Abstract
Based on the successful derivation of a hapten, we prepared and optimized a murine monoclonal antibody against imidocarb, with an IC50 of 2.22 ng/mL and a limit of detection (LOD) of 0.45 ng/mL. Cross-experiment results showed that the cross-over rate for 4,4′-dinitrocarbanilide was 18.12%, and the cross-reactivity with other analogues when using the ic ELISA was less than 0.1%. We used the developed ic-ELISA to detect the addition and recovery of imidocarb in milk and beef samples, and values were 86.0–93.5 and 84.5–101.2%, respectively. The preparation of an immunochromatographic test strip based on gold nanoparticles was used for the rapid identification of imidocarb in milk and beef samples. When assessed by the naked eye, the visual LOD for imidocarb in milk and beef samples was 5 and 10 ng/mL, and the cut-off values were 20 and 50 ng/mL, respectively. Because of its high sensitivity, specificity, and simplicity, the test strip can be used for on-site testing and rapid screening of imidocarb in food samples.
1. Introduction
One of the most common intestinal protozoans, which is widely spread in the livestock and poultry industries causes coccidiosis, which represents a global and persistent disease and is responsible for the loss of over two billion dollars annually. Anti-coccidiosis drugs can effectively reduce coccidiosis infection and alleviate economic losses. Imidocarb is a type of dinitro anticoccidiosis drug currently in use. Dinitro anticoccidiosis drugs work by preventing access to an energy source during the process of oocyst sporulation in the parasite to bring about inhibition of coccidioides.1
Imidocarb, is a derivative of homodiphenylurea and is an animal-specific antiprotozoan compound (Figure 1A). It causes great damage to the liver function of cattle, sheep, and other animals, and it may lead to death of animals because of cardiovascular and neuromuscular side effects. Although the toxicity and side effects of imidocarb on the human body are still difficult to find out, dairy products with resides of imidocarb are potentially dangerous to the human body.2 In addition, drug residing in livestock products have recently received increasing public attention, which makes the use of anticoccidiosis drugs unsustainable. Detection of imidocarb residues in pork tissue has been found using HPLC–UV, and they were found to localize mainly to the liver, kidneys, and muscle.3 The leading cause of residue formation from imidocarb is the conversion of antibiotics, where they combine tightly with tissues having a high DNA content. According to the European Drug Evaluation Agency, the maximum residue limit (MRL) of imidocarb in cow, sheep, and chicken tissues is 300 μg/kg in muscle, 50 μg/kg in fat, 200 μg/kg in liver, and 500 μg/kg in kidney.4−6
Figure 1.
Chemical structural formula of imidocarb (A) and DNC (B).
The 4,4′-dinitrocarbanilide (DNC) is the residue marker for nicarbazin, which is an anticoccidial drug with excellent performance, broad spectrum, and high efficiency, commonly used in the poultry industry (Figure 1B). The Ministry of Agriculture of China issued announcement no. 235 in 2002, stipulating the MRL of nicarbazin in chicken and beef to be 200 μg/kg.7,8
A large number of methods have been developed for the quantitative detection of imidocarb in various substrates. These include liquid chromatography–mass spectrometry (LC–MS/MS),6,9,10 high-performance liquid chromatography–mass spectrometry,11,12 and immunoassays.6,13 The methods above have a good sensitivity; however, they generally need complicated sample pretreatment procedures, well-equipped laboratories, and relatively long assay times. Therefore, we need a more rapid, convenient, and less expensive method for the detection of imidocarb residues in the field. For the last few years, immunoassays for sample analysis have become increasingly significant in the area of food biology and medicine. When compared to the methods mentioned above, immunochromatographic assays based on gold nanoparticles (GNP) have obvious advantages, including ease of operation, high throughout, and rapidity (within 5–10 min).14 The objective of this study was to develop a monoclonal antibody (mAb) based on a new hapten design and establish GNP-based lateral-flow strips for the rapid detection of imidocarb in milk and beef samples.
2. Results and Discussion
2.1. Design and Screening of the Haptens
An immunoassay is a trace analysis method based on specific recognition and reversible binding reactions between the antigen and antibody. For veterinary anticoccidial drugs such as imidocarb, which are small-molecular compounds (molecular weight less than 1000 Da), the key to establishing an immunoassay method was the ability to prepare antibodies with high affinity and high selectivity for small-molecular compounds. Therefore, the design of the hapten represented the most important step. The derivatization steps for hapten A are shown in Figure 2. LC–MS/MS was used to verify that the target product obtained was the product required for the study. The results of LC–MS/MS are shown in Figure 4C. The molecular formula of the desired target product was C9H11N3, and the formula weight was 161.1 g/mol. Because of the amino group in its structure, it could be measured using positive-ion mass spectrometry, and [hapten A + H+] was 162.1 g/mol. LC–MS results showed that the desired hapten A was successfully derived.
Figure 2.
Flow chart of hapten derivation.
Figure 4.
(A) UV/vis spectra of the hapten A immunogen (hapten A–BSA); (B) UV/vis spectra of the hapten A-coating antigen (hapten A–OVA); (C) LC–MS/MS spectrum of hapten A.
The hapten A derivative had no immunogenicity at this stage, meaning that the absence of T cell epitopes could not directly induce the production of specific antibodies in animals, and therefore, it was necessary to use its end, which had an active group and bound to a macromolecular carrier to indirectly induce the proliferation and differentiation of B cells, thereby producing specific antibodies. Qualitative analysis of all hapten A–protein conjugates was performed using UV/vis spectroscopy at wavelengths of 225–500 nm. According to Figure 4A, the maximum absorption peaks of hapten A and BSA were at 310 and 278 nm, respectively. Hapten A–BSA had absorption peaks at 357 and 279 nm. The protein concentration of hapten A was 0.5 mg/mL measured using a Bio-Rad Protein Assay, and this was used in the BSA concentration–absorbance linear regression equation to calculate the absorbance of pure protein and was found to be A1 = 0.2519, and the corresponding absorbance value of hapten A at the maximum absorption peak was A2 = 0.4123. On the basis of the principle of absorbability additivity, the calculated concentration of hapten A was 49.81 μg/mL, and the coupling ratio was 46:1. As seen in Figure 4B, the maximum absorption peaks of hapten A and OVA were at 310 and 280 nm. After calculation, the concentration of hapten A was 44.73 μg/mL, and the coupling ratio was 27:1. Therefore, this provided evidence for successful coupling between hapten A and the BSA and OVA proteins.
2.2. Preparation and Characterization of Monoclonal Antibodies
The mice immunized with immunogen hapten A–BSA, having the highest titer and inhibition, were used for cell fusion. Mouse spleen cells were fused with myeloma cells SP2/0 and polyethylene glycol and subcloned three times. Hybridoma cells with strong positive reactions and showing drug-inhibition effects to imidocarb were selected and purified from mice ascites, to obtain monoclonal antibodies. Based on indirect competitive ELISA (ic-ELISA) test results of the four cell lines obtained, the mAb 2E9 showed the highest titer and the best sensitivity. Therefore, for the follow-up study, we selected mAb 2E9 for optimization. After testing with a commercial isotyping kit, it was determined that the isotype of mAb 2E9 belonged to the IgGa2 subtype, and results are shown in Figure 5.
Figure 5.
Subtype comparison of mAb 2E9.
2.3. Development and Optimization of the ic-ELISA
Because ELISAs represent an equilibrium binding reaction, appropriate doses of the antigen and antibody and appropriate buffer systems were determined as they are especially important for the sensitivity of the assay. This study optimized the assay based on the pH value of the buffer and its NaCl content. Figure 6A shows the influence of buffer pH on the performance characteristics of the ic-ELISA. The buffer solutions with different pH values already available in our laboratory were selected for study. According to the results based on Bmax/IC50 as seen in Figure 6A, with an increase in the pH value, the Bmax/IC50 value also gradually increased initially but then decreased; however, the highest was at pH = 7.4. It may be that the change in pH changes the number of point-like groups. Changes in pH will inhibit the reaction system to a certain extent and have a significant impact on IC50 values, so it was appropriate to choose a buffer with a phosphate-buffered saline (PBS) of pH 7.4. Figure 6B shows the impact of the NaCl content in the buffer, on the sensitivity to imidocarb. As the NaCl content increased, the ionic strength in the system also increased, and the Bmax/IC50 was gradually increased initially and then decreased, reaching the highest value when the NaCl content was 0.8%. Therefore, the most suitable NaCl content was 0.8% in the buffer. In summary, the conditions for optimal performance were 0.8% NaCl content in the buffer and pH 7.4. Under these conditions, imidocarb conformed to the following standard curve y = 0.204 + 1.429/[1 + (x/2.22)1.54], with an IC50 value of 2.22 ng/mL and a limit of detection (LOD) (IC10) value of 0.45 ng/mL and the linear range of detection (IC20–IC80) was 0.90–5.48 ng/mL.
Figure 6.
Standard curve of imidocarb. (A) Optimization of ic-ELISA based on different pH values; (B) optimization of ic-ELISA based on different NaCl contents; (C) standard inhibition curve of imidocarb.
2.4. Specificity of the ic-ELISA
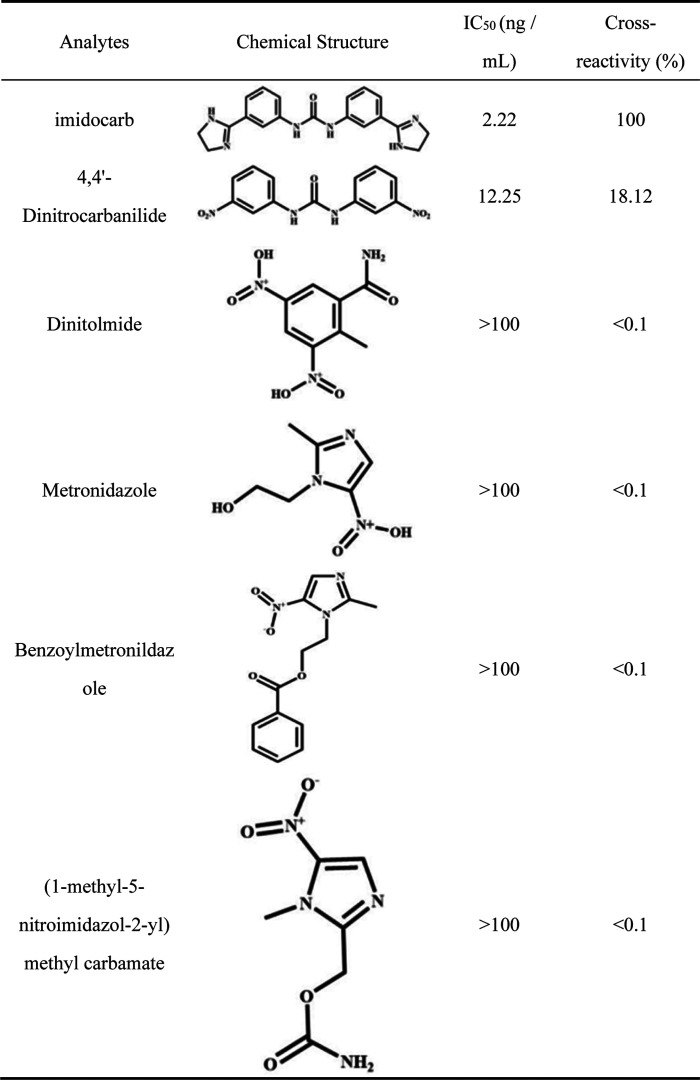
Table 1 depicts the cross-reaction rate of the mAb with several antibiotic drugs and related compounds. It had a 18.12% cross-reactivity with DNC, but did not cross react with other compounds (CR < 0.01%), suggesting that the mAb had high specificity. Therefore, it illustrated the rationality and effectiveness of the hapten designed in our study.
Table 1. Cross-Reactivity of Imidocarb mAb 2E9 with Similar Compounds.
2.5. Optimization of the Lateral-Flow ICA Strip
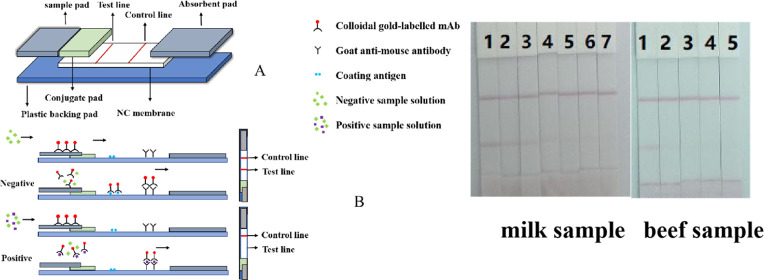
To use the least material but obtain the highest detection sensitivity, we optimized the concentration of the coated and GNP-labeled antibodies on the test strip. In the actual detection process, it was found that when the amount of coating antigen was large, the dilution factor of the GNP-labeled antibody was relatively small. Although the overall color of the test strip could be darker, it affected the detection sensitivity. However, if the detection sensitivity increased, it will affect the overall color development of the test results. As shown in Figure 7A, the optimal concentrations of hapten A–OVA and GNP-labeled antibody were 0.5 mg/mL and 4 μg/mL, respectively. We also optimized the different surfactants in the resuspension, as shown in Figure 7B: basic—no surfactant, 1—PVP, 2—PEG, 4—BSA, 11—Tween 20, 12—Brij-30, and 13—Trixton-100 14—ON-870. However, Brij-30 was finally selected for subsequent experiments. Under these conditions, when the concentration of imidocarb was 0 ng/mL, both the C line and T line were visible. When the concentration of imidocarb reached 10 ng/mL, the color of the T line was not visible. Under conditions where we have selected the antigen and antibody concentration, a series of imidocarb dilutions in PBS were used as standards with concentrations of 0, 0.125, 0.25, 0.5, 1.0, 2.5, 5, and 10 ng/mL which were used to further verify the sensitivity of the developed lateral-flow ICA strip (Figure 7C).
Figure 7.
Optimization and detection of the lateral flow ICA test strip. (A) Optimization of the lateral flow ICA test strip with different concentrations of the coating antigen (0.5 and 1.0 mg/mL) and GNP-labeled mAb (by optimizing 1 mL of GNP with 4 μL K2CO3 and 4 or 8 μg/mL antibody). Strip 1: negative sample without imidocarb (0 ng/mL); strip 2: positive sample with imidocarb (1 ng/mL). (B) Optimization of the different surfactants in the resuspension,: basic—no surfactant, 1 = PVP, 2 = PEG, 4 = BSA, 11 = Tween 20, 12 = Brij-30, 13 = Trixton-100 14 = ON-870. Strip 1: negative sample without imidocarb (0 ng/mL); strip 2: positive sample with imidocarb (1 ng/mL). (C) Imidocarb detection by the lateral flow ICA test strip in PBS samples. The PBS samples: 1 = 0 ng/mL, 2 = 0.25 ng/mL, 3 = 0.5 ng/mL, 4 = 0.5 ng/mL, 5 = 1.0 ng/mL, 6 = 2.5 ng/mL, 7 = 5 ng/mL, and 8 = 10 ng/mL.
In general, we defined the visual detection limit (vLoD) of the lateral-flow ICA strip as the lowest concentration of imidocarb that caused a decrease in the T line brightness when compared to the negative sample and the cut-off limit as the threshold concentration of imidocarb. Therefore, under these concentration conditions, the T line disappeared completely. As shown in Figure 7C, the vLoD for imidocarb was 1.0 ng/mL and the cut-off limit was 10 ng/mL.
2.6. Sensitivity Analysis of the Lateral-Flow ICA Strip
The matrix of the actual sample will affect the sensitivity of the ICA test strip to some extent. For example, beef and milk contain complex matrix compounds including proteins and fats. First, we confirmed by LC–MS/MS that all samples were negative for imidocarb (data not shown). A series of imidocarb standard solutions were added to the milk (0, 0.5, 1.0, 2.5, 5.0, 10, and 20 ng/mL) and beef samples (0, 5.0, 10, 25, and 50 ng/mL). As shown in Figure 8, when the concentration of imidocarb increased to 20 and 50 ng/mL, the T line in milk and beef samples were absent. When the concentration of imidocarb increased to 5 and 10 ng/mL, compared with the negative milk and beef sample, the T line color was obviously weak. In summary, the test strip detection method developed by our research represented a qualitative and semiquantitative method, which could be used for the rapid detection of imidocarb.
Figure 8.

Lateral flow ICA test strip analysis image of imidocarb in milk (A) and beef (B) samples. The concentration of imidocarb added to the milk sample was (1 = 0 ng/mL, 2 = 0.5 ng/mL, 3 = 1.0 ng/mL, 4 = 2.5 ng/mL, 5 = 5.0 ng/mL, 6 = 10 ng/mL, 7 = 20 ng/mL). The concentration of imidocarb added to the beef sample was (1 = 0 ng/mL, 2 = 5.0 ng/mL, 3 = 10 ng/mL, 4 = 25 ng/mL, and 5 = 50 ng/mL).
As shown in Table 2, using ic-ELISA and LC–MS/MS methods to verify and compare the ability of adding and recovering imidocarb in milk and beef samples, this experiment was repeated four times. When the ic-ELISA was used, the recovery rates of imidocarb in milk and beef were 86.0–93.5 and 84.5–101.2%, respectively. When LC–MS/MS was used the recovery rates in beef and milk were 93.0–95.6 and 88.5–100.9%, respectively. When the two methods above were compared, it was not difficult to see that the test results were similar. However, the ICA test strip and ic-ELISA method were more convenient and more suitable for rapid on-site detection of imidocarb in milk and beef samples.
Table 2. Analysis of Artificially Added Imidocarb Milk and Beef Samples by ic-ELISA, LC–MS/MS, and the Immunochromatographic Assay (n = 4)a,b,c.
| IC-Elisa |
LC–MS/MS |
|||||||
|---|---|---|---|---|---|---|---|---|
| samples | spiked | detection level (ng/mL) mean ± SD | recovery rate (%) | CV (%) | detection level (ng/mL) mean ± SD | recovery rate (%) | CV (%) | ICA strip |
| milk samples | 0 | ND | NC | NC | ND | NC | NC | – |
| 2 | 1.72 ± 0.04 | 86.0 ± 2.0% | 6.94 | 1.86 ± 0.03 | 93.0 ± 1.5% | 4.56 | ± | |
| 5 | 4.63 ± 0.09 | 92.6 ± 1.8% | 4.28 | 4.75 ± 0.07 | 95 ± 1.4% | 3.07 | ± | |
| 20 | 18.70 ± 0.29 | 93.5 ± 1.5% | 2.37 | 19.12 ± 0.25 | 95.6 ± 1.3% | 1.97 | + | |
| beef samples | 0 | ND | NC | NC | ND | NC | NC | – |
| 5 | 4.23 ± 0.16 | 84.5 ± 3.2% | 7.13 | 4.43 ± 0.15 | 88.5 ± 3.0% | 5.37 | ± | |
| 10 | 9.16 ± 0.17 | 91.6 ± 1.7% | 5.17 | 9.34 ± 0.14 | 93.4 ± 1.4% | 3.25 | ± | |
| 50 | 50.61 ± 0.63 | 101.2 ± 1.3% | 3.02 | 50.45 ± 0.59 | 100.9 ± 1.2% | 2.07 | + | |
ND, not detectable.
NC, not calculated.
–, negative: the concentration of imidocarb was below 5 μg/kg; ±, weakly positive: the concentration of imidocarb was in the range of 5–20 μg/kg; +, positive: the concentration of imidocarb exceed 20 μg/kg.
3. Conclusions
We developed an anti-imidocarb mAb 2E9 with high sensitivity and specificity and further fabricated a fast and convenient lateral flow ICA test strip to detect imidocarb in milk and beef samples. The ICA test strip could therefore be used for the rapid determination of imidocarb in milk and beef samples. The results of the ICA test strip were consistent with the results from the ic-ELISA and LC–MS/MS, and the ICA test strip could quickly obtain results within 5–10 min and could be applied on site at the market to detect imidocarb in a large number of agricultural and food samples.
4. Experimental Section
4.1. Reagents and Chemicals
The standards used in this study were imidocarb (98%), nicarbazin (98%), dinitolmide (98%), metronidazole (98%), benzoylmetronildazole (98%), and (1-methyl-5-nitroimidazol-2-yl) methyl carbamate (98%). Goat anti-mouse immunoglobulin G horseradish peroxidase conjugate (IgG-HRP), bovine serum albumin (BSA MW = 67,000), ovalbumin (OVA MW = 45,000), N,N-dimethylformamide (DMF), sodium nitrite, 3,3′,5,5′-tetramethylbenzidine (TMB), Tween 20, and Freund’s complete and incomplete adjuvants were all from Sigma-Aldrich Chemical Co. (St. Louis, MO). Hypoxanthine, aminopterin, and thymidine, hypoxanthine and thymidine, and polyethylene glycol 2000 (PEG-2000) were obtained from Sigma-Aldrich Jackson Immuno Research Laboratories (West Grove, PA, USA). Myeloma cells SP2/0 were provided by the China Center for Type Culture Collection (CCTCC). Other reagents were of analytical grade and were purchased from the National Pharmaceutical Group Chemical Reagent Co., Ltd (Shanghai, China).
4.2. Materials and Instruments
Hapten structures were identified by combining electrospray ionization (ESI) (Waters Co., Ltd., Milford, MA, USA). Haptens and antigens were characterized using a UV/vis scanner (Bokin Instruments, Tsushima, Japan). Cell culture plates (24 and 96 wells) and culture flasks were purchased from Costar Inc. (Cambridge, Mass, USA). The strip scan reader was provided by Huaan Magnetic Industry Biotechnology Co., Ltd. (Beijing, China). All buffer solutions were prepared using ultrapure water (Milli-Q Purification System, Millipore, Bedford, Mass, USA). Other instruments used in this study were the CM 4000 paper cutter (Gene, Shanghai, China) and water bath (Shanghai Instrument Group Co, Ltd., supply and marketing company, Shanghai, China).
4.3. Buffers and Solutions
The buffers used in this study were as follows: (1) 0.05 mol/L carbonate buffer (pH 9.6) as the coating buffer. (2) 0.01 mol/L PBS (pH 7.4), (3) PBS with 0.05% Tween 20 as the washing buffer (PBST), (4) coating buffer containing 5% gelatin as the blocking buffer. (5) PBS with 0.05% (v/v) Tween 20 and 0.1% (w/v) gelatin was used as the antibody dilution buffer. (6) Substrate buffer consisted of solutions A (citric acid, H2O2, and Na2HPO4) and B (0.06% v/v TMB in glycol) at a 5:1 (v/v) ratio. (7) 2 mol/L H2SO4 was the stop solution.
4.4. Design and Synthesis of Haptens
Extra consideration to the structure of imidocarb was needed, as it has no groups capable of coupling to proteins. Therefore, this study focused on the design and synthesis of a new hapten. As shown in Figure 2, methyl alcohol (100 mL), m-nitrobenzonitrile (148 g), sulfur (1.6 g), and ethylenediamine (8 mL) were reacted using a reflux device while stirring and heated slowly. After the reflux was stabilized, the temperature of the reaction was maintained at about 67 °C; the samples were taken after a cyclization reaction between m-nitrobenzonitrile and ethylenediamine under sulfur catalysis for 24 h. Approximately, 2 mL of ethylenediamine was added, and again, the reaction was continued for a further 8–10 h until the m-nitrobenzonitrile residue was below 2%. After the reaction, most of the methanol was distilled off, and the temperature was lowered to 0 °C, and suction filtration was performed to obtain 2-(3-nitrophenyl)imidazoline. Next, 2-(3-nitrophenyl)imidazoline (6 g), palladium–carbon (0.6 g), and methanol solution (60 mL) was put into an enamel reaction kettle and stirred at 60 °C for 15 min to obtain the mixed solution M. Nine grams of formic acid was added dropwise to solution M for 30 min, and the solution N was obtained after a thermal reaction for 2 h. Results showed that no 2-(3-nitrophenyl)imidazoline remained in solution N. It was then cooled down to below 40 °C and filtered, and the palladium–carbon catalyst was washed with pure water. The filtrate was put into a distillation kettle, to evaporate the organic solvent methanol in the filtrate, to obtain a pure aqueous solution of 2-(3-aminophenyl)imidazoline. The above aqueous solution was stirred and cooled down to below 30 °C, and hydrochloric acid was added dropwise until neutrality was reached. Finally, it was dried under reduced pressure at 60 °C for 6 h to obtain 5.52 g of the hapten A.
4.5. Preparation of Antigen
The hapten A was combined with BSA using the diazo method, and the resulting hapten (4.38 mg) was dissolved into 400 μL anhydrous DMF, and hydrochloric acid (1 M) was added to acidify the solution; it was then stirred in an ice bath for 1 h. Next, 10 μL of 30% sodium nitrite solution (100 mg dissolved in 500 μL pure water) was added and stirred in the ice bath for 6 h to get activation solution. Then, the activated mixture was added dropwise to a protein solution (6 mg of BSA was dissolved in 2 mL carbonate–bicarbonate buffer, pH 9.6) with stirring overnight at 4 °C. Lastly, the resulting conjugate hapten A–BSA was dialyzed with PBS for 2–3 days and then stored at −20 °C for future use.15−17
The hapten A was combined with OVA using the glutaraldehyde method to use as the coating antigen. Similarly, the resulting hapten A (3.65 mg) was dissolved into 400 μL anhydrous DMF, and 5% glutaraldehyde solution (100 μL 25% glutaraldehyde solution diluted five times with pure water) was added in order to activate the solution, and the mixture was stirred under ice bath conditions for 30 min. Next, the activated solution was added dropwise to a solution of protein (6 mg of OVA dissolved in 2 mL carbonate–bicarbonate buffer, pH 9.6) with stirring at room temperature for 6 h. Finally, the resultant conjugate hapten A–OVA was dialyzed with PBS for 2–3 days and stored at −20 °C for future use.18,19
4.6. Production of Monoclonal Antibodies
A total of 15 BALB/c female mice (6–8 weeks old) were immunized subcutaneously in the back.20,21 The hapten A–BSA immunoconjugate (100 μg) was dissolved in physiological saline solution (0.9% NaCl), emulsified with Freud’s complete adjuvant, and used for the first immunization. Next, 50 μg of immunoconjugate was emulsified with equal amounts of incomplete Freud’s adjuvant to undergo booster immunizations at 3 week intervals. The tail blood from these mice was then collected 1 week after each booster immunization, and the antiserum titer and inhibition were tested via indirect ELISA. After the final booster immunization, the spleen cells from those mice exhibiting the optimal antiserum titer and inhibition were used for the preparation of hybridoma cells, and the mice underwent intraperitoneal injection with 25 μg of immunoconjugate dissolved in physiological saline solution.
Three days later, after the final sprint immunization of the target mouse, its spleen was dissected out and fused with myeloma cells using PEG 2000 in a sterile ultra clean environment. The specific method was performed as previously described from our laboratory.22−24 One week after the fusion, the supernatant from the hybridoma cell culture from 96-well microculture plates was tested using an ELISA. The wells with high antiserum titer and inhibition were screened out for cell subcloning. After subcloning three times, the resultant positive hybridoma cell lines were expanded in six-well microculture plates. When the cell density covered the bottom of the well, the cells were expanded by culturing in a flask and then collected when they were almost fully confluent. They were then injected into five female BALB/c mice, and ascites fluid was gathered and purified by salting out (with caprylic acid ammonium sulfate) and then stored at −20 °C for future use.
4.7. Establishment and Optimization of ic-ELISA
A total of 100 μL/well of the hapten A–OVA coating antigen diluted by coating buffer (0.05 M CB buffer) was added into a 96-well microtiter plate and incubated at 37 °C for 2 h. After washing the plates three times to remove excess coating of the antigen, 200 μL/well of the block buffer was added by incubation at 37 °C for 2 h. After the same washing procedure, 50 μL of gradient diluted sera in antibody dilution buffer and the same amount of gradient diluted imidocarb standards in 0.01 M PBS were incubated in the 96-well microtiter plates at 37 °C for 30 min. Next, 100 μL of the goat anti-mouse IgG HRP (1:3000 dilution) was diluted with antibody dilution buffer and incubated in the plates at 37 °C for 30 min. After one more washing step, 100 μL/well of the TMB/peroxide-based substrate solution was added by incubation at 37 °C for 15 min. Finally, 50 μL/well of the 0.01 M H2SO4 solution was used to stop the color reaction, and the absorbance was measured at 450 nm using a microplate reader.25,26
Several parameters can affect the performance and sensitivity of the ic-ELISA, and this study optimized two of the important terms of the working solution, namely, pH and ionic strength.27−29 A series of standard dilution buffers at different pH values (pH 4.0, 5.5, 7.4, 8.8, and 9.6) were used to dilute the imidocarb standards. Likewise, a series of standard dilution buffers at different NaCl concentrations (NaCl 0.4, 0.8, 1.6, 2.4, and 3.2%) were prepared to dilute the imidocarb standards. Bmax, IC50, and Bmax/IC50 values were then determined and used to evaluate the immunoassay performance, and a standard curve using an S-shaped curve fitting was constructed.
4.8. Cross Reactivity
Imidocarb is an example of a phenylurea antibiotic, which has many similar antibiotic compounds. These analogues including DNC, dinitolmide, metronidazole, benzoylmetronildazole, and (1-methyl-5-nitroimidazol-2-yl) methyl carbamateand were detected using ic-ELISA to determine the specificity of our mAb. The presence or absence of cross-reactivity can be determined from the IC50 ratio of imidocarb and the above analogues, and the formula for its calculation is given below.
4.9. Production of Colloidal Gold-Labeled mAb
GNPs were prepared by reduction with sodium citrate as previously described in our laboratory.30,31 One percent sodium citrate solution was added to chloroauric acid solution and stirred quickly until the mixture turned a fuchsia color. Then, the mixture was stored at 4 °C for future use. It can be known from the literature measurement published in our laboratory that colloidal gold particles have a maximum absorption peak at a wavelength of 525 nm, and their diameter is about 20 nm.
A solution of 0.1 M K2CO3 was used to adjust the pH of freshly prepared colloidal gold solution to pH 8.2; then, the purified imidocarb mAb was added to bring the antibody concentration up to 30 μg/mL. After slowly stirring for 1 h, the BSA solution was added for 2 h to stabilize the reaction. This colloidal gold-antibody BSA solution was centrifuged at 10,000 rcf for 60 min at 4 °C, and the pellet was resuspended in 0.02 M PBS buffer (0.1% Tween, 0.1% PEG, 5% sucrose and 0.2% BSA, pH 7.8); meanwhile, the supernatant was discarded. This was repeated as mentioned above, and the solution was centrifuged at 10,000 rcf for 60 min at 4 °C; then, the pellet was resuspended in resuspension buffer (0.02 M PBS including 5% trehalose, 1% BSA and 0.05% NaN3) and stored at 4 °C.
4.10. Assembly of the Immunochromatographic Test Strip
Based on the schematic in Figure 3A, the immunochromatographic test strips were assembled using colloidal gold-labeled sample pad, nitrocellulose membrane (NC), PVC backing board, and absorbent pad.30,32,33 First, the glass fiber was fully infiltrated with the anti-imidocarb gold-labeled antibody and then made into the gold-labeled sample pad after drying at 37 °C. Next, the coating antigen (hapten A–OVA) and the goat anti-mouse IgG were used to draw lines separately on the NC membrane as detection (T line) and control lines (C line) using a membrane dispenser and then blocked with PBS (including 1% BSA and 0.2% Tween 20) after drying at room temperature. After washing and drying, the NC film detection layer was produced. The absorption pad and the sample pad were fixed on both sides of a soft PVC liner, and an NC film was placed between the two pads with a 2 mm overlap. The above was pressed and cut into 3 mm wide test strips with a paper cutter. The cut test strips were then placed in an aluminum foil bag together with a desiccant and sealed and stored at 4 °C for subsequent experiments.34
Figure 3.
(A) Composition of the lateral-flow ICA strip. (B) Principle of the lateral-flow ICA strip.
4.11. Principle of the Immunochromatographic Test Strip
Based on the schematic in Figure 3B, the principle of the competitive immunochromatographic test strip involved an artificial antigen and goat anti-mouse IgG, which were fixed and coated onto a NC in a strip-like orientation, as detection lines and quality control lines (C line). Here, the test substance competed with the coating antigen immobilized on the NC membrane to bind the gold-labeled antibody.35−37 Next, the sample solution was added dropwise to the sample pad, and under capillary action caused by the glass fiber, it flowed upward through the dissolved gold-labeled antibody and migrated upward chromatographically. When migrating to the detection line (T line), the coating antigen immobilized on the NC membrane, competed with the test substance to bind the limited amount of gold-labeled antibody. After this, the gold-labeled antibody not bound to the coated antigen immobilized on the NC membrane continued to move up to the C line, specifically bound to the goat anti-mouse IgG immobilized on the NC membrane producing a red color. The intensity of the detection line was inversely related to the concentration of the test substance in the sample. In the negative sample, when the concentration of the test substance was low, or absent, a larger amount of the gold-labeled antibody was bound to the NC membrane, and the color of the detection layer was closer to the blank sample. In the positive sample, the concentration of the test substance increased, and the limited binding sites on the gold-labeled antibody became occupied by the test substance, so that the amount of the antigen-bound antibody fixed on the NC membrane decreased and the color of the T line became lighter. When the analyte in the positive sample reached a certain concentration, the analyte completely occupied the binding sites on the gold-labeled antibody, and the coating antigen fixed on the NC membrane did not compete with the gold-labeled antibody; at this point, the T line had no color.
4.12. Sample Pretreatment
Milk and beef samples were obtained from a local market in Wuxi, China. Milk samples were directly diluted with no additional complicated sample processing steps. Five aliquots of homogenized beef samples (5 g each) were weighed and placed into 50 mL centrifuge tubes, and 10 mL of 1% formic acid in methanol was added, and then, the centrifuge tube was shaken for 10 min on a shaker to obtain beef homogenates. After centrifugation at 5000 rcf for 10 min, 3 mL of N-hexane solution was added and left to stand, and then, the upper layer was discarded. This process was repeated three times to degrease the sample. It was then dried under nitrogen, and a PBST solution (PBS solution containing 0.2% Tween 20) was added to reconstitute the samples for subsequent experiments.
4.13. LC–MS/MS Conditions and Methods
In order to compare the ability of using ic-ELISA and LC–MS/MS to add and recover imidocarb in milk and beef samples, the conditions and methods of LC–MS/MS in this experiment are based on the previous report.38 The LC–MS was performed on Waters Xevo-TQD system (Waters, USA), equipped with an ESI source. The analytical column used was a BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm). The operation conditions are as follows: flow rate, 0.3 mL/min; injection volume, 1 μL; column temperature, 45 °C. The mobile phases were 100% acetonitrile (A) and 0.1% formic acid in ultrapure water (v/v) (B): 0 min, 95% B; 12 min, 79% B; 15 min, 40% B; 17 min, 10% B. All chromatographic separation processes are carried out under a gradient elution program.
The MS detection was performed by electrospray ionization in positive-ion mode (ESI–). The ions were detected in multiple reaction monitoring. The parameters are set as follows: the ion source block temperature, 100 °C; capillary voltage, 4500 V; desolvation gas temperature, 400 °C; desolvation gas flow, 700 L/h; the cone voltage, 30 V; the collision gas volume, 0.15 mL/min; and the [M – H]+ at m/z 349 was the parent ion of imidocarb. The daughter ion at m/z 349 → 188 was used as the quantification transition, the daughter ion m/z 349 → 162 was selected as a qualitative ion, and the collision energy was 6 and 20, respectively.
Acknowledgments
This work is financially supported by National Key R&D Program (2018YFC1602303).
The authors declare no competing financial interest.
References
- Chen H.-L.; Zhao X.-Y.; Zhao G.-X.; Huang H.-B.; Li H.-R.; Shi C.-W.; Yang W.-T.; Jiang Y.-L.; Wang J.-Z.; Ye L.-P.; Zhao Q.; Wang C.-F.; Yang G.-L. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasites Vectors 2020, 13, 56. 10.1186/s13071-020-3897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atty S. A.; Zaazaa H. E.; Morsy F. A.; Naguib D. M.; Sedik G. A. Nano Green Voltammetric Determination of Imidocarb Dipropionate and Its Residues in Bovine Meat, Milk and Urine Samples. J. Electrochem. Soc. 2020, 167, 047510. 10.1149/1945-7111/ab7115. [DOI] [Google Scholar]
- Wang Z.; Xiubo L.; Dan S.; Yijing L.; Lianyong W.; Yongjian W.; Wenxue W. Residue depletion of imidocarb in swine tissue. J. Agric. Food Chem. 2009, 57, 2324–2328. 10.1021/jf803251j. [DOI] [PubMed] [Google Scholar]
- Atty S. A.; Zaazaa H. E.; Morsy F. A.; Naguib D. M.; Sedik G. A. Nano Green Voltammetric Determination of Imidocarb Dipropionate and Its Residues in Bovine Meat, Milk and Urine Samples. J. Electrochem. Soc. 2020, 167, 047510. 10.1149/1945-7111/ab7115. [DOI] [Google Scholar]
- Isik N.; Ekici O. D.; Ilhan C.; Coskun D. Safety of Antitheilerial Drug Buparvaquone in Rams. Acta Sci. Vet. 2018, 46, 1678. 10.22456/1679-9216.86688. [DOI] [Google Scholar]
- Traynor I. M.; Thompson C. S.; Armstrong L.; Fodey T.; Danaher M.; Jordan K.; Kennedy D. G.; Crooks S. R. H. Determination of imidocarb residues in bovine and ovine liver and milk by immunobiosensor. Food Addit. Contam., Part A 2013, 30, 1108–1114. 10.1080/19440049.2013.779752. [DOI] [PubMed] [Google Scholar]
- Bampidis V.; Azimonti G.; Bastos M. d. L.; Christensen H.; Dusemund B.; Durjava M. K.; Lopez-Alonso M.; Puente S. L.; Marcon F.; Mayo B.; Pechova A.; Petkova M.; Ramos F.; Sanz Y.; Villa R. E.; Woutersen R.; Bories G.; Brantom P.; Gropp J.; Finizio A.; Focks A.; Teodorovic I.; Holczknecht O.; Tarres-Call J.; Vettori M. V.; Kouba M.; Us E. P. A. P. S. Modification of the terms of authorisation regarding the maximum inclusion level of Maxiban (R) G160 (narasin and nicarbazin) for chickens for fattening. EFSA J. 2019, 17, e05786 10.2903/j.efsa.2019.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bampidis V.; Azimonti G.; Bastos M. D. L.; Christensen H.; Dusemund B.; Kouba M.; Durjava M. K.; Lopez-Alonso M.; Puente S. L.; Marcon F.; Mayo B.; Pechova A.; Petkova M.; Ramos F.; Sanz Y.; Villa R.; Woutersen R.; Aquilina G.; Bories G.; Brantom P.; Cocconcelli P. S.; Halle I.; Kolar B.; Beelen P. v.; Wester P.; Holczknecht O.; Vettori M. V.; Gropp J.; Additives E. P. o. Products or Substances used in Animal, F., Safety and efficacy of Monimax (monensin sodium and nicarbazin) for chickens for fattening and chickens reared for laying. EFSA J. 2018, 16, e05459 10.2903/j.efsa.2018.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K.; Nunome M.; Hino T.; Oka H. DETERMINATION OF IMIDOCARB IN BOVINE TISSUES AND MILK SAMPLES BY LC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2149–2156. 10.1080/10826076.2011.585484. [DOI] [Google Scholar]
- Ishii R.; Takahashi K.; Matsumoto R. Analysis of Imidocarb in Livestock and Seafood Products Using LC-MS/MS. Food Hyg. Saf. Sci. 2011, 52, 34–39. 10.3358/shokueishi.52.34. [DOI] [PubMed] [Google Scholar]
- Milnes E. L.; Delnatte P.; Woodbury M.; Hering A.; Lee S.; Smith D. A.; Nemeth N. M.; Gu Y.; Gehring R.; Johnson R. Pharmacokinetics of imidocarb dipropionate in white-tailed deer (Odocoileus virginianus) after single intramuscular administration. J. Vet. Pharmacol. Ther. 2020, 43, 33–37. 10.1111/jvp.12760. [DOI] [PubMed] [Google Scholar]
- Xiao-Juan L.; Wei L.; Dong-Dong C.; Lian-Feng A.; Chun-Hai G.; Rui-Chun C.; Feng-Chi W. Determination of imidocarb residue in animal tissues and milk by HPLC and HPLC-MS/MS. J. Food Saf. Qual. 2014, 5, 352–358. [Google Scholar]
- Park J. A.; Cho H. J.; Cho S. M.; Jo K.; Kim N. H.; Kim H. S.; Park H. R.; Kwon K. S.; Shin H. C. Development of a direct competitive ELISA for the detection of furazolidone metabolite in foodstuff of animal origin. J. Vet. Pharmacol. Ther. 2012, 35, 91–92. [Google Scholar]
- Li Y.; Xu X.; Liu L.; Kuang H.; Xu L.; Xu C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst 2020, 145, 2143–2151. 10.1039/d0an00170h. [DOI] [PubMed] [Google Scholar]
- Na G.; Hu X.; Sun Y.; Kwee S.; Xing G.; Xing Y.; Zhang G. A highly sensitive monoclonal antibody–based paper sensor for simultaneously detecting valnemulin and tiamulin in porcine liver. J. Food Sci. 2020, 85, 1681–1688. 10.1111/1750-3841.15136. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wu X.; Liu L.; Xu L.; Kuang H.; Xu C. Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chem. 2020, 312, 126116. 10.1016/j.foodchem.2019.126116. [DOI] [PubMed] [Google Scholar]
- Xu X.; Liu L.; Wu X.; Kuang H.; Xu C. Ultrasensitive immunochromatographic strips for fast screening of the nicarbazin marker in chicken breast and liver samples based on monoclonal antibodies. Anal. Methods 2020, 12, 2143–2151. 10.1039/d0ay00414f. [DOI] [Google Scholar]
- Chen Y.; Wang Z.; Wang Z.; Tang S.; Zhu Y.; Xiao X. Rapid Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunoassay for Kanamycin and Tobramycin in Swine Tissues. J. Agric. Food Chem. 2008, 56, 2944–2952. 10.1021/jf703602b. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liu L.; Song S.; Kuang H.; Xu C. A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues. Nanomaterials 2018, 8, 144. 10.3390/nano8030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Wu X.; Liu L.; Xu L.; Kuang H.; Xu C. Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chem. 2020, 312, 126116. 10.1016/j.foodchem.2019.126116. [DOI] [PubMed] [Google Scholar]
- Guo M.; Wu X.; Song S.; Zheng Q.; Luo P.; Kuang H.; Sun J.; Ye L. Ultrasensitive anti-melamine monoclonal antibody and its use in the development of an immunochromatographic strip. Food Agric. Immunol. 2019, 30, 462–474. 10.1080/09540105.2019.1590318. [DOI] [Google Scholar]
- Na G.; Hu X.; Yang J.; Sun Y.; Kwee S.; Tang L.; Xing G.; Xing Y.; Zhang G. A rapid colloidal gold-based immunochromatographic strip assay for monitoring nitroxynil in milk. J. Sci. Food Agric. 2020, 100, 1860–1866. 10.1002/jsfa.10074. [DOI] [PubMed] [Google Scholar]
- Ling F.; Liu L.; Kuang H.; Cui G.; Xu C. Development of Indirect Competitive Enzyme-Linked Immunosorbent Assay and Lateral-Flow Immunochromatographic Strip for the Detection of Digoxin in Human Blood. ACS Omega 2020, 5, 1371–1376. 10.1021/acsomega.9b02254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.; Wu X.; Liu L.; Xu L.; Kuang H.; Xu C. Production of a monoclonal antibody for the detection of vitamin B1 and its use in an indirect enzyme-linked immunosorbent assay and immunochromatographic strip. J. Mater. Chem. B 2020, 8, 1935–1943. 10.1039/c9tb02839k. [DOI] [PubMed] [Google Scholar]
- Lin L.; Song S.; Wu X.; Liu L.; Kuang H. RETRACTED ARTICLE: A colloidal gold immunochromatography test strip based on a monoclonal antibody for the rapid detection of triadimefon and triadimenol in foods. Food Agric. Immunol. 2020, 31, 475–488. 10.1080/09540105.2020.1736010. [DOI] [Google Scholar]
- Ling S.; Xiulan L.; Qiang Z.; Rongzhi W.; Tao T.; Shihua W. Preparation of monoclonal antibody against penicillic acid (PA) and its application in the immunological detection. Food Chem. 2020, 319, 126505. 10.1016/j.foodchem.2020.126505. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wu X.; Liu L.; Xu L.; Kuang H.; Xu C. An immunochromatographic strip sensor for sildenafil and its analogues. J. Mater. Chem. B 2019, 7, 6383–6389. 10.1039/c9tb00280d. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Song S.; Zheng Q.; Luo P.; Wu X.; Kuang H. Development of a sandwich ELISA and immunochromatographic strip for the detection of shrimp tropomyosin. Food Agric. Immunol. 2019, 30, 606–619. 10.1080/09540105.2019.1609912. [DOI] [Google Scholar]
- Lin L.; Wu X.; Luo P.; Song S.; Zheng Q.; Kuang H. IC-ELISA and immunochromatographic strip assay based monoclonal antibody for the rapid detection of bisphenol S. Food Agric. Immunol. 2019, 30, 633–646. 10.1080/09540105.2019.1612330. [DOI] [Google Scholar]
- Lu T.; Zhu K.-D.; Huang C.; Wen T.; Jiao Y.-J.; Zhu J.; Zhang Q.; Ding S.-N. Rapid detection of Shiga toxin type II using lateral flow immunochromatography test strips of colorimetry and fluorimetry. Analyst 2020, 145, 76–82. 10.1039/c9an01996k. [DOI] [PubMed] [Google Scholar]
- Chao M.; Liu L.; Song S.; Wu X.; Kuang H. Development of a gold nanoparticle-based strip assay for detection of clopidol in the chicken. Food Agric. Immunol. 2020, 31, 489–500. 10.1080/09540105.2020.1737655. [DOI] [Google Scholar]
- Li Y.; Liu L.; Kuang H.; Xu C. Visible and eco-friendly immunoassays for the detection of cyclopiazonic acid in maize and rice. J. Food Sci. 2020, 85, 105–113. 10.1111/1750-3841.14976. [DOI] [PubMed] [Google Scholar]
- Costa E.; Climent E.; Ast S.; Weller M. G.; Canning J.; Rurack K. Development of a lateral flow test for rapid pyrethroid detection using antibody-gated indicator-releasing hybrid materials. Analyst 2020, 145, 3490–3494. 10.1039/d0an00319k. [DOI] [PubMed] [Google Scholar]
- Teepoo S.; Wongtongdee U.; Phapugrangkul P. Development of qualitative and quantitative immunochromatographic strip test assay for rapid and simple detection of leucomalachite green residual in aquatic animals. Food Chem. 2020, 320, 126613. 10.1016/j.foodchem.2020.126613. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Fu Q.; Xie J.; Wang H.; Tang Y. Development of a high sensitivity quantum dot-based fluorescent quenching lateral flow assay for the detection of zearalenone. Anal. Bioanal. Chem. 2019, 411, 2169–2175. 10.1007/s00216-019-01652-1. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wu X.; Xu L.; Kuang H.; Xu C. Detection of triclabendazole and three metabolites in bovine muscle samples with a gold nanoparticle-based lateral flow immunoassay. Anal. Methods 2019, 11, 5478–5486. 10.1039/c9ay01795j. [DOI] [Google Scholar]
- Wang Z.; Wu X.; Liu L.; Xu L.; Kuang H.; Xu C. An immunochromatographic strip sensor for sildenafil and its analogues. J. Mater. Chem. B 2019, 7, 6383–6389. 10.1039/c9tb00280d. [DOI] [PubMed] [Google Scholar]
- Guo L.; Wu X.; Liu L.; Kuang H.; Xu C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2018, 14, 1701782. 10.1002/smll.201701782. [DOI] [PubMed] [Google Scholar]