Abstract
BACKGROUND:
Individual differences in cortical gray matter (GM) structure are associated with cognitive function and psychiatric disorders with developmental origins. Identifying when individual differences in cortical structure are established in childhood is critical for understanding the timing of abnormal cortical development associated with neuropsychiatric disorders.
METHODS:
We studied the development of cortical GM and white matter (WM) volume, cortical thickness (CT) and surface area (SA) using structural magnetic resonance imaging (MRI) in two unique cohorts of singleton (121 male/131 female) and twin (99 male/83 female) children imaged longitudinally from birth to 6 years.
Results:
Cortical GM volume increases rapidly in the first year of life, with more gradual growth thereafter. Between ages 1 and 6 years, total SA expands 29%, while average CT decreases about 3.5%. In both cohorts, a large portion of individual variation in cortical GM volume (81-87%) and total SA (73-83%) at 6 years is present by age 1 year. Regional heterogeneity of CT observed at age 6 is largely in place at age 1.
CONCLUSIONS:
These findings indicate that individual differences in cortical GM structure are largely established by the end of the first year of life, following a period of rapid postnatal GM growth. This suggests that alterations in GM structure associated with psychiatric disorders with developmental origins may largely arise in the first year of life, and that interventions to normalize or mitigate abnormal GM development may need to be targeted to very early childhood.
Keywords: neurodevelopment, cortex, neuroimaging, infant, gray matter, sex differences
Introduction
Alterations in cortical morphology are present in neuropsychiatric illnesses with prenatal and early childhood origins. For example, cortical gray matter (GM) volumes, cortical thickness (CT) and surface area (SA) are all reduced in schizophrenia (1, 2); these abnormalities are present in the early stages of clinical illness and in children at risk (3–5). Autism is also associated with alterations of cortical GM structure, including increased CT and SA (6–8). Cortical GM abnormalites are present in attention deficit-hyperactivity disorder (9), bipolar disorder (10), and conduct disorders (11) and major depression (12). In addition, cognitive ability in infants, children and adults is associated with individual differences in regional and cortical GM volumes, CT, and SA (13–18). While the development of cortical GM structure in older childhood, adolescence and adulthood has been well studied (19, 20), little is known about how cortical structure develops in early childhood, a period of rapid cognitive development and risk for neuropsychiatric disorders. Further, it is not known when in development individual differences in cortical GM structure are established. This information is critical for understanding the timing of abnormal cortical development associated with neuropsychiatric disorders and suboptimal cognitive development, as well as for identifying early imaging biomarkers of risk.
We studied two cohorts of children imaged longitudinally from birth: a cohort of 252 term-born, typically developing singleton children, and a replication sample of 182 twins in which one twin from each pair was included in analyses. Brain development in twins is similar to that of singletons except in the period right after birth (21, 22). We focused on cortical GM and WM, and global and regional CT and SA, which have been associated with cognitive development and psychiatric illness. We hypothesized that a large portion of individual variation in these components of cortical structure would present be by 1 year, after the period of rapid postnatal brain growth.
Methods
Participants
Participants were part of the University of North Carolina (UNC) Early Brain Development Study, approved by the Institutional Review Boards of UNC and Duke University. Mothers of singletons and twins were recruited at the prenatal diagnostic clinics of UNC Hospitals and Duke University Medical Center, as well as by local advertising. Informed consent was obtained on enrollment from each mother and from a parent at each postnatal imaging visit. Exclusion at enrollment included major maternal medical or psychiatric illness, substance use during pregnancy, or abnormalities on prenatal ultrasound. Exclusions for this analysis included gestational age at birth less than 37 weeks for singletons, less than 33 weeks for twins, neonatal intensive care unit stay greater than 24 hours, abnormality on MRI other than a minor subdural, subarachnoid, or other bleed or other minor abnormality which is common in the neonatal period (23), major medical/surgical illness including head injury or seizures, neurodevelopmental disorders including autism, attention deficit - hyperactivity disorder, or learning disorders, assessed by parent report and medical record review. Only one child from a set of singleton siblings or twins were included; the sibling with the greatest number of successful scans was included, and randomly chosen if each sibling had the same number of scans. Of the 252 singleton neonates, 56 had a minor subdural or subarachnoid hemorrhage, 1 had a tiny periventricular blood spot, and 1 had a small parenchymal bleed. Of the 182 twin neonates, 11 had a minor subdural or subarachnoid hemorrhage and 1 had a tiny germinal matric hemorrhage.
Image acquisition
All MRIs were acquired at UNC using either a Siemens Allegra head-only 3T scanner or a Siemens TIM Trio 3T scanner, which replaced the Allegra in 2011 (Siemens Medical System, Inc., Erlangen, Germany). Infants were scanned during natural sleep after being fitted with earplugs and secured using a vacuum-fixed immobilization device after birth, and at ages 1 and 2 years. At 4 and 6 years, children were scanned awake watching a movie after being trained in a mock scanner. Scanner sequences are detailed in Supplemental Methods. We have previously shown that scanner platform, but not scan sequence within the platform, is significantly related to neonate GM volume (24); therefore scanner is used as a covariate in analyses.
Image analysis
T1 and T2 weighted images were rated for motion artifacts on a scale of 1 to 4; images with a rating of 4 were excluded if artifacts were present in more than a few slices. Representative images and rating details are presented in Supplemental Figure 1.
Neonatal global tissue volumes were determined using an atlas-based expectation-maximization segmentation algorithm based on both T1 and T2 weighted images specifically adapted to the neonate brain (25). Tissues were automatically segmented into GM, white matter (WM), and cerebrospinal fluid (CSF), and cortical tissue volumes were derived from a 28 region parcellation of the cerebrum achieved by nonlinear warping of a parcellation atlas template as previously described (26).
Structural analysis of scans at ages 1 through 6 years was performed in several steps. Scans were corrected for intensity inhomogeneity via N4 (27), co-registered into pediatric MNI reference space (28), and initial brain masks extracted (all steps via AutoSeg 3.10) (29). Final brain masks were determined via multi-atlas (majority vote) based segmentation using a multi-atlas database with 14 individual atlases. Each mask was visually checked for accuracy, manually edited, then used to skull strip the T1 weighted image. Global tissue and regional volumetric results for 1, 2, 4 and 6 year scans were obtained by atlas-based expectation-maximization segmentation (30) based on T1 weighted images in AutoSeg 3.10. Intracranial volume (ICV) was calculated for all subjects (ICV = GM+WM+CSF) and cortical tissue volumes were determined with an 83-region subdivision (31). All segmentations were visually checked and cases that failed quality control (QC) were removed from the analysis.
CT and SA measures for 1-6 year old datasets were obtained via a CIVET v2.1 workflow (32, 33) adapted for pediatric data using an age-specific atlas (http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET). Neonates were not included as the processing pipeline is not currently optimized for neonatal scans. CIVET includes cortical WM surface reconstruction, calibration of image intensities, local Laplacian based gray matter/pial surface warp, CT and local SA extraction, surface registration using cortical surface features, and extraction of regional measurements. SA was measured at the mid-cortical surface averaged from the white and pial surfaces. Both white and pial surfaces underwent visual QC with surface cut overlay. Cases that failed QC were removed from the analysis. Regional CT and SA measures were averaged for all vertices within each brain region using a geometric sulcal/gyral based parcellation of 148 regions (34) and summed over all vertices of each region for a total regional surface area.
Statistical Analysis
All analysis was done in R version 3.5.2. Alpha=0.05 level of significance was used for all significance testing, and a Benjamini-Hochberg false discovery rate correction (35) was used for all regional analyses of CT or SA. A repeated measures ANOVA showed a significant difference in motion ratings between ages in each cohort (Supplemental Tables 1, 2, 3); motion score was included as a covariate in all further analyses. All primary analyses were conducted first in the singleton cohort (n = 252) and replicated in the twin cohort (n = 182; only one twin from each pair included in analyses).
To quantify associations between measures of cortical structure at earlier ages and at 6 years, separate linear models were used to generate residual GM, WM, CT, and SA measures for neonate, 1-, 2-, and 4-year scans adjusted for motion rating, scanner and age at scan (in days). The GM, WM, CT, and SA residuals from these models were used as covariates in a linear model to estimate these same cortical measures at age 6. Other covariates in these models included birthweight, gestational age at birth, sex, mother’s education (in years), height at age 6, weight at 6, scanner used for the age 6 scan, age in days for the age 6 scan, and motion score for the age 6 scan. Bootstrapped confidence intervals for the partial r-squared were constructed using 1,000 bootstrap samples. Regional CT and SA at ages 1, 2, and 4 were used to estimate regional CT and SA age 6 using a similar approach. Average regional CT across all subjects at ages 1, 2, and 4 was used to estimate average regional CT at age 6 in separate univariate linear models.
Sex differences were tested using a linear model controlling for scanner, age at scan, motion scores, and brain size (cube root of cortical tissue volume [WM + GM] for CT and cortical tissue volume [WM + GM] for SA). Sex differences across development were tested using linear mixed effects models comparing trajectories for males and females. For cortical GM and WM, four different models were fit using each of the consecutive pairs of measurements (neonate – 1 year, 1 – 2 years, 2 – 4 years, 4 – 6 years); for CT and SA, three were fit (1 – 2 years, 2 – 4 years, 4 – 6 years). In each model, age, sex, scanner, motion, gestational age at birth, as well as the interaction between sex and age were included as fixed effects and a random intercept was included for each child.
The trajectories for GM and WM were compared with linear mixed effect models in which age, volume type, sex, scanner, motion, gestational age at birth, as well as the interaction between volume type and age were included as fixed effects and a random intercept was included for each child. For each region the null hypothesis that there is no difference between CT and SA at age one and age six was tested using a linear mixed effects model that controlled for gestational age at birth, scanner, and motion. P-values were adjusted using an FDR correction to account for the 148 cortical regions.
RESULTS
The study sample included 252 singletons and 182 twins; subject characteristics are presented in Table 1. All subjects had at least one successful scan between birth; the percent of subjects with more than one scans were: 2 scans (% of singletons/% of twins: 24%/19%), 3 scans (16%/19%), 4 scans (13%/18%), 5 scans (6%/11%). Sample sizes for each cortical measure across ages can be found in Supplemental Table 1. Subject retention is presented in Supplemental Table 4.
Table 1.
Sample Characteristics.
| Cohort 1 (Singletons) | Cohort 2 (Twins) | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | P-Value | |
| Birthweight (grams) | 252 | 3458.3 | 414.32 | 182 | 2616.11 | 351.65 | 1.18E-75 |
| Gestational age at birth (days) | 252 | 278.1 | 7.21 | 182 | 257.81 | 7.96 | 4.32E-90 |
| Mother’s education (years)* | 251 | 16.28 | 2.97 | 182 | 15.17 | 3.74 | 1.05E-03 |
| Father’s education (years)* | 248 | 15.95 | 3.37 | 176 | 14.98 | 3.99 | 0.01 |
| Total household income ($)* | 236 | 82444.53 | 105282.9 | 171 | 75901.16 | 60603.9 | 0.43 |
| Age at scan, neonate (days) | 181 | 21.13 | 9.35 | 157 | 33.15 | 15.76 | 4.54E-15 |
| Age at scan, 1 year (days) | 111 | 379.87 | 21.44 | 109 | 400.28 | 28.07 | 6.77E-09 |
| Age at scan, 2 year (days) | 70 | 738.84 | 19.7 | 80 | 764.86 | 28.22 | 7.36E-10 |
| Age at scan, 4 year (days) | 92 | 1480.05 | 34.4 | 56 | 1505.96 | 69.55 | 0.01 |
| Age at scan, 6 year (days) | 90 | 2210.52 | 34.04 | 56 | 2228 | 52.6 | 0.03 |
| N | Allegra | Trio | N | Allegra | Trio | ||
| Scanner, Neonate | 181 | 151 | 30 | 157 | 123 | 34 | 0.29 |
| Scanner, Year 1 | 111 | 87 | 24 | 109 | 90 | 19 | 0.54 |
| Scanner, Year 2 | 70 | 56 | 14 | 80 | 51 | 29 | 0.04 |
| Scanner, Year 4 | 92 | 44 | 48 | 56 | 9 | 47 | 1.91E-04 |
| Scanner, Year 6 | 90 | 32 | 58 | 56 | 7 | 49 | 4.11E-03 |
| N | Male | Female | N | Male | Female | ||
| Sex | 252 | 121 | 131 | 182 | 99 | 83 | 0.22 |
| White | Black | Other | White | Black | Other | ||
| Mother’s Ethnicity (N) | 207 | 39 | 6 | 143 | 33 | 6 | 0.63 |
| Mother’s Ethnicity (%) | 82.14 | 15.48 | 2.38 | 78.57 | 18.13 | 3.3 | |
| Father’s Ethnicity (N) | 197 | 45 | 8 | 133 | 37 | 9 | 0.46 |
| Father’s Ethnicity (%) | 78.8 | 18 | 3.2 | 74.3 | 20.67 | 5.03 | |
| N | Yes | No | N | Yes | No | ||
| Mother smoking during pregnancy | 252 | 9 | 243 | 182 | 11 | 171 | 0.33 |
(indicates assessed at time of enrollment).
Cortical gray and white matter development
In singletons, there is rapid growth of cortical GM (149% increase) and WM (42% increase) volume from birth to age 1, with more gradual growth of cortical GM (25% increase) and WM (43% increase) between ages 1 and 6 years in each cohort (Figure 1, Supplemental Table 5). Cortical GM increases significantly faster than WM between each age studied except between 4 and 6 years (Supplemental Table 6).
Figure 1.
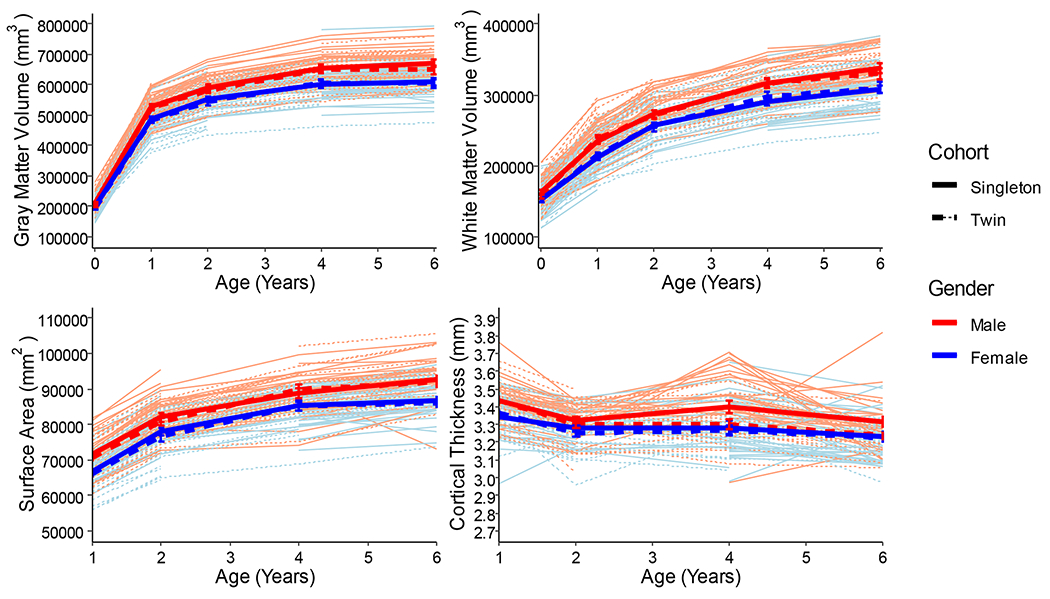
Individual spaghetti plots of growth in cortical GM and WM volume (birth to 6 years), and total SA and average CT (1 to 6 years). Error bars represent standard error.
Average CT decreases 3.5% from age 1 to 6 years in the singleton cohort (Figure 1, Supplemental Table 5). CT decreased significantly in 54 of 148 cortical regions (range 2.0-16.7%), and increased significantly in 33 regions (2.1-6%) between ages 1 and 6 years (Figure 2, Supplemental Table 7). Regions of greatest thinning between 1 and 6 years were frontal, orbital frontal, parietal, inferior temporal and anterior cingulate regions. Regions of significant CT increase include sensori-motor regions, including bilateral central, paracentral and calcarine, and occipital pole regions.
Figure 2.
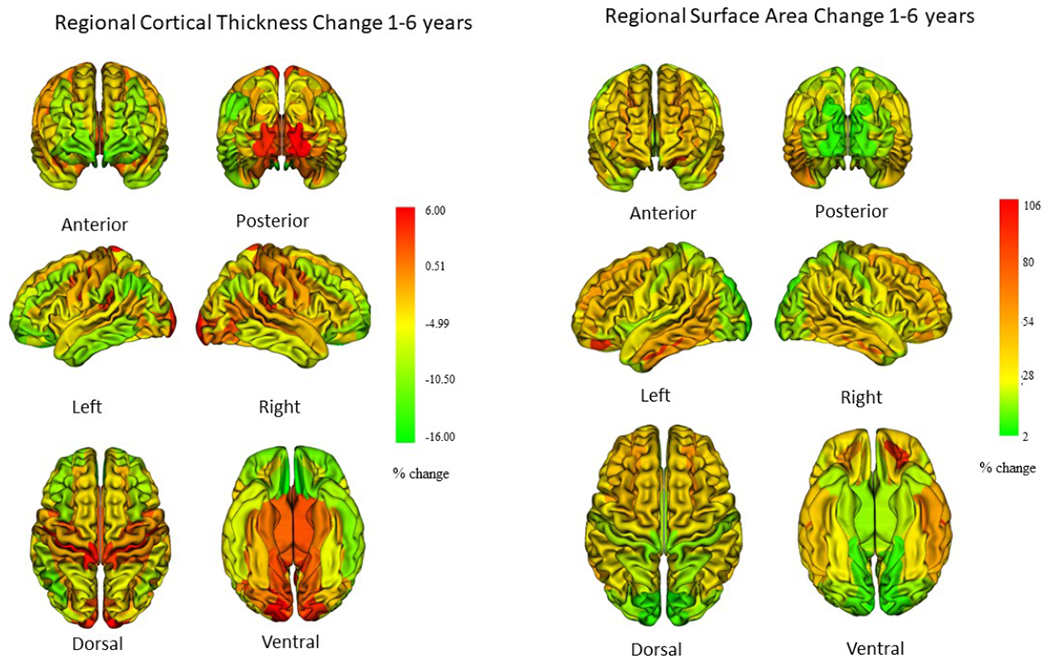
Change in regional cortical thickness and surface area from age 1 to 6 years. Change in cortical thickness tends to be modest in this age range, with most regions, especially association cortices undergoing thinning, while sensorimotor regions experience increases in cortical thickness (data in Supplemental Table 7). Most cortical regions experience surface area expansion (data in Supplemental Table 8).
Total SA increases in a pattern similar to that of GM volume, increasing 29% from 1 to 6 years in singletons (Figure 1; Supplemental Table 5). Almost all cortical regions experienced significant expansion between age 1 and 6 years in both singleton and twin cohorts (Figure 2; Supplemental Table 8). Regions of high expansion include frontal and temporal regions, regions of relatively lower expansion include sensorimotor regions.
Individual variation at age 6 accounted for by variation at earlier ages
In singletons, neonatal cortical GM volume explains 46% of the variance of cortical GM volume at age 6 (Figure 3, Table 2). Cortical GM volumes at ages 1, 2, and 4 each explain 83-87% of the variance in cortical GM at age 6. In contrast, only 25% of the variance of WM volume at age 6 is explained by neonatal cortical WM volume; the variance explained increases to 55% by age one and to 87% at age 4 (Figure 4, Table 2). This indicates that individual variation of GM volume at age 6 years is determined earlier in childhood than that of WM volume, consistent with the faster growth trajectory of GM volume compared to WM volume.
Figure 3.
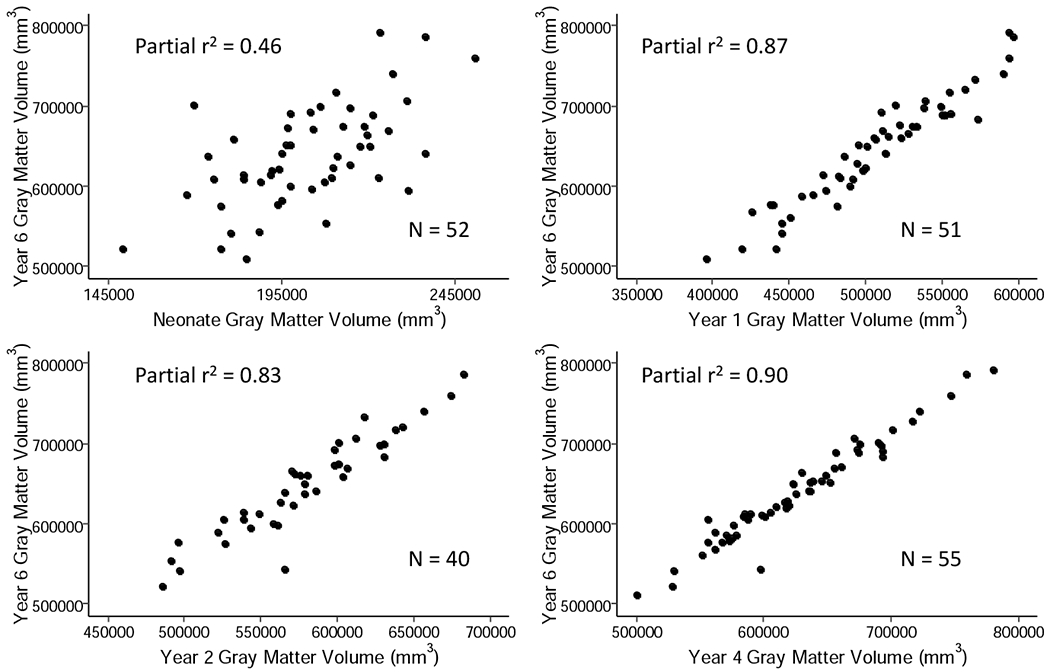
Relationship of cortical GM volume after birth and at ages 1, 2, and 4 years with cortical GM volume at age 6 years. Partial r2 correlations were determined using a linear model; covariates included were birthweight, gestational age at birth, gender, mother’s education, height at six, weight at six, motion score at six, scanner and age at scan.
Table 2.
Variance in cortical structure at age 6 accounted for by earlier ages.
| Singletons | Twins | ||||||
|---|---|---|---|---|---|---|---|
| r2 | CI | N | r2 | CI | N | ||
| Year 0 | WM | 0.25 | (0.05, 0.53) | 52 | 0.27 | (0.05, 0.58) | 45 |
| GM | 0.46 | (0.26, 0.66) | 52 | 0.45 | (0.22, 0.69) | 45 | |
| Year 1 | WM | 0.55 | (0.30, 0.76) | 51 | 0.61 | (0.41, 0.77) | 48 |
| GM | 0.87 | (0.78, 0.94) | 51 | 0.81 | (0.64, 0.93) | 48 | |
| SA | 0.83 | (0.72, 0.91) | 49 | 0.73 | (0.52, 0.88) | 46 | |
| CT | 0.13 | (0.00, 0.40) | 49 | 0.37 | (0.08, 0.76) | 46 | |
| Year 2 | WM | 0.60 | (0.31, 0.82) | 40 | 0.70 | (0.49, 0.87) | 40 |
| GM | 0.83 | (0.67, 0.94) | 40 | 0.83 | (0.65, 0.93) | 40 | |
| SA | 0.91 | (0.82, 0.97) | 40 | 0.78 | (0.53, 0.90) | 38 | |
| CT | 0.45 | (0.13, 0.75) | 40 | 0.59 | (0.31, 0.85) | 38 | |
| Year 4 | WM | 0.87 | (0.74, 0.96) | 55 | 0.93 | (0.85, 0.98) | 32 |
| GM | 0.90 | (0.76, 0.97) | 55 | 0.97 | (0.93, 0.99) | 32 | |
| SA | 0.66 | (0.41, 0.92) | 52 | 0.93 | (0.85, 0.98) | 30 | |
| CT | 0.00 | (0.00, 0.16) | 52 | 0.55 | (0.20, 0.86) | 30 | |
Separate models were used to generate residual cortical GM, WM, average CT, and total SA for neonate, 1, 2, and 4 year scans adjusting for motion rating, scanner and age at scan (in days). Residual cortical measures were then entered as independent variables in a linear model to estimate cortical GM and WM at age 6. Covariates in these models were birthweight, gestational age at birth, sex, mother’s education (in years), height at 6, weight at 6, scanner for the age 6 scan, age in days for the age 6 scan, and motion score for the age 6 scan. A confidence interval for the partial r-squared was determined from 1,000 bootstrap samples using the percentile method.
Figure 4.
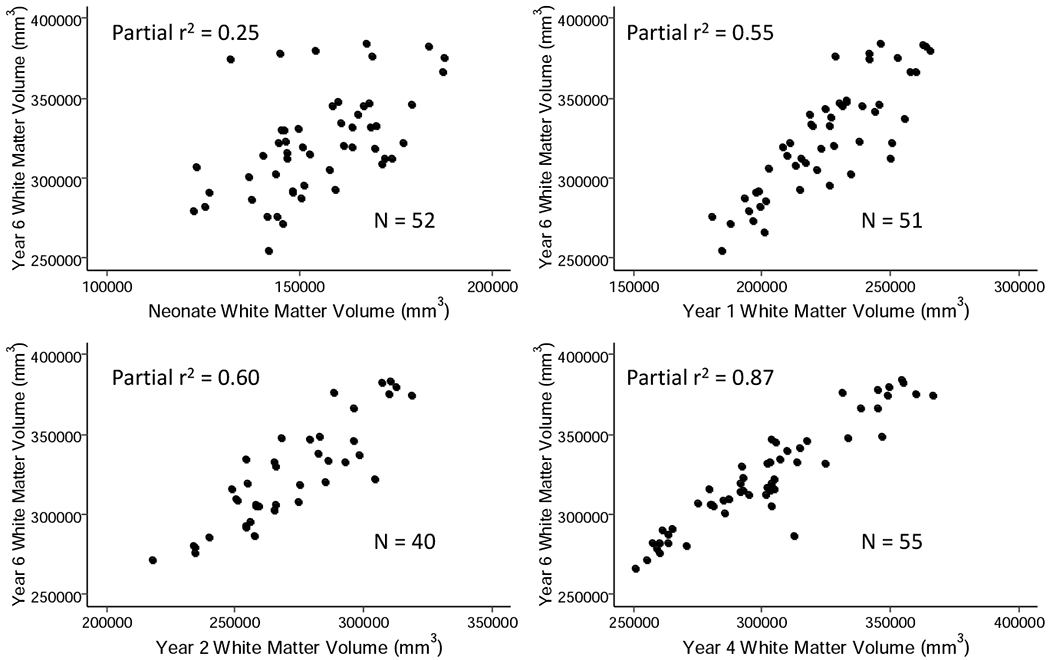
Relationship of cortical WM volume after birth and at ages 1, 2, and 4 years with cortical WM volume at age 6 years. Partial r2 correlations were determined using a linear model; covariates included were birthweight, gestational age at birth, gender, mother’s education, height at six, weight at six, motion score at six, scanner and age at scan.
The variance of whole brain average CT at age 6 was significantly explained by CT at age 2 years (45%) in singletons, but not at other ages (Table 2). There were a few regions of significant variance explanation, most at age 2 years (Supplemental Table 9). This lack of variance explained by earlier CT is likely due to the small magnitude of change from 1 to 6 years in relation to variation of CT at each age across subjects. However, variance of the group mean of CT across the 148 cortical regions at 6 years is highly explained by group mean in regional CT at ages 1, 2, and 4 years, (98%; Supplemental Table 10; Figure 5), indicating that overall regional heterogeneity is well established by age 1.
Figure 5.
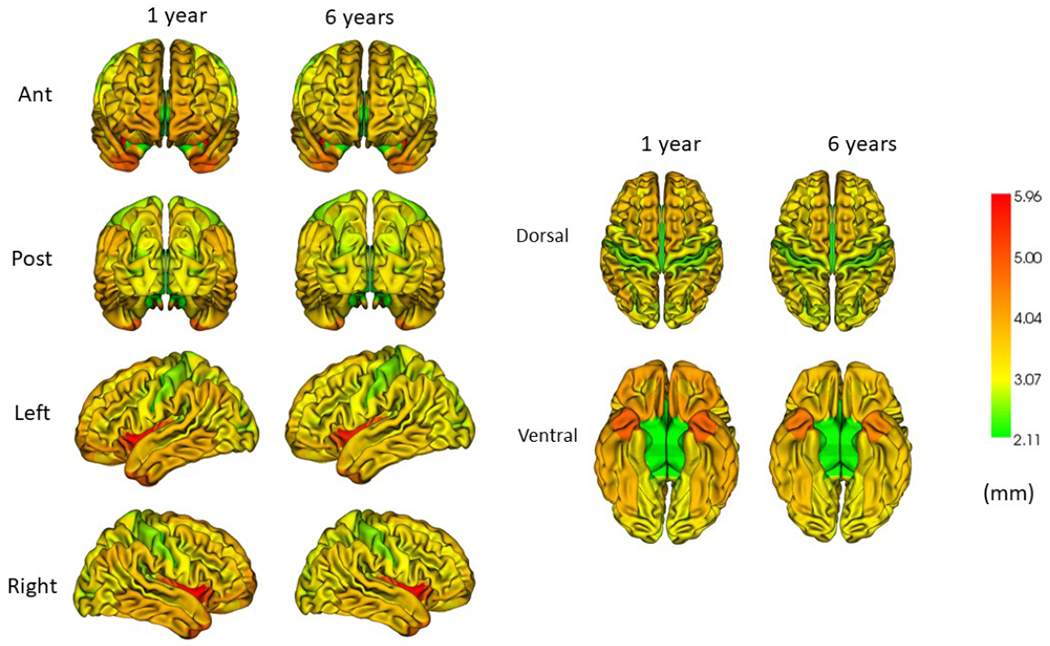
Regional cortical thickness at ages 1 and 6 years. Note that overall patterns of regional thickness present at age 6 are present at age 1 year (Data in Supplemental Table 7).
Individual variation in total SA at age 6 is highly explained by total SA at age 1 (83%), age 2 (91%), with somewhat weaker associations at age 4 (66%; Table 2). Variation in regional SA at age 6 is also highly explained by previous regional SA (Figure 6, Supplemental Table 11).
Figure 6.
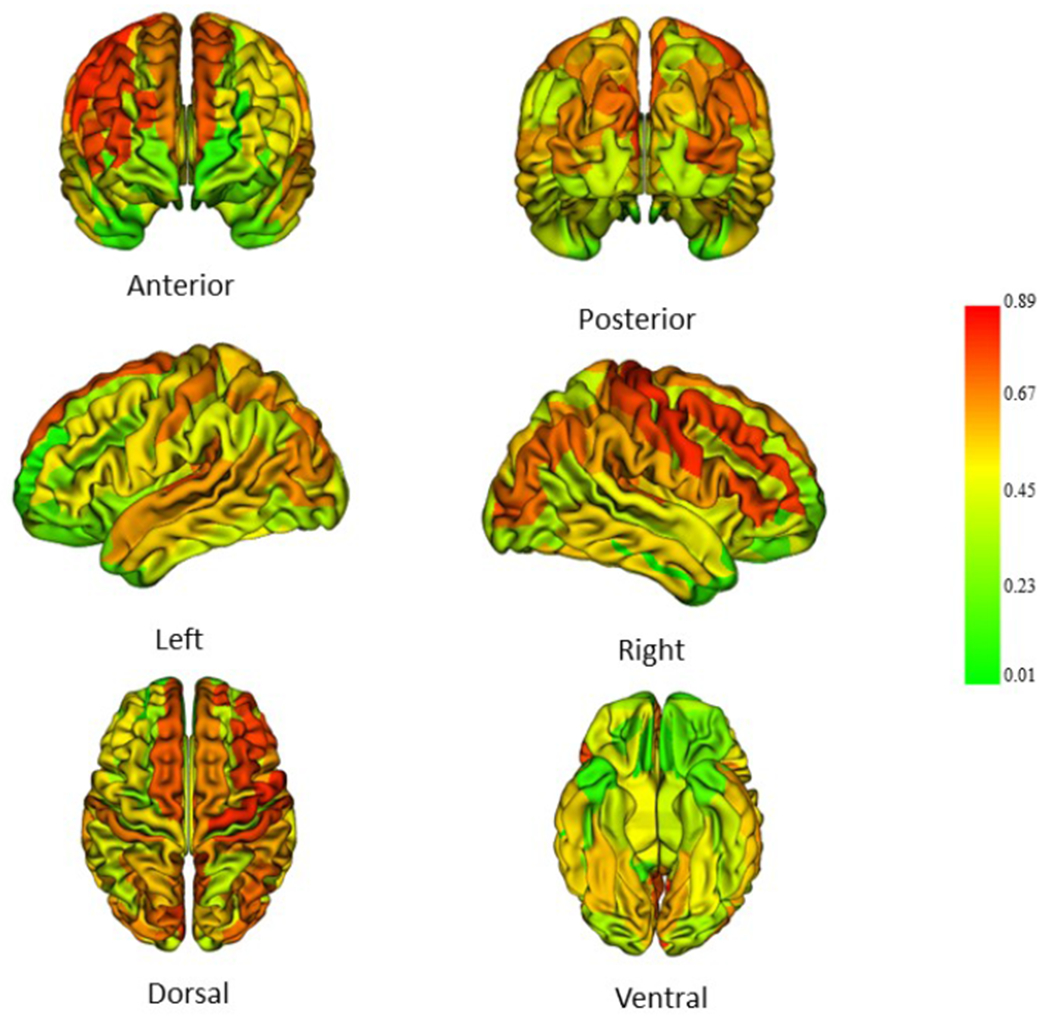
Regional SA at age 1 and its relationship to regional SA at age 6. Partial r2 values were determined using a linear model; covariates included were birthweight, gestational age at birth, gender, mother’s education, height at six, weight at six, motion score at six, scanner and age at scan (Data in Supplemental Table 11).
Sex Differences
Males have larger cortical GM and WM volumes than females at each age, unadjusted for brain size, (Supplemental Table 12), though there are no significant sex differences in growth trajectories except in the first year of life, when growth of cortical GM and WM was accelerated in males compared to females (Supplemental Table 13). There were no sex differences in average CT at any age or in growth trajectory when controlling for sex differences in brain size (Supplemental Tables 12, 13), nor were there sex differences in regional CT at any age (Supplemental Table 7). Total SA, unadjusted for brain size, was larger in males than females (Supplemental Table 12), though the growth trajectory was not significantly different (Supplemental Table 13). Regional SA was larger in males, but not significantly different when controlling for overall brain size (Supplemental Table 8). Variance of 6-year cortical GM and WM volume, average CT, and total SA explained by values at earlier ages was similar in males and females (Supplemental Table 14).
Birthweight, gestational age at birth, and maternal education
To explore the contribution of these variables to cortical structure, we fit a linear model for using birthweight, gestational age at birth, maternal education (a proxy for socio-economic status), scanner, age at scan, and motion as covariates. In singletons, birthweight was significantly associated with cortical GM and WM volumes at all ages (Supplemental Table 15). Birthweight was also significantly associated with average CT at 2 and 4 years, and with total SA at 1 and 6 years (Supplemental Table 16). Birthweight was marginally significantly associated with mean SA at age 2 and 4 (p = 0.06). Gestational age at birth was significantly associated with cortical GM and WM volume at birth, but not at any other age; it was also not significantly associated with average CT or total SA at any age. Birthweight associations did not hold after controlling for ICV, but remained significant after controlling for height (Supplemental Tables 17, 18, 19, 20). This suggests that birthweight is a biomarker of subsequent cortical development independent of gestational age at birth. Maternal education was only significantly associated with cortical WM volume at ages 1, and 6; there were no significant relationships with GM volume, mean CT, or total SA at any age.
Twin replication sample
Analysis of the twin replication sample revealed very similar trajectories of cortical GM, cortical WM, average CT and total SA development (Figure 1), as well as the magnitude and timing of the establishment of individual differences in these measures (Table 2). Sex differences (Supplemental Table 21) and developmental patterns of regional CT and SA were also very similar (Supplemental Tables 22, 23, 24).
Sensitivity Analyses
To determine if the presence of a minor bleed on the neonatal scan influence the main results, we re-analyzed the sample excluding these bleeds (Supplemental Table 25); partial r2 values were similar to the main analysis. A sensitivity analysis was also performed on a subsample of twins excluding subjects born before 37 weeks, similar to the singleton cohort. While the sample was smaller, partial r2 values were similar to the larger twin sample (Supplemental Table 26). To determine the contribution of scan motion, we analyzed a sample with motion ≤2.5, results were similar to the main analysis (Supplemental Table 27). We also compared GM and WM volumes, SA and CT derived from Allegra and Trio scanners; there were no significant differences except for CT at ages 4 and 6 (Supplemental Table 28; Supplemental Figure 2).
Discussion
In the present report, we extend over a decade of work studying early postnatal brain development to reveal within-subject longitudinal trajectories of cortical maturation from birth through early childhood. We found that individual differences in cortical GM volume and total SA at age 6 are largely in place by the end of the first year, a period of rapid postnatal cortical GM growth, with GM volume and SA at age 1 accounting for 83-87% of the variance in the same measures five years later at age 6. Cortical GM volumes decrease after late childhood (1012 years) and are stable or only slightly increased between ages 6 and 12 (19), so it is likely that variance explained at age 6 will be similar to that observed later in development; though a longitudinal study through adolescence is needed to confirm this. Neonatal images were processed using a different pipeline than images in older children; this may be partially responsible for the lower correlation between neonate and 6 year GM and WM values.
Average CT decreases slightly from age 1 to 6 years, generally consistent with a previous cross-sectional study in this age range (36), though we found several cortical regions that experienced a significant increase in CT, especially sensorimotor regions. Interestingly, these areas tended to be regions of lower SA expansion. Overall, the pattern of differential growth trajectories of sensorimotor compared to higher-order association cortices described in older childhood and adolescence (33, 37) is apparent in early childhood as well. It is not clear why these sensorimotor regions would have different trajectory of cortical thickness growth in early childhood, though there are several possibilities, including differences in the composition of astrocytes, microglia and neurons (38), genetic architecture (39), and critical period timing (33). Regional heterogeneity of CT evident at 1 year persists though age 6 years. Using a different methodology to determine CT in neonates and infants, we previously found that CT increases an average of 31% in the first year of life, with little change in the second year to reach 97% of adult values at age 2 years and that adult patterns of regional heterogeneity of CT were largely present at birth (40). A recent study in the first 2 years of life found that average CT peaks about 14 months of age (41).
Cortical WM undergoes a relatively more gradual postnatal expansion compared to cortical GM. This differential trajectory is present before birth, as fetal imaging finds that WM expands linearly from 20 weeks while GM expands after 30 weeks (42). Thus, cortical GM undergoes rapid development from about 30 weeks gestational age until age 1 year, with a more gradual increase until adolescence, while WM volume expands more gradually from 20 weeks gestational age until late adolescence (19, 20, 43). This mirrors a body of work demonstrating that the expression profiles of genes involved in regulating neurogenesis, synaptogenesis, and dendritic arborization peak during gestation and plateau during early infancy (44–46), while myelination occurs rapidly in the first year of life (47), but continues to be modified by experience thereafter (48).
These findings have important implications for the timing and nature of early interventions to normalize trajectories of brain growth. Institutionalized children placed in high quality foster care before the age of 2 years have better cognitive outcomes than those placed after 2 years, suggesting a possible sensitive period for cognitive development (49). Interestingly, cortical GM is reduced at 8-11 years in these children, regardless of foster care, while foster care was associated with normalized WM volumes (50). These results are consistent with our finding that cortical WM volume has a slower developmental trajectory compared to GM, and that a large portion of individual differences in cortical GM are determined in the first year of life, while individual differences in WM volume are determined later in childhood. This suggests that interventions aimed at regulating cortical GM and SA growth will likely be most effective if implemented within the first year of life, whereas interventions targeted to WM may have a wider window of treatment.
Pre- and perinatal environmental factors have long-lasting effects on brain structure. Premature birth is associated with reduced GM and WM volumes that are evident in early adulthood (52, 52), with GM reductions linked to psychiatric symptoms in late adolescence (53). Greater birthweight in term born children, a proxy for an optimal prenatal environment, is associated with larger SA, but not CT, later in life (54, 55). Lower birthweight is also associated with smaller GM and WM volumes in late life (56). In our sample, larger birthweight was associated with larger GM and WM volumes, and larger SA at most ages, and with increased CT at ages 2 and 4, generally consistent with previous studies, indicating that this association is present very early in childhood and persists through development. Overall, this suggests that birthweight is an indicator of prenatal factors that have long lasting influences on cortical morphology. _In neonates, gestational age at birth and birthweight, though highly correlated, have independent influences on GM, WM and SA, though birthweight was not associated with neonatal CT (57, 58). We did not detect effects of gestational age at birth on GM, WM, CT or SA beyond the neonatal time point, suggesting that birthweight provides unique information for later brain development, at least in the case of normative variations in gestational age at birth; extreme preterm birth has been shown to impact brain volumes in childhood and adolescence (51, 59). We found that the relationship between birthweight and cortical structure held when controlling for height, suggesting that the association is not directly one of larger body size being carried through early childhood development. We also found few associations between maternal education level and brain structure in offspring, limited to cortical WM volume at ages 1 and 6 years; this may be a random finding or due to a small effect size. These findings are somewhat in line with studies demonstrating the effects of socioeconomic status on childhood brain structure, with some evidence that effects become more pronounced with age (60–63). We previously found that the effect of maternal education on neonatal GM and WM was mediated in part by birthweight (57), so maternal education effects on early childhood brain volumes are likely mediated to some extent by its influence on birthweight.
This study demonstrates that the first year of life marks a critical period for postnatal cortical development that is likely to have lasting impacts on human brain and behavioral development. Importantly, our results have implications for early risk detection. Recent work has suggested it is possible to predict diagnostic outcomes in infants at risk for autism by age 2 using cortical features from MRI scans in the first year of life (31). Our findings highlight the potential predictive utility of imaging biomarkers of infant cortical structure for even later-emerging neurodevelopmental disorders associated with alterations in GM in late childhood. Future work should seek to identify genetic and environmental contributions to individual variation in cortical development in the first year of life, as these factors likely set the stage for later developmental refinement.
Supplementary Material
Acknowledgements
We thank the members of the Gilmore and Styner labs. This work is supported by NIH grants MH064065, MH070890, and HD053000 to JHG; MH091645 and HD079124 to MS; HD40127 to JBG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Haijma SV; Van Haren N; Cahn W; Koolschijn PC; Hulshoff Pol HE; Kahn RS Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophrenia Bulletin 39, 1129–1138, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Erp TGM; Walton E; Hibar DP; Schmaal L; Jiang W; Glahn DC et al. Cortical brain abnormalities in 4474 Individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta-analysis (ENIGMA) consortium. Biological Psychiatry 84, 644–654, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong Q; Lui S; Sweeney JA A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. The American Journal of Psychiatry 173, 232–243, (2016). [DOI] [PubMed] [Google Scholar]
- 4.Thermenos HW; Keshavan MS; Juelich RJ; Molokotos E; Whitfield-Gabrieli S; Brent BK et al. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. American journal of medical genetics. Part B, Neuropsychiatric 162b, 604–635, (2013). [DOI] [PubMed] [Google Scholar]
- 5.Gilmore JH; Kang C; Evans DD; Wolfe HM; Smith JK; Lieberman JA et al. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. The American Journal of Psychiatry 167, 1083–1091, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagnozzi AM; Conti E; Calderoni S; Fripp J; Rose SE A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. International Journal of Developmental Neuroscience 71, 68–82, (2018). [DOI] [PubMed] [Google Scholar]
- 7.Khundrakpam BS; Lewis JD; Kostopoulos P; Carbonell F; Evans AC Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: A large-scale MRI study. Cerebral Cortex 27, 1721–1731, (2017). [DOI] [PubMed] [Google Scholar]
- 8.van Rooij D; Anagnostou E; Arango C; Auzias G; Behrmann M; Busatto GF et al. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. The American Journal of Psychiatry 175, 359–369, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenz AA; Villemonteix T; Massat I Structural and functional neuroimaging in attention-deficit/hyperactivity disorder. Developmental Medicine and Child Neurology, 61, 399–405, (2018). [DOI] [PubMed] [Google Scholar]
- 10.Hanford LC; Nazarov A; Hall GB; Sassi RB Cortical thickness in bipolar disorder: a systematic review. Bipolar Disorders 18, 4–18, (2016). [DOI] [PubMed] [Google Scholar]
- 11.Rogers JC; De Brito SA Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry 73, 64–72, (2016). [DOI] [PubMed] [Google Scholar]
- 12.Li Q; Zhao Y; Chen Z; Long J; Dai J; Huang X et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depression. Neuropsychopharmacology 45: 703–712, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narr KL; Woods RP; Thompson PM; Szeszko P; Robinson D; Dimtcheva T et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex 17, 2163–2171, (2007). [DOI] [PubMed] [Google Scholar]
- 14.Shaw P; Greenstein D; Lerch J; Clasen L; Lenroot R; Gogtay N et al. Intellectual ability and cortical development in children and adolescents. Nature 440, 676–679, (2006). [DOI] [PubMed] [Google Scholar]
- 15.Karama S; Ad-Dab’bagh Y; Haier RJ; Deary IJ; Lyttelton OC Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence 37, 145–155, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidlitz J; Vasa F; Shinn M; Romero-Garcia R; Whitaker KJ; Vertes PE et al. Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron 97, 231–247, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spann MN; Serino D; Bansal R; Hao X; Nati G Morphological features of the neonatal brain following exposure to regional anesthesia during labor and delivery. Magnetic Resonance Imaging 33, 213–221, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girault JB; Cornea E; Goldman BD; Jha S; Murphy V; Li G et al. Cortical structure and cognition in infants and toddlers. Cerebral Cortex 30, 786–800, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills KL; Goddings AL; Herting MM; Meuwese R; Blakemore SJ; Crone EA et al. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage 141, 273–281, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamnes CK; Herting MM; Goddings AL; Meuwese R; Blakemore SJ; Dahl RE et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. The Journal of Neuroscience 37, 3402–3412, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordaz SJ; Lenroot RK; Wallace GL; Clasen LS; Blumenthal JD; Schmitt JE et al. Are there differences in brain morphology between twins and unrelated singletons? A pediatric MRI study. Genes, Brain and Behavior 9, 288–295, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knickmeyer RC; Kang C; Woolson S; Smith JK; Hamer RM; Lin W et al. Twin-singleton differences in neonatal brain structure. Twin Research and Human Genetics 14, 268–276, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Looney CB; Smith JK; Merck LH; Wolfe HM; Chescheir NC; Hamer RM et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology 242, 535–541, (2007). [DOI] [PubMed] [Google Scholar]
- 24.Knickmeyer RC; Xia K; Lu Z; Ahn M; Jha SC; Zou F et al. Impact of demographic and obstetric factors on infant brain volumes: a population science study. Cerebral Cortex 27, 5616–5625, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prastawa M; Gilmore JH; Lin W; Gerig G Automatic segmentation of MR images of the developing newborn brain. Medical Image Analysis 9, 457–466, (2005). [DOI] [PubMed] [Google Scholar]
- 26.Gilmore JH; Lin W; Prastawa MW; Looney CB; Vetsa YS; Knickmeyer RC et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience 27, 1255–1260 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tustison NJ; Avants BB; Cook PA; Zheng Y; Egan A; Yushkevich PA et al. N4ITK: improved N3 bias correction. IEEE Transactions on Medical Imaging 29, 1310–1320, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonov V; Evans AC; Botteron K; Almli CR; McKinstry RC; Collins DL et al. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 54, 313–327, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J; Vachet C; Rumple A; Gouttard S; Ouziel C; Perrot E et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Frontiers in Neuroinformatics 8, 7, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH; Fonov VS; Dietrich C; Vachet C; Hazlett HC; Smith RG et al. Adaptive prior probability and spatial temporal intensity change estimation for segmentation of the one-year-old human brain. Journal of Neuroscience Methods 212, 43–55, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazlett HC; Gu H; Munsell BC; Kim SH; Styner M; Wolff JJ et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542, 348–351, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw P et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry 72, 191–197, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw P; Malek M; Watson B; Sharp W; Evans A; Greenstein D Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience 28, 3586–3594, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Destrieux C; Fischl B; Dale A; Halgren E Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1–15, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y; Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society 57, 289–300, (1995). [Google Scholar]
- 36.Remer J; Croteau-Chonka E; Dean DC 3rd; D’Arpino S; Dirks H; Whiley D et al. Quantifying cortical development in typically developing toddlers and young children, 1-6 years of age. NeuroImage 153, 246–261, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krongold M; Cooper C; Bray S Modular development of cortical gray matter across childhood and adolescence. Cerebral Cortex 27, 1125–1136, (2017). [DOI] [PubMed] [Google Scholar]
- 38.Shin J; French L; Xu T; Leonard G; Perron M; Pike GB et al. Cell-specific gene-expression profiles in cortical thickness in the human brain. Cerebral Cortex 28, 3267–3277, (2018). [DOI] [PubMed] [Google Scholar]
- 39.Grasby KL; Jahanshad N; Painter JN; Colodro-Conde L; Bralten J; Hibar DP et al. The genetic architecture of the human cerebral cortex. Science 367, 1340, (2020). [Google Scholar]
- 40.Lyall AE; Shi F; Geng X; Woolson S; Li G; Wang L et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex 25, 2204–2212, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F; Wang F; Lian C; Wu Z; Zhang H; Li T; Meng Y et al. Developmental topography of cortical thickness during infancy. Proceedings of the National Academy of Sciences of the United States of America 116, 15855–15860, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andescavage NN; du Plessis A; McCarter R; Serag A; Evangelou I; Vezina G et al. Complex trajectories of brain development in the healthy human fetus. Cerebral Cortex 27, 5274–5283, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilmore JH; Knickmeyer RC; Gao W Imaging structural and functional brain development in early childhood. Nature Reviews. Neuroscience 19, 123–137, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang HJ; Kawasawa YI; Cheng F; Zhu Y; Xu X; Li M et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pletikos M; Sousa AM; Sedmak G; Meyer KA; Zhu Y; Cheng F et al. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron 81, 321–332, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silbereis JC; Pochareddy S; Zhu Y; Li M; Sestan N The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brody BA; Kinney HC; Kloman AS; Gilles FH Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology & Experimental Neurology 46, 283–301, (1987). [DOI] [PubMed] [Google Scholar]
- 48.Fields RD A new mechanism of nervous system plasticity: activity-dependent myelination. Nature Reviews Neuroscience 16, 756–767, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson CA 3rd ; Zeanah CH; Fox NA; Marshall PJ; Smyke AT; Guthrie D Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science (New York, N.Y.) 318, 1937–1940, (2007). [DOI] [PubMed] [Google Scholar]
- 50.Sheridan MA; Fox NA; Zeanah CH; McLaughlin KA; Nelson CA 3rd. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America 109, 12927–12932, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Kieviet JF; Zoetebier L; van Elburg RM; Vermeulen RJ; Oosterlaan J Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Developmental Medicine and Child Neurology 54, 313–323, (2012). [DOI] [PubMed] [Google Scholar]
- 52.Nosarti C; Nam KW; Walshe M; Murray RM; Cuddy M; Rifkin L et al. Preterm birth and structural brain alterations in early adulthood. NeuroImage. Clinical 6, 180–191, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botellero VL; Skranes J; Bjuland KJ; Haberg AK; Lydersen S; Brubakk AM et al. A longitudinal study of associations between psychiatric symptoms and disorders and cerebral gray matter volumes in adolescents born very preterm. BMC Pediatrics 17, 45, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walhovd KB; Fjell AM; Giedd J; Dale AM; Brown TT Neurodevelopmental origins of lifespan changes in brain and cognition. Proceedings of the National Academy of Sciences of the United States of America 113, 9357–9362, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raznahan A; Greenstein D; Lee NR; Clasen LS; Giedd JN Prenatal growth in humans and postnatal brain maturation into late adolescence. Proceedings of the National Academy of Sciences of the United States of America 109, 11366–11371, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller M; Sigurdsson S; Kjartansson O; Gunnarsdottir I; Thorsdottir I; Harris TB et al. Late-life brain volume: a life-course approach. The AGES-Reykjavik study. Neurobiology of Aging 41, 86–92, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knickmeyer RC; Xia K; Lu Z; Ahn M; Jha SC; Zou F et al. Impact of demographic and obstetric factors on infant brain volumes: a population neuroscience study. Cerebral Cortex 27, 5616–5625, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jha SC; Xia K; Ahn M; Girault JB; Li G; Wang L et al. Environmental influences on infant cortical thickness and surface area. Cerebral Cortex. 29, 1139–1149, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padilla N; Alexandrou G; Blennow M; Lagercrantz H; Aden U Brain growth gains and losses in extremely preterm infants at term. Cerebral Cortex 25, 1897–1905, (2015). [DOI] [PubMed] [Google Scholar]
- 60.Noble KG; Houston SM; Brito NH; Bartsch H; Kan E; Kuperman JM et al. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience 18, 773–778, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanson JL; Hair N; Shen DG; Shi F; Gilmore JH; Wolfe BL et al. Family poverty affects the rate of human infant brain growth. PLoS One 8, e80954, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luby J; Belden A; Botteron K; Marrus N; Harms MP; Babb C et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics 167, 1135–1142, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spann MN; Bansal R; Hao X; Rosen T; Peterson B Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychology 26, 170–188, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


