Abstract
Unambiguous assignments have been made for each individual pKa value of the amino group and guanidine substituents on 2-deoxystreptamine, neamine, neomycin, paromomycin, and streptomycin by pH-titration evaluation of their 1H, 13C, and 15N (by 1H-15N heteronuclear multiple-bond correlation (HMBC) spectra) NMR chemical shifts (δXs) as the reporter nuclei. These data require minor revisions of the literature data in terms of the assignment order for neomycin and paromomycin. In situ titrations and NMR spectroscopy are shown to be a powerful combination for rapidly (minutes) obtaining each distinct pKa value of the similar amine and guanidine functional groups, which decorate aminoglycoside antibiotics.
Introduction
Aminoglycosides are polyamine natural products consisting of amino sugars linked by glycosidic bonds, hence their class name.1 Potentiometric titration and UV methods have been used to determine ionization constants (pKa). However, in polyamines, no direct link can be drawn from an individual amino group to a specific pKa value by those techniques.2 In this study, we determine the individual pKa values of four selected aminoglycoside alkaloids from Streptomyces and Micromonospora by detailed multinuclear nuclear magnetic resonance (NMR) spectroscopy, using 1H, 13C, and 15N as the reporter nuclei. This combination, including 1H-15N HMBC spectroscopy, is a rapid and accurate method to gain quantitative data and thereby important molecular structural information.
The first aminoglycoside, streptomycin, was isolated from Streptomyces griseus in 1943 by Waksman. Indeed, Waksman and his research group also isolated the second aminoglycoside, neomycin, from Streptomyces fradiae in 1949 and, in the following years, another naturally occurring aminoglycoside, paromomycin, was discovered.3−6 Aminoglycosides contain an aminocyclitol, a 1,3-diaminoinositol moiety; 2-deoxystreptamine, as found in neamine, neomycin, and paromomycin; or a streptidine ring as found in streptomycin, linked by glycosidic bonds to sugars, such as L-neosamine, D-neosamine, and D-ribose as found in neamine, neomycin, and paromomycin; or to streptose and glucosamine as found in streptomycin.7−9 Aminoglycosides are primarily used for the treatment of bacterial infection caused by Gram-negative (aerobic) or Gram-positive bacteria.1,6,10,11 They act on the 16S fragment of ribosomal RNA (rRNA) located in the 30S subunit of the 70S bacterial ribosome, leading to cell death.12−16 The amino functional group substituents around the different rings, including the similar, but slightly different two monosubstituted guanidines in streptomycin, are key to the biological activities of these natural product alkaloids. Knowing the pKa values of these polyamine alkaloids will afford a better understanding of their structure–activity relationships (SARs) especially the order in which these similar functional groups gain/lose protons, i.e., ammonium ions/free amine bases. Such data will potentially help in understanding the order of target rRNA binding of key basic functional groups.
It is clear that for polyamine molecules, when they are studied in aqueous solutions of different acidity, the 15N nuclei are the primary pH/pD-dependent NMR detectors, i.e., so-called “NMR probes”, while the 1H and 13C nuclei are only secondary probes. Hence, the 15N NMR spectral data are the most important in this case, but unfortunately they are also much more difficult to obtain. This issue has been addressed previously using the methodology of 15N observation, i.e., the determination of midpoints of 15N NMR titration curves vs pH, and comparison of the pKa values found classically, by potentiometry or UV spectroscopic methods,2 with those from the analysis of 15N NMR data. After Coxon’s work on the 15N NMR spectroscopy of amino sugars,17 Botto and Coxon reported on neomycin B and related aminoglycosides.18 Paschal and Dorman reported on the nebramycin aminoglycosides19 comparing the pKa values derived from 15N and 13C NMR spectroscopies, completing the assignment of the 15N resonances of apramycin.20 They showed that hydroxylation adjacent to one of the basic nitrogen atoms of apramycin changed the pKa values of all five amino functional groups in the molecule.20 Serpersu and co-workers have reported solution NMR spectroscopic studies and conformational comparisons between isepamicin (from the kanamycin group of aminoglycosides), butirosin A, and ribostamycin, a neomycin group aminoglycoside.21,22 They also made a detailed NMR study of neomycin B binding to the aminoglycoside phosphotransferase (3′)-IIIa.23 Likewise, the order of the pKa values of tobramycin has been studied by 15N NMR spectroscopy.16,24 Studying whether aminoglycosides adopt similar conformations when bound to RNA and protein targets may have significant implications in the design of enzyme inhibitors and/or antibiotics. Therefore, we are studying new combinations of multinuclear NMR spectroscopic data as a rapid analytical method to deconvolute accurately the individual pKa data of these polyamines in order to know what chemical structure each molecule has at physiological pH.16
Results
1. pKa Values of the Individual Amino Groups of 2-Deoxystreptamine
2-Deoxystreptamine is the central moiety of neamine, neomycin, and paromomycin. It is a cyclohexane ring substituted with two primary amines with a plane of symmetry. As this 4,5-di-O-substituted moiety is central to our aminoglycosides of interest, it was chosen as a model compound on which to determine the accuracy and precision of measuring the pKa values of these polyamine alkaloids in situ using 1H, 13C, and 1H-15N HMBC NMR spectroscopies. The pKa values of the two individual nitrogen atoms of 2-deoxystreptamine, shown in Figure 1 and Table 1, were extracted from the inflection points of the nonlinear sigmoidal curves (using Prism 7). Figure 1 clearly shows NMR titration curves (each n = 3) in (A) each 1H, (B) each 13C, and (C) 1H-15N HMBC of N-1/3 chemical shifts in 99.97% D2O at 25 °C.
Figure 1.

NMR titration curves (each n = 3) for (A) 1H, (B) 13C, and (C) 1H-15N HMBC of N-1/3 chemical shifts of 0.631–0.369 M 2-deoxystreptamine in 99.97% D2O at 25 °C, and (D) pKa values of individual nitrogen atoms of 2-deoxystreptamine determined using 1H (red), 13C (blue), and 15N HMBC (green) NMR spectroscopies. Expansions of NMR titration curves of (E) H-1/3 and (F) C-1/3 (error bars from each n = 3) in 99.97% D2O at 25 °C showing the inflection points of each curve.
Table 1. pKa Values of Individual Nitrogen Atoms of 2-Deoxystreptamine Determined Using 1H, 13C, and 1H−15N HMBC NMR Spectroscopies (n = 3).
The error bars in these determinations about the mean of each chemical shift and of the pH meter values are from MS Excel, and the tiny spread in the calculated pKa values, typically ±0.05, is from Prism (n = 3). Using the NMR technique, these errors are, perhaps not surprisingly, small when titrating in situ and then measuring both the chemical shift and the local pD and then converting the latter into pH (pH = pD – 0.5). Here, we chose to follow the IUPAC Technical Report Guidelines of Popov et al., subtracting 0.5 from the measured pD value,25 rather than subtracting 0.4,26 or applying the equation pH = 0.929pD + 0.42 determined experimentally by Krȩżel and Bal.27 Typically, these errors are <0.1 ppm in 1H, 13C, and 15N and likewise the differences in pH were <0.1, giving rise to measured pKa values of individual nitrogen atoms with an error typically <0.05 obtained with each of the three chosen nuclei. The effects of temperature on 1H and 13C NMR chemical shifts at 25, 35, and 50 °C and of dilution were also shown to be minimal, if any, at the typical concentrations used in these NMR studies. After these initial studies (n = 3), all three of the NMR experiments were repeated (n = 2), and it was shown that this gave similarly reproducibly accurate data. Subsequent in situ NMR spectroscopic titrations of aminoglycosides in D2O were therefore performed at n = 1 providing accurate data.
Due to the symmetrical structure of 2-deoxystreptamine, the signals of H-1/3, C-1/3, and N-1/3 have the same chemical shifts (δX). Therefore, there are two inflections in the H-1/3, C-1/3, and N-1/3 sigmoidal curves. Each inflection point of each curve can be read as a pKa value. These two points are less easily seen in Figure 1A,B, but they are clearly seen using the expanded scale in Figure 1E,F from the reporter nuclei C-1/3 substituted with N-1/3 and from H-1/3 on those carbon atoms shown as NMR titration curves of H-1/3 and C-1/3.
In this work, the amino groups on 2-deoxystreptamine were numbered by comparing them to the 2-deoxystreptamine ring of neamine, neomycin, and paromomycin. The labeling of the symmetrical ring makes sense after a protonation event. In this context, the first pKa is easier to understand. The second less so, but it gives valuable information, which is relevant for the analysis of other molecules in the study. These amino group protons are highly fluctional on the NMR time scale. It is important to observe that the relative direction of travel of the chemical shifts is in the opposite directions from 1H and 15N to 13C.16 The two pKa values of 2-deoxystreptamine were determined to be 7.00 ± 0.05 and 9.26 ± 0.05.
2. pKa Values of the Individual Amino Groups of Neamine
Neamine has four primary amino group substituents on two rings, one of which is 2-deoxystreptamine, the other is a D-amino sugar on a D-glucose hexose template, D-neosamine. All the pKa determinations using 1H, 13C, and 1H-15N HMBC NMR spectroscopies were repeated twice. The average values of the chemical shifts of 1H, 13C, and 1H-15N HMBC of neamine, at different pHs, were plotted against the in situ measured pH values of the solution. The pKa values of each individual nitrogen atom of neamine, shown in Figure 2 and Table 2, were then extracted from the inflection points of the nonlinear sigmoidal curves.
Figure 2.
NMR titration curves (each n = 2) for (A) 1H, (B) 13C, and (C) 1H-15N HMBC chemical shifts (δX) of 0.243–0.155 M neamine in 99.97% D2O at 25 °C, and (D) pKa values of individual nitrogen atoms of neamine determined using 1H (red), 13C (blue), and 15N HMBC (green) NMR spectroscopies.
Table 2. pKa Values of Individual Nitrogen Atoms of Neamine Determined Using 1H, 13C, and 1H−15N HMBC NMR Spectroscopies in this Work (n = 2) and Compared with the Published Data, as Indicateda,b,c.
pKa values of individual nitrogen atoms of neamine determined using 1H NMR spectroscopy in D2O relative to the HDO peak at δ = 4.8 ppm, at 25 °C.28
This work (n = 2).
The pKa value of N-6′ of neamine determined using 1H NMR spectroscopy (in this work) is the average pKa of the values of N-6′ obtained using 1H NMR spectroscopic data for H-6′a (8.33) and 6′b (8.35).
After calculating, the average pKa values, using 1H, 13C, and 1H-15N HMBC NMR spectroscopic data, for each individual nitrogen atom on neamine are as follows: N-1 = 7.60, N-3 = 6.51, N-2′ = 7.11, and N-6′ = 8.31. The assignment order of the average ionization constants is N-6′ > N-1 > N-2′ > N-3. These pKa values are consistent in magnitude and in assignment order with those reported in the literature.28
3. pKa Values of the Individual Amino Groups of Neomycin
Neomycin is the most basic aminoglycoside with its six primary amino group substituents on two amino sugar rings, D-neosamine and L-neosamine, and the central cyclohexane ring (a 4,5-O-disubstituted 2-deoxystreptamine). These three six-membered rings are themselves O-substituents pendant from a D-ribose furanose ring. Using the selected reporter nuclei of neomycin, whose 1H, 13C, and 1H-15N HMBC chemical shifts (ppm) are measured in 99.97% D2O at 25 °C and pD 2.55 are shown in Figure 3A; we have followed the in situ titrations. The spectra obtained with neomycin are representative of the analytical data we have collected in each aminoglycoside titration experiment, e.g., from pD = 2.55 to 12.30 as a 1H stack plot (Figure 3B), the 1H-15N HMBC overlay at pD 5.25 (black) and pD 12.12 (red) (Figure 3C), and 13C-1H heteronuclear single quantum correlation (HSQC) overlay at pD 6.12 (black) and pD 12.12 (red) (Figure 3D). The average (n = 2) of the chemical shifts of 1H, 13C, and 1H-15N HMBC of neomycin at different pHs were plotted against the pH values of the solution. The pKa values of individual nitrogen atoms of neomycin (Figure 3 and Table 3) were then extracted from the inflection points of the nonlinear sigmoidal curves using each of 1H, 13C, and 1H-15N HMBC NMR spectroscopies.
Figure 3.

(A) 1H, 13C, and 1H-15N-HMBC chemical shifts (δX) of 1, 3, 2′, 6′, 2‴, and 6‴ of neomycin were measured relative to TMSP, for 1H and 13C, and CH3NO2, for 1H-15N HMBC, in 99.97% D2O at 25 °C at pD 2.55. (B) 1H stack plot for neomycin. (C) Neomycin 1H-15N HMBC overlay at pD 5.25 (black) and pD 12.12 (red). (D) Neomycin 13C-1H HSQC overlay at pD 6.12 (black) and pD 12.12 (red). In (C) and (D), some peaks have been labeled and arrows are added to indicate the change in the chemical shift upon titrating to 12.12. (E) NMR titration curves for the 1H chemical shifts (δH) (500 MHz) of 200 μM neomycin were measured relative to TMSP in 99.97% D2O at 25 °C. (F) NMR titration curves for the 1H chemical shifts (δH) (500 MHz) of 0.215–0.120 M neomycin were measured relative to TMSP in 100% H2O at 25 °C. (G) pKa values of individual nitrogen atoms of neomycin are determined using 1H (red), 13C (blue), and 1H-15N HMBC (green) NMR spectroscopies.
Table 3. pKa Values of Individual Nitrogen Atoms of Neomycin Determined Using 1H, 13C, and 1H−15N HMBC NMR Spectroscopies in this Work (n = 2) and Compared with the Published Data, as Indicateda,b,c,d,e,f.
pKa values of individual nitrogen atoms of neomycin determined using 15N NMR spectroscopy in H2O/D2O (90:10 v/v) relative to 15NH4Cl at 25 °C.23
pKa values of individual nitrogen atoms of neomycin determined using 15N NMR spectroscopy in H2O/D2O (85:15 v/v) relative to 15NH4NO3 at 25 °C.18
pKa values of individual nitrogen atoms of neomycin determined using 15N NMR spectroscopy in H2O/D2O (90:10 v/v) relative to NH3 by using 1.0 M 15N urea in DMSO at 25 °C.29
pKa values of individual nitrogen atoms of neomycin determined using 1H and 13C HSQC NMR spectroscopies in D2O at 25 °C.30
This work (n = 2).
The pKa value of N-6′ of neomycin determined using 1H NMR spectroscopy (in this work) is the average pKa of the values of N-6′ obtained using 1H NMR spectroscopic data for 6′a (8.65) and 6′b (8.70), and the pKa value of N-6‴ of neomycin determined using 1H NMR spectroscopy (in this work) is the average pKa of the values of N-6‴ obtained using 1H NMR spectroscopic data for 6‴a (8.72) and 6‴b (8.75).
The average pKa values, after calculating using 1H, 13C, and 1H-15N HMBC NMR spectroscopic data, of each individual nitrogen atom on neomycin are as follows: N-1 = 8.08, N-3 = 6.86, N-2′ = 7.98, N-6′ = 8.65, N-2‴ = 8.03, and N-6‴ = 8.76. The assignment order of the average ionization constants is as follows: N-6‴ > N-6′ > N-1 ≈ N-2′ ≈ N-2‴ > N-3, a revision of the assignment order reported in the literature.18,23,29,30
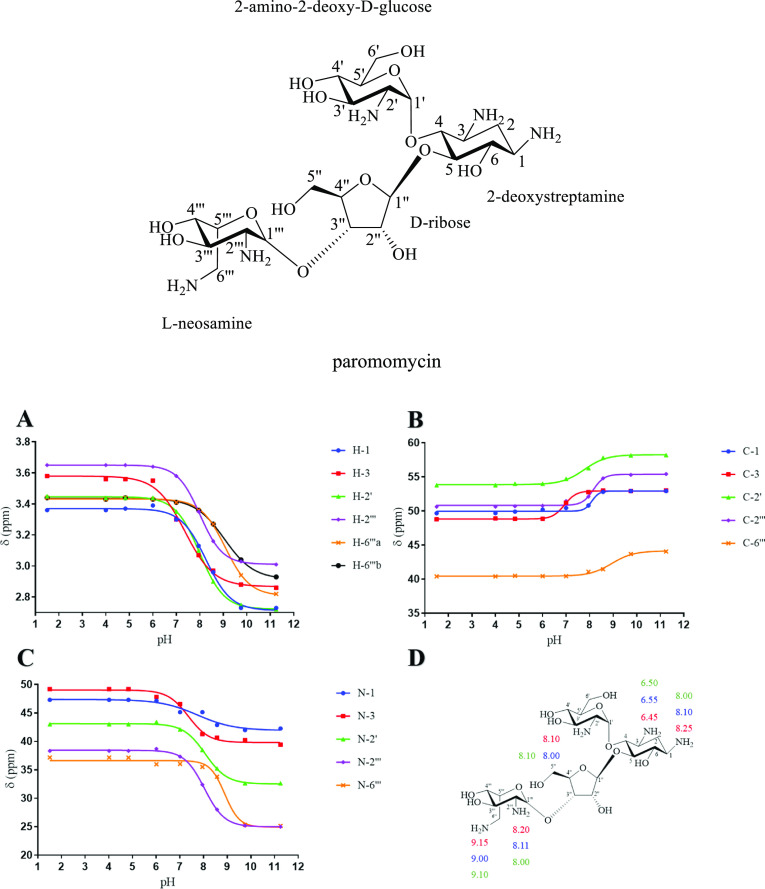
4. pKa Values of the Individual Amino Groups of Paromomycin
Paromomycin is a 4,5-O-disubstituted 2-deoxystreptamine with five primary amino groups which are substituents on two amino sugar rings: 2-amino-2-deoxy-D-glucose and L-neosamine 4,5-O-disubstituted on the central cyclohexane ring (2-deoxystreptamine). These three 6-membered rings are themselves O-substituents pendant from a D-ribose furanose ring. The chemical shifts of 1H, 13C, and 1H-15N HMBC of paromomycin at different pHs were plotted against the pH values of the solution (n = 1) (Figure 4). The pKa values of individual nitrogen atoms of paromomycin (Figure 4 and Table 4) were then extracted from the inflection points of the nonlinear sigmoidal curves.
Figure 4.
NMR titration curves for (A) 1H, (B) 13C, and (C) 1H-15N HMBC chemical shifts (δX) of 0.335–0.208 M paromomycin in 99.97% D2O at 25 °C, and (D) pKa values of individual nitrogen atoms of paromomycin determined using 1H (red), 13C (blue), and 15N HMBC (green) NMR spectroscopies.
Table 4. pKa Values of Individual Nitrogen Atoms of Paromomycin Determined Using 1H, 13C, and 1H−15N HMBC NMR Spectroscopies in this Work and Compared with the Published Data, as Indicateda,b,c,d.
pKa values of individual nitrogen atoms of paromomycin free-base determined using 15N NMR spectroscopy in H2O/D2O (85: 15 v/v) relative to NH3 using 1 M [15N] urea in DMSO at 25 °C.31
pKa values of individual nitrogen atoms of paromomycin sulfate determined using 15N NMR spectroscopy in H2O/D2O (85:15 v/v) relative to NH3 using 1 M [15N] urea in DMSO at 25 °C.29
This work (n = 1).
The pKa value of N-6‴ of paromomycin determined using 1H NMR spectroscopy (in this work) is the average pKa of the values of N-6‴ obtained using 1H NMR spectroscopic data for 6‴a (9.14) and 6‴b (9.16).
The average pKa values, calculated using 1H, 13C, and 1H-15N HMBC NMR spectroscopic data, for each individual nitrogen atom on paromomycin are as follows: N-1 = 8.11, N-3 = 6.50, N-2′ = 8.06, N-2″’ = 8.10, and N-6″’ = 9.08, the new assignment order of the average ionisation constants is as follows: N-6″’ > N-1 ≈ N-2′ ≈ N-2″’ > N-3. These pKa values require a minor revision of the assignment order reported in the literature.29,31
5. pKa Values of the Individual Amino Groups of Streptomycin
Streptomycin has seven nitrogen atoms distributed within two guanidine groups on streptidine and secondary N-methylamine on an N-methyl-1-glucosamine ring. The streptose ring with its six-carbon atoms is a furanose, not a pyranose, and it is not a linear sugar with its methyl and aldehyde functional groups. The chemical shifts of 1H, 13C, and 1H-15N HMBC of streptomycin at different pHs were plotted against the pH values of the solution (n = 1) (Figure 5). The individual pKa values of the two guanidine groups (N-1 and N-3) and the secondary amine (N-methyl) of streptomycin (Figure 5 and Table 5) were extracted from the inflection points of the nonlinear sigmoidal curves.
Figure 5.
NMR titration curves for (A) 1H, (B) 13C, and (C) 1H-15N HMBC chemical shifts (δX) of 0.738–0.527 M streptomycin in 99.97% D2O at 25 °C, and (D) pKa values of individual nitrogen atoms of streptomycin determined using 1H (red), 13C (blue), and 15N HMBC (green) NMR spectroscopies. (E) 1H, 13C, and 1H-15N-HMBC chemical shifts (δX) of 1, 3, and 2′ of streptomycin were measured relative to TMSP, for 1H and 13C, and CH3NO2, for 1H-15N HMBC, in 99.97% D2O at 25 °C at pD 5.10.
Table 5. pKa Values of the Two Guanidine Groups (N-1 and N-3) and the Secondary Amine (N-Methyl) N-2′ of Streptomycin Determined Using 1H, 13C, and 1H−15N HMBC NMR Spectroscopies in this Work and Compared with the Published Data, as Indicateda,b.
pKa values of individual nitrogen atoms of streptomycin determined using 1H NMR spectroscopy in H2O/D2O (95:05 v/v) relative to DSS at 25 °C.32
This work (n = 1).
The three average pKa values after calculating using 1H, 13C, and 1H-15N HMBC NMR spectroscopic data of the two guanidine groups (N-1 and N-3) and the secondary amine (N-methyl, N-2′) on streptomycin are as follows: N-1 (guanidine) = 13.06, N-3 (guanidine) = 12.06, and N-2′ = 8.16. The assignment order of the average (δx) ionization constants is as follows: N-1 > N-3 > N-2′. These pKa values are consistent in the assignment order with those reported in the literature.32
Discussion
The nitrogen atoms are flipping between protonated and unprotonated states, so they are sensitive to the pH of the environment and will naturally shift significantly in the NMR experiment. For carbon atoms, we are primarily detecting shifts on carbon atoms that are attached by one bond to a nitrogen atom that is either protonated or not. The NMR shifts seen are explained by inductive effects (the increased charge at the nitrogen atom causes a change in the carbon–nitrogen bond), but note that this is not simply the intuitive inductive effect, as the shift for 13C goes in the “wrong” direction, as discussed previously.16 We still see the shifts on titration in the proton chemical shifts that are two bonds away from the affected nitrogen atoms. In this case, the inductive effect matches our expectation and can be explained akin to swapping an electronegative atom for a proton in a simple alkane. Furthermore, the magnitude of the shift observed in titration also reflects the relative parts per million scale range (1H = 20 ppm, 13C = 200 ppm, and 15N = 100 ppm).
The short total time taken for the experiment in this pKa by the in situ titration NMR spectroscopic method depends on the concentration. The accuracy and resolution depend upon having suitable signals present to monitor and follow in that titration. It is ideal to use well-resolved 1H spectra right across the pH range under investigation, although it is possible to follow individual peaks as they move during the titration. If 13C or 15N data are needed, then samples will need to be of reasonable concentrations, more so for 15N data, even though both HSQC and HMBC are 1H-detected NMR spectroscopic experiments. While detecting 1H alone is rapid down to concentrations of a few millimolar, this will not be true for 13C or 15N. However, for molecules of low molecular weight (perhaps under 500 Da), a few milligrams of the sample in a standard 5 mm NMR tube, typically in 0.4 mL of solvent, will give suitable 1H-13C HSQC and 1H-15N HMBC data to allow a pKa to be determined in only a few hours. At such concentrations, the 1H measurement only takes minutes; indeed, titrating (adjusting) and then measuring the pH is the rate-limiting step that can still be achieved in minutes. Typically, the 1H spectra are obtained in 1–2 min per data point (assuming either 8 or 16 scans per spectrum). For 1H-13C HSQC data, the standard experimental setup is ns = 2 with the number of increments as td = 256. This gives an acquisition time of ∼20 min per data point, although this can be reduced (e.g., ns = 1 and td = 128 would require ∼5 mins, but with reduced data quality). Similarly, the 1H-15N HMBC experiment uses ns = 8 and td = 128 requiring ∼40 min per data point. However, improvements in modern NMR spectrometers are routinely reducing the time needed for such experiments, with advances such as nonuniform sampling (NUS) and parallel acquisition (acquiring more than one data set simultaneously). Broadly, we have shown that the experimental pKa value derived from each of the three nuclei is similar, and thus if only 1H can be used spectroscopically, that will suffice to provide accurate pKa values.
The chemical shifts corresponding to the H-1/3 of 2-deoxystreptamine when measured at 0.630 M and 0.157 M, at low pD (∼2), did not shift with the change in concentration. Also, pKa data determined over a 1000-fold dilution using a sample of neomycin (218 and 0.200 mM) at four pH values were not significantly different when measured by 1H NMR spectroscopy (Figure 3E and Table 3). Likewise, there was no significant difference studying dissociation constants in D2O instead of pure H2O (Figure 3F). Furthermore, NMR experiments at 25, 35, and 50 °C measuring the proton and carbon chemical shifts gave constant, not temperature-dependent, responses. Therefore, we concluded that the pKa values of these aminoglycosides are not affected by changes in concentration or in the temperature at these typical NMR experiment ranges.
The measurement of the individual ionization constants of polybasic compounds is known to be complex when the separate functional groups have similar constants.33 These problems are particularly acute when measuring, e.g., in streptomycin, the basicity of two different monoalkyl guanidines, the most basic functional group in medicinal chemistry. This study is a continuation of our pKa investigations by multinuclear NMR spectroscopy on other aminoglycosides, tobramycin, kanamycin, amikacin, sisomicin, and netilmicin, recently published in this Journal.16 The correct assignment of the pKa values of amines of an aminoglycoside is important for the characterization of the binding mode of those antibiotics to the RNA. pKa determination by the NMR spectroscopy method has also been studied in significant detail by the Hägele research group for other functional groups, including different organophosphorus compounds, aminophosphonates, and phosphinic acids, whilst not for aminoglycosides.34−36
Using potentiometric titrations, the pKa values of neamine have been measured: pKa1, pKa2, and pKa3 are 6.35 ± 0.2, 7.73 ± 0.15, and 8.62 ± 0.08, respectively, but care must be taken in determining thermodynamic properties.37 Note that for the four amines, the potentiometric method could only resolve three values, presumably N-2′ is overlapping. The dependence of the solution structure of neamine on pH has also been determined by NMR and AMBER molecular dynamics analyses at pD 3.3, 6.5, and 7.4 in D2O at 25 °C. In acid, it essentially showed only one conformer.28 The pKa values determined by NMR titration experiments are as follows: pKa1 = 6.44 ± 0.13 for N-3 of ring-II, pKa2 = 7.23 ± 0.09 for N-2′ of ring-I, pKa3 = 7.77 ± 0.19 for N-1 of ring-II, and pKa4 = 8.08 ± 0.15 for N-6′ of ring-I.28 In this work, using δx, we determined the pKa values of neamine to be N-3 = 6.51, N-2′ = 7.11, N-1 = 7.60, and N-6′ = 8.31. Therefore, neamine exists as a mixture of tetra–/tri–/diprotonated species between pD 4.5 and 7.4, but it really is only a diprotonated species around physiological pDs. This may facilitate the binding of neamine-like aminoglycosides by favorable entropy of binding to the A-site of 16S rRNA, suggesting that novel aminoglycoside compounds carrying such a neamine-based pharmacophore can be developed.28 Such binding complexes are stabilized by the formation of multiple interactions with receptors. The crystal structures of complexes between aminoglycoside antibiotics, e.g., neamine and neomycin B, and oligonucleotides containing the decoding A-site of bacterial ribosomes have been reported at resolutions of 2.2–3.0 Å with up to eight conserved hydrogen bonds in the observed complexes.13 In a good example of the interplay between sterics and electronics, in the binding of streptomycin derivatives to nucleotides, the electrostatic forces are not always the dominant factor; the conformational fit is also important.38
Westhof and co-workers have clearly established the importance of structural and physicochemical parameters, e.g., pKa, in aminoglycoside–rRNA interactions.13,39 Not that every amino group must be (fully) charged, but that in such complexes the number of rings and hence the positions of positive charges promotes specific binding. Indeed, unlike in the example of streptomycin,38 the strength of the interaction of neomycin with RNA is dominated by electrostatics, with the positively charged aminoglycoside displacing metal ions.27 Asensio and co-workers have reported30 that a complete characterization of the protonation equilibrium that accompanies the molecular recognition of neomycin B by a specific 23-mer RNA receptor has been achieved by employing a simple NMR analysis. There were dramatic alterations in the neomycin B amino functional group protonation state upon aptamer binding measured using uniformly enriched 15N-neomycin.30 Some values rose by ∼1 pKa unit, one only by 0.3, and the pKa of N6‴ remained unaltered. The formation of a neomycin–phosphotransferase enzyme complex was determined by isothermal titration calorimetry (ITC). The enthalpy of binding became more favorable (negative) at a higher pH where binding-linked protonation was attributable mostly to the amino groups. Multiple interactions may affect the affinity of the ligand for the enzyme and changes in pH will affect this. Therefore, it remains important to determine the thermodynamic parameters of aminoglycoside–target interactions under different experimental conditions before making attributions to specific sites and their effects on these global parameters.28
Neomycin is the most basic aminoglycoside with its six primary amino groups. A primary alcohol replaces the N6′ primary amine (ammonium ion) in paromomycin whose X-ray crystal structure docked into the eubacterial ribosomal decoding A-site has been reported by Vicens and Westhof.40 In this work, using δX, the average pKas of neomycin are shown to be N-3 = 6.86, N-2′ = 7.98, N-2‴ = 8.03, N-1 = 8.08, N-6′ = 8.65, and N-6‴ = 8.76. Likewise, the average pKas of paromomycin are N-3 = 6.50, N-2′ = 8.06, N-2‴ = 8.10, N-1 = 8.11, and N-6‴ = 9.08. Significantly, in terms of the total number of positive charges, the number of aminoglycoside–RNA contacts is about the same in neomycin and paromomycin.13 Other X-ray crystal structures of aminoglycoside complexes and the decoding A-site oligonucleotides serve to emphasize the roles of the number of rings and the positive charges in the specific binding leading to miscoding.13 Barbieri and Pilch have reported a complete thermodynamic characterization of the multiple protonation equilibria of paromomycin as its binding to different macromolecular targets is coupled to selective protonation of its five amino functional groups.31 ITC studies conducted at a drug concentration of 45 mM revealed that the extent of paromomycin protonation showed that the binding of the drug to its pharmacologically relevant target, the 16S rRNA A-site, is consistent with the pKa values of the free base. With their co-workers, they concluded that it is important to study the protonation equilibria of the aminoglycoside amino groups as well as any concentration/ionic strength or temperature dependence, physicochemical analysis being a critical component of any drug design strategy.29,31
The individual ionization constants, the pKa values of the individual amino groups of 2-deoxystreptamine, neamine, neomycin, paromomycin, and streptomycin, were therefore determined using chemical shift variation with 1H, 13C, and 1H-15N HMBC NMR spectroscopies (Table 6). This was experimentally verified to be a rapid, accurate, precise, and reproducible technique. 1H NMR spectroscopy was the least time consuming (2 min for each data point), then 13C (30 min for each data point), and then 1H-15N HMBC (∼45 min for each data point) NMR spectroscopy. This technique is both accurate (typically, errors of ±0.1 ppm for each nucleus gave rise to measured pKa values of ±0.05) and precise as followed from the small error bars and the results tallying with those obtained in other laboratories and by potentiometric methods. Also, experimentally determined was the reproducibility where the error bars were typically smaller than the (typical, small) symbols used in plotting the curves; again, this is shown to be accurate for each of the three nuclei. This allowed n = 3 to be reduced to n = 1 with no loss of accuracy across a range of concentrations (see Experimental Section, Figure 3E and Table 3). Using the NMR reporter nuclei, the average pKa value for the primary amino groups attached directly to an amino sugar ring (R–NH2) was 7.63, and for the primary aminomethylene groups (R-CH2NH2), it was 8.70 (calculated from Table 6). This observation might result from the primary aminomethylene groups being less sterically hindered than the primary amino groups attached directly to the amino sugar rings.41
Table 6. Average pKa Values of Individual Nitrogen Atoms of the Indicated Aminoglycosides Using 1H, 13C, and 1H-15N HMBC NMR Spectroscopies.
| individual
nitrogen atoms |
||||||
|---|---|---|---|---|---|---|
| aminoglycoside | N-1 | N-3 | N-2′ | N-6′ | N-2‴ | N-6‴ |
| 2-deoxystreptamine (n = 3) | 9.26 | 7.00 | ||||
| 1H | 9.25 ± 0.05 | 6.97 ± 0.04 | ||||
| 13C | 9.35 ± 0.10 | 7.03 ± 0.05 | ||||
| 15N | 9.20 ± 0.05 | 7.01 ± 0.05 | ||||
| neamine (n = 2) | 7.60 | 6.51 | 7.11 | 8.31 | ||
| 1H | 7.62 ± 0.03 | 6.50 ± 0.04 | 7.10 ± 0.05 | 8.34 ± 0.05 | ||
| 13C | 7.60 ± 0.05 | 6.55 ± 0.05 | 7.05 ± 0.05 | 8.25 ± 0.05 | ||
| 15N | 7.60 ± 0.05 | 6.50 ± 0.05 | 7.20 ± 0.10 | 8.35 ± 0.05 | ||
| neomycin (n = 2) | 8.08 | 6.86 | 7.98 | 8.65 | 8.03 | 8.76 |
| 1H | 8.10 ± 0.07 | 6.90 ± 0.02 | 8.00 ± 0.05 | 8.67 ± 0.05 | 8.05 ± 0.05 | 8.73 ± 0.05 |
| 13C | 8.00 ± 0.05 | 6.90 ± 0.05 | 8.00 ± 0.05 | 8.70 ± 0.04 | 8.15 ± 0.06 | 8.86 ± 0.10 |
| 15N | 8.15 ± 0.10 | 6.80 ± 0.05 | 7.95 ± 0.07 | 8.60 ± 0.05 | 7.90 ± 0.10 | 8.71 ± 0.07 |
| paromomycin | 8.11 | 6.50 | 8.06 | 8.10 | 9.08 | |
| 1H | 8.25 | 6.45 | 8.10 | 8.20 | 9.15 | |
| 13C | 8.10 | 6.55 | 8.00 | 8.11 | 9.00 | |
| 15N | 8.00 | 6.50 | 8.10 | 8.00 | 9.10 | |
| streptomycin | 13.06 | 12.06 | 8.16 | |||
| 1H | 13.20 | 12.10 | 8.38 | |||
| 13C | 13.02 | 11.95 | 8.11 | |||
| 15N | 12.97 | 12.15 | 8.01 | |||
Conclusions
1H, 13C, and 1H-15N HMBC NMR spectroscopies with in situ pH titration is shown to be a powerful technique for the measurement of distinct pKa values that are accurate to <±0.1 and sometimes even down to ±0.02 (Table 6). Thus, unambiguous assignments have been made for each individual amino group and guanidine substituent on 2-deoxystreptamine, neamine, neomycin, paromomycin, and streptomycin using variations in the measured NMR chemical shifts (δx). These results demonstrate that, even in complex polyamines, individual amino group basicity can be rapidly (minutes) quantified, and therefore reactivity can be predicted by NMR techniques, which can lead to selective functionalization. A better understanding of SARs will follow a more precise knowledge of the actual functional groups on a molecule at physiological pH.
Experimental Section
Materials and General Methods
Deuterium oxide (99.97% D2O), DCl (20% concentration solution in D2O), and NaOD (30% concentration solution in D2O) were purchased from Goss Scientific (U.K.). Neamine free-base was purchased from Carbosynth (U.K.). 2-Deoxystreptamine dihydrobromide, neomycin sulfate, paromomycin sulfate, streptomycin potassium hydrogen phthalate, disodium tetraborate, sodium trimethylsilylpropanoate (TMSP), and nitromethane (CH3NO2) were purchased from Sigma–Aldrich (U.K.).
Instrumentation
NMR spectra including 1H, 13C, HSQC, HMBC, NOESY, and 1H-15N HMBC were recorded on a Bruker Avance III (operating at 500.13 MHz for 1H, 125.77 MHz for 13C, and 50.67 MHz for 15N) spectrometer at 25 °C (unless otherwise indicated). MestReNova and Bruker Topspin have been used for processing the spectra. 1H and 13C chemical shifts (δx) were observed and are reported in parts per million (ppm) relative to sodium trimethylsilylpropanoate (TMSP) at 0.00 ppm as an internal reference, and 1H-15N HMBC chemical shifts were measured relative to external nitromethane (CH3NO2 in CDCl3 (1:1, v/v)), recorded, and set at δN 379.8 ppm, and the sr value (correction factor) is measured as −511.72 on our spectrometer.42 The recording time differed for each isotope as follows: 2, 30, and ∼45 min per data point for 1H, 13C, and 1H-15N HMBC NMR spectroscopies, respectively, with NMR ns for 1H = 16, 13C = 512, 1H-13C HSQC = 2, and 1H-15N HMBC = 4.
Calibration of 5 mm NMR Tube-pH Electrode
A 5 mm NMR tube-pH electrode purchased from Sigma–Aldrich (U.K.) was used for measuring pH values. The electrode easily fitted into the 5 mm NMR tube. Standard buffers consisting of 0.40 M potassium hydrogen phthalate in H2O, pH 4.00, and 0.01 M disodium tetraborate in H2O, pH 9.18, were used to calibrate the 5 mm NMR tube-pH electrode. All the calibrations were carried out at 25 °C.
pKa Determinations Using 1H, 13C, and 1H-15N HMBC NMR Spectroscopies
Aminoglycoside analyte solutions (in 99.97% D2O) were typically prepared at ∼430 to 205 mg/mL, ∼0.7 to 0.3 M analyte, beginning from acidic pH and adjusting with 0.5 M NaOD (∼10 × 20 μL) to pH = 14, when the final concentration will have been diluted by ∼30% from ∼0.5 to 0.2 M. Specifically, the in situ titrations performed were as follows: 2-deoxystreptamine from 204.45 mg/mL across the titration range of 0.631–0.369 M; neamine from 78.33 mg/mL across 0.243–0.155 M; neomycin from 240.94 mg/mL across 0.392–0.218 M; paromomycin from 206.23 mg/mL across 0.335–0.208 M; and streptomycin from 429.20 mg/mL across 0.738–0.527 M. The pH values were adjusted using 0.5 M NaOD and 0.5 M DCl. MestReNova and Bruker Topspin were used for the analysis of the recorded spectra. 1H and 13C NMR chemical shifts and 1H-15N HMBC spectra of each aminoglycoside were obtained in 99.97% D2O at 25 °C starting, as set out above, in 0.4 mL in a 5 mm diameter NMR tube; then, data were collected at varying pH values diluted by titration with 0.5 M NaOD to a final volume of 0.6 mL. Both the change in solvent column height above the Bruker probe and the dilution makes essentially no difference to the quality of data collected. The transformed data were then plotted against the in situ pH values. The nonlinear sigmoidal curve and the inflection point of the sigmoidal curve were determined using GraphPad Prism 7 (version 2017), after the subtraction of 0.5 (following the IUPAC Technical Report Guidelines) in order to convert the measured pD values into pH values.25 The individual pKa values of each basic functional group on each aminoglycoside are these inflection points.
The data obtained from these 1H, 13C, and 1H-15N HMBC NMR spectroscopic experiments were determined to be precisely reproducible by repetitions (n = 3) starting with the simple symmetrical diamine, 2-deoxystreptamine, as a model compound. Similarly, each of the different NMR experiments was then repeated for neomycin (n = 2) to calculate the errors in the measurement of the following: pH values, chemical shifts (δ), and pKa values. The majority of the error bars for the pH values and for the chemical shifts were of a similar size as the (small, typical) symbols used to plot the points on the nonlinear sigmoidal curves. Typically, pKa values were accurate to <±0.1 and sometimes even down to ±0.02. Therefore, having determined by experiment that the typical size of the errors is small, it was judged that n = 1 was sufficient for obtaining further NMR spectroscopic data for each of paromomycin and streptomycin.
Acknowledgments
We thank the Government of the Kingdom of Saudi Arabia for fully funding this studentship to A.H.A. NMR equipment was provided by the Material and Chemical Characterization Facility at the University of Bath. We also acknowledge with gratitude the careful reading of a referee who alerted us to our omission16 that the external nitromethane reference standard was recorded and set at δN = 379.8 ppm,42 and the measured sr correction value was −511.72 on our spectrometer.
The authors declare no competing financial interest.
References
- Seiler N.; Hardy A.; Moulinoux J. P. Aminoglycosides and polyamines: Targets and effects in the mammalian organism of two important groups of natural aliphatic polycations. Prog. Drug Res. 1996, 46, 183–241. 10.1007/978-3-0348-8996-4_5. [DOI] [PubMed] [Google Scholar]
- Blagbrough I. S.; Metwally A. A.; Geall A. J.. Measurement of polyamine pKa values. In: Polyamines: Methods and Protocols, Methods in Molecular Biology, Springer Science LLC. 2011, 720, 493–503, 10.1007/978-1-61779-034-8_32. [DOI] [PubMed] [Google Scholar]
- Schatz A.; Bugle E.; Waksman S. A. Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Exp. Biol. Med. 1944, 55, 66–69. 10.3181/00379727-55-14461. [DOI] [PubMed] [Google Scholar]
- Waksman S. A.; Lechevalier H. A. Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science 1949, 109, 305–307. 10.1126/science.109.2830.305. [DOI] [PubMed] [Google Scholar]
- Davidson R. N.; den Boer M.; Ritmeijer K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 653–660. 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Armstrong E. S.; Kostrub C. F.; Cass R. T.; Moser H. E.; Serio A. W.; Miller G. H.. Aminoglycosides. In: Antibiotic Discovery and Development; 2nd ed; Dougherty T. J.; Pucci M. J., Eds.; Springer: Boston, 2012; pp. 229–270, 10.1007/978-1-4614-1400-1_7. [DOI] [Google Scholar]
- Hooper I. R.The Naturally Occurring Aminoglycoside Antibiotics. In: Aminoglycoside Antibiotics; Hooper I. R., Umezawa H., Eds.; Springer Verlag: New York, 1982; pp. 1–27. [Google Scholar]
- Maviglia R.; Nestorini R.; Pennisi M. Role of old antibiotics in multidrug resistant bacterial infections. Curr. Drug Targets 2009, 10, 895–905. 10.2174/138945009789108846. [DOI] [PubMed] [Google Scholar]
- Avent M. L.; Rogers B. A.; Cheng A. C.; Paterson D. L. Current use of aminoglycosides: Indications, pharmacokinetics and monitoring for toxicity. Intern. Med. J. 2011, 41, 441–449. 10.1111/j.1445-5994.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- Ramirez M. S.; Tolmasky M. E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins D.; Norris F. A.; Kumar S.; Arya D. P. A fluorescence-based screen for ribosome binding antibiotics. Anal. Biochem. 2013, 434, 300–307. 10.1016/j.ab.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D.; Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 1987, 327, 389–394. 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- François B.; Russell R. J. M.; Murray J. B.; Aboul-ela F.; Masquida B.; Vicens Q.; Westhof E. Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 2005, 33, 5677–5690. 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J. M.; Ramakrishnan V. Structural insights into translational fidelity. Annu. Rev. Biochem. 2005, 74, 129–177. 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- Kulik M.; Goral A. M.; Jasiński M.; Dominiak P. M.; Trylska J. Electrostatic interactions in aminoglycoside–RNA complexes. Biophys. J. 2015, 108, 655–665. 10.1016/j.bpj.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhzem A. H.; Woodman T. J.; Blagbrough I. S. Individual pKa values of tobramycin, kanamycin B, amikacin, sisomicin, and netilmicin determined by multinuclear NMR spectroscopy. ACS Omega 2020, 5, 21094–21103. 10.1021/acsomega.0c02744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon B. Nitrogen-15 n.m.r. spectroscopy of amino sugars. Carbohydr. Res. 1974, 35, C1–C3. 10.1016/S0008-6215(00)84858-2. [DOI] [Google Scholar]
- Botto R. E.; Coxon B. Nitrogen-15 nuclear magnetic resonance spectroscopy of neomycin B and related aminoglycosides. J. Am. Chem. Soc. 1983, 105, 1021–1028. 10.1021/ja00342a062. [DOI] [Google Scholar]
- Dorman D. E.; Paschal J. W.; Merkel K. E. Nitrogen-15 Nuclear magnetic resonance spectroscopy. The nebramycin aminoglycosides. J. Am. Chem. Soc. 1976, 98, 6885–6888. 10.1021/ja00438a020. [DOI] [PubMed] [Google Scholar]
- Paschal J. W.; Dorman D. E. Determination of pKa values using 15N and 13C nuclear magnetic resonance spectroscopy. The case of apramycin. Org. Magn. Reson. 1978, 11, 632–634. 10.1002/mrc.1270111210. [DOI] [Google Scholar]
- DiGiammarino E. L.; Draker K.; Wright G. D.; Serpersu E. H. Solution studies of isepamicin and conformational comparisons between isepamicin and butirosin A when bound to an aminoglycoside 6′-N-acetyltransferase determined by NMR spectroscopy. Biochemistry 1998, 37, 3638–3644. 10.1021/bi972778b. [DOI] [PubMed] [Google Scholar]
- Cox J. R.; Ekman D. R.; DiGiammarino E. L.; Akal-Strader A.; Serpersu E. H. Aminoglycoside antibiotics bound to aminoglycoside-detoxifying enzymes and RNA adopt similar conformations. Cell Biochem. Biophys. 2000, 33, 297–308. 10.1385/CBB:33:3:297. [DOI] [PubMed] [Google Scholar]
- Özen C.; Malek J. M.; Serpersu E. H.. Dissection of aminoglycoside–enzyme interactions: A calorimetric and NMR study of neomycin B binding to the aminoglycoside phosphotransferase (3′)-IIIa, J. Am. Chem. Soc. 2006, 128, 15248–−15254.;J. Am. Chem. Soc., 2007, 129, 11872. 10.1021/ja0643220 [DOI] [PubMed] [Google Scholar]
- Pagano T. G.; Gong Y.; Kong F.; Tsao R.; Fawzi M.; Zhu T. Structural characterization of the tobramycin–piperacillin reaction product formed at pH 6.0. J. Antibiot. 2011, 64, 673–677. 10.1038/ja.2011.72. [DOI] [PubMed] [Google Scholar]
- Popov K.; Rönkkömäki H.; Lajunen L. H. J. Guidelines for NMR measurements for determination of high and low pKa values (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 663–675. 10.1351/pac200678030663. [DOI] [Google Scholar]
- Glasoe P. K.; Long F. A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 1960, 64, 188–190. 10.1021/j100830a521. [DOI] [Google Scholar]
- Krȩżel A.; Bal W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. 10.1016/j.jinorgbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Andac C. A.; Stringfellow T. C.; Hornemann U.; Noyanalpan N. NMR and Amber analysis of the neamine pharmacophore for the design of novel aminoglycoside antibiotics. Bioorg. Chem. 2011, 39, 28–41. 10.1016/j.bioorg.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Kaul M.; Barbieri C. M.; Kerrigan J. E.; Pilch D. S. Coupling of drug protonation to the specific binding of aminoglycosides to the A site of 16S rRNA: Elucidation of the number of drug amino groups involved and their identities. J. Mol. Biol. 2003, 326, 1373–1387. 10.1016/S0022-2836(02)01452-3. [DOI] [PubMed] [Google Scholar]
- Freire F.; Cuesta I.; Corzana F.; Revuelta J.; González C.; Hricovini M.; Bastida A.; Jiménez-Barbero J.; Asensio J. L. A simple NMR analysis of the protonation equilibrium that accompanies aminoglycoside recognition: Dramatic alterations in the neomycin-B protonation state upon binding to a 23-mer RNA aptamer. Chem. Commun. 2007, 174–176. 10.1039/B611597G. [DOI] [PubMed] [Google Scholar]
- Barbieri C. M.; Pilch D. S. Complete thermodynamic characterization of the multiple protonation equilibria of the aminoglycoside antibiotic paromomycin: A calorimetric and natural abundance 15N NMR study. Biophys. J. 2006, 90, 1338–1349. 10.1529/biophysj.105.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgován G.; Noszál B. NMR analysis and site-specific protonation constants of streptomycin. J. Pharm. Biomed. Anal. 2012, 59, 78–82. 10.1016/j.jpba.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Nys P. S.; Savitskaya E. M.; Kolygina T. S. Determination of ionization constants of streptomycin by the indicator method. Pharm. Chem. J. 1971, 5, 576–579. 10.1007/BF00771672. [DOI] [Google Scholar]
- Bier A.; Failla S.; Finocchiaro P.; Hägele G.; Latronico M.; Libertini E.; Ollig J. Pyridin-2-yl-(I-propylamino)-methane phosphinic acid – Protonation and metal complex formation - NMR-controlled titration. Phosphorus, Sulfur Silicon Relat. Elem. 1999, 155, 89–100. 10.1080/10426509908044973. [DOI] [Google Scholar]
- Hägele G.; Szakács Z.; Ollig J.; Hermens S.; Pfaff C. NMR-controlled titrations: Characterizing aminophosphonates and related structures. Heteroat. Chem. 2000, 11, 562–582. . [DOI] [Google Scholar]
- Hägele G. Protolysis and complex formation of organophosphorus compounds — Characterization by NMR-controlled titrations. Molecules 2019, 24, 3238. 10.3390/molecules24183238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrisno; Baran Y.; Lawrance G. A.; von Nagy-Felsobuki E. I. Determination of acid dissociation constants of neamine by potentiometric and electrospray mass spectral techniques. Struct. Chem. 2001, 12, 189–195. 10.1023/A:1016604828024. [DOI] [Google Scholar]
- Fuentes-Martíneza J. P.; Gutiérrez-Rodrigueza D.; Garcia E. R.; Rivera-Mirqueza K. I.; Medrano F.; Torres-Ángeles O.; Castillo-Vargas E.; Duque Montaño B. E.; Godoy-Alcántar C. Streptomycin hydrazone derivatives: Synthesis and molecular recognition in aqueous solution. Nat. Prod. Commun. 2014, 9, 1449–1455. [PubMed] [Google Scholar]
- Walter F.; Vicens Q.; Westhof E. Aminoglycoside–RNA interactions. Curr. Opin. Chem. Biol. 1999, 3, 694–704. 10.1016/S1367-5931(99)00028-9. [DOI] [PubMed] [Google Scholar]
- Vicens Q.; Westhof E. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure 2001, 9, 647–658. 10.1016/S0969-2126(01)00629-3. [DOI] [PubMed] [Google Scholar]
- Krężel A.; Szczepanik W.; Świątek M.; Jeżowska-Bojczuk M. Acid–base versus structural properties of an aminoglycoside antibiotic – sisomicin: NMR and potentiometric approach. Bioorg. Med. Chem. 2004, 12, 4075–4080. 10.1016/j.bmc.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Wishart D. S.; Bigam C. G.; Yao J.; Abildgaard F.; Dyson H. J.; Oldfield E.; Markley J. L.; Sykes B. D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR 1995, 6, 135–140. 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]