Abstract
We review many of the recent findings concerning mechanisms and pathways for pain and its modulation, emphasizing sensitization and the modulation of nociceptors and of dorsal horn nociceptive neurons. We describe the organization of several ascending nociceptive pathways, including the spinothalamic, spinomesencephalic, spinoreticular, spinolimbic, spinocervical, and postsynaptic dorsal column pathways in some detail and discuss nociceptive processing in the thalamus and cerebral cortex. Structures involved in the descending analgesia systems, including the periaqueductal gray, locus ceruleus, and parabrachial area, nucleus raphe magnus, reticular formation, anterior pretectal nucleus, thalamus and cerebral cortex, and several components of the limbic system are described and the pathways and neurotransmitters utilized are mentioned. Finally, we speculate on possible fruitful lines of research that might lead to improvements in therapy for pain.
Rapid advances in our knowledge of the pain system have been made in the recent past. Discoveries have increased our understanding of nociceptors and of the processing of nociceptive information in the spinal cord, brainstem, thalamus, and cerebral cortex. Furthermore, there have been new findings concerning the descending pathways that modulate nociceptive activity. In this review, we highlight some of these new findings and, when feasible, indicate how this information might lead to improvements in patient care.
Keywords: Pain, Pain modulation, Sensitization, Nociceptive pathways, Analgesia systems
NOCICEPTORS
Nociceptors have now been described in most of the structures of the body that give rise to pain sensation, including the skin, muscle, joints, and viscera (reviewed in Willis, 1985; Willis and Coggeshall, 1991). Human studies involving microneurography and microstimulation in peripheral nerves have demonstrated that repetitive activation of cutaneous mechanoreceptors at high rates does not lead to pain (Ochoa and Torebjork, 1983; Torebjork et al., 1987), whereas activation of nociceptors at even relatively low rates (e.g., >=3 Hz) does result in pain (Ochoa and Torebjork, 1989). The quality of the pain sensation depends on the tissue innervated by the nociceptors being stimulated; e.g., stimulation of cutaneous A[delta] nociceptors leads to pricking pain (Konietzny et al., 1981), whereas stimulation of cutaneous C nociceptors results in burning or dull pain (Ochoa and Torebjork, 1989). Activation of nociceptors in muscle nerves by electrical stimulation produces aching pain (Torebjork et al., 1984). Electrical stimulation of visceral nerves at low intensities results in vague sensations of fullness and nausea, but higher intensities cause a sensation of pain (reviewed in Ness and Gebhart, 1990). Unless the experiments using microstimulation of single afferents are shown to be unsound technically, it is evident that pain sensation normally results from the activity of nociceptors and not from overactivation of other kinds of receptors (Wall and McMahon, 1985; Torebjork et al., 1987) and that there are distinct sensory channels for different qualities of pain (Willis and Coggeshall, 1991). However, pain can also result from activation of central nociceptive pathways without involving peripheral nociceptors, e.g., in cases of central pain which may follow damage to the central nervous system (Boivie et al., 1989). Motivational-affective circuits can also mimic pain states, most notably in patients with anxiety, neurotic depression, or hysteria (Chaturvedi, 1987; Merskey, 1989).
A major discovery in the 1980s was that many nociceptors, possibly most, are inactive and rather unresponsive under normal circumstances. This observation was first made in recordings from the nerves supplying the knee joint (Schaible and Schmidt, 1983a,b) and led to the description of these afferents as “silent” or “sleeping” nociceptors. However, inflammation can cause the sensitization of these nerve fibers, after which they “awaken,” by developing spontaneous discharges and becoming much more sensitive to peripheral stimulation (Fig. 1)(Schaible and Schmidt, 1985, 1988). Silent nociceptors have now been described not only in joint nerves but also in cutaneous and visceral nerves (Habler et al., 1990; Handwerker et al., 1991; Davis et al., 1993). Sensitization of nociceptors depends on the activation of second-messenger systems by the action of inflammatory mediators released in the damaged tissue, such as bradykinin (BK), prostaglandins, serotonin, and histamine (Dray et al., 1988; Schepelmann et al., 1992; 1993; Birrell et al., 1993; Davis et al., 1993).
FIG. 1.
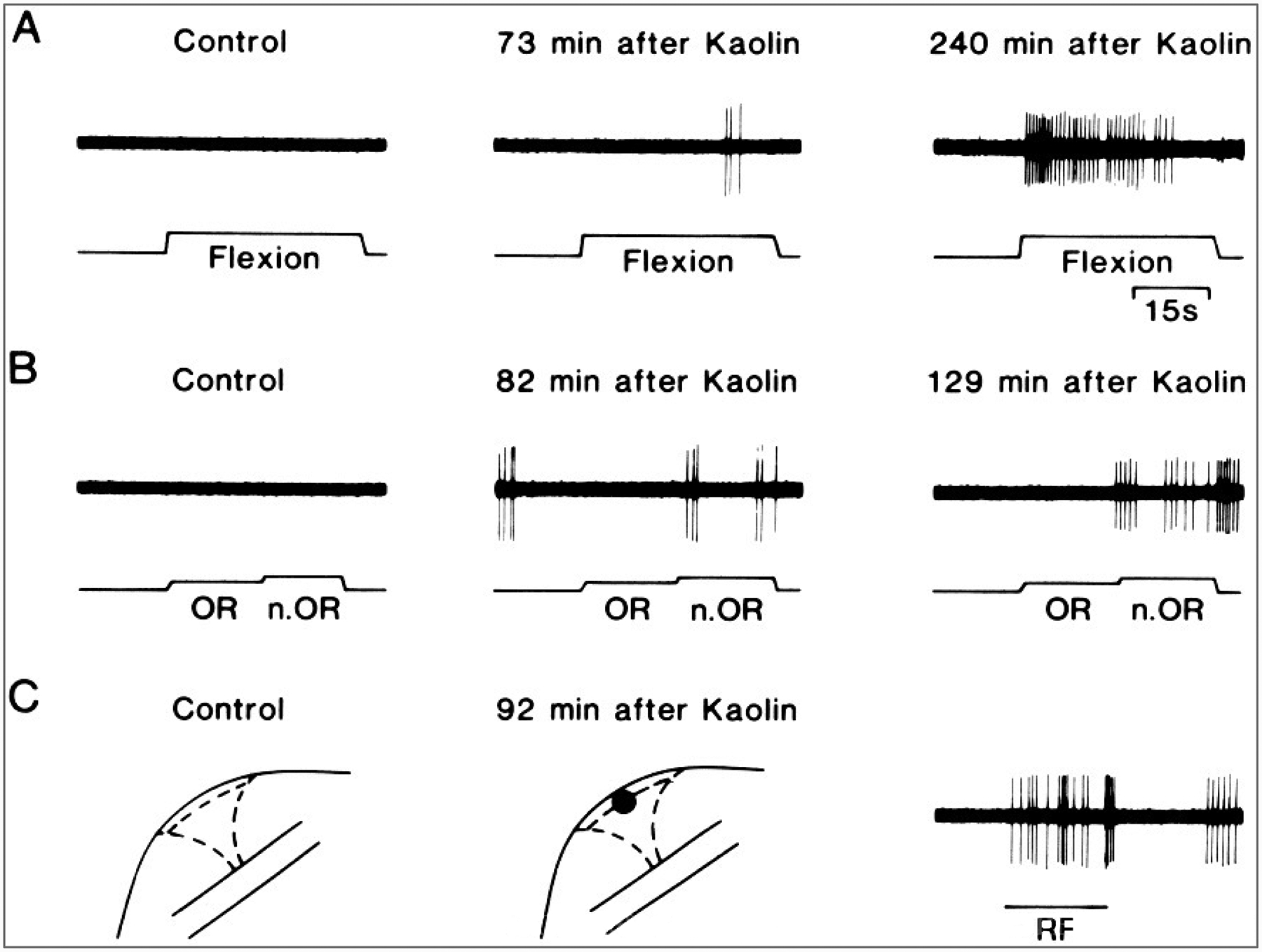
Sensitization of a “silent” C-nociceptor supplying the knee joint. A and B: Left: Absence of responses to flexion of the knee or to innocuous (OR) and noxious (n.OR) outward rotation of the knee in a cat before initiation of experimental arthritis by injection of kaolin and carrageenan into the knee joint. At this time, no receptive field could be demonstrated in the joint by mechanical probing (C: Left). Middle (A-C): A response to flexion and noxious outward rotation of the knee and a receptive field to probing the joint developed by 90 min after initiation of inflammation. The responses continued to increase (A and B: Right). The response to stimulation of the receptive field is shown in C (right). (Reprinted fromSchaible and Schmidt, 1988, with permission.)
Primary hyperalgesia is believed to be a consequence of the sensitization of nociceptors during the process of inflammation (Meyer and Campbell, 1981; LaMotte et al., 1982; 1983). Hyperalgesia can be defined as an increase in the painfulness of a noxious stimulus and a reduced threshold for pain (see Bonica, 1992). Whereas primary hyperalgesia is enhanced pain felt at the site of injury, secondary hyperalgesia is felt at a site remote from the original injury (Lewis, 1942; Hardy et al., 1952) (described herein).
Modulation of Nociceptors
The activity of nociceptors can be affected not only by adequate stimuli, such as strong mechanical, thermal, or chemical stimuli (see Willis, 1985; Willis and Coggeshall, 1991), but also by chemical actions on surface membrane receptors of their axons. For example, primary afferent nociceptors normally have several types of pharmacological receptors on their surface membranes, including opiate, [gamma]-aminobutyric acid (GABA), BK, histamine, serotonin, and capsaicin receptors (Dray, 1994). In addition, the effectiveness of a population of pharmacological receptors on nociceptors can be changed in certain circumstances; e.g., opiate receptors are ineffective in modulating the normal activity of joint nociceptors, but they were shown to become effective after the development of inflammation (Stein, 1994). After peripheral nerve injury, many afferent fibers express newly formed adrenoreceptors (Sato and Perl, 1991; Campbell et al., 1992; Bossut and Perl, 1995; Xie et al., 1995). Furthermore, afferent fibers supplying the knee joint and also the skin have excitatory amino acid receptors (Fig. 2), and intraarticular administration of excitatory amino acid receptor antagonists can reduce hyperalgesia in rats with experimental arthritis (Lawand, W. D. Willis, and K. N. Westlund, unpublished observations, 1996).
FIG. 2.
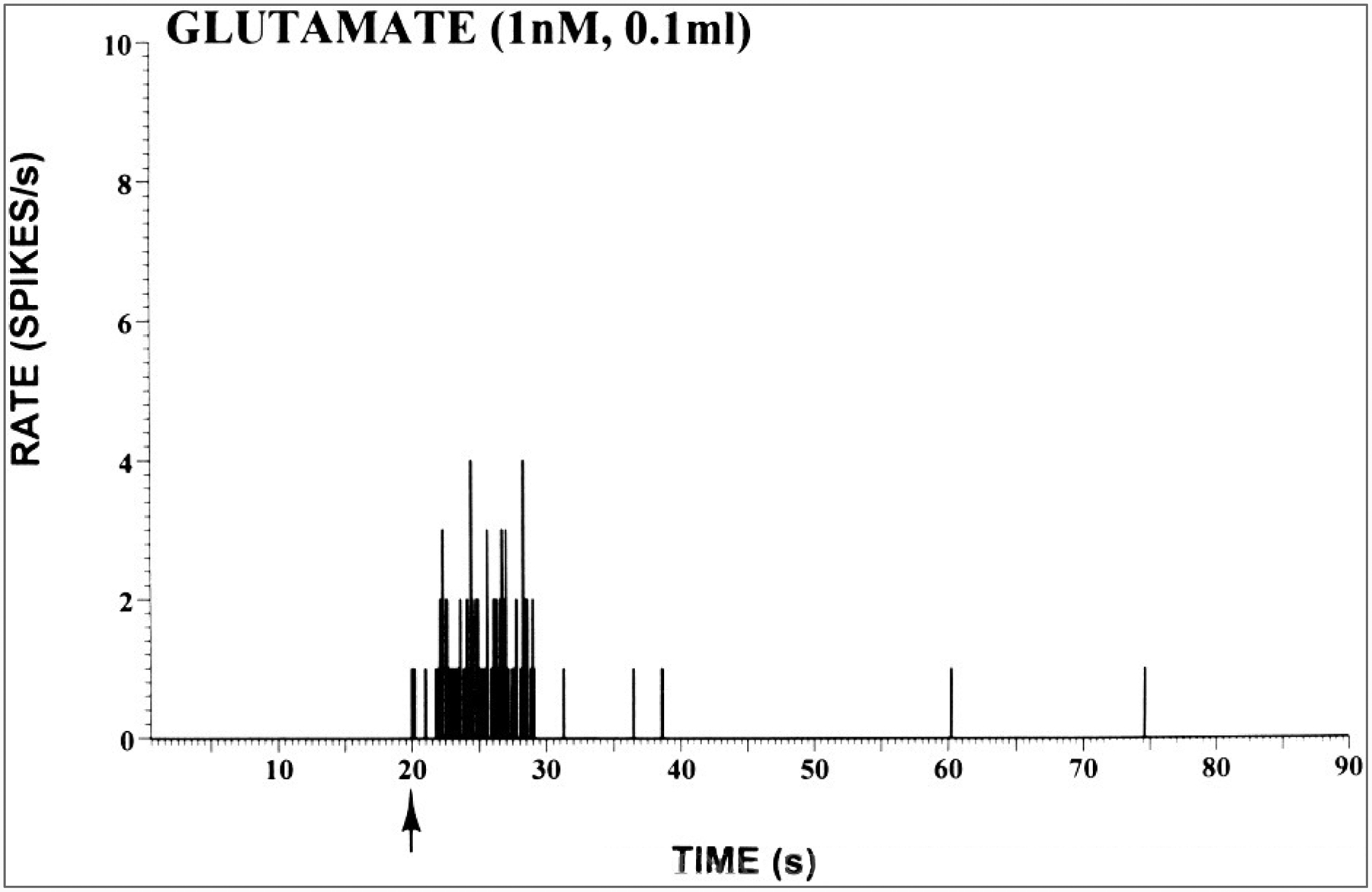
Peristimulus time histogram showing the response of afferent fibers in the medial articular nerve of a rat to intraarticular injection of glutamate. A dose of 0.1 ml of a 1-nM solution of glutamate was injected into the knee joint at the time indicated by the arrow. (From Lawand, W. D. Willis and K. N. Westlund, unpublished observations).
Damage to a peripheral nerve can cause the upregulation of several neuropeptides, including galanin and vasoactive intestinal polypeptide (VIP) in dorsal root ganglion cells and their central branches, but downregulation of others, such as substance P (SP), somatostatin, and calcitonin gene-related peptide (CGRP) (reviewed in Zhang et al., 1993). In addition, Schwann cells in damaged nerves produce increased amounts of messenger RNA for neurotrophins and their receptors (Ernfors et al., 1993; Funakoshi et al., 1993). Presumably neurotrophins play a role in regenerative events. However, growth factors may well cause aberrant forms of regeneration and abnormal pain states; e.g., they may be responsible for the in-growth of sympathetic postganglionic axons into dorsal root ganglia, where they encircle the cell bodies of dorsal root ganglion cells (Chung et al., 1993; McLaughlin et al., 1993). In addition, in the case of peripheral nerve injury, large myelinated afferents, presumably supplying mechanoreceptors, grow into lamina II after peripheral nerve injury (Woolf et al., 1992, 1995). Abnormal connections of these large afferents to the nociceptive processing circuits of the dorsal horn may contribute to allodynia, a sensation of pain that is provoked in pathological circumstances by innocuous stimuli (Bonica, 1992).
Dorsal Horn
The central pathways for processing nociceptive information begin at the level of the spinal cord (and medullary) dorsal horn. Interneuronal networks in the dorsal horn are responsible not only for the transmission of nociceptive information to neurons that project to the brain, but also help modulate that information and pass it on to other spinal cord neurons, including flexor motoneurons and nociceptive projection neurons; e.g., certain patterns of stimulation can lead to enhanced reflex actions and to sensitization of projection neurons and increased nociceptive transmission. Other inputs result in the inhibition of projection neurons. The balance of these excitatory and inhibitory processes is the basis of the gate theory of pain transmission (Melzack and Wall, 1965) and of the mechanism referred to by LeBars et al.(1979a,b) as “diffuse noxious inhibitory controls” or DNIC.
The neurotransmitters contained in the terminals of nociceptive afferent fibers in the dorsal horn include excitatory amino acids, particularly glutamate (De Biasi and Rustioni, 1988), as well as neuropeptides, such as SP, CGRP, VIP, somatostatin, and others (reviewed in Willis and Coggeshall, 1991). These can be demonstrated by immunohistochemical staining of sections of the spinal cord. Certain experimental conditions, such as peripheral nerve damage, can lead to an upregulation or downregulation of these transmitters; e.g., after the sciatic nerve is cut, the levels of stainable galanin in the dorsal horn increase (Zhang et al., 1993). However, the increased stores of galanin do not appear to exist in the synaptic terminals but rather in axons coursing through the dorsal horn (Carlton and Coggeshall, 1996). Another example is the increased stores of glutamate in the dorsal horn that occur after the development of experimental arthritis (Fig. 3A and top panels) (Sluka et al., 1992; Sluka and Westlund, 1993). There is an initial decrease in staining for SP (Fig. 3B)(Sluka et al., 1992), presumably due to release of this peptide (and also of CGRP) into the dorsal horn during the onset of arthritis (Schaible et al., 1990, 1994). However, SP and CGRP stores increase as these neuropeptides are produced and transported from dorsal root ganglion cells to the dorsal horn (Fig. 3B and C) (Sluka and Westlund, 1993). The increase in glutamate stores helps explain the higher concentration of glutamate that can be detected by microdialysis in the dorsal horn in experimental arthritis (Sluka et al., 1994).
FIG. 3.
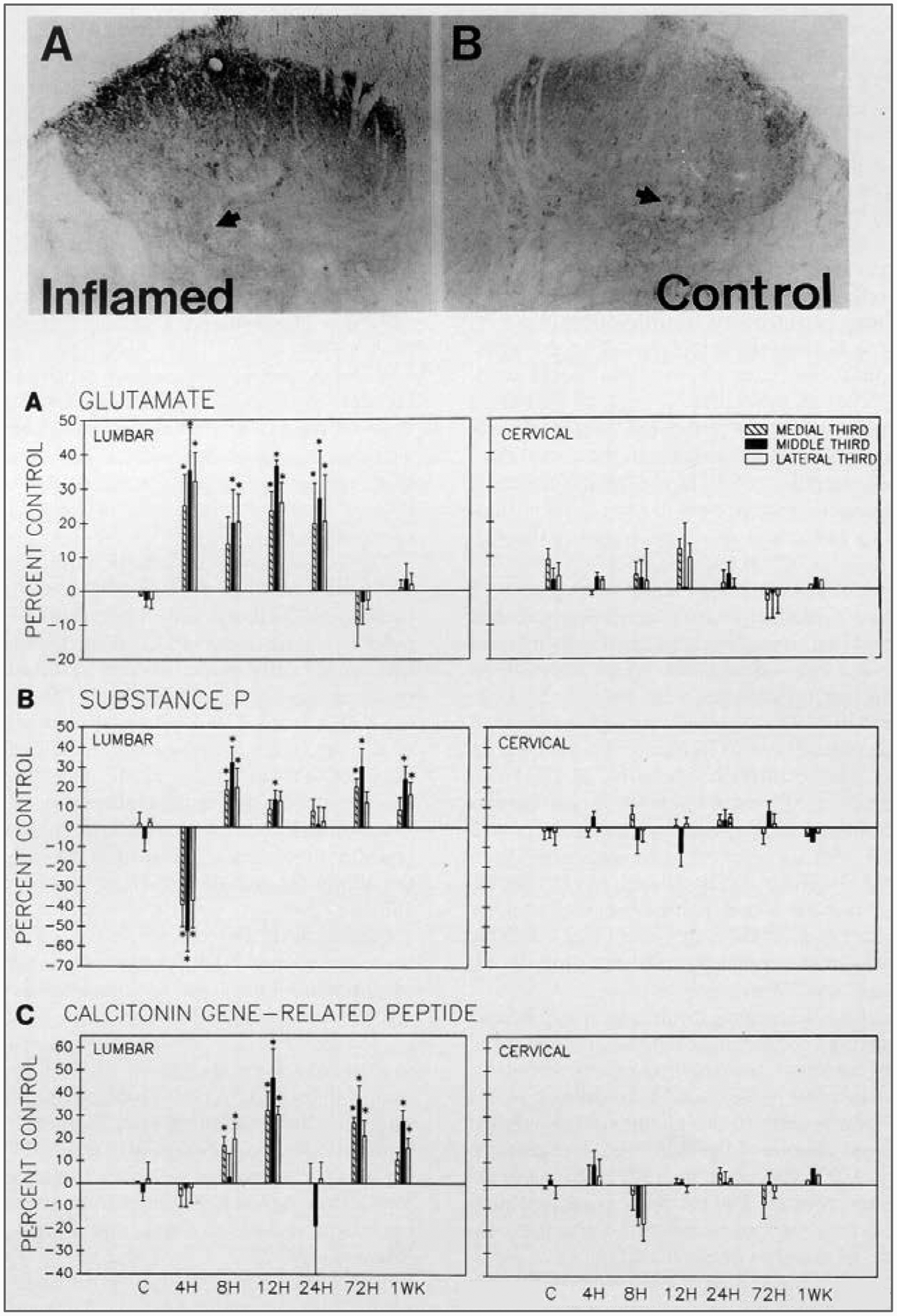
Changes in glutamate and peptide content in the dorsal horn during the development of experimental arthritis.Top: The photomicrographs show a section of the lumbar dorsal horn 24 h after the induction of arthritis by injection of kaolin and carrageenan into the capsule of the knee joint. The section is immunohistochemically stained for glutamate, which is increased on the inflamed side(left) as compared with the normal side (right).Bottom: The bar graphs in A-C show the changes in immunoreactivity of the dorsal horn for glutamate, substance P, and calcitonin gene-related peptide at different times after the induction of arthritis. Lumbar spinal cord (left). Staining density in the cervical spinal cord did not change (right). *Statistically significant changes. (From Sluka and Westlund, 1993.)
After strong or damaging stimuli, such as repeated noxious heating or squeezing of the skin, intradermal injection of capsaicin, or induction of acute arthritis, nociceptive neurons in the dorsal horn develop an enhanced responsiveness to peripheral stimuli applied to undamaged regions of the skin (Fig. 4) (Kenshalo et al., 1979, 1982; Owens et al., 1992; Simone et al., 1991; Dougherty et al., 1992b). Sensitization of neurons in the dorsal horn has been attributed to the combined effects of excitatory amino acids (such as glutamate and aspartate) and peptides (such as SP and CGRP) released into the dorsal horn (Randic et al., 1990; Dougherty et al., 1991, 1992a,b; 1993, 1994, 1995; Dougherty and Willis, 1992; Neugebauer et al., 1993, 1995; Thompson et al., 1994; Urban et al., 1994). Nociceptive dorsal horn neurons, including spinothalamic tract neurons, receive synaptic connections from glutamate-containing terminals (Westlund et al., 1992) as well as from peptide-containing endings (Carlton et al., 1990). Coapplication of excitatory amino acids and SP by iontophoresis onto dorsal horn neurons results in an enhanced responsiveness of these cells (Randic et al., 1990; Dougherty and Willis, 1991). Furthermore, sensitization can be blocked by antagonists of glutamate receptors (Dougherty et al., 1992b) or of neurokinin 1 (SP) receptors (Dougherty et al., 1994).
FIG. 4.
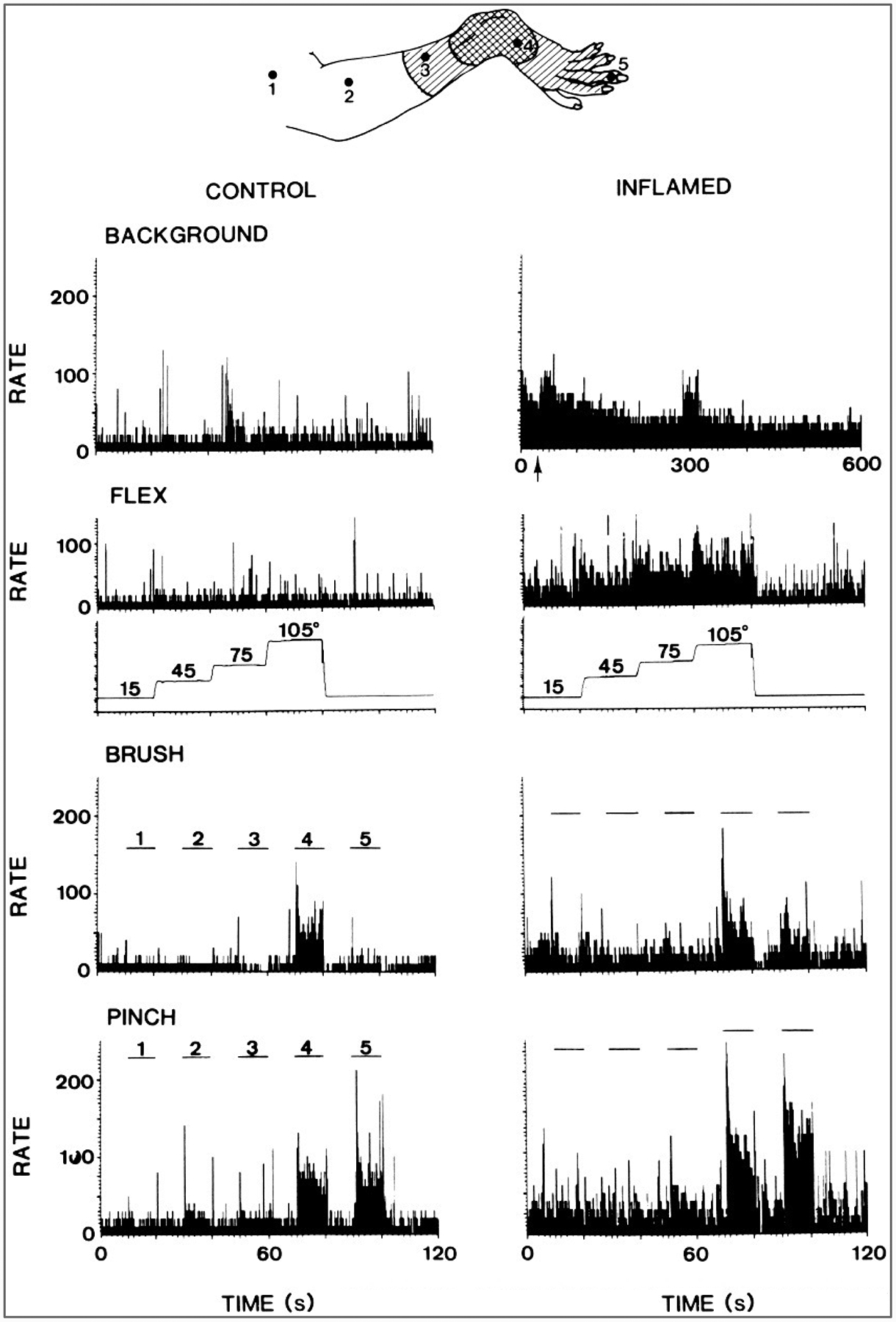
Increased responses of a primate spinothalamic tract cell after acute arthritis was induced by injection of kaolin and carrageenan into the knee joint. Top: Receptive field of the neuron on the ankle and foot before (doubly hatched area) and after(hatched area) the development of arthritis. Left columns: Peristimulus histograms show the background activity of the neuron and its responses to flexion of the knee, to brushing the skin at the points labeled 1–5 in the drawing, and to pinching the skin at the same points. Right columns: Histograms show the enhanced background activity and response after the development of arthritis. The increased background activity would presumably result in pain in an unanesthetized animal, and the increased response to knee flexion would be an indication of primary hyperalgesia. The increased responses to stimulation of the foot would presumably represent secondary mechanical allodynia and hyperalgesia. (From Dougherty et al., 1992b.)
As for peripheral nociceptors, sensitization of dorsal horn nociceptive neurons appears to result from the activation of second messenger systems. Particular signal transduction cascades that are involved include the protein kinase C system (Mao et al., 1993; Palecek et al., 1994; Lin et al., 1996b) and probably other pathways as well.
The consequences of central sensitization include allodynia and hyperalgesia of the secondary type (Lewis, 1942; Hardy et al., 1952; Bonica, 1992). The nociceptors in an area of secondary hyperalgesia show no change in their responsiveness to stimulation (Baumann et al., 1991; LaMotte et al., 1992; Schmelz et al., 1996). Instead, the increased responses of dorsal horn neurons result in the enhancement of behavioral nociceptive responses and of pain. Increased responses of wide dynamic range neurons, i.e., neurons that respond not only to noxious but also to innocuous stimuli, to tactile stimulation can account for allodynia in the area of secondary hyperalgesia (Willis, 1993).
Inhibition in the nociceptive circuits of the dorsal horn is mediated by a number of neurotransmitters, including inhibitory amino acids, such as GABA and glycine (Curtis et al., 1968, 1971a,b; Willcockson et al., 1984a; Lin et al., 1994), as well as neuropeptides, such as enkephalin (Duggan et al., 1977; Willcockson et al., 1984b). GABA is involved in both pre- and postsynaptic inhibition and glycine in postsynaptic inhibition (Eccles et al., 1963; Curtis et al., 1971a,b; 1977; Carlton and Hayes, 1990; Todd, 1990; Carlton et al., 1992). Presynaptic receptors that mediate presynaptic inhibition include GABAA and GABAB receptors (Curtis et al., 1977; Curtis and Lacey, 1994). These same receptor types can also be involved in postsynaptic inhibition, along with glycine receptors. Inhibition of a spinothalamic tract by activation of GABAB receptors is shown in Fig. 5.
FIG. 5.
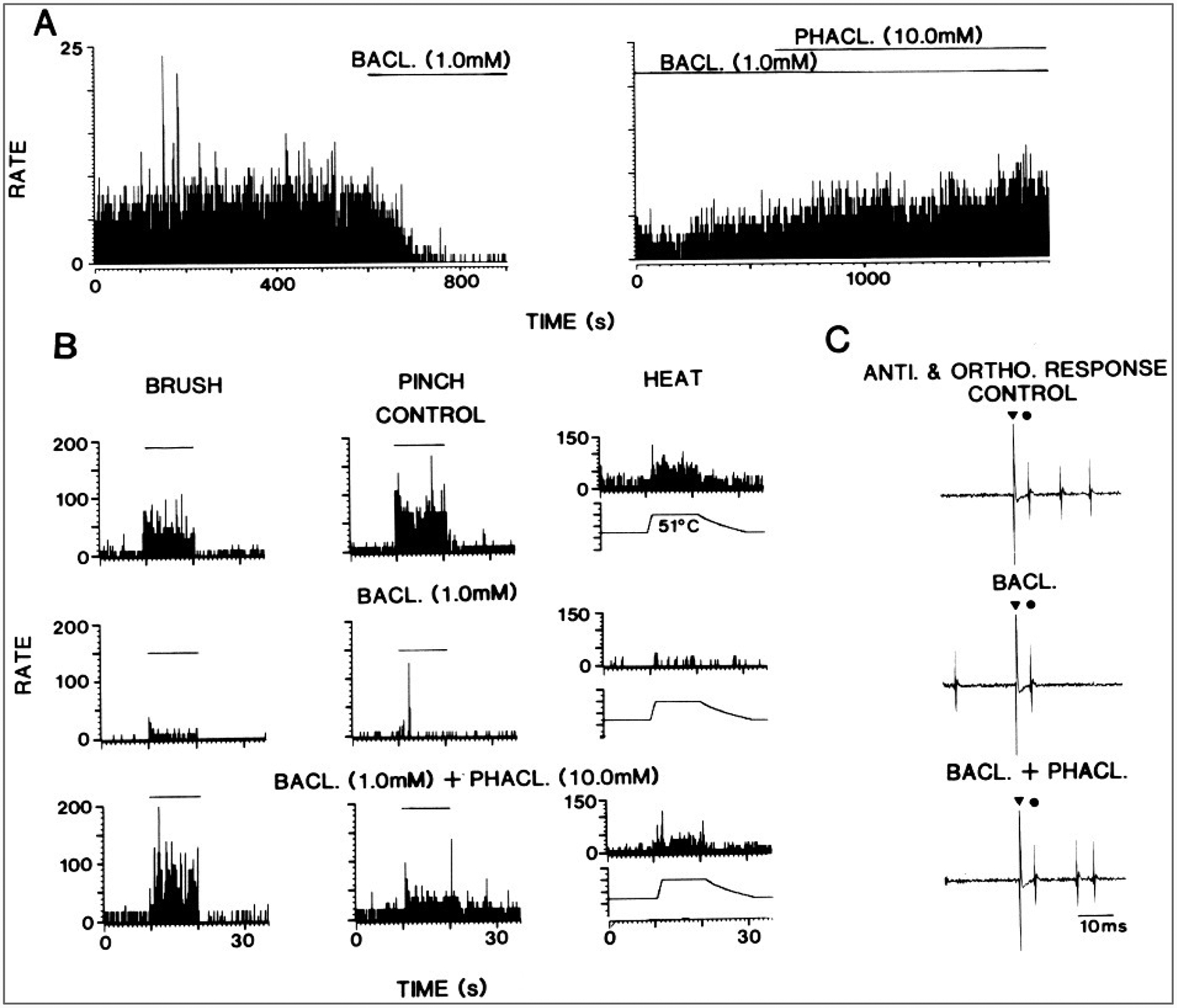
Inhibition of a primate spinothalamic tract cell by baclofen administered in the spinal cord by microdialysis. A: Background activity of the neuron is shown before and during the administration of the GABAB receptor agonist baclofen and (left) right during the coadministration of baclofen and the GABAB receptor antagonist, phaclofen (right).B: Responses of the cell to brush, pinch, and heat stimuli applied to the receptive field before and during baclofen administration and during coadministration of baclofen and phaclofen. C: Antidromic and orthodromic action potentials of the neuron at different times during the experiment. (From Lin et al., 1996c.)
Projection Neurons
Nociceptive projection neurons in the spinal cord transmit information to a number of regions of the brainstem and diencephalon, including the thalamus, periaqueductal gray, parabrachial region, and bulbar reticular formation, as well as to limbic structures in the hypothalamus, amgydaloid nucleus, septal nucleus, and other sites (see Willis, 1985). Recently, existence of a visceral nociceptive pathway in the dorsal columns involving the postsynaptic dorsal column pathway was also demonstrated (Al-Chaer et al., 1996a,b; Hirshberg et al., 1996). In the following sections, the best studied of these pathways are reviewed. Available information about primates, will be emphasized.
PATHWAYS IN THE ANTEROLATERAL QUADRANT
Spinothalamic Tract
The spinothalamic tract in humans is believed to help mediate the sensations of pain, cold, warmth, and touch (Willis, 1985; Gybels and Sweet, 1989; Willis and Coggeshall, 1991). This belief is based largely on the results of anterolateral cordotomies performed to relieve pain (Spiller and Martin, 1912; Foerster and Gagel, 1932; White and Sweet, 1969) or deficits due to damage to the spinal cord by disease or trauma (Gowers, 1878; Spiller, 1905; Head and Thompson, 1906; Noordenbos and Wall, 1976). However, results of experimental studies of primates in which changes in behavioral responses to noxious stimuli before and after spinal lesions were measured are consistent with the clinical evidence (Yoss, 1953; Vierck and Luck, 1979; Vierck et al., 1990).
The cells of origin of the spinothalamic tract have been mapped in monkeys, cats, and rats (Willis and Coggeshall, 1991). Presumably, the pattern in monkeys will prove to be closest to that in human organization. In monkeys, a large fraction of spinothalamic tract cells is located in the lumbar and sacral enlargements, and these cells are concentrated in the marginal zone and neck of the dorsal horn in laminae I and IV-VI (Fig. 6) (Willis et al., 1979; Apkarian and Hodge, 1989a). However, some spinothalamic cells are located in other laminae, including lamina X, which is around the central canal, and in the ventral horn. Comparison of the populations of spinothalamic tract cells projecting to the lateral thalamus, including the ventral posterior lateral nucleus, and those projecting to the medial thalamus, including the central lateral nucleus, show clear differences between the two (Willis et al., 1979). Laterally projecting spinothalamic neurons are more likely to be situated in laminae I and V (Fig. 6), whereas medially projecting cells are more likely to be situated in the deep dorsal horn and in the ventral horn (Fig. 7). Most of the cells project to the contralateral thalamus, although a small fraction projects ipsilaterally. A large group of spinothalamic tract cells is also located in segments C1 and C2 (Apkarian and Hodge, 1989a), in lamina VIII bilaterally and in laminae I-VII contralateral to the thalamic target.
FIG. 6.
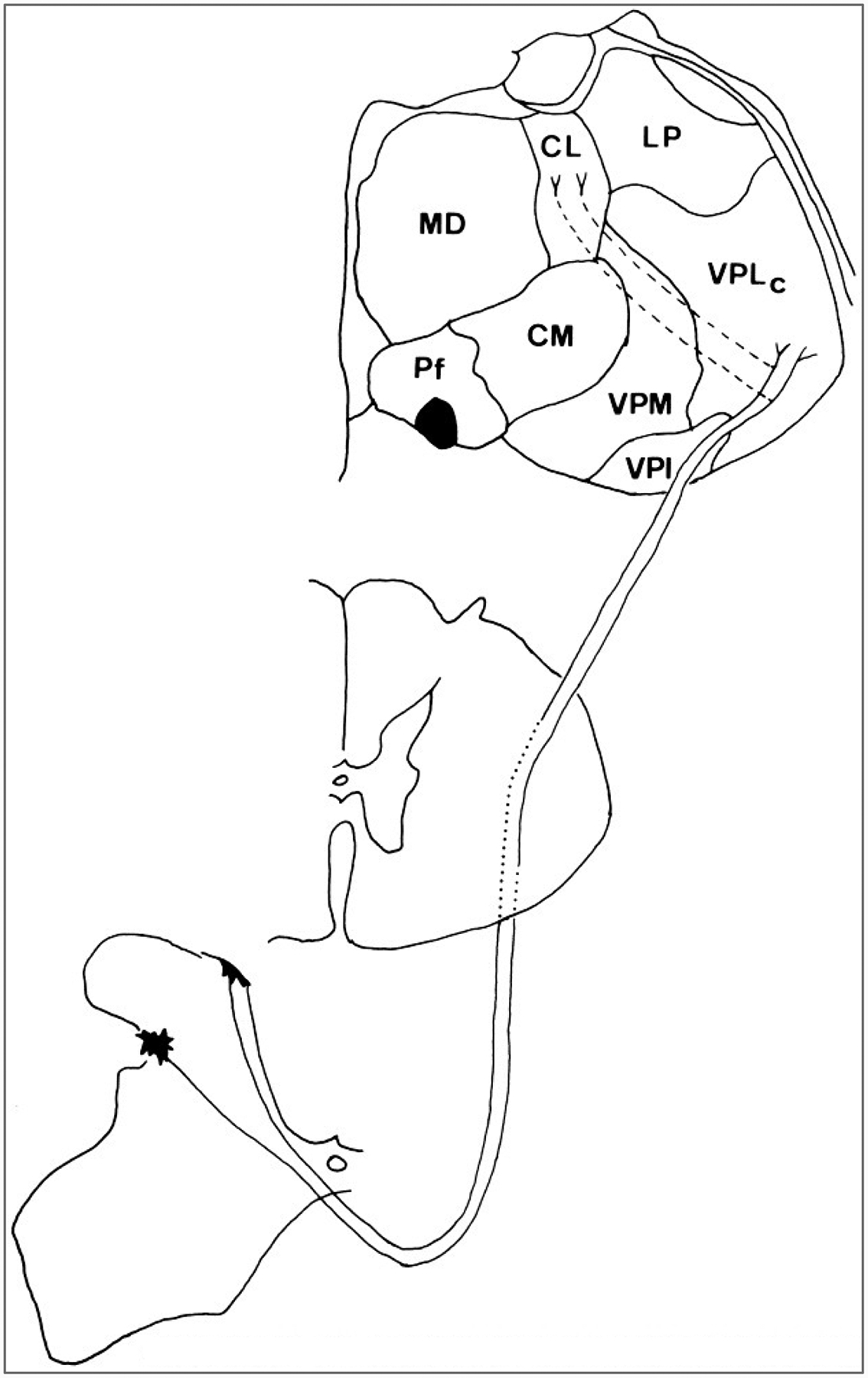
Course of the laterally projecting component of the spinothalamic tract in a macaque monkey. The cells of origin of the part of the spinothalamic tract that projects to the lateral thalamus are concentrated in laminae I and V of the spinal cord dorsal horn. The axons cross the midline in the ventral gray commissure at a level near that of the cell bodies of the neurons. The axons then ascend in the ventral and then in the ventrolateral quadrant. After passing through the brainstem, the axons terminate synaptically in the lateral thalamus. The nuclei of termination include the caudal part of the ventral posterior lateral nucleus (VPLc) and also the ventral posterior inferior (VPI) and the medial part of the posterior group (POm; data not shown). Some of the laterally projecting spinothalamic tract neurons send collaterals to the medial thalamus, where they end in the central lateral(CL) nucleus (dashed lines).
FIG. 7.
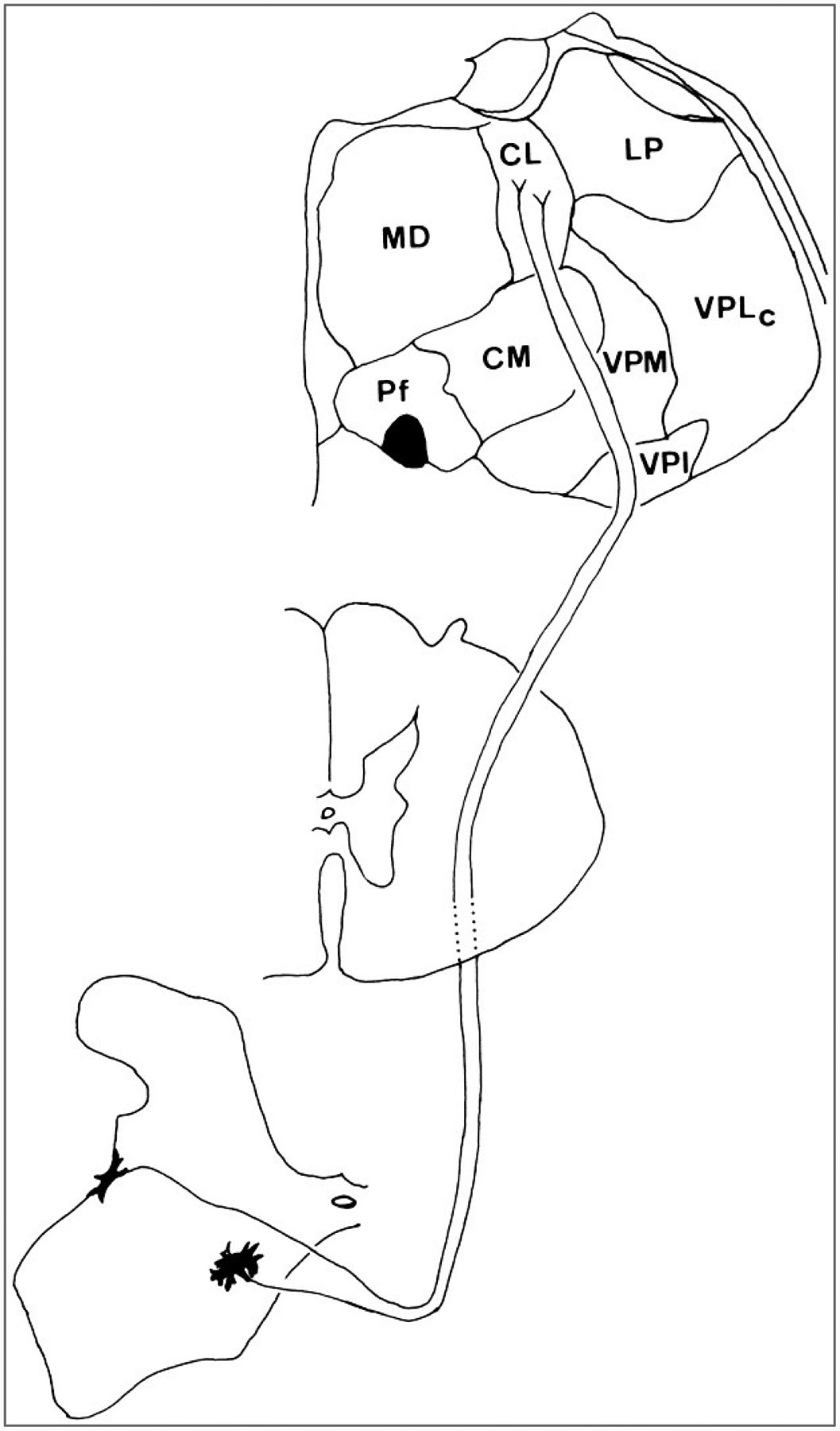
Course of the medially projecting component of the spinothalamic tract of a macaque monkey. The cells of the spinothalamic tract that project just to the intralaminar nuclei of the medial thalamus originate in the deep dorsal horn and the ventral horn of the spinal cord. The axons decussate immediately and then ascend in the ventral and then in the ventrolateral white matter. After passing through the brainstem, they terminate in the intralaminar nuclei, especially the central lateral (CL) nucleus.
The projections of the spinothalamic tract have been traced to the thalamus in humans, as well as in monkeys, cats, rats, and other experimental animals (Willis and Coggeshall, 1991). In primates, terminals exist in the following nuclei: the caudal and oral parts of the ventral posterior lateral nucleus (VPLc and VPLo of Olszewski, 1952), the ventral posterior inferior nucleus (VPI), the medial part of the posterior complex (POm), the central lateral (CL) nucleus, and other intralaminar and medial thalamic nuclei (Figs. 6 and 7) (Mehler et al., 1960; Mehler, 1962; Kerr, 1975; Boivie, 1979; Berkley, 1980; Mantyh, 1983; Apkarian and Hodge, 1989c; Gingold et al., 1991; Apkarian and Shi, 1994). A projection from lamina I has been traced to a medial thalamic nucleus, the posterior part of the ventral medial nucleus, VMpo (Craig et al., 1994).
The axons of spinothalamic neurons often decussate through the ventral white commissure at a very short distance from the cell body (Fig. 6) (Willis et al., 1979). They initially enter the ventral funiculus and then shift into the lateral funiculus as they ascend. Axons from spinothalamic tract cells of lamina I ascend more dorsally in the lateral funiculus than do the axons of spinothalamic tract cells in deeper layers of the dorsal horn (Apkarian and Hodge, 1989b). Clinical evidence from anterolateral cordotomies indicates that spinothalamic axons in the anterolateral quadrant of the spinal cord are arranged somatotopically. At cervical levels, spinothalamic axons representing the lower extremity and caudal body are placed more laterally and those representing the upper extremity and rostral body more anteromedially (Hyndman and Van Epps, 1939; Walker, 1940). Recordings from spinothalamic axons in monkeys are consistent with this scheme (Applebaum et al., 1975). How the somatotopic arrangement and the segregation of axons from lamina I as compared with deeper laminae remain consistent is unclear.
Primate spinothalamic tract cells that project to the lateral thalamus generally have receptive fields on a restricted area of the contralateral skin and are thus well suited to a function in signaling the sensory-discriminative aspects of pain (Willis et al., 1974). Most of the neurons show their best responses when the skin is stimulated mechanically at a noxious intensity. However, many spinothalamic tract cells also respond, although less effectively, to innocuous mechanical stimuli, and some respond best to innocuous mechanical stimuli (Willis et al., 1974; Price and Mayer, 1975; Price et al., 1978; Chung et al., 1979; Ferrington et al., 1987). A large fraction of spinothalamic tract cells also respond to noxious heating of the skin (Kenshalo et al., 1979; Surmeier et al., 1986a,b). Some spinothalamic neurons respond to stimulation of receptors in muscle (Foreman et al., 1979), joints (Dougherty et al., 1992c), or viscera (Milne et al., 1981; Blair et al., 1982, 1984; Ammons, 1989a,b). Spinothalamic neurons with a dominant input from viscera or muscle are situated in segments just rostral and caudal to segments containing spinothalamic tract cells with a dominant cutaneous input from the distal part of an extremity (Hobbs et al., 1992).
Primate spinothalamic tract cells that project to the region of the CL nucleus in the medial thalamus may also collateralize to the lateral thalamus (Fig. 6); these cells have response properties identical to those of spinothalamic tract cells that project just to the lateral thalamus (Giesler et al., 1981). However, spinothalamic tract cells that project just to the CL nucleus (Fig. 7) have very large receptive fields, often encompassing the entire surface of the body and face (Giesler et al., 1981). Some of these have input from visceral structures, as well as from the skin (Ammons et al., 1985). The large receptive fields suggest that these neurons would be more suited for a role in the motivational-affective aspects of pain, rather than in sensory discrimination.
Spinothalamic tract cells have not only excitatory but also inhibitory receptive fields (Gerhart et al., 1981b). The strongest inhibition from stimulation of the skin occurs when noxious intensities of stimulation are used, suggesting that a mechanism similar to DNIC is involved. Inhibition of spinothalamic tract cells is prominent when the stimuli are applied contralaterally or to dermatomes remote from those of the excitatory receptive field (Gerhart et al., 1981b; Hobbs et al., 1992). Spinothalamic tract cells can be inhibited effectively by repetitive electrical stimulation of peripheral nerves (Chung et al., 1984a). The inhibition can outlast stimulation by 20–30 min. Some inhibition can be evoked by stimulation of large myelinated axons of a peripheral nerve, but the inhibition is much more powerful if small myelinated or unmyelinated afferents are included in the volleys (Chung et al., 1984b). The best inhibition is produced by stimulation of a peripheral nerve in the same limb as the excitatory receptive field, but some inhibition occurs when nerves in other limbs are stimulated. A similar inhibition results when high-intensity stimuli are applied to the skin with a clinical transcutaneous electrical nerve stimulator (TENS unit) in place of direct stimulation of a peripheral nerve (Lee et al., 1985).
Several other pathways accompany the spinothalamic tract in the white matter of the ventrolateral quadrant of the spinal cord. These include the spinomesencephlic tract, the spinoreticular tracts, and several recently described spino-limbic tracts (Willis, 1985; Willis and Coggeshall, 1991).
Spinomesencephalic Tract
The spinomesecephalic tract includes several projection systems that terminate in different areas in the midbrain. The cells of origin of the spinomesecephalic tract are distributed in the spinal cord in a manner similar to that of the cells of origin of the spinothalamic tract. In primates, most of the cells are in laminae I and IV-VI, although some are in the ventral horn and lamina X (Fig. 8) (Trevino, 1976; Willis et al., 1979; Mantyh, 1982; Wiberg et al., 1987). Some spinomesencephlic tract cells give off collaterals that end in the lateral thalamus (Yezierski et al., 1987; Zhang et al., 1990).
FIG. 8.
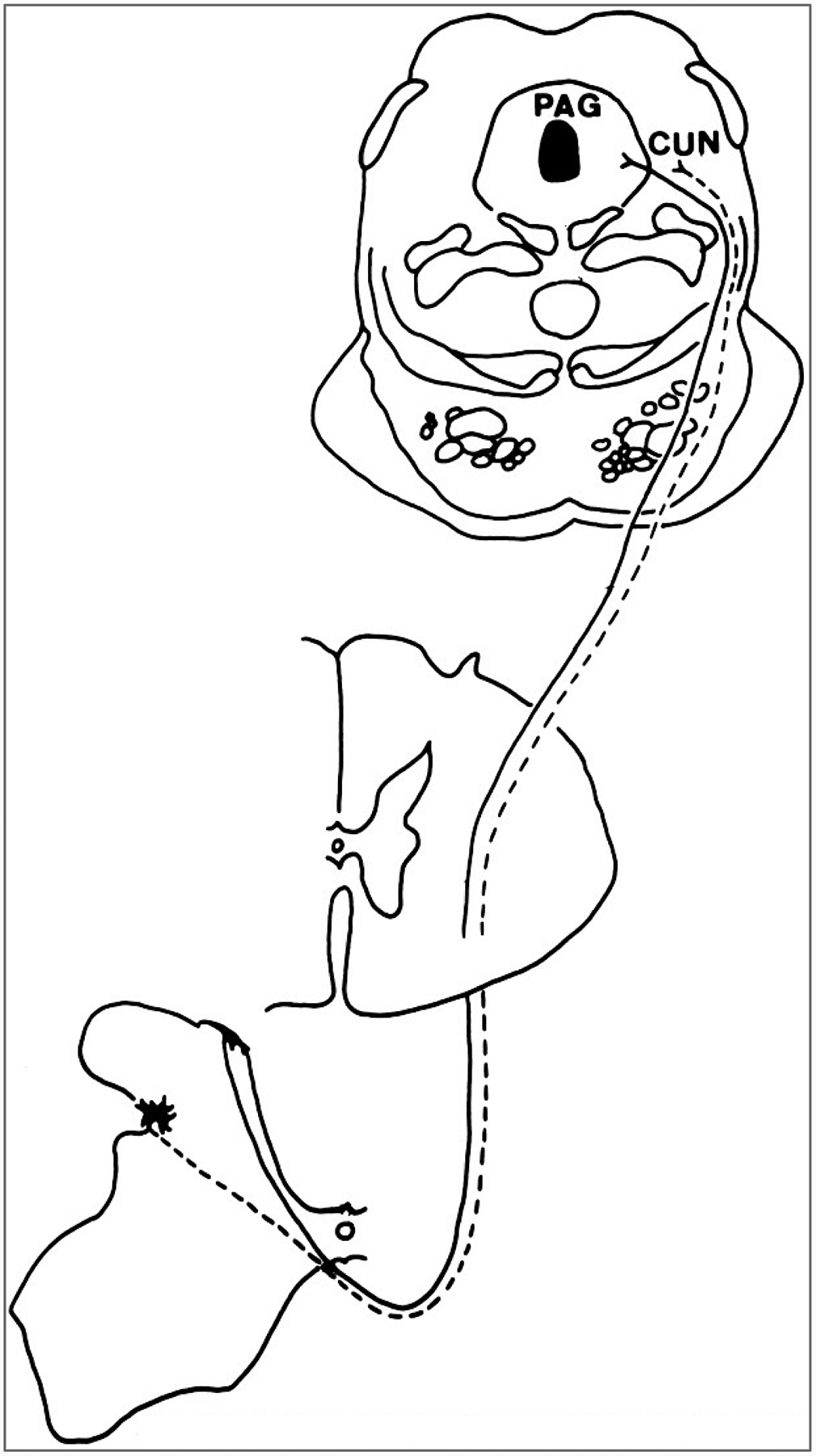
Course of the spinomesencephalic tract in a macaque monkey. The cells of origin of the tract are concentrated in laminae I and V. The axons decussate in the ventral white commissure and ascend to the midbrain in the lateral funiculus. They end in several midbrain nuclei, including the periaqueductal gray (PAG) and cuneiform nucleus(CUN).
The spinomesencephalic projections are to the following midbrain nuclei: periaqueductal gray, nucleus cuneiformis (Fig. 8), intercolliculus nucleus, deep layers of the superior colliculus, nucleus of Darkschewitsch, anterior and posterior pretectal nuclei, red nucleus, Edinger-Westphal nucleus, and interstitial nucleus of Cajal (Mehler et al., 1960; Mehler, 1969; Kerr, 1975; Wiberg et al., 1987; reviewed in Willis, 1985; Willis and Coggeshall, 1991). A rough somatotopic organization exists, in that spinomesencephalic projections from more caudal parts of the body terminate more caudally in the midbrain, whereas projections from more rostral parts of the body end more rostrally in the midbrain (Wiberg et al., 1987).
Spinomesencephlic neurons are nociceptive, responding either to noxious stimuli only or best to noxious but also to innocuous stimuli (Willis and Coggeshall, 1991). Recordings from spinomesecephalic tract cells in monkeys show that these cells often have complex receptive fields on widely separated areas of the body, in contrast to spinothalamic cells projecting to the lateral thalamus, which generally have receptive fields on a restricted area of the contralateral limb (Yezierski et al., 1987).
The different components of the spinomesencephalic tract may have different functions; e.g., the projections to the periaqueductal gray(PAG) could contribute to aversive behavior (Skultety, 1963; Nashold et al., 1969) as well as activate the descending analgesia system that arises from the PAG (described below). The projections to the nucleus cuneiformis could access the midbrain locomotor center (see Brooks, 1986) and the ascending reticular activating system (Magoun, 1963). Input to the deep layers of the superior colliculus are likely to play a role in orienting. Because the anterior pretectal nucleus is another locus that produces analgesia when stimulates (Rees and Roberts, 1993) (described below), the projections here may serve to limit nociception.
Spinoreticular Tracts
Many of the cells of origin of the spinoreticular tracts are located in the deep layers of the dorsal horn and in laminae VII and VIII of the ventral horn (Fig. 9) (Kevetter et al., 1982 reviewed in Willis, 1985; Willis and Coggeshall, 1991). Spinoreticular neurons have been identified by antidromic activation (Fields et al., 1975; Haber et al., 1982) and by retrograde labeling. The spinoreticular projections to the caudal medulla end in several nuclei, including the retroambiguus and superspinalis nuclei as well as dorsal and ventral parts of the nucleus medullae oblongatae centralis (Mehler et al., 1960). More rostral projections are to the lateral reticular nucleus, the nucleus gigantocellularis (Fig. 9), the nuclei paragigantocellularis dorsalis and lateralis, and the nuclei pontis caudalis and oralis. Enkephalin-containing cells with projections into the medulla have been described (Nahin and Micevych, 1986). The spinoreticular and spinothalamic tracts ascend together in the ventrolateral spinal cord and brainstem (Mehler et al., 1960). Both physiological (Haber et al., 1982; Giesler et al., 1981) and anatomic studies (Kevetter and Willis, 1982) have shown that some spinoreticular neurons are collateral branches of spinothalamic tract cells. There is no obvious somatopic organization of the spinoreticular tracts.
FIG. 9.
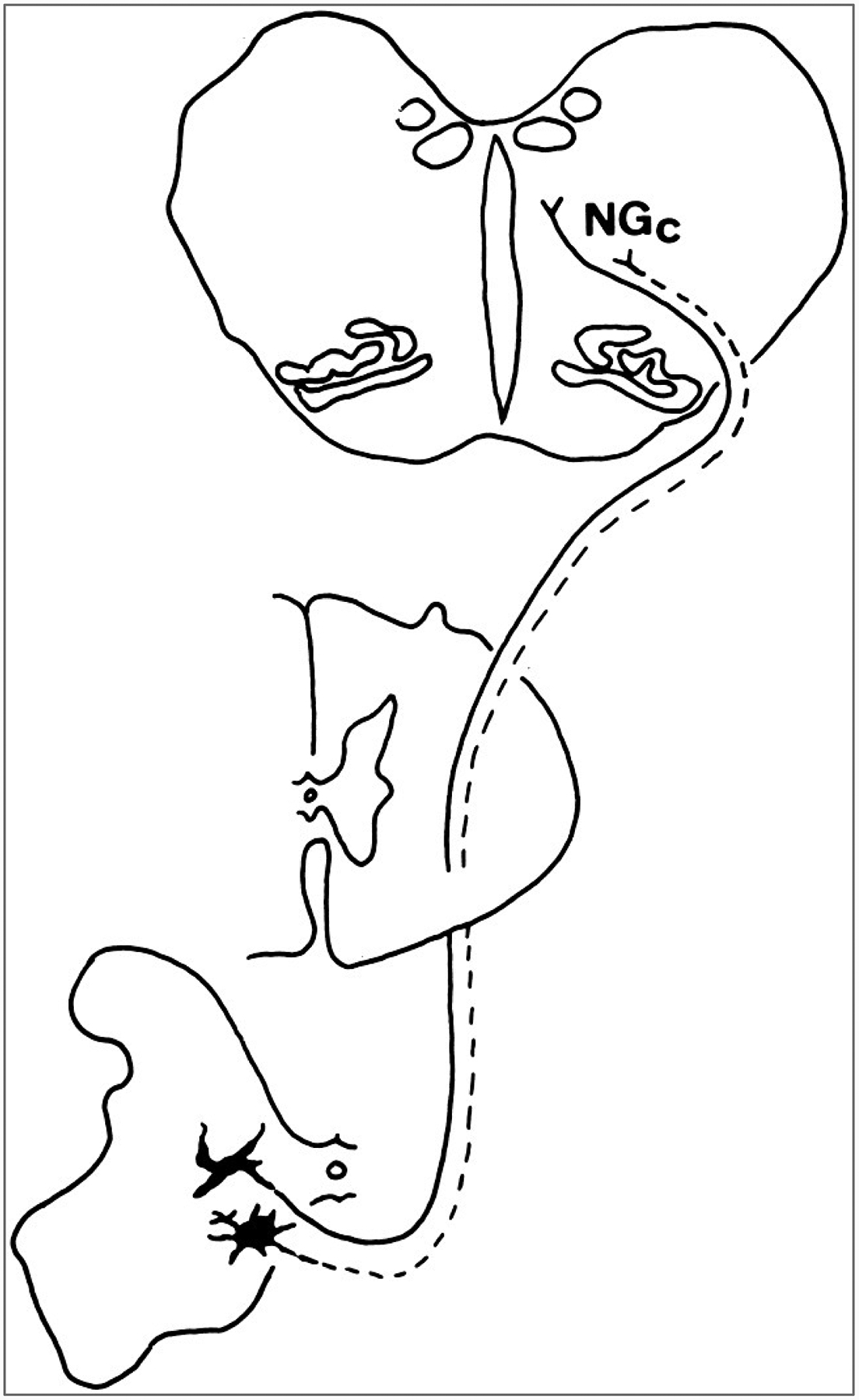
Course of the component of the spinoreticular tract that projects to the caudal reticular formation in a macaque monkey. The cells of origin are concentrated in the ventral horn in laminae VII and VIII. The axons decussate and ascend in the lateral funiculus and terminate in several nuclei of the reticular formation of the medulla and pons, including the nucleus gigantocellularis (NGc).
Another major termination of spinoreticular fibers in the brainstem is in the parabrachial region (Fig. 10) (Cechetto et al., 1985; Hylden et al., 1985; Hylden et al., 1986; Standaert et al., 1986; Menetrey and Basbaum, 1987; Wiberg et al., 1987; Blomqvist et al., 1989; Bernard and Besson, 1990; Lima et al., 1991; Craig, 1992, 1995; Kitamura et al., 1993; Slugg and Light, 1994; Feil and Herbert, 1995).
FIG. 10.
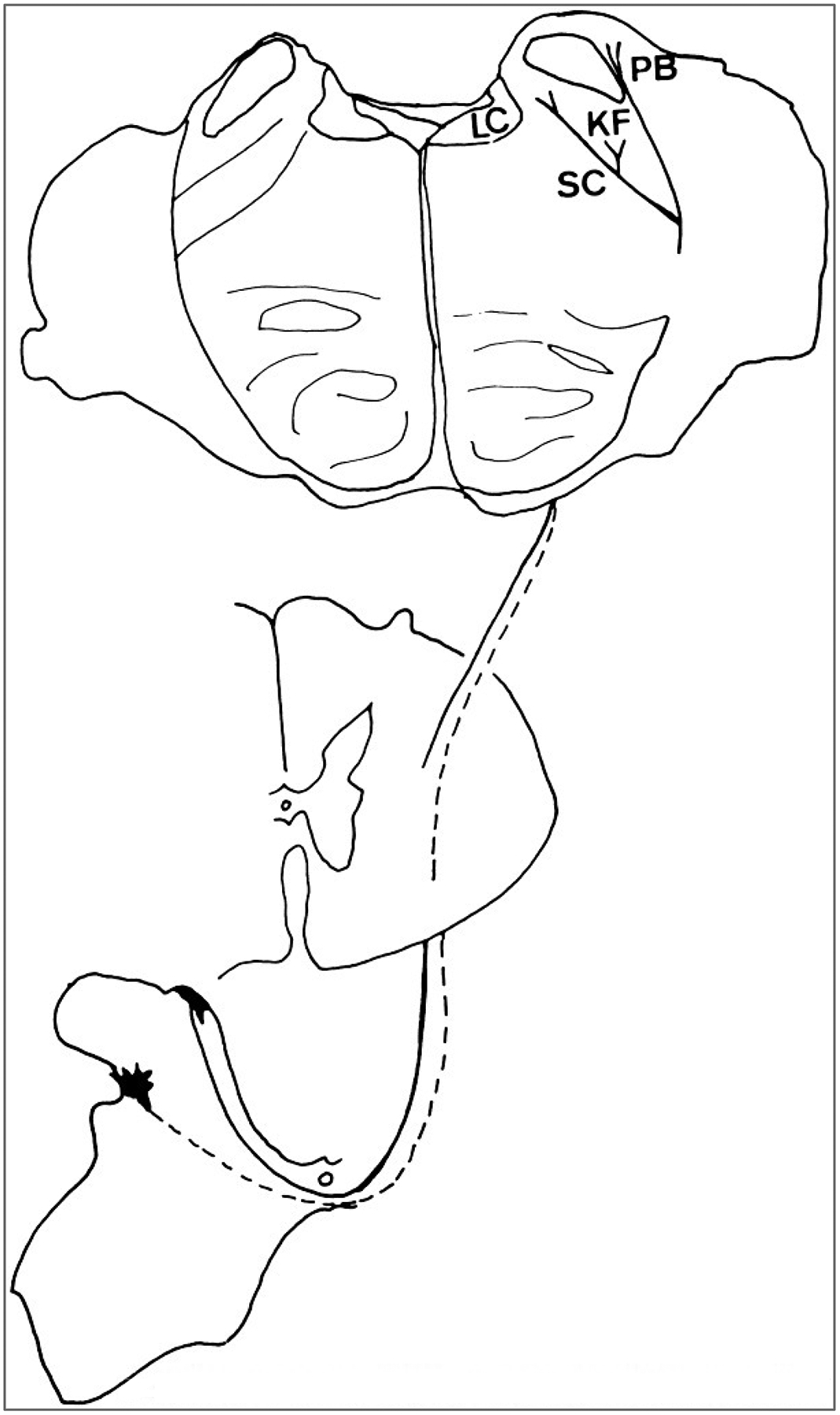
Course of the component of the spinoreticular tract that projects to the parabrachial region. The spinoreticular neurons are in the dorsal horn, including laminae I and V, and project to several nuclei in the parabrachial region, including the locus ceruleus, the Kölliker-Fuse nucleus, and the parabrachial nuclei.
An additional source of spinoreticular input was recently described in experiments in which ascending pathways from lamina I cells of the spinal cord identified in monkeys and cats were traced with an anterograde label (Craig, 1995). The ascending fibers of the lamina I cells are scattered throughout the lateral white matter (Craig, 1991). The lamina I projections ascend through the brainstem among the neurons of the catecholamine cell column in the ventrolateral medulla (Craig, 1995; Westlund and Craig, 1996). The pathway arches dorsally to travel among the cells of the ventral subcoeruleus, Kolliker-Fuse, and dorsal parabrachial nuclei in the dorsolateral pons. The pathway sends collaterals to terminate among almost all of the catecholamine cell groups of the medulla and pons (Fig. 11), including the locus ceruleus (Craig, 1992). Some synaptic contacts were observed at the electron microscopic level for cells of the A5 and A7 cell groups, and many other contacts were made with noncatecholaminergic structures (Westlund and Craig, 1996). Spinoparabrachial and other spinoreticular cells have been shown to contain an assortment of immunoreactive peptides, including SP, VIP, bombesin, dynorphin, and enkephalin (Leah et al., 1988).
FIG. 11.
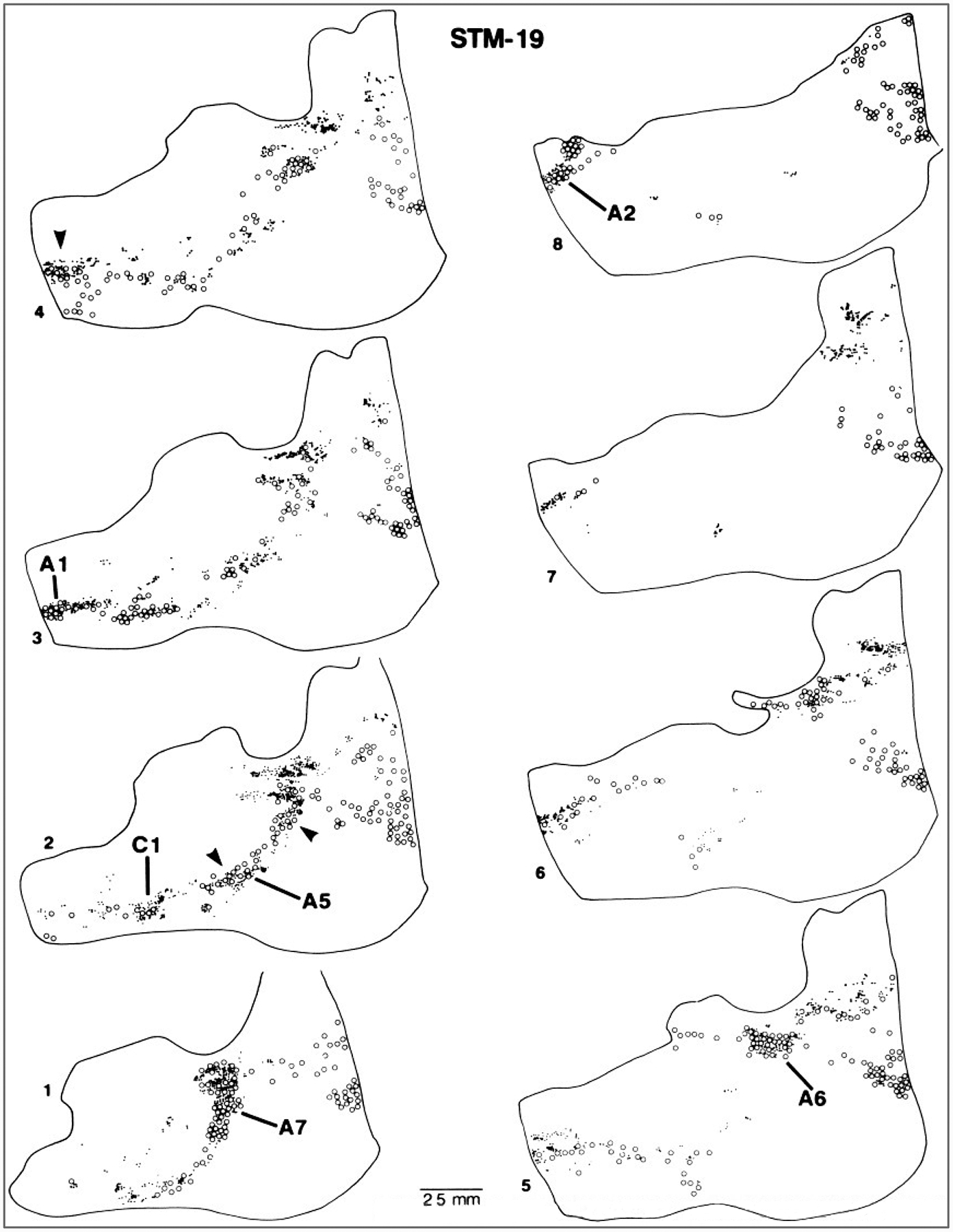
Spinoreticular projection with connections to brainstem catecholamine cell groups. Sagittal sections of a monkey brainstem are shown from the side contralateral to an injection site in lamina I of the cervical spinal cord where an anterograde tracer was placed. The catecholamine cell groups (A1, A2, A5, A6, A7, C1; open circles) were demonstrated by immunocytochemical staining for tyrosine hydroxylase. Locations of the terminals of spinal projection neurons (small dots). (From Westlund and Craig, 1996.)
Many reticular neurons respond preferentially to noxious stimuli (Wolstencroft, 1964; Casey, 1969, 1971a; Guilbaud et al., 1973; Foote et al., 1991). The primary functional significance of this input is undoubtedly to signal homeostatic changes to brainstem autonomic centers, but other brainstem responses include activation of endogenous analgesia systems and relay of information that triggers motivational-affective responses.
Spino-Limbic Tracts
A multisynaptic pathway is proposed as the means of carrying information about noxious inputs to the medial thalamus, where it is relayed to the limbic system (Bishop, 1959); this is sometimes termed the spinoreticulothalamic pathway. A possible anatomic substrate for this pathway would include the spinoreticular tracts described in the previous section. Ascending projections from the reticular formation have been reported to the medial thalamus, hypothalamus, and limbic structures (Fig. 12) (Nauta and Kuypers, 1958; Scheibel and Scheibel, 1958; Bowsher et al., 1968; Robertson et al., 1973; Bowsher, 1975; Blomqvist et al., 1989; Ma et al., 1989; Bernard and Besson, 1990; Carstens et al., 1990).
FIG. 12.
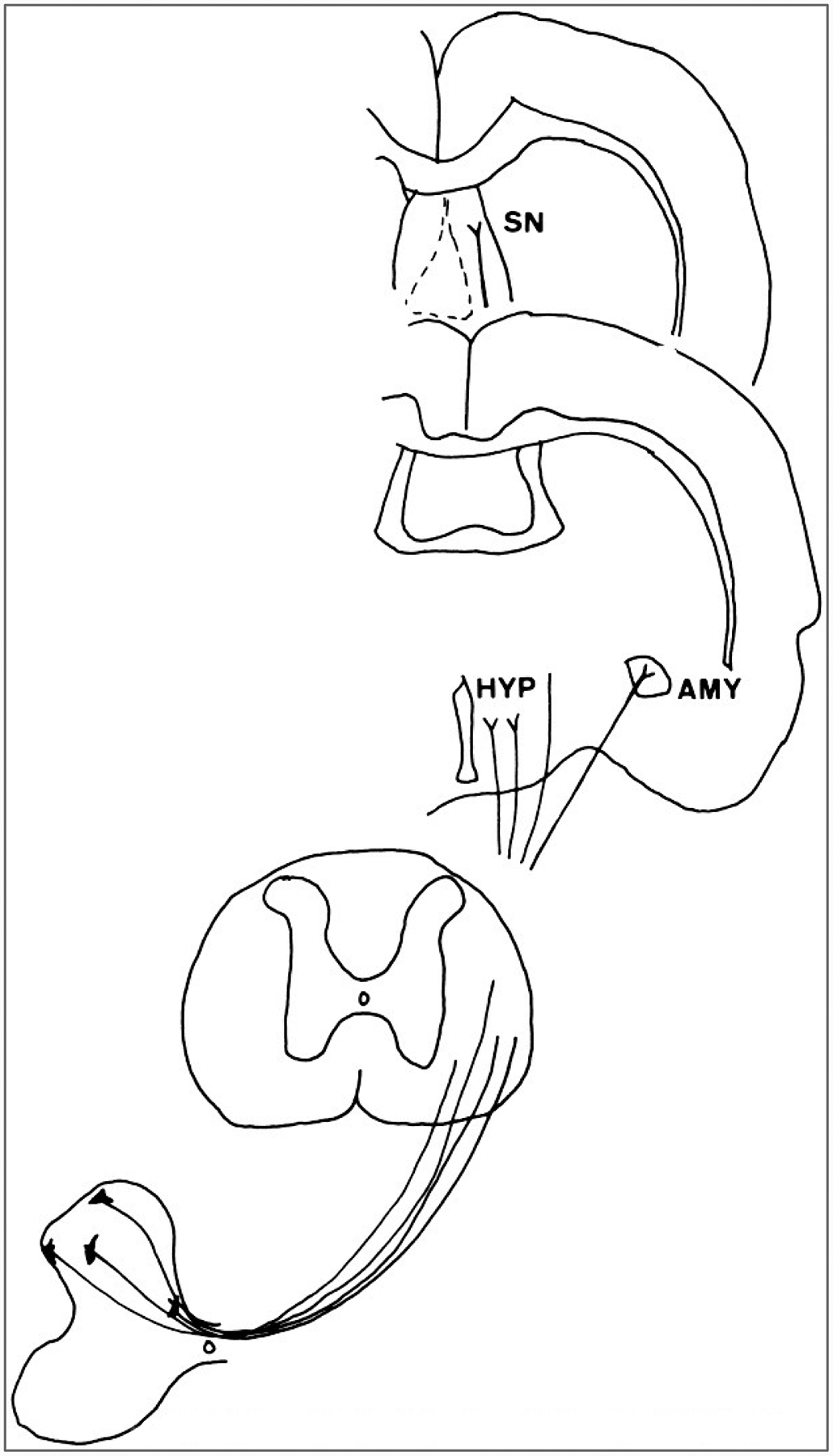
Course of spino-limbic projections in the rat. Neurons in the dorsal horn and also in the region of the central canal project through the lateral funiculus to the hypothalamus(HYP), amygdaloid nucleus (AMY), and the septal nuclei (SN).
In addition, direct spinohypothalamic (Burstein et al., 1987, 1990) and spinoamygdalar (Bernard and Besson, 1990; Burstein and Potrebic, 1993; Menetrey and de Pommery, 1991) pathways have been described. The spinohypothalamic tract is a major bilateral projection to both the medial and lateral hypothalamus arising primarily from cells of the deep dorsal horn and lateral spinal nucleus (Fig. 12). Other cells are localized in laminae I, VII, and X. Some spinal projections also innervate the nucleus accumbens and the septal nuclei, suggesting that a component of this pathway is relevant to the motivational aspects of pain (Burstein and Giesler, 1989).
The spinopontoamygdalar pathway (Bernard and Besson, 1990; Menetrey and de Pommery, 1991; Burstein and Potrebic, 1993) arises from cells situated bilaterally in the lateral reticulated area of the deep dorsal horn and in the gray matter surrounding the central canal. Anterograde tracing studies indicate that the central nucleus of the amygdala is directly innervated innervated by terminals arising from the spinal cord (Fig. 12)(Cliffer et al., 1991).
PATHWAYS IN THE DORSAL QUADRANT
Spinocervicothalamic Pathway
The spinocervicothalamic pathway originates from neurons in the spinal cord dorsal horn and relays in the lateral cervical nucleus in segments C1 and C2 (reviewed in Willis, 1985; Willis and Coggeshall, 1991). The axons of neurons of the lateral cervical nucleus decussate and then ascend with the medial lemniscus to the thalamus (Ha, 1971). A lateral cervical nucleus has been identified in several species, including rat, cat, dog, raccoon, and monkey(for the last, see Mizuno et al., 1967). A comparable nucleus has been observed in at least some human spinal cords (Truex et al., 1965).
The cells of origin of the spinocervical tract have not yet been mapped with anatomic techniques in monkeys. In cats, the cells are situated mostly in lamina IV, although some are situated in adjacent laminae of the dorsal horn and a few are scattered in deeper layers (Craig, 1978; Brown et al., 1980). Antidromic mapping of spinocervical tract cells in monkeys is in general agreement with this distribution (Bryan et al., 1974).
The axons of spinocervical tract neurons ascend in the dorsal part of the lateral funiculus to the upper cervical level (Nijensohn and Kerr, 1975) and then terminate in the lateral cervical nucleus. Cervicothalamic neurons have been mapped in the lateral cervical nucleus of monkeys by the retrograde tracing technique (Smith and Apkarian, 1991). The projections of these cells are to the contralateral ventral posterior lateral nucleus and the medial part of the posterior complex (Berkley, 1980; Boivie, 1980). Many of the cells also give off collaterals to the midbrain (Willis and Coggeshall, 1991).
Numerous electrophysiological studies have been made of spinocervical tract neurons in the cat, particularly by the laboratory of A. G. Brown(reviewed in Willis and Coggeshall, 1991). Many spinocervical tract cells have tactile responses and thus would not be candidates for a nociceptive role. However, some spinocervical tract cells do respond to noxious stimuli, both in cats (Brown and Franz, 1969; Cervero et al., 1977) and in monkeys (Bryan et al., 1974; Downie et al., 1988). Therefore, the spinocervical tract is a potential pathway through which nociceptive signals can reach the lateral thalamus.
Postsynaptic Dorsal Column Pathway
Although dogma holds that the dorsal column subserves graphesthesia, two-point discrimination, and position sense, recent interest in the reasons for the great effectiveness of limited midline myelotomy in reducing intractable pelvic cancer pain in humans (Fig. 13)(Hitchcock 1970, 1972a,b; Schwarcz, 1976, 1978; Gildenberg and Hirshberg, 1984; Hirshberg et al., 1996) has stimulated renewed interest in an additional functional role of the dorsal column in the relay of visceral pain.
FIG. 13.
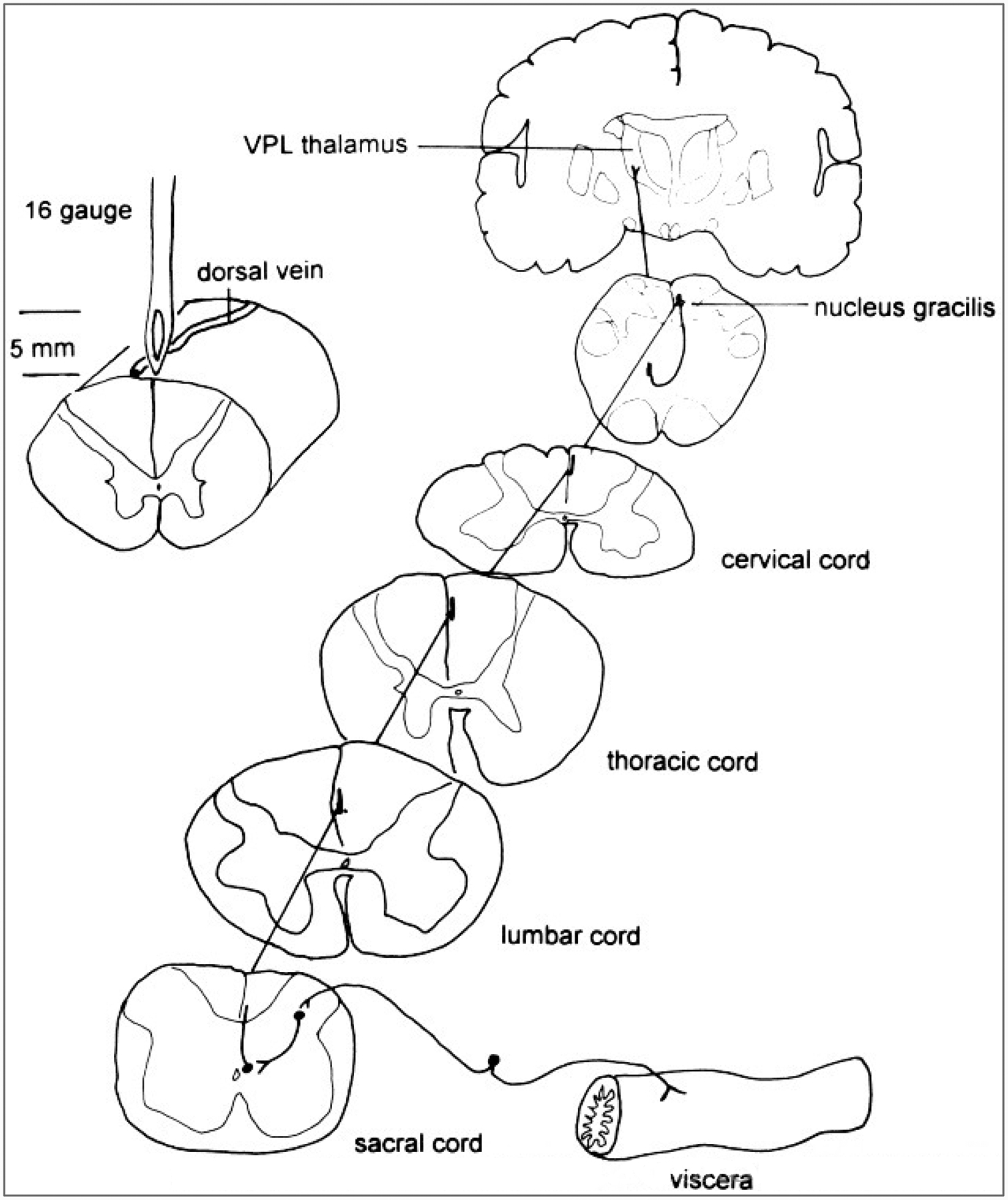
Course of the component of the postsynaptic dorsal column pathway that may mediate visceral pain. The afferent input to the sacral spinal cord from a pelvic visceral organ is shown by a drawing of a dorsal root ganglion cell and its peripheral and central processes. The afferent connects with a circuit that activates a projection neuron located in the central gray region (lamina X). The projection neurons sends its axon rostrally near the midline of the dorsal column to synapse in the nucleus gracilis. The gracile neuron projects to the contralateral ventral posterior lateral (VPL) nucleus in the thalamus.Left: Procedure for a limited midline myelotomy to interrupt this visceral pain pathway (H. J. W. Nauta, E. Hewitt, K. W. Westlund, W. D. Willis, unpublished observations).
In an early report speculating on the passage of visceral nociceptive fibers in the dorsal column, Foerster and Gagel (1932) noted that awake human subjects experience unbearable, excruciating pain when the dorsal column or medial aspect of the nucleus gracilis is probed mechanically. The pain in the latter case is referred to the sacral region and perineum. Nociceptive activity, including responses to uterine and vaginal distension, has been demonstrated in neurons of the dorsal column nuclei (Angaut-Petit, 1975b; Ferrington et al., 1988; Cliffer et al., 1992; Berkley and Hubscher, 1995). These nociceptive responses could be triggered by unmyelinated primary afferent fibers that have been shown to ascend in the dorsal column directly to the dorsal column nuclei (Patterson et al., 1989, 1990; Conti et al., 1990); alternatively, they could be mediated through the postsynaptic dorsal column pathway (Uddenberg, 1968; Angaut-Petit, 1975a,b; Bennett et al., 1984; Noble and Riddell, 1988).
The postsynaptic dorsal column pathway arises from cells distributed medial to laterally in lamina III in the dorsal horn, as well as from a few cells just lateral to lamina X (Fig. 13)(Rustioni, 1973, 1974; Rustioni et al., 1979; Bennett et al., 1983; Giesler et al., 1984). The postsynaptic dorsal column cells, in contrast to spinothalamic tract cells, are innervated by “serotonin-only” fibers, meaning that no coexisting peptide content could be identified in the serotonin-containing synapses (Wu and Wessendorf, 1992).
Although the postsynaptic dorsal column pathway in rats may not have a role in cutaneous pain (Giesler et al., 1984; Al-Chaer et al., 1996a,b), the postsynaptic dorsal column cells and cells of the gracile nucleus were shown to respond to both mechanical and chemical irritation of viscera (Al-Chaer et al., 1996b). The trajectories of postsynaptic dorsal column fibers are somatotopically organized in the dorsal column (Cliffer and Giesler, 1989; Hirshberg et al., 1996). Presumably, the visceral information is relayed together with cutaneous epicritic information in the medial lemniscus to the thalamus.
Thalamus
The spinothalamic tract projects to both the lateral and medial thalamus. The particular nuclei of termination include the VPL, VPI, and POm nuclei in the lateral thalamus and CL and other intralaminar nuclei, as well as VMpo in the medial thalamus. The spinomesencephalic and spinoreticular pathways relay in the reticular formation in areas that project heavily to medial thalamic nuclei, especially the intralaminar nuclei. The spinocervical tract relays in the lateral cervical nucleus, which projects to the VPL and POm nuclei. The postsynaptic dorsal column pathway projects to the dorsal column nuclei, which in turn project to the VPL and POm nuclei.
Electron microscopic studies have shown convergence of medial lemniscal and spinothalamic tract input onto the proximal dendritic trees of thalamocortical neurons (Ralston and Ralston, 1994). This would account for the observation that thalamic neurons respond to input conveyed through both the dorsal column and the lateral funiculus (Al-Chaer et al., 1996a).
Lateral Thalamus
Recordings have been made of nociceptive responses in the VPL and VPM nuclei of the monkey thalamus by many investigators (Gaze and Gordon, 1954; Perl and Whitlock, 1961; Pollin and Albe-Fessard, 1979; Kenshalo et al., 1980; Casey and Morrow, 1983, 1987; Chung, et al., 1986a,b; Bushnell and Duncan, 1987; Yokota et al., 1988; Bushnell et al., 1993; Chandler et al., 1992; Duncan et al., 1993; Apkarian and Shi, 1994; Bruggemann et al., 1994). In some of these experiments, the monkeys were examined in behavioral experiments without the potentially confounding effects of anesthesia. The proportion of nociceptive neurons, as compared with neurons activated only by innocuous stimuli, was low in unanesthetized preparations (~10%). However, strong stimuli could not be applied in these animals; therefore, more neurons may have been judged nociceptive than were reported if intense noxious stimuli could have been used (Casey and Morrow, 1983, 1987; Bushnell et al., 1993). In anesthetized monkeys, a much larger percentage of nociceptive neurons is situated in the VPL nucleus (Chung et al., 1986a,b). The larger proportion of nociceptive neurons in the VPL nucleus in anesthetized animals may result from the more intense stimuli that can be used or possibly from sensitization of nociceptive neurons because of repeated strong stimuli.
Nociceptive neurons in the VPL nucleus generally respond weakly to innocuous cutaneous mechanical stimuli and maximally to noxious mechanical stimuli (Kenshalo et al., 1980; Casey and Morrow, 1983, 1987; Chung et al., 1986a,b). They also respond to noxious heat and to C fiber volleys (Chung et al., 1986b). The receptive fields are restricted in size and are situated on the contralateral side, and the location of the neurons in the VPL nucleus is somatotopic. Almost all of those tested were shown by antidromic activation to project to the SI cortex (Kenshalo et al., 1980). A surprising finding is that most neurons (85%) in the VPL nucleus respond to both cutaneous and visceral stimuli (Chandler et al., 1992; Bruggemann et al., 1994). The cutaneous input may be innocuous or noxious. Although the cutaneous input is somatotopic, the visceral input is not viscerotopic (Bruggemann et al., 1994).
The properties of the nociceptive neurons in the VPL nucleus are appropriate for a role of these neurons in the sensory-discriminative aspects of pain perception. Their projection to the SI cortex, implies that the SI cortex is also involved in the discriminative aspects of pain sensation.
Nociceptive neurons also exist in the VPI and POm nuclei (Pollin and Albe-Fessard, 1979; Casey and Morrow, 1987; Apkarian and Shi, 1994). The cutaneous receptive fields of neurons in the VPI nucleus are somatotopically organized but tend to be larger than those of the VPL nociceptive neurons. Presumably the nociceptive neurons in the VPI nucleus project to the SII cortex. The SII cortex is believed to be involved in memory processing through its connections to the limbic system (Friedman et al., 1986). The cells studied in the monkey POm nucleus had small, contralateral nociceptive receptive fields. The POm nucleus projects to the retroinsular cortex in monkeys (Burton and Jones, 1976).
Recordings have also been made from nociceptive neurons in the human ventral posterior thalamus, presumably including VPL, VPM, and VPI; stimulation at the same sites often evoked pain (Lenz et al., 1993a,b, 1994b). In one patient with angina pectoris, stimulation in the VPL nucleus caused anginal pain (with no accompanying cardiovascular changes), strongly suggesting that the VPL nucleus is involved in visceral and referred pain (Lenz et al., 1994a).
Medial Thalamus
Bushnell and Duncan (1989) made recordings from several intralaminar nuclei, including the CL, center median (CM), and parafascicular (Pf) nuclei, in awake trained monkeys. Several of the neurons were shown to be nociceptive. These had large, usually bilateral receptive fields, suggesting that they do not play an important role in sensory discrimination. However, the responses of the neurons to two intensities of noxious heat showed that the neurons were able to distinguish these stimulus intensities as well as the behaving animal could, indicating that the neurons might indeed contribute to at least some aspects of sensory discrimination, although they may also play a role in motivational-affective behavior.
Craig et al. (1994) recorded from neurons in the VMpo nucleus in monkeys. Most of the neurons examined responded either to noxious or to cold stimuli. The cells had small, somatotopically organized receptive fields. The nucleus is believed to project to the insula, suggesting that it participates in motivational-affective responses to pain and may contribute to memory processing.
Cerebral Cortex
Although early in this century doubts were cast on the role of the cerebral cortex in pain (Head and Holmes, 1911; Holmes, 1927; Penfield and Boldrey, 1937), most recent evidence favors the participation of both the cerebral cortex and the thalamus, not only in the sensory-discriminative aspects of pain, but also in the motivational-affective aspects.
Although not much experimental work has been done on the involvement of the cerebral cortex in pain, responses have been recorded from nonciceptive cortical neurons in animal subjects (Robinson and Burton, 1980a–c; Kenshalo and Isensee, 1983; Kenshalo et al., 1988; Dong et al., 1989; Chudler et al., 1990; see review by Kenshalo and Willis, 1991).
Evidence that the human cerebral cortex participates in nonciceptive derives from imaging studies (Jones et al., 1991; Talbot et al., 1991; Casey et al., 1994; Coghill et al., 1994). Cortical areas most prominently involved include the SI and SII cortex, the anterior insula, and the anterior cingulate gyrus.
DESCENDING MODULATORY PATHWAYS
In 1969, Reynolds reported that he found it possible to perform abdominal surgery on rats without chemical anesthesia during stimulation in the region of the midbrain PAG. The rats did not have motor impairment, and they showed normal responses to innocuous stimuli. Since then, numerous investigations have been made of what became known as the”descending analgesia systems” (see Willis, 1982; Besson and Chaouch, 1987; Fields and Besson, 1988; Light, 1992). These pathways have been shown to utilize several different neurotransmitters, including opioids, serotonin, and/or catecholamines, and the anatomic structures giving rise to them include not only the PAG, but also the locus ceruleus, subceruleus, and Kolliker-Fuse nuclei, the nucleus raphe magnus (NRM), and several nuclei of the bulbar reticular formation. In addition, structures at higher levels of the nervous system, including the cerebral cortex, and various limbic structures, including the hypothalamus, contribute to the analgesia pathways. The major descending systems are reviewed in the following sections.
PAG
Investigators have explored the antinociceptive effect of stimulating in the PAG in awake, behaving animals (Mayer and Liebeskind, 1974; Oliveras et al., 1974; Gebhart and Toleikis, 1978; Hayes et al., 1979; Besson et al., 1991). The PAG has now been shown to have a rostrocaudal columnar organization (Beitz, 1985; Bandler et al., 1991; Shipley et al., 1991; Cameron et al., 1995a,b). Stimulation in the lateral column of the PAG evokes a defense response, which consists of avoidance behavior in rats, retraction of the ears or arching of the back in cats, sympathetic activation, vocalization, and sometimes a flight reaction (Bandler and Depaulis, 1991; Lovick, 1991), as well as analgesia. By contrast, stimulation in the ventrolateral PAG results in immobility and sympathoinhibition, as well as analgesia (Lovick, 1992). Therefore, the PAG is believed to be involved in complex behavioral responses to stressful or life-threatening situations or to promote recuperative behavior after a defense reaction.
These various complex behaviors are mediated by activation of the complex ascending and descending projections of the PAG (Cameron et al., 1995a,b). However, the analgesia is generally attributed to the action of the PAG on spinal cord neurons. Although some neurons in the PAG project directly to the spinal cord (Castiglioni et al., 1978), most of the connections between the PAG and the spinal cord are indirect; e.g., the PAG projects to the NRM and adjacent reticular formation, to several nuclei in the parabrachial area, including the locus ceruleus, and to the A5 cell group (Fig. 14) (Cameron et al., 1995b; Mantyh, 1983).
FIG. 14.
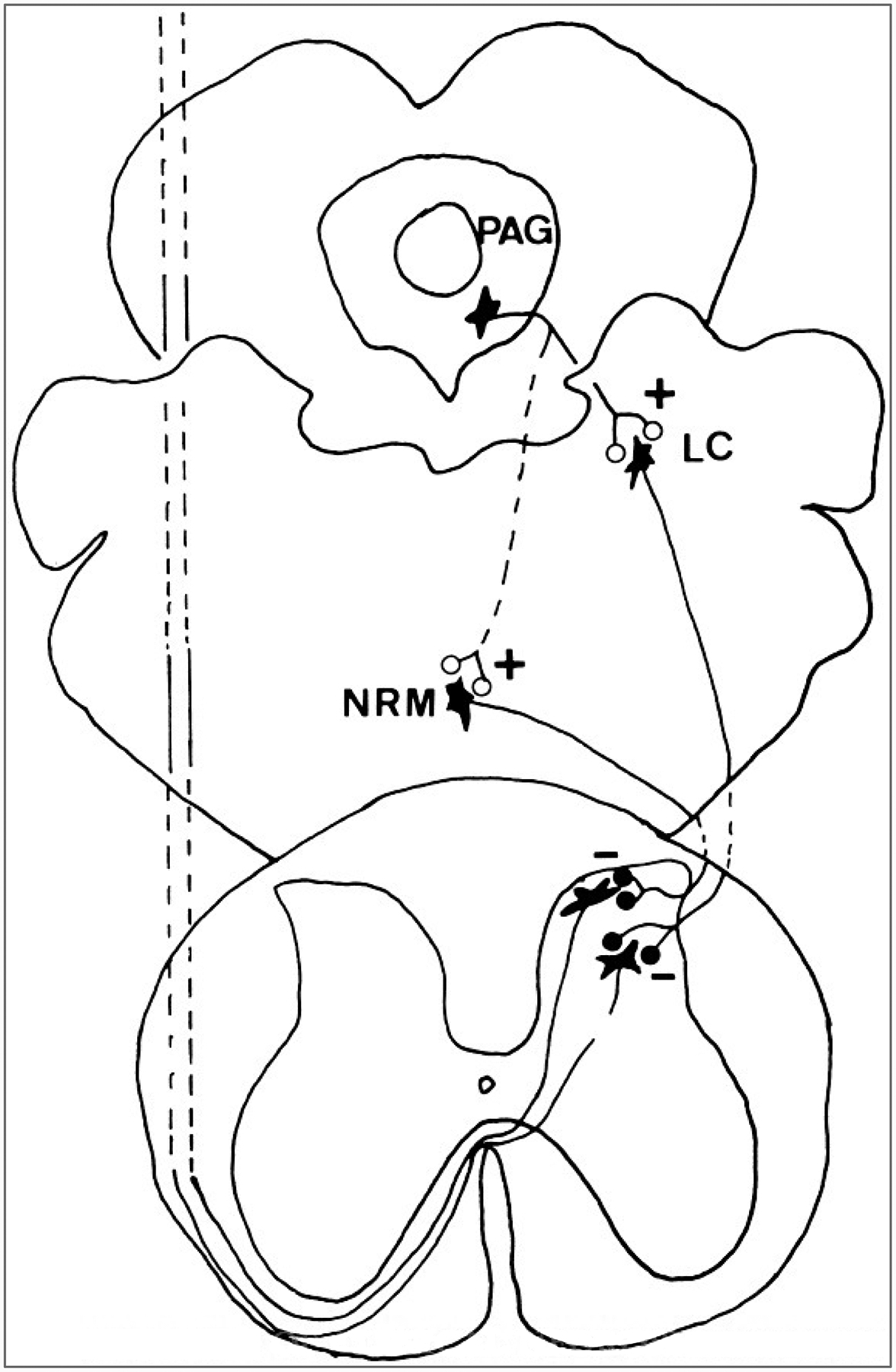
Descending analgesia systems. Two projection neurons in the spinal cord dorsal horn that receive descending inhibitory synapses (minus signs) from brainstem neurons are shown. The descending axons originate in the nucleus raphe magnus (NRM) and the locus ceruleus (LC) and adjacent nuclei of the parabrachial region. The periaqueductal gray (PAG) is shown to have excitatory connections (plus signs) to the NRM and LC.
Stimulation in the PAG causes inhibition of nociceptive dorsal horn neurons (Carstens et al., 1979, 1980, 1981; Lin et al., 1996a; Peng et al., 1996a,b), including spinothalamic tract cells (Hayes et al., 1979; Yezierski et al., 1982; Gerhart et al., 1984; Lin et al., 1994). This inhibition can be at least partially blocked by antagonists of several different receptors, including 5-HT3 and 5HT1A receptors, [alpha]2-adrenoceptors, and GABAA and glycine receptors (Lin et al., 1996a,b; Peng et al., 1996a-c). Presumably, the actions on different classes of 5-hydroxytryptamine (5-HT) receptors are mediated by release of 5-HT from raphespinal axons, and those on adrenoceptors are mediated by NE, released from noradrenergic axons descending from the locus ceruleus/parabrachial complex or the A5 group. Whether the GABA and glycine actions are mediated by release of inhibitory amino acids from long descending axons from the rostral ventromedial medulla (Antal et al., 1996) or are released by inhibitory interneurons in the spinal cord is not clear. The inhibitory interneurons in the dorsal horn could be activated at least in part through an action of 5-HT on 5-HT3 receptors (Peng et al., 1996a), since 5-HT3 receptors open cation channels and thus are excitatory.
Locus Ceruleus, Subceruleus, Parabrachial Area
Stimulation in the dorsolateral pons is reported to produce both a catecholaminergic and a nonadrenergic antinociception (Duggan and North, 1984; Hodge et al., 1986; Proudfit, 1992). Because most of the spinally projecting neurons in the pons are noradrenergic, the nonadrenergic antinociception is most likely relayed through the many sites innervated by these cell groups, including the raphe magnus and pallidus, the PAG, and regions of the ventrolateral reticular formation (Westlund and Coulter, 1980; Holstege, 1988; Kwiat and Basbaum, 1990).
Noradrenergic projections to all regions of the spinal cord arise almost entirely from the dorsolateral pontine catecholamine cell groups A5, A6, and A7. This includes the locus ceruleus, the subceruleus, and the Kolliker-Fuse nucleus (Fig. 14) (Westlund and Coulter, 1980; Stevens et al., 1982; Proudfit, 1992; Westlund et al., 1983, 1984). In monkeys and cats, bilateral projections descend from the locus ceruleus and subcerulear region to innervate laminae I, II, and V, primarily contralaterally (Westlund and Coulter, 1980; Holstege and Kuypers, 1982). Another dense input to the dorsal horn originates from the ipsilateral Kolliker-Fuse nucleus (Stevens et al., 1982; Westlund et al., 1983, 1984; Clark and Proudfit, 1991). Physiological studies have suggested that the subceruleus and Kolliker-Fuse nuclei are primary antinociceptive regions affecting spinal transmission (Yeomans and Proudfit, 1992).
Electrical or chemical stimulation of the dorsolateral pons produced analgesic effects mediated by [alpha]2-adrenoceptors that can be differentiated from cardiovascular effects, and such stimulation causes inhibition of nociceptive neurons in the deep dorsal horn (Segal and Sandberg, 1977; Margalit and Segal, 1979; Mokha et al., 1985, 1986; Hodge et al., 1986; Jones and Gebhart, 1988; Zhao and Duggan, 1988; Proudfit, 1992; Yeomans and Proudfit, 1992).
Noradrenergic terminals in the spinal cord have been demonstrated by immunocytochemical localization of NE in rats (Rajaofetra et al., 1992), cats (Lackner, 1980), and monkeys (Westlund et al., 1984). Such terminals make direct contacts on dorsal horn neurons (Doyle and Maxwell, 1991), including electrophysiologically characterized spinothalamic neurons in laminae V and retrogradely labeled spinothalamic cells in lamina I in monkeys (Westlund et al., 1990).
NE and [alpha]2-agonists applied to the spinal cord inhibit the responses of dorsal horn neurons, including spinothalamic tract neurons (Headley et al., 1978; Fleetwood-Walker et al., 1983; Fleetwood-Walker et al., 1985; Willcockson et al., 1984). These agents also reduce the stimulation-induced release of SP in the dorsal horn (Kuraishi et al., 1985). Intrathecal or direct spinal administration of NE has also been shown to produce antinociception, measurable as increased latencies in hot-plate and tail-flick tests (Yaksh, 1986) and, after inflammation, by increased paw withdrawal times (Hylden et al., 1991) or decreased dorsal horn neuronal activity (Stanfa and Dickenson, 1994). More specific receptor antagonist studies recently showed that after induction of knee joint inflammation, it was an imidazaline ligand(I2) rather than a selective [alpha]2-adrenoceptor antagonist that further reduced paw withdrawal latency (Houghton and Westlund, 1996). This study indicates that previous studies attributing antinociceptive effects to an[alpha]2-adrenoceptor may need to be reassessed in light of the possibility that the effects may be attributable to an I2 imidazoline receptor.
NRM
The brainstem raphe nuclei are a group of nuclei located near the midline in the medulla, pons and midbrain (reviewed in Willis, 1984). They include the nuclei raphe obscurus, raphe pallidus, and NMR in the medulla; the nuclei raphe pontis and the median raphe nucleus in the pons; and the dorsal raphe nucleus in the midbrain. Projections to the spinal cord originate from the nuclei raphe magnus, pallidus, obscurus, and pontis. A striking feature of the raphe nuclei is that a large proportion of the neurons contain 5-HT (Dahlstrom and Fuxe, 1964, 1965). The serotonergic projections from the raphe nuclei to the spinal cord (Bowker et al., 1981, 1983) are the major source of 5-HT in the spinal cord, although some intrinsic spinal cord serotonergic neurons exist (LaMotte et al., 1982; Bowker, 1986).
One of the key structures in the descending analgesia pathway from the PAG is the NRM and the adjacent reticular formation. The PAG has excitatory connections with the NRM (Fig. 14) (Behbehani and Fields, 1979; Pomeroy and Behbehani, 1979; Shah and Dostrovsky, 1980; Willis et al., 1984), suggesting that the antinociceptive effects of stimulation in the PAG are mediated by the NRM.
Stimulation in the NRM was shown to be antinociceptive in behavioral experiments (Oliveras et al., 1975; Basbaum et al., 1976). The antinociceptive effects of stimulation in the NRM have been attributed to the inhibition of nociceptive dorsal horn neurons (;Fields et al., 1977; Guilbaud et al., 1977 see reviews by Basbaum and Fields, 1978; Besson and Chaouch, 1987), including spinothalamic tract cells (Beall et al., 1976; Willis et al., 1977; Gerhart et al., 1981a; Yezierski et al., 1982).
Neurotransmitters involved in the antinociceptive actions of the pathway from the PAG through the NRM and adjacent reticular formation include endogenous opiates, 5-HT, and NE (reviewed in Willis, 1982; Akil and Lewis, 1987; Besson and Chaouch, 1987; Fields and Besson, 1988; Willis and Coggeshall, 1991).
Reticular Formation
Reticular formation neurons are selectively excited by noxious input, and one result of stimulation in the reticular formation in awake monkeys is aversive behaviour (Casey, 1971b). Strong antinociception, however, is evoked by stimulation of the ventrolateral medulla.
Stimulation of the ventromedial or ventrolateral medulla results in[alpha]2-adrenoceptor-mediated analgesia (Sagen and Proudfit, 1981; Barbaro et al., 1985; Hammond and Yaksh, 1984). Retrograde horseradish peroxidase (HRP) studies have also demonstrated nonnoradrenergic spinally projecting neurons in the ventrolateral medulla that are scattered among and sometimes immediately adjacent to the noradrenergic cells of the A1 and A2 cell groups (Westlund et al., 1983, 1984; Tucker et al., 1987). Adrenergic spinally projecting neurons in the rostral ventrolateral medulla (C1 adrenergic cell group) have also been identified (Ross et al., 1984; Carlton et al., 1989) and may have some involvement in the modulation of pain perception since they provide a small projection to the dorsal horn.
Anterior Pretectal Nucleus
Stimulation in the anterior pretectal nucleus results in long-lasting antinociception without aversive side effects, in contrast to stimulation in many other brainstem sites, including the PAG (Rees and Roberts, 1986, 1993; Roberts and Rees, 1986; Prado, 1989; Prado and Roberts, 1985). The anterior pretectal nucleus is located in the pretectal region near several pretectal nuclei that belong to the visual system. However, the main connections of the anterior pretectal nucleus indicate that it is part of the somatosensory system rather than the visual system (Wiberg and Blomqvist, 1984; Berkley et al., 1986; Wiberg et al., 1987; Yoshida et al., 1992).
Nociceptive dorsal horn neurons are affected in different ways after stimulation in the anterior pretectal nucleus, depending on the laminar position of the neurons. Nociceptive specific neurons in the superficial dorsal horn are excited by anterior pretectal stimulation, whereas nociceptive neurons in the deeper layers of the dorsal horn are inhibited (Rees and Roberts, 1987). Excitation of lamina I neurons has been proposed to activate a positive feedback loop whose inhibitory output to the deep dorsal horn neurons results in analgesia (Rees and Roberts, 1993).
Which descending pathways to the spinal cord and which neurotransitters are utilized by the anterior pretectal nucleus is still unclear. There are connections from the anterior pretectal nucleus to the rostral ventral medulla and to the C1 and A5 catecholamine cell groups (Zagon et al., 1995). Important relays include the deep mesencephalic nucleus (Wang et al., 1992), the parabrachial Ch5 group (Terenzi et al., 1992), and the ventrolateral medulla (Terenzi et al., 1991). A noradrenergic link appears to exist in the descending pathway, and 5-HT may also be involved (Rees et al., 1987; Prado, 1989; Rees and Roberts, 1993).
Thalamus and Cerebral Cortex
In human patients, stimulation of the VPL or VPM thalamic nuclei results in a reduction in pain in facial anesthesia dolorosa, postherpetic neuralgia, and the thalamic syndrome (Mazars et al., 1974, 1976; Hosobuchi et al., 1973; Turnbull et al., 1980). Thalamic stimulation also results in antinociception in monkeys (Goodman and Holcombe, 1976).
Stimulation in the VPL nucleus has been shown to cause inhibition of primate spinothalamic tract neurons (Gerhart et al., 1983). The inhibition was suggested to result from antidromic activation of the axons of spinothalamic tract neurons that send collaterals to such brainstem nuclei as the PAG or the NRM. Recordings from neurons in the NRM show that neurons in this nucleus can be excited when stimuli are applied in the VPL nucleus (Tsubokawa et al., 1982; Willis et al., 1984), and 5-HT is released in the spinal cord by such stimulation (Sorkin et al., 1992).
Another possible explanation is that the spinal cord inhibition resulting from stimulation in the VPL nucleus occurs through a cortical loop. Stimulation of the SI region of the cerebral cortex in monkeys causes the inhibition of spinothalamic tract cells (Coulter et al., 1974; Yezierski et al., 1983), whereas stimulation of the motor cortex excites spinothalamic tract cells (Yezierski et al., 1983). The excitation can be monosynaptic (Zhang et al., 1991a). However, the cortical inhibition acts mainly on the responses to innocuous mechanical stimulation (Coulter et al., 1974; Yezierski et al., 1983; Zhang et al., 1991b), whereas the inhibition produced by stimulation in the PAG or NRM powerfully reduces nociceptive responses (Willis et al., 1977; Gerhart et al., 1981, 1984; Yezierski et al., 1982).
Limbic Structures
Pain is quite often accompanied by motivational-affective and autonomic responses. Examples are increased heart rate and blood pressure, endocrine changes, increased attention, arousal, anxiety, and suffering. The neural pathways that mediate these changes are likely to parallel those relaying information about somatic pain sensations, but include additional structures of the limbic system. Much of the information about painful experiences may be relayed through brainstem reticular systems receiving spinoreticular inputs, as detailed previously in the section on the spinoreticular tracts. Some of these brainstem sites then project to higher centers where they have impact on hypothalamic, limbic and neocortical function; e.g., the A1-A6 noradrenergic cell groups receive input from lamina I neurons (discussed in Westlund and Craig, 1996). Some of these regions then project rostrally to the paraventricular hypothalamic nucleus as well as to the central nucleus of the amygdala (Petrov et al., 1993). Catecholaminergic modulation of the hypothalamic-pituitary-adrenal stress axis was reviewed by Plotsky et al. (1989). Direct input to the amygdala from parabrachial regions that receive spinal projections has also been reported (Fallon et al., 1978). Focusing attention on painful stimuli and incorporation of information relevant to the stimuli has been discussed as another role of noxious input relayed through the locus ceruleus to the cortex (Foote et al., 1991; Segal et al., 1991). A pathway serving this role might include the lamina I projections to the locus ceruleus (Craig, 1995). Cortical and hippocampal regions receive their noradrenergic input exclusively from the locus ceruleus (Ungerstedt, 1971).
Dopaminergic inhibition of nociceptive dorsal horn cells, including spinothalamic tract neurons, has been reported (Willcockson et al., 1984; Fleetwood-Walker et al., 1988). This might arise from the All cell group (Blessing and Chalmers, 1979).
CLINICAL IMPLICATIONS OF RECENT FINDINGS ON THE PAIN AND PAIN MODULATION SYSTEMS
New findings in pain research often have the potential for clinical application. The following thoughts about potentially useful lines of investigation are derived from recent work.
The observation that most nociceptors are normally “sleeping” but “awaken” when they are sensitized, e.g., by inflammation, suggests that the pain of inflammation should be reduced if sensitization is minimized. A traditional approach has been use of nonsteroidal antinflammatory agents, such as aspirin, to block the synthesis of prostaglandins, which contribute to the sensitization of nociceptors. However, many other substances also contribute to peripheral sensitization, including BK, serotonin, and a variety of cytokines released from immune cells. Presumably, pharmacological agents directed against the actions of these agents should prove as useful as aspirin, at least under some conditions.
The function of nociceptors clearly can be modulated by drugs that act on their surface membrane receptors, which include not only BK and serotonin receptors, but also opiate, GABA, and capsaicin receptors. Activation of opiate receptors usually is not effective in reducing pain, but this approach becomes effective in inflammation. Whether peripheral administration of opiates will have an important clinical application is unclear, but it may be possible to design opioid-like drugs that have an effect that is restricted to the periphery. Adrenoceptors may be expressed in neuropathic pain states, such as causalgia. Antagonists to these adrenoceptors may prove useful in the treatment of neuropathic pain. Recently, a role for excitatory amino acid receptors on peripheral nerve fibers was proposed, and evidence indicates that blocking these receptors can reduce hyperalgesia (Lawand et al., 1996). Peripherally acting excitatory amino acid receptor antagonists may have a therapeutic use. Manipulations of capsaicin receptors are already being explored; e.g., the capsaicin receptor agonist resiniferotoxin has been shown to produce an excitotoxic action on C nociceptors through its action on capsaicin receptors. Resiniferotoxin very effectively desensitizes capspaicin receptors and may be able to destroy these nociceptors without causing the pain typically produced by capsaicin.
Peripheral nerve damage causes changes in the concentrations of several peptides in dorsal root ganglia and in the dorsal horn of the spinal cord. These changes may contribute to neuropathic or other pain states. Antagonists of the appropriate receptors may be a useful approach to treatment of these pain states.
Aberrant growth of sympathetic nerve fibers or of afferent fibers entering the dorsal horn may result from changes in growth factors after nerve damage. Control of this aberrant growth by the use of antibodies to the growth factors or by antagonists of the growth factor receptors should be explored in such conditions.
Central sensitization is produced after peripheral injuries and is likely to be a major factor in postoperative pain. Central sensitization can be prevented by several agents, including antagonists ofN-methyl-D-aspartate (NMDA) glutamate receptors and NK1 neurokinin receptors. To avoid the cognitive changes that NMDA receptor antagonists produce when they reach the brain, it may be possible to develop NMDA receptor antagonists that are selective for the spinal cord. Neurokinin receptor antagonists are becoming available and may block sensitization without the cognitive effects produced by NMDA antagonists.
Enhancement of pre- or postsynaptic inhibition of dorsal horn nociceptive circuits by drugs should be another useful means of treating pain. Spinal administration of morphine has already proved a successful therapy in certain types of pain. GABA receptor agonists, such as baclofen, are also useful.
Interruption of nociceptive tracts in the spinal cord has a long history. Anterolateral cordotomy has at least a limited success rate when used to treat cancer pain originating from one side of the body. However, it is less effective for visceral pain, since bilateral cordotomies are required and have many side effects. Cordotomies are often of limited usefulness for the treatment of pain when the patient survives for a long time, since pain may return after several months. The pain of pelvic visceral cancer can be effectively relieved by a limited midline dorsal myelotomy; prior to the recent demonstration of a visceral nociceptive pathway that ascends in the dorsal column, there was no good explanation of this observation. This neurosurgical procedure is likely to be a convenient method of reducing pelvic cancer pain that is insufficiently controlled by morphine.
Our knowledge of the descending endogenous analgesia system is still very incomplete. Recent studies indicate that further work is needed to determine in detail the neural connections and the neurotransmitters of this system. That some structures, such as the PAG, produce mixed aversive and analgesic effects is consistent with the observation that electrical stimulation of these sites in human patients is not a useful therapeutic approach. However, other structures, such as the VPL and VPM nuclei, can be stimulated to produce an effective analgesia in certain types of patients. If a means for stimulating the anterior pretectal nucleus could be found, this region might be a suitable target for deep brain stimulation. However, a better approach may be to determine a more physiological way of utilizing this and other analgesia systems through suitable drug therapy.
REFERENCES
- Akil H, Lewis JW, eds. Neurotransmitters and pain control. Karger: Basel, 1987. [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 1996a;76:2661–74. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol 1996b;76:2675–90. [DOI] [PubMed] [Google Scholar]
- Ammons WS. Primate spinothalamic cell responses to ureteral occlusion. Brain Res 1989a;496:124–30. [DOI] [PubMed] [Google Scholar]
- Ammons WS. Electrophysiological characteristics of primate spinothalamic neurons with renal and somatic inputs. J Neurophysiol 1989b;60:1121–30. [DOI] [PubMed] [Google Scholar]
- Ammons WS, Girardot MN, Foreman RD. T2-T5 spinothalamic neurons projecting to medial thalamus with viscerosomatic input. J Neurophysiol 1985;54:73–89. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: I. Existence of long ascending postsynaptic fibres in the cat’s fasciculus gracilis. Exp Brain Res 1975a;22:457–70. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp Brain Res 1975b;22:471–93. [DOI] [PubMed] [Google Scholar]
- Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal cord dorsal by fibres descending from the rostral ventromedial medulla. Neuroscience 1996;73:509–18. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ. Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol 1989a;288:447–73. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ. Primate spinothalamic pathways: II. The cells of origin of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 1989b;288:474–92. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hodge CJ. Primate spinothalamic pathways: III. Thalamic terminations of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 1989c;288:493–511. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 1994;14:6779–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum AE, Beall JE, Foreman RD, Willis WD. Organization and receptive fields of primate spinothalamic tract neurons. J Neurophysiol 1975;38:572–86. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P, Depaulis A. Emerging principles of organization of the midbrain periaqueductal gray matter In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray matter. New York: Plenum Press, 1991:1–8. [Google Scholar]
- Bandler R, Depaulis A. Midbrain periaqueductal gray control of defensive behavior in the cat and the rat In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray matter. New York: Plenum Press, 1991:175–87. [Google Scholar]
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res 1985;343:223–9. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci USA 1976;73:4685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 1978;4:451–62. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 1991;66:212–27. [DOI] [PubMed] [Google Scholar]
- Beall JE, Martin RF, Applebaum AE, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in the region of the nucleus raphe magnus. Brain Res 1976;114:328–33. [DOI] [PubMed] [Google Scholar]
- ehbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 1979;170:85–93. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The midbrain periaqueductal gray in the rat. I. Nuclear volume, cell number, density, orientation, and regional subdivision. J Comp Neurol 1985;237:445–59. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Seltzer Z, Lu GW, Nishikawa N, Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosensory Res 1983;1:131–49. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Nishikawa N, Lu GW, Hoffert MJ, Dubner R. The morphology of dorsal column postsynaptic (DCPS) spino-medullary neurons in the cat. J Comp Neurol 1984;224:568–78. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol 1980;193:283–317. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Budell RJ, Blomqvist A, Bull M. Output systems of the dorsal column nuclei in the cat. Brain Res 1986;396:199–25. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nature Medicine 1995;1:766–73. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 1990;63:473–90. [DOI] [PubMed] [Google Scholar]
- Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev 1987;67:67–186. [DOI] [PubMed] [Google Scholar]
- Besson JM, Fardin V, Oliveras JL. Analgesia produced by stimulation of the periaqueductal gray matter: true antinociceptive effects versus stress effects In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray matter. New York: Plenum Press, 1991:121–38. [Google Scholar]
- Birrell GJ, McQueen DS, Iggo A, Grubb BD. Prostanoid-induced potentiation of the excitatory and sensitizing effects of bradykinin on articular mechanonociceptors in the rat ankle joint. Neuroscience 1993;54:537–44. [DOI] [PubMed] [Google Scholar]
- Bishop GH. The relation between nerve fiber size and sensory modality: phylogenetic implications of the afferent innervation of cortex. J Nerv Ment Dis 1959;128:89–114. [PubMed] [Google Scholar]
- Blair RW, Ammons WS, Foreman RD. Responses of thoracic spinothalamic and spinoreticular cells to coronary artery occlusion. J Neurophysiol 1984;51:636–48. [DOI] [PubMed] [Google Scholar]
- Blair RW, Wenster RN, Foreman RD. Responses of thoracic spinothalamic neurons to intracardiac injection of bradykinin in the monkey. Circ Res 1982;51:83–94. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Chalmers JP. Direct projection of catecholamine (presumably dopamine)-containing neurons from hypothalamus to spinal cord. Neurosci Lett 1979;11:35–40. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Ma W, Berkley KJ. Spinal input to the parabrachial nucleus in the cat. Brain Res 1989;480:29–36. [DOI] [PubMed] [Google Scholar]
- Boivie J. An anatomical reinvestigation of the termination of the spinothalamic tract in the monkey. J Comp Neurol 1979;186:343–70. [DOI] [PubMed] [Google Scholar]
- Boivie J. Thalamic projections from lateral cervical nucleus in monkey. A degeneration study. Brain Res 1980;198:13–26. [DOI] [PubMed] [Google Scholar]
- Boivie J, Leijon G, Johansson I. Central post-stroke pain-a study of the mechanisms through analyses of the sensory abnormalities. Pain 1989;37:173–85. [DOI] [PubMed] [Google Scholar]
- Bonica JJ. Clinical importance of hyperalgesia In: Willis WD, ed. Hyperalgesia and allodynia. New York: Raven Press, 1992:17–43. [Google Scholar]
- Bossut DF, Perl ER. Effects of nerve injury on sympathetic excitation of A[delta] mechanical nociceptors. J Neurophysiol 1995;73:1721–23. [DOI] [PubMed] [Google Scholar]
- Bowker RM. Intrinsic 5HT-immunoreactive neurons in the spinal cord of the fetal non-human primate. Dev Brain Res 1986;28:137–43. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Origin of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res 1981;226:187–99. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Sullivan MC, Wilbur JF, Coulter JD. Descending serotonergic, peptidergic and cholinergic pathways form the raphe nuclei: a multiple transmitter complex. Brain Res 1983;288:33–48. [DOI] [PubMed] [Google Scholar]
- Bowsher D. Diencephalic projections from the midbrain reticular formation. Brain Res 1975;95:211–20. [DOI] [PubMed] [Google Scholar]
- Bowsher D, Mallart A, Petit D, Albe-Fessard D. A bulbar relay to the centre median. J Neurophysiol 1968;31:288–300. [DOI] [PubMed] [Google Scholar]
- Brooks VB. The neural basis of motor control. New York: Oxford University Press, 1986. [Google Scholar]
- Brown AG, Franz DN. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res 1969;7:231–49. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe REW, Noble R, Rose PK, Snow PJ. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol 1980;300:409–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci 1994;14:6796–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RN, Coulter JD, Willis WD. Cells of origin of the spinocervical tract in the monkey. Exp Neurol 1974;42:574–86. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ Jr. Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci 1987;7:4159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Cliffer KD, Giesler GJ Jr. Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol 1990;291:329–44. [DOI] [PubMed] [Google Scholar]
- Burstein R, Giesler GJ Jr. Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res 1989;497:149–54. [DOI] [PubMed] [Google Scholar]
- Burstein R, Potrebic S. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J Comp Neurol 1993;335:469–85. [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol 1976;168:249–302. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Mechanical response properties of ventroposterior medial thalamic neurons in the alert monkey. Exp Brain Res 1987;67:603–14. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 1989;78:415–8. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Tremblay N. Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol 1993;69:739–52. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: aPhaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol 1995a;351:568–84. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol 1995b;351:585–601. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA, Raja SN. Is nociceptor activation by alpha-1 adrenoreceptors the culprit in sympathetically maintained pain? Am Pain Soc J 1992;1:3–11. [Google Scholar]
- Carlton SM, Coggeshall RE. Stereological analysis of galanin and CGRP synapses in the dorsal horn of neuropathic primates. Brain Res 1996;711:16–25. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hayes ES. Light microscopic and ultrastructural analysis of GABA-immunoreactive profiles in the monkey spinal cord. J Comp Neurol 1990;300:162–82. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Honda CN, Denoroy L. Distribution of phenylethanolamine N-methyltransferase cell bodies, axons, and terminals in monkey brainstem: an immunohistochemical mapping study. J Comp Neurol 1989;287:273–85. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Westlund KN, Zhang D, Sorkin LS, Willis WD. Calcitonin gene-related peptide containing primary afferent fibers synapse on primate spinothalamic tract cells. Neurosci Lett 1990;109:76–81. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Westlund KN, Zhang D, Willis WD. GABA-immunoreactive terminals synapse on primate spinothalamic tract cells. J Comp Neurol 1992;322:528–37. [DOI] [PubMed] [Google Scholar]
- Carstens E, Bihl H, Irvine DRF, Zimmermann M. Descending inhibition from medial and lateral midbrain of spinal dorsal horn neuronal responses to noxious and nonnoxious cutaneous stimuli in the cat. J Neurophysiol 1981;45:1029–42. [DOI] [PubMed] [Google Scholar]
- Carstens E, Klumpp D, Zimmermann M. Time course and effective sites for inhibition from midbrain periaqueductal gray of spinal dorsal horn neuronal responses to cutaneous stimuli in the cat. Exp Brain Res 1980;38:425–30. [DOI] [PubMed] [Google Scholar]
- Carstens E, Leah J, Lechner J, Zimmermann M. Demonstration of extensive brainstem projections to medial and lateral thalamus and hypothalamus in the rat. Neuroscience 1990;35:609–26. [DOI] [PubMed] [Google Scholar]
- Carstens E, Yokota T, Zimmermann M. Inhibition of spinal neuronal responses to noxious skin heating by stimulation of mesencephalic periaqueductal gray in the cat. J Neurophysiol 1979;42:558–68. [DOI] [PubMed] [Google Scholar]
- Casey KL. Somatic stimuli, spinal pathways, and size of cutaneous fibers influencing unit activity in the medial medullary reticular formation. Exp Neurol 1969;25:35–56. [DOI] [PubMed] [Google Scholar]
- Casey KL. Responses of bulboreticular units to somatic stimuli eliciting escape behavior in the cat. Int J Neurosci 1971a;2:15–28. [DOI] [PubMed] [Google Scholar]
- Casey KL. Escape elicited by bulboreticular stimulation in the cat. Int J Neurosci 1971b;2:29–34. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 1994;71:802–7. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ. Ventral posterior thalamic neurons differentially responsive to noxious stimulation of the awake monkey. Science 1983;221:675–7. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ. Nociceptive neurons in the ventral posterior thalamus of the awake squirrel monkey: observations on identification, modulation, and drug effects In: Besson JM, Guilbaud G, Peschanski M, eds. Thalamus and pain. Amsterdam: Exerpta Medica, 1987:211–26. [Google Scholar]
- Castiglioni AJ, Gallaway MC, Coulter JD. Spinal projections from the midbrain in monkey. J Comp Neurol 1978;178:329–46. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 1985;240:153–60. [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A, Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol 1977;267:537–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MJ, Hobbs SF, Fu QG, Kenshalo DR, Blair RW, Foreman RD. Responses of neurons in the ventroposterolateral nucleus of primate thalamus to urinary bladder distension. Brain Res 1992;571:26–34. [DOI] [PubMed] [Google Scholar]
- Chaturvedi SK. Prevalence of chronic pain in psychiatric patients. Pain 1987;29:231–7. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Anton F, Dubner R, Kenshalo DR. Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neurophysiol 1990;63:559–69. [DOI] [PubMed] [Google Scholar]
- Chung JM, Fang ZR, Hori Y, Lee KH, Willis WD. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain 1984a;19:259–75. [DOI] [PubMed] [Google Scholar]
- Chung JM, Kenshalo DR, Gerhart KD, Willis WD. Excitation of primate spinothalamic neurons by cutaneous C-fiber volleys. J Neurophysiol 1979;42:1354–69. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain 1984b;19:277–93. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Surmeier DJ, Sorkin LS, Kim J, Willis WD. Response characteristics of neurons in the ventral posterior lateral nucleus of the monkey thalamus. J Neurophysiol 1996b;56:370–90. [DOI] [PubMed] [Google Scholar]
- Chung JM, Surmeier DJ, Lee KH, et al. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol 1986a;56:308–27. [DOI] [PubMed] [Google Scholar]
- Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosci Lett 1993;162:85–8. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res 1991;547:279–88. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ Jr. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 1991;11:852–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ Jr. Postsynaptic dorsal column pathway of the rat. III. Distribution of ascending afferent fibers. J Neurosci 1989;9:3146–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: basic characterization and antidromic activation from the thalamus. J Neurophysiol 1992;68:818–32. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci 1994;14:4095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, De Biasi S, Giuffrida R, Rustioni A. Substance P-containing projections in the dorsal columns of rats and cats. Neuroscience 1990;34:607–21. [DOI] [PubMed] [Google Scholar]
- Coulter JD, Maunz RA, Willis WD. Effects of stimulation of sensorimotor cortex on primate spinothalamic neurons. Brain Res 1974;65:351–6. [DOI] [PubMed] [Google Scholar]
- Craig AD. Spinal and medullary input to the lateral cervical nucleus. J Comp Neurol 1978;181:729–44. [DOI] [PubMed] [Google Scholar]
- Craig AD. Spinal distribution of ascending lamina I axons anterogradely labeled with Phaseolus vulgaris leucoagglutinin(PHA-L) in the cat. J Comp Neurol 1991;313:377–93. [DOI] [PubMed] [Google Scholar]
- Craig AD. Spinal and trigeminal lamina I input to the locus coeruleus anterogradely labeled with Phaseolus vulgaris leucoagglutinin (PHA-L) in the cat and the monkey. Brain Res 1992;584:325–28. [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 1995;361:225–48. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature 1994;372:770–3. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Felix D, Johnston GAR. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res 1971a;32:69–96. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Johnston GAR. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res 1971b;12:547–65. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Hosli L, Johnston GAR. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res 1968;6:1–18. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lacey G. GABA-B receptor-mediated spinal inhibition. NeuroReport 1994;5:540–2. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lodge D, Brand SJ. GABA and spinal afferent terminal excitability in the cat. Brain Res 1977;130:360–3. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurones. Acta Physiol Scand 1964;62(suppl 232):1–55. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neurons systems. Acta Physiol Scand 1965;64(suppl 247):1–36. [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol 1993;69:1071–81. [DOI] [PubMed] [Google Scholar]
- De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA 1988;85:7820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res 1989;484:314–24. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhancement of spinothalamic neuron responses to chemical and mechanical stimuli following combined micro-iontophoretic application of N-methyl-D-aspartic acid and substance P. Pain 1991;47:85–93. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci 1992;12:883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci 1992a;12:3025–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol 1994;72:1464–75. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Willis WD. Infusion of substance P or neurokinin A by microdialysis alters responses of primate spinothalamic tract neurons to cutaneous stimuli and to iontophoretically released excitatory amino acids. Pain 1995;61:411–25. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Zorn S, Willis WD. Combined application of excitatory amino acids and substance P produces long-lasting changes in responses of primate spinothalamic tract neurons. Brain Res Rev 1993;18:227–46. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Sluka KA, Sorkin LS, Westlund KN, Willis WD. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res Rev 1992b;17:1–13. [DOI] [PubMed] [Google Scholar]
- Downie JW, Ferrington DG, Sorkin LS, Willis WD. The primate spinocervicothalamic pathway: responses of cells of the lateral cervical nucleus and spinocervical tract to innocuous and noxious stimuli. J Neurophysiol 1988;59:861–85. [DOI] [PubMed] [Google Scholar]
- Doyle CA, Maxwell DJ. Ultrastructural analysis of noradrenergic nerve terminals in the cat lumbosacral spinal dorsal horn: a dopamine-[beta]-hydroxylase immunocytochemical study. Brain Res 1991;563:329–33. [DOI] [PubMed] [Google Scholar]
- Dray A. Chemical activation and sensitization of nociceptors In: Besson JM, Guilbaud G, Ollat H, eds. Peripheral neurons in nociception: physio-pharmacological aspects. Paris: John Libbey Eurotext, 1994:49–70. [Google Scholar]
- Dray A, Bettaney J, Forster P, Perkins MN. Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neuroscience Lett 1988;91:301–7. [DOI] [PubMed] [Google Scholar]
- Duggan AW, North RA. Electrophysiology of opioids. Pharmacol Rev 1984;35:219–81. [PubMed] [Google Scholar]
- Duggan AW, Hall JG, Headley PM. Enkephalins and dorsal horn neurones of the cat: effects on responses to noxious and innocuous skin stimuli. Br J Pharmacol 1977;61:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GH, Bushnell MC, Oliveras JL, Bastrash N, Tremblay N. Thalamic VPM nucleus in the behaving monkey. III. Effects of reversible inactivation by lidocaine on thermal and mechanical discrimination. J Neurophysiol 1993;70:2086–96. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Pharmacological studies on presynaptic inhibition. J Physiol 1963;168:500–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Rosario JP, Merlio JP, Grant G, Aldskogius H, Persson H. Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development and following peripheral or central axotomy. Mol Brain Res 1993;17:217–26. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Koziel DA, Moore RY. Catecholamine innervation of the basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol 1978;180:509–32. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol 1995;353:506–28. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Downie JW, Willis WD. Primate nucleus gracilis neurons: responses to innocuous and noxious stimuli. J Neurophysiol 1988;59:866–907. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Sorkin LS, Willis WD. Responses of spinothalamic tract cells in the superficial dorsal horn of the primate lumbar spinal cord. J Physiol 1987;388:681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Clanton CH, Anderson SD. Nucleus raphe magnus inhibition of spinal cord dorsal horn neurons. Brain Res 1977;126:4411–53. [DOI] [PubMed] [Google Scholar]
- Fields HL, Besson JM, eds. Pain modulation Progress in brain research vol. 77 Amsterdam: Elsevier, 1988. [Google Scholar]
- Fields HL, Wagner GM, Anderson SD. Some properties of spinal neurons projecting to the medial brain-stem reticular formation. Exp Neurol 1975;47:118–34. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Hope PJ, Iggo A, Mitchell R, Molony V. The effects of iontophoretically applied noradrenaline on the cutaneous sensory responses of identified dorsal horn neurones in the cat. J Physiol (Lond) 1983;483:63–4. [Google Scholar]
- Fleetwood-Walker SM, Hope PJ, Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J Physiol 1988;399:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Mitchell R, Hope PJ, Molony V, Iggo A. An [alpha]2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res 1985;334:243–54. [DOI] [PubMed] [Google Scholar]
- Foerster O, Gagel O. Die Vorderseitenstrangdurchschneidung beim Menschen. Eine klinisch-patho-physiologisch-anatomische Studie. Z Gesampte Neurol Psychiatr 1932;138:1–92. [Google Scholar]
- Foote SL, Berridge CW, Adams LM, Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending In: Barnes CD, Pompeiano O, eds. Neurobiology of the locus coeruleus. Amsterdam: Elsevier, 1991:521–32. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Schmidt RF, Willis WD. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol 1979;286:215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DR, Murray EA, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol 1986;252:323–47. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 1993;123:455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze RM, Gordon G. The representation of cutaneous sense in the thalamus of the cat and monkey. J Exp Physiol 1954;39:279–304. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Toleikis JR. An evaluation of stimulation-produced analgesia in the cat. Exp Neurol 1978;62:570–9. [DOI] [PubMed] [Google Scholar]
- Gerhart KD, Wilcox TK, Chung JM, Willis WD. Inhibition of nociceptive and nonnociceptive responses of primate spinothalamic cells by stimulation in medial brain stem. J Neurophysiol 1981a;45:121–36. [DOI] [PubMed] [Google Scholar]
- Gerhart KD, Yezierski RP, Fang ZR, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in ventral posterior lateral (VPLc)thalamic nucleus: possible mechanisms. J Neurophysiol 1983;49:406–23. [DOI] [PubMed] [Google Scholar]
- Gerhart KD, Yezierski RP, Wilcox TK, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in periaqueductal gray or adjacent midbrain reticular formation. J Neurophysiol 1984;51:450–66. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat. I. Anatomical studies. J Neurophysiol 1984;51:260–75. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Yezierski RP, Gerhart KD, Willis WD. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol 1981;46:1285–308. [DOI] [PubMed] [Google Scholar]
- Gildenberg PL, Hirshberg RM. Limited myelotomy for the treatment of intractable cancer pain. J Neurol Neurosurg Psychiatry 1984;47:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol 1991;308:467–90. [DOI] [PubMed] [Google Scholar]
- Goodman SJ, Holcombe V. Selective and prolonged analgesia in monkey resulting from brain stimulation In: Bonica JJ, Albefessard D, eds. Advances in pain research and therapy, vol. I New York: Raven Press, 1976:495–502. [Google Scholar]
- Gowers WR. A case of unilateral gunshot injury to the spinal cord. Trans Clin Lond 1878;11:24–32. [Google Scholar]
- Guilbaud G, Besson JM, Oliveras JL, Wyon-Maillard MC. Modifications of the firing rate of bulbar reticular units (nucleus gigantocellularis) after intra-arterial injection of bradykinin into the limbs. Brain Res 1973;63:131–40. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Oliveras JL, Giesler G, Besson JM. Effects induced by stimulation of the centralis inferior nucleus of the raphe on dorsal horn interneurons in cat’s spinal cord. Brain Res 1977;126:355–60. [DOI] [PubMed] [Google Scholar]
- Gybels JM, Sweet WH, eds. Neurosurgical treatment of persistent pain. Basel: Karger, 1989. [PubMed] [Google Scholar]
- Ha H. Cervicothalamic tract in the Rhesus monkey. Exp Neurol 1971;33:205–12. [DOI] [PubMed] [Google Scholar]
- Haber LH, Moore BD, Willis WD. Electrophysiological response properties of spinoreticular neurons in the monkey. J Comp Neurol 1982;207:75–84. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 1990;425:545–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res 1984;298:329–37. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. J Physiol 1991;435:229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JD, Wolff HG, Goodell H, eds. Pain sensations and reactions. New York: Williams & Wilkins, 1952; reprinted by New York: Hafner, 1967. [Google Scholar]
- Hayes RL, Price DD, Ruda M, Dubner R. Suppression of nociceptive responses in the primate by electrical stimulation of the brain or morphine administration: behavioral and electrophysiological comparisons. Brain Res 1979;167:417–21. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 1911;34:102–254. [Google Scholar]
- Head H, Thompson T. The grouping of afferent impulses within the spinal cord. Brain 1906;29:537–741. [Google Scholar]
- Headley PM, Duggan AW, Griersmith BT. Selective reduction by noradrenaline and 5-hydroxytryptamine of nociceptive responses of cat dorsal horn neurones. Brain Res 1978;145:185–89. [DOI] [PubMed] [Google Scholar]
- Hirshberg RM, Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Is there a pathway in the posterior funiculus that signals visceral pain? Pain 1996;67:291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock ER. Stereotactic cervical myelotomy. J Neurol Neurosurg Psychiatry 1970;33:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock ER. Electrophysiological exploration of the cervicomedullary region In: Somjen G, ed. Neurophysiology studied in man. Amsterdam: Excerpta Medica, 1972a;237–45. [Google Scholar]
- Hitchcock ER. Stereotaxis of the spinal cord. Conf Neurol 1972b;34:229–310. [DOI] [PubMed] [Google Scholar]
- Hobbs SF, Chandler MJ, Bolser DC, Foreman RD. Segmental organization of visceral and somatic input onto C3-T6 spinothalamic tract cells of the monkey. J Neurophysiol 1992;68:1575–88. [DOI] [PubMed] [Google Scholar]
- Hodge CJ, Apkarian AV, Stevens RT. Inhibition of dorsal-horn cell responses by stimulation of the Kolliker-Fuse nucleus. J Neurosurg 1986;65:825–33. [DOI] [PubMed] [Google Scholar]
- Holmes G. Disorders of sensation produced by cortical lesions. Brain 1927;50:413–27. [Google Scholar]
- Holstege G. Anatomical evidence for a strong ventral parabrachial projection to nucleus raphe magnus and adjacent tegmental field. Brain Res 1988;447:154–8. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study In: Kuypers HGJM, Martin GF, eds. Descending pathways to the spinal cord. Amsterdam: Elsevier, 1982:145–75. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anaesthesia dolorosa. Arch Neurol 1973;29:158–61. [DOI] [PubMed] [Google Scholar]
- Houghton A, Westlund KN. An I2 imidazoline ligand, RS 45041, potentiates hyperalgesia in acute arthritis. NeuroReport 1996;7:1497–1501. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Hayashi H, Bennett GJ. Lamina I spinomesencephalic neurons in the cat ascend via the dorsolateral funiculi. Somatosensory Res 1986;4:31–41. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Hayashi H, Bennett GJ, Dubner R. Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Res 1985;336:195–8. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Thomas DA, Iodorola MJ, Nahin RL, Dubner R. Spinal Opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol 1991;194:135–43. [DOI] [PubMed] [Google Scholar]
- Hyndman OR, Van Epps C. Possibility of differential section of the spinothalamic tract. Arch Surg 1939;38:1036–53. [Google Scholar]
- Jones AKP, Brown WD, Friston KJ, Qi LY, Frackoowiak RSJ. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc R Soc Lond[B] 1991;244:39–44. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res 1988;460:281–96. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Chudler EH, Anton F, Dubner R. SI cortical nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res 1988;454:378–82. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Giesler GJ, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 1980;43:1594–614. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 1983;50:1479–96. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Leonard RB, Chung JM, Willis WD. Responses of primate spinothalamic neurons to graded and to repeated noxious heat stimuli. J Neurophysiol 1979;42:1370–89. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Leonard RB, Chung JM, Willis WD. Facilitation of the responses of primate spinothalamic cells to cold and to tactile stimuli by noxious heating of the skin. Pain 1982;12:141–52. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Willis WD. The role of the cerebral cortex in pain sensation In: Peters A, Jones EG, eds. Cerebral cortex, vol. 9, normal and altered states of function New York: Plenum Press, 1991:153–212. [Google Scholar]
- Kerr FWL. The ventral spinothalamic tract and other ascending systems of the ventral funiculus of the spinal cord. J Comp Neurol 1975;159:335–56. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Haber LH, Yezierski RP, Chung JM, Martin RF, Willis WD. Cells of origin of the spinoreticular tract in the monkey. J Comp Neurol 1982;207:61–74. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Willis WD. Spinothalamic cells in the rat lumbar cord with collaterals to the medullary reticular formation. Brain Res 1982;238:181–5. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Yamada J, Sato H, Yamashita K. Cells of origin of the spinoparabrachial fibers in the rat: a study with fast blue and WGA-HRP. J Comp Neurol 1993;328:449–61. [DOI] [PubMed] [Google Scholar]
- Konietzny F, Perl ER, Trevino D, Light A, Hensel H. Sensory experiences in man evoked by intraneural electrical stimulation of intact cutaneous afferent fibers. Exp Brain Res 1981;42:219–22. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res 1985;359:177–82. [DOI] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. Organization of tyrosine hydroxylase- and serotonin-immunoreactive brainstem neurons with axon colaterals to the periaqueductal gray and the spinal cord in the rat. Brain Res 1990;528:83–94. [DOI] [PubMed] [Google Scholar]
- Lackner KJ. Mapping of monoamine neurones and fibres in the cat lower brainstem and spinal cord. Anat Embryol 1980;161:169–95. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Johns DR, De Lanerolle NC. Immunohistochemical evidence of indolamine neurons in monkey spinal cord. J Comp Neurol 1982;206:359–70. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LER, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 1992;448:749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol 1983;50:1–26. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci 1982;2:765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawand NB, Westlund KN, Willis WD. The effects of excitatory amino acids (EAAs) on the discharge of articular sensory receptors in rats. Neurosci Abstr 1996;22:1369. [Google Scholar]
- Leah J, Menetrey D, de Pommery J. Neuropeptides in long ascending spinal tract cells in the rat: evidence for parallel processing of ascending information. Neuroscience 1988;24:195–207. [DOI] [PubMed] [Google Scholar]
- LeBars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurons in the rat. Pain 1979a;6:283–304. [DOI] [PubMed] [Google Scholar]
- LeBars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II, Lack of effect on non-convergent neurons, supraspinal involvement and theoretical implications. Pain 1979b;6:305–27. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chung JM, Willis WD. Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg 1985;62:276–87. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Hope EJ, et al. The sensation of angina can be evoked by stimulation of the human thalamus. Pain 1994a;59:119–25. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Rowland LH, Dougherty PM. A population of cells in the human thalamic principal sensory nucleus respond to painful mechanical stimuli. Neurosci Lett 1994b;180:46–50. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Lin YC, et al. Neurons in the area of human thalamic nucleus ventralis caudalis respond to painful heat stimuli. Brain Res 1993a;623:235–40. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Richardson RT, et al. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 1993b;70:200–12. [DOI] [PubMed] [Google Scholar]
- Lewis T. Pain. London: Macmillan Press, 1942. [Google Scholar]
- Light AR. The initial processing of pain and its descending control: spinal and trigeminal systems. Basel: Karger, 1992. [Google Scholar]
- Lima D, Mendes-Ribeiro JA, Coimbra A. The spino-latero-reticular system of the rat: projections from the superficial dorsal horn and structural characterization of marginal neurons involved. Neuroscience 1991;45:137–52. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Glycine and GABAA antagonists reduce the inhibition of primate spinothalamic tract neurons produced by stimulation in periaqueductal gray. Brain Res 1994;654:286–302. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Antinociception and inhibition from the periaqueductal gray are mediated in part by spinal 5HT1A receptors. JPET 1996a;276:958–67. [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J Neurosci 1996b;16:3026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Willis WD. Role of GABA receptor subtypes in inhibition of primate spinothalamic tract neurons: difference between spinal and periaqueductal gray inhibition. J. Neurophysiol 1996c;75:109–23. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Interactions between descending pathways from the dorsal and ventrolateral periaqueductal gray matter in the rat In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray. New York: Plenum Press, 1991:101–20. [Google Scholar]
- Lovick TA. Inhibitory modulation of the cardiovascular defense response by the ventrolateral periaqueductal grey matter in rats. Exp Brain Res 1992;89:133–9. [DOI] [PubMed] [Google Scholar]
- Ma W, Blomqvist A, Berkley KJ. Spino-diencephalic relays through the parabrachial nucleus in the cat. Brain Res 1989;480:37–50. [DOI] [PubMed] [Google Scholar]
- Magoun HW. The waking brain, 2nd ed. Springfield: Charles C Thomas, 1963. [Google Scholar]
- Mantyh PW. The ascending input to the midbrain periaqueductal gray of the primate. J Comp Neurol 1982;211:50–64. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. The spinothalamic tract in the primate: a re-examination using wheatgerm agglutinin conjugated to horseradish peroxidase. Neuroscience 1983;9:847–62. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ, Hayes RL, Price DD. Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in 14C-2-deoxyglucose metabolic activity in rats with painful peripheral mononeuropathy. J Neurophysiol 1993;70:470–81. [DOI] [PubMed] [Google Scholar]
- Margalit D, Segal M. A pharmacological study of analgesia produced by stimulation of the nucleus locus coeruleus. Psychopharmacology 1979;62:169–73. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Liebeskind JC. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res 1974;68:73–93. [DOI] [PubMed] [Google Scholar]
- Mazars G, Merienne L, Cioloca C. Traitement de certains types de doulers par des stimulateurs thalamiques implantes. Neurochirurgie 1974;20:117–24. [PubMed] [Google Scholar]
- Mazars GJ, Merienne L, Cioloca C. Contribution of thalamic stimulation to the physiopathology of pain In: Bonica JJ, Albe-Fessard D, eds. Advances in pain research and therapy, vol. 1 New York: Raven Press, 1976:483–5. [Google Scholar]
- McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 1993;363:543–6. [DOI] [PubMed] [Google Scholar]
- Mehler WR. The anatomy of the so-called “pain tract” in man: an analysis of the course and distribution of the ascending fibers of the fasciculus anterolateralis In: French JD, Porter RW, eds. Basic research in paraplegia. Springfield: Charles C Thomas, 1962:26–55. [Google Scholar]
- Mehler WR, Feferman ME, Nauta WJH. Ascending axon degeneration following anterolateral cordotomy. An experimental study in the monkey. Brain 1960;83:718–51. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol 1987;255:439–50. [DOI] [PubMed] [Google Scholar]
- Menetrey D, de Pommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and viscenoroception in the rat. Eur J Neurosci 1991;3:249–59. [DOI] [PubMed] [Google Scholar]
- Merskey H. Pain and psychological medicine In: Wall PD, Melzack R, eds. Textbook of pain, 2nd ed. Edinburgh: Churchill-Livingstone, 1989:656–66. [Google Scholar]
- Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science 1981;213:1527–9. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Foreman RD, Giesler GJ, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain 1981;11:163–83. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nakano K, Imaizumi M, Okamoto M. The lateral cervical nucleus of the Japanese monkey (Macaca fuscata). J Comp Neurol 1967;129:375–84. [DOI] [PubMed] [Google Scholar]
- Mokha SSD, McMillan JA, Iggo A. Descending control of spinal nociceptive transmission. Actions produced on spinal multireceptive neurones from the nuclei locus coeruleus (LC) and raphe magnus (NRM). Exp Brain Res 1985;58:213–26. [DOI] [PubMed] [Google Scholar]
- Mokha SS, McMillan JA, Iggo A. Pathways mediating descending control of spinal nociceptive transmission from the nuclei locus coeruleus (LC) and raphe magnus (NRM) in the cat. Exp Brain Res 1986;61:597–606. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Micevych PE. A long ascending pathway of enkephalin-like immunoreactive spinoreticular neurons in the rat. Neurosci Lett 1986;65:271–6. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg 1969;30:14–24. [DOI] [PubMed] [Google Scholar]
- Nauta HJW, Kuypers HGJM. Some ascending pathways in the brainstem reticular formation In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT, eds. Reticular formation of the brain. Boston: Little, Brown, 1958:3–30. [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain 1990;41:167–234. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Lucke T, Schaible HG. N-Methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat’s knee joint. J Neurophysiol 1993;70:1365–77. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Weiretter F, Schaible HG. Involvement of substance P and neurokinin-1 receptors in the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat’s knee joint. J Neurophysiol 1995;73:1574–83. [DOI] [PubMed] [Google Scholar]
- Nijensohn DE, Kerr FWL. The ascending projections of the dorsolateral funiculus of the spinal cord in the primate. J Comp Neurol 1975;161:459–70. [DOI] [PubMed] [Google Scholar]
- Noble R, Riddell JS. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J Physiol 1988;396:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordenbos W, Wall PD. Diverse sensory functions with an almost totally divided spinal cord. A case of spinal cord transection with preservation of part of one anterolateral quadrant. Pain 1976;2:185–95. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol 1983;342:633–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol 1989;415:583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras JL, Redjemi F, Guilbaud G, Besson JM. Analgesia induced by electrical stimulation of the inferior centralis nucleus of the raphe in the cat. Pain 1975;1:139–45. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Woda A, Guilbaud G, Besson JM. Inhibition of the jaw opening reflex by electrical stimulation of the periaqueductal gray matter in the awake, unrestrained cat. Brain Res 1974;72:328–31. [DOI] [PubMed] [Google Scholar]
- Olszewski J. The thalamus of Macaca mulatta. New York: Karger, 1952. [Google Scholar]
- Owens CM, Zhang D, Willis WD. Changes in the response states of primate spinothalamic tract cells caused by mechanical damage of the skin or activation of descending controls. J Neurophysiol 1992;67:1509–27. [DOI] [PubMed] [Google Scholar]
- Palacek J, Paleckova PM, Dougherty PM, Willis WD. The effect of phorbol esters on the responses of primate spinothalamic neurons to mechanical and thermal stimuli. J Neurophysiol 1994;71:529–37. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Coggeshall RE, Lee WT, Chung K. Long ascending unmyelinated primary afferent axons in the rat dorsal column: immunohistochemical localizations. Neurosci Lett 1990;108:6–10. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Head PA, McNeill DL, Chung K, Coggeshall RE. Ascending unmyelinated primary afferent fibers in the dorsal funiculus. J Comp Neurol 1989;290:384–90. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937;60:389–443. [Google Scholar]
- Peng YB, Lin Q, Willis WD. The role of 5HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. JPET 1995a;276:116–24. [PubMed] [Google Scholar]
- Peng YB, Lin Q, Willis WD. Involvement of[alpha]2-adrenoreceptors in the periaqueductal gray-induced inhibition of dorsal horn cell activity in rats. JPET 1996b;00:00–00. [PubMed] [Google Scholar]
- Peng YB, Lin Q, Willis WD. Effects of GABA and glycine receptor antagonists on the activity and PAG-induced inhibition of rat dorsal horn neurons. Brain Res 1996c;00:00–00. [DOI] [PubMed] [Google Scholar]
- Perl ER, Whitlock DG. Somatic stimuli exciting spinothalamic projections to thalamic neurons in cat and monkey. Exp Neurol 1961;3:256–96. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res 1993;609:81–92. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor role in adrenocorticotropin secretion. Endocrinol Rev 1989;10:437–58. [DOI] [PubMed] [Google Scholar]
- Pollin B, Albe-Fessard D. Organization of somatic thalamus in monkeys with and without section of dorsal spinal tracts. Brain Res 1979;173:431–49. [DOI] [PubMed] [Google Scholar]
- Pomeroy SL, Behbehani MM. Physiologic evidence for a projection from periaqueductal gray to nucleus raphe magnus in the rat. Brain Res 1979;176:143–7. [DOI] [PubMed] [Google Scholar]
- Post C, Persson M-L, Archer T, Minor BG, Danysz W, Sundstrom E. Increased antinociception by [alpha]-adrenoceptor drugs after spinal cord noradrenaline depletion. Eur J Pharmacol 1987;137:107–16. [DOI] [PubMed] [Google Scholar]
- Prado WA. Antinociceptive effect of agonists microinjected into the anterior pretectal nucleus of the rat. Brain Res 1989;493:147–54. [DOI] [PubMed] [Google Scholar]
- Prado WA, Roberts MHT. An assessment of the antinociceptive and aversive effects of stimulating identified sites in the rat brain. Brain Res 1985;340:219–28. [DOI] [PubMed] [Google Scholar]
- Price DD, Hayes RL, Ruda MA, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensation. J Neurophysiol 1978;41:933–47. [DOI] [PubMed] [Google Scholar]
- Price DD, Mayer DJ. Neurophysiological characterization of the anterolateral quadrant neurons subserving pain in M. mulatta. Pain 1975;1:59–72. [DOI] [PubMed] [Google Scholar]
- Proudfit HK. The behavioral pharmacology of the noradrenergic descending system In: Besson J-M, Guilbaud G, eds. Towards the use of noradrenergic agonists for the treatment of pain. New York: Elsevier, 1992:119–36. [Google Scholar]
- Rajaofetra N, Ridet J-L, Poulat P, et al. Immunocytochemical mapping of noradrenergic projections to the rat spinal cord with an antiserum against noradrenaline. J Neurocytol 1992;21:481–94. [DOI] [PubMed] [Google Scholar]
- Ralston HJ III, Ralston DD. Medial lemniscal and spinal projections to the macaque thalamus: an electron microscopic study of differing GABAergic circuitry serving thalamic somatosensory mechanisms. J Neurosci 1994;14:2485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randic M, Hecimovic H, Ryu PD. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurones. Neurosci Lett 1990;117:74–80. [DOI] [PubMed] [Google Scholar]
- Rees H, Roberts MHT. The antinociceptive effects of stimulating the pretectal nucleus of the rat. Pain 1986;25:83–93. [DOI] [PubMed] [Google Scholar]
- Rees H, Roberts MHT. Anterior pretectal stimulation alters the responses of spinal dorsal horn neurones to cutaneous stimulation in the rat. J Physiol 1987;385:415–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Roberts MHT. The anterior pretectal nucleus: a proposed role in sensory processing. Pain 1993;53:121–35. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 1969;164:444–5. [DOI] [PubMed] [Google Scholar]
- Roberts MHT, Rees H. The antinociceptive effects of stimulating the anterior pretectal nucleus of the rat. Pain 1986;25:83–93. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Lynch GS, Thompson RF. Diencephalic distributions of ascending reticular systems. Brain Res 1973;55:309–22. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatotopographic organization in the second somatosensory cortex of M. fascicularis. J. Comp Neurol 1980a;192:43–67. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory, and granular insula of M. fascicularis. J Comp Neurol 1980b; 192:69–92. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory I(SII), retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol 1980c;192:93–108. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol 1984;228:168–85. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the nucleus gracilis from the lumbar cord of the cat. Brain Res 1973;51:81–95. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the cuneate nucleus in the brachial dorsal funiculus of the cat. Brain Res 1974;75:247–59. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Hayes NL, O’Neill S. Dorsal column nuclei and ascending spinal afferents in macaques. Brain 1979;102:95–125. [DOI] [PubMed] [Google Scholar]
- Sagen J, Proudfit HK. Hypoalgesia induced by blockage of noradrenergic projections to the raphe magnus: reversal by blockade of noradrenergic projections to the spinal cord. Brain Res 1981;223:391–6. [DOI] [PubMed] [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 1991;251:1608–10. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Freudenberger U, Neugebauer V, Stiller RU. Intraspinal release of immunoreactive calcitonin gene-related peptide during development of inflammation in the joint in vivo-a study with antibody microprobes in cat and rat. Neuroscience 1994;62:1293–305. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the cat spinal cord during development of acute arthritis in cat’s knee: a study with antibody bearing microprobes. Brain Res 1990;529:214–23. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol 1983a;49:35–44. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Responses of fine medial articular nerve afferents to passive movements of knee joint. J Neurophysiol 1983b;49:1118–26. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol 1985;54:1109–22. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol 1988;60:2180–95. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Structural substrates for integrative patterns in the brain stem reticular core In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RC., eds. Reticular formation of the brain. Boston: Little, Brown, 1958:31–55. [Google Scholar]
- Schepelmann K, Messlinger K, Schaible HG, Schmidt RF. Inflammatory mediators and nociception in the joint: excitation and sensitization of slowly conducting afferent fibers of cat’s knee by prostaglandin I2. Neuroscience 1992;50:237–47. [DOI] [PubMed] [Google Scholar]
- Schepelmann K, Messlinger K, Schmidt RF. The effects of phorbol ester on slowly conducting afferents of the cat’s knee joint. Exp Brain Res 1993;92:391–8. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Ringkamp M, Forster C, Handwerker HO, Torebjork HE. Limitation of sensitization to injured parts of receptive fields in human skin C-nociceptors. Exp Brain Res 1996;109:141–7. [DOI] [PubMed] [Google Scholar]
- Schwarcz JR. Stereotactic extralemniscal myelotomy. J Neurol Neurosurg Psychiat 1976;39:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz JR. Spinal cord stereotactic techniques, trigeminal nucleotomy and extralemniscal myelotomy. Appl Neurophysiol 1978;41:99–112. [DOI] [PubMed] [Google Scholar]
- Segal M, Sandberg D. Analgesia produced by electrical stimulation of catecholamine nuclei in the rat brain. Brain Res 1977; 123:369–72. [DOI] [PubMed] [Google Scholar]
- Segal M, Markram H, Richter-Levin G. Actions of norepinephrine in the rat hippocampus. Prog Brain Res 1991;88:323–30. [DOI] [PubMed] [Google Scholar]
- Shah Y, Dostrovsky JO. Electrophysiological evidence for a projection of the periaqueductal gray to the nucleus raphe magnus in cat and rat. Brain Res 1980;193:534–8. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M, Rizvi TA, Behbehani MM. Topographical specificity of forebrain inputs to the midbrain periaqueductal gray: evidence for discrete longitudinally organized input columns In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray matter. New York: Plenum Press, 1991:417–47. [Google Scholar]
- Simone DA, Sorkin LS, Oh U, et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol 1991;66:228–46. [DOI] [PubMed] [Google Scholar]
- Skultety FM. Stimulation of the periaqueductal gray and hypothalamus. Arch Neurol 1963;8:608–20. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Light AR. Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated withPhaseolus vulgaris leucoagglutinin. J Comp Neurol 1994;339:49–61. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Dougherty PM, Sorkin LS, Willis WD, Westlund KN. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res Rev 1992;17:29–38. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Jordan HH, Willis WD, Westlund KN. Differential effects of N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists on spinal release of amino acids after development of acute arthritis in rats. Brain Res 1994;664:77–84. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain 1993;55:367–77. [DOI] [PubMed] [Google Scholar]
- Smith MV, Apkarian AV. Thalamically projecting cells of the lateral cervical nucleus in monkey. Brain Res 1991;555:10–8. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, McAdoo DJ, Willis WD. Stimulation in the ventral posterior lateral nucleus of the primate thalamus leads to release of serotonin in the lumbar spinal cord. Brain Res 1992;581:307–10. [DOI] [PubMed] [Google Scholar]
- Spiller WG. The occasional clinical resemblance between caries of the vertebrae and lumbothoracic syringomyelia, and the location within the spinal cord of the fibres for the sensations of pain and temperature. Univ Pennsylvania Med Bull 1905; 18:147–54. [Google Scholar]
- Spiller WG, Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. JAMA 1912;58:1489–90. [Google Scholar]
- Standaert DG, Watson SJ, Houghten RA, Saper CB. Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J Neurosci 1986;6:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfa LC, Dickenson AH. Enhanced alpha-2 adrenergic controls and spinal morphine potency in inflammation. NeuroReport 1994;5:469–72. [DOI] [PubMed] [Google Scholar]
- Stein C. Peripheral opioid analgesia: mechanisms and therapeutic applications In: Besson JM, Guilbaud G, Ollat H, eds. Peripheral neurons in nociception: physio-pharmacological aspects. Paris: John Libbey Eurotext, 1994:157–65. [Google Scholar]
- Stevens RT, Hodge CJ Jr, Apkarian AV. Kolliker-Fuse nucleus: The principal source of pontine catecholaminergic cells projecting to the lumbar spinal cord of cat. Brain Res 1982;239:589–94. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Honda CN, Willis WD. Responses of primate spinothalamic neurons to noxious thermal stimulation of glabrous and hairy skin. J Neurophysiol 1986a;56:328–50. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Honda CN, Willis WD. Temporal features of the responses of primate spinothalamic neurons to noxious thermal stimulation of hairy and glabrous skin. J Neurophysiol 1986b;56:351–68. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science 1991;251:1355–8. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Rees H, Morgan SJ, Foster GA, Roberts MHT. The antinociception evoked by anterior pretectal nucleus stimulation is partially dependent upon ventrolateral medullary neurones. Pain 1991;47:231–9. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Rees H, Roberts MHT. The pontine parabrachial region mediates some of the descending inhibitory effects of stimulating the anterior pretectal nucleus. Brain Res 1992;594:205–14. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, Dray A, Urban L. Injury-induced plasticity of spinal reflex activity: NK1 neurokinin receptor activation and enhanced A- and C-fiber mediated responses in the rat spinal cord in vitro. J Neurosci 1994;14:3672–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience 1990;39:387–94. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Ochoa JL, Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain 1984;18:145–56. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Vallbo AB, Ochoa JL. Intraneural microstimulation in man; its relation to specificity of tactile sensations. Brain 1987;110:1509–29. [DOI] [PubMed] [Google Scholar]
- Trevino DL. The origin and projections of a spinal nociceptive and thermoreceptive pathway In: Zotterman Y, ed. Sensory functions of the skin in primates, with special reference to man. New York: Pergamon Press, 1976:367–76. [Google Scholar]
- Truex RC, Taylor MJ, Smythe MQ, Gildenberg PL. The lateral cervical nucleus of cat, dog and man. J Comp Neurol 1965;139:93–104. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Yamamoto T, Katayama Y, Noriyaso N. Clinical results and physiological basis of thalamic relay nucleus stimulation for relief of intractable pain with morphine tolerance. Appl Neurophysiol 1982;45:143–55. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J Comp Neurol 1987;259:591–603. [DOI] [PubMed] [Google Scholar]
- Turnbull IM, Shulman R, Woodhurst WB. Thalamic stimulation for neuropathic pain. J Neurosurg 1980;52:486–93. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res 1968;4:377–82. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand 1971;(suppl 367):1–48. [DOI] [PubMed] [Google Scholar]
- Urban L, Naeem S, Patel IA, Dray A. Tachykinin induced regulation of excitatory amino acid responses in the rat spinal cord in vitro. Neurosci Lett 1994;168:185–8. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci 1990;10:2077–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Luck MM. Loss and recovery of reactivity to noxious stimuli in monkeys with primary spinothalamic cordotomies, followed by secondary and tertiary lesions of other cord sectors. Brain 1979;102:233–48. [DOI] [PubMed] [Google Scholar]
- Walker AE. The spinothalamic tract in man. Arch Neurol Psychiatry 1940;43:284–98. [Google Scholar]
- Wall PD, McMahon SB. Microneurography and its relation to perceived sensation. A critical review. Pain 1985;21:209–29. [DOI] [PubMed] [Google Scholar]
- Wang XM, Yuan B, Hou ZL. Role of the deep mesencephalic nucleus in the antinociception induced by stimulation of the anterior pretrectal nucleus in rats. Brain Res 1992; 17:321–5. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res 1983;263:15–31. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Origins and terminations of descending noradrenergic projections to the spinal cord of monkey. Brain Res 1984;292:1–16. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Carlton SM, Zhang D, Willis WD. Direct catecholaminergic innervation of primate spinothalamic tract neurons. J Comp Neurol 1990;299:178–86. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Carlton SM, Zhang D, Willis WD. Glutamate-immunoreactive terminals synapse on primate spinothalamic tract cells. J Comp Neurol 1992;322:519–27. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Coulter JD. Descending projection of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunochemistry. Brain Res Rev 1980;2:235–64. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Craig AD. Association of spinal lamina I projections with brainstem catecholamine neurons in the monkey. Exp Brain Res 1996;00:00–00. [DOI] [PubMed] [Google Scholar]
- White JC, Sweet WH. Pain and the neurosurgeon. Springfield: Charles C Thomas, 1969. [Google Scholar]
- Wiberg M, Blomqvist A. The projection to the mesencephalon from the dorsal column nuclei. An anatomical study in the cat. Brain Res 1984;311:225–44. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol 1987;264:92–117. [DOI] [PubMed] [Google Scholar]
- Willcockson WS, Chung JM, Hori Y, Lee KH, Willis WD. Effects of iontophoretically released amino acids and amines on primate spinothalamic tract cells. J Neurosci 1984a;4:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcockson WS, Chung JM, Hori Y, Lee KH, Willis WD. Effects of iontophoretically released peptides on primate spinothalamic tract cells. J Neurosci 1984b;4:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD. Control of nociceptive transmission in the spinal cord In: Ottoson D, ed. Progress in sensory physiology 3. Berlin: Springer-Verlag, 1982. [Google Scholar]
- Willis WD. The raphe-spinal system In: Barnes CD, ed. Brainstem control of spinal cord function. Research topics in physiology New York: Academic Press, 1984:141–214. [Google Scholar]
- Willis WD. The pain system. Basel: Karger, 1985. [Google Scholar]
- Willis WD. Mechanical allodynia; a role for sensitized nociceptive tract cells with convergent input from mechanoreceptors and nociceptors? Am Pain Soc J 1993;2:23–33. [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord, 2nd ed. New York: Plenum Press, 1991. [Google Scholar]
- Willis WD, Gerhart KD, Willcockson WS, Yezierski RP, Wilcox TK, Cargill CL. Primate raphe- and reticulospinal neurons: effects of stimulation in periaqueductal gray or VPLc nucleus. J Neurophysiol 1984;51:467–80. [DOI] [PubMed] [Google Scholar]
- Willis WD, Haber LH, Martin RF. Inhibition of spinothalamic tract cells and interneurons by brain stem stimulation in the monkey. J Neurophysiol 1977;40:968–81. [DOI] [PubMed] [Google Scholar]
- Willis WD, Kenshalo DR, Leonard RB. The cells of origin of the primate spinothalamic tract. J Comp Neurol 1979;188:543–74. [DOI] [PubMed] [Google Scholar]
- Willis WD, Trevino DL, Coulter JD, Maunz RA. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol 1974;37:358–72. [DOI] [PubMed] [Google Scholar]
- Wolstencroft JH. Reticulospinal neurones. J Physiol 1964;174:91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 1992;355:75–8. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol 1995;360:121–34. [DOI] [PubMed] [Google Scholar]
- Wu W, Wessendorf MW. Organization of the serotonergic innervation of spinal neurons in rats-I. Neuropeptide coexistence in varicosities innervating some spinothalamic tract neurons but not in those innervating postsynaptic dorsal column neurons. Neuroscience 1992;50:885–98. [DOI] [PubMed] [Google Scholar]
- Xie J, Yoon YW, Yom SS, Chung JM. Norepinephrine rekindles mechanical allodynia in sympathectomized neuropathic rat. Analgesia 1995;1:107–13. [Google Scholar]
- Yaksh TL. The effects of intrathecally administered opioid and adrenegic agents on spinal function In: Yaksh TL, ed. Spinal afferent processing. New York: Plenum Press, 1986:505–40. [Google Scholar]
- Yeomans DC, Proudfit HK. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience 1992;49:681–91. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Gerhart KD, Schrock BJ, Willis WD. A further examination of effects of cortical stimulation in primate spinothalamic tract cells. J Neurophysiol 1983;49:424–41. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Sorkin LS, Willis WD. Response properties of spinal neurons projecting to midbrain or midbrain-thalamus in the monkey. Brain Res 1987;437:165–70. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Wilcox TK, Willis WD. The effects of serotonin antagonists on the inhibition of primate spinothalamic tract cells produced by stimulation in nucleus raphe magnus or the periaqueductal gray. JPET 1982;220:266–77. [PubMed] [Google Scholar]
- Yokota T, Nishikawa Y, Koyama N. Distribution of trigeminal nociceptive neurons in nucleus ventralis posteromedialis of primates In: Dubner R, Gebhart GF, Bond MR, eds. Pain research and clinical management, vol. 3 Amsterdam: Elsevier, 1988:555–9. [Google Scholar]
- Yoshida A, Sessle BJ, Dostrovsky JO, Chiang CY. Trigeminal and dorsal column nuclei projections to the anterior pretectal nucleus in the rat. Brain Res 1992;590:81–94. [DOI] [PubMed] [Google Scholar]
- Yoss RE. Studies of the spinal cord. Part 3. Pathways for deep pain within the spinal cord and brain. Neurology 1953;3:163–75. [DOI] [PubMed] [Google Scholar]
- Zagon A, Terenzi MG, Roberts MHT. Direct projections from the anterior pretectal nucleus to the ventral medulla oblongata in rats. Neuroscience 1995;65:253–72. [DOI] [PubMed] [Google Scholar]
- Zhang D, Carlton SM, Sorkin LS, Willis WD. Collaterals of primate spinothalamic tract neurons to the periaqueductal gray. J Comp Neurol 1990;296:277–90. [DOI] [PubMed] [Google Scholar]
- Zhang D, Owens CM, Willis WD. Short-latency excitatory postsynaptic potentials are evoked in spinothalamic tract neurons by corticospinal tract volleys. Pain 1991a;45:197–201. [DOI] [PubMed] [Google Scholar]
- Zhang D, Owens CM, Willis WD. Two forms of inhibition of spinothalamic tract neurons produced by stimulation of the periaqueductal gray and cerebral cortex. J Neurophysiol 1991b;65:1567–79. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ju G, Elde R, Hokfelt T. Effect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galanin. J Neurocytol 1993;22:342–81. [DOI] [PubMed] [Google Scholar]
- Zhao Z-Q, Duggan AW. Idazoxan blocks the action of noradrenaline but not spinal inhibition from electrical stimulation of the locus coeruleus and nucleus Kolliker-Fuse of the cat. Neuroscience 1988;25:997–1005. [DOI] [PubMed] [Google Scholar]


