Abstract

Supercritical carbon dioxide (SC-CO2) has been progressively used in the development of shale oil and gas. However, the interaction between CO2 and shale can change the mineral composition and the pore structure, thus affecting the mechanical properties of shale. To study the influence of SC-CO2 on shale, shale samples collected from the Songliao Basin in China are treated with SC-CO2 at various time intervals. Then, a series of tests are performed, such as the mineral composition analysis test, the pore structure analysis test, and the macro mechanics test. The results show that the mechanical properties of shale gradually decrease exponentially with the increase of SC-CO2 treatment time. The loss of elastic modulus reaches about 47% after the treatment of 14 d. X-ray diffraction analysis shows that the mineral (except quartz) content decreases after SC-CO2 treatment, and in particular, the proportions of carbonate minerals significantly decrease by about 12%. The primary pores and fractures are eroded through dissolution, and new pores and fracture structures are developed on the surface microstructure. In addition, the proportion of micropores and mesopores decreases, while the proportion of macropores increases after SC-CO2 treatment. The specific surface area and average pore size present upward trends during SC-CO2 treatment. The changes of mineral assemblage and pore structure lead to the obvious decline of mechanical properties in shale reservoirs. This study contributes to understanding the evolution characteristics of mechanical properties under SC-CO2 treatment, which is of great significance for the efficient exploitation in shale reservoirs.
1. Introduction
As the world’s demand for oil and gas resources gradually increases, shale oil has become an important supplement to conventional energy sources and has started to receive attention from countries all over the world.1 In the past few years, the large-scale development and utilization of shale oil in the United States were mainly based on horizontal drilling and the multi-stage hydraulic fracturing technology.2−5 Although the traditional hydraulic fracturing technology has achieved great success in developing shale gas, it also caused many problems such as reservoir damage and clay mineral expansion.6,7 SC-CO2 has the characteristics of a low-viscosity gas and a high-density liquid.8 Moreover, the research shows that these characteristics can be used to promote the development of oil and gas resources.9−12 However, the interaction between SC-CO2 and shale leads to changes in mineral composition, pore structure, and mechanical properties. It is of great significance to study the interaction mechanism of SC-CO2 and shale for the major development of shale oil and gas.
In the process of using SC-CO2 to develop shale oil, the injected CO2 gradually enters the internal pore structure of shale through a wellbore or fracture and finally forms the CO2 reservoir. In the reservoir, CO2 interacts with shale to change the shale mineral composition and pore structure, which affects the various properties of shale. Mehic et al.13 show that the permeability and strength of coal appear to decrease significantly after CO2 adsorption. Lahann et al.14 have found that SC-CO2 can corrode shale, extract and dissolve organic matter in pores and microcracks, and finally form a new microstructure. The organic matter in the fissures forms a new microstructure. Considering the effects of time, pressure, and temperature, Jiang et al.15 obtain the action mechanism of CO2 treatment on the shale micropore structure. Busch et al.16 use Australian shale samples for SC-CO2 treatment, and the test shows that CO2 has an impact on shale porosity. Yin et al.17 study the mineral composition and pore structure of shale after SC-CO2 treatment and find that SC-CO2 can dissolve some organic minerals and clay minerals in shale. At the same time, the impact on micropores is more significant.
Due to the injection of SC-CO2, the mechanical properties of shale have also been greatly affected, which affect shale oil extraction. Lyu et al.18 tested the mechanical properties of shale treated with CO2 and found that after 10 days of CO2 saturation, the uniaxial compressive strength of shale for parallel bedding and vertical bedding is reduced. Verton et al.19 used CO2 and the hydraulic fracturing technology to conduct fracturing experiments and found that CO2 can achieve the same fracturing effect as the hydraulic technology. Zhang et al.20 use 200 mm × 200 mm × 200 mm cubic shale to carry out experimental research on SC-CO2 fracturing shale under different stress levels. It is found that the fracture initiation pressure of the SC-CO2 fracturing technology is 50% lower than that of the hydraulic fracturing technology in shale.
Although several studies have been conducted on the effect of SC-CO2 treatment on shale mechanical properties, the factors that affect these changes differ and have not been adequately studied. This paper conducts a series of experimental studies to explore the mechanism of changing the mechanical properties of shale under SC-CO2 treatment. The influence of mineral composition and microstructure on shale’s mechanical properties is the article’s leading research content.
2. Methodology
2.1. Samples
The sample in this study is shale obtained from the Songliao Basin in China. It was formed in the Cretaceous with a burial depth of 2000–3000 m and a perfect bedding trend. The porosity is 4.6–5.3%, and the permeability is 0.003–0.006 md. Furthermore, it is characterized by low porosity, low permeability, poor cementation, and strong heterogeneity. In order to avoid the interference of rock anisotropy and rock strata, the cores of the same formation in the same block are selected. Then, the two ends of the shale sample are ground flat on the grinding machine to ensure that the two ends are smooth. Then, they are processed into two kinds of standard cylindrical samples of ⌀50 mm × 25 mm and ⌀50 mm × 100 mm (Figure 1a,b). Some shale samples need to be crushed before treatment in SC-CO2, as shown in Figure 1c.
Figure 1.
Samples used in the experiment; (a) shale samples for uniaxial compression, (b) shale samples for Brazilian split, and (c) shale samples for adsorption.
2.2. Experimental Method
The experiments in the article include rock mechanics (the uniaxial compression test and Brazilian test), mineral composition [X-ray diffraction (XRD) analyses and scanning electron microscopy (SEM)], and pore structure (the computerized tomography test and low-pressure nitrogen gas adsorption tests). Through these experiments, the influence of CO2 on rock characteristic parameters and the relationship between parameters are studied.
2.2.1. Mechanical Characteristic Measurements
The test equipment of shale mechanical properties adopts an XTYE-2000 hydraulic pressure testing machine. The maximum axial pressure of the equipment is 2000 kN, and the maximum lateral pressure is 100 MPa. It mainly measures the tensile strength and uniaxial compressive strength of shale under different SC-CO2 treatment times and indirectly obtains the elastic modulus and Poisson’s ratio of shale.
2.2.2. Mineral Composition Analysis
The mineral composition analysis test mainly uses the combination of XRD analyses and SEM. The X-ray diffractometer used in the XRD test is a Shimadzu XRD-7000, and the scanning angle is 10–80°. The angle measurement accuracy is 0.001°, and the continuous scanning method is adopted. The step angle is 0.02° to obtain the internal mineral composition and content of the shale under different SC-CO2 treatment times. The Japanese JEOL JSM-7800F field emission scanning electron microscopes are used for the SEM test. The instrument’s minimum point resolution is 3 nm, and the magnification range is 4–100,000 to obtain the shale surface micromorphology under different treatment times.
2.2.3. Pore Structure Tests
Computer tomography (CT) is performed with Siemens Somatom plus a medical spiral CT scanner. The spatial resolution is 0.35 mm × 0.35 mm, the minimum recognizable volume is 0.12 mm2, and the density contrast resolution is 0.3%. After obtaining the CT scanning images of different core sections, the fracture distribution of different sections is reconstructed to obtain the core’s 3d fracture distribution model. The experimental device for the nitrogen adsorption test is the BELSORP-max II gas adsorption instrument produced by Mecchik Bayer Ltd. of Japan. Its pore diameter ranges from 0.35 to 500 nm; the minimum specific surface area can be measured to 0.0005 m2/g, and the minimum pore volume can be detected to 0.0001 cm2/g. According to the static capacity method, it can measure shale’s nitrogen adsorption capacity under different equilibrium pressures, and the nitrogen adsorption curve can be drawn.
2.2.4. SC-CO2 Treatment Test
The SC-CO2 treatment test device is shown in Figure 2. The CO2 pressurization system is a set of self-developed experimental devices, which controls temperature and pressure to convert carbon dioxide into a supercritical state. The experimental device can simulate the change of micropore structure and mechanical properties of the rock after CO2 is injected into the rock. The immersion temperature is 60 °C, and the pressure is 25 MPa.
Figure 2.

Diagram of the experimental device.
The specific practical steps are as follows:
-
(1)
According to the international rock mechanics standards, the experiment’s standard shale samples are obtained by wire cutting. The remaining shale samples are taken to prepare particles with a particle size of 100–200 mesh. The standard shale samples and shale sample particles are placed in an oven at 110° C for 24 h and cooled to room temperature.
-
(2)
Before SC-CO2 treatment, the equipment draws vacuum. Then, CO2 is injected to reach a certain pressure, and the temperature is controlled to make the CO2 enter the supercritical state.
-
(3)
The samples are divided into four groups (0 d, 3 d, 7 d, and 14 d). Shale particles and standard shale samples are put into the SC-CO2 treatment chamber.
-
(4)
After treatment, the samples with different treatment times are tested by XRD and SEM.
-
(5)
The pore structure experiment and rock mechanics experiment after SC-CO2 treatment are carried out by using the BELSORP-max II gas adsorption instrument and XTYE-2000 hydraulic testing machine.
3. Results
3.1. Mechanical Properties
The difference between the shale samples can affect the experimental results. Therefore, six groups of samples are selected for the repeated test, and the average of these data is used for mechanical property analysis. Figure 3 shows each sample’s fracture photos which have been tested by the Brazilian splitting test and the uniaxial compression test.
Figure 3.
Shale failure mode (a) Brazil split test and (b) uniaxial compression test.
3.1.1. Mechanical Strength Changes
Figure 4 shows the contrast diagram of shale peak strength under the different treatment times of SC-CO2. It can be seen from Figure 4 that the average tensile strength of the untreated shale is about 5.33 MPa. After 3 d, 7 d, and 14 d SC-CO2 treatment, the average tensile strengths of shale are 4.89, 4.69, and 4.34 MPa, respectively. Under the three treatment time levels, the tensile strength losses are 8.25, 12, and 18.57%, respectively. The uniaxial compressive strength of the untreated shale is about 61.71 MPa. The uniaxial compressive strengths of shale treatment in SC-CO2 for 3 d, 7 d, and 14 d are 51.01, 47.02, and 42.99 MPa, respectively. The loss rates of uniaxial compressive strength are 17.34, 23.8, and 30.34%. Further, the tensile strength and uniaxial compressive strength are fitted. At the same time, the function fitting curve of tensile strength and uniaxial compressive strength with treatment time is obtained
| 1 |
| 2 |
where σbt is the tensile strength, MPa; σ1 is the uniaxial compressive strength, MPa; and t is the treatment time of SC-CO2, d. The correlation coefficients R2 of tensile strength and uniaxial compressive strength are 0.9555 and 0.9987, respectively, and the fitting effect is good.
Figure 4.
Analysis of uniaxial compressive strength and tensile strength of shale after treatment; (a) tensile strength curve and (b) uniaxial compressive strength curve.
The results show that SC-CO2 treatment deteriorates the strength of shale to a certain extent. As the treatment time increases, the tensile strength and uniaxial compressive strength of shale after treatment are significantly lower than those of untreated shale. The peak intensity decreases gradually, and the rate of decline slows down.
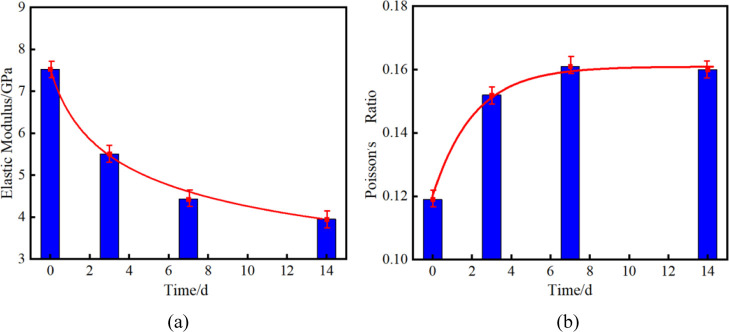
3.1.2. Deformation Characteristic Changes
Figure 5 shows the comparison of the elastic modulus and Poisson’s ratio of shale under different treatment times in SC-CO2. As shown in Figure 5, it can be seen that the elastic modulus of shale without SC-CO2 treatment is about 7.53 GPa, and the elastic moduli of shale after 3 d, 7 d, and 14 d SC-CO2 treatment are 5.51, 4.33, and 3.95 GPa, respectively. The losses of elastic modulus are 26.82, 42.49, and 47.54%, respectively. Poisson’s ratio of shale without SC-CO2 treatment is about 0.119. After 3 d, 7 d, and 14 d SC-CO2 treatment of shale, Poisson’s ratios are 0.152, 0.161, and 0.16, respectively. The growths of Poisson’s ratio are 27.73, 35.29, and 34.45%, respectively. Further, the elastic modulus and Poisson’s ratio are fitted. At the same time, the function-fitting curve of elastic modulus and Poisson’s ratio with treatment time is obtained
| 3 |
| 4 |
where E is the modulus of elasticity, GPa; μ is Poisson’s ratio; and t is the treatment time of SC-CO2, d. The correlation coefficients R2 of tensile strength and uniaxial compressive strength are 0.9892 and 0.9946, respectively.
Figure 5.
Analysis of elastic modulus and Poisson’s ratio after shale treatment; (a) elastic modulus and (b) Poisson’s ratio.
The results show that the elastic modulus decreases and Poisson’s ratio increases significantly after treatment in SC-CO2, which may be due to the expansion of natural fractures and bedding and the expansion of the shale matrix.
3.2. Microscopic Mineral Composition Characteristics
3.2.1. Mineral Composition Based on XRD
The results of XRD analysis are shown in Table 1 and Figure 6a. The results show that the main mineral contents of shale samples are quartz, carbonate minerals, and clay minerals. Compared with the shale without SC-CO2 treatment, the mineral composition of the treated shale has changed. Except for the increase of quartz content after treatment, other mineral components decrease gradually with the treatment time, especially the content of carbonate minerals. It can be seen from Figure 6b that carbonate minerals decline by 12.16% with SC-CO2 treatment. This shows that SC-CO2 and shale have been dissolved to a certain extent during the treatment. Quartz does not react with CO2. The increase in its content results from a decrease in the overall quality of shale. The higher the percentage of quartz after treatment, the greater the reaction consumption of other components with SC-CO2.
Table 1. XRD Test Results.
| treatment | mineral content (%) |
|||||
|---|---|---|---|---|---|---|
| time | quartz | feldspar | carbonate | clay minerals | pyrite | siderite |
| 0 d | 30.8 | 10.1 | 31.3 | 24.1 | 1.2 | 2.5 |
| 3 d | 36.6 | 8.6 | 28.3 | 23.4 | 1 | 2.1 |
| 7 d | 37.9 | 8.3 | 27.8 | 23.1 | 0.9 | 2 |
| 14 d | 38.7 | 8.1 | 27.5 | 23 | 0.8 | 1.9 |
Figure 6.
Changes of mineral content under SC-CO2 treatment; (a) changes in all minerals and (b) changes in carbonate minerals.
3.2.2. Mineral Composition Evolution Process Analysis Based on SEM
Mineral composition analysis based on SEM is a part of rock microstructure analysis. The previous samples and treated samples are tested by SEM. The distribution of micropores and microcracks is observed by SEM images. Figure 7 shows the surface morphology under different treatment times obtained using a scanning electron microscope. It can be seen from the figure that the surface of the sample without SC-CO2 treatment is relatively smooth, but there are also a few primary pores and primary fractures. After 3 d of SC-CO2 treatment, the mineral components of shale begin to react with CO2 and produce honeycomb-shaped pores. After 7 d of SC-CO2 treatment, clay particles in shale begin to separate from the binding water, and the particles become smaller. SC-CO2 extracts a lot of mineral solute from the shale interior, so the number of new pores is obviously increased. The surface of shale becomes very rough. The primary pores and primary fractures develop further, and the new pores generated after treatment for 3 days also have different degrees of development. After 14 days of treatment, the shale surface has undergone significant changes. For example, more pores and crack widths have been further expanded, and more and more obvious honeycomb pores have appeared. These pores can become better storage spaces for shale gas.
Figure 7.

SEM analysis results; (a) untreated, (b) treatment for 3 days, (c) treatment for 7 days, and (d) treatment for 14 days.
3.3. Microscopic Pore Structure
3.3.1. Mesopore Distribution Characteristics
The nitrogen adsorption experiment is carried out on a BELSORP-max II gas adsorption instrument. In this experiment, 100–200 mesh shale particles with a weight of 0.5–1 g and a nitrogen concentration purity greater than 99.99% are used for nitrogen adsorption analysis. During the experiment, shale samples are placed in a 77.4 K liquid nitrogen environment. According to the Langmuir adsorption isotherm model, the adsorption and desorption of nitrogen under different relative pressures are measured. The adsorption capacity, specific surface area, and pore structure distribution of nitrogen are obtained. The mathematical expression is as follows
| 5 |
where nad-dry is the adsorption amount of nitrogen on the surface of dry clay, mmol/g; Aslit is the area of a single clay crystal layer, m2/g; 2Aslit is the specific surface area of the pore, m2/g; K is the maximum adsorption per unit area, mmol/m2; PL is the Langmuir pressure, MPa; and P is the gas pressure, MPa.
Around the whole experimental process, the desorption curve is above the adsorption curve, with the obvious adsorption hysteresis phenomenon. The obvious adsorption hysteresis is the most obvious when P/P0 = 0.63. According to the IUPAC standard classification of gas adsorption types, the shale adsorption type meets type IV nitrogen adsorption. When P/P0 < 0.78, the adsorption capacity increases by 42% and the adsorption curve increases slowly. At this stage, the adsorption is mainly monolayer and multilayer nitrogen molecules. When P/P0 > 0.78, the adsorption capacity of nitrogen increased significantly, and the adsorption curve gradually became concave. In the end, the adsorption capacity still increases, but the nitrogen molecules do not reach saturation, resulting in capillary condensation of nitrogen molecules. According to the above analysis, it can be judged that the shale contains a lot of mesopores (2–50 nm), as shown in Figure 8a.
Figure 8.
Nitrogen adsorption curve; (a) desorption and adsorption curve and (b) change curve of nitrogen adsorption content.
It can be seen from Figure 8b that the adsorption capacity is also significantly different for different CO2 treatment times. Specifically, with the increase of treatment time, the adsorption amount of nitrogen increases by 30.79% in 0–3 days, 12.41% in 3–7 days, and 4.51% in 7–14 days. Therefore, the increase in nitrogen adsorption gradually tends to be flat, which shows that the erosion of CO2 on the pores of the rock and the ability to generate pores are the most obvious during the initial immersion. As time increases, the new cracks or pore erosion appear within 3–7 days, but the erosion rate and the formation of cracks are slower than 0–3 days, and the adsorption capacity does not change much within 7–14 days. This indicates that the ability to form corrosion and pores gradually slows down. This phenomenon is reflected in the increase of nitrogen adsorption capacity and specific surface area, and the distribution of pore area is more extensive in the pore structure. It shows that the higher the degree of pore development, the stronger the ability to form complex pore networks.
Figure 9a shows the pore structure changes of shale after treatment in CO2 for different times. It can be seen from Figure 9a that with the increase of treatment time, the volume of shale pore structure increases. This indicates that the pore structure of shale is eroded under the action of SC-CO2. It is difficult for the pore structure of shale to change rapidly in the presence of only CO2. However, even under drying conditions, there are water molecules in the shale’s pore structure, mostly the bound water and a small amount of free water in micropores and mesopores. SC-CO2 reacts with water molecules to form carbonate or bicarbonate, which corrodes the framework of the shale structure.
Figure 9.
Pore structure diagram; (a) change curve of pore structure under different treatment times and (b) pore structure change curve.
Figure 9b shows that with the increase of treatment time, the mesoporous structure volume increases obviously. This is related to the increase in the pore size of some micropores to mesopores after CO2 corrosion. With the SC-CO2 treatment, clay minerals are prone to swell after acid-sensitive reaction, clog nanopores, and affect the permeability of the shale reservoir. However, the corrosion effect of CO2 is obviously greater than that of swelling. This is because there are more carbonate minerals than clay minerals, and the water in the clay minerals combines with CO2 to make the clay particles smaller. The diameter of CO2 molecules is 0.33 nm, which can quickly enter the micropores. In micropores, bound water is the main form because molecular forces such as hydrogen bonds and van der Waals forces between water molecules and clay minerals play a more critical role.21 Therefore, the micropores are corroded by SC-CO2. The macropores’ pore volume hardly changes, which is related to the less carbonate or bicarbonate formed in the macropores.
3.3.2. Macropore Distribution Characteristics
It is not comprehensive enough to rely solely on SC-CO2 to analyze the micro–nano pore structure. Therefore, the experimental study of micro CT is carried out in this paper. Figure 10 shows the comparison of the development of shale microcracks after SC-CO2 treatment. The experiment uses micron CT scans to explore the degree of development of shale fractures as the SC-CO2 treatment time increases. When the core is scanned for the second time, it is controlled to scan the same position, and the magnification used simultaneously is the same before and after the experiment. It can be seen from Figure 10 that the longer the CO2 treatment time, the more obvious the crack growth.
Figure 10.
Crack changes in different treatment times; (a) untreated, (b) treatment for 3 days, (c) treatment for 7 days, and (d) treatment for 14 days.
There are microcracks in the rock before SC-CO2 has been treated. By comparing the crack growth law of different times after SC-CO2 treatment, it is found that the longitudinal cracks and transverse cracks tend to cross. Under the action of CO2, not only the pore structure of the rock at the micropore scale has undergone significant changes but also the crack propagation at the macropore scale has also been greatly promoted.
4. Discussion
It is not difficult to find that shale mechanical properties’ change is closely related to shale mineral composition and pore structure changes by analyzing the above test results. From a different perspective, the reasons for the changes in the mechanical properties of rocks under SC-CO2 immersion are analyzed.
4.1. Influence of Mineral Composition on Mechanical Properties
The shale mineral composition is mainly composed of quartz, carbonate, and clay minerals. XRD results show that the contents of other minerals decrease during the SC-CO2 treatment process except for quartz. The increase in the percentage of quartz can be considered the result of the decrease in other minerals. In the process of SC-CO2 treatment shale, the chemical reactions with feldspar and calcite are as follows22
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
Figure 11 shows the change in mechanical properties corresponding to the content of a single mineral. Under the SC-CO2 treatment, the contents of all minerals except quartz have decreased to varying degrees. The mechanical properties of each single mineral content are analyzed. It is found that quartz has a positive correlation with tensile strength, uniaxial compressive strength, and Young’s modulus. Moreover, it is negatively correlated with Poisson’s ratio. The reason for the analysis is that the immersion of SC-CO2 reduces the content of carbonate minerals in the rock, and this in turn weakens the connection between mineral particles, reduces friction, and reduces the mechanical strength of the rock.
Figure 11.
Mechanical properties corresponding to different mineral contents; (a) relationship curve between mineral content and strength parameters and (b) relationship curve between mineral content and elastic parameters.
4.2. Influence of Pore Structure on Mechanical Properties
In the nitrogen adsorption test, the amount of nitrogen adsorption characterizes the degree of pore structure development. The higher the nitrogen adsorption content, the higher the degree of pore development and the larger the volume of the pore structure. Figures 12 and 13 show the nitrogen adsorption capacity, macroscopic fracture characteristics, and mechanical performance curves under CT scans. It can be seen from the figure that the higher the nitrogen adsorption capacity, the higher the degree of fracture development. The reason is that with the extension of SC-CO2 treatment time, some mineral components in shale are dissolved, which increase the original porosity and specific surface area of shale. At the same time, new pores and cracks are created at the microlevel, which increase structural connectivity. Figure 14 is a schematic diagram of shale pore development under SC-CO2 immersion. It can be seen that the change of pore structure makes shale more prone to microcracks under an external load, which is manifested as the deterioration of shale mechanical properties.
Figure 12.
Relationship curve between nitrogen adsorption content and mechanical properties; (a) tensile strength, (b) uniaxial compressive strength, (c) elastic modulus, and (d) Poisson’s ratio.
Figure 13.
Relationship curve between crack characteristics and mechanical properties; (a) tensile strength, (b) uniaxial compressive strength, (c) elastic modulus, and (d) Poisson’s ratio.
Figure 14.
Development of internal fissures in shale.
5. Conclusions
In this paper, the shale in China is selected for SC-CO2 treatment experiments under different treatment time conditions. The influence of SC-CO2 on the mechanical properties of shale is analyzed through the Brazil splitting experiment and uniaxial compression experiment. Also, the changes in shale microstructure are analyzed by XRD and SEM tests. In addition, the development law of shale pore structure is studied through nitrogen adsorption and the CT scanning technology. The main conclusions are as follows:
-
(1)
The tensile strength, uniaxial compressive strength, and elastic modulus of shale after SC-CO2 treatment all decrease to varying degrees. Furthermore, as the treatment time increases, the loss of shale mechanical parameters increases, but the loss rate shows a decreasing trend. Among them, the loss effect of the elastic modulus is the most obvious. At the same time, Poisson’s ratio increases with the increase of treatment time, but the increasing rate gradually decreases and finally is generally stable. It shows that SC-CO2 has a significant deteriorating effect on the mechanical properties of shale.
-
(2)
According to the results of XRD and SEM, it can be seen that after SC-CO2 treatment, the mineral composition of shale has changed to different degrees, and the surface morphology of shale has been changed. The carbonate minerals react with CO2, and the content significantly decreases. At the same time, under SEM observation, there are more honeycomb pores on the shale surface. The analysis shows that treatment of SC-CO2 reduces the brittle mineral content of shale, thereby weakening the strength characteristics of shale.
-
(3)
After SC-CO2 treatment, the pore structure of shale is developed, especially micropores and mesopores. With the increase of treatment time, the decrease of SC-CO2 reaction minerals on the pore surface of shale leads to the gradual slowing down of the development speed of pores. However, in micron-level fractures, as the CO2 action time increases, the degree of fracture development accelerates, and the rate of fracture development does not decrease significantly. The generation of new fractures continuously exposes minerals that can react with CO2, which promotes the development of shale fractures. On the other hand, the continuous development of pore structure reduces the strength characteristics of shale.
Acknowledgments
This research was funded by Central Program of Basic Science of National Natural Science Foundation of China (72088101), National Key Research and Development Program of China (2018YFE0196000), and Foundation Program for Directly Affiliated Institutions of CNPC (2019D-500808).
The authors declare no competing financial interest.
References
- Ren J.; Tan S.; Goodsite M. E.; Sovacool B. K.; Dong L. Sustainability, shale gas, and energy transition in China: assessing barriers and prioritizing strategic measures. Energy 2015, 84, 551–562. 10.1016/j.energy.2015.03.020. [DOI] [Google Scholar]
- Rutqvist J.; Rinaldi A. P.; Cappa F.; Moridis G. J. Modeling of fault activation and seismicity by injection directly into a fault zone associated with hydraulic fracturing of shale-gas reservoirs. J. Pet. Sci. Eng. 2015, 127, 377–386. 10.1016/j.petrol.2015.01.019. [DOI] [Google Scholar]
- Ikonnikova S.; Gülen G.; Browning J.; Tinker S. W. Profitability of shale gas drilling: a case study of the Fayetteville shale play. Energy 2015, 81, 382–393. 10.1016/j.energy.2014.12.051. [DOI] [Google Scholar]
- Westwood R. F.; Toon S. M.; Cassidy N. J. Sensitivity analysis of the effect of pumping parameters on hydraulic fracture networks and local stresses during shale gas operations. Fuel 2017, 203, 843–852. 10.1016/j.fuel.2017.05.004. [DOI] [Google Scholar]
- Yuan B.; Zheng D.; Moghanloo R. G.; Wang K. A novel integrated workflow for evaluation, optimization, and production predication in shale plays. Int. J. Coal Geol. 2017, 180, 18–28. 10.1016/j.coal.2017.04.014. [DOI] [Google Scholar]
- Jia Y.; Lu Y.; Elsworth D.; Fang Y.; Tang J. Surface characteristics and permeability enhancement of shale fractures due to water and supercritical carbon dioxide fracturing. J. Pet. Sci. Eng. 2018, 165, 284–297. 10.1016/j.petrol.2018.02.018. [DOI] [Google Scholar]
- Yu W.; Lashgari H. R.; Wu K.; Sepehrnoori K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. 10.1016/j.fuel.2015.06.092. [DOI] [Google Scholar]
- Wang J.; Elsworth D.; Wu Y.; Liu J.; Zhu W.; Liu Y. The influence of fracturing fluids on fracturing processes: a comparison between water, oil and SC-CO2.. Rock Mech. Rock Eng. 2018, 51, 299–313. 10.1007/s00603-017-1326-8. [DOI] [Google Scholar]
- Middleton R. S.; Carey J. W.; Currier R. P.; Hyman J. D.; Kang Q.; Karra S.; Jiménez-Martínez J.; Porter M. L.; Viswanathan H. S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. 10.1016/j.apenergy.2015.03.023. [DOI] [Google Scholar]
- Tang J.; Lu Y.; Chen Y.; Zhang X.; Ao X.; Jia Y.; Li Q. Experimental study on the influence of supercritical carbon dioxide treatment pressure on the mechanical properties of shale. Rock Soil Mech. 2018, 39, 797–802. [Google Scholar]
- Guo X.; Ni H.; Li M.; Zhang L.; Wang Y.; Ding L. Experimental study on the influence of supercritical carbon dioxide treatment pressure on the mechanical properties of shale. Indian Geotech. J. 2018, 48, 384–391. 10.1007/s40098-017-0289-8. [DOI] [Google Scholar]
- Jia B.; Tsau J.-S.; Barati R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs.. Fuel 2019, 236, 404–427. 10.1016/j.fuel.2018.08.103. [DOI] [Google Scholar]
- Mehic M. R.; Ranjith P. G.; Choi S. K.. The Geomechanical Behavior of Australian Black Coal under the Effects of CO2 Injection: Uniaxial Testing. Geoshanghai International Conference, 2006; pp 290–297.
- Lahann R.; Mastalerz M.; Rupp J. A.; Drobniak A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. 10.1016/j.coal.2011.05.004. [DOI] [Google Scholar]
- Jiang Y.; Luo Y.; Lu Y.; Qin C. Effects of SC-CO2 treatment time, pressure, and temperature on microstructure of shale. Energy 2016, 97, 173–181. 10.1016/j.energy.2015.12.124. [DOI] [Google Scholar]
- Busch A.; Alles S.; Gensterblum Y.; Prinz D.; Dewhurst D.; Raven M.; Stanjek H.; Krooss B. Carbon dioxide storage potential of shales. Int. J. Greenhouse Gas Control 2008, 2, 297–308. 10.1016/j.ijggc.2008.03.003. [DOI] [Google Scholar]
- Yin H.; Zhou J.; Jiang Y.; Xian X.; Liu Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. 10.1016/j.fuel.2016.07.028. [DOI] [Google Scholar]
- Lyu Q.; Ranjith P.; Long X.; Ji B. Experimental investigation of mechanical properties of black shales after CO2-Water-Rock interaction. Materials 2016, 9, 663. 10.3390/ma9080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon J.; Kendall J.-M.; Maxwell S. C. A coMParison of passive seismic monitoring of fracture stimulation from water and SC-CO2 injection. Geophysics. 2010, 75, MA1. 10.1190/1.3377789. [DOI] [Google Scholar]
- Zhang X.; Lu Y.; Tang J.; Zhou Z.; Liao Y. Experimental study on fracture initiation and propagation in shale using supercritical carbon dioxide fracturing. Fuel 2017, 190, 370–378. 10.1016/j.fuel.2016.10.120. [DOI] [Google Scholar]
- Yang L.; Ge H.; Shi X.; Cheng Y.; Zhang K.; Chen H.; Shen Y.; Zhang J.; Qu X. The effect of microstructure and rock mineralogy on water imbibition characteristics in tight reservoirs. J. Nat. Gas Sci. Eng. 2016, 34, 1461–1471. 10.1016/j.jngse.2016.01.002. [DOI] [Google Scholar]
- Nešić S. Key issues related to modelling of internal corrosion of oil and gas pipelines – A review. Corros. Sci. 2007, 49, 4308–4338. 10.1016/j.corsci.2007.06.006. [DOI] [Google Scholar]