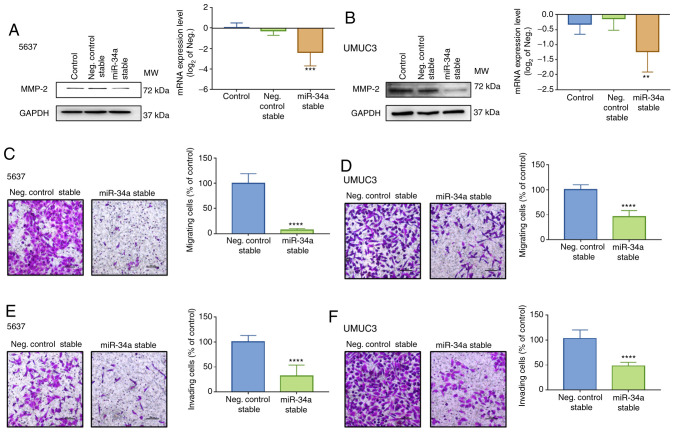
To confirm that MMP-2 is a critical mediator involved in hsa-miR-34a-regulated cell migration and invasion, BC cell lines 5637 and UMUC3 that stably expressed control or hsa-miR-34a were established. Low levels of MMP-2 expression were measured in hsa-miR-34a stable cells (Fig. 4A and B) and hsa-miR-34a inhibitor reversed the MMP-2 downregulation (Fig. S1C). The successful transfection of hsa-miR-34a inhibitor was verified (Fig. S1D). Moreover, hsa-mir-34a stable cells exhibited low mobility (Fig. 4C and D) and invasiveness (Fig. 4E and F). Thus, hsa-miR-34a regulates cell migration and invasion by suppressing MMP-2 expression in BC cells.
Figure 4.
Inhibition of MMP-2 expression suppresses cell motility in BC cells stably expressing hsa-miR-34a. (A and B) BC cells stably expressing control or hsa-miR-34a were established. The levels of MMP-2 protein and mRNA expression were determined using western blot analysis and qPCR assay, respectively. (C-F) Cell migration and invasion abilities in stable cells were determined using Transwell assays. The number of migrated or invaded cells were quantified. All data are expressed as the mean ± SD of triplicate samples. **P<0.01, ***P<0.001, ****P<0.0001 compared with the control group. MMP-2, matrix metalloproteinase-2; BC, bladder cancer.
Clinicopathologic characteristics of MMP-2 in patients with BC
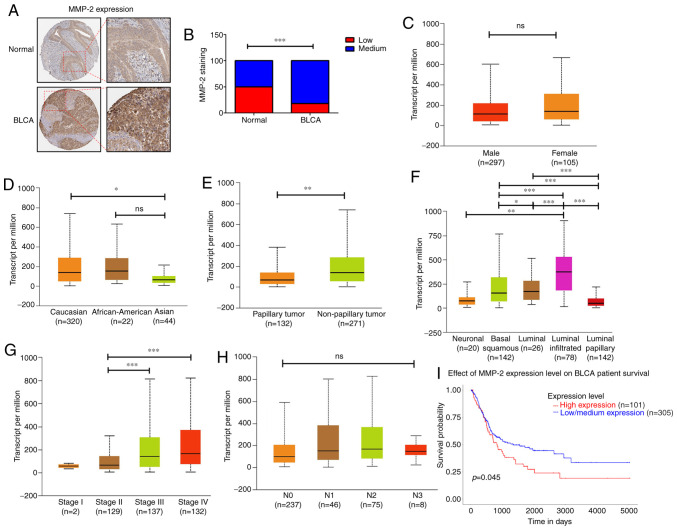
To further investigate the role of MMP-2 in human BC, we analyzed the clinical importance of MMP-2 in human BC tissue samples derived from the Human Protein Atlas database. MMP-2 protein levels were higher in tumor specimens than in normal tissues (Fig. 5A and B). The data presented in Fig. 5C-H revealed that, in BC patients, MMP-2 expression differed according to sex, race, tumor histology, molecular subtype, tumor stages, and regional lymph node metastasis. Overexpression of MMP-2 was higher in Caucasians than in Asians (P=0.012; Fig. 5D). Similarly, MMP-2 expression was significantly elevated in BC tissues of nonpapillary (P=0.0043; Fig. 5E) or luminal-infiltrated subtypes (luminal-infiltrated vs. luminal-papillary: P=1.76E-12; Fig. 5F). Additionally, the mRNA levels of MMP-2 in patients with stage III and IV BC were clearly higher than those in patients with stage II (stage II vs. stage III: P=0.00002; stage II vs. stage IV: P=0.00001; Fig. 5G). We also found that high levels of MMP-2 expression were significantly correlated with low survival rates for patients with BC (P=0.045; Fig. 5I). However, no relationship was observed between MMP-2 expression and other clinical features such as sex (Fig. 5C) and regional lymph node metastasis (Fig. 5H). Taken together, our results indicate that increased MMP-2 might predict a poor prognosis for patients with BC, and MMP-2 is an excellent target for developing BC treatments.
Figure 5.
Levels of MMP-2 expression correlate with clinicopathologic features of BC. (A and B) Representative immunohistochemistry images of MMP-2 protein expression in BC tissues and normal bladder tissues. MMP-2 staining intensity was quantified. (C) Comparison of MMP-2 expression between male and female individuals in BC samples. (D-H) MMP-2 expression for different race, tumor histology, molecular subtypes, clinical stages, and nodal metastasis status of BC samples. (I) Survival probability between patients with BC with high and low MMP-2 expression. All data are expressed as the mean ± SD of triplicate samples. *P<0.05, **P<0.01, ***P<0.001 compared with the control group; ns, not significant. MMP-2, matrix metalloproteinase-2; BC, bladder cancer.
Discussion
Bladder cancer (BC) is a common urologic cancer (31). Surgery and chemotherapy are useful standard treatments; however, tumor recurrence rates (an estimated 70% within 5 years) remain high, and progression to invasive disease is extremely common (32). The poor prognosis in patients with BC is related to high local recurrence rates and distant metastases (33). Developing a novel and safer BC treatment that improves patient outcomes and recurrence rates is essential. This study investigated the therapeutic potential of hsa-miR-34a in inhibiting BC cell migration and invasion.
The extracellular matrix (ECM) in the tumor microenvironment plays a vital role in regulating cancer progression (34). Notably, MMPs are involved in every step of tumor progression, such as promoting angiogenesis (35), tumor growth, and distant metastasis (36). MMP-14 silencing significantly reduced tumor cell migration and invasion ability (37). Similarly, MMP-9 silencing was found to diminish cell migration and invasion in glioma cells (38). Furthermore, in vivo analysis revealed that increased MMP-2 levels are correlated with melanoma progression (39). MMP-9 knockdown significantly inhibited tumor growth and metastasis in a mouse xenograft model of triple-negative breast cancer (40). In the present study, we found that high MMP-2 expression levels were significantly correlated with low survival rates in patients with BC. The mRNA levels of MMP-2 in patients with stage III and IV BC were markedly higher than those in patients with stage II. We further demonstrated that MMP-2 mediates BC cell migration and invasion. From a clinical perspective, MMP expression is correlated with tumor aggressiveness, disease stage, and poor prognosis in patients with cancers (41,42). Together, these findings indicate that MMPs are an attractive target for cancer therapeutics. Cancer clinical trials have been conducted with numerous MMP inhibitors, including peptidomimetics, nonpeptidomimetics inhibitors, and tetracycline derivatives, which target MMPs in the extracellular space (43). However, the results have been disappointing and associated complications, such as musculoskeletal pain and inflammation, have been of great concern (43). Therefore, new drugs for the treatment of BC should be developed.
As crucial modulators in cellular pathways, microRNAs are instrumental in tumorigenesis and metastasis by targeting numerous oncogenes simultaneously through translation repression or mRNA degradation (44). A thorough understanding of associated downstream and upstream miRNAs is essential for successful treatment. In BC, hsa-miR-34a has been reported to attenuate metastasis and chemoresistance by reducing the levels of TCF1 and LEF1 (45). Also, hsa-miR-34a was found to inhibit BC cell migration and invasion by upregulating tumor-suppressor gene PTEN (46). Our study results concur with these findings, by showing that hsa-miR-34a inhibits MMP-2 expression and thereby suppresses cell migration and invasion in BC cells. The relevant results indicate that the hsa-miR-34a/MMP-2 axis is involved in the regulation of the antitumor effect of esophageal squamous cell carcinoma and glioma (47,48). In addition, we also found that hsa-miR-34a was significantly correlated with clinical and histopathologic characteristics in human BC. However, hsa-miR-34a-mediated MMP-2 expression and tumor invasiveness should be evaluated in vivo to confirm these findings. In multiple myeloma, intratumor or exogenous delivery of lipidic-formulated synthetic hsa-miR-34a mimic triggered antitumor action in an in vivo animal model (49,50). Related results revealed that systemic transfer of hsa-miR-34a mimic to tumors in an in vivo lung cancer animal model resulted in extraordinary suppression of tumor growth, suggesting the effectiveness of hsa-miR-34a replacement therapy in lung cancer (51,52). Notably, MRX34, a liposomal formulation of nanoparticles packed with hsa-miR-34a mimics, is progressing to a phase I clinical trial (NCT01829971) for primary liver cancer, several solid tumors, and hematopoietic malignancies (27). Therefore, hsa-miR-34a is attractive as a potential treatment in cancers.
Although prior studies have emphasized the potential benefit of miRNA expression profiles in the prognosis for BC patients (53,54), their value concerning ethnicity is unfamiliar. Caucasians are about twice as likely to develop BC as African-Americans, and Asians have slightly lower rates of BC (55). The reasons for these differences are not well understood. In the present investigation, we found that tumor-suppressor hsa-miR-34a has higher expression in Asians than Caucasians and African-Americans. This result suggests a racial-association for hsa-miR-34a in BC progression. hsa-miR-34a may repress tumorigenicity, especially in the Asian population, leading to a lower prevalence of BC. However, further investigation is still needed to prove the specificity of interaction between hsa-miR-34a and ethnicity.
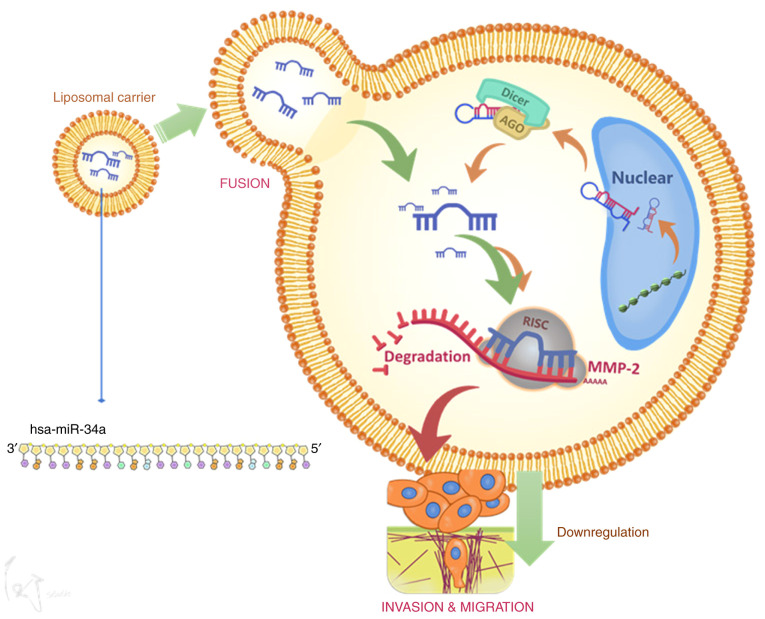
In conclusion, hsa-miR-34a inhibits MMP-2 expression and thus suppresses cell migration and invasion (Fig. 6). These results may provide significant therapeutic opportunities for human BC.
Figure 6.
Schematic image depicting the mechanism by which hsa-miR-34a modulates cell motility through MMP-2 downregulation in BC cells. Modeling revealed that hsa-miR-34a directly targets the 3′-UTR of MMP-2 mRNA and thus suppresses MMP-2 translation. Overexpression of hsa-miR-34a in human BC cells may represent a potential treatment strategy. MMP-2, matrix metalloproteinase-2; BC, bladder cancer.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of the TCGA Research Network: https://www.cancer.gov/tcga. We thank Jiun-Lin Lai for his preparation of the schematic summary of Fig. 6.
Funding Statement
This work was supported by the Ministry of Science and Technology, Taiwan (MOST-108-2314-B-341-001-) and Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-106-DR-09).
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST-108-2314-B-341-001-) and Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-106-DR-09).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
Conceptualization of the study was achieved by KYC and TISH. Data curation was conducted by ACC and PCC. Formal analysis was carried out by TFT and YCL. Investigation of the data and results was conducted by KYC and ACC. Methodology was overseen by KYC. Project administration was carried out by HEC and CYH. Resources were provided by PCC and TFT. Software analysis was overseen by ACC and PCC. Supervision was carried out by CYH, YCL, HEC and TISH. Writing of the original draft was carried out by KYC and ACC. Writing of the review and editing were conducted by KYC and TISH.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors of this article declare that they do not have any competing interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian J Urol. 2015;31:289–296. doi: 10.4103/0970-1591.166445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2,596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. Discussion 475-467. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T. Understanding the biology of urothelial cancer metastasis. Asian J Urol. 2016;3:211–222. doi: 10.1016/j.ajur.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer-roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15:1085–1091. doi: 10.7314/APJCP.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 8.Nannuru KC, Futakuchi M, Varney ML, Vincent TM, Marcusson EG, Singh RK. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010;70:3494–3504. doi: 10.1158/0008-5472.CAN-09-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Kesh K, Gupta A, Swarnakar S. An overview of matrix metalloproteinase 9 polymorphism and gastric cancer risk. Asian Pac J Cancer Prev. 2015;16:7393–7400. doi: 10.7314/APJCP.2015.16.17.7393. [DOI] [PubMed] [Google Scholar]
- 10.Malcherczyk D, Heyse TJ, El-Zayat BF, Kunzke V, Moll R, Fuchs-Winkelmann S, Paletta JRJ. Expression of MMP-9 decreases metastatic potential of Chondrosarcoma: An immunohistochemical study. BMC Musculoskelet Disord. 2018;19:9. doi: 10.1186/s12891-017-1920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett. 2017;14:5865–5870. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis ST, Leite KR, Piovesan LF, Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP, Srougi M, Dall'Oglio MF. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012;12:18. doi: 10.1186/1471-2490-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou W, Yu X, Sun S, Zhang X, Yang W, Zhang J, Zhang X, Jiang Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed Pharmacother. 2019;118:109369. doi: 10.1016/j.biopha.2019.109369. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan K, Sivasankar V. MicroRNAs-Biology and clinical applications. J Oral Maxillofac Pathol. 2014;18:229–234. doi: 10.4103/0973-029X.140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichhorn SW, Guo H, McGeary SE, Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villén J, Bartel DP. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol Cell. 2014;56:104–115. doi: 10.1016/j.molcel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond SM. MicroRNAs as oncogenes. Curr Opin Genet Dev. 2006;16:4–9. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 18.Chim CS, Wong KY, Qi Y, Loong F, Lam WL, Wong LG, Jin DY, Costello JF, Liang R. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis. 2010;31:745–750. doi: 10.1093/carcin/bgq033. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H, Wang B, Ouyang J, Zhang X. Inhibitory effects of microRNA-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting Notch1. J Huazhong Univ Sci Technolog Med Sci. 2012;32:375–382. doi: 10.1007/s11596-012-0065-z. [DOI] [PubMed] [Google Scholar]
- 21.Yu G, Yao W, Xiao W, Li H, Xu H, Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014;33:779. doi: 10.1186/s13046-014-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Zhou W, Kong F, Xiao X, Kuang H, Zhu Y. microRNA-34a overexpression inhibits cell migration and invasion via regulating SIRT1 in hepatocellular carcinoma. Oncol Lett. 2017;14:6950–6954. doi: 10.3892/ol.2017.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Li Y, Gao J, Zhang T, Li S, Luo A, Chen H, Ding F, Wang X, Liu Z. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–4303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 24.Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- 25.Lin JF, Lin YC, Yang SC, Tsai TF, Chen HE, Chou KY, Hwang TI. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des Devel Ther. 2016;10:1501–1513. doi: 10.2147/DDDT.S95900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Liao Y, Tang L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inamura K. Bladder cancer: New insights into its molecular pathology. Cancers (Basel) 2018;10:100. doi: 10.3390/cancers10040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Yuan L, Luo J, Gao J, Guo J, Xie X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med. 2013;13:109–117. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 30.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 31.Yaxley JP. Urinary tract cancers: An overview for general practice. J Family Med Prim Care. 2016;5:533–538. doi: 10.4103/2249-4863.197258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Z, Shen T, Wientjes MG, O'Donnell MA, Au JL. Intravesical treatments of bladder cancer: Review. Pharm Res. 2008;25:1500–1510. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pierro GB, Gulia C, Cristini C, Fraietta G, Marini L, Grande P, Gentile V, Piergentili R. Bladder cancer: A simple model becomes complex. Curr Genomics. 2012;13:395–415. doi: 10.2174/138920212801619232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Xiong G, Trinkle C, Xu R. Integrated extracellular matrix signaling in mammary gland development and breast cancer progression. Histol Histopathol. 2014;29:1083–1092. doi: 10.14670/hh-29.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, Ramírez-Camacho MA, Alvarez-Sánchez ME. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda J, Kajita M, Suenaga N, Fujii K, Seiki M. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: Importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003;22:8716–8722. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- 38.Lakka SS, Rajan M, Gondi C, Yanamandra N, Chandrasekar N, Jasti SL, Adachi Y, Siddique K, Gujrati M, Olivero W, et al. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–8019. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000;191:245–256. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH632>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5:2736–2749. doi: 10.18632/oncotarget.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 42.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 43.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 44.Skaftnesmo KO, Prestegarden L, Micklem DR, Lorens JB. MicroRNAs in tumorigenesis. Curr Pharm Biotechnol. 2007;8:320–325. doi: 10.2174/138920107783018390. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Liu X, Wu Y, Fang Z, Wu Q, Wu C, Hao Y, Yang X, Zhao J, Li J, et al. MicroRNA-34a attenuates metastasis and chemoresistance of bladder cancer cells by targeting the TCF1/LEF1 axis. Cell Physiol Biochem. 2018;48:87–98. doi: 10.1159/000491665. [DOI] [PubMed] [Google Scholar]
- 46.Ding ZS, He YH, Deng YS, Peng PX, Wang JF, Chen X, Zhao PY, Zhou XF. MicroRNA-34a inhibits bladder cancer cell migration and invasion, and upregulates PTEN expression. Oncol Lett. 2019;18:5549–5554. doi: 10.3892/ol.2019.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Song X, Zhu J, Li M, Ji Y, Wu F, Chen Y, Cui X, Hu J, Wang L, et al. Tumor suppressor microRNA-34a inhibits cell migration and invasion by targeting MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J Oncol. 2017;51:378–388. doi: 10.3892/ijo.2017.4015. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Xing F, Yuan J, Li Z, Zhang W. Sevoflurane inhibits migration and invasion of glioma cells via regulating miR-34a-5p/MMP-2 axis. Life Sci. 2020;256:117897. doi: 10.1016/j.lfs.2020.117897. [DOI] [PubMed] [Google Scholar]
- 49.Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli E, et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: In vitro and in vivo evidence. Clin Cancer Res. 2012;18:6260–6270. doi: 10.1158/1078-0432.CCR-12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan D, Zhou X, Chen X, Hu DN, Dong XD, Wang J, Lu F, Tu L, Qu J. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest Ophthalmol Vis Sci. 2009;50:1559–1565. doi: 10.1167/iovs.08-2681. [DOI] [PubMed] [Google Scholar]
- 51.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyrskjøt L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL, Andersen CL, Zieger K, et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y, Ma X, Chen L, Li H, Gu L, Gao Y, Zhang Y, Li X, Fan Y, Chen J, Zhang X. MicroRNAs with prognostic significance in bladder cancer: A systematic review and meta-analysis. Sci Rep. 2017;7:5619. doi: 10.1038/s41598-017-05801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Husseini MJ, Kunbaz A, Saad AM, Santos JV, Salahia S, Iqbal M, Alahdab F. Trends in the incidence and mortality of transitional cell carcinoma of the bladder for the last four decades in the USA: A SEER-based analysis. BMC Cancer. 2019;19:46. doi: 10.1186/s12885-019-5267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.