Abstract
VSP-17, a novel peroxisome proliferator-activated receptor γ (PPARγ) agonist, has been previously demonstrated to suppress the metastasis of triple-negative breast cancer (TNBC) by upregulating the expression levels of E-cadherin, which is a key marker of epithelial-mesenchymal transition (EMT). However, the mechanism of action of VSP-17, in particular whether it may be associated with the EMT process, remains unknown. The present study investigated the ability of VSP-17 to inhibit the invasiveness and migratory ability of TNBC cell lines (MDA-MB-231 and MDA-MB-453) performed in in vitro experiments. including cell migration assay, cell invasion assay, cell transfection, RT-qPCR, western blot (WB) analysis and immunofluorescence. The present study aimed to ascertain whether and how the PPARγ/AMP-activated protein kinase (AMPK) signaling pathway serves a role in the inhibitory effects of VSP-17 on cell migration and invasion. The results revealed that both treatment with compound C (an AMPK inhibitor) and transfection with small interfering RNA (si)AMPK notably diminished the inhibitory effect of VSP-17 treatment on the migration and invasion of MDA-MB-231 and MDA-MB-453 cells, indicating that VSP-17 may, at least partly, exert its effects via AMPK. Furthermore, both compound C and siAMPK markedly diminished the VSP-17-induced downregulation of vimentin expression levels and upregulation of E-cadherin expression levels, further indicating that the VSP-17-induced inhibition of the EMT process may be dependent on AMPK. The combination of GW9662 (a PPARγ antagonist) or siPPARγ diminished the inhibitory effect of VSP-17 treatment on the migration and invasion of the TNBC cells, indicating that PPARγ may serve an important role in the VSP-17-induced inhibition of the migration and invasion of TNBC cells. In addition, both GW9662 and siPPARγ significantly reversed the VSP-17-induced downregulation of vimentin expression levels and upregulation of E-cadherin expression levels, implying that the VSP-17-induced inhibition of the EMT process may be dependent on PPARγ. VSP-17 treatment also upregulated the expression levels of p-AMPK, which could be reversed by either GW9662 or siPPARγ, indicating that the VSP-17-induced activation of the AMPK signaling pathway was PPARγ-dependent. In conclusion, the findings of the present study indicated that VSP-17 treatment may inhibit the migration and invasion of TNBC cells by suppressing the EMT process via the PPARγ/AMPK signaling pathway.
Keywords: adenosine 5′-monophosphate-activated protein kinase, VSP-17, epithelial-mesenchymal transformation, triple-negative breast cancer, metastasis, peroxisome proliferator-activated receptor γ
Introduction
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that lacks the expression of the human epidermal growth factor receptor 2 (HER2), estrogen and progesterone receptors (1,2). Metastasis is a major clinical feature of late stage TNBC, and is the main cause of death in patients (3,4). It has been widely recognized that the migration and invasion of TNBC cells are considered to be key steps in the metastatic process (5). One study revealed that TNBC cells with high migratory and high invasive properties are closely associated with a poor prognosis in TNBC (6). In a previous study, thymoquinone, an active ingredient of Nigella sativa, markedly inhibited cell growth, migration and invasion in both TNBC cells and orthotopic TNBC model mice by regulating the elongation factor 2 kinase (eEF-2K) signaling axis (7). Furthermore, in another study, luteolin, a natural flavonoid compound, reportedly suppressed the epithelial-mesenchymal transition (EMT) and migration of TNBC cells by inhibiting YAP/TAZ activity (8). These findings suggest that preventing the migration and invasion of TNBC cells may inhibit TNBC metastasis and represent a target for TNBC treatment.
EMT is a biological process in which epithelial cells lose key proteins (such as E-cadherin) involved in cell junctions and obtain a mesenchymal phenotype, which is evidenced by upregulated protein expression levels of mesenchymal markers (such as vimentin) (9–11). The activation of EMT has been found to serve an important role in the initial stages of the metastatic cascade by enhancing the migratory and invasive abilities of cancer cells (12). It has been demonstrated that irisin (a novel myokine) could inhibit the EMT process by suppressing the migration and invasion of MIA PaCa-2 and Panc03.27 cells (13). In addition, isorhamnetin could inhibit migration and invasion by suppressing AKT/ERK-mediated EMT in A549 human non-small cell lung cancer cells (14). These aforementioned findings suggest that blocking the process of EMT may represent a potential approach to inhibit the migration and invasion of TNBC cells.
AMP-activated protein kinase (AMPK), a vital metabolic energy sensor that regulates protein and lipid metabolism responses, has been demonstrated to serve an important role in the EMT process associated with tumor metastasis (15–19). Increasing evidence has revealed that AMPK is inactivated in TNBC cells, and that the absence of AMPK in patients predicted a poor prognosis (20,21). Conversely, the activation of AMPK could inhibit the EMT process of tumor cells by regulating the expression levels of EMT-related markers (22). For example, metformin could suppress the EMT process in TNBC cells by activating the AMPK signaling pathway (23). Similarly, rosmarinic acid was found to suppress the metastatic characteristics of colorectal cancer (CRC) cells by activating AMPK (24). These previous studies suggest that the activation of AMPK may be a target for the inhibition of metastasis and the EMT process in TNBC.
Peroxisome proliferator-activated receptor γ (PPARγ), a ligand-dependent transcription factor that regulates lipid metabolism, has been proven to participate in tumor metastasis (25). Accumulating evidence indicates that PPARγ is inactivated in TNBC cells, and its activation may inhibit the metastasis of breast cancer cells (26,27). VSP-17 has been identified as a novel PPARγ agonist, and it has been shown to suppress the metastasis of TNBC by upregulating the expression levels of E-cadherin (28). However, the mechanism through which VSP-17 may inhibit TNBC metastasis remains unclear. The present study aimed to determine the mechanism of action of the VSP-17-induced upregulation of E-cadherin levels in inhibiting TNBC metastasis, with a focus on the EMT and PPARγ/AMPK signaling pathways.
Materials and methods
Reagents
VSP-17 (C23H18F3N3O, MW: 409.1402, purity ≥98%) was designed and synthesized by our team (Guangxi Colleges and Universities Key Laboratory of Pharmacology), the details of which have been described in our previous study (28). siAMPK (cat. no. 203834) and siPPARγ (cat. no. 202738) were obtained from Sangon Biotech; Lipofectamine 3000 reagents (cat. no. 2149659) were purchased from Thermo Fisher Scientific, Inc., and compound C (cat. no. 866405-64-3) and GW9662 (cat. no. 22978-75-2) were purchased from MedChemExpress. Primary antibodies against the following targets: AMPK (cat. no. BS6271), p-AMPK (cat. no. BS4010), E-cadherin (cat. no. BS1098) and vimentin (cat. no. BS1491) were supplied by Bioworld Technology, Inc.; glyceraldehyde-3-phosphate dehydrogenase (GAPDH, cat. no. 60004-1-Ig) was obtained from ProteinTech Group, Inc. HiScript II One-Step RT-PCR Kit (cat. no. R323-01) and ChamQ SYBR qPCR Master Mix (cat. no. Q311-02) were purchased from Vazyme; 4′,6-diamidino-2-phenylindole (DAPI; cat. no. C0060) was obtained from Beijing Solarbio Science & Technology Co., Ltd. Other analytical reagent grade chemicals were purchased from Chemical Reagent Co. Ltd.
Cell culture
MDA-MB-231 and MDA-MB-453 cells were obtained from the American Type Culture Collection (ATCC) and supplemented with Leibovitz's (L-15) medium (cat. no. 11415064; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in 5% CO2, 95% air cell culture at 37°C. The cells were starved for 2 h before the experiments.
Cell migration assay
According to the manufacturer's protocol, the cell migration assay was carried out using Transwell plates. Post-treated cells or compound C co-cultured cells (1×105) were detached and suspended in the culture medium. Then, the cells were added to the upper chamber of the Transwell plates, while the lower chamber was filled with 600 µl of culture medium containing 10% FBS as a chemical attractant. After being incubated for 6 h, the non-migratory cells on the upper surface of the membrane were removed with a soaked cotton swab. In addition, the cells that migrated to the bottom face of the membranes were counted after being stained with 0.5% crystal violet solutions at 37°C for 30 min. Then, 5 microscopic fields per filter were captured randomly with the help of an inverted microscope (Olympus, Tokyo, Japan; ×200 magnification).
Cell invasion assay
The invasion potential of the MDA-MB-231 and MDA-MB-453 cells was evaluated using a Matrigel-coated Transwell chamber with 8.0-µm pore size. Post-transfected cells or compound C co-cultured cells (1×105) were resuspended with L-15 medium and plated into the upper chamber, and L-15 medium containing 20% FBS was added to the lower chambers as an attractant. The suspension was discarded after 24 h, counting the cells which invaded the lower chamber under an inverted microscope (Olympus, Tokyo, Japan; ×200 magnification).
Cell transfection
Under the guidance of the manufacturer's instructions, Lipofectamine 2000 and small interfering RNA (siRNA) duplex specific for AMPK and PPARγ were used to transiently transfect MDA-MB-231 and MDA-MB-453 cells for 6 h at 37°C, and then the cells were incubated with L-15 medium. At 24 h post-infection, the cells were used for subsequent experiments. The sequences of AMPK- and PPARγ-specific siRNAs were as follows: SiAMPK: sense, 5′-GUUGCCUACCAUCUCAUAAUATT-3′ and antisense, 5′-UAUUAUGAGAUGGUAGGCAACTT-3′; and siPPARγ: Sense, 5′-GACAGUGACUUGGCUAUAUTT-3′ and antisense, 5′-GCGAUCUUGACAGGAAAGATT-3′.
Quantitative real-time polymerase chain reaction (qPCR)
For verifying the results of RNA sequencing, qPCR assay was performed. Total RNA was extracted from the cells with TRIzol reagent. The mRNA was reverse transcribed to cDNA with the help of HiScript II One-Step RT-PCR Kit. ChamQ SYBR qPCR Master Mix and quantitative fluorescence analysis were used to analyze the expression of EMT-related factors, and the analysis system was CFX96 Touch optical system (Bio-Rad Laboratories, Inc.). The primer sequences of E-cadherin are as follows: sense, 5′-GCCATCGCTTACACCATCCTCAG-3′ and antisense, 5′-CTCTCTCGGTCCAGCCCAGTG-3′. PCR conditions were as follows: 95°C for 5 min (1×), followed by 95°C for 10 sec, 63°C for 30 sec (40×), 60°C for 10 sec (1×), and then 95°C for 10 sec (1×). Relative quantification was calculated with the 2−ΔΔCq method as described by Livak and Schmittgen (29).
Western blot (WB) analysis
Total proteins were extracted from the MDA-MB-231 and MDA-MB-453 cells using RIPA lysis buffer with phosphatase inhibitor and PMSF for 30 min on ice. The proteins were quantified using the BCA protein concentration determination kit, separated by 8% SDS-PAGE gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk and incubated overnight at 4°C with specific primary antibodies (vimentin, E-cadherin, p-AMPK and AMPK) at a dilution rate of 1:1,000 (v/v). After washing 3 times with TBST for 5 min, the membranes were incubated with HRP-conjugated secondary antibodies and then the membrane was visualized on a gel imager after adding Immobilon Western Chemiluminescent HRP substrate (cat. no. SQ201; Shanghai EpiZyme Biotechnology, Inc.). GAPDH was used as a normalized control. Images were captured by Bio-Rad Gel-Doc XR+ system with Image Lab (version 4.1) software and processed with Quantity One software (Bio-Rad Laboratories, Inc.).
Immunofluorescence (IF)
The suspended cells were seeded in a 96-well plate at a density of 5×103 per well. At 80–90% confluence, the cells were treated with compound C or siAMPK for 24 h, fixed with 4% buffered paraformaldehyde, and permeabilized with 1% Triton X-100. After being blocked using 5% non-fat milk solution for 30 min, the cells were incubated with primary anti-E-cadherin or anti-vimentin monoclonal antibody at 37°C for 2 h and subsequently incubated with secondary antibody (cat. no. AMJ-AB2005, 200 µl; Wuhan AmyJet Scientific, Inc.) at 37°C for 1 h. Then the cells were incubated with DAPI at 37°C for 5 min. Finally, the cells were visualized with an Olympus fluorescence microscope (Olympus).
Statistical analysis
Data are expressed as the means ± SEM. Statistical analyses were performed by a one-way analysis of variance (ANOVA), followed by Tukey's test. A value of P<0.05 was accepted as a statistically significant difference.
Results
VSP-17 treatment suppresses the migration and invasion of TNBC cells in an AMPK-dependent manner
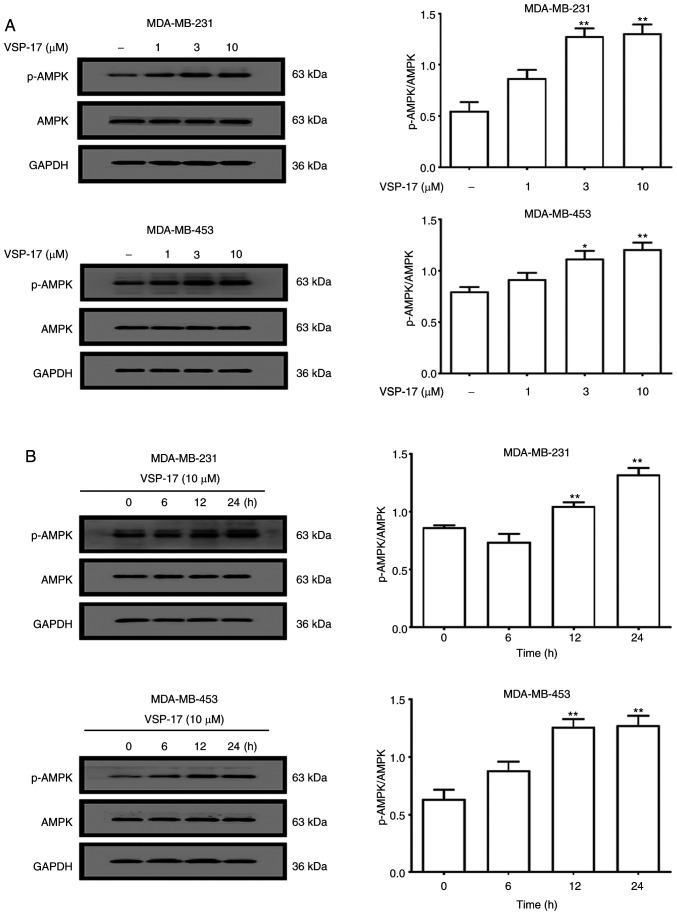
To determine whether AMPK serves a key role in mediating the anti-migratory and anti-invasive effects of VSP-17, the expression levels of AMPK and p-AMPK in MDA-MB-231 and MDA-MB-453 cells were determined using western blotting. As shown in Fig. 1A, the treatment with 1, 3 or 10 µM VSP-17 significantly upregulated the expression levels of p-AMPK in a dose-dependent manner, while VSP-17 had no significant effect on the expression levels of AMPK in the MDA-MB-231 and MDA-MB-453 cells. Moreover, the expression levels of p-AMPK in both cell lines were upregulated by 10 µM VSP-17 treatment in a time-dependent manner (Fig. 1B).
Figure 1.
Effect of VSP-17 treatment on the activation of AMPK. (A) MDA-MB-231 and MDA-MB-453 cells were treated with 1, 3 or 10 µM VSP-17 for 24 h. The protein expression levels of p-AMPK and AMPK were analyzed using western blotting. (B) MDA-MB-231 and MDA-MB-453 cells were treated with 10 µM VSP-17 for 6, 12 or 24 h, and the expression levels of p-AMPK and AMPK were analyzed using western blotting. The data are expressed as the mean ± SEM of three independent experiments. *P<0.05, **P<0.01 vs. control. AMPK, AMP-activated protein kinase; p-, phosphorylated.
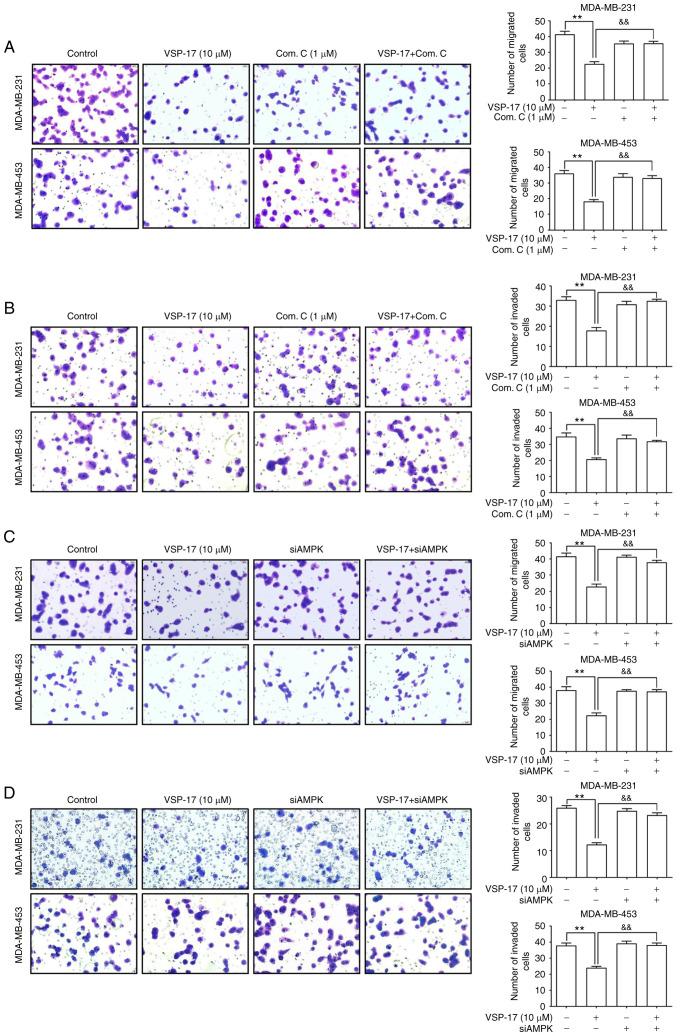
Subsequently, to further investigate the role of AMPK in the inhibitory effect of VSP-17 on cell migration and invasion, MDA-MB-231 and MDA-MB-453 cells were treated with compound C or transfected with siAMPK. The data revealed that both compound C (Fig. 2A and B) and siAMPK (Fig. 2C and D) significantly reversed the inhibitory effect of VSP-17 on cell migration and invasion.
Figure 2.
Effects of compound C and siAMPK on the VSP-17-induced inhibition of migration and invasion in MDA-MB-231 and MDA-MB-453 cells. (A and B) Cells were treated with 1 µM compound C, 10 µM VSP-17 or 10 µM VSP-17 + 1 µM compound C for 24 h. Transwell assays were used to evaluate the (A) migratory and (B) invasive abilities in MDA-MB-231 and MDA-MB-453 cells. (C and D) Cells were treated with 10 µM VSP-17 or transfected with siAMPK. Transwell assays were used to evaluate the (C) migratory and (D) invasive abilities in MDA-MB-231 and MDA-MB-453. The data are expressed as the mean ± SEM of three independent experiments. **P<0.01 vs. control; &&P<0.01 vs. VSP-17. Com. C, compound C; si, small interfering RNA; AMPK, AMP-activated protein kinase.
VSP-17 treatment inhibits the EMT process in TNBC cells in an AMPK-dependent manner
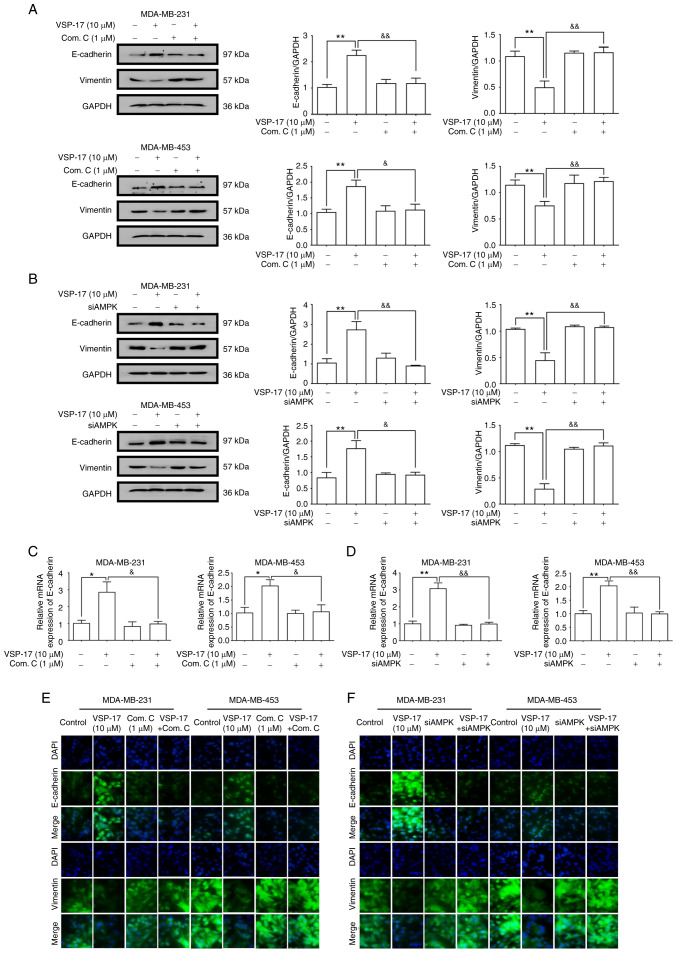
E-cadherin is a key marker of EMT (30). A previous study reported that VSP-17 treatment could upregulate the mRNA expression levels of E-cadherin in TNBC cells (28). To further determine the relationship between the activation of AMPK and the inhibition of the EMT process by VSP-17, MDA-MB-231 and MDA-MB-453 cells were treated with VSP-17 (10 µM) in combination with compound C (1 µM) or siAMPK transfection, respectively. As determined by western blot analysis (Fig. 3A and B), both compound C and siAMPK markedly abolished the VSP-17-induced downregulation of vimentin expression levels and upregulation of E-cadherin expression levels, and reverse transcription-quantitative PCR analysis (Fig. 3C and D) and immunofluorescent analysis (Fig. 3E and F) further confirmed this result, suggesting that AMPK may play an important role in the VSP-17-induced inhibition of EMT in MDA-MB-231 and MDA-MB-453 cells.
Figure 3.
Effects of compound C and siAMPK on the VSP-17-induced inhibition of the epithelial-mesenchymal transition (EMT) process in MDA-MB-231 and MDA-MB-453 cells. (A and B) Expression levels of E-cadherin and vimentin in MDA-MB-231 and MDA-MB-453 cells treated with 10 µM VSP-17 and/or 1 µM compound C (A) and/or transfected with siAMPK (B) were analyzed using western blotting. (C and D) mRNA expression levels of E-cadherin in MDA-MB-231 and MDA-MB-453 cells treated with 10 µM VSP-17 and/or 1 µM compound C (C) and/or transfected with siAMPK (D) were analyzed using reverse transcription-quantitative PCR. (E and F) Cells were cultured with 10 µM VSP-17 and/or 1 µM compound C (E) and/or transfected with siAMPK (F) and the expression levels of E-cadherin and vimentin were measured by immunofluorescence. The data are expressed as the mean ± SEM of three independent experiments. *P<0.05, **P<0.01 vs. control; &P<0.05, &&P<0.01 vs. VSP-17. Com. C, compound C; si, small interfering RNA; AMPK, AMP-activated protein kinase.
VSP-17 treatment suppresses the migration and invasion of TNBC cells in a PPARγ-dependent manner
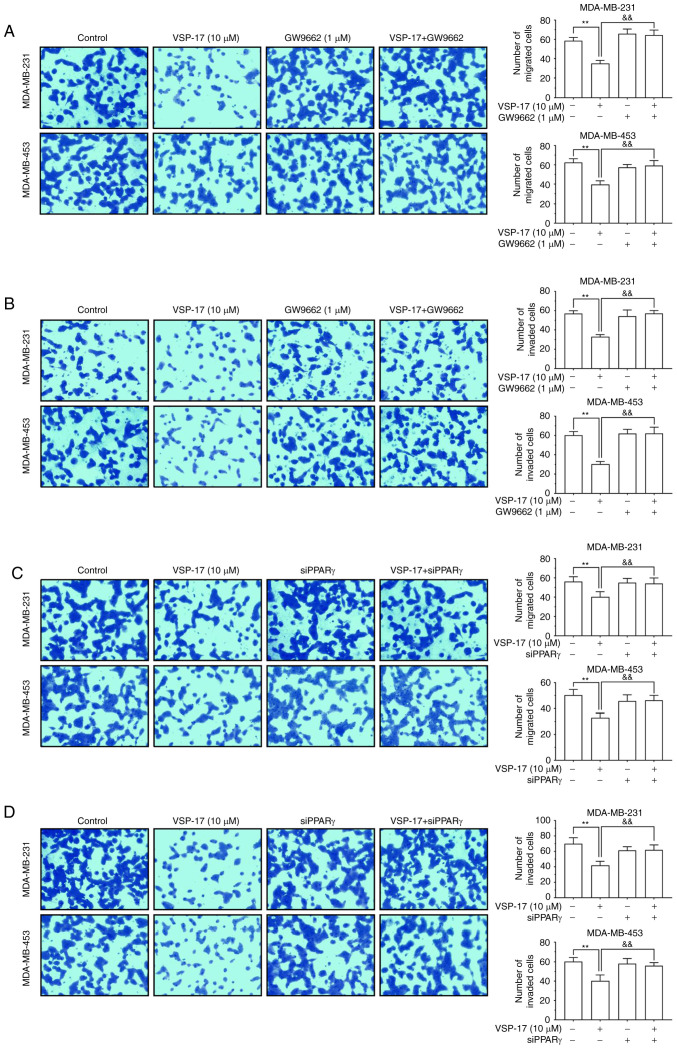
To identify the role of PPARγ in the inhibitory effect of VSP-17 on migration and invasion, the PPARγ antagonist, GW9662 and siPPARγ were used. The findings demonstrated that both GW9662 and siPPARγ reversed the inhibitory effect of VSP-17 on cell migration and invasion (Fig. 4A-D), indicating that PPARγ may also serve an important role in the VSP-17-induced inhibition of migration and invasion in TNBC cells.
Figure 4.
Effects of GW9662 and siPPARγ on the VSP-17-induced inhibition of migration and invasion in MDA-MB-231 and MDA-MB-453 cells. (A and B) Cells were treated with 1 µM GW9662, 10 µM VSP-17 or 10 µM VSP-17 + 1 µM GW9662 for 24 h. Transwell assays were used to evaluate the (A) migratory and (B) invasive abilities of MDA-MB-231 and MDA-MB-453 cells. (C and D) Cells were treated with 10 µM VSP-17 and/or transfected with siPPARγ. Transwell assays were used to evaluate the (C) migratory and (D) invasive abilities of MDA-MB-231 and MDA-MB-453 cells. The data are expressed as the mean ± SEM of three independent experiments. **P<0.01 vs. control; &&P<0.01 vs. VSP-17. si, small interfering RNA; PPARγ, peroxisome proliferator-activated receptor γ.
VSP-17 treatment inhibits the EMT process in TNBC cells in a PPARγ-dependent manner
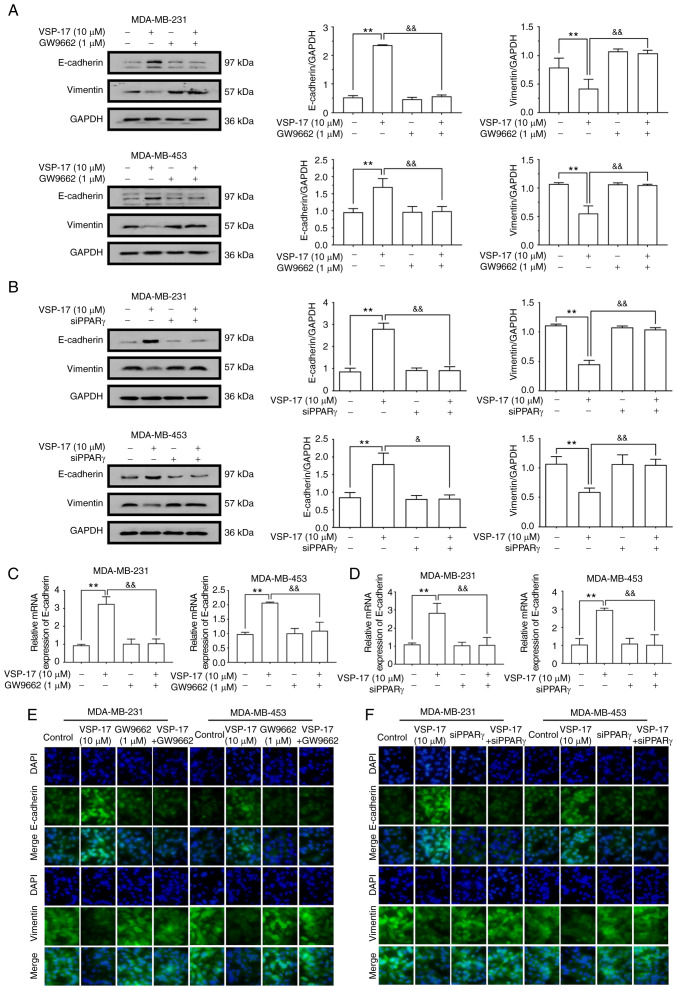
To further determine the relationship between the activation of PPARγ and the inhibition of the EMT process by VSP-17, MDA-MB-231 and MDA-MB-453 cells were co-treated with VSP-17 (10 µM) and GW9662 (1 µM) or treated with VSP-17 (10 µM) and transfected with siPPARγ. As determined by western blot analysis (Fig. 5A and B), both GW9662 and siPPARγ significantly reversed the VSP-17-induced downregulation of vimentin expression levels and upregulation of E-cadherin expression levels, and reverse transcription-quantitative PCR analysis (Fig. 5C and D) and immunofluorescent analysis (Fig. 5E and F) further confirmed this result, suggesting that PPARγ may serve an important role in the VSP-17-induced inhibition of EMT in TNBC cells.
Figure 5.
Effects of GW9662 and siPPARγ on the VSP-17-induced inhibition of the epithelial-mesenchymal transition (EMT) process in MDA-MB-231 and MDA-MB-453 cells. (A and B) Expression levels of E-cadherin and vimentin in MDA-MD-231 and MDA-MD-453 cells treated with 10 µM VSP-17 and/or 1 µM GW9662 (A) and/or transfected with siPPARγ (B) were analyzed using western blotting. (C and D) mRNA expression levels of E-cadherin in MDA-MB-231 and MDA-MB-453 treated with 10 µM VSP-17 and/or 1 µM GW9662 (C) and/or transfected with siPPARγ (D) were analyzed using reverse transcription-quantitative PCR. (E and F) Cells were cultured with 10 µM VSP-17 and/or 1 µM GW9662 (E) and/or transfected with siPPARγ (F) and the expression levels of E-cadherin and vimentin were measured by immunofluorescence. The data are expressed as the mean ± SEM of three independent experiments. **P<0.01 vs. control; &P<0.05, &&P<0.01 vs. VSP-17. si, small interfering RNA; PPARγ, peroxisome proliferator-activated receptor γ.
VSP-17 treatment activates AMPK in a PPARγ-dependent manner
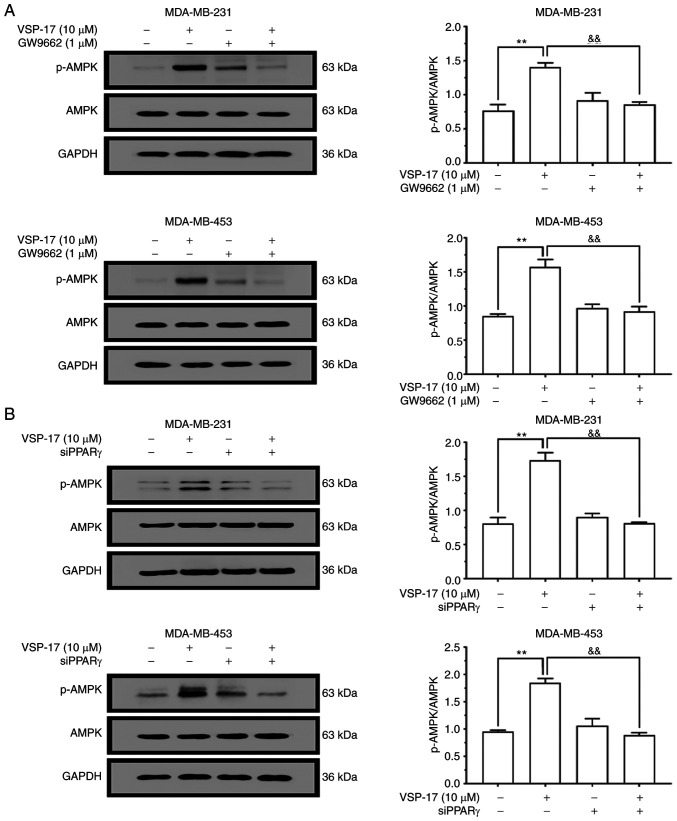
To determine whether the AMPK signaling pathway is involved in the effects of VSP-17, the effect of VSP-17 treatment on the activation of AMPK in MDA-MB-231 and MDA-MB-453 cells was investigated. As shown in Fig. 6A and B, the expression levels of p-AMPK in MDA-MB-231 and MDA-MB-453 cells were markedly upregulated following the treatment with 10 µM VSP-17. In addition, both GW9662 treatment (Fig. 6A) and siPPARγ transfection (Fig. 6B) reversed the VSP-17-induced upregulation in p-AMPK expression levels, indicating that the VSP-17-induced activation of the AMPK signaling pathway may be mediated by PPARγ.
Figure 6.
Effects of GW9662 and siPPARγ on the VSP-17-induced activation of the AMPK signaling pathway in MDA-MB-231 and MDA-MB-453 cells. (A) Cells were treated with 1 µM GW9662, 10 µM VSP-17 or 10 µM VSP-17 + 1 µM GW9662 for 24 h. Western blotting was used to analyze the protein expression levels of AMPK and p-AMPK. (B) Cells transfected with siPPARγ were treated with 10 µM VSP-17 for 24 h. The protein expression levels of AMPK and p-AMPK were analyzed using western blotting. The data are expressed as the mean ± SEM of three independent experiments. **P<0.01 vs. control; &&P<0.01 vs. VSP-17. si, small interfering RNA; PPARγ, peroxisome proliferator-activated receptor γ; AMPK, AMP-activated protein kinase; p-, phosphorylated.
Discussion
Emerging evidence has revealed that triple-negative breast cancer (TNBC) metastasis involves the migration and invasion of TNBC cells (28,31), and TNBC cells with increased migratory and invasive abilities are closely associated with tumor metastasis (32). Therefore, the inhibition of metastasis may represent a potential strategy for treating TNBC (33). Previous studies have demonstrated that PPARγ agonists, such as rosiglitazone, could inhibit the migration and invasion of breast cancer cells (34–37). However, it is worth noting that the long-term use of these drugs has been found to promote numerous adverse reactions, such as hepatotoxicity, weight gain and heart disease (38–40). Therefore, the development of a novel PPARγ agonist with low toxicity and high selectivity may represent a potential strategy to inhibit the metastasis of TNBC. In previous studies, the new PPARγ agonist, VSP-17, synthesized by our team, was found to suppress the metastasis of TNBC (28). However, the mechanism of action remains unclear. Therefore, the present study sought to further clarify the mechanism of action of VSP-17 with the aim of progressing VSP-17 into clinical trials.
Previous studies have reported that epithelial-mesenchymal transition (EMT) is a crucial initial step for cancer invasion (41,42) and it has been proven to promote the metastasis of tumors (43,44). The expression levels of EMT markers are significantly altered following the occurrence of EMT in tumor cells. In particular, the downregulation of E-cadherin expression levels and upregulation of vimentin expression levels are the main characteristics of EMT (45,46). A previous study revealed that puerarin could inhibit hepatocellular carcinoma metastasis by upregulating the expression levels of epithelial markers and downregulating the expression levels of mesenchymal markers (47). Similarly, rosmarinic acid, an abundant phenolic ester found in Prunella vulgaris, could inhibit the EMT process by upregulating the expression levels of E-cadherin and downregulating the expression levels of vimentin, and then inhibited the development of colorectal cancer (24). These findings indicate that the inhibition of the EMT process may be a potential strategy for suppressing TNBC metastasis. In the present study, VSP-17 treatment significantly upregulated the mRNA and protein expression levels of E-cadherin, and downregulated the protein expression levels of vimentin, which suggest that VSP-17 treatment may inhibit the EMT process in TNBC cells.
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated nuclear receptor, which belongs to the nuclear receptor gene superfamily (25). After ligand binding, PPARγ forms a heterodimer with retinoid X receptors, which then binds to the promoter of target genes, including NF-κB, nuclear factor erythroid 2-related factor 2 (Nrf2) and AMPK, to regulate the expression levels (48–50). In a previous study, the PPARγ agonist pioglitazone was found to prevent hypoxia/reoxygenation (H/R) damage through enhancing autophagy via the AMPK/mTOR signaling pathway (51). In addition, ciglitazone-mediated PPARγ activation led to the downregulation of oxidized low-density lipoprotein receptor 1 (LOX-1) expression levels and the activation of the AMPK signaling pathway (52). These previous studies suggest that PPARγ agonists may exert their biological roles by activating AMPK. An increasing number of studies have also reported that the activation of PPARγ could reverse the EMT process by upregulating the expression levels of E-cadherin and downregulating the expression levels of vimentin (53,54), which suggests that the activation of PPARγ may also be considered as a potential strategy for inhibiting the EMT process. In the present study, GW9662 treatment or the transfection with siPPARγ reversed the effect of VSP-17 treatment on the inhibition of metastasis, and reversed the upregulation of E-cadherin expression levels and downregulation of vimentin expression levels in TNBC cells. In addition, the VSP-17-induced upregulation of p-AMPK expression levels was blocked by GW9662 treatment or siPPARγ transfection. These findings suggest that VSP-17 treatment may suppress the EMT process and induce the AMPK signaling pathway in a PPARγ-dependent manner.
Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), a major mediator in cellular energy and metabolic control, has been proven to be closely associated with the EMT process (55,56). Experimental studies have found that AMPK activators could inhibit the EMT transition in tumors through suppressing the TGF-β-induced phosphorylation of SMAD2/3 (57). Increasing evidence has also revealed that the AMPK activator, metformin, could reverse the EMT process in breast cancer cells by activating the AMPK signaling pathway (58). It was also previously reported that the activation of AMPK inhibited the metastasis of melanoma cells (59). The results of the present study demonstrated that VSP-17 treatment could inhibit the EMT process in an AMPK-dependent manner. It was worth noting that multiple targets, such as mTOR, cyclooxygenase-2 (COX-2) and acetyl-CoA carboxylase (ACC), are also known to be regulated by AMPK activation (60). Based on these findings, it was hypothesized that VSP-17 may regulate proteins in the downstream signaling pathway of AMPK, such as mTOR, COX-2 and ACC, thereby leading to the regulation of the expression levels of EMT-related markers. However, further studies are required to identify the exact mechanism of action.
In conclusion, the findings of the present study suggest that VSP-17 may inhibit the migration and invasion of TNBC cells by suppressing the EMT process via the PPARγ/AMPK signaling pathway.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ACC
acetyl-CoA carboxylase
- AKT
protein kinase B
- AMPK
adenosine 5′-monophosphate (AMP)-activated protein kinase
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- DAPI
4,6-diamidino-2-phenylindole
- EMT
epithelial-mesenchymal transformation
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GAPDH
glycer-aldehyde-3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma
- H/R
hypoxia/reoxygenation
- HRP
horseradish peroxidase
- LOX-1
low-density lipoprotein receptor-1
- mTOR
mechanistic target of rapamycin
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- PMSF
phenylmethanesulfonyl fluoride
- PPARγ
peroxisome proliferator-activated receptor γ
- PVDF
polyvinylidene fluoride
- RA
rosmarinic acid
- TNBC
triple-negative breast cancer
- TGF-β
transforming growth factor-β
Funding Statement
The present study was supported by the National Natural Science Fund of China (no. 81660604), the Natural Science Fund of Guangxi (nos. 2017GXNSFBA198104 and 2018GXNSFAA138098), the Special Fund for Talents of Guangxi (no. AD18281016), the special funding for 2017 Guangxi BaGui Scholars, and the special funding for 2018 Guangxi Basic Skills of Young Teachers in the University (nos. 2018KY0408 and 2018KY0421).
Funding
The present study was supported by the National Natural Science Fund of China (no. 81660604), the Natural Science Fund of Guangxi (nos. 2017GXNSFBA198104 and 2018GXNSFAA138098), the Special Fund for Talents of Guangxi (no. AD18281016), the special funding for 2017 Guangxi BaGui Scholars, and the special funding for 2018 Guangxi Basic Skills of Young Teachers in the University (nos. 2018KY0408 and 2018KY0421).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
XX and XD conceived and designed the experiments. ML and YY performed the experiments. CW and XZ analyzed the data. HS was involved in project management and supervised the study. YW contributed to revising the manuscript for intellectual content and language editing. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no financial competing interests.
References
- 1.da Silva JL, Cardoso Nunes NC, Izetti P, de Mesquita GG, de Melo AC. Triple negative breast cancer: A thorough review of biomarkers. Crit Rev Oncol Hematol. 2020;145:102855. doi: 10.1016/j.critrevonc.2019.102855. [DOI] [PubMed] [Google Scholar]
- 2.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Z, Xu Q, Wang X, Wang J, Mu X, Cai Y, Qian Y, Shao W, Shao Z. RPLP1 promotes tumor metastasis and is associated with a poor prognosis in triple-negative breast cancer patients. Cancer Cell Int. 2018;18:170. doi: 10.1186/s12935-018-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braicu C, Chiorean R, Irimie A, Chira S, Tomuleasa C, Neagoe E, Paradiso A, Achimas-Cadariu P, Lazar V, Berindan-Neagoe I. Novel insight into triple-negative breast cancers, the emerging role of angiogenesis, and antiangiogenic therapy. Expert Rev Mol Med. 2016;18:e18. doi: 10.1017/erm.2016.17. [DOI] [PubMed] [Google Scholar]
- 5.Koedoot E, Fokkelman M, Rogkoti VM, Smid M, van de Sandt I, de Bont H, Pont C, Klip JE, Wink S, Timmermans MA, et al. Uncovering the signaling landscape controlling breast cancer cell migration identifies novel metastasis driver genes. Nat Commun. 2019;10:2983. doi: 10.1038/s41467-019-11020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Xia D, Li Z, Zhou T, Chen T, Wu Z, Zhou W, Li Z, Li L, Xu J. Aurora-A/ERK1/2/mTOR axis promotes tumor progression in triple-negative breast cancer and dual-targeting Aurora-A/mTOR shows synthetic lethality. Cell Death Dis. 2019;10:606. doi: 10.1038/s41419-019-1855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabil N, Bayraktar R, Kahraman N, Mokhlis HA, Calin GA, Lopez-Berestein G, Ozpolat B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res Treat. 2018;171:593–605. doi: 10.1007/s10549-018-4847-2. [DOI] [PubMed] [Google Scholar]
- 8.Cao D, Zhu GY, Lu Y, Yang A, Chen D, Huang HJ, Peng SX, Chen LW, Li YW. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed Pharmacother. 2020;129:110462. doi: 10.1016/j.biopha.2020.110462. [DOI] [PubMed] [Google Scholar]
- 9.Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39. doi: 10.1002/1878-0261.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savagner P. Leaving the neighborhood: Molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 11.Heimes AS, Schmidt M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs. 2019;28:1–5. doi: 10.1080/13543784.2019.1552255. [DOI] [PubMed] [Google Scholar]
- 12.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Song N, Huang Y, Chen Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci Rep. 2018;8:15247. doi: 10.1038/s41598-018-33229-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Liu Q, Jiang N, Li M, Shi L. Isorhamnetin inhibited migration and invasion via suppression of Akt/ERK-mediated epithelial-to-mesenchymal transition (EMT) in A549 human non-small-cell lung cancer cells. Biosci Rep. 2019;39:BSR20190159. doi: 10.1042/BSR20190159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Green AS, Chapuis N, Lacombe C, Mayeux P, Bouscary D, Tamburini J. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: From metabolism to cancer cell biology. Cell Cycle. 2011;10:2115–2120. doi: 10.4161/cc.10.13.16244. [DOI] [PubMed] [Google Scholar]
- 17.He K, Guo X, Liu Y, Li J, Hu Y, Wang D, Song J. TUFM downregulation induces epithelial-mesenchymal transition and invasion in lung cancer cells via a mechanism involving AMPK-GSK3β signaling. Cell Mol Life Sci. 2016;73:2105–2121. doi: 10.1007/s00018-015-2122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: Therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–513. doi: 10.1016/j.cytogfr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Si Y, Wang J, Liu X, Zhou T, Xiang Y, Zhang T, Wang X, Feng T, Xu L, Yu Q, et al. Ethoxysanguinarine, a novel direct activator of AMP-activated protein kinase, induces autophagy and exhibits therapeutic potential in breast cancer cells. Front Pharmacol. 2020;10:1503. doi: 10.3389/fphar.2019.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba Y, Nosho K, Shima K, Meyerhardt JA, Chan AT, Engelman JA, Cantley LC, Loda M, Giovannucci E, Fuchs CS, Ogino S. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer. Br J Cancer. 2010;103:1025–1033. doi: 10.1038/sj.bjc.6605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazarin JM, Coelho RG, Hecht F, Andrade BM, Carvalho DP. 5′-AMP-activated protein kinase regulates papillary (TPC-1 and BCPAP) thyroid cancer cell survival, migration, invasion, and epithelial-to-mesenchymal transition. Thyroid. 2016;26:933–942. doi: 10.1089/thy.2015.0440. [DOI] [PubMed] [Google Scholar]
- 23.Qu C, Zhang W, Zheng G, Zhang Z, Yin J, He Z. Metformin reverses multidrug resistance and epithelial-mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol Cell Biochem. 2014;386:63–71. doi: 10.1007/s11010-013-1845-x. [DOI] [PubMed] [Google Scholar]
- 24.Han YH, Kee JY, Hong SH. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front Pharmacol. 2018;9:68. doi: 10.3389/fphar.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamers C, Schubert-Zsilavecz M, Merk D. Therapeutic modulators of peroxisome proliferator-activated receptors (PPAR): A patent review (2008-present) Expert Opin Ther Pat. 2012;22:803–841. doi: 10.1517/13543776.2012.699042. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan A, Nair SA, Pillai MR. Biology of PPAR gamma in cancer: A critical review on existing lacunae. Curr Mol Med. 2007;7:532–540. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]
- 27.Nunez-Anita RE, Cajero-Juarez M, Aceves C. Peroxisome proliferator-activated receptors: Role of isoform gamma in the antineoplastic effect of iodine in mammary cancer. Curr Cancer Drug Targets. 2011;11:775–786. doi: 10.2174/156800911796798931. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Zhu M, Yuan B, Zhang K, Zhong M, Yi W, Xu X, Duan X. VSP-17, a New PPARγ agonist, suppresses the metastasis of triple-negative breast cancer via upregulating the expression of E-Cadherin. Molecules. 2018;23:121. doi: 10.3390/molecules23010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Jolly MK, Ware KE, Xu S, Gilja S, Shetler S, Yang Y, Wang X, Austin RG, Runyambo D, Hish AJ, et al. E-Cadherin represses anchorage-independent growth in sarcomas through both signaling and mechanical mechanisms. Mol Cancer Res. 2019;17:1391–1402. doi: 10.1158/1541-7786.MCR-18-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang DY, Sp N, Kim DH, Joung YH, Lee HG, Park YM, Yang YM. Salidroside inhibits migration, invasion and angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int J Oncol. 2018;53:877–885. doi: 10.3892/ijo.2018.4430. [DOI] [PubMed] [Google Scholar]
- 32.Chien YC, Liu LC, Ye HY, Wu JY, Yu YL. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am J Cancer Res. 2018;8:422–434. [PMC free article] [PubMed] [Google Scholar]
- 33.Zardavas D, Baselga J, Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013;10:191–210. doi: 10.1038/nrclinonc.2013.29. [DOI] [PubMed] [Google Scholar]
- 34.Bojková B, Garajová M, Kajo K, Péc M, Kubatka P, Kassayová M, Kisková T, Orendás P, Ahlersová E, Ahlers I. Pioglitazone in chemically induced mammary carcinogenesis in rats. Eur J Cancer Prev. 2010;19:379–384. doi: 10.1097/CEJ.0b013e32833ca233. [DOI] [PubMed] [Google Scholar]
- 35.Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012;38:475–484. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Fogo AB. Potential for peroxisome proliferator-activated receptor-gamma agonists in progression: Beyond metabolism. Curr Opin Nephrol Hypertens. 2008;17:282–285. doi: 10.1097/MNH.0b013e3282f9b1c0. [DOI] [PubMed] [Google Scholar]
- 37.Xu YY, Liu H, Su L, Xu N, Xu DH, Liu HY, Spaner D, Bed-David Y, Li YJ. PPARγ inhibits breast cancer progression by upregulating PTPRF expression. Eur Rev Med Pharmacol Sci. 2019;23:9965–9977. doi: 10.26355/eurrev_201911_19563. [DOI] [PubMed] [Google Scholar]
- 38.Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: A phase II study. Breast Cancer Res Treat. 2003;79:391–397. doi: 10.1023/A:1024038127156. [DOI] [PubMed] [Google Scholar]
- 39.Spence JD, Viscoli CM, Inzucchi SE, Dearborn-Tomazos J, Ford GA, Gorman M, Furie KL, Lovejoy AM, Young LH, Kernan WN, IRIS Investigators Pioglitazone therapy in patients with stroke and prediabetes: A post Hoc analysis of the IRIS randomized clinical trial. JAMA Neurol. 2019;76:526–535. doi: 10.1001/jamaneurol.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheen AJ. Thiazolidinediones and liver toxicity. Diabetes Metab. 2001;27:305–313. [PubMed] [Google Scholar]
- 41.Barriga EH, Mayor R. Adjustable viscoelasticity allows for efficient collective cell migration. Semin Cell Dev Biol. 2019;93:55–68. doi: 10.1016/j.semcdb.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Zeng Y, Xing X, Zeng J, Gao Y, Cai Z, Xu B, Liu X, Huang A, Liu J. Quantitative proteomics analysis of early recurrence/metastasis of huge hepatocellular carcinoma following radical resection. Proteome Sci. 2014;12:22. doi: 10.1186/1477-5956-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT) Breast Cancer Res Treat. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 45.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 46.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Xue R, Wang J, Ren H. Puerarin inhibits hepatocellular carcinoma invasion and metastasis through miR-21-mediated PTEN/AKT signaling to suppress the epithelial-mesenchymal transition. Braz J Med Biol Res. 2020;53:e8882. doi: 10.1590/1414-431x20208882erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juge-Aubry CE, Gorla-Bajszczak A, Pernin A, Lemberger T, Wahli W, Burger AG, Meier CA. Peroxisome proliferator-activated receptor mediates cross-talk with thyroid hormone receptor by competition for retinoid X receptor. Possible role of a leucine zipper-like heptad repeat. J Biol Chem. 1995;270:18117–18122. doi: 10.1074/jbc.270.30.18117. [DOI] [PubMed] [Google Scholar]
- 49.Xia H, Ge Y, Wang F, Ming Y, Wu Z, Wang J, Sun S, Huang S, Chen M, Xiao W, Yao S. Protectin DX ameliorates inflammation in sepsis-induced acute lung injury through mediating PPARγ/NF-κB pathway. Immunol Res. 2020;68:280–288. doi: 10.1007/s12026-020-09151-7. [DOI] [PubMed] [Google Scholar]
- 50.Rani N, Arya DS. Chrysin rescues rat myocardium from ischemia-reperfusion injury via PPAR-γ/Nrf2 activation. Eur J Pharmacol. 2020;883:173389. doi: 10.1016/j.ejphar.2020.173389. [DOI] [PubMed] [Google Scholar]
- 51.Xi X, Zou C, Ye Z, Huang Y, Chen T, Hu H. Pioglitazone protects tubular cells against hypoxia/reoxygenation injury through enhancing autophagy via AMPK-mTOR signaling pathway. Eur J Pharmacol. 2019;863:172695. doi: 10.1016/j.ejphar.2019.172695. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Wang S, Li B, Sun A, Zou Y, Ge J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARγ-dependent AMPK/eNOS pathway. J Cell Mol Med. 2015;19:92–102. doi: 10.1111/jcmm.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kao HF, Chang-Chien PW, Chang WT, Yeh TM, Wang JY. Propolis inhibits TGF-β1-induced epithelial-mesenchymal transition in human alveolar epithelial cells via PPARγ activation. Int Immunopharmacol. 2013;15:565–574. doi: 10.1016/j.intimp.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Reka AK, Kurapati H, Narala VR, Bommer G, Chen J, Standiford TJ, Keshamouni VG. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol Cancer Ther. 2010;9:3221–3232. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YC, Li H, Wang J. Mechanisms of metformin inhibiting cancer invasion and migration. Am J Transl Res. 2020;12:4885–4901. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang ZG, Zhang HS, Sun HL, Liu HY, Liu MY, Zhou Z. KDM5B promotes breast cancer cell proliferation and migration via AMPK-mediated lipid metabolism reprogramming. Exp Cell Res. 2019;379:182–190. doi: 10.1016/j.yexcr.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Lin H, Li N, He H, Ying Y, Sunkara S, Luo L, Lv N, Huang D, Luo Z. AMPK inhibits the stimulatory effects of TGF-β on Smad2/3 activity, cell migration, and epithelial-to-mesenchymal transition. Mol Pharmacol. 2015;88:1062–1071. doi: 10.1124/mol.115.099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee P, Surendran H, Chowdhury DR, Prabhakar K, Pal R. Metformin mediated reversal of epithelial to mesenchymal transition is triggered by epigenetic changes in E-cadherin promoter. J Mol Med (Berl) 2016;94:1397–1409. doi: 10.1007/s00109-016-1455-7. [DOI] [PubMed] [Google Scholar]
- 59.Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P, Bertolotto C, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605–1615. doi: 10.1158/1535-7163.MCT-12-1226-T. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6:7365–7378. doi: 10.18632/oncotarget.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.