Abstract
Platelet activation and the risk of thrombosis are increased in cancer patients, especially after chemotherapy. Our previous studies indicated that chemotherapy-induced platelet activation is largely due to endothelial cell damage. Thus, simple in vitro tests, such as aggregometry, are not desirable tests to predict platelet responsiveness to different chemotherapeutic agents because other contributory factors, such as tumor cells, endothelial cells, and the flow rate of platelets, also contribute to the formation of cancer-associated thrombosis. Therefore, developing a platelet detection system, which includes all possible risk parameters, is necessary. In the present study, we described a microengineered microfluidic system that contained a drug concentration generator, cancer cell culture chip, and three-dimensional (3D) circular microvascular model covered with a confluent endothelial layer and perfused with human platelets at a stable flow rate. Doxorubicin was injected through two injection sites. Endothelial cell injury was evaluated by counting, cell cytoskeleton observation, and the level of IACM1 and ET-1 in endothelial cells or a culture medium. Prestained platelets were perfused into the artificial blood vessel, and platelet-endothelial cell adhesion was measured. We found that (i) MCF7 cell-released factors had a cytotoxicity effect on both endothelial cells and platelets. (ii) We confirmed that doxorubicin-induced platelet activation was endothelial cell-dependent. (iii) A lower dosage of doxorubicin (0–2.0 μM) induced platelet activation, while a higher dosage of doxorubicin (2.0–4.0 μM) led to platelet death. Our findings indicated that platelet-endothelial cell adhesion could be used as a diagnostic marker of platelet activation, providing a simple and rapid detective way to predict platelet responsiveness before or during chemotherapy.
1. Introduction
Cancer-associated thrombosis is one of the most severe complications in cancer patients. It is reported that about 1–10% of cancer patients develop thrombus before chemotherapy, and up to 20% of patients develop thrombus after chemotherapy;1−4 those patients are more likely to have shortened survival compared to patients without thrombus.5,6 The tumor itself can cause thrombosis, and this effect is largely exacerbated after receiving chemotherapy drugs. Therefore, monitoring platelet responsiveness before or during chemotherapy would be an important strategy to improve the quality of life and prolong the survival time.
Doxorubicin is one of the most widely used chemotherapeutic drugs for the treatments of several kinds of tumors. Clinical and experimental studies, including ours, have revealed that platelets are activated in response to doxorubicin, indicating that evaluating platelet activation is extremely important during the whole period of chemotherapy.7−9 Aggregometry is a common detection way to test platelet aggregation in clinic. However, the aggregometry assay for platelets extracted from vehicle- or doxorubicin-injected mice indicated that platelet aggregation was similar between two groups.9,10 Our previous results further reveled that doxorubicin-induced endothelial cell damage was the primary mechanism for platelet activation.11 Thus, when assessing platelet function, it is inadequate if we only focus on platelets themselves without considering endothelial cells. Under this circumstance, simple in vitro tests, such as aggregometry or flow cytometry, are not desirable tests to predict platelet responsiveness to different chemotherapeutic agents because other contributory factors, such as tumor cells, endothelial cells, and flowed platelets, also contribute to the formation of cancer-associated thrombosis. Therefore, developing a platelet detection system, which includes all possible risk parameters, is necessary.
In recent years, microfluidic devices have emerged as novel platforms for a range of applications in agricultural, environmental, and basic biological studies.12−14 Advances in microfluidic techniques have enabled the construction of newly engineered in vitro models that simulate the complex structure and physiological functions of human tissues and organs.15 To date, microfluidic chips are widely used in tumor microenvironmental,16 vascular,17,18 or platelet studies.19,20 However, combining all these three microfluidic chips into one system has not been reported.
In the present study, we developed a microfluidic chip to study cancer-associated platelet activation. First, we constructed a circular cross-sectional microvascular model with a perfusion platform, conforming the biological characteristics of vessels. We introduced tumor cells into the culture system, setting up the coculture system of cancer cells, endothelial cells, and platelets. Then, we loaded different concentrations of doxorubicin, determining the effects of tumor cells and chemotherapy drugs on vascular-dependent platelet functions. We hypothesized that the microfluidic chip could be an important diagnosis platform for cancer patients who received chemotherapy.
2. Results
2.1. Construction of the Microfluidic System
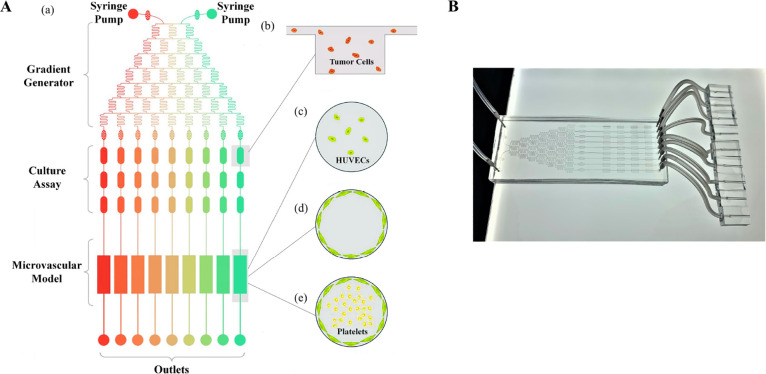
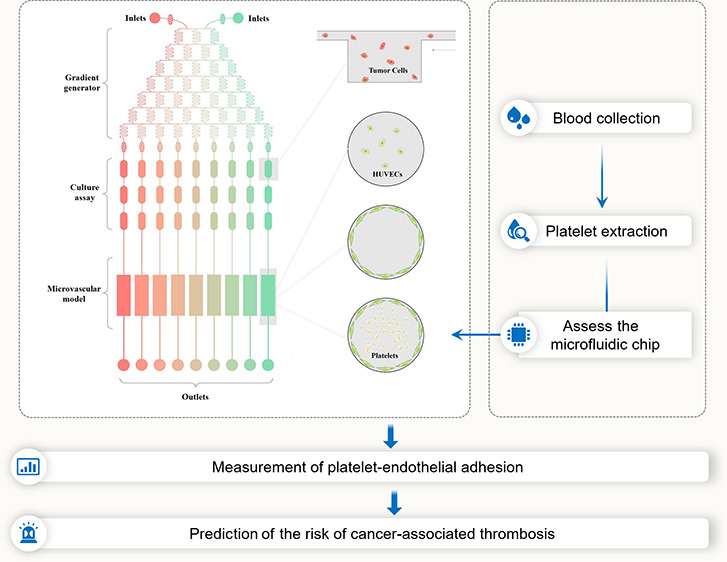
The whole microfluidic system, including the drug injection sites, tumor cell culture assay, and microengineered vessel consisting of three parts, was constructed to simulate the in vivo conditions (Figure 1A-a). Two different concentrations or two different drugs were injected to the gradient generator chip to generate the expected drug concentrations. MCF7 cells were seeded into the tumor cell culture assay (Figure 1A-b). A three-dimensional (3D) circular microvascular model was established by culturing HUVECs on the surface, which formed a confluent monolayer (Figure 1A-c,d). Platelets were perfused into the artificial blood vessel at a flow rate of 30 μL/min (Figure 1A-e).
Figure 1.
Construction of the microfluidic system. Schematic representation of the microfluidic chip showing inlets, the drug concentration gradient generator, tumor cell culture assay, microvascular model, and outlet port (A). The image of the actual microfluidic chip (B).
2.2. Validation of the Drug Gradient Generation
To validate drug gradient generation, we injected rhodamine through the two injection sites into the chip. After the concentration gradient generator was shunted down, we observed that the rhodamine solution color in microchannels of the chip gradually faded from left to right (Figure 2A). Meanwhile, the fluorescence intensity of each microchannel decreased correspondingly, which was visualized under a fluorescence microscope (Figure 2B). Furthermore, by measuring the fluorescence intensity value of each channel, we found that there was a strong correlation between the fluorescence intensity values and drug concentrations (R2 = 0.9832; Figure 2C). To validate drug gradient generation, we injected two different concentrations of doxorubicin (0 and 4 μM) through two injection sites, which generated the concentration gradient of doxorubicin, including 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 μM. MCF7 cells were seeded on the tumor cell culture assay. When the cells reached 80% confluence, cells were treated with different concentrations of doxorubicin for 24 h. The cytotoxicity of doxorubicin on MCF7 cells was examined by calcein AM and PI staining. With the increase of doxorubicin concentration, the number of dead cells increased gradually (Figure 2D). Cell viability versus doxorubicin concentrations are plotted in Figure 2E.
Figure 2.
Validation of the drug gradient generation. Rhodamine was injected through the two injection sites into the chip (A). Fluorescent image in each channel after injection of rhodamine (B). Fluorescence intensities versus drug concentrations were plotted (C). Representative images of MCF7 cells stained by calcein AM and PI (D). Green cells represented live cells, while red cells represented dead cells. Quantitation of cell viability is presented in panel (E). n = 3 per group. Dox: doxorubicin. Statistical analysis was performed by one-way analysis of variance (ANOVA).
2.3. Representation of 3D Microvascular Model
HUVECs on the microvascular model were stained by a green fluorescent dye. Figure 3A,B shows the longitudinal section and the cross-sectional views of the 3D vascular model under the fluorescence microscope. These results confirmed that the endothelial cells were able to grow well on the curved surface and form a confluent monolayer. Then, the prestained platelets (orange fluorescent dye) were added to the microvascular model to induce platelet-endothelial cell adhesion (Figure 3C).
Figure 3.

Representation of the 3D microvascular mode. The endothelial cells were prestained by MitoGreen and cultured on the 3D vascular chips. Representative fluorescent images showing the top view (A) and cross-sectional view (B) of the 3D vessel. Platelets were prestained by orange fluorescence and then perfused into the vascular lumen (C). Bars represent 100 and 200 μm. EC: endothelial cell.
2.4. The Cytotoxic Effect of Doxorubicin on HUVECs
A doxorubicin-induced vascular injury was evaluated by cell cytoskeleton observation and measuring the content of IACM1, eNOS, and ET-1 in cells or a culture medium. HUVECs were treated with increasing concentrations of doxorubicin (from 0 to 4 μM) for 24 h. Under the normal conditions, HUVECs were randomly oriented with clear filaments. When the concentration of doxorubicin reached 1 μM, the actin filaments became apparent. When the concentration of doxorubicin was further increased to 3.0 μM, the number of cells reduced, but the number of actin filaments significantly increased and became irregular. When the doxorubicin concentration reached 4 μM, the cell number was significantly decreased, and cell morphology became round (deattachment) (Figure 4A,B,F). ICAM1 is a cell adhesion protein on the surface of endothelial cells, which is up-regulated in response to endothelial injury. When HUVECs were exposed to the lower concentrations of doxorubicin (≤2.5 μM), the level of ICAM1 was up-regulated, indicating that doxorubicin caused endothelial cell injury. In contrast, when HUVECs were exposed to the higher concentrations of doxorubicin (>2.5 μM), ICAM1 was down-regulated and the fluorescence was restricted to the nucleus, which was likely due to the cell death (Figure 4C,D,G). Additionally, after 24 h of doxorubicin stimulation, we perfused THP1 cells (orange fluorescent dye) into the microengineered vessel to test the adhesion of monocyte cells to endothelial cells. Endothelial cell-monocyte adhesion was enhanced by doxorubicin in the absence of MCF7 cells (≤2.5 μM) (Figure 4E,H). The adhesive ability was further enhanced by coculturing with MCF7 cells. In contrast, when HUVECs were exposed to the higher concentrations of doxorubicin (>1 μM), endothelial cell-monocyte adhesion was disrupted due to the cell death (Figure 5E,H), showing a similar trend to ICAM1 levels. eNOS plays a significant protective role in the endothelial homeostasis, and as our results have shown, the protein level of eNOS in HUVECs decreased in response to doxorubicin stimulation (Figure 4I). ET-1 is released by endothelial cells, working as a potent vasoconstrictor. With the increasing concentrations of doxorubicin, the content of ET-1 in the culture medium decreased gradually (Figure 4J).
Figure 4.
Cytotoxic effect of doxorubicin on HUVECs. Representative fluorescent images of actin filaments (phalloidin, green fluorescence) showing morphology of endothelial cells stimulated by different concentrations of doxorubicin for 24 h at low (A) and high magnifications (B). Representative immunofluorescent images of ICAM1 (red fluorescence) of endothelial cells stimulated by different concentrations of doxorubicin for 24 h at low (C) and high magnifications (D). Representative immunofluorescent images of THP1 cell (orange fluorescence) and endothelial cell adhesion (green fluorescence) stimulated by different concentrations of doxorubicin at high magnifications (E). Quantitation of cell numbers per view is measured and presented in panel (F). Quantitation of mean fluorescence intensity is measured and presented in panel (G). Quantitation of THP1 cell adhesion is shown in panel (H). Levels of eNOS (I) and ET-1 (J) in the culture medium were measured. Bars represent 50 and 100 μm. n = 5 per group. EC: endothelial cell; Dox: doxorubicin. Statistical analysis was performed by one-way ANOVA. n = 5 per group. *P < 0.05, ***P < 0.001, and ****P < 0.0001.
Figure 5.
Accumulative cytotoxic effect of doxorubicin and tumor cells on HUVECs. Representative fluorescent images of actin filaments (phalloidin, green fluorescence) showing morphology of endothelial cells stimulated by different concentrations of doxorubicin as well as MCF7 cells for 24 h at low (A) and high magnifications (B). Representative immunofluorescent images of ICAM1 (red fluorescence) of HUVECs stimulated by different concentrations of doxorubicin as well as MCF7 cells for 24 h at low (C) and high magnifications (D). Representative immunofluorescent images of THP1 (orange fluorescence) and endothelial cell (green fluorescence) adhesion stimulated by different concentrations of doxorubicin in the presence of MCF7 cells at high magnifications (E). Quantitation of cell numbers per view is measured and presented in panel (F). Quantitation of mean fluorescence intensity is measured and presented in panel (G). Corresponding quantitation of THP1 cell adhesion is shown in panel (H). Levels of eNOS (I) and ET-1 (J) in the culture medium were measured. Bars represent 50 and 100 μm. EC: endothelial cell; Dox: doxorubicin. Statistical analysis was performed by one-way ANOVA. n = 5 per group. *P < 0.05, **P < 0.001, ***P < 0.001, and ****P < 0.0001.
2.5. The Accumulative Cytotoxic Effect of Doxorubicin and Tumor Cells on HUVECs
To identify the combined cytotoxic effect of tumor cells and doxorubicin on HUVECs, MCF7 cells were seeded in a tumor cell culture assay chip, and a medium for tumor cell culture can be transferred to HUVECs via the vascular lumen. Without doxorubicin stimulation, MCF7 cells significantly increased the number of HUVECs, and the actin filaments became apparent and disordered, as compared with HUVEC culture alone (Figure 5A,B). After stimulating with doxorubicin for 24 h (from 0 to 4 μM), we found that tumor cells made HUVECs less resistant to doxorubicin, as assessed by the cell number, cell morphology, and endothelial cell-derived proteins (Figure 5C–J).
2.6. Measurement of Platelet-Endothelial Cell Adhesion on Chip
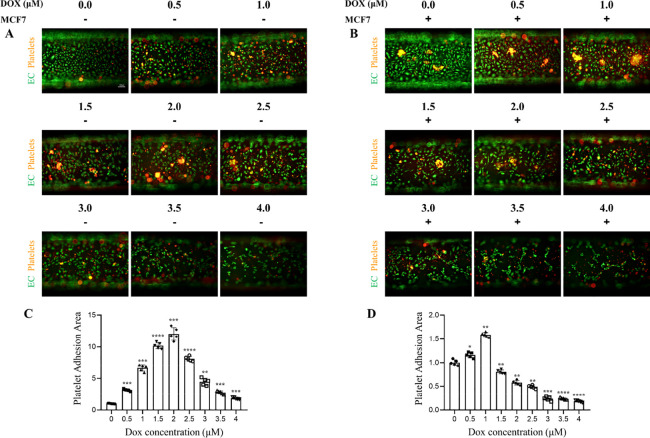
To test platelet-endothelial cell adhesion, we perfused freshly isolated and prestained platelets (orange fluorescent dye) from donors through the syringe injection sites into the lumen of the microengineered vessel. After 90 min of constant perfusion, the chip was immediately imaged by fluorescence microscopy. Under the normal physiological conditions, endothelial cells provided an antithrombotic surface, with minimal adhesive ability. We injected doxorubicin into the 3D microvascular model for 24 h to mimic intravenous injection. When the concentrations of doxorubicin were lower than 2.5 μM, doxorubicin led to enhanced platelet adhesion and aggregation on the surface of HUVECs, as assessed by the area of platelet coverage, and such an effect was dosage-dependent. When the concentrations of doxorubicin were bigger than 2.5 μM, platelet adhesion and aggregation were reduced or disrupted due to death happening on both platelets and HUVECs (Figure 6A,C).
Figure 6.
Measurement of platelet-endothelial cell adhesion on the microfluidic chip. Representative images of platelet-endothelial cell adhesion stimulated by different concentrations of doxorubicin in the absence (A) or presence (B) of MCF7 cells. Corresponding quantitation is shown in panels (C, D). Bars represent 100 μm. EC: endothelial cell; Dox: doxorubicin. Statistical analysis was performed by one-way ANOVA. n = 5 per group. *P < 0.05, **P < 0.001, ***P < 0.001, and ****P < 0.0001.
We previously demonstrated that MCF7 cells induced HUVEC damage and further aggravated doxorubicin-induced HUVEC injury, and we next investigated whether MCF7 cells could aggravate doxorubicin-induced platelet-endothelial cell adhesion. Consistently, without doxorubicin stimulation, MCF7 cells significantly increased platelet adhesion and aggregation, as compared with HUVEC culture alone. After adding different concentrations of doxorubicin, we found that tumor cells made platelets less resistant to doxorubicin by reducing the maximum concentration (doxorubicin concentrations for inducing the maximum platelet adhesion) from 2.5 to 1.0 μM. When the concentrations of doxorubicin were bigger than 1.0 μM, enhanced cell death caused by doxorubicin disrupted platelet adhesion and aggregation (Figure 6B,D).
3. Discussion
In the present study, we described a microengineered microfluidic system that contains a drug concentration generator, cancer cell culture chip, and 3D circular microvascular model covered with a confluent endothelial layer and perfused with human platelets at a stable flow rate, which are all critical factors for cancer-associated thrombus formation. By using this microfluidic system, several novel findings have been made. First, MCF7 cell-released factors had a cytotoxicity effect on both endothelial cells and platelets. Second, we confirmed that doxorubicin-induced platelet activation is endothelial cell-dependent. Third, a lower dosage of doxorubicin induced platelet activation, while a higher dosage of doxorubicin led to platelet apoptosis. We used platelet-endothelial cell adhesion as the endpoint of platelet activation, which is more physiologically relevant compared to in vitro platelet aggregation assays. Collectively, our findings indicate that such a microfluidic system is a simple and rapid detective way to predict platelet responsiveness before or during chemotherapy.
Cancer-associated thrombosis is a complicated process, mainly through the following two fundamental mechanisms. First, malignancy per se is able to induce thrombus formation.21 Second, many chemotherapeutic or tumor-targeted drugs such as doxorubicin, 5-Fu, vascular endothelial growth factor pathway inhibitors, and tyrosine kinase inhibitors reportedly increase the risk for thrombosis in cancer patients.9,22−24 Although the mechanisms have been poorly elucidated, platelets are likely the potential candidates to be affected by tumors and drugs. P2Y receptors, including P2Y1 and P2Y12, are G-protein coupled receptors that control critical steps of platelet activation. ADP initially binds P2Y1 and induces transient activation of platelets, which is subsequently strengthened and sustained by interaction with P2Y12.25,26 Generation of ADP has been documented in several types of cancers and induces platelet activation.27,28 Previous in vitro studies performed using isolated platelets showed that chemotherapeutic drugs, such as doxorubicin, had no direct effect on platelet activity.9,10 Due to intravenous injection, most chemotherapeutic drugs could cause harmful effects to endothelial cells prior to exerting their effects to other tissues. Endothelial cells are important cellular sources of platelet agonists.29 We previously used animal models to demonstrate that doxorubicin-induced endothelial cell injury was a major mechanism for platelet activation in response to doxorubicin treatment.11 Under this circumstance, simple in vitro tests, such as aggregometry or flow cytometry, are not desirable tests to predict platelet responsiveness to different chemotherapeutic agents because other contributory factors, such as tumor cells, endothelial cells, and flow rate of platelets, also contribute to thrombus formation. Therefore, there is a need for designing a test that includes all relevant factors contributing to cancer-associated thrombosis.
It is well known that cancer cells stimulate endothelial cell proliferation by releasing a vascular endothelial growth factor (VEGF) or other growth factors.30−33 Consistent with previous findings, by using our microfluidic system, we confirmed that tumor cells promoted endothelial cell proliferation. The tumor vasculature demonstrates abnormal features, which are characterized by dilated, excessively curved, and disorganized vessels, as compared to normal blood vessels. Vascular immaturity and lack of gap junction proteins lead to excessive permeability and poor perfusion.34,35 Consistently, similar findings were observed from us. Besides proliferation, we further found that HUVECs cocultured with an MCF7 conditioned medium displayed abnormal morphology, with irregular filaments and an elevated level of ICAM, as compared with HUVECs cultured alone, indicating that tumor cell-enhanced proliferative HUVECs lack function. Besides working on HUVECs, we also found that MCF7 cells enhanced platelet-endothelial adhesion, which is likely via both endothelial cell-dependent and independent mechanisms.
Our previous studies found that doxorubicin injection in mice induced severe endothelial cell injury.11 As a consequence, exposure of collagen from subendothelium to the circulating blood initiates platelet adhesion.36,37 We further demonstrated that enhanced generation of thrombin in endothelial cells might be an important mechanism mediating platelet adhesion in response to doxorubicin in mice. The von Willebrand factor (vWF) is an adhesive GP stored in platelets and endothelial cells. Binding of vWF to GPIb-IX-V or GPIIb/IIIa induces platelet adhesion on the endothelium.38 Under the conditions of endothelial injury, the vWF is rapidly exposed in the subendothelia matrix or released into plasma.39,40 Whether doxorubicin induced platelet-endothelial adhesion via the vWF still needs further investigation.
Adhesion of leukocytes to the surface endothelial cells is the essential step of leukocyte infiltration, which is initiated by the selectin family and adhesion proteins.41,42 This attachment is further firmed by ICAM1 on endothelium and MAC-1 on leukocytes.43,44 In the present study, we found that endothelial-monocyte adhesion was also affected by doxorubicin and tumor cells. This may explain that enhanced inflammatory responses are always associated with carcinomas. However, platelet adhesion and aggregation were not affected in the presence of monocytes.
Although anti-platelet drugs are recommended to patients with high risk of thrombosis, the use should be with caution. Thrombocytopenia is a common adverse effect raised by chemotherapy in clinical practice, including doxorubicin.45,46 Consistently, we found that a lower dosage of doxorubicin induced platelet activation, while a higher dosage of doxorubicin led to platelet death. Doxorubicin is administrated by intravenous injection, allowing a direct exposure to circulating platelets. As a consequence, doxorubicin promoted platelet death by inducing phosphatidylserine exposure, microparticle generation, and mitochondrial transmembrane potential change, thereby reducing the platelet count.9,10 Our findings also explain why the risk of thrombosis is higher at the early stage of chemotherapy but lower at the late stage of chemotherapy. Notably, quite a few cancer patients are under risks of both thrombosis and bleeding, and anti-platelet therapy may enhance the incidence of bleeding events. Under these circumstances, the timing of anti-platelet administration needs to be optimized. Hence, a function-guided anti-platelet strategy seems more sensible.
4. Conclusions
Collectively, this is the first study reporting a microengineered microfluidic chip, which contains the drug concentration generator, the cancer cell culture assay, and the 3D circular microvascular model covered with the confluent endothelial layer and perfused with human platelets. By using this chip, platelet-endothelial adhesion could be an important diagnostic marker for monitoring the risk of thrombosis in cancer patients with chemotherapy.
5. Materials and Methods
5.1. Platelet Isolation
Fresh blood samples (10 mL) were collected from healthy donors. The clinical study was approved by the ethics committee of the First Affiliated Hospital of Dalian Medical University, all participants were given a written informed consent, and the study conformed to the principles outlined in the Declaration of Helsinki. Fresh blood samples were mixed with platelet washing buffer (pH 6.5, 4.3 mM K2HPO4, 4.3 mM Na2HPO4, 24.3 mM NaH2PO4, 113 mM NaCl, 5.5 mM glucose, and 0.5% bovine serum albumin) in the presence of prostaglandin E1 (#P5640, 0.1 μg/mL, Sigma-Aldrich) and apyrase (#A6535, 1 U/mL, Sigma-Aldrich) and then centrifuged at 250g for 20 min. Platelet pellets from platelet rich plasma (PRP) were collected by centrifugation at 700g for 5 min and resuspended in modified Tyrode buffer before use.
5.2. Construction of the Whole Microfluidic System
The whole microfluidic system included two syringe pumps (#KDS100, KD Scientific), two syringes, a microfluidic chip, and joints and tubes, which are shown in Figure 1. The syringe pump was used for medium perfusion and the control of flow rate. The microfluidic chip consisted of three parts named the drug concentration gradient generator, tumor cell culture assay, and microvascular model. The gradient generator chip contained a network of 150 μm microchannels that repeatedly split and mixed the injected solutions, two inlets (2 mm in diameter), and nine outlets (1 mm in diameter). The tumor cell culture assay chip contained 36 concave rectangle microwells (3 mm in length, 1.5 μm in width, and 100 μm in depth). The microvascular model is a straight microchannel chip with a circular cross section (1 mm diameters). The chips from all three parts were connected via tubes and blunted needles.
5.3. Fabrication of the Microfluidic Chip
The drug concentration gradient generator and tumor cell culture assay chip were fabricated in polydimethylsiloxane (PDMS) using the standard soft lithography technology. Specifically, first, we had drawn the desired channel pattern using CAD software and printed as a photomask. Then, the negative photoresist SU-8 was spin-coated on a silicon wafer (40 μm thickness of the gradient generator and 100 μm thickness of the tumor cell culture assay). After prebaking, UV light exposure, postbaking, and developing, the negative replicas of the designed microstructure were created on the silicon wafer. The PDMS prepolymer was mixed with a curing reagent (10:1 mass ratio), poured onto the wafer, and cured in a drying oven (80 °C) for 60 min, and the PDMS layer was peeled off from the wafer. Holes were drilled at the inlet and outlet locations by using biopsy punch. Finally, the PDMS layer gradient generator chip was bonded into a clean glass slide for studying the effect of different concentrations of anti-tumor drugs on the blood vessel, or the PDMS layer of the gradient generator chip (80 °C drying oven, 30 min) (top layer) was thermally bonded to the PDMS layer of the tumor cell assay chip (bottom layer) for studying the accumulative effect of different concentrations anti-tumor drugs and the tumor cell on the blood vessel.
For building a circular cross-sectional microvascular model, we used microwire molding technology. This method used a microstainless steel wire (1 mm diameter) with the circular cross section as the mold. PDMS was poured, cured in a drying oven (80 °C) for 60 min, and then, the stainless steel wire was removed and cut into small pieces encompassing the entire channel structure. The straight microchannel with the circular cross section could be completed.
5.4. Validation of Gradient Generator
To quantify the drug concentrations, rhodamine ethanol solution was injected through one of the inlets, while the same volume ethanol solution but without rhodamine was injected through the other inlet. The solutions were injected at a flow rate of 0.5 μL/min, which were controlled by two syringe pumps. Fluorescent images were taken by a microscope after the microfluidic system reached stability.
5.5. Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) were isolated from the neonatal umbilical cord (Dalian Obstetrics and Gynecology Hospital) and cultured in an endothelial cell medium (ECM, ScienCell) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin at 37 °C with 5% CO2. The MCF7 cell line was also cultured in the ECM under the same culture conditions. Cells were resuspended at a density of 1.0 × 106 cells/mL and then seeded on collagen II (2 μg/cm2, Sigma-Aldrich) pretreated microwells. Thereafter, HUVECs were stained by MitoGreen (1/1000 dilution, #KGMP0072, KeyGEN BioTECH) at 37 °C for 30 min, and then, cell suspension (15 μL) was injected into the microvascular model. Then, the chip was incubated at 37 °C with 5% CO2, and the cultural medium in the chip was replaced every 8 h until the cells in the chip reached 80% confluence.
5.6. Effects of Different Anti-Tumor Drugs on MCF7 Cell Viability
The cytotoxicity of doxorubicin on MCF7 cells was tested by calcein AM and propidium iodide (PI) staining. A total of 10 μL of mixed solution containing calcein AM (1/500 dilution, #17783, Sigma-Aldrich) and propidium iodide (PI, 1/1500 dilution, #P4170, Sigma-Aldrich) was added to each well of the tumor cell assay chip. Calcein AM and PI fluorescence were observed by using a fluorescence microscope. Cell viability was determined as the number of live cells relative to the total cell number.
5.7. Immunofluorescence
HUVECs were fixed with 4% paraformaldehyde for 15 min. After a 20 min permeabilization with 0.1% Triton X-100 and 1 h of blocking with 10% goat serum, an anti-intercellular adhesion molecule1 (ICAM1, #ab2213, Abcam) antibody diluted at a ratio of 1:100 was injected into the chip channel and then incubated at 4 °C overnight. After washing, Alexa Fluor 546 goat anti-mouse antibody solution diluted in 1/200 was incubated at 37 °C in the dark for 2 h. HUVECs were also stained with 100 μL of Alexa Fluor 488-conjugated phalloidin (#P5285, Sigma-Aldrich) solution for 1 h in the dark at room temperature followed by infusing DAPI (#C1005, Beyotime) solution for 2 min. After washing, images were captured using a microscope with a CCD camera.
5.8. ET-1/eNOS Assay
The endothelin-1 (ET-1) (#CSB-E08322h, CUSABIO) and eNOS (#CSB-E07007h, CUSABIO) level from the HUVEC culture medium in each sample were measured using a commercial enzyme immunoassay technique-based kit. The plate was read for absorbance at 450 nm on a microplate spectrophotometer (GENios Plus, Tecan), and samples were compared to the obtained standard curve.
Acknowledgments
H.L. was supported by the State Natural Science Foundation of Liaoning (2019-MS-081). Y.L. was supported by the scientific research project of the Education Department of Liaoning Province (LZ2019017). Y.-L.X. was supported by the Dalian Talents Innovation Supporting Project (2018RD09).
Author Contributions
§ Z.H. and H.L. contributed equally to this work.
The authors declare no competing financial interest.
References
- Cronin-Fenton D. P.; Søndergaard F.; Pedersen L. A.; Fryzek J. P.; Cetin K.; Acquavella J.; Baron J. A.; Sørensen H. T. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br. J. Cancer 2010, 103, 947–953. 10.1038/sj.bjc.6605883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana A. A.; Dalal M.; Lin J.; Connolly G. C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119, 648–655. 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- Goodnough L. T.; Saito H.; Manni A.; Jones P. K.; Pearson O. H. Increased incidence of thromboembolism in stage IV breast cancer patients treated with a five-drug chemotherapy regimen. Cancer 1984, 54, 1264–1268. . [DOI] [PubMed] [Google Scholar]
- Zamorano J. L.; Lancellotti P.; Rodriguez Muñoz D.; Aboyans V.; Asteggiano R.; Galderisi M.; Habib G.; Lenihan D. J.; Lip G. Y. H.; Lyon A. R.; Lopez Fernandez T.; Mohty D.; Piepoli M. F.; Tamargo J.; Torbicki A.; Suter T. M.; 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- Khorana A. A.; Francis C. W.; Culakova E.; Kuderer N. M.; Lyman G. H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemostasis 2007, 5, 632–634. 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- Takasaki K.; Miyamoto M.; Takano M.; Soyama H.; Aoyama T.; Matsuura H.; Iwahashi H.; Ishibashi H.; Sakamoto T.; Furuya K. Thrombotic events induce the worse prognosis in ovarian carcinomas and frequently develop in ovarian clear cell carcinoma. Int. J. Clin. Oncol. 2019, 24, 1273–1283. 10.1007/s10147-019-01464-4. [DOI] [PubMed] [Google Scholar]
- Baz R.; Li L.; Kottke-Marchant K.; Srkalovic G.; McGowan B.; Yiannaki E.; Karam M. A.; Faiman B.; Jawde R. A.; Andresen S.; Zeldis J.; Hussein M. A. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin. Proc. 2005, 80, 1568–1574. 10.4065/80.12.1568. [DOI] [PubMed] [Google Scholar]
- Kedzierska M.; Czernek U.; Szydłowska-Pazera K.; Potemski P.; Piekarski J.; Jeziorski A.; Olas B. The changes of blood platelet activation in breast cancer patients before surgery, after surgery, and in various phases of the chemotherapy. Platelets 2013, 24, 462–468. 10.3109/09537104.2012.711866. [DOI] [PubMed] [Google Scholar]
- Kim S.-H.; Lim K.-M.; Noh J.-Y.; Kim K.; Kang S.; Chang Y. K.; Shin S.; Chung J.-H. Doxorubicin-induced platelet procoagulant activities: an important clue for chemotherapy-associated thrombosis. Toxicol. Sci. 2011, 124, 215–224. 10.1093/toxsci/kfr222. [DOI] [PubMed] [Google Scholar]
- KIM E.-J.; LIM K.-M.; KIM K.-Y.; BAE O.-N.; NOH J.-Y.; CHUNG S.-M.; SHIN S.; YUN Y.-P.; CHUNG J.-H. Doxorubicin-induced platelet cytotoxicity: a new contributory factor for doxorubicin-mediated thrombocytopenia. J. Thromb. Haemostasis 2009, 7, 1172–1183. 10.1111/j.1538-7836.2009.03477.x. [DOI] [PubMed] [Google Scholar]
- Lv H.; Tan R.; Liao J.; Hao Z.; Yang X.; Liu Y.; Xia Y. Doxorubicin contributes to thrombus formation and vascular injury by interfering with platelet function. Am. J. Physiol.: Heart Circ. Physiol. 2020, 319, H133–H143. 10.1152/ajpheart.00456.2019. [DOI] [PubMed] [Google Scholar]
- Jiang Q.; Wu J.; Yao K.; Yin Y.; Gong M. M.; Yang C.; Lin F. Paper-Based Microfluidic Device (DON-Chip) for Rapid and Low-Cost Deoxynivalenol Quantification in Food, Feed, and Feed Ingredients. ACS Sens. 2019, 4, 3072–3079. 10.1021/acssensors.9b01895. [DOI] [PubMed] [Google Scholar]
- Stanley C. E.; Grossmann G.; i Solvas X. C.; DeMello A. J. Soil-on-a-Chip: microfluidic platforms for environmental organismal studies. Lab Chip 2016, 16, 228–241. 10.1039/C5LC01285F. [DOI] [PubMed] [Google Scholar]
- Hamon M.; Hong J. W. New tools and new biology: Recent miniaturized systems for molecular and cellular biology. Mol. Cells 2013, 36, 485–506. 10.1007/s10059-013-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H.; Sung J. H. Organ-on-a-Chip Technology for Reproducing Multiorgan Physiology. Adv. Healthcare Mater. 2018, 7, 1700419. 10.1002/adhm.201700419. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Varghese S. Ex Vivo Tumor-on-a-Chip Platforms to Study Intercellular Interactions within the Tumor Microenvironment. Adv. Healthcare Mater. 2018, 1801198. 10.1002/adhm.201801198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Kim W.; Lim S.; Jeon J. S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4, 8. 10.3390/bioengineering4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P. F.; Albers H. J.; Linssen J. E. A.; Middelkamp H. H. T.; van der Hout L.; Passier R.; van den Berg A.; Malda J.; van der Meer A. D. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. 10.1039/C7LC00202E. [DOI] [PubMed] [Google Scholar]
- Li M.; Ku D. N.; Forest C. R. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 2012, 12, 1355. 10.1039/c2lc21145a. [DOI] [PubMed] [Google Scholar]
- Thon J. N.; Mazutis L.; Wu S.; Sylman J. L.; Ehrlicher A.; Machlus K. R.; Feng Q.; Lu S.; Lanza R.; Neeves K. B.; Weitz D. A.; Italiano J. J. Jr. Platelet bioreactor-on-a-chip. Blood 2014, 124, 1857–1867. 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga A.; Russo L.; Milesi V.; Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 79–83. 10.1016/j.critrevonc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Otten H.-M. M. B.; Mathijssen J.; Ten Cate H.; Soesan M.; Inghels M.; Richel D. J.; Prins M. H. Symptomatic Venous Thromboembolism in Cancer Patients Treated With Chemotherapy: An Underestimated Phenomenon. Arch. Intern. Med. 2004, 164, 190–194. 10.1001/archinte.164.2.190. [DOI] [PubMed] [Google Scholar]
- Nalluri S. R.; Chu D.; Keresztes R.; Zhu X.; Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008, 300, 2277–2285. 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- Choueiri T. K.; Schutz F. A. B.; Je Y.; Rosenberg J. E.; Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J. Clin. Oncol. 2010, 28, 2280–2285. 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- Jin J.; Kunapuli S. P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 8070–8074. 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T.; Nurden P. Advantages of Fast-Acting ADP Receptor Blockade in Ischemic Heart Disease. Arterioscler., Thromb., Vasc. Biol. 2003, 23, 158–159. 10.1161/01.ATV.0000053387.06709.32. [DOI] [PubMed] [Google Scholar]
- Boukerche H.; Berthier-Vergnes O.; Penin F.; Tabone E.; Lizard G.; Bailly M.; McGregor J. L. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. Br. J. Haematol. 1994, 87, 763–772. 10.1111/j.1365-2141.1994.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Bastida E.; Escolar G.; Almirall L.; Ordinas A. Platelet activation induced by a human neuroblastoma tumor cell line is reduced by prior administration of ticlopidine. Thromb. Haemostasis 1986, 55, 333–337. [PubMed] [Google Scholar]
- George J. N. Platelets. Lancet 2000, 355, 1531–1539. 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- Greenberg J. I.; Shields D. J.; Barillas S. G.; Acevedo L. M.; Murphy E.; Huang J.; Scheppke L.; Stockmann C.; Johnson R. S.; Angle N.; Cheresh D. A. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M.; Vignoli A.; Russo L.; Balducci D.; Pagnoncelli M.; Barbui T.; Falanga A. Endothelial capillary tube formation and cell proliferation induced by tumor cells are affected by low molecular weight heparins and unfractionated heparin. Thromb. Res. 2008, 121, 637–645. 10.1016/j.thromres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Krishnapriya S.; Sidhanth C.; Manasa P.; Sneha S.; Bindhya S.; Nagare R. P.; Ramachandran B.; Vishwanathan P.; Murhekar K.; Shirley S.; Ganesan T. S. Cancer stem cells contribute to angiogenesis and lymphangiogenesis in serous adenocarcinoma of the ovary. Angiogenesis 2019, 22, 441–455. 10.1007/s10456-019-09669-x. [DOI] [PubMed] [Google Scholar]
- An J.; Du Y.; Fan X.; Wang Y.; Ivan C.; Zhang X.-G.; Sood A. K.; An Z.; Zhang N. EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene 2019, 38, 2123–2134. 10.1038/s41388-018-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard C.; Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- Shang B.; Cao Z.; Zhou Q. Progress in tumor vascular normalization for anticancer therapy: challenges and perspectives. Front. Med. 2012, 6, 67–78. 10.1007/s11684-012-0176-8. [DOI] [PubMed] [Google Scholar]
- Massberg S.; Gawaz M.; Grüner S.; Schulte V.; Konrad I.; Zohlnhöfer D.; Heinzmann U.; Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 2003, 197, 41–49. 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B.; Watson S. P. Platelet-collagen interaction: is GPVI the central receptor?. Blood 2003, 102, 449–461. 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- Dong J.-F.; Moake J. L.; Nolasco L.; Bernardo A.; Arceneaux W.; Shrimpton C. N.; Schade A. J.; McIntire L. V.; Fujikawa K.; Lopez J. A. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002, 100, 4033–4039. 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M. Von Willebrand factor, platelets and endothelial cell interactions. J. Thromb. Haemostasis 2003, 1, 1335–1342. 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Sadler J. E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 1998, 67, 395–424. 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 7390–7397. 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N. G. The immune system and cardiac repair. Pharmacol. Res. 2008, 58, 88–111. 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W.; Lawler J. Integrins as dynamic regulators of vascular function. Faseb J. 1994, 8, 929–938. 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- Li W.; Lam M. S.; Birkeland A.; Riffel A.; Montana L.; Sullivan M. E.; Post J. M. Cell-based assays for profiling activity and safety properties of cancer drugs. J. Pharmacol. Toxicol. Methods 2006, 54, 313–319. 10.1016/j.vascn.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Wang S.; Konorev E. A.; Kotamraju S.; Joseph J.; Kalivendi S.; Kalyanaraman B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells via Distinctly Different Mechanisms. J. Biol. Chem. 2004, 279, 25535–25543. 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]