SUMMARY
Context:
There is substantial intraindividual variation in cortisol and dietary sodium intake.
Objective:
To assess the influence of a dietary sodium intake intervention on cortisol measurements within the general population.
Design:
Cross-over intervention.
Patients and Measurements:
630 adults without known Cushing syndrome, cardiovascular or renal disease completed a restricted dietary sodium diet (10 mmol/day, 230 mg/day) followed by cross-over to a liberalized dietary sodium diet (200 mmol/day, 4600 mg/day). 24-hour urine collection and biochemical investigations were performed at the end of each dietary intervention.
Results:
Mean 24-hour urinary free cortisol increased with liberalized sodium intake when compared with restricted sodium intake (178.0 ± 89.7 vs. 121.3 ± 65.6 nmol/day, p<0.001). Nearly all participants (84%) had an increase in the urinary free cortisol following liberalized sodium intake. This translated to a substantial difference in the proportion of participants exceeding categorical thresholds of urinary cortisol on liberalized vs restricted sodium intake: 62% vs 27% for 138 nmol/day (50 mcg/day), 46% vs 17% for 166 nmol/day (60 mcg/day), 32% vs 10% for 193 nmol/day (70 mcg/day), 23% vs 6% for 221 nmol/day (80 mcg/day), 17% vs 4% for 248 nmol/day (90 mcg/day). In parallel, there was a small decrease in morning total serum cortisol with liberalized sodium intake (303.0 ± 117.3 vs. 326.4 ± 162.5 nmol/L, p<0.001).
Conclusions:
Increased dietary sodium intake increases urinary free cortisol excretion and may increase the risk for false-positive results. Variations in dietary sodium intake may influence the interpretations of cortisol measurements performed to evaluate for hypercortisolism.
Keywords: sodium, dietary sodium, cortisol, urinary free cortisol
INTRODUCTION
The average dietary sodium intake of adults living in the United States is estimated to be 3600 mg per day.[1] This is higher than the 2019 Institute of Medicine guidelines that recommend an upper limit of 2300 mg of sodium per day in adults and substantially greater than American Heart Association guidelines, which target an upper limit of 1500 mg per day.[2 3] Higher dietary sodium intake induces a range of physiological changes including intra-vascular volume expansion, increased glomerular filtration rate, and suppression of renin-angiotensin-aldosterone-system (RAAS) activity.[4] Although the mean dietary sodium intake in the United States may be high, studies have consistently shown that there is large intra-individual variability in day-to-day sodium intake and excretion.[1 5 6] This variability implies that downstream physiological processes that are influenced by sodium intake may also be variable.
24-hour urinary free cortisol (24hUFC) is a screening test commonly used to assess for endogenous hypercortisolism.[7] Previous studies suggest that intra-individual variability of 24hUFC can be as high as 38%.[8] Many factors contribute to this degree of variability, including physiological fluctuations in daily cortisol secretion, inconsistent patient practice during the urine collection, physical and emotional stressors, renal impairment, and more. Variability in 24hUFC for the screening of hypercortisolism can contribute to false-positive results, which may trigger more testing, healthcare expenditure, and anxiety.
Although many factors affect 24hUFC, few studies have directly studied the influence of dietary factors, in particular dietary sodium intake, in the general population. The objective of the current study was to assess the influence of dietary sodium intake on 24hUFC within the general population.
METHODS
Study Design
The International HyperPath (Hypertensive Pathotype) is a cohort study that was designed to include dietary sodium modulation to evaluate phenotypic and genotypic determinants of blood pressure. Details of the study design have previously been reported [9–11]; pertinent details are outlined below.
Participants
Participants were included if they were generally healthy adults with normal blood pressure or stage I hypertension. Participants were excluded if they had known secondary hypertension, cardiac disease (coronary artery disease or electrocardiogram evidence of heart block or ischemia), cerebrovascular disease, serum creatinine > 133 μmol/L and known kidney disease, abnormal thyroid function tests or liver function tests, current oral contraceptive use, current tobacco use, illicit drug use or heavy alcohol intake, and current glucocorticoid use. None of the participants had known or suspected Cushing syndrome. Institutional review board approval was obtained from the Human Research Committee which also monitored safety and protocol adherence. Participants provided informed written consent prior to study enrolment.
Interventions
All participants completed a cross-over of two dietary sodium interventions lasting 5–7 days each: a restricted intake approximating 10 mmol/day (230 mg/day) and liberalized sodium intake approximating 200 mmol/day (4600 mg/day). Dietary potassium (100 mmol/day) and calcium (20 mmol/day) intake were stable during both diet phases. Diets and dietary counselling were provided by professional research staff in the Clinical Research Center.
Measurements
A 24-hour urine collection was performed at the end of each dietary phase to measure urinary sodium and electrolytes, creatinine, and 24hUFC. Participants were admitted to the Clinical Research Center in the evening and slept there overnight. The following morning, while maintaining a supine posture, blood samples were collected at 8 AM to measure morning total serum cortisol, glucose, insulin, plasma aldosterone, and renin activity. All blood sampling was performed after 1–3 months of cessation of anti-hypertensive medications, if applicable. [9 11]
Blood pressure was measured at five-minute intervals using an automated blood pressure device (Dinamap, Critikon, Tampa, FL); the average of five consecutive readings was used in analysis.
All laboratory assays were performed at a central research laboratory. Urine and serum sodium and potassium were assayed by flame phytometry (Nova Biomedical, Waltham, MA). Insulin was assayed by chemiluminescence Access Immunoassay System (Beckman Coulter, Chaska, MN). Urine cortisol was measured using Coat-A-Count radio-immunoassay (Siemens, Los Angeles, CA) with sensitivity 5.52 nmol/L and precision of 3.0 – 6.4%. Since this urine cortisol assay was conducted by a high-throughput research laboratory, and not a U.S. CLIA-approved clinical laboratory, a pre-specified reference range was not established. The default uncalibrated manufacturer guidelines suggested an upper limit of the reference range of 248 nmol/day (90 mcg/day); however, we also evaluated other thresholds. Serum cortisol was measured with the Access Chemiluminescent Immunoassay (Beckman Coulter, Chaska MN), with sensitivity of 11.0 nmol/L and precision of 6.4 – 7.9%. Plasma renin activity (PRA) was measured using radioimmunoassay as previously described.[12] Urine and plasma aldosterone were measured using radio-immunoassay (Siemens, Los Angeles, CA).
Statistical Analysis
The current analyses included only those participants who had complete data for 24hUFC and morning total serum cortisol on both dietary interventions (n=630). Data were summarized using means and standard deviation. Paired t-tests were used to evaluate for differences in variables between liberalized and restricted dietary sodium intakes. Statistical analysis was carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Influence of Dietary Sodium Intervention on General Characteristics
Mean participant age was 46.1 ± 10.6 years, 56% of participants were men and the majority of participants (83.6%) self-identified as Caucasian. Mean 24-hour urinary sodium excretion was 13.9 ± 9.8 mmol/day (approximately 320 mg/day) on restricted sodium intake and increased to 243.9 ± 68.1 mmol/day (approximately 5600 mg/day) during liberalized sodium intake, along with increases in body-mass index, blood pressure, and estimated glomerular filtration rate, confirming the expected changes induced by the dietary sodium intervention (Table 1).[3] The remaining participant characteristics following liberalized and restricted sodium intake are summarized in Table 1.
Table 1:
Clinical and biochemical variables on restricted versus liberal sodium diet (Mean ± SD).
| Liberal Sodium Diet | Restricted Sodium Diet | P value | |
|---|---|---|---|
| N= 630 (unless otherwise indicated) | |||
| BMI (kg/m2) | 27.7 ± 4.4 | 27.0 ± 4.4 | <0.0001 |
| 24h urinary sodium (mmol/day) | 243.9 ± 68.1 | 13.9 ± 9.8 | <0.0001 |
| Serum sodium (mmol/L) | 140.9 ± 5.4 | 139.7 ± 7.6 | 0.01 |
| Systolic blood pressure (mmHg) | 135 ± 23 | 123 ± 19 | <0.0001 |
| Diastolic blood pressure (mmHg) | 80 ± 14 | 74 ± 12 | <0.0001 |
| Fasting plasma glucose (mmol/L) | 5.07 ± 0.85 | 5.28 ± 0.99 | <0.0001 |
| Fasting plasma insulin (μIU/L) | 10.4 ± 8.4 | 11.8 ± 8.3 | <0.0001 |
| Total serum cortisol (nmol/L) | 303.0 ± 118.6 | 326.4 ± 162.8 | 0.0003 |
| Serum creatinine (μmol/L)* | 74.2 ± 15.2 | 83.3 ± 27.4 | <0.0001 |
| Estimated glomerular filtration rate (mL/min/1.73m2)* | 91.6 ± 19.6 | 81.5 ± 18.0 | <0.0001 |
| 24h urinary free cortisol (nmol/day) | 178.0 ± 89.7 | 121.3 ± 65.6 | <0.0001 |
| Urinary cortisol:creatinine ratio (nmol/μmol) | 0.014 ± 0.01 | 0.010 ± 0.01 | <0.0001 |
| 24h urinary creatinine excretion (μmol/day) | 12769 ± 3885 | 13046 ± 3914 | <0.01 |
| 24h urine volume (ml) | 2323 ± 1028 | 2178 ± 934 | 0.0001 |
| Supine Plasma renin activity (μg/L/h) | 0.47 ± 0.71 | 2.51 ± 2.39 | <0.0001 |
| Supine Serum aldosterone (pmol/L) | 130.6 ± 92.3 | 476.4 ± 305.7 | <0.0001 |
paired values on both diets available in 236 participants
Effect of dietary sodium variation on cortisol measurements
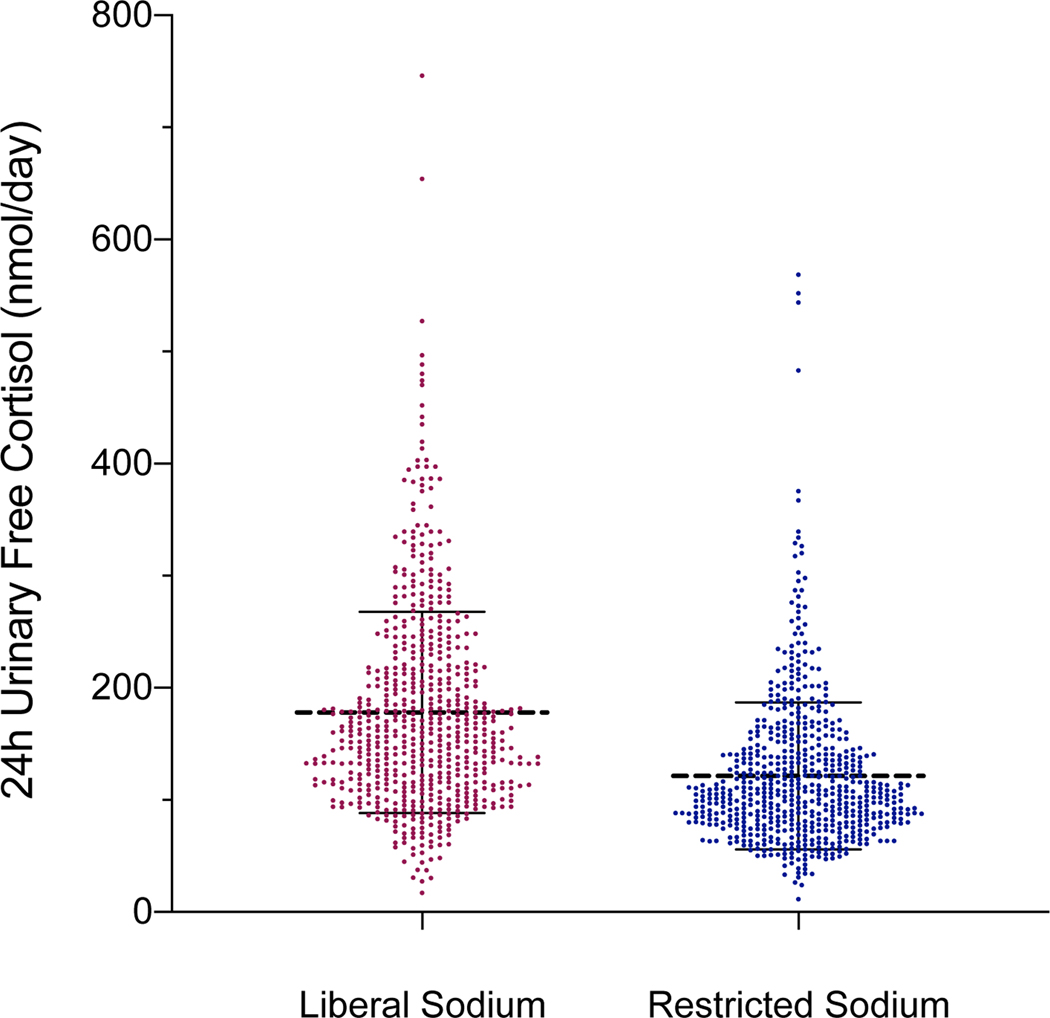
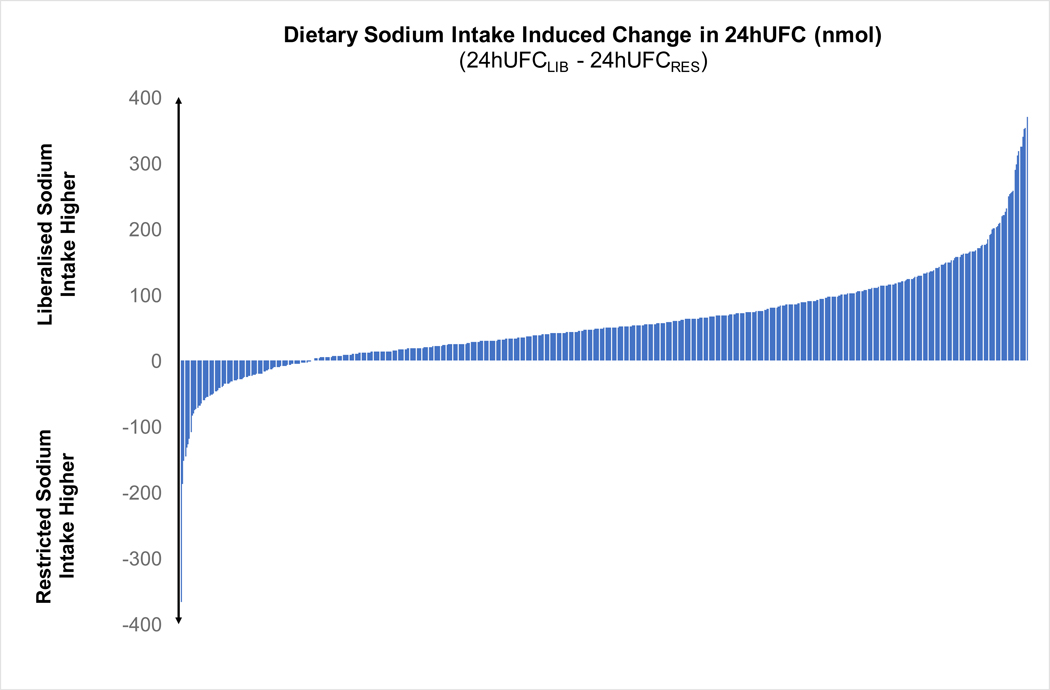
The mean 24hUFC was significantly higher on a liberalized dietary sodium intake when compared to a restricted dietary sodium intake (178.0 ± 89.7 vs. 121.3 ± 65.6 nmol/day, p<0.001, Figure 1A). Consistently, the urinary cortisol-to-creatinine excretion ratio was higher on liberalized sodium intake when compared to restricted dietary sodium intake (0.014 ± 0.01 vs. 0.010 ± 0.01 nmol/μmol, p<0.0001, Table 1). The vast majority, 84% (532/630), of participants had a higher 24hUFC when they were maintained on a liberalized sodium intake when compared to restricted sodium intake (Figure 1B). Higher 24hUFC excretion was seen on liberalized sodium intake, when compared to restricted sodium intake, regardless of blood pressure status (normotensive participants: 154.1 ± 76.7 vs. 107.7 ± 61.1 nmol/day, p<0.0001; hypertensive participants: 189.7 ± 93.3 vs. 127.9 ± 66.7 nmol/day, p<0.0001). Positive, though weak, correlations between urinary cortisol excretion and the extremes of urinary sodium excretion were seen on each dietary intervention (r=0.21, p<0.001 for liberalized sodium intake and r=0.13, p<0.001 for restricted sodium intake).
Figure 1:
A) Scatter plots of 24-hour urinary free cortisol during restricted vs. liberal sodium diet (dashed line represents the mean and hash marks represent the standard deviation); B) Waterfall plots of the change in 24-hour urinary free cortisol induced by dietary sodium intervention for each participant (24h UFCLIB – 24hUFCRES).
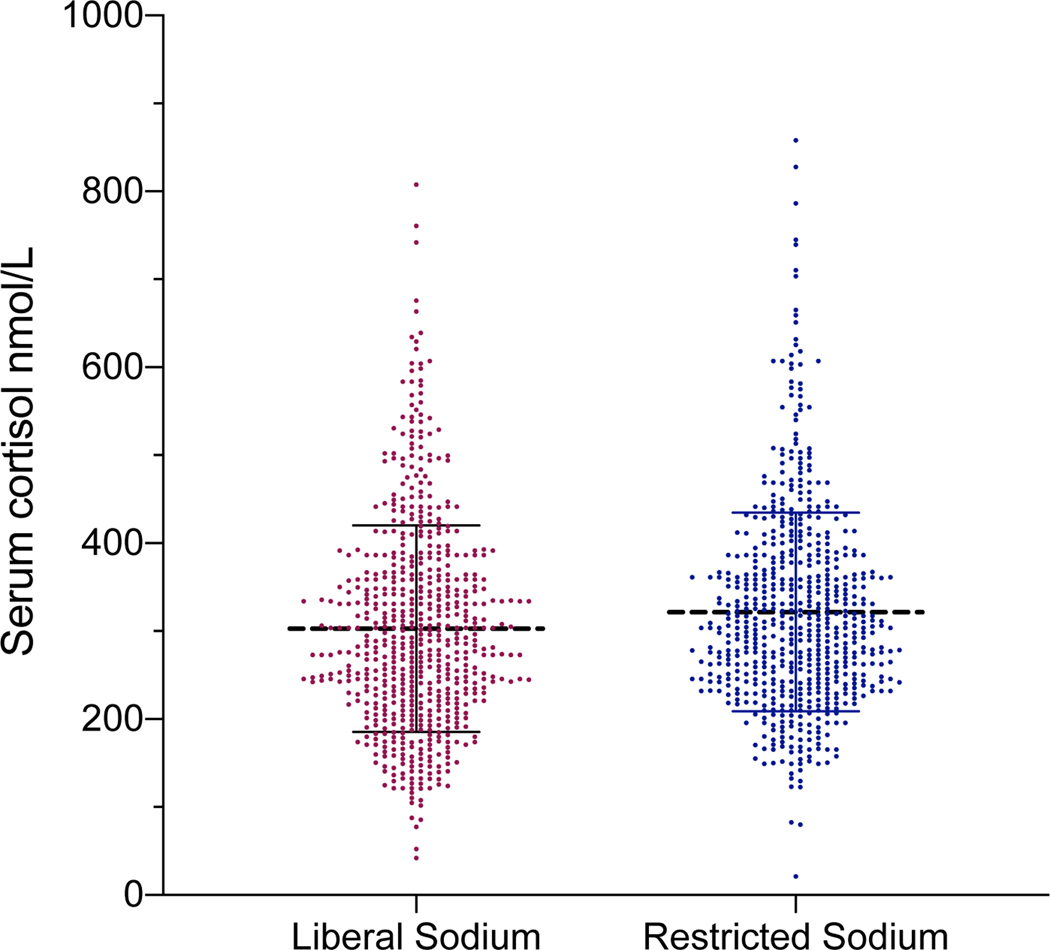
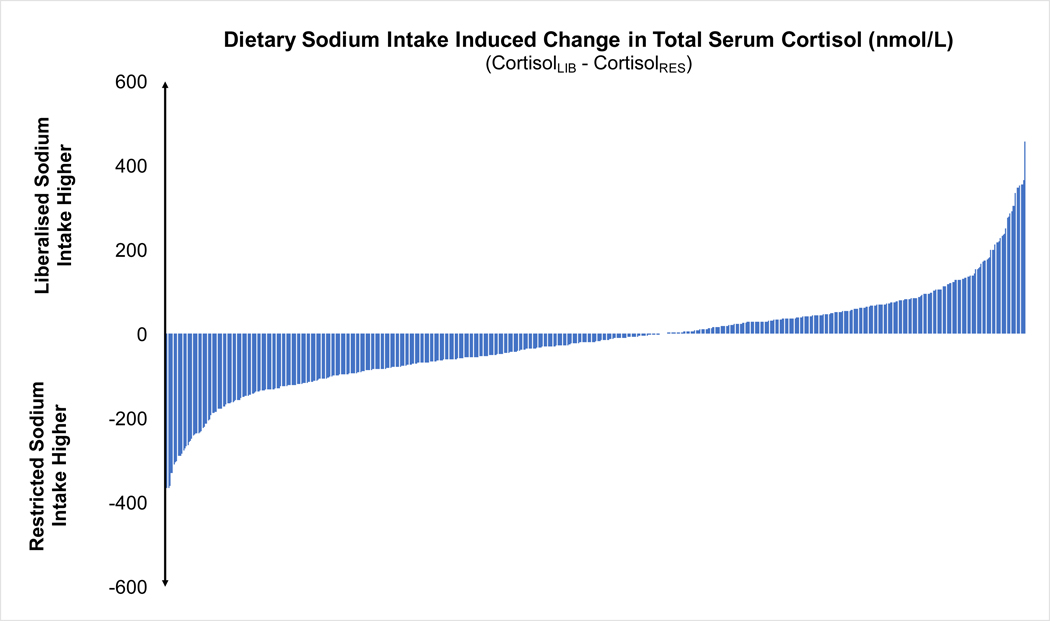
In contrast, morning total serum cortisol concentration was significantly lower during the liberalized dietary sodium intake diet when compared to the restricted dietary sodium intake, although the magnitude of difference was very small (303.0 ± 118.6 vs. 326.4 ± 162.8 nmol/L, p<0.001: Figure 2A). The majority, 59% (369/630), of participants had lower morning total serum cortisol concentration when they were maintained on liberalized sodium intake when compared to restricted sodium intake (Figure 2B).
Figure 2:
A) Scatter plots of total serum cortisol during restricted vs. liberal sodium diet (dashed line represents the mean and hash marks represent the standard deviation); B) Waterfall plots of the change in total serum cortisol induced by dietary sodium intervention for each participant (CortisolRES – CortisolLIB).
Effect of dietary sodium intake on proportion of participants above different 24hUFC thresholds
Compared with restricted dietary sodium intake, the proportion of participants exceeding every 24hUFC threshold was 2–4-fold greater during liberalized dietary sodium intake. Regardless of the threshold used, when participants were maintained on liberalized dietary sodium intake they had higher 24hUFC values (Table 2).
Table 2:
Proportion of participants above 24hUFC thresholds on liberal vs. restricted sodium intake.
| 24-hour UFC threshold in SI units (nmol/day) | 24-hour UFC thresholds in conventional units (mcg/day) | Participants above threshold on liberal sodium intake (n/%) | Participants above threshold on restricted sodium intake (n/%) | Population Discordance (number of additional cases from total population exceeding threshold on liberal sodium intake) (n/%) | Relative Discordance (percent difference exceeding threshold on liberal sodium intake compared to restricted sodium intake) (%) |
|---|---|---|---|---|---|
| 138 | 50 | 389 61.7% | 173 27.4% | 216 34.3% | 225% |
| 166 | 60 | 291 46.2% | 105 16.7% | 186 29.5% | 277% |
| 193 | 70 | 202 32.1% | 64 10.2% | 138 21.9% | 316% |
| 221 | 80 | 143 22.7% | 38 6.0% | 105 16.7% | 376% |
| 248* | 90 | 108 17.1% | 26 4.1% | 82 13.0% | 415% |
The default upper limit of reference range is presumed to be 248 nmol/day based on the uncalibrated manufacturer guidelines since this assay was conducted in a research laboratory and had not been calibrated for a reference population by a certified clinical laboratory.
DISCUSSION
In this study involving cross-over dietary sodium modulations with paired intra-individual comparisons in otherwise healthy participants without known or suspected Cushing syndrome, we observed that a high and liberalized dietary sodium intake was associated with significantly higher 24hUFC compared with restricted dietary sodium intake. These increases in 24hUFC resulted in a greater proportion of participants exceeding thresholds for 24hUFC when compared to measurements performed on a restricted sodium diet. Importantly, these changes were induced within one week of dietary sodium modulation and occurred consistently in nearly all participants, suggesting that short-term variations in dietary sodium intake can meaningfully contribute to variability in 24hUFC measurements. In contrast, morning total serum cortisol concentrations were slightly, albeit significantly, lower when dietary sodium intake was liberalized.
Although it is well-recognised that many different factors contribute to intra- and inter-individual variation in 24hUFC measurements, our understanding of the contribution and clinical implications of variable dietary sodium intake on cortisol assessments is limited.[7 13] Prior interventional studies with significantly smaller sample sizes have suggested that modulating dietary sodium intake can change 24hUFC levels by 40–50% [11 14 15]. Similarly, prior cross-sectional studies have consistently observed a positive association between urinary sodium and cortisol excretion.[16–18] Herein, we first validate these prior observations in a much larger sample size, and further, we interpret how the results may translate to the clinical care of patients. We demonstrate that a liberalized sodium diet induced a mean 47% increase in 24hUFC compared with restricted sodium diet, and resulted in a 2–4-fold greater number of individuals exceeding thresholds of 24hUFC, depending on the threshold used. Using the most conservative upper limit threshold of 248 nmol/day (90 mcg/day), the number of participants with elevated 24hUFC was 4-fold higher on liberalized sodium diet, with 17% of participants exceeding this threshold as opposed to only 4% when on a restricted sodium diet. Taken together, these physiologic observations underscore the importance of performing several different measures of systemic cortisol during the clinical evaluation of hypercortisolism and highlight the need to consider dietary sodium intake in the interpretation of findings. Although dietary sodium intake variations in the real-world are not as extreme as the differences induced by the intervention in this study, our findings are consistent with previous studies that have shown that a high sodium diet is positively correlated with 24hUFC and cortisol metabolites.[16 17] In a cross-sectional study of 220 adults with chronically elevated dietary sodium intake, Baudrand et al. demonstrated a strong positive correlation between high dietary sodium intake and elevated 24hUFC.[17] This observation was also found in a cohort of children and adults, where urinary sodium was positively correlated with urinary cortisol metabolites.[18] Collectively, these studies suggest that both acute and chronic changes in sodium intake may influence cortisol measurements.
Interestingly, in addition to changes in urinary cortisol excretion, a restricted sodium diet was associated with higher morning total serum cortisol than liberalized sodium diet in the current analysis, although the magnitude of this difference was very small. This likely reflects the challenges of measuring serum cortisol. Firstly, due to the diurnal variation of cortisol secretion, a random or single morning serum cortisol measurement in itself, is an inadequate measure of total daily cortisol and not part of the evaluation recommended by the Endocrine Society.[19] Secondly, serum cortisol also demonstrates wide inter-individual variability.[20] Finally, acute increases in dietary sodium intake is known to induce intravascular volume expansion, increase blood pressure and glomerular filtration rate, and suppress the renin-angiotensin-aldosterone system; in contrast, acute dietary sodium restriction can contract intravascular volume, lower blood pressure and glomerular filtration rate, and stimulate the renin-angiotensin-aldosterone system.[21 22] Though our study was not specifically designed to interrogate the mechanisms underlying our observations, we hypothesize that the known hemodynamic changes induced by increasing dietary sodium intake may have the dual impact of inducing a small but significant dilutional decrement in circulating serum cortisol and increasing urinary filtration and excretion of cortisol. Indeed, during liberalized sodium intake, participants had significantly higher BMI and estimated glomerular filtration rates, when compared to the restricted sodium diet, where volume contraction was induced [23]. In healthy individuals, high sodium intake modulates a positive correlation between GFR, effective renal plasma flow and BMI, particularly when BMI > 25kg/m2.[21 23] Mean BMI was > 25kg/m2 on both sodium diets, thus, hyperfiltration during liberalized sodium is likely to have contributed, in part, to 24hUFC differences during the dietary phases. Other mechanisms, such as changes in cortisol metabolism, changes in corticotropin or hypothalamic-pituitary stress responses, may also be operative; however, our study was not designed to evaluate these directly.[15]
To ensure patients with endogenous hypercortisolism are appropriately detected, screening tests should have high sensitivity. To account for the physiological variability in cortisol secretion and the differences in assays used to measure 24hUFC, the Endocrine Society recommends that the threshold for a “positive” 24hUFC value be based on the reference range for individual laboratories.[7] While many factors are recognized to contribute to this variability, our observations suggest that high dietary sodium intake may increase the risk for a false-positive 24hUFC result regardless of what pre-specified threshold is designated as “positive”. Further, assay manufacturers and reference laboratories should consider specifying or controlling dietary sodium intake when defining reference ranges and calibrating assay performance. Until such revised reference standards are available, clinicians may want to consider measuring 24-hour urinary sodium excretion at the time of 24hUFC, or consider advising a moderate dietary sodium intake in the days preceding the urine collection. We emphasize, that an isolated, mildly elevated 24hUFC result is not indicative of pathological endogenous hypercortisolism. However, since false-positive screening results can lead to additional, costly, and generally unnecessary investigations, the potential clinical implications of dietary sodium associated false-positive 24hUFC screening results should be considered.
One of the strengths of this study is that it is the largest in size to investigate the effect of acute changes in dietary sodium intake on systemic measures of cortisol. Moreover, dietary sodium interventions were carried out in a carefully controlled environment. A further strength was the use of 24-hour urinary sodium excretion, rather than spot urine measurements, to evaluate dietary sodium excretion.[24] However, we recognise several study limitations. Dietary sodium intake modulation was greater than what may occur in the real-world. For example, our liberal sodium diet contained ~25% more sodium than the average American consumes, and the restricted sodium diet contained less than 10% of what the average American consumes. Though this was helpful in evaluating the physiologic range of our findings, real-world dietary sodium intake variation usually occurs within a narrower range. Our dietary interventions were not blinded to participants or investigators since this would have been logistically and practically challenging or unfeasible. Thus, we cannot exclude the possibility that knowledge of the intervention could have introduced some bias. Newer cortisol assays increasingly rely on LC-MS/MS technology which may reduce assay variability; however, the differences we saw were systematic and unlikely to be due to assay variation alone. We did not have measurements of corticotropin, DHEA-S, or urinary protein excretion, and therefore cannot comment on how this may have influenced the findings or cortisol measurements.
CONCLUSION
We observed that increasing dietary sodium intake substantially increased urinary cortisol excretion and mildly decreased total circulating serum cortisol, when compared with dietary sodium restriction. These variations in dietary sodium intake could modify the classification of individuals as having an elevated urinary free cortisol, regardless of the threshold utilized. Dietary sodium intake may be an important clinical consideration, and contributor to cortisol variability, when evaluating for endogenous hypercortisolism in the general population.
Acknowledgments:
We are grateful for funding from the following grants from the National Institutes of Health: 5R01 HL136567 (GHW), 1R01 HL144779 (GHW), 1R01 HL114765 (GHW), RM-07-2002 (GHW), RO1 HL085224 (GHW), RO1 HL086907 (GHW), 5 P50 HL55000 (GHW), R01 DK115392 (AV), R01 DK107407 (AV), R01 DK16618 (AV), F32 HL147453 (AVH).
Footnotes
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request
Conflicts of Interest: AV reports consulting fees unrelated to the contents of this work from Corcept Therapeutics, HRA Pharma, Orphagen Pharmaceuticals, Catalys Pacific. AV, AXC, AVH and GWH have nothing to disclose
REFERENCES
- 1.Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour Urinary Sodium and Potassium Excretion in US Adults. JAMA 2018;319(12):1209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stallings VA, Harrison M, Oria M. Dietary Reference Intakes for Sodium and Potassium: National Academies Press; (US: ), 2019. [PubMed] [Google Scholar]
- 3.Arnett DK, Blumenthal RS, Albert MA, et al. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J Am Coll Cardiol 2019:26029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 2015;65(10):1042–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver CM, Martin BR, McCabe GP, et al. Individual variation in urinary sodium excretion among adolescent girls on a fixed intake. J Hypertension 2016;34(7):1290–97. [DOI] [PubMed] [Google Scholar]
- 6.Campbell NRC, He FJ, Tan M, et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J Clin Hypertension 2019;21(6):700–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman LK, Biller BMK, Findling JW, et al. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersenn S, Newell-Price J, Findling JW, et al. High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol 2014;80(2):261–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 2007;92(11):4472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pojoga L, Kolatkar NS, Williams JS, et al. Beta-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension2006;48(5):892–900. [DOI] [PubMed] [Google Scholar]
- 11.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism 2011;60(7):965–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown NJ, Agirbasli MA, Williams GH, Litchfield WR, Vaughan DE. Effect of activation and inhibition of the renin-angiotensin system on plasma PAI-1. Hypertension 1998;32(6):965–71 [DOI] [PubMed] [Google Scholar]
- 13.Pecori Giraldi F, Ambrogio AG. Variability in laboratory parameters used for management of Cushing’s syndrome. Endocrine 2015;50(3):580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wambach G, Bleienheuft C, Bonner G. Sodium loading raises urinary cortisol in man. J Endocrinol Invest 1986;9(3):257–9. [DOI] [PubMed] [Google Scholar]
- 15.Lewicka S, Nowicki M, Vecsei P. Effect of sodium restriction on urinary excretion of cortisol and its metabolites in humans. Steroids 1998;63(7–8):401–5. [DOI] [PubMed] [Google Scholar]
- 16.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 1996;81(7):2468–73. [DOI] [PubMed] [Google Scholar]
- 17.Baudrand R, Campino C, Carvajal C, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol 2014;80(5):677–84 [DOI] [PubMed] [Google Scholar]
- 18.Torres SJ, Grimes C, Nowson CA, et al. Urinary sodium is positively associated with urinary free cortisol and total cortisol metabolites in a cross-sectional sample of Australian schoolchildren aged 5–12 years and their mothers. Br J Nutrition 2018:1–8 [DOI] [PubMed] [Google Scholar]
- 19.El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva–are our assays good enough? Ann Clin Biochemistry 2017;54(3):308–22 [DOI] [PubMed] [Google Scholar]
- 20.El-Farhan N, Pickett A, Ducroq D, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol 2013;78(5):673–80 [DOI] [PubMed] [Google Scholar]
- 21.Krikken J, Lely A, Bakker S, Navis G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 2007;71(3):260–65 [DOI] [PubMed] [Google Scholar]
- 22.Nomura K, Asayama K, Jacobs L, Thijs L, Staessen JA. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int 2017;92(1):67–78. [DOI] [PubMed] [Google Scholar]
- 23.Bankir L, Perucca J, Norsk P, Bouby N, Damgaard M. Relationship between Sodium Intake and Water Intake: The False and the True. Ann Nutrition Metabol 2017;70(suppl 1)(Suppl. 1):51–61. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook J, Patterson K, Bodner J, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutrition 1984;40(4):786–93 [DOI] [PubMed] [Google Scholar]