Abstract
IMPORTANCE
Axicabtagene ciloleucel, an anti-CD19-CD28-CD3ζ chimeric antigen receptor T-cell therapy, was the first US Food and Drug Administration–approved, genetically engineered T-cell therapy for adults with relapsed or refractory large B-cell lymphoma (LBCL) after 2 or more lines of systemic therapy. There has not been a US Food and Drug Administration–approved product for these cancers in more than 4 decades.
OBSERVATIONS
Unlike traditional anticancer therapies, axicabtagene ciloleucel is a patient-specific, live-cell product that has unique requirements for manufacturing, shipping, and storage, as well as for its administration and management of its adverse events. In addition, axicabtagene ciloleucel has demonstrated efficacy in patients with refractory LBCL. This review presents a timeline of the rapid clinical development of axicabtagene ciloleucel from bench to bedside, highlights how axicabtagene ciloleucel satisfies an unmet medical need for treatment of refractory LBCL, outlines the logistics of the production process and administration of axicabtagene ciloleucel, describes its mechanism of action, and summarizes the results of the pivotal study. This review also provides a survey of adverse events, with attention to the kinetics of their clinical presentation; discusses the management of adverse events; and offers suggestions for appropriate patient selection for safe administration of axicabtagene ciloleucel.
CONCLUSIONS AND RELEVANCE
The integration of axicabtagene ciloleucel therapy into standard-of-care practice for relapsed/refractory LBCL is the beginning of a paradigm shift in the treatment of patients with LBCL and is likely to lead to improvements in their survival and curability. Timely referral to centers offering the therapy is necessary for optimal patient outcomes.
Axicabtagene ciloleucel is an autologous, anti-CD19 chimeric antigen receptor (CAR) T-cell therapy with significantly improved efficacy in patients with refractory large B-cell lymphoma (LBCL) compared with other currently available treatment options.1 Although standard-of-care therapies can cure approximately 60% of patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL),2,3 outcomes for patients with relapsed or refractory LBCL are poor.4–8 A large, international, retrospective patient-level analysis of outcomes in refractory LBCL prior to the availability of CAR T-cell therapy, SCHOLAR-1, reported an objective response rate (ORR) of 26%, a complete response (CR) rate of 7%, and a short median overall survival of 6.3 months,1 highlighting the need for innovative therapeutic approaches.
The antigen CD19 is an optimal target for CAR T-cell therapy given its expression across a wide range of B-cell cancers and its restricted expression in healthy tissues.9,10 However, targeting CD19 can lead to B-cell aplasia and hypogammaglobulinemia, similar to routinely used CD20-targeting monoclonal antibody therapies, and immunoglobulin supplementation can mitigate the increased risk of infections attributable to B-cell aplasia.11 B-cell recovery has been observed 2 years after receiving anti-CD19 CAR T-cell therapy in patients with ongoing CR.12
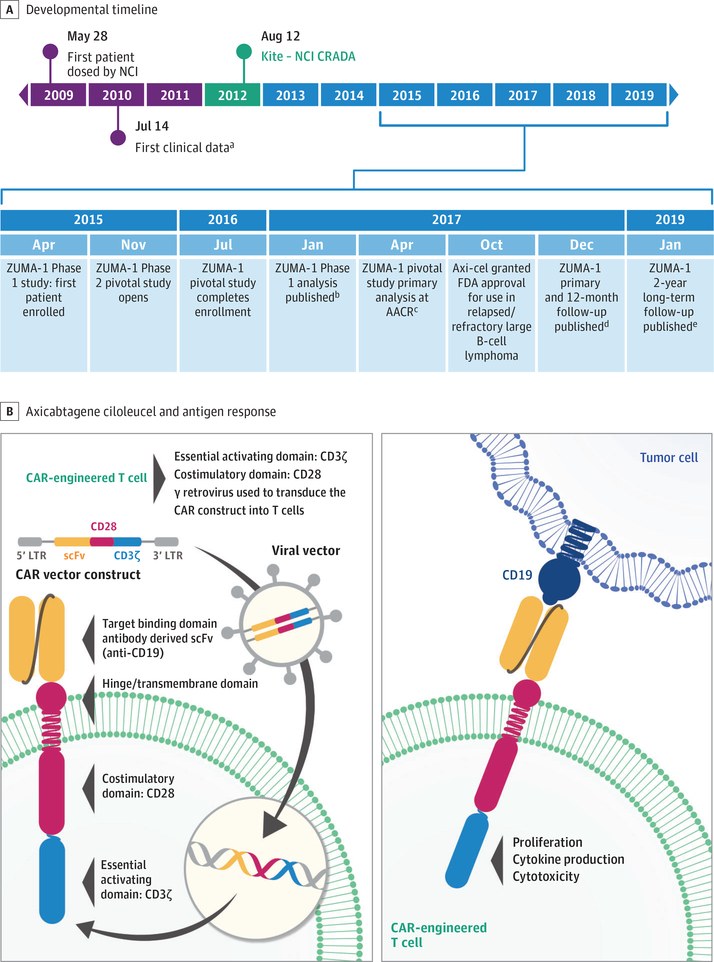
The cell product that became axicabtagene ciloleucel was collaboratively developed with Kite, a Gilead Company, and the National Cancer Institute (Figure 1A).13–17 Early work revealed that, although first-generation CAR T cells (ie, CARs with only a single T-cell signaling domain) demonstrated cytotoxic activity, they lacked functional persistence.18–20 For optimal T-cell activity, antigen recognition must occur in conjunction with appropriate costimulation, such as through CD28.21 In 2009, Kochenderfer and colleagues22 described the development of the CAR construct used in axicabtagene ciloleucel, which consists of an extracellular CD19-targeting, single-chain variable fragment, an intracellular CD28 costimulatory domain, and a CD3ζ signaling domain (Figure 1B). This development was quickly followed in 2010 by, to our knowledge, the first reported successful use of this CAR in a patient with B-cell lymphoma.13
Figure 1. Axicabtagene Ciloleucel Developmental Timeline and Structure.
Developmental timeline (A) with structure of axicabtagene ciloleucel and antigen response (B). AACR indicates American Association of Cancer Research; CAR, chimeric antigen receptor; CRADA, cooperative research and development agreement; LTR, long terminal repeat; NCI, National Cancer Institute; scFv, single-chain variable fragment; ZUMA, long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma.
aFirst clinical data reported by Kochenderfer et al.13
bAnalysis reported by Locke et al.14
cAnalysis reported by Locke et al.15
dFollow-up reported by Neelapu et al.16
eFollow-up reported by Locke et al.17
Additional studies from the National Cancer Institute using the CD19-CD28-CD3ζ CAR demonstrated the importance of conditioning therapy with cyclophosphamide and fludarabine before CAR T-cell infusion. The conditioning regimen was systematically investigated and optimized in 14 different conditioning dose cohorts in early phase trials.23,24 Conditioning therapy likely induces depletion of healthy lymphocytes, eliminating cytokine sinks and making homeostatic cytokines available for the survival and proliferation of adoptively transferred T cells,25 as well as possibly eradicating immunosuppressive cells, that is, regulatory T cells and myeloid-derived suppressor cells, leading to improved CAR T-cell function.25 Subsequent studies have confirmed the importance of the homeostatic cytokine environment after conditioning, notably that increased serum IL-15 promotes response to CAR T-cell therapy.12,26,27
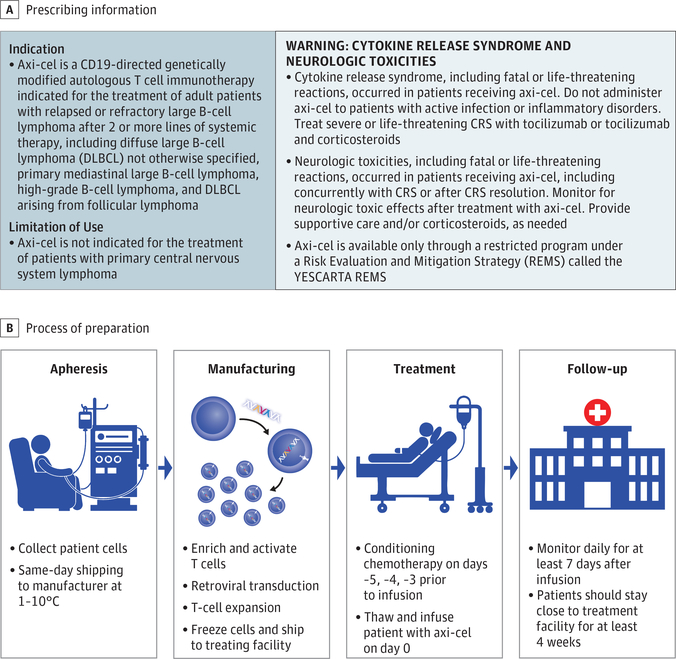
The high response rates observed in these early studies led to further development of this CAR construct as axicabtagene ciloleucel, which uses a closed manufacturing process. ZUMA-1, the pivotal, multicenter, phase 1/2 trial, investigated axicabtagene ciloleucel in refractory LBCL.16,17 Axicabtagene ciloleucel demonstrated unprecedented efficacy in ZUMA-1 and was approved by the US Food and Drug Administration (FDA) in October 2017 for the treatment of adult patients with relapsed or refractory LBCL after 2 or more lines of systemic therapy.28 Axicabtagene ciloleucel was the first product approved by the FDA for relapsed/refractory LBCL (Figure 2A).
Figure 2. Axicabtagene Ciloleucel Prescribing Information and Delivery.
A, Indication, limitations of use, and warning from the prescribing information.28 B, Axicabtagene ciloleucel (axi-cel) process from patient to manufacturer, and back to the patient at 1 °C to 10 °C. CRS indicates cytokine release syndrome; G1, grade 1; G2, grade 2; G3, grade 3.
Manufacturing and Administration
The ZUMA-1 study demonstrated that the centralized manufacturing of axicabtagene ciloleucel was rapid and reliable with coordination of leukapheresis and shipping from multiple centers across the United States (Figure 2B). Axicabtagene ciloleucel manufacturing was initiated by harvesting patient T cells using outpatient leukapheresis of peripheral blood mononuclear cells without mobilization. Following leukapheresis, no bridging chemotherapy was allowed. Fresh apheresis material was transported at 1 °C to 10 °C to the central cell-processing facility where mononuclear cells were enriched with a closed Ficoll gradient (Sepax 2; Biosafe) system and activated with recombinant anti-CD3 monoclonal antibody and IL-2.29 Activated T cells were transduced with a γ-retroviral vector containing the CAR gene and expanded in culture. Once the target dose was achieved (2 × 106 CAR T cells/kg; maximum dose, 2 × 108 CAR T cells), the product was washed and cryopreserved, assessed by quality control testing, and shipped to the treatment site. Before administration, the patient received conditioning chemotherapy: 3 days of cyclophosphamide, 500 mg/m2/d, and fludarabine, 30 mg/m2/d.
In ZUMA-1, this process successfully manufactured axicabtagene ciloleucel for 99% (110/111) of patients enrolled, with 91% (101/111) of patients receiving treatment despite interpatient variability in apheresis material composition and baseline lymphocyte counts, including patients with severe lymphopenia (<300 lymphocytes/μL in the peripheral blood).16,30,31 The median turnaround time from apheresis to delivery of the final axicabtagene ciloleucel product to the treating center was 17 days. Short turnaround time was necessary for patients with rapidly growing disease and high tumor burden. The manufacturing process for commercial axicabtagene ciloleucel product is the same Good Manufacturing Practice–adherent process used for ZUMA-1.
Efficacy
In ZUMA-1, axicabtagene ciloleucel demonstrated durable efficacy in patients with refractory LBCL (Table).16,17,24,32–34 Eligible patients must have had progressive or stable disease as the best response to their last line of systemic therapy or have relapsed within 12 months from high-dose chemotherapy and autologous stem-cell transplantation. Phase 1 results in 7 patients with DLBCL (ORR, 71% with 4 of 7 [57%] having a CR) led to the pivotal, phase 2 portion of the trial.14,15 The expanded phase 2 trial assessed patients in 2 cohorts: 77 patients with DLBCL (cohort 1) and 24 patients with transformed follicular lymphoma or primary mediastinal B-cell lymphoma (cohort 2).15 Cancer in these patients was highly refractory, with 26% (26/101) having a history of primary refractory disease and 53% (54/101) having a history of resistance to 2 or more consecutive lines of previous therapy.
Table.
Anti–CD-19 CAR T Cells in Clinical Trials
| Study | NCI24 | ZUMA-116,17 | JULIET32 | TRANSCEND33 |
|---|---|---|---|---|
| ClinicalTrials.gov identifier | NCT00924326 | NCT02348216 | NCT02445248 | NCT02631044 |
| CAR T investigated | Anti-CD19 CAR T | Axicabtagene ciloleucel |
Tisagenlecleucel | Lisocabtagene maraleucel |
| Patient population | 15 Enrolled; 15 treated; DLBCL (n = 9); CLL (n = 4); indolent lymphoma (n = 2) | 111 Enrolled; 108 treated; DLBCL (n = 84); PMBCL/TFL (n = 24) | 165 Enrolled; 111 treated; 79% DLBCL; 19% TFL | 134 Leukapheresed; 114 treateda; 102 treated and evaluable; DLBCL (n = 63); TFL (n = 23); TMCL/TCLL (n = 12); FL/PMBCL (n = 4) |
| Bridging therapy allowed | No | No | Yes | Yes |
| Length of follow-up | Not reported | Median, 27.1 mo | Median, 14 mo | Median, 12 mo (8 mo for duration of response) |
| Overall population ORR/CR, % | 80/53 | 83/58 | 52/40 | 75/55b |
| Relapsed/refractory ORR | Chemorefractoryc: 63%; relapse after ASCT:100% | Refractory to ≥ second-line: 83%; relapse <12 mo after ASCT: 76% | Refractory to last line: 55%; relapsed to last line: 45%; prior transplant: 49% | Chemorefractoryc: 41%; relapse <12 mo after ASCT: 53% |
| Median duration of response, mo | Not reported | 11.1 | NR | NR |
| Range | 1–23 mo | Not reported | Not reported | Not reported |
| 95% CI | Not reported | 4.2-NE | 10.0-NR | 5-NR |
| Median PFS, mo (95% CI) | Not reported | 5.9(3.3–15.0) | 2.9 (2.2–4.2)34 | Not reported |
| Median OS | Not reported | NR (12.8-NE) | 12 (7-NR) | NR (10.4-NR) |
| Cytokine release syndromed | Not reported | 11% Grade ≥3 | 22% Grade 3–4 | 1% Grade 3–4 |
| Neurologic events | 40% Grade ≥3 | 32% Grade ≥3 | 12% Grade 3–4 | 13% Grade 3–4 |
| Manufacturing success rate; median turnaround time | 100% (15/15); not reported | 99% (117/118); 17 de | Not reported; 54 df | 99% (including 12 nonconforming); not reported |
Abbreviations: ASCT, autologous stem-cell transplant; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CR, complete response; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NCI, National Cancer Institute; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PMBCL, primary mediastinal B-cell lymphoma; TCLL, transformed chronic lymphocytic leukemia; TFL, transformed follicular lymphoma; TMCL, transformed mantle cell lymphoma.
Twelve patients received nonconforming product.
Responses in the pivotal, core population, that is, patients with not otherwise specified DLBCL or high-grade B-cell lymphoma.
No achievement of partial response or CR after most recent chemotherapy in patient with DLBCL.
Cytokine release syndrome (CRS) was graded using the Penn CRS grading scale35 in JULIET and modified Lee criteria36 in ZUMA-1 and TRANSCEND.
Time from leukapheresis to delivery at infusion center.
Time from enrollment to infusion.
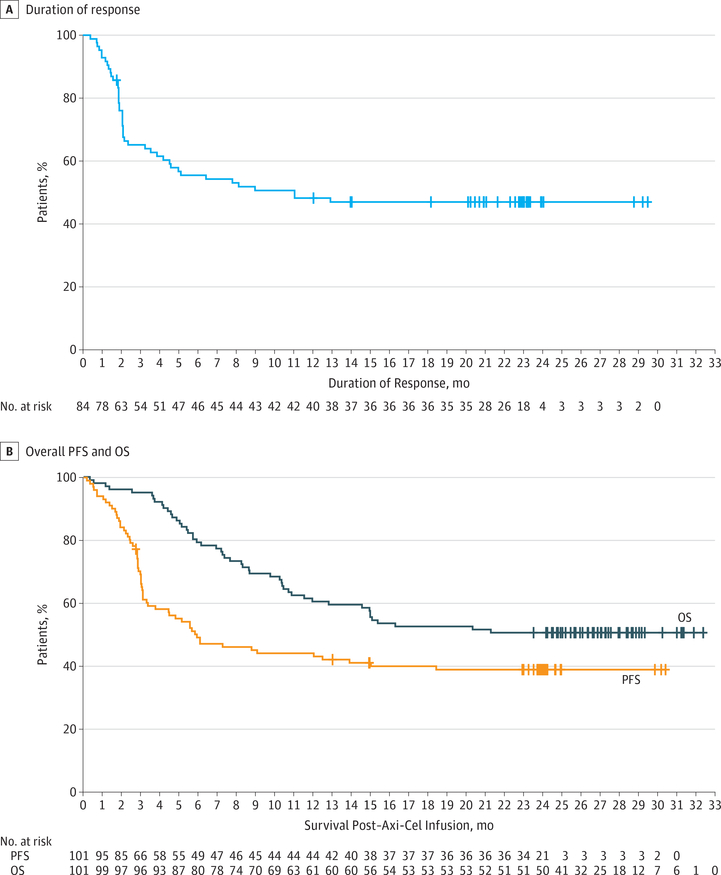
Among the patients in the phase 2 trial who received axicabtagene ciloleucel, the best ORR was 83% and the highest CR rate was 58%.17 With a median follow-up of 27.1 months, 39% (39/101) of the patients remained in response, and 37% (37/101) remained in CR (Figure 3A). The median progression-free survival was 5.9 months, and the median overall survival was not reached (Figure 3B). Responses were dynamic, particularly in the first 3 months following infusion, with the emergence of a plateau in the progression-free survival curve at approximately 6 months. The median time to response was 1 month, and over 50% of progression events occurred by 3 months. The 1-month restaging was valuable to identify early progression necessitating alternative therapy, but 11 of 33 patients (33%) with partial response and 11 of 24 patients (46%) with stable disease at 1 month converted to CR up to 12 months after axicabtagene ciloleucel treatment, suggesting that these patients should be observed closely for deepening responses. Patients in CR or partial response at 3 months after axicabtagene ciloleucel infusion had an approximately 75% likelihood of remaining in response at 2 years without any subsequent intervention.
Figure 3. Efficacy Outcomes for Patients Treated With Axicabtagene Ciloleucel (Axi-Cel).
A, Median duration of response, 11.2 months (95% CI, 4.2-not reached [NR]). B, Median overall survival (OS), NR (95% CI, 12.8-NR); progression-free survival (PFS), 5.9 (95% CI, 3.3-15.0).
In addition, the National Cancer Institute has reported ongoing responses beyond 4 years with the same CD19-CD28-CD3ζ CAR construct as axicabtagene ciloleucel.12 Thus,these combined results suggest that a single axicabtagene ciloleucel treatment, without consolidation or maintenance, may lead to long-term control of refractory LBCL for patients without curative options.17,37 Although not vali-dated in the relapsed/refractory setting, analysis of first-line studies suggests that 2-year progression-free survival may be able to estimate the probability of durable remissions beyond 5 years.38
Data from other anti-CD19 CAR T-cell therapies show comparable efficacy in related populations (Table). Tisagenlecleucel (previously called CTL019), targets CD19 using the same single-chain variable fragment as axicabtagene ciloleucel, a similar CD3ζ-signaling domain, but instead contains a 4–1BB costimulatory domain.39 JULIET, the phase 2 study of tisagenlecleucel in relapsed/refractory DLBCL, demonstrated a 52% ORR (40% CR) with a median follow-up of 14 months (range, 0.1–26).32 Lisocabtagene maraleucel (formerly called JCAR017) targets CD19, also using the same single-chain variable fragment as axicabtagene ciloleucel, and contains a 4–1BB costimulatory domain and CD3ζ signaling domain and is manufactured with a defined 1:1 CD4:CD8 T-cell ratio. TRANSCEND NHL 001, the phase 1 study of lisocabtagene maraleucel in patients with relapsed/refractory, aggressive B-cell non-Hodgkin lymphoma, demonstrated a 75% ORR (55% CR) with a median follow-up of 12 months in its pivotal population.33 Direct comparisons of efficacy results between the pivotal trials are impractical owing to differences in patient populations, trial de-signs, and follow-up periods. Studies coordinated by a multicenter outcome registry, such as the database managed by the Center for International Blood and Marrow Transplant Research,40 may provide insights on the relative clinical activity of different CAR T-cell therapies.
Safety
Acute Adverse Events
Chimeric antigen receptor T-cell therapy is associated with serious and potentially life-threatening class-specific acute adverse events (AEs), namely cytokine release syndrome (CRS) and neurologic events.36,41 ZUMA-1 demonstrated that, with appropriate AE management training of clinicians, axicabtagene ciloleucel can be safely and effectively administered at multiple sites, including many sites without prior CAR T-cell experience. Vigilant monitoring for early AE detection and rapid intervention using preestablished management guidelines can decrease the likelihood of more severe AEs. In ZUMA-1, although nearly all patients developed any grade CRS (94/101 [93%]) most commonly manifesting as pyrexia (77/101 [76%)], severe, grade 3 or higher CRS was observed in only 12 of 108 patients (11%).16,17 The incidence of grade 3 or higher of CRS decreased from 17% in the first 69 patients to 0% in the final 39 patients treated in ZUMA-1. This improvement was likely owing to revised management guidelines with earlier use of tocilizumab for grade 2 CRS implemented during the latter part of the trial. Of 108 patients, 72 patients (67%) experienced any-grade neurologic events, with 32% having grade 3 or higher events.17 This symptom complex is also known as CAR-related encephalopathy syndrome or, most recently, immune effector-cell-associated neurotoxicity syndrome.42,43 Similar to CRS, there was a modest decrease in grade 3 or higher neurologic events over the course of ZUMA-1, from 38% of the first 69 patients to 23% of the final 39 patients.17 There were no new axicabtagene ciloleucel–related cases of CRS or neurologic events after the primary (6-month) analysis.16,17 With the exception of those ongoing at the time of death (n = 6), all CRS and neurologic events resolved.16
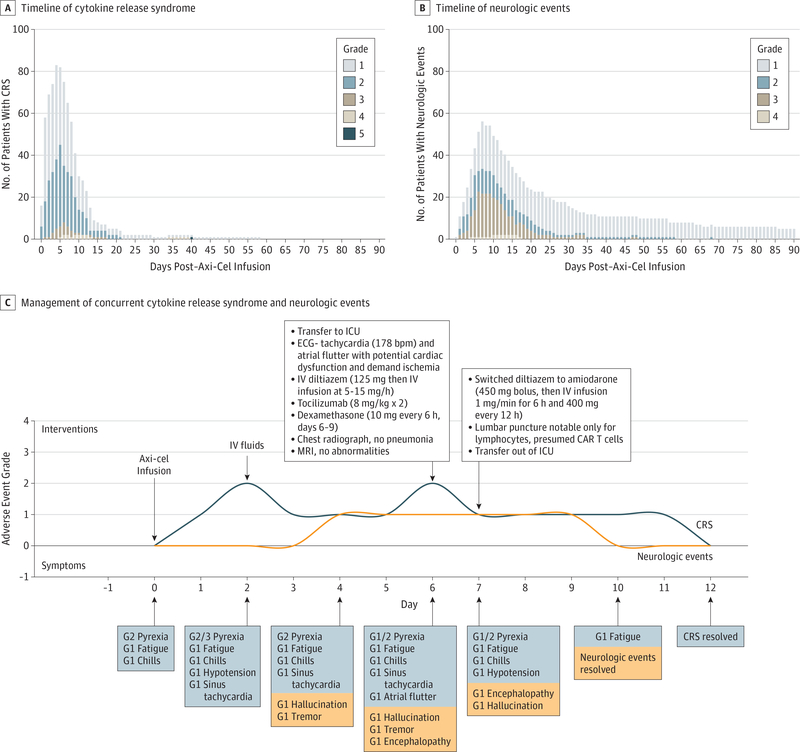
For the practicing clinician, understanding the timing of CRS and neurologic events is important to patient management. In ZUMA-1, following axicabtagene ciloleucel infusion on day 0, the median onset of CRS was on day 2 (range, 1–12), and resolution occurred at a median of day 8.16 The onset of neurologic events was slightly later, occurring at a median of day 5 (range, 1–17) with a median resolution by day 17.16 In addition, understanding the severity of CRS and neurologic events over time is helpful for understanding the clinical course of these events (Figure 4A, B). Between days 30 and 90, 3 patients were classified as having CRS: 2 with grade 1 events ongoing from before day 30 (fatigue [n = 1] and decreased appetite [n = 1], both of which resolved) and 1 death due to hemophagocytic lymphohistiocytosis. The most common neurologic events between days 30 and 90 were grade 1: encephalopathy, tremor, and memory impairment. The onset of nearly all late neurologic events began in the first 30 days post axicabtagene ciloleucel infusion, and all events resolved except for those ongoing at the time of death due to other causes.
Figure 4. Timeline of Adverse Events.
Timeline of cytokine release syndrome (A) and neurologic events (B) after axicabtagene ciloleucel therapy in ZUMA-1, with exact day of the event shown, and a visual case study illustrating management of concurrent cytokine release syndrome and neurologic events (C). Time-to-onset is calculated as (day of infusion, ie, day 0) + 1. Five patients experienced grade 1 neurologic events that were ongoing at day 90 (1 patient each with encephalopathy, hypoesthesia, tremor, and memory impairment; 1 patient with encephalopathy and leukoencephalopathy), and all events subsequently resolved. Axi-Cel indicates axicabtagene ciloleucel; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; ECG, electrocardiogram; G1, grade 1; G2, grade 2; G3, grade 3; ICU, intensive care unit; IV, intravenous; and MRI, magnetic resonance imaging.
Cytokine release syndrome (64/111 [58%]) and neurologic events (23/111 [21%]) were also observed with tisagenlecleucel in the JULIET study with grade 3 to 4 in 24 patients (22%) and 13 patients (12%) patients, respectively.32 In the TRANSCEND evaluation of lisocabtagene maraleucel, CRS (27/73 [37%]) and neurologic events (18/73 [25%]) occurred, with 1 patient (1%) and 11 patients (15%) experiencing grade 3 or higher events, respectively.33 The Penn CRS grading scale35 was used in JULIET compared with ZUMA-1 and TRANSCEND, which used modified Lee criteria for CRS, and the trials had different guidelines for AE management.16,36 A consensus grading system for CRS and neurologic toxic effects was recently published, and there are ongoing efforts to harmonize management of toxic effects.42,43 As with efficacy data, final results from these trials and larger data sets are needed before adequate comparisons are possible.
Late AEs
Late AEs, occurring beyond 12 months and up to 2 years in ZUMA-1, were similar to those in previous reports.12,16,17 In ZUMA-1, 52% of all patients had B-cell aplasia and hypogammaglobulinemia at study entry due to prior anti-CD20 antibody therapy, and cumulatively, 31% of patients received intravenous immunoglobulin therapy to mitigate the risk of infections. Twenty-four of 32 patients (75%) with ongoing responses had detectable B cells by 2 years post axicabtagene ciloleucel infusion. The cumulative incidence of severe grade 3 or higher infectious complications after axicabtagene ciloleucel administration was 28%.17 Between the 12- and 24-month analyses, infections were reported in only 4 patients experiencing 6 infections,and overall, 4 patients developed new serious AEs, but none were related to axicabtagene ciloleucel (grade 3 mental status changes, grade 3 lung infection, grade 3 bacteremia, and grade 4 myelodysplastic syndrome).17,34 Follow-up for the JULIET and TRANSCEND trials is still limited, but similar long-term AE profiles are anticipated. There were no events of insertional mutagenesis reported with the use of the retroviral or lentiviral constructs in any of the 3 trials.16,33,44
AE Management
Adverse events associated with axicabtagene ciloleucel therapy, specifically CRS and neurologic events, are generally manageable and reversible with appropriate treatment strategies. Education for multi disciplinary care teams, patients, and caregivers on CRS and neurologic events symptoms is critical to early intervention and positive outcomes.42 Thorough baseline assessments enable accurate detection of new-onset symptoms, and practitioners should be familiar with available grading scales and recommended treatments.28,36,42 A comprehensive review on the assessment and management of CRS and CAR-related encephalopathy syndrome based on multiple clinical trial experiences across different anti-CD19 CAR T-cell therapies provides detailed, practical clinical guidance for practitioners managing patients treated with CAR T-cell therapy, including axicabtagene ciloleucel.42 There is currently a general consensus that low-grade CRS and CAR-related encephalopathy syndrome may be managed mostly with supportive care and severe events will additionally need tocilizumab, an anti–IL-6 receptor antagonist, and/or corticosteroids.28,42 Tocilizumab was recently FDA approved for the treatment of CAR T-cell–induced CRS.45 In ZUMA-1, 43 of 101 patients (43%) received tocilizumab and 27 of 101 patients (27%) received corticosteroids for AE management. However, the use of tocilizumab or corticosteroids did not affect the ORR, CR rate, or durability of the responses.16 The symptoms of CRS and neurologic events can often wax and wane, which can complicate their management. The case study shown in Figure 4C illustrates this concept as well as timely and appropriate escalation of care. Stage 4 refractory DLBCL in a 42-year-old man with an Eastern Cooperative Oncology Group performance score of 0 and an International Prognostic Index of 3 had progressed after 4 previous treatment regimens. This patient experienced concurrent CRS and neurologic events that, with appropriate management, fully resolved by day 12 after axicabtagene ciloleucel infusion.
Correlative Biomarkers and Mechanisms of Resistance
Correlative studies on ZUMA-1 have provided mechanistic insights into response, resistance, and AEs after axicabtagene ciloleucel therapy. Axicabtagene ciloleucel expansion peaks approximately 7 days after infusion followed by a partial contraction within the first month and a more gradual contraction thereafter.46 Greater CAR T-cell expansion correlates with durable responses and severe neurologic events, but not grade 3 or higher CRS.46,47 However, the lack of correlation between CAR T-cell expansion and grade 3 or higher CRS may be confounded by evolving recommendations to intervene at grade 2 CRS as opposed to previous recommendations to intervene at grade 3. In addition, because only 12 of 108 patients (11%) in ZUMA-1 had grade 3 or higher CRS,17 small patient numbers may further confound correlative analysis. Elevated IL-15 levels following conditioning chemotherapy are associated with higher CART-cell expansion and remission as well as grade 3 or higher neurologic events.12,46 Early upregulation (day 0 and day 1 post infusion) of some myeloid-associated cytokines, such as granulocyte-macrophage colony-stimulating factor and IL-6, has also been associated with severe neurologic events.48
Analyses of tumor samples pretreatment and at progression suggested CD19 loss as one potential mechanism of resistance.16 Expression of immune checkpoint molecules, such as CTLA-4, LAG-3, and programmed death 1 on CAR T cells and programmed death ligand 1 on tumor cells may also be involved in mediating resistance.16,49 Additional biomarker studies may elucidate targets for AE prophylaxis while preserving CAR T-cell function and strategies for further enhancing efficacy.
Patient Selection
Axicabtagene ciloleucel represents an important treatment option for patients with relapsed or refractory LBCL. Understanding patient eligibility, and critically, identifying those who may not be appropriate candidates based on baseline factors, is important for selecting patients. In ZUMA-1, patients with previous T-cell therapy; allogeneic stem-cell transplantation; central nervous system disorders; significant cardiac disease; active autoimmune disease; history of HIV, hepatitis B, or hepatitis C infection; or active infection requiring intravenous antimicrobials were excluded. Simple urinary tract infections and uncomplicated bacterial pharyngitis that were responding to treatment did not preclude eligibility. Other than the presence of active infection and inflammatory disorders, the FDA-approved label does not list any of the above conditions as contraindications and practitioners must carefully evaluate the risks of treating patients outside the ZUMA-1 eligibility criteria. Commercially, at H Lee Moffitt Cancer Center & Research Institute and MD Anderson Cancer Center, it has been the practice to select patients for axicabtagene ciloleucel treatment based on ZUMA-1 eligibility criteria with some caveats. For example, thrombocytopenia may be the result of recent chemotherapy, so a platelet count less than 75 × 103/μL (to convert to ×109 per liter, multiply by 1) but increasing at the time of cell collection might be considered. Similarly, patients with mild cardiac, hepatic, and renal dysfunction; previous allogeneic stem-cell transplantation without active graft-vs-host-disease; and previous T-cell therapy may be considered by experienced practitioners after careful evaluation. In a small study at the National Cancer Institute, treatment of patients with prior allogeneic stem-cell transplantation with CAR T cells generated from the matched donor was found to be safe and did not increase the risk of graft-vs-host-disease.50 Patients with active central nervous system lymphoma could benefit from CAR T-cell therapy as evidenced by case reports51; however, without stronger safety data, we recommend not to treat patients with active central nervous system lymphoma, and these patients should be an important focus of ongoing investigation.
It is necessary to reassess patients for infection and inflammation throughout the treatment journey, specifically before apheresis, conditioning chemotherapy, and axicabtagene ciloleucel infusion. Patients with preexisting active infection and inflammatory conditions are at risk of developing severe CRS and therefore should not be treated with axicabtagene ciloleucel, as cautioned in the prescribing information.14,28,42 Axicabtagene ciloleucel administration can and should be delayed in patients with new fever or symptoms of active infection to allow diagnostic workup and antimicrobial treatment (Figure 4). In cases in which fever or other significant constitutional symptoms are believed to be due to rapidly progressive disease rather than infections, therapies to reduce the tumor burden and tumor-induced inflammatory state should be considered before axicabtagene ciloleucel infusion, even if the product has already been manufactured. In ZUMA-1, patients with the lowest quartile of tumor burden demonstrated a high ongoing response rate (67%) with minimal grade 3 or higher CRS (4%) and neurologic events (7%); as such, debulking bridging therapy may improve the efficacy and safety profiles in patients with high disease burden.52 Considering the risks of the therapy and given current safety data, we do not believe that axicabtagene ciloleucel should be used in patients with major comorbidities or very poor performance status.
Cost of Care and Economic Viability
Chimeric antigen receptor T-cell therapy for DLCBL is a single-use therapy with the potential for long-term disease control. While the product price of CAR T-cell therapy exceeds the 1-time cost of most other oncologic agents, almost all of the other agents require repeated use with diminishing returns for prolonged remission. Additional costs are incurred from CAR T-cell administration, including those associated with patient safety testing, apheresis, product shipping and receiving, conditioning chemotherapy, outpatient visits, inpatient hospitalization, and supportive care. Further hidden costs include the maintenance of the FDA Risk Evaluation and Mitigation Strategies program for commercial therapy, infrastructure necessary for long-term safety reporting, and the maintenance of professional accreditation.
However, CAR T-cell therapy offers value to patients with refractory LBCL who otherwise face a lifespan measured in months.53 Two different post hoc analyses of cost-effectiveness of ZUMA-1 indicated a cost-effective gain in survival compared with salvage chemotherapy,54,55 although longer follow-up is needed to confirm the cost-effectiveness of this therapy.
There is concern among medical experts and hospital administrators that Medicare & Medicaid rules do not currently allow for full cost recovery following inpatient CAR T-cell therapy administration. This restriction places hospitals at risk as the product cost must be payed upfront and carried over until the payer provides reimbursement. The amount effectively reimbursed for all required services by payers and the economic burden that the therapy might place on patients should be explored in future studies.
Future Directions
CAR T-cell therapy represents the beginning of a paradigm shift in the management of relapsed or refractory LBCL. Axicabtagene ciloleucel, with its ability to provide long-term disease control, is a much-needed therapy in this setting as patients lack curative treatment options. In an ongoing, international randomized ZUMA-7 study,56 the efficacy of axicabtagene ciloleucel is being directly compared with the existing standard of care of salvage chemotherapy followed by autologous stem-cell transplantation in patients with DLBCL at first relapse, essentially second-line therapy.57 Other registrational studies are evaluating this anti-CD19 CAR construct in indolent B-cell lymphoma,58 mantle cell lymphoma,59 and acute lymphoblastic leukemias.60,61 In addition, combination therapy with axicabtagene ciloleucel and immune checkpoint blockade with anti-programmed death ligand 1 antibody is currently being investigated in ZUMA-6 to overcome a potential mechanism of tumor resistance.62 In the future, switch technologies may give practitioners the ability to precisely control CAR T-cell levels and activity to improve patient safety.63,64 In addition, bispecific and multispecific CAR T-cell products targeting multiple tumor antigens are in development.65,66 It is hoped that these advancements will overcome mechanisms of resistance, improve the safety profile, and continue to enhance the efficacy of CAR T-cell therapy.
Acknowledgments
Conflict of Interest Disclosures: Dr Locke reported personal fees and nonfinancial support from Kite/Gilead and Novartis, and personal fees from Cellular BioMedicine Group during the conduct of the study; in addition, Dr Locke had a patent to Survivin vaccine pending. Dr Go reported receiving other from Kite, a Gilead Company, other from Gilead Biosciences during the conduct of the study, and other from A2 Biotherapeutics outside the submitted work. Dr Go was previously employed by Kite, a Gilead Company, is currently employed by A2 Biotherapeutics Inc, and has equity ownership in Gilead Sciences Inc and in A2 Biotherapeutics Inc. Dr Neelapu reported receiving grants, personal fees, and nonfinancial support from Kite, a Gilead Company, during the conduct of the study; personal fees from Novartis, grants and personal fees from Allogene, Celgene, Merck, and Unum Therapeutics; grants from BMS, Cellectis, Poseida, Karus, and Acerta; and personal fees from Pfizer, Precision Biosciences, Cell Medica, and Incyte outside the submitted work; in addition, Dr Neelapu had a patent to chimeric antigen receptor against CD79b pending. No other disclosures were reported.
Additional Contributions: We thank the patients who participated in the studies discussed and their families, friends, and caregivers, and the study staff and health care professionals at the clinical study sites included in this review. Medical writing support was provided by Christopher Waldapfel, PharmD (Kite, a Gilead Company), and Skye Geherin, PhD (Nexus Global Group Science LLC), which was funded by Kite, a Gilead Company.
Contributor Information
Frederick L. Locke, Department of Blood and Marrow Transplant and Cellular Immunotherapy, H Lee Moffitt Cancer Center & Research Institute, Tampa, Florida.
William Y. Go, Kite, a Gilead Company, Santa Monica, California.
Sattva S. Neelapu, The University of Texas MD Anderson Cancer Center, Houston, Texas.
REFERENCES
- 1.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817–1826. doi: 10.1016/S0140-6736(13)60313-X [DOI] [PubMed] [Google Scholar]
- 3.Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood. 2016;128(22):469. [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20+ diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol 2012;30(36):4462–4469. doi: 10.1200/JCO.2012.41.9416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88(10):890–894. doi: 10.1002/ajh.23524 [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. doi: 10.1182/blood-2006-08-038257 [DOI] [PubMed] [Google Scholar]
- 8.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. doi: 10.1038/bmt.2015.213 [DOI] [PubMed] [Google Scholar]
- 9.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20(2):149–157. doi: 10.1016/j.coi.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uckun FM, Jaszcz W, Ambrus JL, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71(1):13–29. [PubMed] [Google Scholar]
- 11.Roberts ZJ, Better M, Bot A, Roberts MR, Ribas A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk Lymphoma. 2018;59(8):1785–1796. doi: 10.1080/10428194.2017.1387905 [DOI] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017;35(16):1803–1813. doi: 10.1200/JCO.2016.71.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295. doi: 10.1016/j.ymthe.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke FL, Neelapu SS, Bartlett NL, et al. Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Proc 107th Annu Meet Am Assoc Cancer Res 2017:CT019. [Google Scholar]
- 16.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964 [DOI] [PubMed] [Google Scholar]
- 20.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. doi: 10.1097/CJI.0b013e3181ac6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26(2):111–117. doi: 10.1016/j.it.2004.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bot A, Rossi J, Yizhou J, et al. Cyclophosphamide and fludarabine conditioning chemotherapy induces a key homeostatic cytokine profile in patients prior to CAR T cell therapy. American Society of Hematology Annual Meeting Abstracts. 2015;126(23):4426. [Google Scholar]
- 27.Rossi J, Sherman M, Xue A, Wei-Shen Y, Navale L, Rosenberg S. Low dose conditioning chemotherapy and CD19-directed CAR T cells may elicit distinct immune programs associated with clinical responses. Society for Immunotherapy of Cancer Meeting Abstracts; 2016. [Google Scholar]
- 28.Yescarta [package insert] Santa Monica, CA: Kite Pharma Inc; 2017. [Google Scholar]
- 29.Better M, Chiruvolu V, Sabatino M. Overcoming challenges for engineered autologous T cell therapies. Cell & Gene Therapy Insights 2018;4(4): 173–186. doi: 10.18609/cgti.2018.014 [DOI] [Google Scholar]
- 30.Better M, Chiruvolu V, Oliver J, et al. Production of KTE-C19 (Anti-CD19 CAR T Cells) for ZUMA-1: a phase 1/2 multi-center study evaluating safety and efficacy in subjects with refractory aggressive non-Hodgkin lymphoma (NHL). Mol Ther. 2016;24(suppl 1):S115. doi: 10.1016/S1525-0016(16)33096-9 [DOI] [Google Scholar]
- 31.Better M, Chiruvolu V, Oliver J, et al. Manufacturing and characterization of KTE-C19 in a multicenter trial of subjects with refractory aggressive non-Hodgkin lymphoma (NHL) (ZUMA-1) [abstract]. Proc 105th Annu Meet Am Assoc Cancer Res 2015:2308. [Google Scholar]
- 32.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 33.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 2018;36(suppl):7505. doi: 10.1200/JCO.2018.36.15_suppl.7505 [DOI] [Google Scholar]
- 34.Neelapu SS, Locke FL, Bartlett NL, et al. Long-term follow-up ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130(suppl 1):578 10.1182/blood.V130.Suppl_1.578.578 [DOI] [Google Scholar]
- 35.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303): 303ra139. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavez JC, Locke FL. A possible cure for refractory DLBCL: CARs are headed in the right direction. Mol Ther. 2017;25(10):2241–2243. doi: 10.1016/j.ymthe.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10): 1066–1073. doi: 10.1200/JCO.2013.51.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kymriah [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corp; 2018. [Google Scholar]
- 40.ClinicalTrials.gov Protocol for a Research Database for Hematopoietic Stem Cell Transplantation, Other Cellular Therapies and Marrow Toxic Injuries. NCT01166009 https://clinicaltrials.gov/ct2/show/NCT01166009. Accessed October 8, 2019.
- 41.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DW, Santomasso BD, Locke FL, et al. ASTCTconsensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 44.Borchmann P, Tam CS, Jäger U, et al. An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). European Hematology Association Annual Abstracts 2018:S799 https://www.primeoncology.org/app/uploads/hematology-updates-stockholm-2018-dlbcl-abstract-s799-salles.pdf. Accessed October 9, 2019. [Google Scholar]
- 45.Actemra (tocilizumab) [package insert] South San Francisco, CA: Genentech: I; 2018. [Google Scholar]
- 46.Locke FL, Rossi J, Xue A, et al. Immune signatures of cytokine release syndrome and neurologic events in a multicenter registrational trial (ZUMA-1) in subjects with refractory non-Hodgkin lymphoma treated with axicabtagene ciloleucel (KTE-C19). In: Proceedings of the 107th Annual Meeting of the American Association of Cancer Research; 2017:CT020. [Google Scholar]
- 47.Neelapu SS, Locke FL, Bartlett NL, et al. KTE-C19 (anti-CD19CAR T cells)induces complete remissions in patients with refractory diffuse large B-cell lymphom (DLBCL): results from the pivotal phase 2 ZUMA-1. Blood. 2016;128(22):LBA–6. https://www.semanticscholar.org/paper/Kte-C19-(anti-CD19-CAR-TCells)-Induces-Complete-in-Neelapu-Locke/e2bdf41ecc720520e84352d69e55b290cffdff00. [Google Scholar]
- 48.Locke FL, Sherman M, Rossi J, et al. Early biomarker correlates of severe neurologic events and cytokine release syndrome in ZUMA-1, a multicenter trial evaluating axicabtagene ciloleucel in refractory aggressive non-Hodgkin lymphoma. Society for Immunotherapy of Cancer Meeting Abstracts. 2017:92. [Google Scholar]
- 49.Zhang E, Gu J, Xu H. Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors. Mol Cancer. 2018;17(1):7. doi: 10.1186/s12943-018-0759-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–4139. doi: 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–784. doi: 10.1056/NEJMc1704610 [DOI] [PubMed] [Google Scholar]
- 52.Locke FL, Ghobadi A, Lekakis LJ, et al. Outcomes by prior lines of therapy (LoT) in ZUMA-1, the pivotal phase 2 study of axicabtagene ciloleucel (axi-cel) in patients (pts) with refractory large B-cell lymphoma [published online June 1, 2018]. J Clin Oncol doi: 10.1200/JCO.2018.36.15_suppl.3039 [DOI] [Google Scholar]
- 53.Tice JA, Walsh JME, Otuonye I, et al. Final Evidence Report—CAR-T Therapies for B-Cell Cancers. Boston, MA: Institute for Clinical and Economic Review; 2018. [Google Scholar]
- 54.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238–1245. doi: 10.1080/13696998.2018.1529674 [DOI] [PubMed] [Google Scholar]
- 55.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2(2):e190035. doi: 10.1001/jamanetworkopen.2019.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ClinicalTrials.gov Efficacy of Axicabtagene Ciloleucel Compared to Standard of Care Therapy in Subjects With Relapsed/Refractory Diffuse Large B Cell Lymphoma. NCT03391466 https://clinicaltrials.gov/ct2/show/NCT03391466. Accessed October 8, 2019.
- 57.Oluwole OO, Bishop MR, Gisselbrecht C, et al. ZUMA-7: a phase 3 randomized trial of axicabtagene ciloleucel (Axi-Cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL) [published online June 1, 2018]. J Clin Oncol. doi: 10.1200/JCO.2018.36.15_suppl.TPS7585 [DOI] [Google Scholar]
- 58.ClinicalTrials.gov A Phase 2 Multicenter Study of Axicabtagene Ciloleucel in Subjects With Relapsed/Refractory Indolent Non-Hodgkin Lymphoma NCT03105336 https://clinicaltrials.gov/ct2/show/NCT03105336. Accessed October 8, 2019.
- 59.ClinicalTrials.gov A Phase 2 Multicenter Study Evaluating Subjects With Relapsed/Refractory Mantle Cell Lymphoma NCT02601313 https://clinicaltrials.gov/ct2/show/NCT02601313. Accessed October 8, 2019.
- 60. [Accessed October 8, 2019];A Study Evaluating KTE-X19 in Adult Subjects With Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia (ZUMA-3) ClinicalTrials.gov. NCT02614066. https://clinicaltrials.gov/ct2/show/NCT02614066.
- 61.ClinicalTrials.gov Study Evaluating KTE-C19 in Pediatric and Adolescent Subjects With Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia NCT02625480 https://clinicaltrials.gov/ct2/show/NCT02625480. Accessed October 8, 2019.
- 62.Locke FL, Westin JR, Miklos D, et al. Phase 1 results from ZUMA-6: axicabtagene ciloleucel (axi-cel; KTE-C19) in combination with atezolizumab for the treatment of patients with refractory diffuse large B-cell lymphoma. American Society of Hematology Annual Meeting Abstracts 2017:2628. [Google Scholar]
- 63.Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6(8):e458. doi: 10.1038/bcj.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodgers DT, Mazagova M, Hampton EN, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad SciUSA. 2016;113(4):E459–E468. doi: 10.1073/pnas.1524155113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4 (6):498–508. doi: 10.1158/2326-6066.CIR-15-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. Addendum: T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4(7):639–641. doi: 10.1158/2326-6066.CIR-16-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]